Abstract
Background
Patients with skin of color are at risk for skin cancer, pigmentary disorders, and photo-exacerbated conditions but find it challenging to use sunscreens on the market that leave an obvious residue on their skin.
Objective
The objective of this study was to examine sunscreen recommendations from the popular press and from practicing dermatologists for patients with skin of color.
Methods
We queried the Google search engine with the following search terms: “Sunscreen” with “skin of color,” “dark skin,” “black skin.” For comparison, we also searched for “sunscreen” with “white skin,” “pale skin,” and “fair skin.” We conducted an anonymous survey regarding sunscreen recommendations among dermatology trainees and board-certified dermatologists.
Results
Websites with recommendations on sunscreens for patients with skin of color compared with sunscreens for white or fair skin were more likely to recommend chemical sunscreens (70% vs. 36%) and more expensive products (median: $14 vs. $11.3 per ounce), despite the lower sun protection factor level (median: 32.5 vs. 50). In our survey study, dermatologists were overall cost-conscious and felt that sun protection factor level, broad spectrum (ultraviolet A/B protection), and price were the most important features of sunscreens for their patients. Cosmetic elegance was deemed least important. Dermatologists overall counseled patients with skin of color less on sunscreen use, and 42.9% reported that they either never, rarely, or only sometimes take patients’ skin type into account when making sunscreen recommendations.
Conclusion
These data represent an area for growth within dermatology to improve culturally competent care by gaining familiarity with sunscreen types and formulations that are geared toward patients with skin of color.
Keywords: Sunscreen, Chemical, Physical, Skin of color, Dark skin, White skin, Pale skin
Introduction
Patients with skin of color are at risk for skin cancer and photoaging and are uniquely predisposed to pigmentary disorders that are worsened by ultraviolet exposure (Agbai et al., 2014). Importantly, photo-exacerbated pigmentary disorders are one of the most common conditions leading to dermatology consultation in patients with darker skin tones (Alexis et al., 2007, Davis et al., 2012).
Prior consumer studies have shown that cosmetic elegance is the most important feature of sunscreen. In our clinical experience, patients with darker skin tones cite the undesirable white residue that is left on their skin as an impediment to regular sunscreen use (Xu et al., 2016). Dermatology is one of the least diverse specialties in the field of medicine, which may in turn have an impact on dermatologists’ ability to address such unique needs in patients with skin of color (Pandya et al., 2016). The objective of this study was to examine sunscreen recommendations from the popular press and from practicing dermatologists for patients with skin of color.
Methods
Methods for sunscreen recommendations from popular press
We queried the Google search engine with the following search terms: “Sunscreen” with “skin of color,” “dark skin,” “black skin” and for comparison also searched for “sunscreen” with “white skin,” “pale skin,” and “fair skin.” We limited data extraction regarding sunscreen recommendations and associated characteristics (e.g., physical sunscreens containing zinc oxide and titanium dioxide, and chemical sunscreen containing ingredients such as avobenzone) to the first page of results, excluding commercial websites of specific retailers, such as Sephora. The sunscreens were further narrowed to products that were recommended by at least two unique websites. The size and price of sunscreen products were obtained from the brand company’s original website or, if not available, from a major well-known retailer website. The sunscreen recommendations were compared for the two groups using t tests. Sunscreens that were not commercially available were excluded from the study.
Methods for survey of dermatologists
We also conducted an anonymous survey regarding sunscreen recommendations among dermatology trainees and board-certified dermatologists across multiple tertiary institutions associated with Harvard Medical School in Boston, Massachusetts. The results were captured and analyzed using Research Electronic Data Capture (Harris et al., 2009). The study was deemed exempt by the Beth Israel Deaconess Medical Center’s Committee on Clinical Investigations.
Statistical analysis
Patient characteristics are presented as a number (percentage) for categorical variables and as a mean ± standard deviation (or as a median where appropriate) for continuous variables. A t test was used to compare difference in the means of the two groups. A p-value of <.05 was considered statistically significant. The statistical analysis was performed using SAS, version 9.4.
Results
Sunscreen recommendations in popular press
There were 14 unique websites with sunscreen recommendations for patients with skin of color, of which only two websites had the input of a board-certified dermatologist, and none represented a medical or scientific source. There were 88 distinct sunscreens, with 20 sunscreens that were recommended by at least two separate sources (Table 1, Table 2).
Table 1.
Characteristics of sunscreen recommendations in popular press.
| Sunscreen characteristics | Dark/skin of color % (n) | White/pale skin % (n) |
|---|---|---|
| Chemical | 60 (12) | 28.5 (4) |
| Mixture | 10 (2) | 7.1 (1) |
| Physical | 30 (6) | 64.3 (9) |
| Clear | 15 (3) | 0 (0) |
| Tinted | 15 (3) | 21.4 (3) |
| Not tinted | 70 (14) | 78.6 (11) |
| Sun protection factor range | 30–60 | 30–100 |
| Sun protection factor mean | 39 | 47.9 |
| Sun protection factor median | 32.5 | 50 |
| Dollar/ounce range | 6–40 | 2–22.4 |
| Dollar/ounce mean | 17.2 | 11.32 |
| Dollar/ounce median | 14 | 11.33 |
| Spray | 5 (1) | 0 |
| Powder | 5 (1) | 7.1 (1) |
| Cream/lotion | 90 (18) | 92.9 (13) |
| Total | 20 | 14 |
Table 2.
Characteristics of sunscreen recommendations for patients with darker skin tones.
| No. of unique mentions | Sunscreen | Ingredients (physical, chemical, mixture) | Tinted | Sun protection factor | Price | Size, oz | Price per ounce | Vehicle |
|---|---|---|---|---|---|---|---|---|
| 9 | Glossier Invisible Shield | Chemical | Transparent | 35 | $25.00 | 1 | $25.00 | Cream/lotion |
| 9 | Supergoop! Unseen Sunscreen | Chemical | Transparent | 40 | $34.00 | 1.7 | $20.00 | Cream/lotion |
| 7 | Black Girl Sunscreen | Chemical | No | 30 | $18.99 | 3 | $6.33 | Cream/lotion |
| 6 | EltaMD UV Clear Broad-Spectrum | Mixture | No | 46 | $35.00 | 1.7 | $20.59 | Cream/lotion |
| 6 | Mario Bdescu Oil-Free Moisturizer SPF | Chemical | No | 30 | $28.00 | 2 | $14.00 | Cream/lotion |
| 5 | Kate Somerville UncompliKated | Chemical | No | 50 | $38.00 | 3.4 | $11.18 | Spray |
| 3 | Bolden Brightening Moisturizer SPF 30 | Chemical | No | 30 | $28.00 | 2 | $14.00 | Cream/lotion |
| 3 | Colorescience Sunforgettable Brush-On Sunscreen SPF 30 | Physical | Yes | 50 | $65.00 | 0.21 | N/A | Powder |
| 3 | Drunk Elephant Umbra Sheer Physical Daily Defense Broad Spectrum Sunscreen SPF 30 | Physical | No | 30 | $34.00 | 3 | $11.33 | Cream/lotion |
| 3 | La Roche-Posay Anthelios Clear Skin Sunscreen | Chemical | No | 60 | $19.99 | 1.7 | $11.76 | Cream/lotion |
| 3 | Murad City Skin | Physical | Yes | 50 | $68.00 | 1.7 | $40.00 | Cream/lotion |
| 3 | NYDG Chem-Free Active Defense | Physical | No | 30 | $98.00 | 4 | $24.50 | Cream/lotion |
| 3 | Specific Beauty Active Radiance Day Broad Spectrum Facial Moisturizers | Chemical | No | 30 | $40.00 | 1.7 | $23.53 | Cream/lotion |
| 3 | Unsun Tinted Mineral Sunscreen | Physical | Yes | 30 | $29.99 | 1.7 | $17.64 | Cream/lotion |
| 2 | Aveeno Positively Radiant Daily Moisturizer Sunscreen SPF 30 | Chemical | No | 30 | $19.49 | 2.5 | $7.80 | Cream/lotion |
| 2 | CeraVe Hydrating Sunscreen Broad Spectrum SPF 50 | Physical | No | 50 | $14.99 | 2.5 | $6.00 | Cream/lotion |
| 2 | EltaMD UV Moisturizing Facial Sunscreen Broad-Spectrum SPF 30 | Mixture | No | 30 | $30.00 | 4 | $7.50 | Cream/lotion |
| 2 | Julep No Excuses Invisible Sunscreen Gel | Chemical | Transparent | 40 | $20.00 | 1 | $20.00 | Cream/lotion |
| 2 | La Roche Posay Anthelios Melt-In-Sunscreen Milk SPF 60 | Chemical | No | 60 | $35.99 | 5 | $7.20 | Cream/lotion |
| 2 | Murad Essential-C Day Moisture Sunscreen | Chemical | No | 30 | $65.00 | 1.7 | $38.24 | Cream/lotion |
N/A: Not applicable
Of the 20 sunscreens that were most commonly recommended for patients with skin of color, 70% were chemical or mixed chemical/physical sunscreens, and 30% were physical sunscreens. The median sun protection factor (SPF) was 32.5, and the median price was $14 (range, $6-$40) per ounce. The top three most frequently recommended products for patients with skin of color were produced by Glossier, Supergoop, and Black Girl, of which three were chemical sunscreens and two had clear/transparent formulations.
For patients with white or pale skin, there were 12 unique websites with sunscreen recommendations, of which four had the input of a board-certified dermatologist, and none represented a medical or scientific source. There were 113 unique sunscreens, with 14 sunscreens recommended by at least two separate sources (Table 3). Of these 14 sunscreens, 64% were physical sunscreens, and 36% were chemical or mixed chemical/physical sunscreens. The median SPF was 47.9, and the median price was $11.30 (range, $2-$22.40) per ounce. The most frequently recommended products for patients with white or pale skin were produced by Blue Lizard, Colorescience, Aveeno, Badger, EltaMD, and LaRoche-Posay.
Table 3.
Characteristics of sunscreen recommendations for patients with lighter skin tones.
| No. of unique mentions | Sunscreen | Ingredients (physical, chemical, mixture) | Tinted | Sun protection factor | Price | Size, oz | Price per ounce | Vehicle |
|---|---|---|---|---|---|---|---|---|
| 5 | Blue Lizard Australian Sunscreen, Sensitive SPF 30 | Physical | No | 30 | $14.98 | 5 | $3.00 | Cream/lotion |
| 4 | Colorescience Sunforgettable Brush-On Sunscreen SPF 30 | Physical | Yes | 50 | $65.00 | 0.21 | N/A | Powder |
| 3 | Aveeno Protect + Hydrate Lotion Sunscreen With Broad Spectrum SPF 30 | Chemical | No | 30 | $12.49 | 3 | $4.16 | Cream/lotion |
| 3 | Badger SPF 30 Unscented Sunscreen Cream | Physical | No | 30 | $16.99 | 2.9 | $5.86 | Cream/lotion |
| 3 | EltaMD UV Clear Broad-Spectrum SPF 46 Facial Sunscreen | Mixture | No | 46 | $35.00 | 1.7 | $20.59 | Cream/lotion |
| 3 | La Roche-Posay Anthelios Ultra-Light Mineral Sunscreen SPF 50 | Physical | No | 50 | $50.00 | 1.7 | $19.71 | Cream/lotion |
| 2 | Drunk Elephant Umbra Sheer Physical Daily Defense Broad Spectrum Sunscreen SPF 30 | Physical | No | 30 | $34.00 | 3 | $11.33 | Cream/lotion |
| 2 | EltaMD UV Elements Tinted Broad Spectrum SPF 44 | Physical | Yes | 44 | $35.00 | 2 | $17.50 | Cream/lotion |
| 2 | Hawaiian Tropic Antioxidant Sunscreen Lotion 50 | Chemical | No | 50 | $11.99 | 6 | $2.00 | Cream/lotion |
| 2 | Isdin Eryfotona Actinica Broad Spectrum SPF 50+ | Physical | No | 50 | $55.00 | 3.4 | $16.18 | Cream/lotion |
| 2 | Kiehl's Super Fluid UV Mineral Defense Titanium Dioxide Sunscreen Broad Spectrum SPF 50+ | Physical | Yes | 50 | $38.00 | 1.7 | $22.35 | Cream/lotion |
| 2 | La roche-posay anthelios clear skin sunscreen SPF 60 | Chemical | No | 60 | $19.99 | 1.7 | $11.76 | Cream/lotion |
| 2 | MDSolarSciences Mineral Moisture Defense SPF 50 | Physical | No | 50 | $39.00 | 4 | $9.75 | Cream/lotion |
| 2 | Neutrogena Ultra Sheer Dry-Touch SPF 100+ | Chemical | No | 100 | $8.99 | 3 | $3.00 | Cream/lotion |
N/A: Not applicable
None of the top three sunscreens recommended for patients with darker skin tones were recommended by multiple sources for patients with white or pale skin. There were only four sunscreens recommended by at least two sources for both patients with white and skin of color, which were produced by Elta-MD, La-Roche Posay, Drunk Elephant, and Colorescience. The average price per ounce was higher for darker skin tones (median: $14 vs. $11.30 per ounce). The average SPF was lower for darker skin tones (median: 32.5 vs. 50).
Sun protection recommendations by dermatologists
There were 218 surveys sent out to dermatology trainees and dermatology faculty across all hospitals affiliated with Harvard Medical School; 83 participants responded, and 77 consented to participate in the survey (response rate: 35.3%). Survey responders mostly were women (n = 55; 71%), had Fitzpatrick skin type II to III (n = 58; 76%), and were evenly distributed across years out of dermatology training (Table 4). The most important reason dermatologists used sunscreen included prevention of photodamage (n = 74; 97.4%), followed by prevention of skin cancer (n = 59; 77.6%).
Table 4.
Characteristics of dermatology survey respondents.
| Characteristic | n (%) |
|---|---|
| Sex | |
| Female | 55 (71.4) |
| Male | 22 (28.6) |
| Years out of training | |
| 0 (in training) | 16 (20.8) |
| 1–4 | 19 (24.7) |
| 5–10 | 17 (22.1) |
| >10 | 25 (32.5) |
| Fitzpatrick skin type | |
| I–II | 38 (50) |
| III–IV | 33 (43.5) |
| V–VI | 5 (6.6) |
| No. of skin cancers | |
| 0 | 73 (94.8) |
| 1 | 2 (2.6) |
| >1 | 2 (2.6) |
| Type of sunscreen use | |
| Mixture of chemical/physical | 44 (57.1) |
| Physical | 21 (27.3) |
| Chemical | 11 (14.3) |
| None | 1 (1.3) |
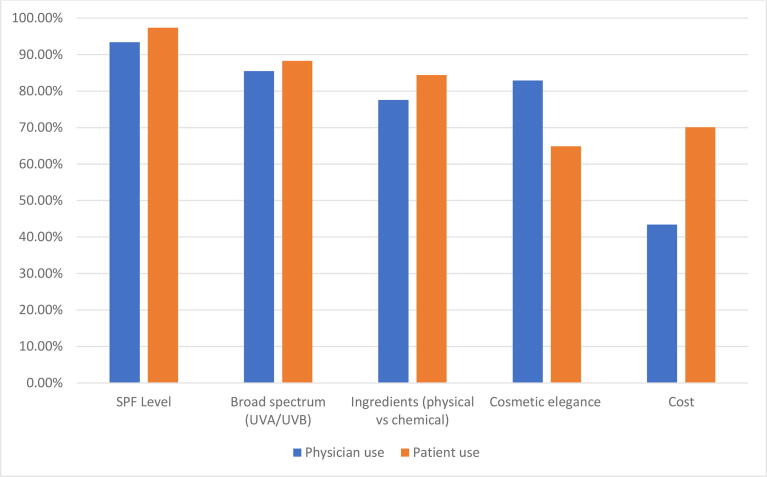
The top criteria for sunscreen use for dermatologists’ personal use included SPF level (n = 71; 93.4%) and broad-spectrum ultraviolet A (UVA)/ultraviolet B (UVB) protection (n = 65; 85.5%), followed by cosmetic elegance/feel (n = 63; 82.9%), and ingredients (n = 59; 77.6%), with cost being the least important (n = 33; 43.4%) criterion (Fig. 1). The top criteria for sunscreen recommendations for patients included SPF level (n = 75; 97.4%), broad spectrum UVA/UVB protection (n = 68; 88.3%), ingredients (n = 65; 84.4%), and cost (n = 54; 70.1%), with cosmetic elegance/feel (n = 50; 64.9%) being the least important criterion. Approximately half of the responding dermatologists (n = 40; 52.6%) reported taking cost into consideration most of the time or always with sunscreen recommendations to their patients.
Fig. 1.
Criteria for sunscreen choice for physician personal use and patient recommendations.
Most dermatologists (n = 75; 97.4%) reported that they counsel patients regarding sunscreen use during most or all visits, but reported that they counsel patients with darker skin types less regarding sun protection sometimes (n = 36; 46.8%), most of the time (n = 14; 18.2%), or always (n = 3; 3.9%). More than half of all dermatologists (n = 44; 57.2%) reported taking patient skin type into account most of the time or always when providing sunscreen recommendations, but the rest (n = 33; 42.9%) reported either never, rarely, or only sometimes taking patient skin type into account. The brands that dermatologists recommend the most frequently to their patients included Neutrogena, Elta MD, Blue Lizard, and CeraVe (Table 5).
Table 5.
Most frequently recommended sunscreen brands by dermatologists.
| Brand | n (%) |
|---|---|
| Neutrogena | 37 (48) |
| Elta MD | 27 (35.1) |
| Cerave | 17 (22.1) |
| La Roche-Posay | 14 (18.2) |
| Vanicream | 9 (11.7) |
Discussion
This study suggests that patients with skin of color encounter beauty magazines or columns when searching for sunscreen recommendations on search engines, which rarely incorporate input from a board-certified dermatologist. It may be helpful for dermatologists to improve their familiarity with the brands and characteristics of these sunscreens geared toward patients with darker skin tones.
In patients with skin of color compared with those with white or pale skin, sunscreen recommendations in the popular press were more likely to contain chemical ingredients (70% vs. 29%, respectively). The top three sunscreens (Glossier, Supergoop, and Black Girl) for darker skin tones were chemical sunscreens, and two had clear or transparent formulations. Therefore, it may be prudent for dermatologists to review the key differences between physical and chemical sunscreens, including increased white residue left on the skin with physical sunscreens, unknown long-term effects of systemic absorption of ingredients in chemical sunscreens, and superior broad UVA/UVB and visible light protection with physical sunscreens for pigmentary or photo-exacerbated conditions (Adamson and Shinkai, 2020, Matta et al., 2020).
Patients with darker skin tones and photo-exacerbated conditions, such as melasma or discoid lupus erythematosus, may seek maximum photoprotection provided by physical sunscreens but find the white residue from physical sunscreen unappealing. Tinted physical sunscreens can be more effective in these scenarios. Most tinted sunscreens recommended by the popular press are produced in one medium color shade, which may still be too light for patients with skin types V to VI. Colorescience’s Sunforgettable brush-on sunscreen was the only physical sunscreen recommended by the popular press that is produced in 4 to 5 different color shades, but it was also one of the most expensive options ($310/oz, excluded from analysis of price comparisons given its powder formulation, which cannot be compared with cream and lotion formulations). Mixing the patient’s foundation with a physical sunscreen is another effective and potentially more cost-effective strategy to decrease the white residue left on the skin.
Despite the lower SPF on average, the price per ounce on average was higher for sunscreens marketed toward patients with darker skin tones. The reasons for the higher prices for this subset of sunscreens are likely multifactorial, including perceived smaller customer base, limited number of companies that market sunscreen products for skin of color, and/or higher manufacturing and distribution costs incurred by smaller companies.
There is tremendous variation in pricing in all sunscreen recommendations for both populations (Xu et al., 2016). If patients use the recommended amount of sunscreen for the head and neck region (0.5 teaspoon), based on the amount of sunscreen needed to achieve the SPF tested at an applied thickness of 2 mg/cm2 (or 2 µL/cm2, assuming a specific gravity of 1), daily use would require 183 teaspoons, 30.5 oz, or approximately 1 L of sunscreen in a year (Schneider, 2002). Daily use of the cheapest sunscreen on the head/neck region recommended for white/pale skin ($2/oz) would lead to an annual cost of $61; for darker skin ($6/oz), the annual cost would be $182. However, daily use of sunscreens produced by Elta-MD, which was the most popular brand recommended by dermatologists in our survey, would lead to an annual cost of $640. Providers should be cognizant of the financial implications or their sunscreen recommendations.
In our survey study, we found that dermatologists try to be cost-conscious when making sunscreen recommendations for patients, despite feeling that cost is less important when considering products for their own use. Dermatologists may not view cost as an obstacle for personal use for a variety of reasons, including but not limited to the following: increased access to sunscreen products that are provided as free samples at national dermatology conferences, increased spending on products that protect against photoaging and skin cancer due to their field of work, and/or decreased financial burden due to their higher socioeconomic status.
As prior studies have shown, the most important criteria for both personal sunscreen use and recommended sunscreens for patients were SPF and broad-spectrum UVA/UVB protection (Fig. 1; Farberg et al., 2017). Interestingly, dermatologists highly valued cosmetic elegance for personal use but viewed cosmetic elegance as the least important factor when making recommendations for patient use. Given that dermatologists require cosmetic acceptability for their own personal use and prior studies demonstrate that cosmetic elegance is the most important feature for consumers, the role of cosmetic elegance for patient use should not be underestimated (Xu et al., 2016). Providers could explore the unique barriers to sunscreen use in each patient; one patient may prefer the most affordable product whereas another would gladly use an expensive sunscreen if certain criteria were met.
More than half of the dermatologists discuss sunscreen use less with patients with darker skin tones, despite 97% of dermatologists reporting that they discuss sun protection in almost all or all visits. This finding is consistent with prior findings that white patients are nine times more likely to be counseled on sunscreen use compared with black patients (Akamine et al., 2014). There may be medical reasons for less counseling, such as the lower incidence of skin cancers in darker skin tones and the unclear role of UV exposure in subtypes of skin cancers (e.g., melanoma in patients with darker skin tones; Agbai et al., 2014, Liu et al., 2016). Nevertheless, decreased counseling may be problematic for several reasons. Skin cancers are caught at a more advanced stage with a worse prognosis in this population (Agbai et al., 2014). Moreover, sun protection is the mainstay of treatment for pigmentary disorders, which have been shown repeatedly to be one of the most common conditions leading to dermatology consultation in this population (Alexis et al., 2007, Davis et al., 2012).
Many dermatologists in our study did not take skin type into consideration when making sunscreen recommendations. This survey statistic is one specific example of the increasingly recognized need for residency training and ongoing learning after graduation to serve the unique needs of patients with darker skin tones, as well as increased representation of dermatology practitioners with skin of color (Imadojemu and James, 2016, Pandya et al., 2016, Taylor, 2019). Patient satisfaction with care during dermatology visits is highly tied to specialized knowledge of black skin and hair, and cursory recommendations to use a physical sunscreen without further discussion of possible associated challenges could make patients feel that providers are unaware of and/or unable to provide individualized treatment recommendations for darker skin (Gorbatenko-Roth et al., 2019, Taylor, 2019). These factors may explain patient preference for race-concordant visits in dermatology, which has also been demonstrated in other specialties (Cooper et al., 2003, Gorbatenko-Roth et al., 2019, LaVeist and Nuru-Jeter, 2002).
Interestingly, the most frequently recommended brands from dermatologists participate in and provide free samples at national dermatology conferences at the American Academy of Dermatology. Whether at the local clinic or national conference level, representation of a wider variety of products may be helpful to allow dermatologists to gain exposure to sunscreen products that are geared toward patients of skin of color.
Limitations
The main study limitation was that this study was not a comprehensive review of all available sunscreen recommendations, especially social media, internet forums, and/or print magazines. Other study limitations include the inability to determine the method by which websites gathered sunscreen recommendations and the limited input of dermatologists in these recommendations. This study also did not evaluate whether the recommended sunscreens for patients with skin of color did indeed leave less of a residue. Study limitations for the survey study include a limited sample size from a restricted geographical region and possible survey response biases. The majority of survey respondents were women, which may have also skewed the study results.
Conclusion
There is a need for increased research and familiarity regarding skin of color in dermatology so that dermatologists may provide knowledgeable, culturally sensitive care. The main sources of information online regarding products, including sunscreen for patients with darker skin tones, are currently beauty magazines and columns created without physician input, which often recommend chemical sunscreens and more expensive products compared with sunscreens for lighter skin tones.
In our survey study, dermatologists were overall cost-conscious and felt that cosmetic elegance was the least important criterion, despite it being the most important criterion for consumers in prior studies (Xu et al., 2016). Dermatologists often counsel patients with skin of color less on sunscreen use and often do not take skin type into consideration when making sunscreen recommendations. These data may represent an area for growth within dermatology to improve culturally competent care.
Conflicts of interest
None.
Funding
None.
Study approval
N/A.
References
- Adamson A.S., Shinkai K. Systemic absorption of sunscreen: balancing benefits with unknown harms. JAMA. 2020;323(3):223–234. doi: 10.1001/jama.2019.20143. [DOI] [PubMed] [Google Scholar]
- Agbai O.N., Buster K., Sanchez M., Hernandez C., Kundu R.V., Chiu M. Skin cancer and photoprotection in people of color: A review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70(4):748–762. doi: 10.1016/j.jaad.2013.11.038. [DOI] [PubMed] [Google Scholar]
- Akamine K.L., Gustafson C.J., Davis S.A., Levender M.M., Feldman S.R. Trends in sunscreen recommendation among US physicians. JAMA Dermatol. 2014;150(1):51–55. doi: 10.1001/jamadermatol.2013.4741. [DOI] [PubMed] [Google Scholar]
- Alexis A.F., Sergay A.B., Taylor S.C. Common dermatologic disorders in skin of color: A comparative practice survey. Cutis. 2007;80(5):387–394. [PubMed] [Google Scholar]
- Cooper L.A., Roter D.L., Johnson R.L., Ford D.E., Steinwachs D.M., Powe N.R. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139(11):907–915. doi: 10.7326/0003-4819-139-11-200312020-00009. [DOI] [PubMed] [Google Scholar]
- Davis S.A., Narahari S., Feldman S.R., Huang W., Pichardo-Geisinger R.O., McMichael A.J. Top dermatologic conditions in patients of color: An analysis of nationally representative data. J Drugs Dermatol. 2012;11(4):466–473. [PubMed] [Google Scholar]
- Farberg A.S., Glazer A.M., Rigel A.C., White R., Rigel D.S. Dermatologists’ perceptions, recommendations, and use of sunscreen. JAMA Dermatol. 2017;153(1):99–101. doi: 10.1001/jamadermatol.2016.3698. [DOI] [PubMed] [Google Scholar]
- Gorbatenko-Roth K., Prose N., Kundu R.V., Patterson S. Assessment of black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155(10):1129–1134. doi: 10.1001/jamadermatol.2019.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imadojemu S., James W.D. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152(1):15–16. doi: 10.1001/jamadermatol.2015.3030. [DOI] [PubMed] [Google Scholar]
- LaVeist T.A., Nuru-Jeter A. Is doctor-patient race concordance associated with greater satisfaction with care? J Health Soc Behav. 2002;43(3):296–306. [PubMed] [Google Scholar]
- Liu L., Zhang W., Gao T., Li C. Is UV an etiological factor of acral melanoma? J Expo Sci Environ Epidemiol. 2016;26(6):539–545. doi: 10.1038/jes.2015.60. [DOI] [PubMed] [Google Scholar]
- Matta M.K., Florian J., Zusterzeel R., Pilli N.R., Patel V., Volpe D.A. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: A randomized clinical trial. JAMA. 2020;323(3):256–267. doi: 10.1001/jama.2019.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya A.G., Alexis A.F., Berger T.G., Wintroub B.U. Increasing racial and ethnic diversity in dermatology: A call to action. J Am Acad Dermatol. 2016;74(3):584–587. doi: 10.1016/j.jaad.2015.10.044. [DOI] [PubMed] [Google Scholar]
- Schneider J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138(6):838–839. doi: 10.1001/archderm.138.6.838-b. [DOI] [PubMed] [Google Scholar]
- Taylor S.C. Meeting the unique dermatologic needs of black patients. JAMA Dermatol. 2019;155(10):1109–1110. doi: 10.1001/jamadermatol.2019.1963. [DOI] [PubMed] [Google Scholar]
- Xu S., Kwa M., Agarwal A., Rademaker A., Kundu R.V. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152(8):920–927. doi: 10.1001/jamadermatol.2016.2344. [DOI] [PubMed] [Google Scholar]