Abstract
Background
Dermoscopy is undoubtedly a useful tool to improve diagnostic accuracy and minimize the number of unnecessary biopsies. However, much of the literature on dermoscopy focuses on findings in lighter-skin phototypes, leaving potential gaps of knowledge regarding its use in skin of color (SoC). As the clinical applications of dermoscopy continue to increase, understanding dermoscopic patterns in SoC is imperative.
Objective
This review discusses the literature on dermoscopic findings of neoplasms in SoC, highlighting unique and characteristic dermoscopic features.
Methods
A literature review was performed using the PubMed database. Case reports, case series, case-control studies, and systematic reviews were included.
Results
A total of 8326 studies were identified based on the selected search terms, and 41 were included in this review based on relevance.
Conclusion
There are specific dermoscopic characteristics in SoC for benign nevi, acral lentiginous melanoma, ethnic melanonychia, and dermatofibroma; however, there is a lack of published data about specific features of cutaneous melanoma, subungual melanoma, pigmented basal cell carcinoma, and pigmented squamous cell carcinoma in SoC. Because pigmented basal cell carcinoma, pigmented squamous cell carcinoma, ethnic melanonychia, and acral lentiginous melanoma are diagnosed at later stages in this population, it is important to understand their dermoscopic features. Further descriptive studies are needed to better characterize unique dermoscopic features in neoplasms in SoC.
Keywords: Dermoscopy, Dermatoscopy, Skin of color, Ethnic skin, Pigmented Bowen’s disease, Pigmented basal cell carcinoma, Nevi, Dermatofibroma, Acral lentiginous melanoma, Melanoma, Longitudinal melanonychia
Introduction
Dermoscopy is a noninvasive tool that can help identify cutaneous malignancies, increase diagnostic accuracy, and minimize the number of unnecessary biopsies. Although the dermoscopic features of melanocytic and keratinocytic malignancies, as well as inflammatory conditions, are relatively well described, much of this literature is based on findings in skin phototypes I through III. Dermatology in skin of color (SoC) is a growing area of interest among clinicians and researchers. Neoplasms in SoC populations often present with features less commonly seen in white populations (Agbai et al., 2014), which may lead to delays in diagnosis. Photographs of benign pigmented lesions in SoC are also limited and likely underutsed in training and in the development of algorithms for artificial intelligence (Adamson and Smith, 2018).
This review summarizes the existing literature on dermoscopic patterns and findings in neoplasms in SoC. Specifically, this article details pigmented basal cell carcinoma (pBCC), pigmented Bowen’s disease (pBD), squamous cell carcinoma in situ (SCCis), squamous cell carcinoma (SCC) nevi, melanoma, acral lentiginous melanoma (ALM), longitudinal melanonychia, subungual melanoma, and dermatofibromas.
Methods
A literature review was performed using the PubMed database and included a search for articles in the English language from inception to July 2019. Search terms included “dermoscopy,” “dermatoscopy,” “ethnic skin,” and “skin of color”. These terms were then searched in association with the terms “pigmented basal cell carcinoma,” “pigmented Bowen’s disease,” “squamous cell carcinoma in situ,” “squamous cell carcinoma,” “nevi,” “melanoma,” “acral lentiginous melanoma,” “longitudinal melanonychia,” “subungual melanoma,” and “dematofibroma”.
All 8326 resulting studies were initially assessed for relevance based on title and/or abstract. The vast majority of studies were found to be repeats. Forty-one articles with a fitting title and/or abstract pertaining to dermoscopic findings were reviewed fully for relevance. Articles cited in the references of these articles were additionally reviewed for relevance. Studies that reported subjects with skin phototypes IV through VI or explicitly mentioned SoC (n = 19) were reviewed, and the findings are described in this review. Twenty-one studies with subjects with skin phototypes I through III or an unclear delineation of phototypes were also included for reference. Dermoscopic photographs were taken with either a Dermlite DL200 dermatoscope, Dermlite DL3 dermatoscope, DermLite photo connector, or a Canon point-and-shoot camera.
Results
Basal cell carcinoma
Although only 6% of all basal cell carcinoma (BCC) cases are pigmented, pigmented BCCs (pBCC) account for at least half of all BCCs in Asians, Blacks, and Hispanics (Bigler et al., 1996, Bradford, 2009). pBCCs are most commonly seen anatomically on sun-exposed areas, including the head and neck in SoC populations, similar to lighter-skin phototypes (Bradford, 2009). BCCs have characteristic dermoscopic features, such as arborizing telangiectasias, ulceration, gray-blue globules, maple leaf-like areas, and spoke-wheel areas (Altamura et al., 2010, Demirtaşoglu et al., 2006, Takahashi et al., 2016), but these latter features are seen more commonly in pBCC (Altamura et al., 2010). Maple leaf-like and spoke-wheel areas, gray-blue globules, in-focus dots, and concentric structures are features from melanin (Fig. 1; Wozniak-Rito et al., 2018). For patients with darker skin, clinicians should be aware that darker pigment is commonly a feature of BCC and can clinically resemble a seborrheic keratosis or melanoma (Abudu and Cohen, 2019, White et al., 2003), warranting evaluation with dermoscopy. The tool also aids in identifying visually undetectable pigment within a BCC by up to 30% (Fabiano et al., 2016), which would be particularly useful in pBCC seen more commonly in SoC. To our knowledge, no unique dermoscopic features have been identified specifically in SoC.
Fig. 1.
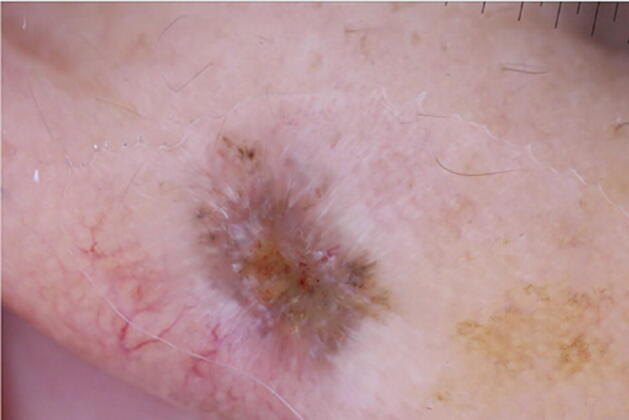
Pigmented basal cell carcinoma in a Cuban patient: scattered blue-gray dots, as well as spoke-wheel and maple-leaf areas.
Squamous cell carcinoma/Bowen’s disease
SCC/SCCis is the most common skin cancer in black and Indian patients and the second most common in Chinese and Japanese patients (Gloster and Neal, 2006). pBD is a clinically pigmented form of SCCis and occurs in 1.7% of all SCCis. However, frequency is increased in black men, particularly on the lower extremities and notably in intertriginous areas (Cavalleiro de Macedo Mota et al., 2014). Clinically, pBD is a well-demarcated brown plaque with overlying scale crust (Cameron et al., 2010). In contrast, SCCis in fairer skin phototypes is typically erythematous with scale crust and most commonly located on the extremities (Reizner et al., 1994). Evidence for specific dermoscopic features of pBD has been limited in several studies because are widely variable in appearance (Cameron et al., 2010).
The largest study to date reviewed 52 pBD cases and described common dermoscopic features (Cameron et al., 2010). Commonly noted features include structureless asymmetric brown areas, combination of structureless patterns and dots, linear arrangement of blue/gray dots, and coiled vessels (Cameron et al., 2010, Yang et al., 2017). The brown areas typically lie on the periphery of the lesion and appear larger than the pBCC’s characteristic maple-leaf areas (Yang et al., 2017). Similarly, nonspecific dermoscopic findings suggesting both melanocytic (e.g., pigment network, streaks, variegated colors, amorphous patterns) and nonmelanocytic (e.g., defined borders, comedo-like openings) neoplasms have been reported in the literature in pBD in SoC (Gutierrez-Mendoza et al., 2010, Cavalleiro de Macedo Mota et al., 2014, Vivan et al., 2017). The anatomic distribution in SoC and likely decreased index of suspicion have the potential to delay diagnosis; therefore, dermoscopic clues should be utilized for additional information.
Dermatofibroma
Dermatofibromas can be diagnosed clinically, featuring a pathognomonic but nonspecific dimple sign (Ferrari et al., 2013). The variability in clinical presentation can require differentiation from other pigmented lesions, melanoma, nonmelanoma skin cancer, and neurofibromas. Peripheral pigmented networks and central shiny white and/or vascular structures are common dermoscopic features (Fig. 2; Ferrari et al., 2013, Zaballos et al., 2008). Kelati et al. (2017) identified dermoscopic findings of dermatofibromas in Moroccan patients with phototypes IV and V. Patients most frequently presented with classic dermoscopic features; however, variants such as pigmented networks with darkened rings surrounding the opening of the follicle, star-shaped white patches in the peripheral network, and annular white structures superimposed on diffuse pigmentation were also noted (Kelati et al., 2017).
Fig. 2.
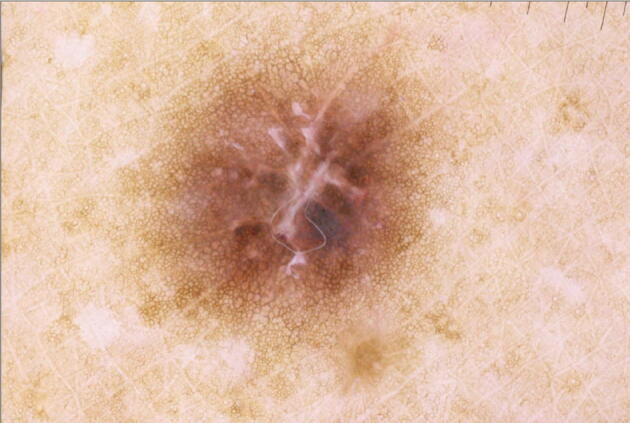
Dermatofibroma in a patient with Fitzpatrick skin type VI: reticular pattern peripherally with shiny white center.
Benign nevi
SoC patients have significantly fewer nevi than patients with lighter skin (Lallas et al., 2014a, Tuma et al., 2015). The location of nevi also differs, with acral nevi more frequently occurring in darker skin but on the trunk in fair skin (Tuma et al.). In both fair skin and SoC, uniform distribution of pigment is most commonly seen in acquired nevi. However, multifocal areas of hyper- and/or hypopigmentation in nevi are more commonly seen in fairer-skinned patients, whereas a darker background with central hyperpigmentation is more common in SoC (Tuma et al., 2015). Studies have also found an increased frequency of reticular pattern in nevi of darker skin phototypes (Scope et al., 2008, Sosa-Seda et al., 2014).
Variations of color and patterns can even differ between phototypes V and VI. Those with phototype V typically have dark brown nevi in a reticular pattern; in contrast, those with phototype VI present more often with a structureless background and black, blue, or gray nevi (Lallas et al., 2014a). Because of these variations, clinicians should familiarize themselves with patients’ normal-appearing nevi to understand each patient’s clinical and dermoscopic patterns and to assess for any lesions that deviate from this.
Melanoma
According to the Surveillance, Epidemiology, and End Result database, the incidence of melanoma among white persons is 27.8 persons per 100,000, followed by American Indian/Alaska Native at 5.6 per 100,000, Hispanic with 4.9 per 100,000, Asian/Pacific Islander with 1.4 per 100,000, and black populations at 1.0 per 100,000 (National Cancer Institute, 2019). Melanoma occurs more commonly on acral sites in black patients (Chan et al., 2016, Kim and Yun, 2016, Weber et al., 2018). In Hispanic patients, melanoma is more likely to occur in sun-exposed areas, including the trunk, arms, and legs (Agbai et al., 2014). In American Indian/Alaska Native subjects, melanoma most frequently occurs on the trunk (Kryatova and Okoye, 2016). Reports of nonacral melanoma in SoC have described clinical and dermoscopic features similar to those in fairer-skinned patients (Gurfinkel et al., 2013, Pellizzari et al., 2013).
Although melanoma has a higher incidence in white populations, its morbidity and mortality is significantly higher in patients with SoC and represents an important health disparity (Baldwin et al., 2016, Ward-Peterson et al., 2016). The cause is likely multifactorial and may include differences in biologic behavior, as well as limitations in access to dermatologic care. However, delays in identification by the patient and/or physician may also contribute to this health gap. De Giorgi et al. (2006) demonstrated that dermoscopy improves differentiation of melanoma and benign nevi in black patients. To our knowledge, no studies have elucidated unique features of melanoma in SoC or described dermoscopic features that are more commonly seen in SoC. This represents a significant gap in our knowledge.
Acral lentiginous melanoma
SoC populations have increased numbers of acral nevi (Madankumar et al., 2016, Tuma et al., 2015) and a disproportionate incidence of ALM with increased morbidity and mortality (Kryatova and Okoye, 2016, Tan and Stein, 2019, Weber et al., 2018). There are three distinct patterns of acquired acral nevi that signify benignity. The parallel furrow pattern has pigment in the furrows (sulci) of the foot or hand (Fig. 3). The lattice pattern is a variant of parallel furrow with perpendicular pigmented lines connecting pigment in the furrows. The fibrillar pattern shows pigmentation tangentially crossing the skin markings. Less common patterns, such as the homogenous pattern, have been identified. The homogenous pattern, most commonly seen in SoC, is described as a uniform brown hyperpigmented macule and can also be a feature of lentigo (Criscito and Stein, 2017). Congenital nevi may be clinically larger, darker, and more atypical appearing. Their dermoscopic features include a crista-dotted pattern, described as a pearl-necklace feature, with linear pigmented globules on the acral ridges (Chuah et al., 2015). When combined with the parallel furrow pattern, it is described as a “peas in a pod” pattern (Chuah et al., 2015, Criscito and Stein, 2017). These aforementioned patterns suggest a benign acral nevus.
Fig. 3.
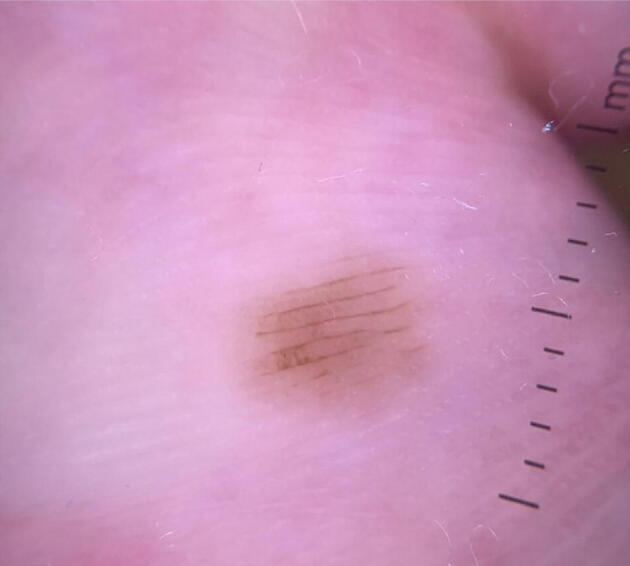
Common acral nevus, demonstrating parallel furrow pattern, as well as subtle area of lattice pattern.
The most specific dermoscopic pattern for ALM is the parallel ridge pattern, which features pigment in the ridges of glabrous skin (Fig. 4; Saida et al., 2011, Weber et al., 2018); however, not all acral melanomas show the parallel ridge pattern. Although the pattern holds a specificity of 99% (Criscito and Stein, 2017), one study found that only 38.2% of ALMs present with the parallel ridge pattern (Lallas et al., 2015). Moreover, a predominant ridge pattern is more frequently associated with the early stages of ALM; therefore, recognition is especially important for early diagnosis. Variations in pigmentation across the lesion, asymmetric structures, and blue/gray veil are all suggestive of a malignant lesion (Lallas et al., 2015, Lallas et al., 2014b). If one is unable to identify whether the pigment is on the ridge or the furrow, the ink furrow test can be used to highlight the furrows. The peripheral edges of the lesion are marked with a green or blue whiteboard marker, and the excess ink is wiped off with a dry towel. The ink marks adjacent furrows and can help the observer identify whether the pigment is in the furrows or ridges within the lesion (Saida et al., 2004, Braun et al., 2008).
Fig. 4.
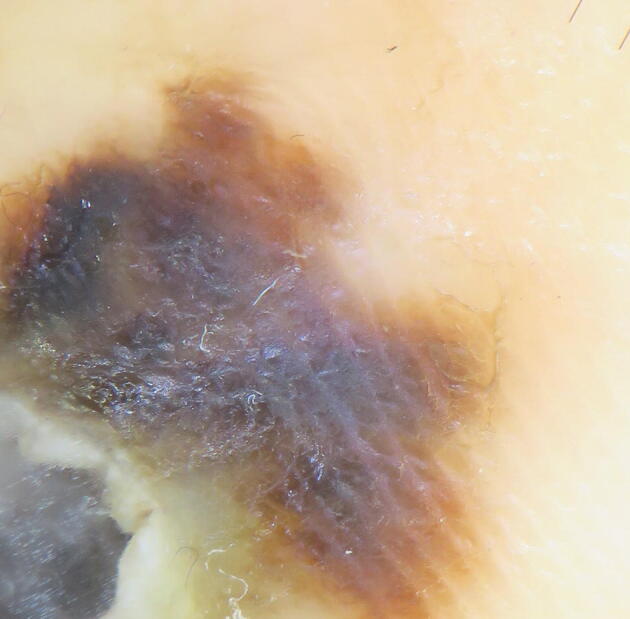
Acral lentiginous melanoma in a patient with Fitzpatrick skin type VI: variegated dark brown and black colors, irregular borders, central gray veil, and predominant parallel ridge pattern.
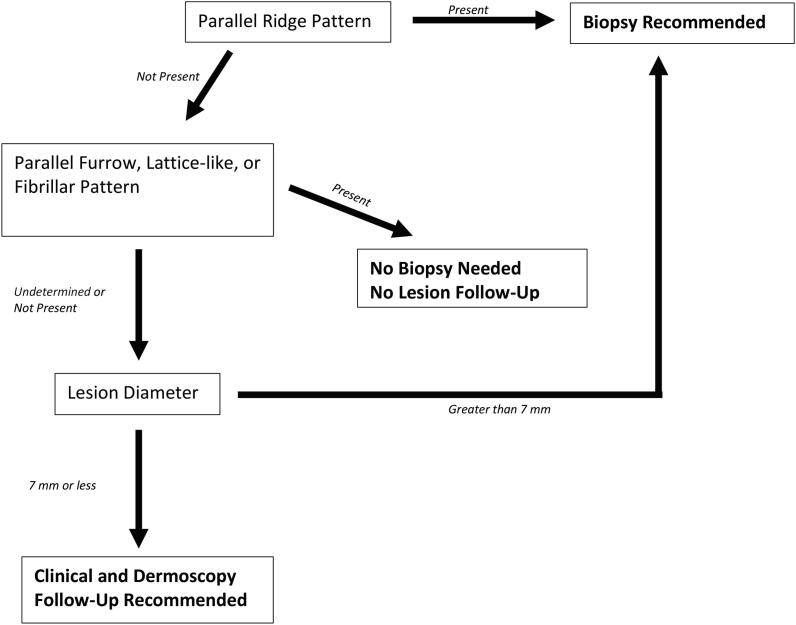
Algorithms have been developed for diagnosis of acral pigmented lesions. The two main algorithms include the three-step algorithm and the BRAAFF (irregular blotching, parallel ridge pattern, asymmetric structures, asymmetric colors, parallel furrow pattern, and fibrillar pattern) checklist. Both algorithms highlight the ridge pattern as a sign of malignancy. The three-step algorithm determines a need for biopsy by lesion pattern and size (Fig. 5). If a parallel ridge pattern is present, a biopsy is needed. If the lesion demonstrates a typical benign pattern (i.e., fibrillar, lattice, or parallel furrow) throughout the entire lesion, no follow-up is needed. If the lesion is not clearly malignant or benign, it is measured. A lesion < 7 mm in diameter requires clinical and dermoscopic follow-up, and those > 7 mm need a biopsy (Koga and Saida, 2011). The BRAAFF checklist is a scoring system to determine whether a lesion needs a biopsy based on particular dermoscopic features (irregular blotch: +1; parallel ridge pattern: +3; asymmetry of structures: +1; asymmetry of colors: +1; parallel furrow pattern: −1; fibrillar pattern: −1). A lesion with a score > 1 should be biopsied (Table 1; Lallas et al., 2015). A significant proportion of ALM research comes from predominantly Japanese patients, and little has been published on ALM dermoscopy of other SoC populations (See Fig. 6).
Fig. 5.
Three-step algorithm for acral pigmented lesion.
Table 1.
BRAAFF checklist.
| Characteristic | Point value |
|---|---|
| Irregular blotch | +1 |
| Parallel ridge pattern | +3 |
| Asymmetric structure | +1 |
| Asymmetric colors | +1 |
| Parallel furrow pattern | –1 |
| Fibrillar pattern | –1 |
| Total | >1: diagnostic of acral lentiginous melanoma |
Fig. 6.

Ethnic melanonychia in a patient with Fitzpatrick skin type IV: two visible light brown-gray bands with parallelism and even spacing and width.
Nail unit: melanonychia
Pigmented lesions in the nail unit can be difficult to diagnose with the naked eye alone. Melanonychia (pigmentation of the nail) is frequently benign and often does not require a biopsy. However, the high morbidity and mortality associated with nail melanoma highlights the need for recognition of clinical and dermoscopic features that can clue clinicians in to the need for a nail unit biopsy. Moreover, most patients do not recognize nail abnormalities associated with a subungual melanoma (Halteh et al., 2017) and may not present for changes regarding the nail unit. Features of pigmented nail lesions are best appreciated with contact dermoscopy, under polarized light, with the use of gel immersion, such as ultrasound gel (Braun et al., 2007).
The main causes of melanonychia include melanocytic activation (benign increased pigment production of melanocytes of the nail unit) and melanocytic hyperplasia, which can be either benign as in a nail unit nevus or malignant as in melanoma.
Melanocytic activation of the nail matrix produces a longitudinal pigmented band of the nail (Carreno et al., 2013). Endogenous and/or exogenous factors may cause melanocytic activation, including ethnic melanonychia, onychomychosis, nail lentigines, and medications (Braun et al., 2007, Koga et al., 2011, Thomas and Dalle, 2007). Ethnic melanonychia (Fig. 5) is common in SoC, occurring in almost 100% of black patients by the age of 50 years and with an overall high prevalence in phototypes IV, V, and VI (Andre and Lateur, 2006, de Magalhães Mariano Astur et al., 2016). Multidigit involvement is common, which is a sign of benignity (Jiyad and Akhras, 2019, Ruben, 2010). Affected sites are most often in grasping fingers (first to third digits) and sites of trauma (first toe; Andre and Lateur, 2006). Ethnic melanonychia typically presents with a gray longitudinal band and may appear on a gray background (Thomas and Dalle, 2007, Koga et al., 2011, Braun et al., 2007) Fig. 6. However, a recent study advocated for reconsideration of this characteristic, with reports of ethnic melanonychia (biopsy-proven melanocytic activation in SoC) presenting with brown or black background pigmentation as opposed to gray (de Magalhães Mariano Astur et al., 2016). Although ethnic melanonychia is a benign condition, any band that looks markedly different from the others should be evaluated (Ishihara et al., 2006).
Nail unit nevi (proliferation of matrix melanocytes) may be congenital or acquired and present with a linear configuration, typically brown or black in color. They occur more frequently during childhood (Andre and Lateur, 2006). They are often smaller in size (less than one-third of the nail width) than melanoma, although this may not be the case in early nail unit melanoma. Melanocytic nevi may also present with a pseudo-Hutchinson sign, caused by nail matrix pigmentation observed through a translucent nail fold. This can be difficult to differentiate from the Hutchinson sign; however, the pseudo-Hutchinson sign on dermoscopy will show a linear brushy pattern on the hyponychium, with furrow pigmentation similar to plantar nevi (Kawabata et al., 2001).
Melanocytic hyperplasia: Nail melanoma
Nail matrix melanoma is a rare malignancy, but it has a higher relative incidence and mortality in SoC (Andre and Lateur, 2006, Lee et al., 2018). One study demonstrated that African-American patients have a 3.5 times higher mortality rate than Caucasian patients (O'Leary et al., 2000). Clinically, nail melanomas occur more frequently on the first fingernail and first toenail (Lee et al., 2018). Both nail melanoma and benign nevi typically present with a brown to black color, and dermoscopy can be used to aide in distinguishing the two. According to the International Dermoscopy Society, nail melanomas have six key differentiating features (Benati et al., 2017). Nail melanoma is more likely to cover more than two-third of the nail. An irregular linear pattern, including variable spacing, width, and nonparallelism, is also a feature of melanoma. Gray or black pigment on a brown background should raise suspicion. Pigmented granules and a positive Hutchinson sign (pigmentation of the periungual skin and nail fold[s]) or a micro-Hutchinson sign (pigmentation only seen through dermoscopy) are also concerning.
Finally, nail disfigurement is a clinical clue. Size and color were shown to be the most important clinical factors reported (Benati et al., 2017). Triangular pigmentation (when pigmentation at the proximal nail plate is wider than at the distal nail plate) is also a sign of early melanoma. The irregularity and obscurity of the pigmented bands can alert a clinician to the diagnosis of melanoma (Di Chiacchio et al., 2013). However, it is important to note that regular brown lines may be present in patients with early ungual melanoma. Because of the similarities between melanocytic nevi and early stage melanoma, regular monitoring with dermoscopy for additional features is useful (Koga et al., 2011). Dermoscopy can also be used to guide biopsy site selection. Visualization of pigment at the superior portion of the nail plate suggests proximal matrix involvement whereas pigment on the inferior portion of the nail plate suggests a distal matrix involvement. Importantly, misdiagnosis of melanoma of the nail occurs in 52% of cases; therefore, utilization of all clinical and dermoscopic clues is prudent (Andre and Lateur, 2006).
Call to action
The dearth of SoC populations featured in clinical and research studies (Higgins et al., 2018, Mahendraraj et al., 2017, Mulcahy et al., 2017) limits our knowledge of dermoscopic features in SoC. This gap is particularly worrisome now that machine learning algorithms are being developed for melanoma detection using training sets of images predominantly composed of lesions from fair skin. This lack of SoC images may lead to limitations in the algorithms’ utility in SoC patients (Adamson and Smith, 2018, Hogarty et al., 2020). This disparity highlights a two-part need: an increased image reservoir of pigmented lesions in SoC and an increased application of dermoscopy in pigmented lesions for this same population to better characterize unique and prevalent features. At the time of print, the International Dermoscopy Society is conducting studies to ameliorate this image gap and potentially better characterize dermoscopic features in SoC.
Limitations
This study has some limitations. With the vast number of publications in our literature review being repeats, we were unable to determine the true percentage of dermoscopy literature focusing on SoC. However, we believe that our search highlights the absolute number of papers discussing neoplasms in SoC (n = 19).
Conclusion
There is limited literature on and findings about dermoscopic features of neoplasms in SoC (Table 2, Table 3). In this review, we have highlighted unique and more frequently encountered features that have been reported, with dermoscopic photographs for reference. Salient dermoscopic features include maple-leaf and spoke-wheel areas in pBCC, parallel ridge pattern in ALM, and Hutchinson sign and triangular longitudinal melanonychia suggestive of nail matrix melanoma. Common nevi are more likely to have reticular or homogenous patterns in SoC. Much information about dermoscopic features in SoC comes from Asia, but there are relatively fewer studies in other SoC populations.
Table 2.
Common dermoscopic features in neoplasms with increased relative incidence in skin of color.
| Lesion type | Common characteristics |
|---|---|
| Pigmented basal cell carcinoma | Maple leaf-like and spoke-wheel areas; concentric structures, blue-gray globules, in-focus dots, ulceration, arborizing vessels |
| Pigmented squamous cell carcinoma/Bowen’s disease | Linear arrangement of brown dots, glomerular/coiled vessels, structureless asymmetric brown areas |
| Nail melanoma | Gray/black pigment on brown background, pigmented granules, positive Hutchinson sign (pigmentation of periungual skin and nail fold[s]), micro-Hutchinson sign (pigmentation only seen through dermoscopy), triangular longitudinal melanonychia |
Table 3.
Identifiable variant characteristics seen in neoplasms in skin of color.
| Lesion | Variant characteristics |
|---|---|
| Dermatofibroma | Central scar-like white area with delicate peripheral network-like structures, ring-like globules, central shiny white lines (crystalline structures) |
| Acquired nevi | Commonly dark brown to black. Blue is sometimes present. In compound nevi, common to see central hyperpigmentation pattern with a peripheral reticular pattern
|
| Acral lentiginous melanoma | Parallel ridge pattern and any nontypical patterns (Fig. 5). Look for non–site-specific melanoma features as well, including blue-white veil. Diameter > 7 mm for lesions with nontypical pattern is concerning. |
| Ethnic melanonychia | Most frequently described as gray longitudinal band and may appear on a gray background; however, has been noted to present with brown or black background pigmentation in patients with Fitzpatrick skin types IV through VI |
Funding
None.
Study approval
The author(s) confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Ekene Ezenwa, Email: Ekene-a-ezenwa@ouhsc.edu.
Loren Krueger, Email: loren.krueger@gmail.com.
References
- Abudu B., Cohen P.R. Pigmented basal cell carcinoma masquerading as a melanoma. Cureus. 2019;11(4) doi: 10.7759/cureus.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson A.S., Smith A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 2018;154(11):1247–1248. doi: 10.1001/jamadermatol.2018.2348. [DOI] [PubMed] [Google Scholar]
- Agbai O.N., Buster K., Sanchez M., Hernandez C., Kundu R.V., Chiu M. Skin cancer and photoprotection in people of color: A review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70(4):748–762. doi: 10.1016/j.jaad.2013.11.038. [DOI] [PubMed] [Google Scholar]
- Altamura D., Menzies S.W., Argenziano G., Zalaudek I., Soyer H.P., Sera F. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol. 2010;62(1):67–75. doi: 10.1016/j.jaad.2009.05.035. [DOI] [PubMed] [Google Scholar]
- Andre J., Lateur N. Pigmented nail disorders. Dermatol Clin. 2006;24(3):329–339. doi: 10.1016/j.det.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Baldwin J., Janitz A.E., Erb-Alvarez J., Snider C., Campbell J.E. Prevalence and mortality of melanoma in Oklahoma among racial groups, 2000–2008. J Okla State Med Assoc. 2016;109(7–8):311–316. [PMC free article] [PubMed] [Google Scholar]
- Benati E., Ribero S., Longo C., Piana S., Puig S., Carrera C. Clinical and dermoscopic clues to differentiate pigmented nail bands: an international dermoscopy society study. J Eur Acad Dermatol Venereol. 2017;31(4):732–736. doi: 10.1111/jdv.13991. [DOI] [PubMed] [Google Scholar]
- Bigler C., Feldman J., Hall E., Padilla R.S. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34(5 Pt 1):751–752. doi: 10.1016/s0190-9622(96)90007-9. [DOI] [PubMed] [Google Scholar]
- Bradford PT. Skin cancer in skin of color. Dermatol Nurs 2009;21(4):170–7, 206; quiz 178. [PMC free article] [PubMed]
- Braun R.P., Baran R., Le Gal F.A., Dalle S., Ronger S., Pandolfi R. Diagnosis and management of nail pigmentations. J Am Acad Dermatol. 2007;56(5):835–847. doi: 10.1016/j.jaad.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Braun R.P., Thomas L., Kolm I., French L.E., Marghoob A.A. The furrow ink test: A clue for the dermoscopic diagnosis of acral melanoma vs nevus. Arch Dermatol. 2008;144(12):1618–1620. doi: 10.1001/archderm.144.12.1618. [DOI] [PubMed] [Google Scholar]
- Cameron A., Rosendahl C., Tschandl P., Riedl E., Kittler H. Dermatoscopy of pigmented Bowen's disease. J Am Acad Dermatol. 2010;62(4):597–604. doi: 10.1016/j.jaad.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Carreno A.M., Nakajima S.R., Pennini S.N., Candido R., Jr, Mendes Schettini A.P. Nail apparatus melanoma: a diagnostic opportunity. An Bras Dermatol. 2013;88(2):268–271. doi: 10.1590/S0365-05962013000200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalleiro de Macedo Mota AN, Piñeiro-Maceira J, de Fatima Guimarães Scotelaro Alves M, Mateus Tarazona MJ. Pigmented Bowen's disease. An Bras Dermatol 2014;89(5):825–827. [DOI] [PMC free article] [PubMed]
- Chan K.K.W., Chan R.C.L., Ho R.S.L., Chan J.Y.W. Clinical patterns of melanoma in Asians: 11-year experience in a tertiary referral center. Ann Plast Surg. 2016;77(Suppl 1):S6–S11. doi: 10.1097/SAP.0000000000000731. [DOI] [PubMed] [Google Scholar]
- Chuah S.Y., Tsilika K., Chiaverini C., Fontas E., Ortonne J.P., Lacour J.P. Dermoscopic features of congenital acral melanocytic naevi in children: a prospective comparative and follow-up study. Br J Dermatol. 2015;172(1):88–93. doi: 10.1111/bjd.13187. [DOI] [PubMed] [Google Scholar]
- Criscito M.C., Stein J.A. Improving the diagnosis and treatment of acral melanocytic lesions. Melanoma Manag. 2017;4(2):113–123. doi: 10.2217/mmt-2016-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Giorgi V., Trez E., Salvini C., Duquia R., De Villa D., Sestini S. Dermoscopy in black people. Br J Dermatol. 2006;155(4):695–699. doi: 10.1111/j.1365-2133.2006.07415.x. [DOI] [PubMed] [Google Scholar]
- de Magalhães Mariano Astur M, Farkas CB, Junqueira JP, Simoes E Silva Enokihara MM, Enokihara MY, Michalany N, et al. Reassessing melanonychia striata in phototypes IV, V, and VI patients. Dermatol Surg 2016;42(2):183–90. [DOI] [PubMed]
- Demirtaşoglu M., Ilknur T., Lebe B., Kuşku E., Akarsu S., Ozkan S. Evaluation of dermoscopic and histopathologic features and their correlations in pigmented basal cell carcinomas. J Eur Acad Dermatol Venereol. 2006;20(8):916–920. doi: 10.1111/j.1468-3083.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- Di Chiacchio N.D., Cadore de Farias D., Piraccini B.M., Hirata S.H., Richert B., Zaiac M. Consensus on melanonychia nail plate dermoscopy. An Bras Dermatol. 2013;88(2):309–313. doi: 10.1590/S0365-05962013000200029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiano A., Argenziano G., Longo C., Moscarella E., Specchio F., Lallas A. Dermoscopy as an adjuvant tool for the diagnosis and management of basal cell carcinoma. G Ital Dermatol Venereol. 2016;151(5):530–534. [PubMed] [Google Scholar]
- Ferrari A., Argenziano G., Buccini P., Cota C., Sperduti I., De Simone P. Typical and atypical dermoscopic presentations of dermatofibroma. J Eur Acad Dermatol Venereol. 2013;27(11):1375–1380. doi: 10.1111/jdv.12019. [DOI] [PubMed] [Google Scholar]
- Gloster H.M., Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55(5):741–760. doi: 10.1016/j.jaad.2005.08.063. quiz 61–4. [DOI] [PubMed] [Google Scholar]
- Gurfinkel P.C., Campos-do-Carmo G., Ishida C.E., Piñeiro-Maceira J., Valiante P.M., Ramos-E-Silva M. A lesion suspected of melanoma by dermoscopy: we must trust this diagnostic tool. J Dermatol Case Rep. 2013;7(3):88–92. doi: 10.3315/jdcr.2013.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Mendoza D., Narro-Llorente R., Karam-Orantes M., Fonte-Avalos V., Martínez-Luna E., Toussaint-Caire S. Dermoscopy clues in pigmented Bowen's disease. Dermatol Res Pract. 2010;2010 doi: 10.1155/2010/464821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halteh P., Scher R., Artis A., Lipner S. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2017;2(3–4):156–161. doi: 10.1159/000452673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S., Nazemi A., Chow M., Wysong A. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44(7):903–910. doi: 10.1097/DSS.0000000000001547. [DOI] [PubMed] [Google Scholar]
- Hogarty D.T., Su J.C., Phan K., Attia M., Hossny M., Nahavandi S. Artificial intelligence in dermatology-Where we are and the way to the future: a review. Am J Clin Dermatol. 2020;21(1):41–47. doi: 10.1007/s40257-019-00462-6. [DOI] [PubMed] [Google Scholar]
- Ishihara Y., Saida T., Miyazaki A., Koga H., Taniguchi A., Tsuchida T. Early acral melanoma in situ: Correlation between the parallel ridge pattern on dermoscopy and mMicroscopic features. Am J Dermatopathol. 2006;28(1):21–27. doi: 10.1097/01.dad.0000187931.05030.a0. [DOI] [PubMed] [Google Scholar]
- Jiyad Z., Akhras V. Incidence of melanoma and outcomes of longitudinal melanonychia in a cohort of cases referred to a London dermatology department. Br J Dermatol. 2019;181(1):204–205. doi: 10.1111/bjd.17607. [DOI] [PubMed] [Google Scholar]
- Kawabata Y., Ohara K., Hino H., Tamaki K. Two kinds of Hutchinson's sign, benign and malignant. J Am Acad Dermatol. 2001;44(2):305–307. doi: 10.1067/mjd.2001.112398. [DOI] [PubMed] [Google Scholar]
- Kelati A., Aqil N., Baybay H., Gallouj S., Mernissi F.Z. Beyond classic dermoscopic patterns of dermatofibromas: a prospective research study. J Med Case Rep. 2017;11(1):266. doi: 10.1186/s13256-017-1429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.Y., Yun S.J. Cutaneous melanoma in Asians. Chonnam Med J. 2016;52(3):185–193. doi: 10.4068/cmj.2016.52.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga H., Saida T. Revised 3-step dermoscopic algorithm for the management of acral melanocytic lesions. Arch Dermatol. 2011;147(6):741–743. doi: 10.1001/archdermatol.2011.136. [DOI] [PubMed] [Google Scholar]
- Koga H., Saida T., Uhara H. Key point in dermoscopic differentiation between early nail apparatus melanoma and benign longitudinal melanonychia. J Dermatol. 2011;38(1):45–52. doi: 10.1111/j.1346-8138.2010.01175.x. [DOI] [PubMed] [Google Scholar]
- Kryatova M.S., Okoye G.A. Dermatology in the North American Indian/Alaska Native population. Int J Dermatol. 2016;55(2):125–134. doi: 10.1111/ijd.12977. [DOI] [PubMed] [Google Scholar]
- Lallas A., Kyrgidis A., Koga H., Moscarella E., Tschandl P., Apalla Z. The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma. Br J Dermatol. 2015;173(4):1041–1049. doi: 10.1111/bjd.14045. [DOI] [PubMed] [Google Scholar]
- Lallas A., Reggiani C., Argenziano G., Kyrgidis A., Bakos R., Masiero N.C.M.S. Dermoscopic nevus patterns in skin of colour: A prospective, cross-sectional, morphological study in individuals with skin type V and VI. J Eur Acad Dermatol Venereol. 2014;28(11):1469–1474. doi: 10.1111/jdv.12316. [DOI] [PubMed] [Google Scholar]
- Lallas A., Sgouros D., Zalaudek I., Tanaka M., Saida T., Thomas L. Palmar and plantar melanomas differ for sex prevalence and tumor thickness but not for dermoscopic patterns. Melanoma Res. 2014;24(1):83–87. doi: 10.1097/CMR.0000000000000037. [DOI] [PubMed] [Google Scholar]
- Lee D.J.R., Arbache S.T., Quaresma M.V., Nico M.M.S., Gabbi T.V.B. Nail apparatus melanoma: Experience of 10 years in a single institution. Skin Appendage Disord. 2018;5(1):20–26. doi: 10.1159/000488722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madankumar R., Gumaste P.V., Martires K., Schaffer P.R., Choudhary S., Falto-Aizpurua L. Acral melanocytic lesions in the United States: prevalence, awareness, and dermoscopic patterns in skin-of-color and non-Hispanic white patients. J Am Acad Dermatol. 2016;74(4) doi: 10.1016/j.jaad.2015.11.035. 724–30.e1. [DOI] [PubMed] [Google Scholar]
- Mahendraraj K., Sidhu K., Lau C.S.M., McRoy G.J., Chamberlain R.S., Smith F.O. Malignant melanoma in African-americans: a population-based clinical outcomes study involving 1106 African-American patients from the Surveillance, Epidemiology, and End Result (SEER) database (1988–2011) Medicine (Baltimore) 2017;96(15) doi: 10.1097/MD.0000000000006258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy A., Mehrotra A., Edison K., Uscher-Pines L. Variation in dermatologist visits by sociodemographic characteristics. J Am Acad Dermatol. 2017;76(5):918–924. doi: 10.1016/j.jaad.2016.10.045. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Cancer stat facts: Melanoma of the skin [Internet]. 2019 [cited xxx]. Available from: https://seer.cancer.gov/statfacts/html/melan.html.
- O'Leary J.A., Berend K.R., Johnson J.L., Levin L.S., Seigler H.F. Subungual melanoma. A review of 93 cases with identification of prognostic variables. Clin Orthop Relat Res. 2000;378:206–212. [PubMed] [Google Scholar]
- Pellizzari G., Magee J., Weedon D., Rosendahl C. A tiny invasive melanoma: a case report with dermatoscopy and dermatopathology. Dermatol Pract Concept. 2013;3(2):49–51. doi: 10.5826/dpc.0302a06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizner G.T., Chuang T.Y., Elpern D.J., Stone J.L., Farmer E.R. Bowen's disease (squamous cell carcinoma in situ) in Kauai, Hawaii. A population-based incidence report. J Am Acad Dermatol. 1994;31(4):596–600. doi: 10.1016/s0190-9622(94)70222-5. [DOI] [PubMed] [Google Scholar]
- Ruben B.S. Pigmented lesions of the nail unit: clinical and histopathologic features. Semin Cutan Med Surg. 2010;29(3):148–158. doi: 10.1016/j.sder.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Saida T., Koga H., Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38(1):25–34. doi: 10.1111/j.1346-8138.2010.01174.x. [DOI] [PubMed] [Google Scholar]
- Saida T., Miyazaki A., Oguchi S., Ishihara Y., Yamazaki Y., Murase S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140(10):1233–1238. doi: 10.1001/archderm.140.10.1233. [DOI] [PubMed] [Google Scholar]
- Scope A., Marghoob A.A., Dusza S.W., Satagopan J.M., Agero A.L.C., Benvenuto-Andrade C. Dermoscopic patterns of naevi in fifth grade children of the Framingham school system. Br J Dermatol. 2008;158(5):1041–1049. doi: 10.1111/j.1365-2133.2008.08510.x. [DOI] [PubMed] [Google Scholar]
- Sosa-Seda I.M., Valentín-Nogueras S., Figueroa L.D., Sánchez J.L., Mercado R. Clinical and dermoscopic patterns of melanocytic nevi in Hispanic adolescents: a descriptive study. Int J Dermatol. 2014;53(3):280–287. doi: 10.1111/j.1365-4632.2012.5784.x. [DOI] [PubMed] [Google Scholar]
- Takahashi A., Hara H., Aikawa M., Ochiai T. Dermoscopic features of small size pigmented basal cell carcinomas. J Dermatol. 2016;43(5):543–546. doi: 10.1111/1346-8138.13173. [DOI] [PubMed] [Google Scholar]
- Tan A., Stein J.A. Dermoscopic patterns of acral melanocytic lesions in skin of color. Cutis. 2019;103(5):274–276. [PubMed] [Google Scholar]
- Thomas L., Dalle S. Dermoscopy provides useful information for the management of melanonychia striata. Dermatol Ther. 2007;20(1):3–10. doi: 10.1111/j.1529-8019.2007.00106.x. [DOI] [PubMed] [Google Scholar]
- Tuma B., Yamada S., Atallah A.N., Araujo F.M., Hirata S.H. Dermoscopy of black skin: a cross-sectional study of clinical and dermoscopic features of melanocytic lesions in individuals with type V/VI skin compared to those with type I/II skin. J Am Acad Dermatol. 2015;73(1):114–119. doi: 10.1016/j.jaad.2015.03.043. [DOI] [PubMed] [Google Scholar]
- Vivan M.M., Hirata S.H., Santos do Nascimento L. Simões E Silva Enokihara MM. A case of pigmented Bowen's disease. An Bras Dermatol. 2017;92(1):124–125. doi: 10.1590/abd1806-4841.20175381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward-Peterson M., Acuña J.M., Alkhalifah M.K., Nasiri A.M., Al-Akeel E.S., Alkhaldi T.M. Association between race/ethnicity and survival of melanoma patients in the United States over 3 decades: a secondary analysis of SEER data. Medicine (Baltimore) 2016;95(17) doi: 10.1097/MD.0000000000003315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber P., Tschandl P., Sinz C., Kittler H. Dermatoscopy of neoplastic skin lesions: recent advances, updates, and revisions. Curr Treat Options Oncol. 2018;19(11):56. doi: 10.1007/s11864-018-0573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E.A., Rabinovitz H.S., Greene R.S., Oliviero M., Kopf A. Pigmented basal cell carcinoma simulating melanoma in a burn scar. Cutis. 2003;71(5):404–406. [PubMed] [Google Scholar]
- Wozniak-Rito A., Zalaudek I., Rudnicka L. Dermoscopy of basal cell carcinoma. Clin Exp Dermatol. 2018;43(3):241–247. doi: 10.1111/ced.13387. [DOI] [PubMed] [Google Scholar]
- Yang Y., Lin J., Fang S., Han S., Song Z. What's new in dermoscopy of Bowen's disease: two new dermoscopic signs and its differential diagnosis. Int J Dermatol. 2017;56(10):1022–1025. doi: 10.1111/ijd.13734. [DOI] [PubMed] [Google Scholar]
- Zaballos P., Puig S., Llambrich A., Malvehy J. Dermoscopy of dermatofibromas: a prospective morphological study of 412 cases. Arch Dermatol. 2008;144(1):75–83. doi: 10.1001/archdermatol.2007.8. [DOI] [PubMed] [Google Scholar]