Significance
A better understanding of how central B cell tolerance operates in human B cells can lead to developing approaches that reduce bone marrow egress of autoreactive B cell clones and their burden in circulation. Using a human immune system mouse model, this study characterizes the phenotype of human autoreactive immature B cells undergoing central tolerance and shows that cells with a similar but more subtle phenotype are present within fresh human bone marrow tissue. Treatment of mice with a pharmacologic antagonist of CXCR4, one of the markers of central B cell tolerance, demonstrates CXCR4 is necessary to prevent autoreactive human and mouse B cell clones from egressing the bone marrow, thus contributing to the establishment of tolerance.
Keywords: B cell tolerance, hu-mice, autoimmunity, CXCR4
Abstract
Central B cell tolerance, the process restricting the development of many newly generated autoreactive B cells, has been intensely investigated in mouse cells while studies in humans have been hampered by the inability to phenotypically distinguish autoreactive and nonautoreactive immature B cell clones and the difficulty in accessing fresh human bone marrow samples. Using a human immune system mouse model in which all human Igκ+ B cells undergo central tolerance, we discovered that human autoreactive immature B cells exhibit a distinctive phenotype that includes lower activation of ERK and differential expression of CD69, CD81, CXCR4, and other glycoproteins. Human B cells exhibiting these characteristics were observed in fresh human bone marrow tissue biopsy specimens, although differences in marker expression were smaller than in the humanized mouse model. Furthermore, the expression of these markers was slightly altered in autoreactive B cells of humanized mice engrafted with some human immune systems genetically predisposed to autoimmunity. Finally, by treating mice and human immune system mice with a pharmacologic antagonist, we show that signaling by CXCR4 is necessary to prevent both human and mouse autoreactive B cell clones from egressing the bone marrow, indicating that CXCR4 functionally contributes to central B cell tolerance.
The bone marrow tissue is the major site of postnatal B cell development in both mice and humans (1, 2). B lymphopoiesis begins when hematopoietic stem cells (HSCs) differentiate into common lymphoid progenitors that commit to the B cell lineage by developing into precursors of B cell progenitors (3). These precursors undergo a step-by-step differentiation process that is accompanied by the rearrangement of V, D, and J gene segments at the immunoglobulin (Ig) heavy chain locus first and then, after a heavy (H) chain is expressed, of V and J segments at the Ig light chain loci, Igκ and Igλ (4). The de novo synthesized H and L Ig chains pair with the signaling molecules CD79A and CD79B to form a B cell receptor (BCR) that is expressed on the cell surface as IgM and that functions as antigen receptor (5). These newly generated IgM+ B cells, which are identified as immature B cells, undergo further differentiation into transitional B cells that enter the circulation and complete their maturation in the spleen (6).
A feature of B cell development is that the assembly of the Ig H and L chain genes occurs through random reassortment of numerous V(D)J gene segments, which facilitates the generation of a vast number of antibody specificities, one per each developing B cell (4). While this feature is crucial to the development and maintenance of a B cell population and antibody repertoire capable of recognizing any pathogen, the disadvantage is that the majority of these V(D)J gene sequences encode antibodies that are self-reactive (7, 8). It is well established that the entry of newly generated autoreactive B cell clones into the peripheral tissue is restricted by the physiological process of tolerance, a process that evolved to reduce the chance of autoantibody responses and autoimmunity. Indeed, antibody repertoire studies in mice and humans as well as studies with Ig transgenic and knock-in mice have amply demonstrated that the transition from the bone marrow immature B cell stage to the peripheral B cell stage is accompanied by a significant decrease (about twofold) in the frequency of autoreactive clones (8, 9), and this is because clones with BCRs that exhibit high avidity for self-antigens are prevented from entering the peripheral B cell population (10–12).
The process of central B cell tolerance has been mainly characterized in mouse models where it operates via the maintenance of RAG1/2-mediated VJ recombination at the light chain loci (i.e., receptor editing) and by inducing cell death (i.e., clonal deletion) in clones in which receptor editing fails to provide a nonautoreactive specificity within a few days (13–16). A major bottleneck in studying central B cell tolerance in humans is the difficulty of acquiring fresh bone marrow samples and the inability to identify and distinguish autoreactive from nonautoreactive B cell clones. Single-cell cloning of Ig genes from newly formed bone marrow B cells that emigrated into the blood, together with the expression and testing of the antibodies they encode, have provided estimates of the efficiency of central B cell tolerance in humans (7, 17). These studies have elegantly shown that central B cell tolerance is significantly less efficient in many autoimmune patients, and particularly in those with systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), type-1 diabetes (T1D), and Sjögren’s syndrome (18–22). These studies have further shown that the genetic variant R620W of the PTPN22 protein tyrosine phosphatase, a variant linked to a higher risk for the development of autoimmunity, is also associated with higher frequencies of autoreactive/polyreactive clones among the new emigrant transitional B cells, revealing a defective central tolerance checkpoint in individuals carrying this risk allele (23).
To elucidate mechanisms of development and tolerance of human B cells, our group has investigated human immune system humanized mice (HIS hu-mice) in which human B lymphocytes develop subsequent to the engraftment of human umbilical cord blood HSCs (24, 25). With this intent, we have previously created a HIS hu-mouse model in which all mouse cells express a synthetic membrane-bound self-antigen (Hcκ) that reacts at high avidity with developing human Igκ+ B cells (26). In this model, all human κ+ B cells are autoreactive and undergo central tolerance in the bone marrow via a mix of receptor editing and clonal deletion (26).
In the present study we aimed to exploit this HIS hu-mouse model to discover markers that distinguish human autoreactive immature B cells from nonautoreactive cells, as well as to identify pathways that contribute mechanistically to the enforcement of central B cell tolerance. Our data show that human autoreactive immature B cells contrast from nonautoreactive cells by up-regulating CD69 and CXCR4 while downmodulating the expression of IgM, CD19, CD81, and BAFFR as well as maintaining lower ERK activation. Cells with a similar phenotype, although more subtle, were also observed within the developing immature B cell population of human bone marrow specimens. Moreover, small differences in the expression of these markers and in the amount of sera autoantibodies were found in HIS hu-mice generated with HSCs from some donors genetically predisposed to autoimmunity. Finally, our data demonstrate that retention of human autoreactive B cells in the bone marrow does not rely on CD69 expression while is critically dependent on CXCR4 signaling, indicating that the CXCR4–CXCL12 pathway enforces central B cell tolerance.
Results
Differential Expression of Several Markers Distinguishes Autoreactive from Nonautoreactive Human Immature B Cell Clones.
To determine diagnostic and functional differences between autoreactive and nonautoreactive newly generated human B cells, we employed the Hcκ+ HIS hu-mouse model in which human B cells develop in the bone marrow of immunodeficient mice following the transplantation of human cord blood HSCs (26). In these Hcκ+ hu-mice, all κ+ immature B cells that emerge at the completion of the IgL VJ recombination process (about 50% of all human immature B cells) are de facto high-avidity autoreactive clones that undergo central tolerance, while λ+ B cells are nonautoreactive toward the Hcκ self-antigen (26).
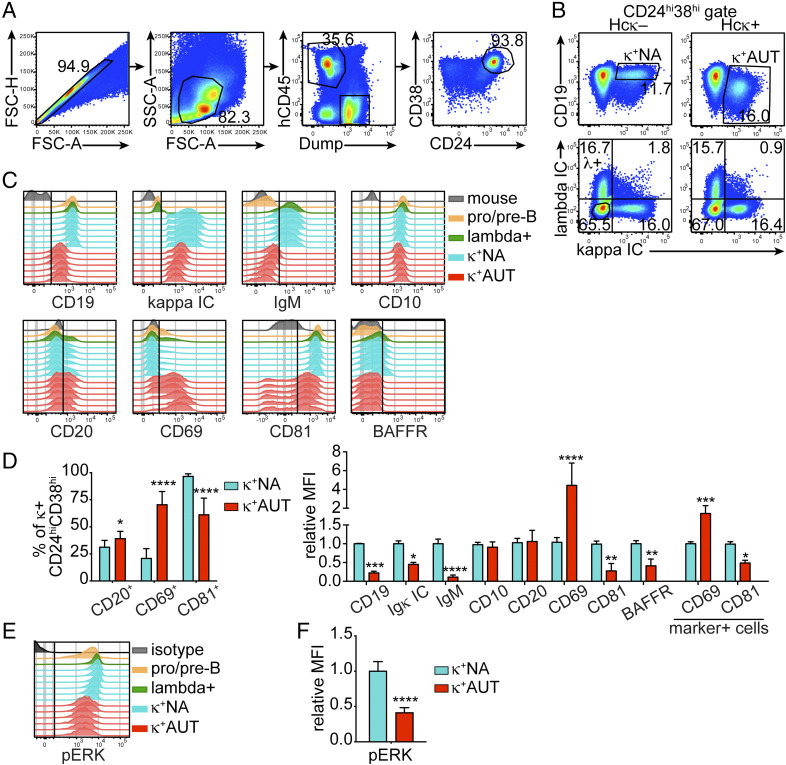
Groups of Hcκ+ and Hcκ− hu-mouse littermates were reconstituted with cord blood HSCs obtained from different donors, and bone marrow cells from these mice were analyzed by flow cytometry 13 to 15 wk after HSCs engraftment. A flow cytometry staining panel was developed to exclude unwanted cells and to gate on hCD45+ CD24hiCD38hi developing human B cells, including pro-B, pre-B, and immature B cells (27) (Fig. 1A and SI Appendix, Fig. S1A). The bone marrow CD24hiCD38hi cell population of HIS hu-mice comprises almost exclusively CD19+ B cells and only few CD19−/low cells (SI Appendix, Fig. S1A). CD20 is expressed only by a minority of these cells, as it is up-regulated asynchronously between the pre-B and the transitional B cell stages (26). As previously shown (26), the autoreactive human κ+ B cells developing in Hcκ+ hu-mice display almost complete internalization of the autoantigen-bound (IgM) BCR and significant downmodulation of CD19, characteristics similar to mouse B cells undergoing central tolerance (28–30). Consequently, we used CD19 surface levels and intracellular (IC) Igκ (corresponding to total surface and IC Igκ) to gate CD19low κ+ autoreactive immature B cells among the CD24hiCD38hi-developing B cells in Hcκ+ hu-mice, and CD19hi κ+ nonautoreactive immature B cells in Hcκ− hu-mice (Fig. 1B). We additionally analyzed human λ+ immature B cells and κ−λ− pro-B/pre-B cells (Fig. 1B, bottom plots) as well as hCD45−Dump+ mouse cells (Fig. 1A) as control populations. These cell populations were investigated for the expression of some molecules known to mark developmental changes in human B cells, mouse immature B cells that engage or do not engage self-antigen, or T cells during thymic selection.
Fig. 1.
Analysis of Hcκ transgenic hu-mice uncovers markers that distinguish human autoreactive from nonautoreactive immature B cells. Bone marrow cells from Hcκ− and Hcκ+ HIS hu-mice were analyzed by flow cytometry 13 to 15 wk after engraftment of hHSCs. (A) Strategy for gating human CD24hiCD38hi developing B cells in the bone marrow of HIS hu-mice (SI Appendix, Fig. S1A). The dump channel excludes cells positive for hCD3 (human T cells), hCD14 and hCD33 (human monocytes and macrophages), mCD45 (mouse hematopoietic cells), and dead cells. (B) Representative analysis of surface CD19 and IC Igκ (Top) and IC Igκ and Igλ (Bottom) in human CD24hiCD38hi B cells from bone marrow of hu-mice. The top plots display the gating of CD19hi κ+ nonautoreactive (κ+NA) B cells in Hcκ− bone marrow and of CD19low κ+ autoreactive (κ+AUT) B cells in Hcκ+ bone marrow. The bottom plots show the gating of pro/pre-B cells (circle gate in the κ–λ−, double-negative quadrant). (C) Histogram plots displaying expression of indicated molecules by the following cell populations: mouse cells (hCD45−Dump+), pro/pre B cells (CD24hiCD38hiκ−λ−), λ+ immature B cells from Hcκ+ (CD24hiCD38hiκ−λ+), κ+NA immature B cells (CD24hiCD38hiCD19hiκ+ from Hcκ−), and κ+AUT immature B cells (CD24hiCD38hiCD19lowκ+ from Hcκ+). Each histogram represents a mouse, and the data are from littermates transplanted with HSCs from the same cord blood donor. (D) Frequencies of marker-positive cells (Left) and relative MFI of indicated markers (Right) among the human κ+NA or κ+AUT CD24hiCD38hi B cells of Hcκ+ and Hcκ− hu-mice generated with different cord bloods. Markers were analyzed as shown in C. (E and F) Representative flow cytometric analysis of pERK in pervanadate-treated human B cell subsets (E) and relative MFI of pERK in κ+NA and κ+AUT immature B cells from Hcκ+ and Hcκ− hu-mice generated with different cord bloods (F). The isotype control–stained sample represents CD38hi cells from a Hcκ− hu-mouse. The MFIs for graphs in D and F were normalized by dividing the MFI values of κ+AUT and κ+NA cells from individual mice to the average MFI of κ+NA cells from Hcκ− mice analyzed on the same day. Results in all graphs are expressed as mean and SD. n = 10 Hcκ+ and 13 Hcκ− hu-mice generated with three or four different CBH samples, respectively. Statistical analysis was performed with two-way ANOVA multiple comparison test. *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
CD10 and CD20, which are up-regulated either before the pro-B cell stage or between the pre-B and transitional B cell stages, respectively (25, 31, 32), were similarly expressed by autoreactive and nonautoreactive human immature B cells (Fig. 1 C and D and SI Appendix, Fig. S1B), although there was a slightly higher frequency of CD20+ B cells within the autoreactive population (Fig. 1 D, Left). By contrast, and in accordance with our previous data (26), IgM was greatly downmodulated from the cell surface of human κ+ autoreactive immature B cells (Fig. 1 C and D and SI Appendix, Fig. S1B). The expression of BAFFR, the main receptor for the B cell growth hormone BAFF, was also evaluated because it is lower on mouse autoreactive immature B cells relative to nonautoreactive cells (33, 34). We similarly observed in hu-mice a diminished expression of BAFFR on autoreactive human B cells compared to nonautoreactive cells (Fig. 1 C and D).
One of the molecules we elected to analyze was CD69, a transmembrane C-type lectin protein that marks thymocytes engaging major histocompatibility complex molecules during positive and negative selection (35–37), but that has not been previously identified as a marker of B cell tolerance. Surprisingly, surface expression of CD69 was greatly increased (by about fourfold) on κ+ autoreactive cells of Hcκ+ hu-mice, and this manifested both as a higher frequency of CD69+ cells and higher amount expressed per cell (Fig. 1 C and D).
As previously indicated, downmodulation of the CD19 coreceptor is a recognized marker of self-antigen engagement during central B cell tolerance both in mice and hu-mice (26, 29, 30). To modulate BCR signaling, CD19 operates in complex with CD21, CD225, and the tetraspanin CD81 (38, 39). While CD225 and CD21 are only minimally expressed in developing B cells, CD81 is present throughout B cell development (40). Moreover, both in mice and humans, the complexing of CD81 and CD19 in the endoplasmic reticulum (ER) or Golgi is required for their traffic to the cell surface (41–45). Our analyses show that, similar to CD19, CD81 was greatly decreased on κ+ autoreactive B cells from Hcκ+ hu-mice relative to the nonautoreactive κ+ cells from Hcκ− animals (Fig. 1 C and D). However, the correlation between CD81 and CD19 was not present in all cells to the same extent (SI Appendix, Fig. S1 C, Left). Interestingly, CD81-negative cells were more prevalently CD20+ (i.e., cells further along development) compared to CD81low/+ cells. CD69 was also expressed by about 30% of nonautoreactive cells that were, for the most part, CD20− (SI Appendix, Fig. S1 C, Center). In addition, CD81 was highly expressed by most pro-B/pre-B cells, but about 20% of these cells displayed low levels of CD19 and 50 to 70% expressed CD69 (SI Appendix, Fig. S1 C, Right).
Because expression of CD81 and CD69 has not been explicitly reported in mouse autoreactive and nonautoreactive immature B cells, we measured CD19, CD69, and CD81 on bone marrow immature B cells from the 3-83Igi mouse model of tolerance (46). CD81 was expressed at lower levels on autoreactive than nonautoreactive B cells (SI Appendix, Fig. S2C), but the difference was smaller than observed in human B cells. In contrast, CD69 was virtually not expressed by mouse immature B cells, although a closer analysis revealed CD69 mean fluorescence intensity (MFI) was almost twofold higher on autoreactive B cells (SI Appendix, Fig. S2C).
We have previously shown that ERK activation, as represented by the amount of phosphorylated ERK (pERK) in cells treated with pervanadate, is higher in nonautoreactive than autoreactive mouse immature B cells (34, 47). In agreement with the murine data, human autoreactive immature B cells displayed less than half the amount of pERK observed in nonautoreactive cells (Fig. 1 E and F).
In summary, human autoreactive immature B cells that develop in HIS hu-mice display lower levels of IgM, CD19, CD81, BAFFR, and pERK, and higher amounts of CD69 relative to nonautoreactive B cells. These differences were consistently observed in animals reconstituted with CD34+ cells from separate cord blood samples (SI Appendix, Fig. S1B), thus representing a distinctive phenotype that potentially enables the identification of human autoreactive B cells undergoing central tolerance.
Human CD19low Immature B Cells in Wild-Type hu-Mice and Human Bone Marrow Display a Phenotype Consistent with Self-Reactivity.
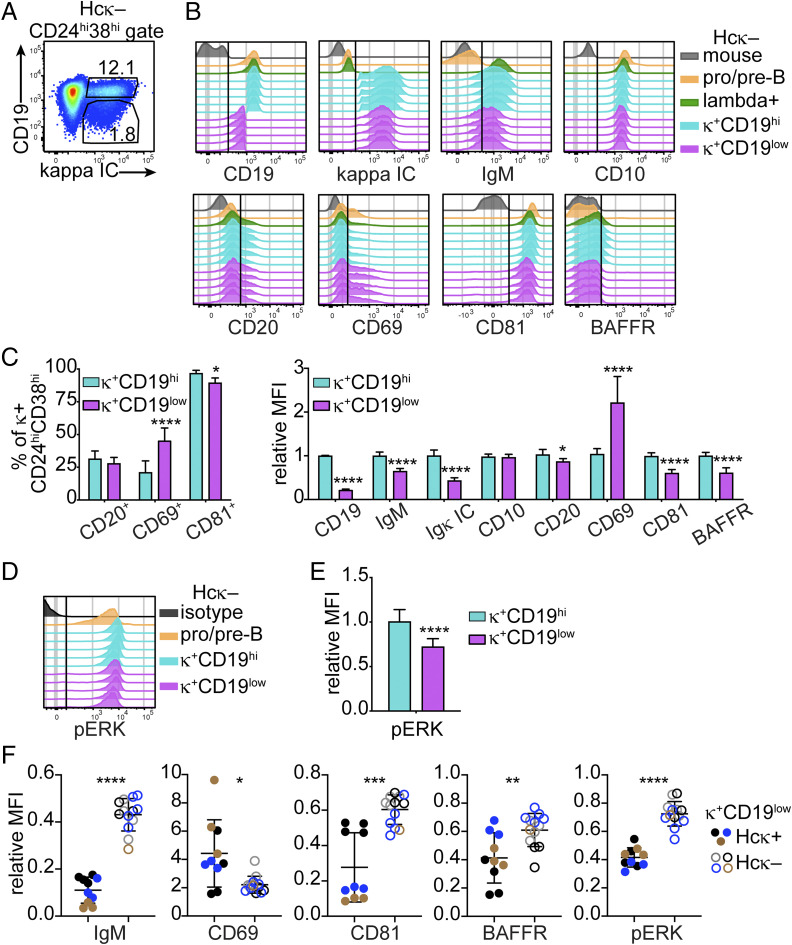
Published studies have estimated that about 30% of human and mouse bone marrow immature B cells produce BCRs with reactivities for natural self-antigens sufficient to trigger central tolerance (7, 48). To investigate the presence of these cells in hu-mice, we compared the expression of tolerance markers by (κ+ or λ+) CD19hi and CD19low immature B cells from wild-type (Hcκ−) hu-mice, assuming that low CD19 marks autoreactive B cells undergoing central tolerance.
The κ+ CD19low immature B cells from Hcκ− hu-mice (Fig. 2A) displayed significantly lower expression of surface IgM, CD81, BAFFR, and total (IC) Igκ compared to CD19hi cells (Fig. 2 B and C). Moreover, the frequency of CD69+ cells and the mean expression of CD69 per cell (i.e., MFI) were significantly higher in the κ+ CD19low than the CD19hi population (Fig. 2 B and C). In agreement with the expression of surface markers, CD19low immature B cells also displayed lower amounts of pERK (Fig. 2 D and E). Notably, these relative phenotypic differences were consistently observed in groups of HIS hu-mice generated with different healthy human cord blood units (SI Appendix, Fig. S3A) as well as between CD19hi and CD19low λ+ B cell populations from the same animals (SI Appendix, Fig. S3 B–F). However, the differences observed between CD19hi and CD19low cells from Hcκ− hu-mice were significantly smaller than those in Hcκ+ hu-mice (Fig. 2F).
Fig. 2.
CD19low human immature B cells from wild-type (Hcκ−) hu-mice show a phenotype consistent with autoreactive κ+ immature B cells from Hcκ+ hu-mice. (A) Gating strategy for CD19low and CD19hi κ+ immature B cells within the human CD24hiCD38hi bone marrow cell population of Hcκ− HIS hu-mice. CD24hiCD38hi cells were gated as shown in Fig. 1A. (B) Representative histogram plots comparing the expression of indicated markers by CD19low and CD19hi κ+ immature B cells and control cell populations from Hcκ− hu-mice. Each histogram represents a mouse from a group of littermates transplanted with the same cord blood HSCs. (C) Frequencies of marker-positive cells (Left) and relative MFI of indicated markers (Right) among the human CD19low and CD19hi κ+ (CD24hiCD38hi) immature B cells from groups of Hcκ− hu-mice generated with distinct cord blood HSCs. (D and E) Representative flow cytometric histograms of pERK analysis in pervanadate-treated human B cell subsets from Hcκ− hu-mice from one cord blood group (D), and statistical analysis of pERK MFI from Hcκ− hu-mice from multiple cord blood groups (E). Each histogram in D represents a mouse, and the isotype control shows CD38hi cells from one mouse. (F) Relative MFI of indicated markers expressed by κ+AUT (CD19low) immature B cells from Hcκ+ hu-mice (filled circles) and by κ+CD19low cells from Hcκ− hu-mice (open circles). Each symbol represents a hu-mouse, and colors identify hu-mice generated with different cord blood samples. The MFIs for graphs in C, E, and F were normalized by dividing the MFI values of CD19hi or CD19low κ+ cells of individual hu-mice to the average MFI of CD19hi κ+ cells from Hcκ− hu-mice analyzed on the same day. Results in all graphs are expressed as mean ± SD. n = 10 Hcκ+ and 13 Hcκ− hu-mice generated with three or four different CBH samples, respectively. Statistical analysis was performed with two-way ANOVA multiple comparison test. *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
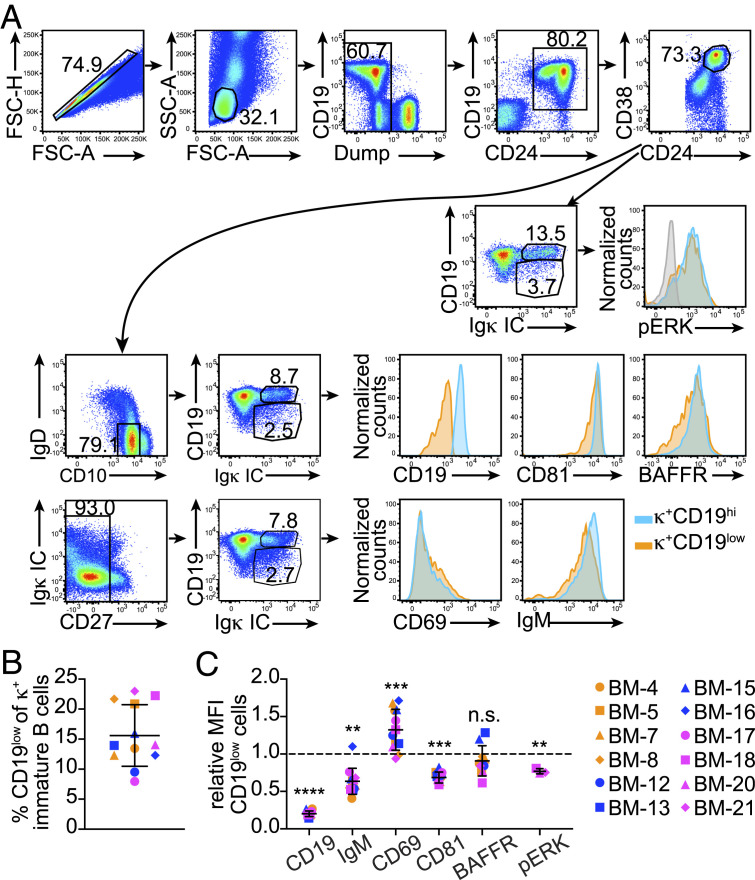
In order to resolve whether the phenotype observed for human autoreactive immature B cells occurs physiologically, we examined B cells from 12 pediatric bone marrow samples isolated from patients ranging in age from 8 mo to 16 y (SI Appendix, Table S1 and Fig. 3A). As shown in Fig. 3B, κ+CD19low cells in human bone marrow were about 15% of all κ+ immature B cells, on average (range: 7.9 to 22.9%). In addition, these κ+CD19low cells expressed up to twofold higher amounts of CD69 and as much as 1.5-fold lower amounts of CD81 and IgM relative to CD19hi cells, with variations from sample to sample (Fig. 3C). Levels of BAFFR were not overall significantly different between CD19low and CD19hi κ+ cells, although they were lower on CD19low cells from 8 out of 10 bone marrow biopsy specimens (Fig. 3C). Furthermore, lower amounts of pERK were observed in CD19low relative to CD19hi κ+ cells in the specimens analyzed for this marker (Fig. 3C).
Fig. 3.
The immature B cell population in human bone marrow samples include immature B cells exhibiting a mild tolerance phenotype. (A) Serial gating strategy for the analysis of human CD19low and CD19hi B cells within the immature B cell population of human bone marrow. This analysis is representative of a total of 12 pediatric bone marrow biopsy specimens (SI Appendix, Table S1). (B) Frequency of κ+CD19low cells among all κ+ cells within the CD24hiCD38hiIgD−CD10+ immature B cell population in human bone marrow biopsy specimens. (C) MFI of indicated molecules in κ+CD19low cells relative to that of κ+CD19hi cells in each human bone marrow sample. Results in graphs are expressed as mean ± SD. Statistical analysis was performed with a two-tailed paired t test. n = 12, except for BAFFR and pERK groups where the n = 10 and 3, respectively. **P < 0.01; ***P < 0.001; ****P < 0.0001; and n.s.= not significant.
Overall, these data show the existence of naturally occurring immature B cells with differential expression of IgM, CD19, CD81, BAFFR, and CD69, a phenotype potentially identifying human immature B cells reacting with natural bone marrow self-antigens.
HSCs from Some Autoimmune-Prone Infants Lead to Differences in the Expression of B Cell Tolerance Markers.
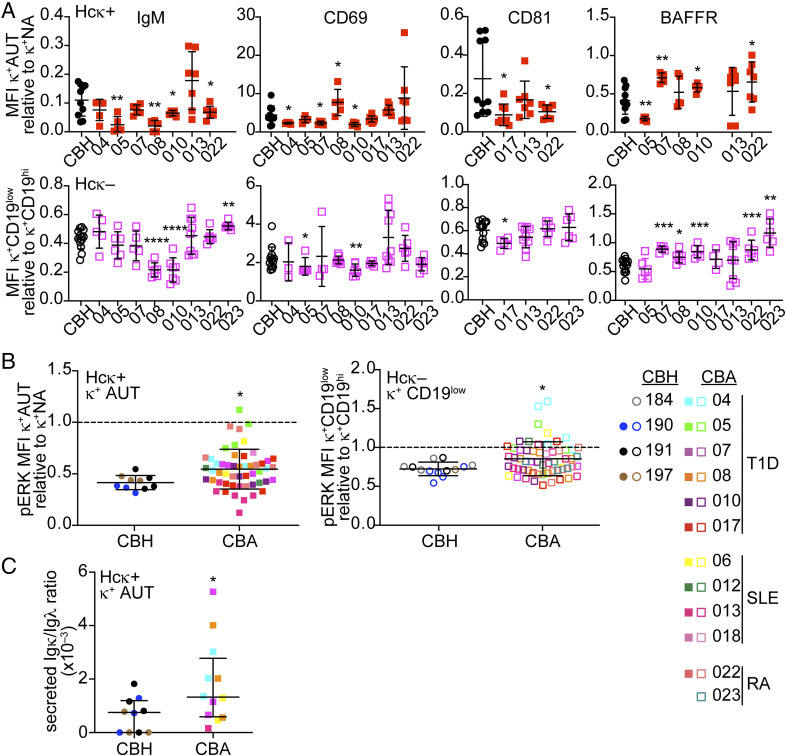
Several autoimmune diseases manifest with a reduced stringency in central B cell tolerance (49). Thus, we next used autoimmune-prone cord blood samples to investigate if these lead to changes in the expression of the B cell tolerance markers in hu-mice. To this aim, we enrolled pregnant women with a diagnosis of T1D, SLE, or RA and collected the umbilical cord blood after they gave birth (SI Appendix, Table S2). Among the 12 autoimmune mothers that donated cord blood to our study, 8 (66.7%) indicated autoimmunity existed among consanguineous family members, supporting the existence of a genetic component in disease onset (50). One of the risk alleles for autoimmunity is the PTPN22 variant R620W, a variant that has been linked to defects in central B cell tolerance (23). One of the cord blood samples was positive for this autoimmune-related genetic variant (CBA-05; SI Appendix, Table S2) upon PCR screening (51). HSCs from the “autoimmune-prone” cord bloods (CBAs) were used to reconstitute Hcκ+ and Hcκ− littermates, and B lineage cells from these HIS hu-mice were analyzed similar to those reconstituted with “healthy” (CBH) cord blood.
Our analyses did not reveal any overall significant difference in the expression of surface markers on bone marrow CD19low κ+ immature B cells when hu-mice were assessed together across all CBA donors (SI Appendix, Fig. S4A). However, we noticed significant differences when we compared the CD19low κ+ immature B cells of individual “autoimmune” cord blood groups (for which we had observations from three or more mice) to cells from the CBH groups. Hu-mice from CBAs 05, 08, 10, and 022 developed B cells with abnormal expression of at least three markers when compared to CBH B cells (Fig. 4A). For CBAs 05, 08, and 10, we also observed abnormal frequencies of CD19low cells within the CD20+ cell population (SI Appendix, Fig. S4B), a parameter with larger intergroup variation in hu-mice generated with “healthy” samples (26). In addition to these differences, CD19low κ+ cells from some CBA hu-mice groups (CBA 5 and 22 in Hcκ+ mice and 4, 5, 10, 13, and 22 in Hcκ− mice) exhibited significantly higher amounts of pERK compared to cells in CBH animals (Fig. 4B). Interestingly, cord blood CBA-05, which carried the autoimmune-associated PTPN22 W620 variant, developed into B cells with altered levels of several markers, such as IgM, CD69, BAFFR, and pERK MFIs, and the frequency of CD20+ cells with CD19-low expression.
Fig. 4.
HSCs from some autoimmune-prone newborn infants lead to differences in the expression of B cell tolerance markers in hu-mice. HIS hu-mice were generated with HSCs enriched from cord blood samples collected from newborns of either healthy or autoimmune mothers. (A) Relative MFI of indicated markers among the CD19low κ+ human immature B cells from Hcκ+ hu-mice (Top) or Hcκ− hu-mice (Bottom) that were generated with cord blood from newborns of either “healthy” (circles) or distinct “autoimmune” (squares) mothers. CBA-05 was a carrier of the PTPN22 W620 genetic variant. Groups of hu-mice generated with different CBA samples are separated in the graphs, while those generated with CBH samples are displayed in the same group. Only CBA groups with 3 or more observations were plotted in the graphs. CD19low CD24hiCD38hi immature B cells were gated as shown in Figs. 1 A and B and 2A. Filled and empty symbols represent Hcκ+ and Hcκ− hu-mice, respectively. (B) Relative MFI of pERK in pervanadate-treated human CD19low κ+ human immature B cells from Hcκ+ (Left) and Hcκ− (Right) hu-mice generated with CBH or CBA cord bloods. Each symbol is a mouse and symbols and colors are the same as in A. The MFIs for graphs in A and B were normalized by dividing the MFI values of CD19low κ+ cells of individual mice to the average MFI of CD19hi κ+ cells from hu-mice analyzed on the same day. (C) Ratio of secreted Igκ to Igλ in serum of individual Hcκ+ hu-mice generated with CBH or CBA cord blood samples. Results in all graphs are expressed as mean ± SD. n = 10 Hcκ+ and 13 Hcκ− hu-mice generated with three or four different CBH samples, respectively. n = 53 Hcκ+ hu-mice and 58 Hcκ− hu-mice generated with HSCs from 11 or 12 CBA individual cord blood samples, respectively. Statistical analysis was performed with two-way ANOVA multiple comparison test. *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
In order to investigate whether a potential genetic predisposition to autoimmunity leads to defects in peripheral tolerance, we compared secreted Igκ in the sera of Hcκ+ hu-mice. Because serum Ig is highly variable and often not detected in this model (26), we normalized the Igκ titers to those of Igλ. In accordance with our previous studies (26), tolerance was generally robust, as we could only measure low amounts of Igκ in sera from Hcκ+ hu-mice. Nevertheless, Hcκ+ hu-mice generated with “autoimmune” cord blood displayed higher Igκ serum titers compared to mice reconstituted with “healthy” cord blood (Fig. 4C).
In summary, these data suggest that HIS hu-mice can reveal individual variations in central and peripheral B cell tolerance that may relate to the development of autoimmunity.
CD69 Is Not Necessary to Retain Human Autoreactive B Cells in the Bone Marrow.
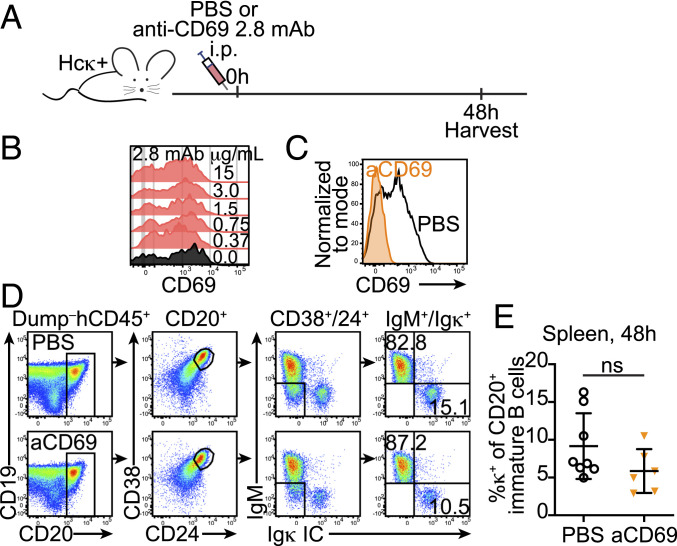
It has been shown that CD69 mediates the retention of developing T cells in the thymus (52–54), and when overexpressed, it leads to a reduction of immature B cells in the blood of mice (55). These findings suggested that the elevated expression of CD69 on autoreactive immature B cells of HIS hu-mice increased their retention in the bone marrow tissue. To test this hypothesis, we used an anti-human CD69 monoclonal antibody (clone 2.8) that causes CD69 down-regulation and promotes hematopoietic cells to egress from the bone marrow (56).
Cohorts of Hcκ+ HIS hu-mice were injected with 500 μg of anti-hCD69 2.8 mAb or phosphate-buffered saline (PBS) as control, and the blood and spleen of mice were analyzed 2 d after treatment (Fig. 5A), a time selected based on past studies (56) and to maximize the chance of observing released immature B cells prior to their potential death. The efficacy of the mAb treatment in causing CD69 down-regulation was verified by staining for CD69 with a different anti-CD69 Ab clone, FN50 (Fig. 5B). CD69 was below detection on cells from hu-mice treated with anti-hCD69 2.8 mAb while it was readily detected on cells of PBS-treated animals (Fig. 5C). At both 24 and 48 h after treatment, κ+ B cells were too scarce in the blood for a reliable analysis. Thus, we focused our analysis on spleen cells and, specifically, on CD20+ B cells. This was done to increase the capture of immature B cells released from the bone marrow and to exclude (CD20−) B cells developing in situ in the spleen (26). CD20+ spleen cells were further discriminated to identify immature CD24hiCD38hi B cells that were positive for surface IgM or for IC Igκ (Fig. 5D). However, despite significant downmodulation of surface CD69, the frequencies of κ+ cells among the splenic immature B cells were similar in PBS and anti-CD69–treated Hcκ+ hu-mice (Fig. 5E).
Fig. 5.
Blocking CD69 does not release human κ+ autoreactive immature B cells from the bone marrow of hu-mice. (A) A schematic of the experiment. Hcκ+ HIS hu-mice (13 to 15 wk after birth and HSC engraftment) were injected IP with either PBS or 500 μg of the anti-hCD69 2.8 mAb. After 48 h following treatment, tissues were harvested for flow cytometric analysis. (B) Bone marrow cells from an Hcκ+ HIS hu-mouse were incubated with different concentrations of the anti-hCD69 2.8 mAb for 30 min in ice, washed, and then stained with the anti-hCD69 clone FN50 and other antibodies for flow cytometric detection. The histograms represent CD24hiCD38hiCD19lowIgκ+ cells and show that CD69 FN50 detection is not blocked by the binding of the anti-CD69 2.8 mAb. (C) Representative histogram overlay showing CD69 detection on bone marrow CD20+ B cells from Hcκ+ HIS hu-mice treated 48 h earlier with either PBS or the anti-hCD69 2.8 mAb. (D) Representative gating strategy for the analysis of κ+ B cells in the spleens of Hcκ+ HIS hu-mice treated with either PBS or anti-hCD69 2.8 mAb. The serial gating was performed to gate CD20+CD24hiCD38hi surface IgM+ or IC Igκ+ immature/transitional B cells. (E) Frequency of κ+ cells among splenic CD20+CD24hiCD38hi immature/transitional B cells from Hcκ+ HIS hu-mice treated 48 h earlier with either PBS or the anti-hCD69 2.8 mAb. Each symbol represents a mouse, and the bars indicate the mean and SD. n = 8 Hcκ+ hu-mice treated with PBS and 6 Hcκ+ hu-mice treated with the anti-hCD69 2.8 mAb from three independent experiments. Statistical analysis was performed with a two-tailed Mann–Whitney U test; ns = not significant.
CD69 mediates tissue retention of lymphocytes by causing downmodulation of the sphingosine-1-phosphate receptor S1PR1 (52, 57, 58). In agreement to studies in mice (57, 58), S1PR1 was expressed reciprocally to surface CD69 on human T cells from hu-mice (SI Appendix, Fig. S5 A–C). In addition, the frequency of κ+ autoreactive B cells expressing S1PR1 was twofold smaller than that in the nonautoreactive population (SI Appendix, Fig. S5 A–D). The proportion of S1PR1+ immature B cells was very small, however, reaching only about 4% of nonautoreactive cells (SI Appendix, Fig. S5D). Moreover, injection of the anti-CD69 2.8 mAb did not increase the frequency of S1PR1+ cells (or the cell level of S1PR1) either among the T or the immature B cells (SI Appendix, Fig. S5 B, C, and E).
These data indicate that surface downmodulation of CD69 does not promote the egress of autoreactive immature B cells from the bone marrow of hu-mice, possibly because of a negligeable expression of S1PR1 in this process.
CXCR4 Is Key to the Retention of Human and Mouse Autoreactive B Cells in the Bone Marrow.
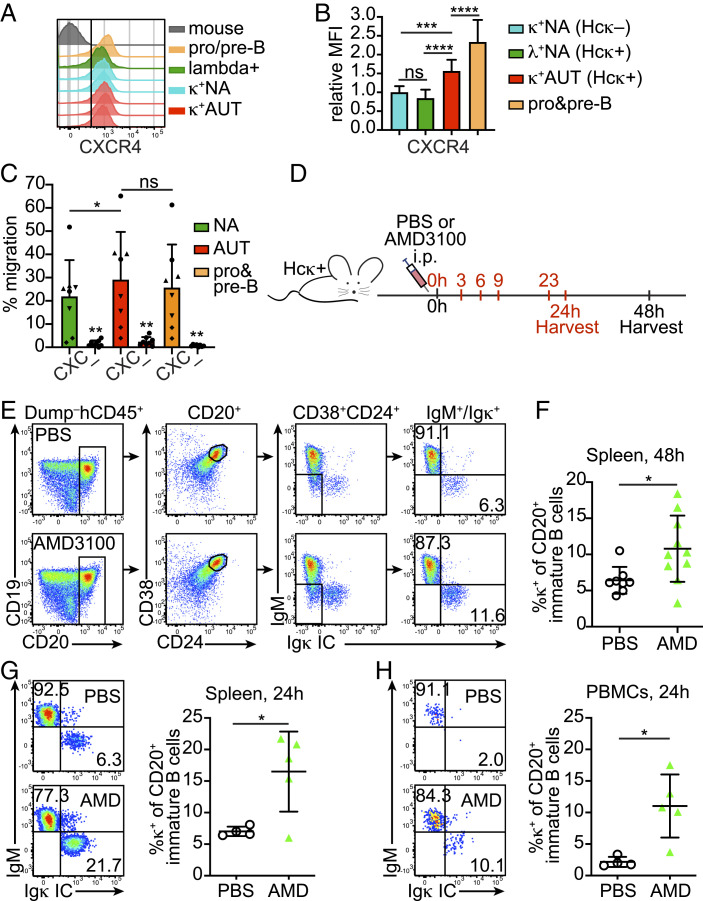
The chemokine receptor CXCR4 and its CXCL12 chemokine ligand play a major role in the retention of hematopoietic cells in the bone marrow of both mice and humans (59–61). Mouse developing B cells rely on CXCR4 to navigate through both cell development and bone marrow niches while their CXCR4 expression decreases from the pre-B to the immature B cell stage, at which point cells become able to egress into sinusoid capillaries (62–64). Moreover, and relevant to this study, immature B cells have higher CXCR4 expression when engaging antigen (64). Thus, we first questioned whether CXCR4 is differentially expressed by human immature B cells that engage or not self-antigen. Indeed, flow cytometric analysis showed that autoreactive κ+ immature B cells from Hcκ+ HIS hu-mice expressed CXCR4 at 1.5-fold the levels of nonautoreactive λ+ cells in the same animals, or κ+ cells in Hcκ− hu-mice (Fig. 6 A and B). In comparison, CXCR4 on (CD19+IgM−) pro-B/pre-B cells was about twofold higher than on nonautoreactive immature B cells (Fig. 6B). Although these represent small differences in expression, they are comparable to those described in mouse bone marrow B cells where they mediate differences in migration to CXCL12 (64–66). Indeed, when tested in a transwell assay, CD19low autoreactive immature B cells from Hcκ+ hu-mice migrated slightly better toward CXL12 than CD19high nonautoreactive B cells (Fig. 6C and SI Appendix, Fig. S6A).
Fig. 6.
CXCR4 blockade leads to the release of human autoreactive immature B cells into the peripheral tissue of hu-mice. (A) Representative analysis of CXCR4 expression by bone marrow κ+AUT (CD19low) and κ+NA (CD19high) CD24hiCD38hi B cells from Hcκ+ and Hcκ− hu-mice, respectively. Mouse cells (hCD45−Dump+), pro/pre-B cells (CD24hiCD38hiκ−λ−), and λ+ B cells are shown for comparison. Cells were gated as shown in Fig. 1 A and B. (B) Relative MFI for CXCR4 staining of CD24hiCD38hi κ+NA cells from Hcκ− hu-mice, κ+AUT cells from Hcκ+ hu-mice, λ NA cells from Hcκ+ hu-mice, and pro/pre-B cells from both Hcκ+ and Hcκ− hu-mice. The relative MFIs were calculated by dividing the MFI values of the indicated cell subsets of individual mice to the average MFI of κ+NA cells from hu-mice analyzed on the same day. The graph shows the mean and SD. n = 13 Hcκ+ and 8 Hcκ−, analyzed during three independent experiments. (C) Chemotaxis of indicated bone marrow B cell populations from Hcκ+ HIS hu-mice toward CXCL12 (CXC) or media (–). Autoreactive and nonautoreactive immature B cells and pro/pre-B cells were gated based on surface marker profile as shown in SI Appendix, Fig. S6A. The bar graph represents % migration (mean and SD) of cells from 4 Hcκ+ hu-mice tested in duplicate wells and over two experiments. Each mouse is identified by a different symbol. Statistical analysis was performed with a two-tailed paired t test. (D) A scheme depicting the timeline of Hcκ+ HIS hu-mice treatment with either one injection (in black) or five injections (in red) of 100 μg of AMD3100 or PBS control. (E and F) Representative gating strategy (E) and analysis (F) of CD20+CD24hiCD38hi surface IgM+ or IC Igκ+ B cells in the spleen of Hcκ+ HIS hu-mice injected once 48 h earlier with either PBS or AMD3100. Each symbol represents a mouse and the bars indicate the mean and SD. n = 8 Hcκ+ hu-mice treated with PBS and 10 Hcκ+ hu-mice treated with AMD3100 from four independent experiments. (G and H) Flow plots showing representative IgM and Igκ staining of CD20+CD24hiCD38hi immature B cells in the spleen (G) and blood (H) of Hcκ+ hu-mice injected five times with either AMD3100 or PBS (gated as in E). Graphs display frequencies of κ+ cells in these populations. Each symbol represents a mouse and the bars are mean and SD. n = 4 Hcκ+ hu-mice treated with PBS and 5 Hcκ+ hu-mice treated with AMD3100 over two independent experiments. Statistical analysis in B, F, G, and H was performed with an unpaired two-tailed t test with Welch’s correction. *P < 0.05, ***P < 0.001; and ****P < 0.0001.
To investigate the contribution of CXCR4 to the retention of newly generated human autoreactive B cells within the bone marrow tissue, we treated Hcκ+ HIS hu-mice with AMD3100, a CXCR4 antagonist effective in both mice and humans and with a half-life of 2 to 3 h (56, 64, 67). The efficacy of AMD3100 was first confirmed by replicating previous findings (68) showing it causes a rapid increase of CD34+ cells and B cells in the blood of wild-type mice within 1 h from injection (SI Appendix, Fig. S6B). Two different approaches were next used to test the effect of CXCR4 blockade on autoreactive immature B cells of hu-mice (Fig. 6D). In the first approach, similar to the studies with the anti-CD69 Ab, Hcκ+ hu-mice were injected once with AMD3100 or PBS and spleens were analyzed 48 h after treatment. As shown (Fig. 6 E and F), a single injection of AMD3100 led to an increase of almost twofold in the frequency of κ+ autoreactive B cells within the splenic immature B cell population. In a second approach, hu-mice were injected five times with either AMD3100 or PBS over the course of 24 h and analyzed 1 h after the last injection and, thus, within the window of drug activity (Fig. 6D). Compared to PBS-injected hu-mice, the frequency of κ+ autoreactive B cells in AMD3100-treated animals was twofold and fivefold higher, on average, within the spleen (Fig. 6G) and blood (Fig. 6H) immature B cell populations, respectively.
Murine immature B cells express higher CXCR4 when they engage self-antigen (SI Appendix, Fig. S6C) (64, 69). To determine whether CXCR4 is also important in the bone marrow retention of mouse autoreactive B cells, 3-83Igi,H-2b mice were treated with AMD3100 or PBS either once or four times every few hours, and circulating B cells were analyzed 1 h after the last injection. Based on past studies, 3-83Ig+ autoreactive immature B cells are generally restricted to the bone marrow and identified as surface IgM−/low, CD2+CD19lowCD24high, and Igλ−IgD− (47, 69), while B cells in peripheral tissue are virtually all edited cells that do not express the 3-83 BCR (34, 46, 47, 69). The proportion of blood B220+CD24high immature B cells with a phenotype corresponding to autoreactive 3-83Ig+ B cells increased 6 to 14-fold after injecting AMD3100 when compared to PBS (SI Appendix, Fig. S6D), which is consistent with augmented release from the bone marrow tissue. These B cells also displayed lower expression of CD19 (SI Appendix, Fig. S6D).
Overall, these data demonstrate that CXCR4 functions to retain both human and mouse autoreactive B cells within the bone marrow tissue.
Discussion
By providing models in which B cells have a predetermined antigen specificity, Ig transgenic and knock-in animals have greatly aided our understanding of the hallmarks and mechanisms that define and mediate B cell tolerance in mice. Comparable knowledge of human B cell tolerance has lagged behind. This is particularly true for central B cell tolerance because this process takes place in the bone marrow, a tissue that is challenging to procure and study in humans. Single-cell antibody analyses of recent bone marrow immature B cell emigrants found in human blood have established that the central tolerance checkpoint is defective in many autoimmune patients (49), suggesting aberrant central B cell tolerance predisposes to autoimmune development. Thus, a better understanding of how central B cell tolerance operates in human B cells have implications for the development of future approaches that reduce bone marrow egress of autoreactive B cell clones and their burden in circulation. Aided by an established HIS mouse model of central tolerance (26), in this study we demonstrate that human autoreactive immature B cells can be discerned from nonautoreactive cells via a phenotype that includes higher expression of CXCR4 and CD69 and lower levels of CD19, CD81, IgM, BAFFR, and pERK. Most importantly we show that the CXCR4 chemokine receptor functions to prevent the egress of human and mouse autoreactive B cells from the bone marrow, thus contributing to our understanding of the mechanisms that regulate central B cell tolerance.
The underlying hypothesis of our study was that by defining molecules differentially expressed by autoreactive and nonautoreactive human immature B cells, we may discover not only a phenotype that defines human autoreactive B cell clones but also new pathways that contribute to human central B cell tolerance. Two molecules with decreased expression in human autoreactive immature B cells of HIS hu-mice are BAFFR and pERK. These molecules are also reduced in mouse autoreactive immature B cells (33, 34, 47), and we have previously shown that the expression of BAFFR positively correlates with ERK activation (33, 34). Thus, our finding that human immature B cells that bind self-antigens exhibit both reduced IgM and BAFFR and generate less active ERK suggests that a similar relationship exist in mouse and human developing B cells. Mechanistic studies in mice have shown that increasing either ERK activation and/or BAFFR expression does not alter the efficiency of central B cell tolerance (33, 34). Thus, these molecules may function more as markers than mediators of bone marrow B cell selection both in mice and humans.
Previous studies have demonstrated that both human (in hu-mice) and mouse autoreactive immature B cells exhibit low expression of the B cell–restricted molecule CD19 (26, 29). CD19 is known to complex with CD81, a tetraspanin protein expressed by B cells at all developmental stages. We show that CD19 and CD81 are both prominently downmodulated (by fourfold on average) on human κ+ autoreactive immature B cells. Reduced CD81 could potentially account for CD19 downmodulation, as surface expression of CD19 depends on its ability to complex with CD81 in the ER and/or Golgi (38, 39). However, the correlation between CD19 and CD81 was not precise, as about 20% of the κ+ autoreactive cells were CD81− although expressing similar or slightly higher CD19 amounts. Interestingly, a higher fraction of the CD81− than the CD81+ cells expressed CD20, a molecule that marks B cells further along in bone marrow development. Based on these findings, we infer that lower CD81 expression may correlate with the time autoreactive immature B cells spend undergoing central tolerance. We were surprised to find that while CD81 was also expressed on mouse immature B cells, the level of expression and the degree of downmodulation on autoreactive cells were much less compelling than those observed on human cells, suggesting CD19 downmodulation might be independent on CD81.
Another molecule that we show markedly defined autoreactive human B cells undergoing central tolerance in HIS hu-mice is CD69. CD69 has not been previously described as being modulated during central B cell tolerance, and it was expressed by human but not mouse autoreactive B cells. CD69 was also expressed by about half of human B lineage cells within the pro-B/pre-B cell fraction and by one-third of CD19hi κ+ immature B cells of Hck− hu-mice. We suggest these were likely immature B cells that had previously expressed an autoreactive BCR and were in the process of, or had successfully terminated, editing their autoreactive light chain. Alternatively, CD69 expression on pre-B cells may result from pre-BCR signaling. Nonetheless, the highest frequency of CD69+ cells as well as the highest degree of expression was observed on human immature B cells that were de facto autoreactive (e.g., κ+ B cells in Hcκ+ hu-mice). Thus, CD69 expression during human B cell development likely marks antigen engagement by the BCR.
Among immature B cells of Hcκ− hu-mice and human bone marrow biopsy specimens, we observed cells displaying a profile similar to that established for autoreactive immature B cells in Hcκ+ hu-mice. Specifically, these cells displayed downmodulation of CD19, IgM, CD81, BAFFR, and pERK and higher levels of CD69 than observed on CD19hi B cells in the same animal or biopsy specimen. This suggests these naturally occurring CD19low cells are human immature B cells that react with natural self-antigen(s). The degree of down or upmodulation of the autoreactive markers by CD19low immature B cells of Hcκ− hu-mice and human bone marrow, was significantly less than that displayed by CD19low κ+ B cells of Hcκ+ hu-mice, and it was also more variable from sample to sample. We hypothesize that, similar to what is described for IgM (70, 71), the degree of marker modulation may relate to the avidity of the BCR for the self-antigen. Expression of IgM, CD81, and pERK showed similar trends in CD19low cells of Hcκ− hu-mice and human bone marrow. Other markers (e.g., CD69 and BAFFR) showed less modulation in bone marrow biopsy specimens, and this may relate to the cell density and length of time at which cells were kept by the pathology laboratory before our analysis. Future studies that include probing the self-reactivity of antibodies cloned from single cells will be needed to test if naturally occurring CD19low cells are indeed autoreactive.
There has been a growing interest in using HIS hu-mice as models of autoimmune responses (reviewed in refs. 72 and 73). The reconstituted HIS in HIS hu-mice cannot recapitulate full-fledged autoimmune diseases (73), but these animal models could be used to dissect immunological processes that underlie the development of autoimmunity, including defects in B cell tolerance (74–76). Here, we used our HIS hu-mouse Hck model to determine whether HSCs isolated from umbilical cord blood of infants born from T1D, SLE, or RA autoimmune mothers developed into B cells with altered modulation of central tolerance markers. Although we did not expect that every newborn from whom we obtained cord blood had inherited a full complement of autoimmune risk alleles, we anticipated that a tangible fraction carried variants related to defects in central B cell tolerance. Indeed, immature B cells from hu-mice generated with 4 of the 12 cord blood units (3 from T1D and 1 from RA) showed small but significant alteration in the expression of multiple tolerance markers. Of notice, three “autoimmune” cord blood donors led to the generation of autoreactive B cells with very low CD69 expression, suggesting a possible defect in BCR signaling. B cells derived from two of these donors also displayed higher expression of BAFFR, which could lead to binding more BAFF and escaping anergy in peripheral tissue (77). Two autoimmune cord blood units were also associated to defective peripheral B cell tolerance, as indicated by higher titers of Igκ secreted antibodies detected in Hcκ+ hu-mice. Notably, among the four cord blood donors showing altered tolerance profile, one carried a copy of the PTPN22 W620 variant that, after HLA, has the strongest association with autoimmune development (78). Overall, these data support the idea that an altered tolerance phenotype of developing B cells may anticipate defects in central B cell tolerance. However, longitudinal studies that follow the development of autoantibodies and autoimmunity in cord blood donors are required to extrapolate the full significance of data collected in hu-mice.
Our study aimed also to explore B cell tolerance at the mechanistic level, and specifically to determine whether some of the autoreactivity markers we revealed represent pathways that function to enforce human central B cell tolerance. Here, we tested CD69 and CXCR4 because of their increased expression by autoreactive cells and their known function in tissue retention. Via mediating the downmodulation of the sphingosine-1-phosphate receptor S1PR1, CD69 helps to restrain developing thymocytes within the thymus (52–54), mature lymphocytes within peripheral lymphoid tissues (57), and developing B cells within the bone marrow parenchyma (55, 56, 79). Mouse studies have shown that both the CD69-dependent S1P receptors S1PR1 (55, 79) and the CD69-independent S1P receptor S1PR3 (80) promote entry of immature B cells into the sinusoids, likely facilitating their ingress into circulation (62). However, results from our studies indicate that CD69 surface expression is not necessary for the bone marrow retention of human autoreactive B cell clones because hu-mice treated or not with downmodulating anti-CD69 mAbs displayed similar frequencies of autoreactive B cells in their spleens. Flow cytometric analyses revealed that only 2 to 5% of immature B cells were S1PR1+ in hu-mice, and this frequency (and the S1PR1 level per cell) was not increased following Ab-mediated downmodulation of CD69. Although this was surprising based on previous reports (57, 58), it suggests that CD69 and S1PR1 do not play a substantive role in the bone marrow retention of autoreactive immature B cells and the egress of nonautoreactive cells. This likely extends to mouse autoreactive immature B cells as they display no meaningful expression of CD69.
Mouse studies have led to propose that downmodulation of CXCR4 and the subsequent decrease of CXCR4 signaling may contribute to the bone marrow egress of immature B cells (62, 64). Upon engaging antigen, mouse bone immature B cells up-regulate CXCR4 (64, 69), but whether variations in CXCR4 signaling are needed to discriminate the ability of nonautoreactive and autoreactive immature B cells to enter the blood has been unclear. Our studies in hu-mice demonstrate that human autoreactive immature B cells express 1.5-fold higher CXCR4 than nonautoreactive cells. By comparison, CXCR4 expression on pre-B cells was twofold higher than nonautoreactive cells, indicating CXCR4 is downmodulated when both pre-BCR and antigen-induced BCR signaling cease. Although these variations in CXCR4 expression are modest, other studies have demonstrated that 1.8 to twofold changes in CXCR4 levels on mouse bone marrow B cells reflect functional differences in chemotaxis toward CXCL12 (43, 64, 65). Indeed, autoreactive immature B cells from Hcκ+ hu-mice displayed slightly higher chemotaxis to CXCL12 in vitro relative to IgM+ nonautoreactive cells from the same tissue. More importantly, treatment of Hcκ+ hu-mice with the CXCR4 inhibitor AMD3100 resulted in a significant elevation of autoreactive B cell clones in the blood and/or spleen of hu-mice, depending on the timing of AMD3100 administration and related analysis. These findings were extended to developing mouse B cells by showing that AMD3100 treatment of autoreactive 3-83Igi,H-2b mice leads to a significant exit of autoreactive immature B cells from the bone marrow. Overall, these data demonstrate that CXCR4 signaling helps to retain (mouse and human) autoreactive B cell clones within the bone marrow tissue and functions as one of the gatekeepers of central tolerance. In mice, CXCR4 signaling also promotes transcription and rearrangements at the Igκ locus and Rag1/2 gene expression (66). Thus, it is also conceivable that the higher expression of CXCR4 on autoreactive B cells conduces to secondary Vκ-Jκ gene rearrangements that, together with Igλ gene rearrangements, are the hallmark of receptor editing. Future studies in people treated with AMD3100 could determine whether these findings extend to human B cells that physiologically develop in their natural host.
Overall, our study provides a set of markers that might distinguish human autoreactive B cell clones within an immature B cell population and offers evidence that the CXCR4 pathway contributes to central human and mouse B cell tolerance by restraining autoreactive immature B cells within the bone marrow tissue. Notably, defects in CXCR4 signaling have been implicated in many autoimmune diseases, mainly with a role in cell trafficking to inflamed tissues (81). Our data suggest the CXCR4–CXCL12 axis has a multifaceted contribution to autoimmunity, starting with central B cell selection.
Materials and Methods
Expanded Material and Methods are provided in SI Appendix, Supporting Materials and Methods. All data supporting the findings of these studies (and associated protocols and material) are available within the paper and SI Appendix.
Human Subjects and Samples.
Human studies were approved by the University of Colorado Institutional Review Board and were performed in accordance with the Declaration of Helsinki. Pediatric bone marrow biopsy specimens were provided by the Pathology laboratory of the Children’s Hospital of Colorado as de-identified samples (SI Appendix, Table S1). Healthy cord blood samples (CBH) were donated by the University of Colorado Cord Blood Bank at ClinImmune Labs (Aurora, CO) as de-identified samples. “Autoimmune” cord blood samples (CBA) were obtained after the consent of subjects (pregnant women) with a diagnosis of systemic lupus erythematosus, rheumatoid arthritis, or type-1 diabetes (described in SI Appendix, Table S2). These subjects were recruited at the Colorado University Hospital (UCH) either among Rheumatology pregnant patients or directly in the labor ward. Diagnostic PCR of the PTPN22 alleles encoding the R620 or W620 variants was performed according to a published protocol (51). CD34+ HSCs were purified from the CB mononuclear cells using the human CD34+ MicroBead Kit (Miltenyi Biotec) and were then expanded in culture for 5 d as previously described (24, 25). Cells were stored at –80 °C and thawed just before their injection into mice.
Development and Treatment of HIS hu-Mice.
BALB/c-Rag2nullIl-2rγnullSirpaNOD (BRGS) mice (82, 83) and Hcκ7-Tg/+ (Hcκ+) BRGS mice (26) were bred to produce Hcκ+ and Hcκ− BRGS littermates. The mice were maintained under Specific Pathogen Free conditions in the Vivarium of the University of Colorado Denver Anschutz Medical Campus in Aurora, CO. For the generation of HIS hu-mice, 1- to 3-d-old Hcκ+ and Hcκ− BRGS pups were sublethally irradiated with 300 rad and injected intravenously in the facial vein or, less frequently, directly into the liver with CD34+ cells isolated from either CBH or CBA human umbilical cord blood samples. The anti-hCD69 2.8 monoclonal antibody (mAb) was generated, tested, and kindly provided by Dr. Pilar Lauzurica (84). Hu-mice were treated with a single intraperitoneal (IP) injection of 500 μg of anti-hCD69 2.8 mAb in PBS; a single or multiple IP injections (given few hours apart) of 100 μg of AMD3100 (Calbiochem) in PBS, or PBS alone. Animal procedures were approved by the University of Colorado Anschutz Medical Campus Institutional Animal Care and Use Committee.
Cell Staining, Chemotaxis, and Flow Cytometry.
Bone marrow, spleen, and blood cells from hu-mice were isolated by standard methods. The cell migration assay (described in detail in SI Appendix, Supporting Materials and Methods) was performed in 5 μm pore transwell plates with the bottom wells containing medium supplemented or not with 0.3 μg/mL of recombinant human CXCL12 (R&D). Cells were stained in staining buffer (PBS, 1% bovine serum albumin, and 0.1% NaN3) with antibodies listed in SI Appendix, Supporting Materials and Methods. For the IC detection of proteins, cells were first stained for surface molecules, then fixed in 2% formaldehyde and permeabilized with 0.5% Saponin (Sigma-Aldrich) before staining with antibodies resuspended in 0.5% Saponin. For detection of pERK, cells were first rested for 1 h at 4 °C in Hanks’ Balanced Salt Solution with Ca+2 and Mg+2 (Cellgro) with 1% fetal bovine serum. Cells were then treated with 60 μM sodium pervanadate for 5 min at 37 °C, fixed in 2% formaldehyde, permeabilized with 90% methanol, and stained with anti-pERK or isotype control antibodies. Live/dead cell staining was performed using Zombie Green Fixable Viability Kit (BioLegend) according to manufacturer’s instructions. For data collection, cells were run on a BD LSRFortessa Flow Cytometer (BD Biosciences), and data were analyzed with FlowJo v10.7.1 software (FlowJo, LLC/BD, formerly Tree Star Inc).
ELISAs.
Total serum Igκ and Igλ of HIS hu-mice were measured by ELISA as previously described (26) and detailed in SI Appendix, Supporting Materials and Methods. Human Igs on plates were detected by incubating for 2 h at 37 °C with alkaline phosphatase (AP)-conjugated mouse anti-human IgM (UHB) or IgG (H2) (Southern Biotechnologies Associates) diluted at 1:500. After washing four times, plates were developed by the addition of AP’s substrate (Sigma-Aldrich) solubilized in 0.1 M diethanolamine and 0.02% NaN3. Light absorbance was measured at OD405 multiple times with a VersaMax plate reader (Molecular Devices). Data were analyzed with the SoftMax Pro software version 6.4.2.
Statistical Analysis.
Statistical significance evaluation was performed with GraphPad Prism software using two-tailed t test when comparing two groups or ANOVA when comparing three or more groups/variables. When ANOVA was used, multiple comparisons between the groups were performed with the uncorrected Fisher’s least significant difference test. Data in graphs is represented as mean ± SD.
Supplementary Material
Acknowledgments
We are deeply indebted to the perinatal nurses of the University of Colorado Clinical Translational Research Center, supported by the Colorado Clinical and Translational Sciences Institute, for their help with procuring the “autoimmune” umbilical cord blood units used in our study. We are also indebted to all the patients who agreed to participate in our study by donating their newborn’s cord blood after delivery. We thank Karen Franklin and the Rheumatology Division for helping to enroll some of the rheumatology patients. We also thank the Immu-Micro Flow Facility and the Vivarium at the University of Colorado Anschutz Medical Campus for maintaining the flow cytometers and the mouse facility, respectively. We are very grateful to all members of our laboratories as well as the Cambier’s laboratory for the numerous useful discussions. This work was supported by the NIH (Grants AI124474 and AI131639 to R.P. and Grant AI136534 to R.M.T.). It was also supported in part by NIH National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Awards Grant UL1 TR002535. The contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2021570118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Melchers F., Checkpoints that control B cell development. J. Clin. Invest. 125, 2203–2210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pieper K., Grimbacher B., Eibel H., B-cell biology and development. J. Allergy Clin. Immunol. 131, 959–971 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Nutt S. L., Kee B. L., The transcriptional regulation of B cell lineage commitment. Immunity 26, 715–725 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Schlissel M. S., Regulating antigen-receptor gene assembly. Nat. Rev. Immunol. 3, 890–899 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Hardy R. R., Hayakawa K., B cell development pathways. Annu. Rev. Immunol. 19, 595–621 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Carsetti R., Rosado M. M., Wardmann H., Peripheral development of B cells in mouse and man. Immunol. Rev. 197, 179–191 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Wardemann H., et al., Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Grandien A., Fucs R., Nobrega A., Andersson J., Coutinho A., Negative selection of multireactive B cell clones in normal adult mice. Eur. J. Immunol. 24, 1345–1352 (1994). [DOI] [PubMed] [Google Scholar]
- 9.Wardemann H., Nussenzweig M. C., B-cell self-tolerance in humans. Adv. Immunol. 95, 83–110 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Nemazee D. A., Bürki K., Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature 337, 562–566 (1989). [DOI] [PubMed] [Google Scholar]
- 11.Hartley S. B., et al., Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature 353, 765–769 (1991). [DOI] [PubMed] [Google Scholar]
- 12.Chen C., et al., The site and stage of anti-DNA B-cell deletion. Nature 373, 252–255 (1995). [DOI] [PubMed] [Google Scholar]
- 13.Goodnow C. C., Vinuesa C. G., Randall K. L., Mackay F., Brink R., Control systems and decision making for antibody production. Nat. Immunol. 11, 681–688 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Shlomchik M. J., Sites and stages of autoreactive B cell activation and regulation. Immunity 28, 18–28 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Nemazee D., Mechanisms of central tolerance for B cells. Nat. Rev. Immunol. 17, 281–294 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelanda R., Torres R. M., Central B-cell tolerance: Where selection begins. Cold Spring Harb. Perspect. Biol. 4, a007146 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meffre E., The establishment of early B cell tolerance in humans: Lessons from primary immunodeficiency diseases. Ann. N. Y. Acad. Sci. 1246, 1–10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yurasov S., et al., Defective B cell tolerance checkpoints in systemic lupus erythematosus. J. Exp. Med. 201, 703–711 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuels J., Ng Y. S., Coupillaud C., Paget D., Meffre E., Impaired early B cell tolerance in patients with rheumatoid arthritis. J. Exp. Med. 201, 1659–1667 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chamberlain N., et al., Rituximab does not reset defective early B cell tolerance checkpoints. J. Clin. Invest. 126, 282–287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saadoun D., et al., Expansion of autoreactive unresponsive CD21-/low B cells in Sjögren’s syndrome-associated lymphoproliferation. Arthritis Rheum. 65, 1085–1096 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinnunen T., et al., Specific peripheral B cell tolerance defects in patients with multiple sclerosis. J. Clin. Invest. 123, 2737–2741 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menard L., et al., The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J. Clin. Invest. 121, 3635–3644 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang J., Weiss N., Freed B. M., Torres R. M., Pelanda R., Generation of hematopoietic humanized mice in the newborn BALB/c-Rag2null Il2rγnull mouse model: A multivariable optimization approach. Clin. Immunol. 140, 102–116 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang J., et al., Studies of lymphocyte reconstitution in a humanized mouse model reveal a requirement of T cells for human B cell maturation. J. Immunol. 190, 2090–2101 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang J., et al., Receptor editing and genetic variability in human autoreactive B cells. J. Exp. Med. 213, 93–108 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bendall S. C., et al., Single-cell trajectory detection uncovers progression and regulatory coordination in human B cell development. Cell 157, 714–725 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelanda R., et al., Receptor editing in a transgenic mouse model: Site, efficiency, and role in B cell tolerance and antibody diversification. Immunity 7, 765–775 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Verkoczy L., et al., Basal B cell receptor-directed phosphatidylinositol 3-kinase signaling turns off RAGs and promotes B cell-positive selection. J. Immunol. 178, 6332–6341 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duong B. H., et al., Negative selection by IgM superantigen defines a B cell central tolerance compartment and reveals mutations allowing escape. J. Immunol. 187, 5596–5605 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanz I., et al., Challenges and opportunities for consistent classification of human B cell and plasma cell populations. Front. Immunol. 10, 2458 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ichii M., et al., The density of CD10 corresponds to commitment and progression in the human B lymphoid lineage. PLoS One 5, e12954 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rowland S. L., Leahy K. F., Halverson R., Torres R. M., Pelanda R., BAFF receptor signaling aids the differentiation of immature B cells into transitional B cells following tonic BCR signaling. J. Immunol. 185, 4570–4581 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greaves S. A., Peterson J. N., Torres R. M., Pelanda R., Activation of the MEK-ERK pathway is necessary but not sufficient for breaking central B cell tolerance. Front. Immunol. 9, 707 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swat W., Dessing M., von Boehmer H., Kisielow P., CD69 expression during selection and maturation of CD4+8+ thymocytes. Eur. J. Immunol. 23, 739–746 (1993). [DOI] [PubMed] [Google Scholar]
- 36.Yamashita I., Nagata T., Tada T., Nakayama T., CD69 cell surface expression identifies developing thymocytes which audition for T cell antigen receptor-mediated positive selection. Int. Immunol. 5, 1139–1150 (1993). [DOI] [PubMed] [Google Scholar]
- 37.Brändle D., Müller S., Müller C., Hengartner H., Pircher H., Regulation of RAG-1 and CD69 expression in the thymus during positive and negative selection. Eur. J. Immunol. 24, 145–151 (1994). [DOI] [PubMed] [Google Scholar]
- 38.Wentink M. W. J., van Zelm M. C., van Dongen J. J. M., Warnatz K., van der Burg M., Deficiencies in the CD19 complex. Clin. Immunol. 195, 82–87 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Levy S., Function of the tetraspanin molecule CD81 in B and T cells. Immunol. Res. 58, 179–185 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Clavarino G., et al., Novel strategy for phenotypic characterization of human B lymphocytes from precursors to effector cells by flow cytometry. PLoS One 11, e0162209 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shoham T., et al., The tetraspanin CD81 regulates the expression of CD19 during B cell development in a postendoplasmic reticulum compartment. J. Immunol. 171, 4062–4072 (2003). [DOI] [PubMed] [Google Scholar]
- 42.van Zelm M. C., et al., CD81 gene defect in humans disrupts CD19 complex formation and leads to antibody deficiency. J. Clin. Invest. 120, 1265–1274 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maecker H. T., Levy S., Normal lymphocyte development but delayed humoral immune response in CD81-null mice. J. Exp. Med. 185, 1505–1510 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyazaki T., Müller U., Campbell K. S., Normal development but differentially altered proliferative responses of lymphocytes in mice lacking CD81. EMBO J. 16, 4217–4225 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsitsikov E. N., Gutierrez-Ramos J. C., Geha R. S., Impaired CD19 expression and signaling, enhanced antibody response to type II T independent antigen and reduction of B-1 cells in CD81-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 94, 10844–10849 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halverson R., Torres R. M., Pelanda R., Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat. Immunol. 5, 645–650 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Teodorovic L. S., et al., Activation of Ras overcomes B-cell tolerance to promote differentiation of autoreactive B cells and production of autoantibodies. Proc. Natl. Acad. Sci. U.S.A. 111, E2797–E2806 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casellas R., et al., Igkappa allelic inclusion is a consequence of receptor editing. J. Exp. Med. 204, 153–160 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meffre E., O’Connor K. C., Impaired B-cell tolerance checkpoints promote the development of autoimmune diseases and pathogenic autoantibodies. Immunol. Rev. 292, 90–101 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gutierrez-Arcelus M., Rich S. S., Raychaudhuri S., Autoimmune diseases–Connecting risk alleles with molecular traits of the immune system. Nat. Rev. Genet. 17, 160–174 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eliopoulos E., et al., Association of the PTPN22 R620W polymorphism with increased risk for SLE in the genetically homogeneous population of Crete. Lupus 20, 501–506 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng C., et al., A potential role for CD69 in thymocyte emigration. Int. Immunol. 14, 535–544 (2002). [DOI] [PubMed] [Google Scholar]
- 53.Nakayama T., et al., The generation of mature, single-positive thymocytes in vivo is dysregulated by CD69 blockade or overexpression. J. Immunol. 168, 87–94 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Matloubian M., et al., Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Allende M. L., et al., S1P1 receptor directs the release of immature B cells from bone marrow into blood. J. Exp. Med. 207, 1113–1124 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Notario L., et al., Anti-CD69 therapy induces rapid mobilization and high proliferation of HSPCs through S1P and mTOR. Leukemia 32, 1445–1457 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Shiow L. R., et al., CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440, 540–544 (2006). [DOI] [PubMed] [Google Scholar]
- 58.Bankovich A. J., Shiow L. R., Cyster J. G., CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J. Biol. Chem. 285, 22328–22337 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma Q., Jones D., Springer T. A., The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity 10, 463–471 (1999). [DOI] [PubMed] [Google Scholar]
- 60.Pozzobon T., Goldoni G., Viola A., Molon B., CXCR4 signaling in health and disease. Immunol. Lett. 177, 6–15 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Zehentmeier S., Pereira J. P., Cell circuits and niches controlling B cell development. Immunol. Rev. 289, 142–157 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fistonich C., et al., Cell circuits between B cell progenitors and IL-7+ mesenchymal progenitor cells control B cell development. J. Exp. Med. 215, 2586–2599 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tokoyoda K., Egawa T., Sugiyama T., Choi B.-I., Nagasawa T., Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity 20, 707–718 (2004). [DOI] [PubMed] [Google Scholar]
- 64.Beck T. C., Gomes A. C., Cyster J. G., Pereira J. P., CXCR4 and a cell-extrinsic mechanism control immature B lymphocyte egress from bone marrow. J. Exp. Med. 211, 2567–2581 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mcheik S., et al., Coexpression of CCR7 and CXCR4 during B cell development controls CXCR4 responsiveness and bone marrow homing. Front. Immunol. 10, 2970 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mandal M., et al., CXCR4 signaling directs Igk recombination and the molecular mechanisms of late B lymphopoiesis. Nat. Immunol. 20, 1393–1403 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J., Tannous B. A., Poznansky M. C., Chen H., CXCR4 antagonist AMD3100 (plerixafor): From an impurity to a therapeutic agent. Pharmacol. Res. 159, 105010 (2020). [DOI] [PubMed] [Google Scholar]
- 68.Paganessi L. A., et al., Effective mobilization of hematopoietic progenitor cells in G-CSF mobilization defective CD26-/- mice through AMD3100-induced disruption of the CXCL12-CXCR4 axis. Exp. Hematol. 39, 384–390 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greaves S. A., Peterson J. N., Strauch P., Torres R. M., Pelanda R., Active PI3K abrogates central tolerance in high-avidity autoreactive B cells. J. Exp. Med. 216, 1135–1153 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goodnow C. C., et al., Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature 334, 676–682 (1988). [DOI] [PubMed] [Google Scholar]
- 71.Tan C., et al., Nur77 links chronic antigen stimulation to B cell tolerance by restricting the survival of self-reactive B cells in the periphery. J. Immunol. 202, 2907–2923 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schinnerling K., Rosas C., Soto L., Thomas R., Aguillón J. C., Humanized mouse models of rheumatoid arthritis for studies on immunopathogenesis and preclinical testing of cell-based therapies. Front. Immunol. 10, 203 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alves da Costa T., Lang J., Torres R. M., Pelanda R., The development of human immune system mice and their use to study tolerance and autoimmunity. J. Transl. Autoimmun. 2, 100021 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cantaert T., et al., Activation-induced cytidine deaminase expression in human B cell precursors is essential for central B cell tolerance. Immunity 43, 884–895 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schickel J. N., et al., PTPN22 inhibition resets defective human central B cell tolerance. Sci. Immunol. 1, aaf7153 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borsotti C., et al., HSC extrinsic sex-related and intrinsic autoimmune disease-related human B-cell variation is recapitulated in humanized mice. Blood Adv. 1, 2007–2018 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lesley R., et al., Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity 20, 441–453 (2004). [DOI] [PubMed] [Google Scholar]
- 78.Bottini N., Peterson E. J., Tyrosine phosphatase PTPN22: Multifunctional regulator of immune signaling, development, and disease. Annu. Rev. Immunol. 32, 83–119 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pereira J. P., Xu Y., Cyster J. G., A role for S1P and S1P1 in immature-B cell egress from mouse bone marrow. PLoS One 5, e9277 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Donovan E. E., Pelanda R., Torres R. M., S1P3 confers differential S1P-induced migration by autoreactive and non-autoreactive immature B cells and is required for normal B-cell development. Eur. J. Immunol. 40, 688–698 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.García-Cuesta E. M., et al., The role of the CXCL12/CXCR4/ACKR3 axis in autoimmune diseases. Front. Endocrinol. (Lausanne) 10, 585 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Legrand N., et al., Functional CD47/signal regulatory protein alpha (SIRP(alpha)) interaction is required for optimal human T- and natural killer- (NK) cell homeostasis in vivo. Proc. Natl. Acad. Sci. U.S.A. 108, 13224–13229 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lang J., et al., Replacing mouse BAFF with human BAFF does not improve B-cell maturation in hematopoietic humanized mice. Blood Adv. 1, 2729–2741 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Esplugues E., et al., Induction of tumor NK-cell immunity by anti-CD69 antibody therapy. Blood 105, 4399–4406 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.