Abstract
Infectious disease (ID) is one of the top-most serious threats to human health globally, further aggravated by antimicrobial resistance and lack of novel immunization options. Andrographis paniculata (Burm. f.) Wall. ex Nees and its metabolites have been long used to treat IDs. Andrographolide, derived from A. paniculata, can inhibit invasive microbes virulence factors and regulate the host immunity. Controlled clinical trials revealed that A. paniculata treatment is safe and efficacious for acute respiratory tract infections like common cold and sinusitis. Hence, A. paniculata, mainly andrographolide, could be considered as an excellent candidate for antimicrobial drug development. Considering the importance, medicinal values, and significant role as antimicrobial agents, this study critically evaluated the antimicrobial therapeutic potency of A. paniculata and its metabolites, focusing on the mechanism of action in inhibiting invasive microbes and biofilm formation. A critical evaluation of the secondary metabolites with the aim of identifying pure compounds that possess antimicrobial functions has further added significant values to this study. Notwithstanding that A. paniculata is a promising source of antimicrobial agents and safe treatment for IDs, further empirical research is warranted.
Keywords: antimicrobial agent, clinical trial, ethnopharmacology, infectious disease, medicinal plant, metabolites, natural products
1. Introduction
Infectious disease (ID) is a serious global health problem that leads to a high mortality rate worldwide every year [1]. The world has recently witnessed a most formidable threat in recent human history, COVID-19, in the modern era of highest advancement of medical sciences. Infectious agents (i.e., invading microbes or pathogens) evolved a variety of strategies, such as modulating their cell surfaces, releasing proteins to inhibit or degrade host immune factors, or even mimicking host molecules to evade the host immunity and ensuring their own survival within a host [2,3,4]. Pathogens, particularly bacteria, are multifaceted in their methods used to escape host immune detection. Due to having the mastery of these camouflaging and precise weaponry techniques by pathogens, developing new vaccines and innovative treatments become challenging, even in some cases almost impossible, for example, treating antibiotic-resistant strains. As a result, the death toll is increasing rapidly. For example, multidrug-resistant tuberculosis (MDR-TB) has recorded around 206,000 new cases in 2019, a 10% increase from 187,000 in 2018, and a total of 1.4 million people died from tuberculosis in 2019 [5]. Furthermore, antibiotics usage causes some common side effects, including hypersensitivity and depletion of beneficial gut microorganism [6,7].
Acute upper respiratory tract infections (URTIs) is another significant cause of antibiotic resistance since physicians needlessly write antibiotic prescriptions [8,9,10,11,12]. Even though the vast majority of URTIs are mild, self-diagnosed and self-treated, they are the most common reason for absenteeism from school or work [13]. URTIs can be mainly caused by viruses, such as a rhinovirus, influenza virus, adenovirus, enterovirus, and respiratory syncytial virus. Bacteria like S. pyogenes, a Group A Streptococcus, may cause roughly 15% of sudden onset pharyngitis presentations [8]. Viral pharyngitis is mainly treated based on the symptoms that appeared, whereas bacterial pharyngitis can be treated with antibiotics. However, current evidence does not support the usefulness of antibiotics treatment in non-specific URTIs [13,14]. Therefore, research is urgently required to find alternatives to conventional medications for eradicating IDs. Natural products based therapy could be an excellent source of antimicrobial agents that would offer symptomatic relief since they have the high potentiality to inhibit the growth of microbes in the host-defence mechanism [4], as well as they offer promising outcomes in the scientific investigations [15,16,17,18,19,20]. Additionally, it would reduce unnecessary antibiotic prescription; therefore, the chances of antibiotic-resistance would be reduced.
Nowadays, the philosophy of drug discovery has transformed into “one drug, multitarget” from “one drug, one target” [16,21,22,23,24,25,26,27,28]. Plant-derived secondary metabolites hold the potential of multi-targeting properties as they need to undergo evolving defence mechanisms of the plant against predators like bacteria, fungi, virus, even insects and herbivores [15,16,21,22,29,30]. A majority of the world population relies on medicinal plants for first-line treatment due to the severe side effects of synthetic drugs [31]. Moreover, plants’ ability to cure diseases and the necessity of their study in sacred texts motivated people to use natural remedies and researchers to study their pharmacology [32,33].
Plant-based secondary metabolites commonly isolated are phenols, tannins, flavonoids, lignans, terpenes, and a wide range of alkaloids [21]. Since natural products are better models with ideal pharmacokinetics/ pharmacodynamics properties [16], often feature biologically relevant molecular scaffolds and pharmacophore patterns that have evolved as preferred ligand-protein binding motif [22], they gained tremendous importance for the development of polypharmacological drugs for IDs, cancers, and neurological disorders [22,34]. Furthermore, about 80% of drugs are either natural products or analogues mimicking them, and steadily increasing approval rate (after the 1990s, the average annual approval rate is 10.3) of natural product-derived drugs from the US Food and Drug Administration (FDA) have encouraged researchers and pharmaceutical industries to search the effective multitarget drugs for various ailments [30,34]. Currently, a number of natural products, including morphine, quinine, reserpine, cocaine, and ephedrine, are now available in pure form as drug substances [15]. Besides, many pure compounds are identified by pharmaceutical scientists worldwide because of having advanced technology that eases and fasten the characterization and structural elucidation of isolated metabolites. Capitalizing on these findings are crucial for medical advancement to overcome unavoidable circumstances happed by synthetic drugs.
One attractive medicinal plant and its metabolites that have gained considerable and progressive interest for decades are Andrographis paniculata (Burm. f.) Wall. ex Nees. This annual plant belongs to the Acanthaceae family and is commonly known as ”King of the bitters” or ”Kalmegh”. It is native to India and Sri Lanka and widely found in Southern and Southeastern Asia, including Bangladesh, China, Hong Kong, Indonesia, Malaysia, Myanmar, Philippines, and Thailand [35,36,37,38,39]. Usually, the aerial parts, roots or leaves of A. paniculata are used separately. These plant parts are used traditionally as powder, infusion, or decoction form either alone or in combination with other medicinal plants for the treatment of leprosy, gonorrhoea, respiratory tract infections, scabies, boils, skin eruptions, chronic and seasonal fevers, griping, irregular bowel habits, loss of appetite, alopecia, general debility, diabetes, jaundice, dyspepsia, hemopathy, cough, oedema, liver complaints, dysentery, malaria, enteritis, helminthiasis, herpes, peptic ulcer, skin infections (topical use), and snake-bites (topical use) [35,36,39,40].
Modern science is focusing on validating the traditional claims of this plant through systemic investigations. Different extracts (i.e., acetone, chloroform, ethanol, hexane, methanolic or aqueous extract) and isolated pure metabolites from A. paniculata have been investigated for pharmacological properties, for example, antibacterial, antiviral, antifungal, antiparasitic, choleretic, hypocholesterolemia, anti-inflammatory, anti-hyperglycemic, hepatoprotective, anticancer, immunomodulatory, cardiovascular, antihyperlipidemic, emollient, anti-snake venom, anti-platelet aggregation, anti-fertility, carminative, and antipyretic properties at in vitro and in vivo conditions [41,42,43,44,45,46,47,48,49,50,51,52,53].
Clinical trials were conducted to validate the in vitro and preclinical antimicrobial pharmacology of A. paniculata. Several clinical trials have justified the anti-infectious activity of A. paniculata against URTIs, influenza, and HIV as well as its effectiveness in treating osteoarthritis and multiple sclerosis [54,55,56,57,58,59]. The clinical studies conducted using the standardized A. paniculata extracts alone or in combination with other medicinal plants (i.e., Kan Jang, KalmCold™). The extracts reported having approximately 4–6% of andrographolide [56,60], responsible for the beneficial effects of the plant extracts. In recent years, there are few more clinical studies conducted [12,61,62,63,64,65,66,67,68,69,70,71,72,73]. However, to date, Andrographolide or A. paniculata extracts have not achieved the human clinical trial stage. This might be because of low bioavailability that is attributed to the fast biotransformation and removal from the body [74] or due to a lack of a well-defined mechanism of action because it is considered as a highly promiscuous compound and engaged in covalent interactions with numerous previously unknown cellular targets in cell type-specific manner [75]. There are two important and typical challenges: (i) poor pharmacokinetics and (ii) limited bioavailability are involved with many natural products, for example, β-sitosterol, quercetin, genistein, rutin etc., prevent their in vitro to preclinical or clinical translation [76]. Nevertheless, there is a numerous investigation reported to have potential efficacy of A. paniculata for IDs as well as other ailments, including clinical efficacy. So, would A. paniculata extracts and their metabolites be a choice of better therapeutics? Would it be undergone for clinical development? Hence, there is a need to explore further the feasibility of A. paniculata with potential use as anti-infective agents for a wide range of invasive microbes. A better understanding of the mode of actions of the active ingredients of A. paniculata would enhance the clinical development of this drug. Additionally, there were several recent in vitro, in vivo, and a few clinical trials reported its potential therapeutic efficacy for antimicrobial pharmacology. To our best knowledge, critical evaluation of A. paniculata’s purported benefits is not substantial yet; hence, it would be important to review them before conducting further research in this area.
Therefore, the objectives of this study were: (i) to establish a comprehensive list of antimicrobial metabolites of A. paniculata, (ii) to discuss the antimicrobial therapeutic potency of A. paniculata and its metabolites, focusing on the mechanisms of actions, (iii) to evaluate the salient achievements of controlled clinical trials systematically to determine its efficacy and safety for the treatment of infections or infection causing agent.
2. Andrographis paniculata—“The King of Bitter”
2.1. Botanical Data of A. paniculata
A. paniculata is an economically important herb of the Andrographis genus. It is an erect and branched and annual flowering herb. This herb grows well in hedgerows throughout the plane lands, hill slopes, waste ground, farms, moist habitat, seashores, and roadsides [38,39]. It can also be grown in the garden as well. For their excellent development, moist and shady places, forests, and wastelands are preferable. It is a salt-sensitive plant [77]; therefore, its growth is limited under stress conditions, particularly salinity stress that drastically affects plant growth and crop productivity [78,79].
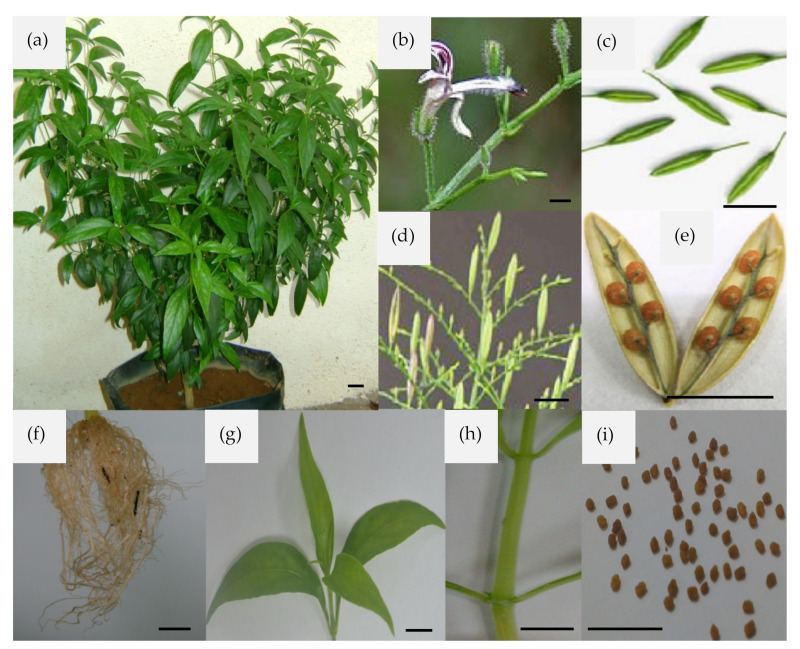
This plant, under cultivation, can reach up to a height of 30 to 110 cm. Its stem is dark green, 30–110 cm in length, 2 to 6 mm in diameter, quadrangular with longitudinal furrows and wings at angles of the young parts, slightly enlarged at the nodes. The leaves are dark green, glabrous, 2–12 cm long and 1–3 cm broad, opposite, decussate, lanceolate, entire margin, and venation pinnate; the petiole is very short. The flowers are small, consist of five linear particle calyx, narrow tube, and about 6 mm long white corolla with rose-purple spots on the petals. December to April is the flowering and fruiting period. The fruits are small 2-celled odourless erected capsules, 1–2 cm long, 2–5 mm wide, linear-oblong, acute at both ends and compressed. The leaf taste is intensely bitter, and seeds are numerous, sub-quadrate and yellowish-brown (Figure 1) [77]. The detailed botanical description has been reviewed somewhere else [39]. Different extracts and their secondary metabolites, particularly andrographolide, are one of the extensively studied natural products. The therapeutically active extracts are prepared, or metabolites isolated from aerial parts, leaves, roots, whole plants (Figure 1) or callus [36,37,80].
Figure 1.
Andrographis paniculata and its different parts. (a) Aerial part, (b) flower, (c) pod stage with panicles: mature capsule, (d) fruit, (e) opened capsule, (f) roots, (g) leaves: opposite arrangement, (h) stem, and (i) seed. Bar = 1 cm. This figure is reproduced from a thesis of the first author of this article [77].
2.2. Recent Progress in Publication of A. paniculata
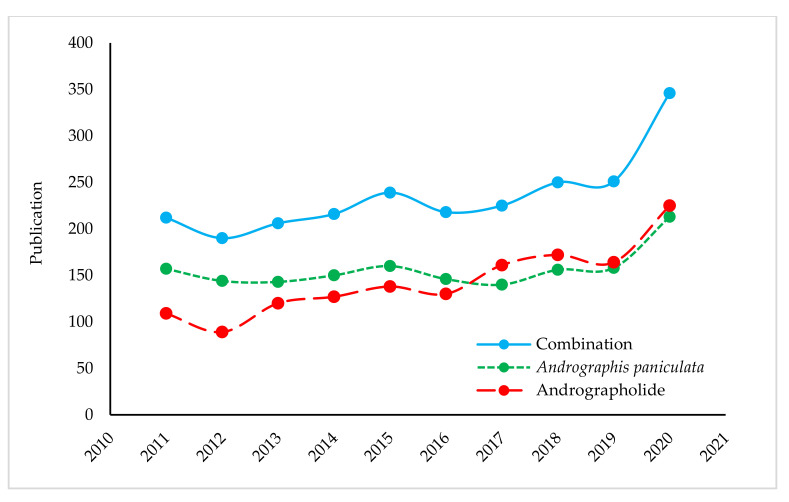
The measurement of scientific interest in a particular topic can be revealed from its trend of publications. Due to having tremendous medicinal importance, the relentless interest in this plant and its versatile molecules have resulted in overwhelming publications over the past ten years. The publication numbers amounted to 3279 (as of 15 February 2021). In other words, we can say, daily, almost one publication (Figure 2), of which about 14% publications were about the antimicrobial study of either A. paniculata extracts or its metabolites, especially andrographolide. This bibliometric data was extracted from the Scopus database using the query of the term “Andrographis paniculata” OR “andrographolide” in titles, abstracts, and keywords. This number might be increased if data can be combined from different databases like PubMed, Web of Science and so on.
Figure 2.
Annual publication statistics on topics covering the A. paniculata and andrographolide from 2011 to 2020 (Scopus).
3. Invasive Microbes Used in the Antimicrobial Study of A. paniculata
There are about 1400 known species of human pathogens. Although this seems like a large number, they are less than 1% of the total number of microbial species on the planet earth [81]. Even though this is less than 1% of total microorganisms, a harmless microbe can sometimes be harmful under a specific condition like an immunocompromised patient. Exploring proper medications for these spontaneous behavioural changing microbes is a continuous effort of the scientific community. A. paniculata extracts and their bioactive molecules were investigated against a wide variety of pathogens, including several antibiotic-resistant species, for example, Staphylococcus aureus, Pseudomonas aeruginosa, Shigella spp., Salmonella spp., Candida spp., Streptococcus pneumoniae. We have identified a total of 59 invasive microbes that have been used to investigate the antimicrobial efficacy of A. paniculata extracts and/or their isolated pure compounds. The categorized microbes included 33 bacterial, four viral, 12 fungal and ten parasite species. The details antimicrobial effectiveness of different extracts has been discussed in the later section. The list of tested microbes, their types, mode of transmission, a disease caused, and infecting organisms are presented in the Supplementary Table S1.
4. Antimicrobial Secondary Metabolites of A. paniculata
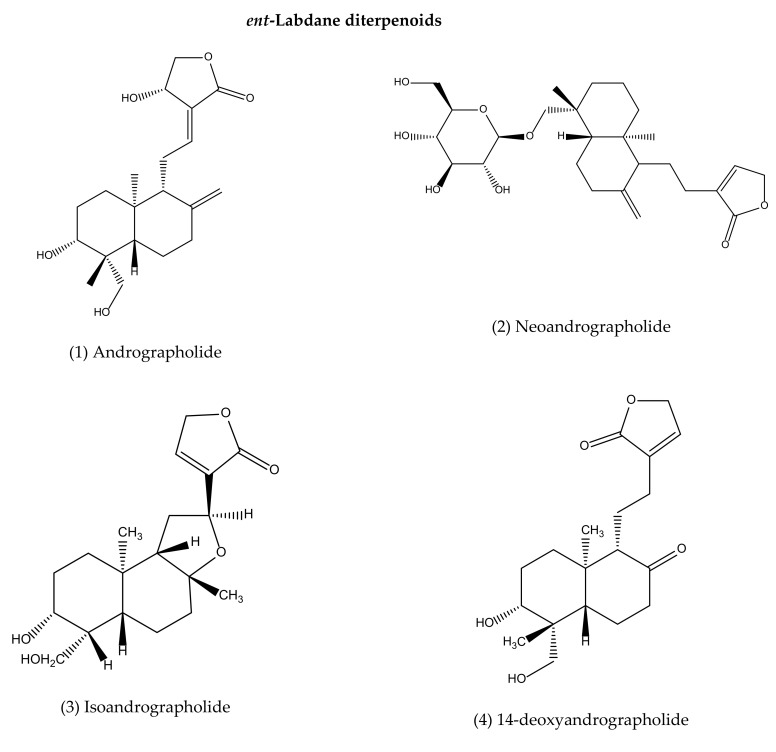
A. Paniculata contains therapeutically active secondary metabolites that include lactones, diterpenes, flavonoids, quinic acid, xanthones, noriridoids, and other compounds. In our previous study [39], we reported more than 55 ent-labdane diterpenoids, 30 flavonoids, eight quinic acid derivatives, four xanthones, and five rare noriridoids in A. paniculata; however, in this study, our extensive review revealed at least 142 secondary metabolites that already isolated from A. paniculata using different plant parts and fractionations of organic solvents (i.e., acetone, butanol, chloroform, ethanol, methanol, and hexane) or water and chromatographic analysis like thin layer chromatography (TLC), high-performance thin-layer chromatography (HPTLC), liquid chromatography, micellar electrokinetic capillary chromatography (MECC), high-speed counter-current chromatography (HSCCC), high-performance liquid chromatography (HPLC), ultra-performance liquid chromatography (UPLC), Silica Gel Chromatography (SGC), and flow injection spectrophotometry (FIS) [65,80,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168]. Among the chromatographic methods, HPLC and TLC are more commonly used. This might be due to easy accessibility and accuracy. The UPLC is good for its selectivity, linearity, precision, accuracy, stability, robustness, the limit of detection and quantification. This method showed good linearity, accuracy and satisfactory precision with a run time of less than 3 min [165].
The identified secondary metabolites included 78 ent-labdane diterpenoids, 41 flavonoids, eight quinic acid derivatives, four xanthones, five rare noriridoids, three steroids and three other compounds (Supplementary Table S2). A recent study also reported a similar number of unique secondary metabolites [169]. Among these compounds, about 80 compounds showed different types of pharmacological activities, including antibacterial [109,139,170,171,172,173,174,175], anti-biofilm [88,176], antiviral (i.e., chikungunya virus [91], Anti-HIV [177,178,179], anti-influenza [99,180], Herpes simplex virus 1 [83,99,181], dengue virus serotype 1 [92,93,94,182], anti-Epstein Barr virus [97], pestivirus and flavivirus [90], human papillomavirus type 16 [96]), anti-fungal [109,183], antiparasitic (i.e., antimalarial [95,184,185], anti-Leishmaniasis [98]), antiproliferative [85,86,87,120,122,124,127,128,177,186], cytotoxicity [84,85,86,101,102,127,186], anti-inflammatory [84,86,136,187], antiplatelet aggregation [102], phagocytosis and anti-complement [84,86,102,104,109,113,114,117,128,133,135,138,142,177]. Some of these metabolites did not exhibit pharmacological properties or showed very week effectiveness. The leads secondary metabolites isolated mainly from aerial parts (majority cases), leaves, whole plant, and some from roots (Supplementary Table S2). The part used, geography, season, and time of harvesting of plant materials significantly influences the quantity and quality of phytoconstituents [39,188].
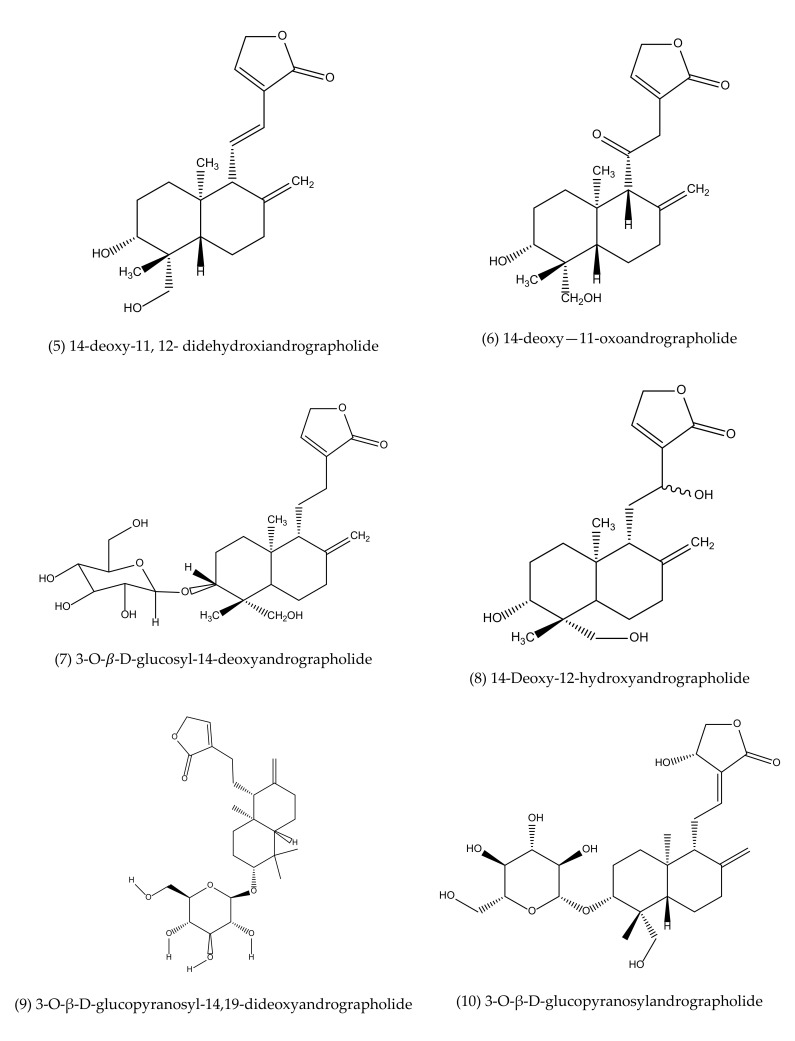
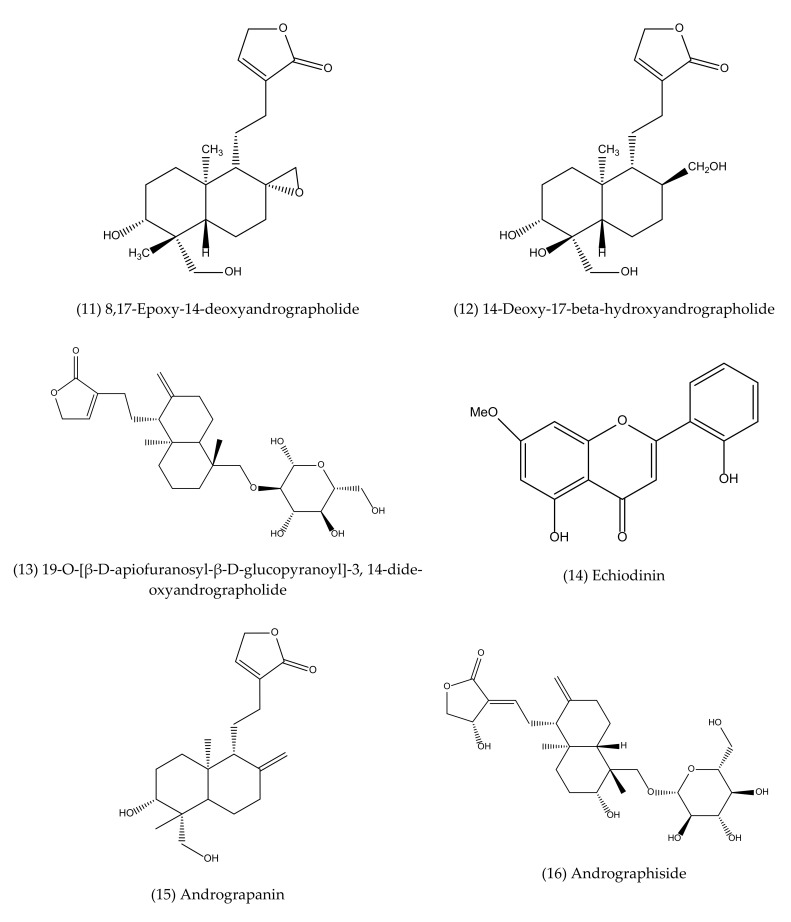
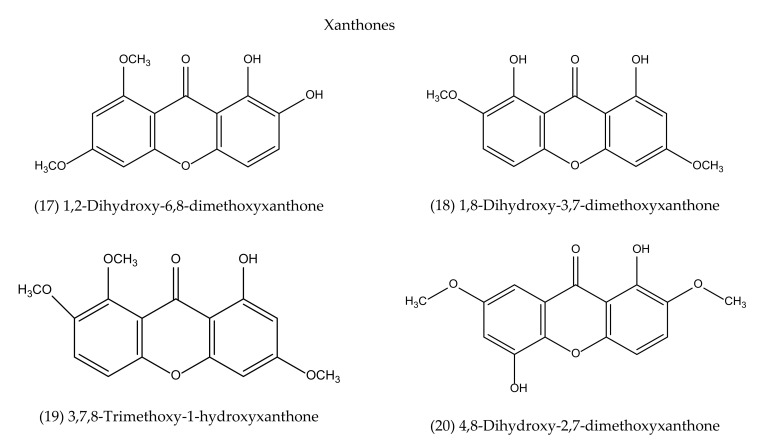
To our best knowledge, a total of 35 isolated compounds have tested for antimicrobial activities, of which 20 secondary metabolites (Figure 3) showed antimicrobial effects (Table 1). Antimicrobial metabolites were extracted from the whole plant, aerial part, leaves and roots (Figure 4). Most of the lead compounds have high potential, particularly compounds (1–7, 14–20) against microbes. However, few compounds (8–13) showed very weak potentiality or were inactive against different strains of bacteria, for example, Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Micrococcus luteus, Sarcina lutea and fungi, such as Candida albicans, Candida sake, and Aspergillus niger [109].
Figure 3.
Antimicrobial agents (pure compounds) of A. paniculata [39,176,192,193].
Table 1.
Isolated secondary metabolites of A. paniculata and their antimicrobial activity.
| No. | Name | Sources | Extraction Solvent |
Analytical Technique |
Antimicrobial Potentiality |
|---|---|---|---|---|---|
| ent-Labdane diterpenoids | |||||
| 1 | Andrographolide | AeP, L, R, WP | AW, E, H, M | HPLC, HPLC-MS, MECC, FIS, UPLC, HPLC-DAD | Antibacterial [139,170,171,172,173], anti-biofilm [88,139], anti-CHIKV [91], Anti-HIV [177,178,179], anti-influenza [99,180], anti-HSV-1 [83,99,181], anti-DVS-1 [92,93,94,182], anti-EBV [97], anti-HPV-16 [96], anti-HBV [186], anti-HCV [194], antimalarial [95,184,185], anti-Leishmaniasis [98], pestivirus and flavivirus [90] |
| 2 | Neoandrographolide | AeP, L, R, WP | AW, E, M | TLC | Anti-HSV-1 [99], antimalarial [184] |
| 3 | Isoandrographolide | AeP, L, R, WP | AW, E, M | HPLC, TLC | Antibacterial [109,174,175], anti-fungal [183] |
| 4 | 14-deoxyandrographolide | AeP, L, WP | AW, E, H, M | HPLC, TLC | Antibacterial [174,175], anti-fungal [183], antimalarial [109,184] |
| 5 | 14-deoxy-11, 12-didehydroxiandrographolide | AeP, L, WP | E, H, M, DCM | HPLC, TLC | Antibacterial [109], anti-biofilm [88], Antifungal [109,183], anti-HIV [177], Anti-HSV [99] |
| 6 | 14-deoxy—11-oxo- andrographolide |
AeP, L | AW, M | SGC | Anti-Leishmaniasis [89,167] |
| 7 | 3-O-β-D-glucosyl-14-deoxy- andrographolide |
AeP, WP | E, M | HPLC, TLC | Antibacterial [174,175], anti-fungal [183] |
| 8 | 14-Deoxy-12-hydroxy- andrographolide |
AeP | AW, M | HPLC, TLC | Antibacterial [109], anti-fungal [109] |
| 9 | 3-O-β-D-glucopyranosyl- 14,19-dideoxyandrographolide | AeP | AW, M | HPLC, TLC | Antibacterial [109], anti-fungal [109] |
| 10 | 3-O-β-D-glucopyranosyl- andrographolide |
AeP | AW, M | HPLC, TLC | Antibacterial [109], anti-fungal [109] |
| 11 | 8,17-Epoxy-14-deoxy-andrographolide | AeP | AW, M | HPLC, TLC | Antibacterial [109], anti-fungal [109] |
| 12 | 14-Deoxy-17-β-hydroxy- andrographolide |
AeP | AW, M | HPLC, TLC | Antibacterial [109], anti-fungal [109] |
| 13 | 19-O-[β-D-apiofuranosyl-β-D-glucopyranoyl]-3,14-dideoxy- andrographolide |
AeP | AW, M | HPLC, TLC | Antibacterial [109], anti-fungal [109] |
| 14 | Echiodinin | Callus | AW, M | TLC | Antibacterial [80] |
| 15 | Andrograpanin | AeP, L | E, H | HPLC, TLC, SGC | Antibacterial [109], anti-fungal [109], Antibiofilm [168,176] |
| 16 | Andrographiside | WP | n-butanol | TLC | Antimalarial [184] |
| Xanthones | |||||
| 17 | 1,2-Dihydroxy-6,8-dimethoxyxanthone | R | CF, M, PE, W | TLC | Antimalarial [190,191] |
| 18 | 1,8-Dihydroxy-3,7-dimethoxyxanthone | R | CF, M, PE, W | TLC | Antimalarial [190,191] |
| 19 | 3,7,8-Trimethoxy-1-hydroxyxanthone | R | CF, M, PE, W | TLC | Antimalarial [190,191] |
| 20 | 4,8-Dihydroxy-2,7-dimethoxyxanthone | R | CF, M, PE, W | TLC | Antimalarial [190,191] |
AeP: Aerial part, AW: Acetone-water, CF: Chloroform, CHIKV: Chikungunya Virus, DCM: Dichloromethane, E: Ethanol, H: Hexane, L: Leaves, M: Methanol. PE: Petroleum ether, R: Root, W: Water, WP: Whole plant, HSV: Herpes Simplex Virus, DVS: Dengue Virus Serotype, EBV: Epstein Barr virus, HPV: Human Papilloma Virus, MECC: micellar electrokinetic capillary chromatography, FIS: flow injection spectrophotometry, TLC: Thin Layer Chromatography, HPLC: High-performance Liquid Chromatography, HPLC-DAD: HPLC with a diode-array detector, HPLC-MS: HPLC-Mass Spectrometry, UPLC: Ultra-performance Liquid Chromatography, SCG: Silica Gel Chromatography.
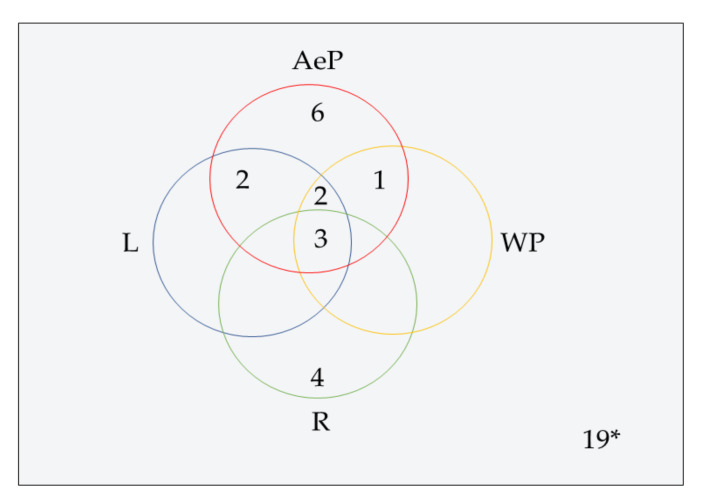
Figure 4.
Venn diagram shows antimicrobial pure metabolites source of part of A. paniculata. AeP: Aerial part, L: Leaves, R: Root, WP: Whole plant. * One metabolite (14) isolated from callus, which is not shown in the diagram.
Andrographolide (1), neoandrographolide (2) and isoandrographolide (3) are the most abundant lead bioactive compounds that can be isolated from any part of A. paniculata for example, aerial apart, leaves, whole plant, and even roots. However, these present in high amounts in leaves [82]. The yield reached the maximum level while the plant materials collected between 110–130 days of cultivation [189]. Compounds 4 and 5 are the next most abundant, followed by 6, 7, 14, 15. The least abundant are compounds 17–20, which are only available in roots [190,191].
5. Antimicrobial Pharmacology
5.1. Antibacterial Effects
For centuries, the traditional use of A. paniculata in treating several IDs caused by bacteria encouraged researchers to study its anti-bacterial properties and how it fights against invasive microbes. Our review found about 33 different types of bacteria that were inhibited by different types of extracts (Table 2 and Supplementary Table S1).
Table 2.
Antibacterial activity of the different types of A. paniculata extracts.
| Plant Part | Extraction Methods | Assay | Number of Test MOs | Most Inhibited Mos | MEIC | ZOI (mm or %) | Remarks | Reference |
|---|---|---|---|---|---|---|---|---|
| AeP | Chloroform | AWDM | 9 | Enterobacter faecalis | 250 μg/mL | 35 | Seven out of 9 pathogens were inhibited that were comparable with antibiotic, amikacin | [203] |
| L | Water | DDM | 5 |
P. aeruginosa S. aureus MRSA |
2 µg/disc 1000 µg/disc 250 µg/disc |
8 ± 0.1 6 ± 0.1 8 ± 0.1 |
No activity against K. pneumoniae and E. coli. | [200] |
| L | 70% Methanol | Two-fold broth MDM | 10 |
Edwardsiella tarda E. coli Flavobacterium sp. P. aeruginosa Vibrio cholerae |
31.5 mg/L | - | All the test MOs were inhibited | [204] |
| WP | DCM | DDM | 12 |
E. faecalis
S. aureus S. saprophyticus |
1000 µg/disc | 21.33 ± 1.53 20.00 ± 1.50 19.33 ± 1.15 |
Gram-negative bacteria were more resistant. | [205] |
| WP | Methanol |
E. faecalis
S. aureus S. saprophyticus |
1000 µg/disc | 24.00 ± 0.00 22.00 ± 0.00 22.00 ± 1.53 |
No activity observed against S. saprophyticus at 250 µg/disc | |||
| WP | Aqueous |
M. luteus
S. pyogenes E. faecalis |
1000 µg/disc | 23.17 ± 0.76 22.67 ± 0.58 22.00 ± 1.00 |
No activity was observed against M. luteus, S. pyogenes, E. faecalis and K. pneumoniae at 250 µg/disc | |||
| R | Hexane | Broth MDM | 4 |
B. pumilus
B. subtilis E. coli Proteus vulgaris |
100 mg/mL 100 mg/mL 200 mg/mL 200 mg/mL |
12 12 13 12 |
Hexane and methanolic extracts were more efficient against all tested MOs | [206] |
| R | Methanol | Broth MDM | 4 |
E. coli
B. subtilis Proteus vulgaris |
100 mg/mL 200 mg/mL 200 mg/mL |
12 12 13 |
||
| WP | Methanol | CPADM | 5 | S. aureus | 1000 μg/mL | 19.67 ± 0.76 | Gram-negative bacteria were more resistant to methanol extracts | [174] |
| AeP | Ethanol | AWDM | 11 |
S. typhi
V. cholerae |
200 μg/mL | 14 13 |
The ethanol extract was efficient | [173] |
| L | Methanol | AWDM | 6 | S. aureus | 50 mg/mL | 24 ± 0.2 | Inhibit both Gram-positive and negative bacteria, but gram-negative bacteria are less susceptible | [201] |
| WP | DCM | DDM | 10 | S. aureus | 1000 μg/disc | 20 ± 1.50 | Aqueous extracts were more effective compared to the DCM and methanol extracts | [207] |
| Methanol |
S. aureus
S.saprophyticus |
1000 μg/disc | 22 ± 0.00 22 ± 1.53 |
|||||
| Aqueous | M. luteus | 1000 μg/disc | 23.17 ± 0.76 | |||||
| WP | Methanol | CPADM | 5 |
S. aureus
M. luteus |
1000 μg/mL | 19.67 ± 0.76 18.50 ± 0.58 |
Effective against all test MOs | [175] |
| L | Chloroform | AWDM | 6 | B. subtilis | 22 ± 0.071 | Chloroform extract of leaves was more efficient to inhibit all tested MOs than other extracts | [208] | |
| Aqueous | 6 |
K. pneumoniae
S. aureus B. subtilis |
12 ± 0.344 12 ± 0.447 12 ± 0.084 |
|||||
| Acetone | 6 | S. aureus | 13 ± 0.416 | |||||
| Ethyl acetate | 6 | B. subtilis | 15 ± 0.152 | |||||
| Petroleum ether | - | - | - | No inhibitory activity | ||||
| R | Chloroform | AWDM | 6 | B. subtilis | 18 ± 0.055 | Chloroform extract of roots was more efficient to inhibit all tested MOs than other extracts | [208] | |
| Aqueous | K. pneumoniae | 14 ± 0.297 | ||||||
| Acetone | S. aureus | 15 ± 0.055 | ||||||
| Ethyl acetate | B. subtilis | 10 ± 0.626 | ||||||
| DMSO | S. aureus | 14 ± 0.187 | ||||||
| Petroleum ether | - | - | - | No inhibitory activity | ||||
| S | Ethyl acetate | AWDM | 6 |
S. aureus
B. subtilis |
8 ± 0.303 8 ± 0.327 |
Chloroform extract of stems was more efficient to inhibit all tested MOs than other extracts | [208] | |
| DMSO | S. aureus | 16 ± 0.332 | ||||||
| Acetone | S. aureus | 16 ± 0.374 | ||||||
| Chloroform | B. subtilis | 24 ± 0.219 | ||||||
| Aqueous | B. subtilis | 13 ± 0.373 | ||||||
| Petroleum ether | - | - | - | No inhibitory activity |
AeP: Aerial part, L: Leaves, R: Root, S: Stem/bark, WP: Whole plant, AWDM: Agar Well Dilution Method, CPADM: Cup-plate Agar Diffusion Method, DCM: Dichloromethane, DDM: Disc Diffusion Method, MEIC: Most Effective Inhibitory Concentration, DMSO: Dimethyl Sulfoxide, FA: Fluorogenic Assay, MDM: Microdilution Method, MOs: Microorganisms, NA: No activity, ZOI: Zone of Inhibition.
5.1.1. A. paniculata Extracts as Antibacterial Agents
Researchers used different types of extracts of A. paniculata to explore their potentiality against numerous invasive microbes, including antibiotic-resistant strains, such as methicillin-resistant S. aureus (MRSA), vancomycin-resistant E. faecalis (VRE), carbapenem-resistant Actinobacillus baumannii, β-Lactamase-negative, ampicillin-resistant (BLNAR) Haemophilus influenzae, P. aeruginosa. A summary of the antibacterial activity of the different types of A. paniculata extracts is shown in Table 2.
The aqueous extract of A. paniculata showed significant antibacterial activity, which was further linked to the presence of andrographolides and arabinogalactan proteins [195]. The role of andrographolide and neoandrographolide in treating bacillary dysentery caused by Shigella sp. was reported in several studies as well [196,197]. In an experiment, A. paniculata was used to treat 1,611 cases of bacterial dysentery and 955 cases of diarrhoea [198]. The efficacy of A. paniculata extracts for dysentery was proved from laboratory stool test with 82.5% and 91.3% [198,199]. However, the crude water extract of A. paniculata leaves exhibited no effect on E. coli and K. pneumoniae. A significant activity against the Gram-positive S. aureus, MRSA, and Gram-negative P. aeruginosa was reported by crude water extracts of leaves sample [200].
In contrast, methanol extract of leaves showed significant activity against E. coli along with P. aeruginosa, K. pneumonia, S. aureus, B. subtilis and Streptococcus epidemidis [201]. Furthermore, no antimicrobial effect of the aqueous extracts of the whole plant and isolated andrographolide on tested common pathogenic bacteria [170]. However, ethanol extracts were found effective against Legionella pneumophila and Bordetella pertussis only [170]. These findings indicated that the extraction process and solvent have a significant role in the efficacy of A. paniculata as the number and yield of pure metabolites greatly differ depending on the types of fractions.
Significant antibacterial activity of subsequent hexane, chloroform, n-butanol, and aqueous fractions of 50% ethyl alcohol-treated extracts of A. paniculata exhibited against E. coli [202]. Similarly, the potent inhibitory effect of ethanol extract of aerial parts on the growth of both gram-positive and gram-negative bacteria, namely, Salmonella typhi, V. cholera, V. alginolyteus, S. aureus, Shigella boydii, Shigella sonnei, E. coli, B. licheniformis, and Salmonella typhimurium. Another study [173] reported that the co-presence of andrographolide and arabinogalactan proteins in the ethanol extract was further acknowledged for its enhanced antibacterial activity compared to andrographolide and arabinogalactan proteins alone. This outcome has been derived maybe because of their synergistic effect.
5.1.2. Isolated Compound as Antibacterial Agent: Mechanisms of Action
A substantial number of evaluations proved the efficacy of different extracts of A. paniculata against many severe pathogenic microbes. Besides this, 13 pure secondary metabolites of A. paniculata were also reported to have significant antibacterial effects (Table 1). These are compounds 1, 3–5, and 7–16. These compounds have been used to evaluate antibacterial potency against a wide range of bacteria. Overall, Gram-positive bacteria were more susceptible to compound 1 than Gram-negative bacteria due to the presence of the outer membrane and the polarity nature of the compound [119,139]. Depending on the bacterial species, the mode of actions of compound 1 differs by a large extent. S. aureus was largely susceptible (MIC is 0.1 mg/mL) to compound 1 among the tested microbes [139]. Healthcare-associated infections are prevalently (10.7%) caused by S. aureus, a major bacterial human pathogen that can form biofilm [209]. It causes a wide variety of clinical manifestations, including pneumonia, mastitis, osteomyelitis, endocarditis, skin infections, abscesses, food poisoning, toxic shock syndrome, and sepsis, and treatment remains challenging due to the emergence of multi-drug resistant strains such as MRSA [210].
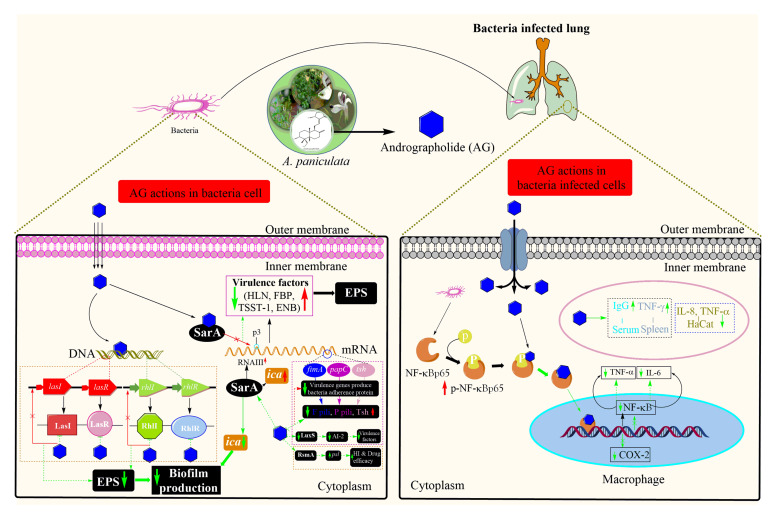
Compound 1 acts on bacteria themselves as well as plays an important role in the regulation of host immunity by regulating macrophage phenotypic polarization and Ag-specific antibody production [211]. When S. aureus infected the lungs, it significantly promotes NF-κB p65 phosphorylation and increases TNF-α and IL-6 production (Figure 5). Compound 1 can downregulate them sufficiently but retain the immune cells at the level that can kill bacteria without serious immune damage. In comparison to penicillin, compound 1 showed better management of bacterial infection and persistent host immunity [212]. Since compound 1 works on immune regulation, there are fewer chances of drug resistance. Therefore, compound 1 would further reduce the problems associated with antibiotic resistance, one of the current severe health crises.
Figure 5.
Schematic representation of a plausible mechanism of actions of andrographolide in bacteria, mainly Staphylococcus aureus. AG: andrographolide ( ), the Black arrow (
), the Black arrow ( ): regular process/pathway, Black dotted rectangle area: AG Induction of Salmonella-specific cell-mediated immune response in Salmonella typhimurum, Blue dotted rectangle area: AG inhibits cellular inflammatory factor in Propionibacterium acnes, COX-2: Cyclooxygenase-2, ENB: Enterotoxin B, FBP: Fibronectin-binding protein, Green dotted arrow (
): regular process/pathway, Black dotted rectangle area: AG Induction of Salmonella-specific cell-mediated immune response in Salmonella typhimurum, Blue dotted rectangle area: AG inhibits cellular inflammatory factor in Propionibacterium acnes, COX-2: Cyclooxygenase-2, ENB: Enterotoxin B, FBP: Fibronectin-binding protein, Green dotted arrow ( ): expected regulation process, Green dotted arrow with a flat bottom (
): expected regulation process, Green dotted arrow with a flat bottom ( ): attachment of AG to the target site, Green down arrow (
): attachment of AG to the target site, Green down arrow ( ): downregulation and expected outcomes, Green up arrow (
): downregulation and expected outcomes, Green up arrow ( ): upregulation and expected outcomes, HaCaT: Human Epidermal Keratinocyte line, HLN: Hemolysin, IFN-γ: Interferon-gamma, IgG: Immunoglobulin G, IL-6: Interleukin 6, IL-8: Interleukin 8, NF-κB: Nuclear factor-kappa-light-chain-enhancer of activated B cell, Orange dotted rectangle area: AG analogue influences the quorum sensing system and inhibits exopolysaccharides generation in Pseudomonas aeruginosa. psl production is also significantly inhibited in this biofilm-forming bacteria, (
): upregulation and expected outcomes, HaCaT: Human Epidermal Keratinocyte line, HLN: Hemolysin, IFN-γ: Interferon-gamma, IgG: Immunoglobulin G, IL-6: Interleukin 6, IL-8: Interleukin 8, NF-κB: Nuclear factor-kappa-light-chain-enhancer of activated B cell, Orange dotted rectangle area: AG analogue influences the quorum sensing system and inhibits exopolysaccharides generation in Pseudomonas aeruginosa. psl production is also significantly inhibited in this biofilm-forming bacteria, ( ): Phosphorylation, Purple dotted rectangle area: AG influences quorum sensing system and reduces the expression of F1 pili, P pili and Tsh by downregulating fimA, papC and tsh in Escherichia coli. All these virulence genes help bacteria to the adherence cell surface. The red arrow (
): Phosphorylation, Purple dotted rectangle area: AG influences quorum sensing system and reduces the expression of F1 pili, P pili and Tsh by downregulating fimA, papC and tsh in Escherichia coli. All these virulence genes help bacteria to the adherence cell surface. The red arrow ( ): Inhibition/downregulation of the process, Red up arrow (
): Inhibition/downregulation of the process, Red up arrow ( ): upregulation (at disease or infection stage), TNF-α: Tumor Necrosis Factor- alpha, TSST-1: Toxic Shock Syndrome Toxin-1.
): upregulation (at disease or infection stage), TNF-α: Tumor Necrosis Factor- alpha, TSST-1: Toxic Shock Syndrome Toxin-1.
In another study, Banerjee et al. [139] reported that compound 1 could strongly inhibit DNA synthesis (approximately 31%) and consequently RNA (about 26% inhibition) and protein (around 36% inhibition) synthesis in S. aureus. This result was similar to that of antibiotic ciprofloxacin (25% incorporation). However, cell wall biosynthesis was not hampered [212]. Secondary metabolites showing antimicrobial can work on a specific target site. Compound 1 can affect the quorum sensing system (QSS), a communication system between bacteria; thereby, it is an effective antibacterial target. This system enhances the production of biofilm by bacteria, such as P. aeruginosa. The antibacterial drug effects in this system resulting in regulates the production of bacterial efflux pumps and virulence factors [213]. Compound 1 effects on the QSS, especially Las and Rhl systems, resulting in reduced production of compositions of extracellular polymeric substance (EPS), such as carbohydrate, nucleotide, and amino acid polymers, as well as inhibiting virulence factors (Figure 5) [214,215]. In addition, compound 1 could restore the antibiotic sensitivity in P. aeruginosa by reducing expression of mexAB-oprM efflux pump [216] and inhibit bacterial adhesion, such as E. coli and S. epidermidis, to the epithelial cells of lungs; therefore, significantly reduced respiratory colonization and level of fimA, papC, and tsh (Figure 5) [217].
5.1.3. Mechanisms of Action Influence on Biofilm Production by Pure Compounds
Among the selected metabolites, compound 1, 5 and 15 exhibited significant anti-biofilm effects against P. aeruginosa [88,176] and S. aureus [139]. Biofilm is a critical part of bacterial pathogenesis in the host. The multilayer structure of biofilm acts as a potential barrier against the host defense and antibiotics action and serves as a sign of antibiotic resistance as well. Biofilm-embedded bacteria are helped to break free from the drugs and weaken as a result [218]. Biofilm forming infections are getting severe health issues daily and significantly enhancing antibiotic resistance; some are relevant to implant-associated infections [219,220]. Exploring efficient drugs are crucial as conventional antibiotics are not sufficient to inhibit the production of biofilm. A. paniculata brought an excellent opportunity to treat biofilm infections as some of its metabolites, for example, compound 5 and 15, possess significant potentiality in inhibiting biofilm formation. Interestingly, A. paniculata metabolites showed synergistic effects with standard antibiotics (i.e., gentamicin and azithromycin), which were unable to reduce biofilm growth alone [88,176]. Compound 5 and 15 showed efficacies in a dose-dependent manner. Compound 15 at 0.125 mM concentration prevented about 54% biofilm formation. Inhibition of biofilm production was further augmented up to 60% while the concentration was used at 0.15 mM [176]. Similarly, compound 5 showed a significant reduction of biofilm growth (about 56%) by P. aeruginosa, whereas compound 1 exhibited only 40% inhibition of biofilm production [88]. Neither gentamicin nor azithromycin was incapable of inhibiting biofilm production of P. aeruginosa. However, the outcome was dramatically changed to about 90% inhibition (p < 0.0001) when the bacteria were treated in a combination of compound 15 and gentamicin [119]. In compound 5, this inhibition becomes about 92% while combined with gentamicin or azithromycin. However, compound 1 showed the least efficacy in inhibiting bacterial biofilm production (about 60% in combination) than the other two compounds [88].
EPS consists of carbohydrate, nucleotide, and amino acid polymers are considered as major components of the biofilm matrix produced by P. aeruginosa [221]. Both compound 5 and 15 significantly reduced the level of biofilm carbohydrate, extracellular DNA, and proteins up to 90% while combined with gentamicin or azithromycin. Unfortunately, compound 1 was not adequate to reduce EPS compositions. They were also efficient to reduce essential virulence factors, the exoprotease activity (up to 93% inhibition), including LasB, rhamnolipid, and pyocyanin in combined treatment as well as inhibit motility movement compared to the antibiotic gentamicin or azithromycin alone [88,176]. This synergism was achieved by inhibiting the process of biofilm development, not by killing microbes. For S. aureus biofilm formation, compound 1 at a concentration of 50 μg/mL diminished biofilm thickness by about 45% on the polystyrene surface compared to the control set after 24 h exposure [139]. Reduction of biofilm thickness was dose-dependent manner, and the outcomes demonstrated that compound 1 has an important role in the inhibition of biofilm production in S. aureus.
5.2. Antiviral Effects
Since the last three decades, researchers have extensively studied the antiviral properties of A. paniculata. Although antiviral activity against a limited number of viruses viz. dengue virus serotype 1 [DENV-1] [182], herpes simplex virus type 1 [HSV-1] [181], influenza A virus [180], HIV [54,177,178,179], hepatitis B [186] and Hepatitis C [194] has been reported, their findings are very encouraging and significant considering the role of these viruses on human morbidity and mortality worldwide.
It is noteworthy that the formation of syncytia in co-culture of HIV-1 infected MOLT cell lines was significantly inhibited by the methanol extracts of A. paniculata [222]. The aqueous bark extracts of A. paniculata was investigated for HIV-1 protease inhibition activity, and this result supports an earlier report by Yao, et al. [223]. They reported positive results, but the extracts were less effective (29.6% and 26.3% inhibition at 250 µg/mL and 25 µg/mL, respectively) against HIV-1 protease [179]. Methanol extracts of A. paniculata showed antiviral effects against dengue virus (DENV) serotype-1 in vitro assay. After treating with the extracts, the viability of DENV-1 infected Vero E6 cells was 113 ± 4.65% with maximum non-toxic dose (0.050 mg/mL), and the percentage of inhibition was 75% [182]. Panraksa, et al. [93] evaluated andrographolide’s anti-viral activity against DENV serotype-2 in HepG2 and HeLa cell lines and DENV serotype-4 in one HepG2 cell line. They found a significant reduction of cellular infection and virus output levels in both cell lines, HepG2 (EC50 = 21.304 μM) and HeLa (EC50 = 22.739 μM) for DENV 2. The anti-viral activity of andrographolide was confined to a post-infection stage [93]. Andrographolide was more potent to inhibit DENV compared to the chikungunya virus (CHIKV). The CHICKV EC50 (77 μM) was about 3.5 fold higher than the DENV, comparable to two different DENV serotypes. In addition, andrographolide affected CHIKV replication [91]. Both cases in HepG2 and HeLa cell lines did not show any toxicity sign after treating with andrographolide at a maximum concentration of 100 μM for 24 h [91,93].
Reddy, et al. [177] have investigated several pure metabolites of A. paniculata, including bis-andrographolide ether, andrographolide, 14-deoxy-11,12-didehydroandrographolide, andrograpanin, 14-deoxyandrographolide, (±)-5-hydroxy-7,8-dimethoxyflavanone and 5-Hydroxy-7,8-dimethoxyflavone against HIV. Among these compounds, only andrographolide and 14-deoxy-11,12-didehydroandrographolide have demonstrated significant anti-HIV properties with (EC50 = 49.0 mg/mL) and (EC50 = 56.8 mg/mL). However, these outcomes were comparatively very less than the standard HIV treatment, azidothymidine (EC50 = 20 ng/mL) used for HIV. They used MT2 cells for the anti-HIV test and found a significant reduction of p24 antigen levels (doses used 5 to 100 mg/mL). The level of p24 antigen is one of the determinants of the anti-HIV effect since it is a viral protein that makes the viral capsid or core, and its expression is highest during the early phase of infection [224]. Andrographolide was also significantly effective to increase CD4+ lymphocytes in HIV patients. HIV RNA copy number was also decreased, but it was not significant [54].
Neoandrographolide, another most potent anti-HIV agent, have a unique C3-O-glucosyl moiety which plays a vital role in the inhibition of furin (IC50 = 53.5 µM). This activity is around 20-fold higher than the andrographolide (IC50 = 1.0 mM and Ki = 200 µM). Moreover, furin is a protease involved in the proteolysis of HIV envelop polyprotein gp120 prior to viral assembly. Gp120 helps viruses to attach to the specific cell surface receptor [225]. A derivative of andrographolide called dehydroandrographolide succinic acid monoester (DASM) was also experimented with in H9 (T-helper-cell line) cells and human peripheral blood mononuclear cells (PBMCs) and reported to have potential anti-HIV activity [178]. DAMS showed immense improved in vitro anti-HIV activity at the concentration of 50–200 (average, 108) µg/mL that was nontoxic to the H9 cells. It also demonstrated the inhibitory effect against HIV-1 and HIV-2 strains. They reported that the subtoxic concentration of DASM (200–400 µg/mL) partially interfered with HIV-induced cell fusion and binding of HIV virions to H9 cells. Probably, DASM might also be interfered with HIV replication by inhibiting HIV infected cell proliferation at another unidentified step(s).
Time- and dose-dependent hepatitis C virus (HCV) replication suppressive effect of andrographolide also reported earlier [194]. The researchers observed the synergistic effect of andrographolide in combination with IFN-α, an inhibitor targeting HCV NS3/4A protease or NS5B polymerase. The andrographolide’s effect was further linked to the up-regulation of heme oxygenase-1, which led to increased amounts of its metabolite (biliverdin) production that promoted the antiviral IFN responses inhibited NS3/4A protease activity which eventually suppressed HCV replication and showed anti-HCV activity [194]. In another study, dehydroandrographolide and andrographolide isolated from A. paniculata reported having hepatitis C virus (HBV) DNA replication suppressive activity with IC50 values of 22.58 54.07 μM and low SI values of 8.7 and 3.7 [186].
Another recent study conducted on human papillomavirus (HPV)-16 pseudovirus (HPV16PsV) to investigate the antiviral effect of andrographolide, it’s derivative- 14-deoxy-11,12-didehydroandrographolide and semi-synthetic analogue- 3,19-isopropylidene andrographolide (IPAD) [226]. They reported that all compounds inhibited HPV16PsV infection, of which 14-deoxy-11,12-didehydroandrographolide showed the highest potency. Additionally, only andrographolide suppressed the long control region (LCR) transcription activity of HPV16 in transiently transfected C33A cells [226].
Chen, et al. [180] reported considerable inhibitory activity (both in vitro and in vivo) of 14-ά-lipoyl andrographolide (AL-1), a synthetic derivative of andrographolide, against influenza A viruses H5N1, H9N2, and H1N1. It successfully prevents mortality in mice against H1N1 infection at the dose of 200 mg/kg/d, and 80% of mice survived at both dosages of 100 gm/kg/d and 200 gm/kg/d against H9N2 and H5N1 infections. It also demonstrated the most effective inhibition of viral adsorption onto red blood cells at the concentration of 5.3 to 16.8 mM, thereby inhibiting virus transmission to the uninfected cells. The similar result was observed by Aromdee, et al. [181] for 14-acetyl analogues of andrographolides (14-acetyl-3,9-isopropylideneandrographolide, 14-acetylandrographolide, 3,14,19-triacetylandrographolide) against HSV-1 in vitro. These three analogues were good for blocking viral entry in the pre-infection step. A cyclic dioxane analogue (3,9-isopropylideneandrographolide) was good for inhibiting viral replication at the post-infection level. However, andrographolide exhibited less effective inhibition activity against influenza A and HSV-1.
Prevention and management of coronavirus disease (COVID-19) have not yet successful since no specific preventive measurement and treatment available. Therefore, searching for potential bioactive compounds from natural sources is an ongoing investigation as medicinal plants possess a tremendous antiviral compound. In recent in silico studies, andrographolide [227], neoandrographolide [228], glycosides 5,4’-dihydroxy-7-O- β-D-pyran-glycuronate butyl ester [229] and glycoside 3-O-β-D-glucopyranosyl- andrographolide [229] have been reported to have a potential role in inhibiting the main protease of SARS-CoV-2, including NSP9, RNA-dependent RNA polymerase, and 6LU7. Andrographolide was docked successfully in the binding site of SARS-CoV-2 Mpro. Their findings revealed that the andrographolide molecule has good solubility, pharmacodynamics property and target accuracy [230]. In another very recent report also stated that andrographolide and its derivative-14-deoxy-11,12-didehydroandrographolide have strong binding affinities with targets. They can modulate the immune system by regulating chemokine signaling, Rap1 signaling, cytokine–cytokine receptor interaction, MAPK signaling, NF-kappa B signaling, RAS signaling, p53 signaling, HIF-1 signaling, and natural killer cell-mediated cytotoxicity [231]. A couple of in silico studies suggest strong interaction of andrographolide and its derivatives against COVID-19 associated target proteins and exhibited different immunoregulatory pathways; thus, these metabolites could be potential candidates for COVID-19 treatment, and further evaluation in vitro and in vivo would be worthy.
5.3. Antifungal Effects
A. paniculata crude extracts have been used for the treatment of fungal infections in folk medicines for centuries. The ethanol crude extract of the whole plant was reported to possess moderate antifungal activity against A. oryzae (60% inhibition) as well as A. niger (<60% inhibition) and Penicillium sp. (<40% inhibition) at 3% (v/v) concentration [232]. The hexane and methanol root extracts were evaluated for their antifungal activity against A. niger and Penicillium chrysogenum. Two concentrations (100 gm/mL and 200 mg/mL) of each extract were studied, which involved determining the inhibition zone diameter for a specific time. It was found that both extracts exhibited significant inhibition, 13 mm and 12 mm at 200 mg/mL concentration against A. niger and Penicillium chrysogenum, respectively. However, these inhibitions were less than the standard fluconazole, 17 mm and 16 mm at 100 µg/mL concentration, respectively [206].
Sule, et al. [183] first reported on the isolation of antifungal bioactive compounds from dichloromethane (DCM) and methanol extracts of A. paniculata whole plant. All the isolated bioactive, 3-O-β-D-glucosyl-14-deoxyandrographolide, 14-deoxyandrographolide and 14-deoxy-11,12-didehydroandrographolide, showed significant antifungal activity against Microsporum canis, A. niger, and C. albicans. MIC values for all antifungal compounds ranged from 50 to 150 μg/mL, and minimum fungicidal concentration (MFC) values ranged from 50 to 200 μg/mL. Among the isolated antifungal substances, 14-deoxyandrographolide exerted the lowest MIC (50 μg/mL) and MFC (50 μg/mL) against M. canis, which indicates the most potent antifungal activity. It is noted that no anti-fungal activity was reported against T. mentagrophytes and T. rubrum at 250 μg/mL. Even though different extracts of A. paniculata reported to have potential anti-fungal activity, the mode of actions yet underreport.
5.4. Anti-Parasitic Effects
A. paniculata and its bioactive compounds have been tested and found extensive anti-parasitic activity against various parasites, such as Ascaris lumbricoidis, Plasmodium falciparum, P. berghei, Trypanosoma cruzi, etc. The extract and fractions reduced parasitaemia level in Mastomys natalensis while used in a dose-dependent manner [184]. Misra, et al. [184] have also studied the anti-malarial activity of the four diterpenes-andrographolide, neoandrographolide, deoxyandrographolide and andrographolide-isolated from A. paniculata and revealed that neoandrographolide (2.5 mg/kg BW) exhibit the highest activity when administered by gastric lavage than other diterpenes.
The fractions of A. paniculata also possessed significant anti-malarial activity [185]. The methanol extract was found to have complete inhibition at a concentration of 2.5 mg/mL by 48 h. Chloroform extract also achieved the same effect by 24 h at only 0.05 mg/mL concentration [233]. The isolated andrographolide also exhibited substantial anti-malarial activity against the MRC-pf-303 strain of P. falciparum and particularly inhibited the parasites at the ring stage [234]. Consequently, the methanol fractions soluble in chloroform were evaluated as significant inhibition of parasitaemia (74%) at the concentration of 1 mg/mL. Additionally, andrographolide showed the highest (53.9%) inhibition of parasitaemia level [235].
It has been found that the alcohol rhizome extract possessed significant in vitro activity against A. lumbricoides [236]. Two reviews were also reported A. paniculata rhizome exhibited extensive activity against A. lumbricoides [35,36]. Dutta and Sukul [237] have studied in vitro anti-filarial activity of leaves decoctions of A. paniculata against Dipetalonema reconditum and found the decoctions kill microfilaria in 40 min. In vivo study revealed that more than 85% of microfilaria in the blood reduced after three subcutaneous injections of the extract into infected dogs at 0.06 mL/kg BW.
6. Controlled Clinical Trials of A. paniculata Treatment: A Systematic Evaluation
A. paniculata and/or its bioactive compounds have been used to treat patients with uncomplicated upper respiratory tract infections (URTIs), including common cold, rhinitis, nasopharyngitis, pharyngitis, and pharyngotonsillitis. The surrounding bacteria and viruses are the usual source of infection for the URTIs. In the treatment of URTIs, pills (made by whole powdered plant and water) and tablets (water extract of the plant) have been used, and the cure rates are 88% and 61%, respectively. The therapeutic effectiveness differed mainly due to the preparation method and duration of treatment [36]. Several randomized, double-blind, placebo-controlled trials proved the efficacy of A. paniculata standardized extracts and/or andrographolide. Some other bioactive compounds treat various infectious diseases associated with cold symptoms and infections caused by virus-like influenza [55,56,57,58,59,60,238,239,240,241,242].
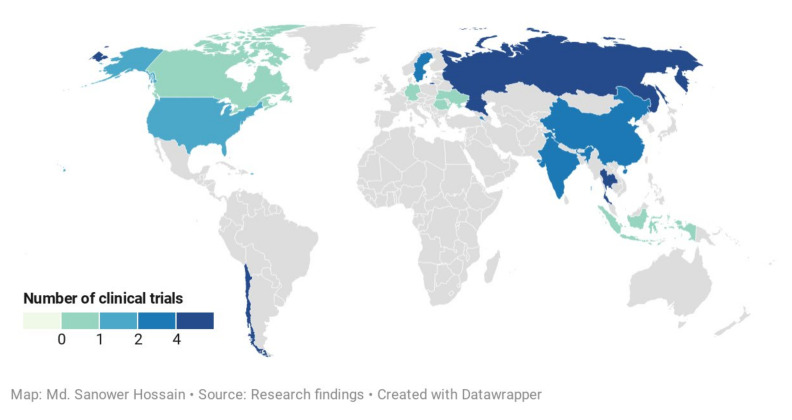
Our systemic investigation revealed a total of 41 individual clinical trials after removing the duplicates from three different databases. After critical evaluation, 23 individual trials (n = 2760) were finally selected for this study which was distributed in 13 different countries (Figure 6). These included 11 controlled clinical trials of the treatment of uncomplicated URTIs (eight studies) [56,58,59,60,238,239,240,242], viral infections (three studies)-influenza (two studies) [55,57] and HIV (1 studies) [54], and one trial focused on the immunity enhancement [241] that prevent the occurrence of the common cold in a rural school-going healthy student (Table 3). The remaining controlled clinical trials are 11 that covered the A. paniculata treatment on the healthy volunteers (three studies)-physiological effects [12], semen quality and fertility [243], pharmacokinetics [244], two studies each for ulcerative colitis [62,63], arthritis pain [66,245], and other studies include one trial each of Familial Mediterranean Fever (FMF) [246], hypertriglyceridemia [64], fatigue [67], type 2 diabetes mellitus [61].
Figure 6.
Global distribution of controlled clinical trials of Andrographis paniculata. (This map indicates the location of clinical studies conducted and color intensity indicated the number of studies frequencies. One trial conducted by Sandborn, et al. [63] covered five countries: the USA, Canada, Germany, Romania, and Ukraine).
Table 3.
Study characteristics and treatment outcomes of controlled clinical trials of Andrographis paniculata in treating uncomplicated upper respiratory tract infections and viral diseases.
| Study ID; Year; Country |
Study Design | Gender & Age | Recruitment(n)/Analyzed(n) | Diagnosis | Study Medications | Daily Dosage (Duration) | Active Ingredients |
Salient Outcomes |
|---|---|---|---|---|---|---|---|---|
| Thamlikitkul, et al. [60]; 1991; Thailand |
R, DB | G: M&F A: 12 y or older |
n = 152/142 CR = (93.43%) AP (LDG) = 48/46 AP (HDG) = 51/47 Paracetamol = 53/49 BCS = NSD (p > 0.05) |
Pharyngotons- illitis |
AP dried leaves extract (250 or 500 mg/capsule) and paracetamol (325 mg/capsule) | 3 capsules 4 xD (7 d) | 6% of AND | There was NSD in the efficacy of relieving fever (p = 0.16) and sore throat (p = 0.49) among 3 groups on day 7. The majority of the Paracetamol and AP (HDG) group patients stopped taking medication on the 3rd day due to relief of symptoms. |
| Hancke, et al. [56]; 1995; Chile |
R, DBPC | G: M&F A: 18–60 y |
n = 59/59 CR = 100% AP = 33/33 P = 28/28 BCS = NSD (p > 0.05) |
Common cold | Monodrug Kan Jang: AP dried extract (100 mg/tablet) |
1.2 g daily (4 d) | 4% of AND | AP extract attenuates the signs of a common cold significantly at day 4 after treatment which is not observed with the placebo (p < 0.05). |
| Caceres, et al. [241]; 1997; Chile |
R, DBPC | G: M&F A: ~18 y |
n = 107/107 CR = 100% AP = 54/54 P = 53/53 |
Healthy volunteer * | Monodrug Kan Jang: AP dried extract (100 mg/tablet) |
Daily 2 tablets, 5 d/w (3 m) | 5.6% of AND | After the third month of treatment, a significant decrease in common colds in the AP group was observed compared to the P group (p < 0.05). |
| Melchior, et al. [58]; 1997; Sweden |
R, DBPC | G: M&F A: 18–55 y |
n = 50/50 CR = 100% AP = 25/25 P = 25/25 BCS = NSD (p > 0.05) |
Common cold sand sinusitis |
AP leaves hydroalcoholic extract (85 mg/tablet) | 4 tablets 3 xD (5 d) | AND & DAND | The total recovery rate was 67.5% and 36% in Kan Jang and placebo group, respectively (p < 0.046). |
| Melchior, et al. [240]; 2000; Sweden |
R, DBPC | G: M&F A: 18–55 y |
n = 47/46 CR = 97.87% AP = 23/23 P = 24/23 BCS = NSD (p > 0.05) |
Uncomplicated acute URTI |
Combination of APE and AS extract (85 mg/tablet) | 3 tablets 4 xD (5–6 d) | 5.25 mg AND & DAND; 9.7 mg per tablet EB and EE | Much improvement in the patient’s overall symptoms cores in TrG was observed compared to P (p = 0.08). |
| Melchior, et al. [240]; 2000; Russia |
R, DBPC | G: M&F A: 18–55 y | n = 180/179 CR = 99.44% AP = 90/89 P = 90/90 BCS = NSD (p > 0.05) |
Uncomplicated acute URTI |
Combination of APE and AS extract (85 mg/tablet) | 3 tablets 4 xD (5–6 d) | 55.25 mg AND & DAND; 9.7 mg per tablet EB and EE | The difference between TrG and P groups was significant for total diagnosis score (p = 0.003) and total symptom score (p = 0.0006). |
| Caceres, et al. [242]; 1999; Chile |
R, DBPC | G: M&F A: 25–50 y | n = 208/158 CR = 87.78% AP = 102/79 P = 106/79 BCS = NSD (p > 0.05) |
Common colds | APE (100 mg/tablet) | 4 tablets 3 xD (5 d) | 5% of total AND & DAND | On day 4 of treatment, the decrease in the intensity and duration of symptoms was highly significant between TrG and P groups (p < 0.001). |
| Gabrielian, et al. [239]; 2002; Armenia |
PG, DBPC | G: M&F A: 15–64 y | n = 200/185 CR = 95.45% AP = 100/95P = 100/90 BCS = NSD (p > 0.05) |
Acute URTIs and sinusitis | Combination of APE and AS extract (85 mg/tablet) | 4 tablets 3 xD (5 d) | 5 mg AND & 10 mg per tablet | Headache, nasal, sore and dry throat, and general malaise showed the most significant improvement (p < 0.001), while cough and eye symptoms did not differ significantly between the groups. |
| Spasov, et al. [238]; 2004; Russia |
RC3PG | G: M&F A: 4–11 y | n = 133/133 CR = 100% Group A (AP) = 53/53 Group B = 41/41 Group C = 39/39 BCS = SD; NSD (BP, p > 0.05) |
Uncomplicated URTI |
Combination of APE and AS extract (85 mg/tablet); Immunal drops: contain EP and ethanol (4:1) | A: 2 tablets 3 xD (10 d); B: 10 drops 3 xD (10 d);C: 500 mg paracetamol 3 xD (10 d) and othersℓ | 5.25 mg AND & DAND; 9.7 mg per tablet EB and EE | Compared with group B (Immunal), the AP extract group considerably improved the development of the disease and intensified children’s recovery with common colds. |
| Saxena, et al. [59]; 2010; India |
R, DBPC | G: M&F A: 18–60 y | n = 223/220 CR = 98.65% AP = 112/112P = 111/108 BCS = NSD (p > 0.05) |
Uncomplicated URTIs | KalmCold™ (dried AP leaves extract; formulated by mixing methanol and water extract) (100 mg/capsule) | 1 capsule 2 xD (5 d) | AND, IAND, NAND, AGN, DDHAND, SCF & MWN |
The intervention group showed 2.1 folds higher improvement in reducing URTI symptoms than the placebo group (p ≤ 0.05). |
| Kulichenko, et al. [57]; 2003; Russia |
RPG | G: M&F A: 19–63 y | n = 540/540 CR = 100% G (AP) = 71/71 Crt = 469/469 BCS = NSD (p > 0.05) |
Influenza | AP: Combination of APE (88.8 mg) and AS (10 mg) extract (100 mg/tablet) Ctr: STD€ |
AP: 2 tablets 3 xD (3–5 d); Ctr: STD€ |
AP: 5.25 mg AND & DAND; 9.7 mg per tablet EB and EE;Ctr: amantadine | Kan Jang treated patients recovered (69.9%) very quickly and reduced the risk of post-influenza complications, while Ctr group recovered 32.2% only (p < 0.001). |
| Kulichenko, et al. [57]; 2003; Russia |
SRC | G: M&F A: 20–63 y | n = 66/66 CR = 100% AP = 35/35 Ctr = 31/31 BCS = NSD (p > 0.05) |
Influenza | AP: Combination of APE (88.8 mg) and AS (10 mg) extract (100 mg/tablet) Ctr: STD€ |
AP: 3 tablets 3 xD (5 d); Ctr: Standard therapy |
AP: 5.25 mg/tablet AND & DAND, EB and EE; Ctr: amantadine | AP extract significantly reduced clinical symptoms and sped up patients’ recovery, and significantly (p < 0.001) decreased the number of days off work and the number of cases with post-influenza complications. |
| Calabrese, et al. [54]; 2000; USA |
NRCT | G: M&F A: 18 y and above | n = 18/17 CR = 94.44% AP = 13/13HV = 5/4 BCS = NSD (p > 0.05) |
HIV | AND | 5, 10, 20 mg/kg BW 3 xD first 3 w, second 3 w and last 3 w, respectively | AND | A dose of 10 mg/kg andrographolide administration significantly (p = 0.002) increased the mean CD4+ lymphocyte count (405 cells/mm3 to 501 cells/mm3) in HIV patients. |
| Chuthaputti, et al. [55]; 2007; Thailand |
RCOL | G: M&F A: 3–15 y | n = 25/25 CR = 100% AP = 15/15 Ctr = 10/10 BCS = NSD (p > 0.05) |
Influenza | AP: Paracetamol and AP aerial part extract (400 mg/capsule); Ctr: Paracetamol (500 mg/tablet) | AP: 4 capsules 4 xD; Ctr: 2 tablets 4 xD (7 d) | 9% of AND, Paracetamol |
The severity of cough, fatigue, and overall symptoms of the TrG were significantly lower than the Ctr group from Day 4 onwards. |
* To improve immunity and diminish the occurrence of common colds among the rural school children; A: Age; AGN: Andrograpanin; AND: Andrographolide; AP: Andrographis paniculata; APE: AP extract; AS: Acanthopanax senticosus; AS: Acanthopanax senticosus; BCS: Baseline characteristics; BP: Bacterial population; BW: Body weight; CR: Compliance rate; CrG: Control group; Ctr: Controlled; d: Day; DAND: Deoxyandrographolide; DAS: Dehydroandrographolide succinate; DBPC: Double-blind, placebo-controlled; DDHAND: 14-deoxy-11,12- didehydroandrographolide; EB: Eleutheroside B; EE: Eleutheroside E; ES: Echinacea purpurea; F: Female; G: Gender; Gr: Group; FMF: Familial Mediterranean Fever, HDG: High dose group; HV: Healthy volunteer; ImmunoGuard®: 370 mg, containing a fixed combination of AP special extract (50 mg) standardized for the content of andrographolide(3-[2-[-decahydro-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylene 1-naphthalenyl] ethyldiene] dihydro-4-hydroxy-2(3H)-furanone)–4 mg, Eleutherococcus senticosus special extract (10 mg) standardized for the content of Eleutherosid E (>0.8 mg), Schizandra chinensis special extract (100 mg) standardized for the content of Schisandrins (>0.8 mg), Glycyrrhiza glabra L extract (10 mg) standardized for the content of Glycyrrhizin (>0.6 mg), 190.3 mg microcristalline cellulose, 7.4 mg Syloid FP, 1.8 mg magnesium stearate, 0.8 mg shellac, 0.1 mg olive oil, and 0.07 mg Macrogol; IAND: Isoandrographolide; IV: Intravenous administration; LDG: Low dose group; M: Male; Mix: Mixture; m: Month; MWN: 7-O-methylwogonin; n: Sample size; NAND: Neoandrographolide; NRCT: Non-Randomized Controlled Trial; NSD: No significant difference; PG: Parallel-group; R: Randomized; R3WC: Randomized, three-way crossover; RC3PG: Randomized Controlled three parallel group; RCOL: randomized controlled open-label; RPG: Randomized Parallel-group; SCF: skullcapflavone I; Rt: Recruitment; SRC: Simple randomization via computer; STD: Standard treatment; STD€: mainly amantadine (antiviral agent) with ascorbic acid as an adjuvant. Each tablet contains 50 mg amantadine. 1st day– 2 tablets 3 xD, 2nd & 3rd day– 2 tablets 2 xD, 4th day– 2 tablets 1 xD; ℓ: standard treatment of lavish warm drinks, throat gargles, antiseptic nose drops, and paracetamol; TD: Treatment duration; TrG: Treatment group; URTIs: Upper respiratory tract infections; VO: Valeriana officinalis; w: Weeks; xD: times daily; Y: Year.
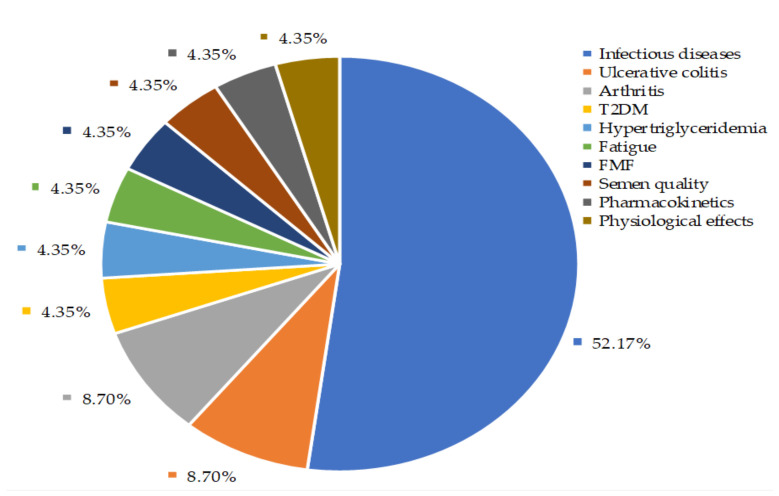
Our findings depicted that about 52% of controlled clinical trials were conducted to investigate the A. paniculata extracts and their bioactive compounds effects on the disappearance of common cold, uncomplicated UTRIs symptoms, as well as recovery from viral infections including influenza and HIV (Figure 7). Among these studies, eight were randomized, double-blind, placebo-controlled studies. The characteristics of these studies and treatment outcomes are shown in Table 3. The details of A. paniculata treatment outcomes on the other health conditions and physiological effects on the healthy volunteer (HV) are given in Supplementary Table S3.
Figure 7.
Controlled clinical trials of Andrographis paniculata. Infectious diseases include uncomplicated upper respiratory infections (UTRI) (i.e., common cold, rhinitis, nasopharyngitis, pharyngitis, and pharyngotonsillitis) and viral diseases (i.e., influenza, HIV). FMF: Familial Mediterranean Fever; T2DM: Type 2 diabetes mellitus.
6.1. Evaluation of A. paniculata Efficacy Against Infections
Eleven randomized, and 1 non-randomized clinical trials (n = 2008) were met inclusion criteria. The compliance rate of this study was 95.97%. Of these, nine were double-blind, of which eight were placebo-controlled. Others were either simple randomized control or randomized parallel-group or randomized controlled open-label. Melchior, et al. [240] and Kulichenko, et al. [57] included one pilot trial and one main trial in one article. Therefore, we split their findings and presented them separately in Table 3.
A comparative, randomized, double-blind study [60] had been done on 152 Thai patients with pharyngotonsillitis to investigate the efficacy of A. paniculata extracts using either paracetamol (3.9 g/day) or encapsulated A. paniculata dried leaves for low dose group (LDG) (3 g/day) or high dose group (HDG) (6 g/day) for seven days. There was no significant difference in efficacy of relieving fever (p = 0.16) and sore throat (p = 0.49) among three groups on day 7. Most of Paracetamol and HDG stopped taking medication on day three because their symptoms had disappeared. However, LDG patients discontinued taking medications due to persistent adverse side effects or worsening symptoms. The incident of mild or self-limiting side effects (i.e., nausea, vomiting, abdominal discomfort, dizziness, drowsiness, and malaise) was not statistically significant among the three groups (p = 0.8), and patients were almost equally satisfied with paracetamol and a high dose of A. paniculata treatment. The findings indicated that daily 6 g of A. paniculata dried leaves extracts that contain at least 6% of andrographolide can be replaced by paracetamol treatment as a standard treatment for the patients with symptoms of pharyngotonsillitis.
In a placebo-controlled study conducted by Caceres, Hancke, Burgos and Wikman [241], Kan Jang tablets, a standardized A. paniculata extract, had been administrated to 107 healthy students in a rural school at a dose of two tablets (200 mg) per day for three months to evaluate the efficacy of Kan Jang to prevent common colds. The common colds were successfully prevented; 2.1-fold higher prevention in the Kan Jang group compared to the placebo group. The incidence of common colds was 30% (16 out of 54) and 62% (33 out of 53) in the Kan Jang group and placebo group, respectively. Melchior, et al. [58] conducted another similar study on 50 volunteers suffering from common colds and sinusitis using Kan Jang tablets for five days. Both subjective symptoms and duration of the symptoms were significantly reduced; 68% of patients recovered entirely in the Kan Jang group. In contrast, only 36% of patients recovered in the placebo group.
Another randomized, double-blind placebo-controlled study on 158 adult patients of both sexes used A. paniculata dried extract SHA-10 for five days to investigate the efficacy in plummeting the prevalence and intensity of sign and symptoms of common colds. The extract (1.2 g/day) showed the ability to reduce the intensity of the symptoms of tiredness, sleeplessness, sore throat, and nasal secretion compared to the placebo group. The intensity of symptoms started to decrease from the second day. All symptoms disappeared on day four without showing any adverse effect [242]. In similar two other Swedenian studies, the Kan Jang, a standardized extract of A. paniculata in a fixed combination with Eleutherococcus senticosus, has been used three times daily for 3–8 days. This study evaluated the efficacy in the treatment of uncomplicated URTIs on 46 and 179 patients, respectively. The treatment group showed highly significant improvement compared to the placebo group in terms of both the total symptom score (p < 0.0006) and total diagnosis score (p < 0.003). In addition, relief of throat symptom was highly significant in both studies [240].
A three-arm clinical study has been carried out on 130 children (ages 4–11 years) over ten days to evaluate the effects of Kan Jang on uncomplicated respiratory disease-common colds. In the three groups, the control group treated with standard common cold treatment and the other two groups adjuvant and adjuvant control group treated with Kan Jang tablets and Immunal (a preparation of Echinacea purpurea L. extract) concomitant to standard treatment, respectively. The Kan Jang showed more significant effects by the rapid recovery process. Using a less standard medication, resulting in the symptoms, particularly nasal secretion and congestion, was less severe at an early stage of uncomplicated common colds in the Kan Jang treatment group. However, Immunal did not exhibit the same efficacy. The use of Kan Jang as an alternative medication was found to be safe and efficacious in treating uncomplicated upper respiratory tract diseases [238].
A randomized, controlled clinical study has also been conducted to investigate Kan Jang’s effects in treating influenza viral infection on 540 patients. In (same as before) group, Kan Jang treated patients recovered very quickly from infections, and Kan Jang reduced the risk of post-influenza complications. Moreover, patients tolerated the Kan Jang very nicely [57]. In addition, the addition of A. paniculata to paracetamol in influenza patients could further reduce the severity of influenza symptoms compared to the control group who was given paracetamol alone [55].
Existing clinical studies showed that A paniculata has a clinical effect on infectious diseases and influences several other diseases. A. paniculata can decrease fasting and postprandial glucose and decrease body mass index in diabetes mellitus patients. A. paniculata also influences mild to moderately active ulcerative patient. Administration of 1800 mg showed a significant clinical response (rectal bleeding) compared to placebo (p = 0.0183) [61].
6.2. Evaluation of Safety of A. paniculata Treatment
The incidents of the adverse effect of treatment with the natural product are sporadic. To our best knowledge, there was no such severe adverse event reported for A. paniculata treatment to the drug safety body. Our critical observations revealed that A. paniculata treatment showed a mild adverse event in some cases. Out of 14 reports (Table 4), six reports stated (n = 579) that they did not observe any adverse effects (or report not provided). These studies cover only 1991–2010. Our systematic investigation did not reveal any clinical studies conducted from 2010 to now. However, several clinical studies conducted using A. paniculata extracts or pure compounds on the healthy volunteer to check semen quality [243], pharmacokinetics and tolerance ability [244], ulcerative colitis [62,63], arthritis [66,245], fatigue [67], Familial Mediterranean Fever [246], hypertriglyceridemia [64], and type 2 diabetes mellitus [61] (Supplementary Table S3).
Table 4.
Adverse events reported in the treatment of human subjects using A. paniculata for uncomplicated upper respiratory tract infections and viral diseases.
| Reference | Study Design | Recruitment(n)/Analyzed(n) | Diagnosis | Adverse Effects (Cases) | |
|---|---|---|---|---|---|
| Treatment | Placebo | ||||
| Thamlikitkul, et al. [60] | R, DB | n = 152/142 | Pharyngotonsillitis | Minimal and self-limiting side effects (i.e., nausea, vomiting, abdominal discomfort, dizziness, drowsiness, and malaise) were found about 20% in treatment (LDG & HDG) and paracetamol groups (9–11). | No placebo groups. |
| Hancke, et al. [56] | R, DBPC | n = 59/59 | Common cold | No adverse event reported | No adverse event reported. |
| Caceres, et al. [241] | R, DBPC | n = 107/107 | HV * | Not reported adverse effect information | Not reported adverse effect information |
| Melchior, et al. [58] | R, DBPC | n = 50/50 | Common colds and sinusitis | Urticaria (2) | No adverse event reported. |
| Melchior, et al. [240] | R, DBPC | n = 47/46 | Uncomplicated acute URTI | No adverse event information reported | No adverse event information reported |
| Melchior, et al. [240] | R, DBPC | n = 180/179 | Uncomplicated acute URTI | Unpleasant sensations in the chest and intensified headache (1) | No adverse event reported. |
| Caceres, et al. [242] | R, DBPC | n = 208/158 | Common colds | No adverse events were observed | No adverse event reported. |
| Gabrielian, et al. [239] | PG, DBPC | n = 200/185 | URTIs and sinusitis | Increase in nasal discharge and epigastric pain (1), nose blocked (1), and severe headache (1). They were excluded from the analysis data. | No adverse event reported. |
| Spasov, et al. [238] | RC3PG | n = 133/133 | Uncomplicated URTI | No side effects were observed | No placebo group |
| Saxena, et al. [59] | R, DBPC | n = 223/220 | URTIs | Mild adverse effect; vomiting (1), epistaxis (1), Urticaria (1) and diarrhoea (3). Except for vomiting (patient in AP group) and urticaria, all other effects stopped spontaneously without any medication. | No adverse event reported. |
| Kulichenko, et al. [57] | RPG | n = 540/540 | Influenza | AP group: Dry cough, rhinitis, and pain in the throat (22). Influenza complications were found in 30.1% of the AP group and 67.8% of the Ctr group (p < 0.01). | No placebo group |
| Kulichenko, et al. [57] | SRC | n = 66/66 | Influenza | Influenza complications were found in 31.43% of AP-treated patients and 70.97% of STD€-treated patients (p < 0.01). | No placebo group |
| Calabrese, et al. [54] | NRCT | n = 18/17 | HIV | Anaphylactic reaction (1). All but one (92%) reported at least one adverse event during the study. ¾ reported an adverse event by the healthy volunteer. All conditions were returned to normal by week 9. | No placebo group |
| Chuthaputti, et al. [55] | RCOL | n = 25/25 | Influenza | No adverse event information reported. | No placebo group |
* To improve immunity and diminish the occurrence of common colds among the rural school children; AP: Andrographis paniculata; DBPC: Double-blind, placebo-controlled; HDG: High dose group; HV: Healthy volunteer; LDG: Low dose group; NRCT: Non-Randomized Controlled Trial; PG: Parallel group; R: Randomized; R3WC: Randomized, three-way crossover; RC3PG: Randomized Controlled three parallel-group; RCOL: randomized controlled open-label; RPG: Randomized Parallel-group; SRC: Simple randomization via computer; STD€: mainly amantadine (antiviral agent) with ascorbic acid as an adjuvant. Each tablet contains 50 mg amantadine; URTIs: Upper respiratory tract infections.
Saxena, et al. [59] (n = 223) reported mild adverse effect (2.73%): vomiting (1 case), epistaxis (1 case), Urticaria (1 case) and diarrhoea (3 case). Except for vomiting (patient in the treatment group) and urticaria, all other effects stopped spontaneously without any medication. Minimal and self-limiting side effects (n = 152) (i.e., nausea, vomiting, abdominal discomfort, dizziness, drowsiness, and malaise) were found about 20% in treatment (LDG & HDG) and paracetamol groups (9–11 cases) [60]. These are pervasive mild effects that usually recovered shortly without any medications. Three cases out of 200 subjects were also reported mild side effects: increase in nasal discharge and epigastric pain (1), nose blocked (1), and severe headache (1) for the treatment of A. paniculata fixed combination Kan Jang in URTIs and sinusitis [239]. For viral infections, the treatment group (Kan Jang) experienced dry cough, rhinitis, and pain in the throat (22 cases out of 540). Control group received antiviral agent amantadine which showed significantly (p < 0.01) higher (67.8% cases) influenza complications compared to treatment group (30.1% cases) [57]. For main trials, influenza complications were found in 31.43% of A. paniculata treated patients and 70.97% of standard medicine (amantadine) treated patients (p < 0.01). one HIV positive experience an anaphylactic reaction in phase I clinical trials [54]. All but one (92%) reported at least one adverse event during the study. About 75% reported an adverse event by the healthy volunteer. All conditions were returned to normal by week 9 [54]. A. paniculata standardized extract treatment of uncomplicated acute URTI patient experienced unpleasant sensations in the chest and intensified headache (1 case out of 180) [240], and for common cold and sinusitis, two patients out of 50 experienced urticaria [58].
Clinical studies other than respiratory infections also reported no adverse effects or mild effects. Only two patients (n = 180) with mild-to-moderate ulcerative colitis experienced severe adverse effects [63]. However, clinical response was significantly higher in the A. paniculata group than in the placebo (p = 0.0183). The best efficacy was observed with the HDG of A. paniculata. In another clinical trial with ulcerative colitis (n = 108), both A. paniculata extract-treated group and control group experiences several side effects, including aphthous ulcer (1), WBC decrease (1), abdominal pain (1), blood in the stool (1), fever (1), elevated glucose (1), rash (1) [treatment group]; blood in the urine (2), elevated CRP (1), WBC decrease (1), blood in the urine (2), fever (1), WBC decrease (1), abdominal pain (1), dry mouth (1), oedema lower extremity (1) cough (2), diarrhoea (2), dizziness and nausea (1), WBC elevated in urine (1) and other [control group], where comparatively treatment groups showed less adverse effects [62]. This indicated that the drug tolerance capacity of ulcerative patients is less regardless type of drugs, and they should be taken precaution measurement before taking any medicine, including A. paniculata.
Considering the type of adverse events and frequency and overall efficacy in treating subjective symptoms of common cold or URTIs either alone or in combination with Acanthopanax senticosus, A. paniculata might be safe for both adults and children. However, the treatment must need to follow the prescribed regime and daily recommended dose. Based on the findings of this study, 90–150 mg andrographolide daily could be recommended as a safe dose for the treatment of URTIs and other similar complications. Additionally, URTIs patients with ulcerative colitis should take extensive precaution taking any medications, and they are not recommended or encouraged for self-medication of any herbal drugs, including A. paniculata.
7. Methodology
The information related to this article was systematically collected from worldwide accepted scientific databases including PubMed (http://www.ncbi.nlm.nih.gov/pubmed (accessed on 27 March 2021)), ScienceDirect (http://www.sciencedirect.com/ (accessed on 27 March 2021)), Scopus (http://www.scopus.com/ (accessed on 27 March 2021)), Web of Science (https://apps.webofknowledge.com/ (accessed on 27 March 2021)), Springer Nature (http://link.springer.com/ (accessed on 27 March 2021)), Wiley Online Library (http://onlinelibrary.wiley.com/ (accessed on 27 March 2021)), and advanced search in Google Scholar (http://scholar.google.com.my/ (accessed on 27 March 2021)), as well as recognized books, abstract, and thesis/dissertation using the keywords “Andrographis paniculata”, “antimicrobial”, “anti-bacterial” “antiviral” “antifungal”. In the aforementioned databases, other relevant papers from the list of references of all available articles were searched. For searching the controlled clinical trials, the following keywords: “antiparasitic”, “clinical trials”, “controlled clinical trials”, and “randomized clinical trials” were used in PubMed, Scopus, and web of science. Controlled clinical trials were systematically screened and selected for further evaluation of their outcomes in this study. The studies were selected for this review (clinical section) if they were controlled clinical trials dealing with A. paniculata to treat infections incredibly uncomplicated upper respiratory tract infection. Used of A. paniculata for other health conditions and healthy volunteers were also selected in this study. English language only restriction was imposed.
8. Conclusions and Recommendations
Human invasive microbes are growing resistant to the available antibiotics for many reasons. As A. paniculata works on immune regulation, there are fewer chances of drug-resistance occurrence. Even though A. paniculata has a potential antimicrobial activity, the detailed study regarding the mode of actions, effects concerning the available antimicrobial agents and specific administration route as well as schedule remain to be explored. The active constituents of A. paniculata could be a potential source of antimicrobial agent, and exploring the therapeutic potentiality of them based on the clinical implications are worthwhile. We have explored substantial antimicrobial agents in A. paniculata (Table 1); however, very little is still known about their molecular mechanisms in response to microbes or host-infected cells. A majority of metabolites are not investigated to identify the molecular target to understand the drug-target-disease network. In addition, our checklist of secondary metabolites of A. paniculata (Supplementary Table S2) can be used for further exploration of their effectiveness because about 44% of metabolites are yet to be evaluated.
The outcomes from clinical effects suggest that A. paniculata offers a promising treatment for alleviating symptoms of infections caused by bacteria or virus that appeared in the URTIs. Nevertheless, a few common mild adverse events were reported from both short term and long term treatments. Therefore, self-medication using A. paniculata should be cautious to avoid potential adverse effects such as vomiting, diarrhea etc. If we consider the overall efficacy of A. paniculata treatment, it would be a worthy consideration as a natural product treatment option for acute URTIs as currently, there is a lack of compelling therapeutic opportunity for IDs.
In some cases, pure compounds from medicinal plants, such as aristolochic acids, possess severe side effects like kidney failure and urinary tract cancers [247]. Therefore, moving forward, the further requires us to take a more comprehensive approach to harness the true potential of A. paniculata for IDs fully. Different disease conditions have diverse responses to the drugs; therefore, to obtain a complete picture of the drug-target-disease network, elucidating each secondary metabolite’s mechanism(s) is crucial. Andrographolide has the potential to target multiple sites since it has shown significant efficacy against different disease conditions. Therefore, this natural product could be considered as a potential candidate for polypharmacology. We would obtain the full advantage of using andrographolide for therapeutics in the near future.
Acknowledgments
Authors are highly thankful to Nazmul Haque and K.M. Hafizur Rahman for their critical review comments and proofreading of this manuscript. Z.U. is pleased to express her profound gratitude to the Institute of Postgraduate Studies, Universiti Malaysia Pahang, to be awarded the Doctoral Research Scheme (DRS) to pursue her PhD in Industrial Biotechnology.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/life11040348/s1, Table S1. Invasive test microbes for the evaluation of antimicrobial efficacy of different extracts of Andrographis paniculata and its isolated metabolites, Table S2. Isolated pure metabolites of Andrographis paniculata and the part used, Table S3. Study characteristics and intervention outcomes of clinical trials of Andrographis paniculata for healthy volunteers and other health complications included familial Mediterranean fever, diabetes mellitus, hypertriglycemia, ulcerative colitis, fatigue and arthritis pain.
Author Contributions
Conceptualization, S.H. and Z.U.; data curation, S.H., H.K. and R.B.M.; writing—original draft preparation, S.H., Z.U., H.K., R.B.M., A.M.Q. and A.M.M.A.; writing—review and editing, S.H., L.C.M., E.P. and R.C.; visualization, S.H.; supervision, S.H. and R.C.; project administration, S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was a in house study, no animal or human subject was involved; therefore, ethical approval was not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bloom D.E., Cadarette D. Infectious Disease Threats in the Twenty-First Century: Strengthening the Global Response. Front. Immunol. 2019;10:549. doi: 10.3389/fimmu.2019.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paczosa M.K., Mecsas J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016;80:629–661. doi: 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Jong M.F., Alto N.M. Cooperative Immune Suppression by Escherichia coli and Shigella Effector Proteins. Infect. Immun. 2018;86:e00517–e00560. doi: 10.1128/IAI.00560-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bizzell E. Microbial Ninja Warriors: Bacterial Immune Evasion. [(accessed on 15 February 2021)]; Available online: https://asm.org/Articles/2018/December/Microbial-Ninja-Warriors-Bacterial-Immune-Evasion.
- 5.WHO Antimicrobial Resistance. [(accessed on 20 October 2020)]; Available online: http://www.who.int/mediacentre/factsheets/fs194/en/
- 6.Namita P., Mukesh R. Medicinal plants used as antimicrobial agents: A review. Int. Res. J. Pharm. 2012;3:31–40. [Google Scholar]
- 7.Levy S.B., Marshall B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004;10:S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 8.Thomas M., Bomar P.A. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2020. Upper Respiratory Tract Infection. [PubMed] [Google Scholar]
- 9.Aabenhus R., Hansen M.P., Saust L.T., Bjerrum L. Characterisation of antibiotic prescriptions for acute respiratory tract infections in Danish general practice: A retrospective registry based cohort study. NPJ Prim. Care Respir Med. 2017;27:37. doi: 10.1038/s41533-017-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connor R., O’Doherty J., O’Regan A., Dunne C. Antibiotic use for acute respiratory tract infections (ARTI) in primary care; what factors affect prescribing and why is it important? A narrative review. Ir. J. Med. Sci. 2018;187:969–986. doi: 10.1007/s11845-018-1774-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Doherty J., Leader L.F.W., O’Regan A., Dunne C., Puthoopparambil S.J., O’Connor R. Over prescribing of antibiotics for acute respiratory tract infections; a qualitative study to explore Irish general practitioners’ perspectives. BMC Fam. Pract. 2019;20:27. doi: 10.1186/s12875-019-0917-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suriyo T., Pholphana N., Ungtrakul T., Rangkadilok N., Panomvana D., Thiantanawat A., Pongpun W., Satayavivad J. Clinical Parameters following Multiple Oral Dose Administration of a Standardized Andrographis paniculata Capsule in Healthy Thai Subjects. Planta Med. 2017;83:778–789. doi: 10.1055/s-0043-104382. [DOI] [PubMed] [Google Scholar]
- 13.Poolsup N., Suthisisang C., Prathanturarug S., Asawamekin A., Chanchareon U. Andrographis paniculata in the symptomatic treatment of uncomplicated upper respiratory tract infection: Systematic review of randomized controlled trials. J. Clin. Pharm. Ther. 2004;29:37–45. doi: 10.1046/j.1365-2710.2003.00534.x. [DOI] [PubMed] [Google Scholar]
- 14.Little A. Review: Antibiotics are not effective for upper respiratory tract infection in children. Evid. Based Nurs. 1999;2:77. doi: 10.1136/ebn.2.3.77. [DOI] [Google Scholar]
- 15.DeCorte B.L. Underexplored Opportunities for Natural Products in Drug Discovery. J. Med. Chem. 2016;59:9295–9304. doi: 10.1021/acs.jmedchem.6b00473. [DOI] [PubMed] [Google Scholar]
- 16.Fang J., Cai C., Wang Q., Lin P., Zhao Z., Cheng F. Systems Pharmacology-Based Discovery of Natural Products for Precision Oncology Through Targeting Cancer Mutated Genes. CPT Pharmacomet. Syst. Pharmacol. 2017;6:177–187. doi: 10.1002/psp4.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazrati S., Govahi M., Sedaghat M., Kashkooli A.B. A comparative study of essential oil profile, antibacterial and antioxidant activities of two cultivated Ziziphora species (Z. clinopodioides and Z. tenuior) Ind. Crop. Prod. 2020;157:7. doi: 10.1016/j.indcrop.2020.112942. [DOI] [Google Scholar]
- 18.De Veras B.O., de Oliveira J.R.S., de Menezes Lima V.L., do Amaral Ferraz Navarro D.M., de Oliveira Farias de Aguiar J.C.R., de Medeiros Moura G.M., da Silva J.W., de Assis C.R.D., Gorlach-Lira K., de Assis P.A.C., et al. The essential oil of the leaves of Verbesina macrophylla (Cass.) S.F.Blake has antimicrobial, anti-inflammatory and antipyretic activities and is toxicologically safe. J. Ethnopharmacol. 2021;265:113248. doi: 10.1016/j.jep.2020.113248. [DOI] [PubMed] [Google Scholar]
- 19.de Araujo M.R.C., Maciel P.P., Castellano L.R.C., Bonan P.R.F., Alves D.D.N., de Medeiros A.C.D., de Castro R.D. Efficacy of essential oil of cinnamon for the treatment of oral candidiasis: A randomized trial. Spec. Care Dent. 2021;9 doi: 10.1111/scd.12570. [DOI] [PubMed] [Google Scholar]
- 20.Freires I.A., Denny C., Benso B., de Alencar S.M., Rosalen P.L. Antibacterial Activity of Essential Oils and Their Isolated Constituents against Cariogenic Bacteria: A Systematic Review. Molecules. 2015;20:7329–7358. doi: 10.3390/molecules20047329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Garcia A., Hosseini S., Martinez-Chapa S.O., Cordell G.A. Multi-target Activities of Selected Alkaloids and Terpenoids. Mini Rev. Org. Chem. 2017;14:272–279. doi: 10.2174/1570193X14666170518151027. [DOI] [Google Scholar]
- 22.Rodrigues T., Reker D., Schneider P., Schneider G. Counting on natural products for drug design. Nat. Chem. 2016;8:531–541. doi: 10.1038/nchem.2479. [DOI] [PubMed] [Google Scholar]
- 23.Keiser M.J., Setola V., Irwin J.J., Laggner C., Abbas A.I., Hufeisen S.J., Jensen N.H., Kuijer M.B., Matos R.C., Tran T.B., et al. Predicting new molecular targets for known drugs. Nature. 2009;462:175–181. doi: 10.1038/nature08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie L., Xie L., Kinnings S.L., Bourne P.E. Novel computational approaches to polypharmacology as a means to define responses to individual drugs. Annu. Rev. Pharmacol. Toxicol. 2012;52:361–379. doi: 10.1146/annurev-pharmtox-010611-134630. [DOI] [PubMed] [Google Scholar]
- 25.Yildirim M.A., Goh K.I., Cusick M.E., Barabasi A.L., Vidal M. Drug-target network. Nat. Biotechnol. 2007;25:1119–1126. doi: 10.1038/nbt1338. [DOI] [PubMed] [Google Scholar]
- 26.Hopkins A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008;4:682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 27.Reddy A.S., Tan Z., Zhang S. Curation and analysis of multitargeting agents for polypharmacological modeling. J. Chem. Inf. Model. 2014;54:2536–2543. doi: 10.1021/ci500092j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy A.S., Zhang S. Polypharmacology: Drug discovery for the future. Expert Rev. Clin. Pharmacol. 2013;6:41–47. doi: 10.1586/ecp.12.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor W.F., Yanez M., Moghadam S.E., Moridi Farimani M., Soroury S., Ebrahimi S.N., Tabefam M., Jabbarzadeh E. 7-epi-Clusianone, a Multi-Targeting Natural Product with Potential Chemotherapeutic, Immune-Modulating, and Anti-Angiogenic Properties. Molecules. 2019;24:4415. doi: 10.3390/molecules24234415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J.W., Vederas J.C. Drug discovery and natural products: End of an era or an endless frontier? Science. 2009;325:161–165. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- 31.Ismail N.A., Hossain M.S., Mustafa N.H.M., Phang I.C. Morpho-physiological characterizatics, selected macronutrient uptak, and oxidative stress level of Andrographis paniculata under salinity condition. J. Teknol. 2015;77:135–140. doi: 10.11113/jt.v77.6721. [DOI] [Google Scholar]
- 32.Hossain M.S., Urbi Z., Evamoni F.Z., Zohora F.T., Rahman K.M.H. A secondary research on medicinal plants mentioned in the Holy Qur’an. J. Med. Plants. 2016;15:81–97. [Google Scholar]
- 33.Urbi Z., Hossain M.S., Rahman K.M.H., Zayed T.M. Grape: A Medicinal Fruit Species in the Holy Qur’an and its Ethnomedinical Importance Department of Basic Medical Sciences, Faculty of Pharmacy. World Appl. Sci. J. 2014;30:253–265. doi: 10.5829/idosi.wasj.2014.30.03.81114. [DOI] [Google Scholar]
- 34.Patridge E., Gareiss P., Kinch M.S., Hoyer D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today. 2016;21:204–207. doi: 10.1016/j.drudis.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Kumar A., Dora J., Singh A., Tripathi R. A review on King of Bitter (Kalmegh) Int. J. Res. Pharm. Chem. 2012;2:116–124. [Google Scholar]
- 36.Akbar S. Andrographis paniculata: A review of pharmacological activities and clinical effects. Altern. Med. Rev. 2011;16:66–77. [PubMed] [Google Scholar]
- 37.Benoy G.K., Animesh D.K., Aninda M., Priyanka D.K., Sandip H. An overview on Andrographis paniculata (Burm. F.) Nees. Int. J. Res. Ayur. Pharm. 2012;3:752–760. [Google Scholar]
- 38.Hossain M.S., Urbi Z. Effect of Naphthalene Acetic Acid on the Adventitious Rooting in Shoot Cuttings of Andrographis paniculata (Burm.f.) Wall. ex Nees: An Important Therapeutical Herb. Int. J. Agron. 2016;2016:1–6. doi: 10.1155/2016/1617543. [DOI] [Google Scholar]
- 39.Hossain M.S., Urbi Z., Sule A., Hafizur Rahman K.M. Andrographis paniculata (Burm. f.) Wall. ex Nees: A review of ethnobotany, phytochemistry, and pharmacology. Sci. World J. 2014;2014:274905. doi: 10.1155/2014/274905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anju D., Jugnu G., Kavita S., Arun N., Sandeep D. A review on medicinal prospectives of Andrographis paniculata Nees. J. Pharm. Sci. Innov. 2012;1:1–4. [Google Scholar]
- 41.Mussard E., Jousselin S., Cesaro A., Legrain B., Lespessailles E., Esteve E., Berteina-Raboin S., Toumi H. Andrographis paniculata and Its Bioactive Diterpenoids Against Inflammation and Oxidative Stress in Keratinocytes. Antioxidants. 2020;9:530. doi: 10.3390/antiox9060530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee D., Baek C.Y., Hwang J.H., Kim M.Y. Andrographis paniculata Extract Relieves Pain and Inflammation in Monosodium Iodoacetate-Induced Osteoarthritis and Acetic Acid-Induced Writhing in Animal Models. Processes. 2020;8:873. doi: 10.3390/pr8070873. [DOI] [Google Scholar]
- 43.Li X., Yuan K., Zhu Q., Lu Q., Jiang H., Zhu M., Huang G., Xu A. Andrographolide Ameliorates Rheumatoid Arthritis by Regulating the Apoptosis-NETosis Balance of Neutrophils. Int. J. Mol. Sci. 2019;20:5035. doi: 10.3390/ijms20205035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu L., Yu Q., Li Q., Zhang L., Lu H., Zhang X. Andrographolide Protects PC12 Cells against β-Amyloid-Induced Autophagy-Associated Cell Death Through Activation of the Nrf2-Mediated p62 Signaling Pathway. Int. J. Mol. Sci. 2018;19:2844. doi: 10.3390/ijms19092844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mussard E., Cesaro A., Lespessailles E., Legrain B., Berteina-Raboin S., Toumi H. Andrographolide, a Natural Antioxidant: An Update. Antioxidants. 2019;8:571. doi: 10.3390/antiox8120571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akhtar M.T., Bin Mohd Sarib M.S., Ismail I.S., Abas F., Ismail A., Lajis N.H., Shaari K. Anti-Diabetic Activity and Metabolic Changes Induced by Andrographis paniculata Plant Extract in Obese Diabetic Rats. Molecules. 2016;21:1026. doi: 10.3390/molecules21081026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qader S.W., Abdulla M.A., Chua L.S., Najim N., Zain M.M., Hamdan S. Antioxidant, total phenolic content and cytotoxicity evaluation of selected Malaysian plants. Molecules. 2011;16:3433–3443. doi: 10.3390/molecules16043433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y.T., Chen H.W., Lii C.K., Jhuang J.H., Huang C.S., Li M.L., Yao H.T. A Diterpenoid, 14-Deoxy-11, 12-Didehydroandrographolide, in Andrographis paniculata Reduces Steatohepatitis and Liver Injury in Mice Fed a High-Fat and High-Cholesterol Diet. Nutrients. 2020;12:523. doi: 10.3390/nu12020523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ismail S., Hanapi N.A., Ab Halim M.R., Uchaipichat V., Mackenzie P.I. Effects of Andrographis paniculata and Orthosiphon stamineus extracts on the glucuronidation of 4-methylumbelliferone in human UGT isoforms. Molecules. 2010;15:3578–3592. doi: 10.3390/molecules15053578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loh S.H., Tsai Y.T., Huang S.F., Yu T.C., Kuo P.C., Chao S.C., Chou M.F., Tsai C.S., Lee S.P. Effects of Andrographolide on Intracellular pH Regulation, Cellular Migration, and Apoptosis in Human Cervical Cancer Cells dagger. Cancers. 2020;12:387. doi: 10.3390/cancers12020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Panossian A., Brendler T. The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections. Pharmaceuticals. 2020;13:236. doi: 10.3390/ph13090236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y., Jiao J., Yang Y., Yang M., Zheng Q. Screening and Identification for Immunological Active Components from Andrographis Herba Using Macrophage Biospecific Extraction Coupled with UPLC/Q-TOF-MS. Molecules. 2018;23:1047. doi: 10.3390/molecules23051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaur R., Sharma P., Gupta G.K., Ntie-Kang F., Kumar D. Structure-Activity-Relationship and Mechanistic Insights for Anti-HIV Natural Products. Molecules. 2020;25:2070. doi: 10.3390/molecules25092070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calabrese C., Berman S.H., Babish J.G., Ma X., Shinto L., Dorr M., Wells K., Wenner C.A., Standish L.J. A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother. Res. 2000;14:333–338. doi: 10.1002/1099-1573(200008)14:5<333::AID-PTR584>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 55.Chuthaputti A., Pornpatkul V., Suwankiri U. The Efficacy of Andrographis paniculata (Burm. f.) Wall. ex Nees for the Relief of the Symptoms of Influenza. J. Thai Tradit. Altern. Med. 2007;5:1–10. [Google Scholar]
- 56.Hancke J., Burgos R., Caceres D., Wikman G. A double-blind study with a new monodrug Kan Jang: Decrease of symptoms and improvement in the recovery from common colds. Phytother. Res. 1995;9:559–562. doi: 10.1002/ptr.2650090804. [DOI] [Google Scholar]
- 57.Kulichenko L.L., Kireyeva L.V., Malyshkina E.N., Wikman G. A randomized, controlled study of Kan Jang versus amantadine in the treatment of influenza in Volgograd. J. Herb. Pharmacother. 2003;3:77–93. doi: 10.1080/J157v03n01_04. [DOI] [PubMed] [Google Scholar]
- 58.Melchior J., Palm S., Wikman G. Controlled clinical study of standardized Andrographis paniculata extract in common cold—A pilot trial. Phytomedicine. 1997;3:315–318. doi: 10.1016/S0944-7113(97)80002-5. [DOI] [PubMed] [Google Scholar]
- 59.Saxena R.C., Singh R., Kumar P., Yadav S.C., Negi M.P., Saxena V.S., Joshua A.J., Vijayabalaji V., Goudar K.S., Venkateshwarlu K., et al. A randomized double blind placebo controlled clinical evaluation of extract of Andrographis paniculata (KalmCold) in patients with uncomplicated upper respiratory tract infection. Phytomedicine. 2010;17:178–185. doi: 10.1016/j.phymed.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 60.Thamlikitkul V., Dechatiwongse T., Theerapong S., Chantrakul C., Boonroj P., Punkrut W., Ekpalakorn W., Boontaeng N., Taechaiya S., Petcharoen S., et al. Efficacy of Andrographis paniculata, Nees for pharyngotonsillitis in adults. J. Med. Assoc. Thai. 1991;74:437–442. [PubMed] [Google Scholar]
- 61.Widjajakusuma E.C., Jonosewojo A., Hendriati L., Wijaya S., Surjadhana A., Sastrowardoyo W., Monita N., Muna N.M., Fajarwati R.P., Ervina M., et al. Phytochemical screening and preliminary clinical trials of the aqueous extract mixture of Andrographis paniculata (Burm. f.) Wall. ex Nees and Syzygium polyanthum (Wight.) Walp leaves in metformin treated patients with type 2 diabetes. Phytomedicine. 2019;55:137–147. doi: 10.1016/j.phymed.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 62.Tang T., Targan S.R., Li Z.S., Xu C., Byers V.S., Sandborn W.J. Randomised clinical trial: Herbal extract HMPL-004 in active ulcerative colitis-a double-blind comparison with sustained release mesalazine. Aliment. Pharmacol. Ther. 2011;33:194–202. doi: 10.1111/j.1365-2036.2010.04515.x. [DOI] [PubMed] [Google Scholar]
- 63.Sandborn W.J., Targan S.R., Byers V.S., Rutty D.A., Mu H., Zhang X., Tang T. Andrographis paniculata extract (HMPL-004) for active ulcerative colitis. Am. J. Gastroenterol. 2013;108:90–98. doi: 10.1038/ajg.2012.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phunikhom K., Khampitak K., Aromdee C., Arkaravichien T., Sattayasai J. Effect of Andrographis paniculata Extract on Triglyceride Levels of the Patients with Hypertriglyceridemia: A Randomized Controlled Trial. J. Med. Assoc. Thai. 2015;98(Suppl. 6):S41–S47. [PubMed] [Google Scholar]
- 65.Islam M.T., Ali E.S., Uddin S.J., Islam M.A., Shaw S., Khan I.N., Saravi S.S.S., Ahmad S., Rehman S., Gupta V.K., et al. Andrographolide, a diterpene lactone from Andrographis paniculata and its therapeutic promises in cancer. Cancer Lett. 2018;420:129–145. doi: 10.1016/j.canlet.2018.01.074. [DOI] [PubMed] [Google Scholar]
- 66.Hancke J.L., Srivastav S., Caceres D.D., Burgos R.A. A double-blind, randomized, placebo-controlled study to assess the efficacy of Andrographis paniculata standardized extract (ParActin(R)) on pain reduction in subjects with knee osteoarthritis. Phytother. Res. 2019;33:1469–1479. doi: 10.1002/ptr.6339. [DOI] [PubMed] [Google Scholar]
- 67.Bertoglio J.C., Baumgartner M., Palma R., Ciampi E., Carcamo C., Caceres D.D., Acosta-Jamett G., Hancke J.L., Burgos R.A. Andrographis paniculata decreases fatigue in patients with relapsing-remitting multiple sclerosis: A 12-month double-blind placebo-controlled pilot study. BMC Neurol. 2016;16:77. doi: 10.1186/s12883-016-0595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barth A., Hovhannisyan A., Jamalyan K., Narimanyan M. Antitussive effect of a fixed combination of Justicia adhatoda, Echinacea purpurea and Eleutherococcus senticosus extracts in patients with acute upper respiratory tract infection: A comparative, randomized, double-blind, placebo-controlled study. Phytomedicine. 2015;22:1195–1200. doi: 10.1016/j.phymed.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 69.Sgorlon S., Colitti M., Asquini E., Ferrarini A., Pallavicini A., Stefanon B. Administration of botanicals with the diet regulates gene expression in peripheral blood cells of Sarda sheep during ACTH challenge. Domest Anim. Endocrinol. 2012;43:213–226. doi: 10.1016/j.domaniend.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 70.Raghavan R., Cheriyamundath S., Madassery J. Andrographolide, a new potential NF-kappa B inhibitor: Docking simulation and evaluation of drug-likeness. Mol. Simulat. 2012;38:582–588. doi: 10.1080/08927022.2011.651138. [DOI] [Google Scholar]
- 71.Novianto F., Zulkarnain Z., Mana T.A. A Clinical Observation to Understand the Safety of Herbs Used for Diabetes mellitus. Media Penelit Pengem. 2018;28:9–14. doi: 10.22435/mpk.v28i1.7009.9-14. [DOI] [Google Scholar]
- 72.He Y., Yang J., Zeng G., Shen T., Fontaine R.E., Zhang L., Shi G., Wang Y., Li Q., Long J. Risk factors for critical disease and death from hand, foot and mouth disease. Pediatr. Infect. Dis. J. 2014;33:966–970. doi: 10.1097/INF.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 73.Ciampi E., Uribe-San-Martin R., Carcamo C., Cruz J.P., Reyes A., Reyes D., Pinto C., Vasquez M., Burgos R.A., Hancke J. Efficacy of andrographolide in not active progressive multiple sclerosis: A prospective exploratory double-blind, parallel-group, randomized, placebo-controlled trial. BMC Neurol. 2020;20:173. doi: 10.1186/s12883-020-01745-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Panossian A., Hovhannisyan A., Mamikonyan G., Abrahamian H., Hambardzumyan E., Gabrielian E., Goukasova G., Wikman G., Wagner H. Pharmacokinetic and oral bioavailability of andrographolide from Andrographis paniculata fixed combination Kan Jang in rats and human. Phytomedicine. 2000;7:351–364. doi: 10.1016/S0944-7113(00)80054-9. [DOI] [PubMed] [Google Scholar]
- 75.Li L., Wijaya H., Samanta S., Lam Y., Yao S.Q. In situ imaging and proteome profiling indicate andrographolide is a highly promiscuous compound. Sci. Rep. 2015;5:11522. doi: 10.1038/srep11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bisson J., McAlpine J.B., Friesen J.B., Chen S.N., Graham J., Pauli G.F. Can Invalid Bioactives Undermine Natural Product-Based Drug Discovery? J. Med. Chem. 2016;59:1671–1690. doi: 10.1021/acs.jmedchem.5b01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hossain M.S. Master’s Thesis. Department of Biotechnology, Kulliyyah of Science, International Islamic University Malaysia; Kuantan, Pahang, Malaysia: 2016. The Effect of Salinity Stress on the Morpho-physiology and Protein Profile of Andrographis Paniculata. [Google Scholar]
- 78.Hossain M.S. Present scenario of global salt affected soils, its management and importance of salinity research. Int. Res. J. Biol. Sci. 2019;1:1–3. [Google Scholar]
- 79.Hossain M.S. Proteomic Studies: Contribution to Understanding Plant Salinity Stress Response. Glob. J. Bot. Sci. 2020;8:1–10. doi: 10.12974/2311-858X.2020.08.1. [DOI] [Google Scholar]
- 80.Arifullah M., Namsa N.D., Mandal M., Chiruvella K.K., Vikrama P., Gopal G.R. Evaluation of anti-bacterial and anti-oxidant potential of andrographolide and echiodinin isolated from callus culture of Andrographis paniculata Nees. Asian Pac. J. Trop. Biomed. 2013;3:604–610. doi: 10.1016/S2221-1691(13)60123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Editorial Microbiology by numbers. Nat. Rev. Microbiol. 2011;9:628. doi: 10.1038/nrmicro2644. [DOI] [PubMed] [Google Scholar]
- 82.Sharma A., Lal K., Handa S.S. Standardization of the Indian Crude Drug Kalmegh by High-Pressure Liquid-Chromatographic Determination of Andrographolide. Phytochem. Anal. 1992;3:129–131. doi: 10.1002/pca.2800030308. [DOI] [Google Scholar]
- 83.Seubsasana S., Pientong C., Ekalaksananan T., Thongchai S., Aromdee C. A potential andrographolide analogue against the replication of herpes simplex virus type 1 in vero cells. Med. Chem. 2011;7:237–244. doi: 10.2174/157340611795564268. [DOI] [PubMed] [Google Scholar]
- 84.Lee S., Morita H., Tezuka Y. Preferentially Cytotoxic Constituents of Andrographis paniculata and their Preferential Cytotoxicity against Human Pancreatic Cancer Cell Lines. Nat. Prod. Commun. 2015;10:1153–1158. doi: 10.1177/1934578X1501000704. [DOI] [PubMed] [Google Scholar]
- 85.Wen Q., Jin X., Lu Y., Chen D.F. Anticomplement ent-labdane diterpenoids from the aerial parts of Andrographis paniculata. Fitoterapia. 2020;142:104528. doi: 10.1016/j.fitote.2020.104528. [DOI] [PubMed] [Google Scholar]
- 86.Koteswara Rao Y., Vimalamma G., Rao C.V., Tzeng Y.M. Flavonoids and andrographolides from Andrographis paniculata. Phytochemistry. 2004;65:2317–2321. doi: 10.1016/j.phytochem.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 87.Reddy M.K., Reddy M.V., Gunasekar D., Murthy M.M., Caux C., Bodo B. A flavone and an unusual 23-carbon terpenoid from Andrographis paniculata. Phytochemistry. 2003;62:1271–1275. doi: 10.1016/S0031-9422(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 88.Majumdar M., Misra T.K., Roy D.N. In vitro anti-biofilm activity of 14-deoxy-11,12-didehydroandrographolide from Andrographis paniculata against Pseudomonas aeruginosa. Braz. J. Microbiol. 2020;51:15–27. doi: 10.1007/s42770-019-00169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lala S., Nandy A.K., Mahato S.B., Basu M.K. Delivery in vivo of 14-deoxy-11-oxoandrographolide, an antileishmanial agent, by different drug carriers. Indian J. Biochem. Biophys. 2003;40:169–174. [PubMed] [Google Scholar]
- 90.Liu R.H., Jacob J.R., Tennant B. Patent No. US8445533B2. Andrographolide Derivatives to Treat Viral Infections. 2013 May 21;
- 91.Wintachai P., Kaur P., Lee R.C., Ramphan S., Kuadkitkan A., Wikan N., Ubol S., Roytrakul S., Chu J.J., Smith D.R. Activity of andrographolide against chikungunya virus infection. Sci. Rep. 2015;5:14179. doi: 10.1038/srep14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paemanee A., Hitakarun A., Wintachai P., Roytrakul S., Smith D.R. A proteomic analysis of the anti-dengue virus activity of andrographolide. Biomed. Pharmacother. Biomed. Pharmacother. 2019;109:322–332. doi: 10.1016/j.biopha.2018.10.054. [DOI] [PubMed] [Google Scholar]
- 93.Panraksa P., Ramphan S., Khongwichit S., Smith D.R. Activity of andrographolide against dengue virus. Antiviral. Res. 2017;139:69–78. doi: 10.1016/j.antiviral.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 94.Edwin E.S., Vasantha-Srinivasan P., Senthil-Nathan S., Thanigaivel A., Ponsankar A., Pradeepa V., Selin-Rani S., Kalaivani K., Hunter W.B., Abdel-Megeed A., et al. Anti-dengue efficacy of bioactive andrographolide from Andrographis paniculata (Lamiales: Acanthaceae) against the primary dengue vector Aedes aegypti (Diptera: Culicidae) Acta Trop. 2016;163:167–178. doi: 10.1016/j.actatropica.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 95.Prakoso N.I., Zakiyah Z.N., Liyanita A., Rubiyanto D., Fitriastuti D., Ramadani A.P., Kamari A., Mow S.K. Antimalarial Activity of Andrographis Paniculata Ness‘s N-hexane Extract and Its Major Compounds. Open Chem. 2019;17:788–797. doi: 10.1515/chem-2019-0086. [DOI] [Google Scholar]
- 96.Fangkham S., Ekalaksananan T., Aromdee C., Seubsasana S., Kongyingyoes B., Patarapadungkit N., Pientong C. The effect of andrographolide on Human papillomavirus type 16 (HPV16) positive cervical cancer cells (SiHa) Int. J. Infect. Dis. 2012;16:E80. doi: 10.1016/j.ijid.2012.05.192. [DOI] [Google Scholar]
- 97.Lin T.P., Chen S.Y., Duh P.D., Chang L.K., Liu Y.N. Inhibition of the epstein-barr virus lytic cycle by andrographolide. Biol. Pharm. Bull. 2008;31:2018–2023. doi: 10.1248/bpb.31.2018. [DOI] [PubMed] [Google Scholar]
- 98.Sinha J., Mukhopadhyay S., Das N., Basu M.K. Targeting of liposomal andrographolide to L. donovani-infected macrophages in vivo. Drug Deliv. 2000;7:209–213. doi: 10.1080/107175400455137. [DOI] [PubMed] [Google Scholar]
- 99.Wiart C., Kumar K., Yusof M.Y., Hamimah H., Fauzi Z.M., Sulaiman M. Antiviral properties of ent-labdene diterpenes of Andrographis paniculata nees, inhibitors of herpes simplex virus type 1. Phytother. Res. 2005;19:1069–1070. doi: 10.1002/ptr.1765. [DOI] [PubMed] [Google Scholar]
- 100.Zhou K.L., Chen L.X., Zhuang Y.L., Wang N.L., Yao X.S., Qiu F. Two new ent-labdane diterpenoid glycosides from the aerial parts of Andrographis paniculata. J. Asian Nat. Prod. Res. 2008;10:939–943. doi: 10.1080/10286020802217432. [DOI] [PubMed] [Google Scholar]
- 101.Zhang L., Liu Q., Yu J., Zeng H., Jiang S., Chen X. Separation of five compounds from leaves of Andrographis paniculata (Burm. f.) Nees by off-line two-dimensional high-speed counter-current chromatography combined with gradient and recycling elution. J. Sep. Sci. 2015;38:1476–1483. doi: 10.1002/jssc.201401458. [DOI] [PubMed] [Google Scholar]
- 102.Wu T.S., Chern H.J., Damu A.G., Kuo P.C., Su C.R., Lee E.J., Teng C.M. Flavonoids and ent-labdane diterpenoids from Andrographis paniculata and their antiplatelet aggregatory and vasorelaxing effects. J. Asian Nat. Prod. Res. 2008;10:17–24. doi: 10.1080/10286020701273627. [DOI] [PubMed] [Google Scholar]
- 103.Wang C.H., Li W., Qiu R.X., Jiang M.M., Li G.Q. A new diterpenoid from the aerial parts of Andrographis paniculata. Nat. Prod. Commun. 2014;9:13–14. doi: 10.1177/1934578X1400900105. [DOI] [PubMed] [Google Scholar]
- 104.Suriyo T., Pholphana N., Rangkadilok N., Thiantanawat A., Watcharasit P., Satayavivad J. Andrographis paniculata extracts and major constituent diterpenoids inhibit growth of intrahepatic cholangiocarcinoma cells by inducing cell cycle arrest and apoptosis. Planta Med. 2014;80:533–543. doi: 10.1055/s-0034-1368399. [DOI] [PubMed] [Google Scholar]
- 105.Tran Q.T.N., Tan W.S.D., Wong W.S.F., Chai C.L.L. Polypharmacology of andrographolide: Beyond one molecule one target. Nat. Prod. Rep. 2020 doi: 10.1039/d0np00049c. [DOI] [PubMed] [Google Scholar]
- 106.Rahman M.M., Ahmad S.H., Mohamed M.T., Ab Rahman M.Z. Antimicrobial compounds from leaf extracts of Jatropha curcas, Psidium guajava, and Andrographis paniculata. Sci. World J. 2014;2014:635240. doi: 10.1155/2014/635240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shao Z.J., Zheng X.W., Feng T., Huang J., Chen J., Wu Y.Y., Zhou L.M., Tu W.W., Li H. Andrographolide exerted its antimicrobial effects by upregulation of human beta-defensin-2 induced through p38 MAPK and NF-kappaB pathway in human lung epithelial cells. Can. J. Physiol. Pharmacol. 2012;90:647–653. doi: 10.1139/y2012-050. [DOI] [PubMed] [Google Scholar]
- 108.Sharma V., Kaushik S., Pandit P., Dhull D., Yadav J.P., Kaushik S. Green synthesis of silver nanoparticles from medicinal plants and evaluation of their antiviral potential against chikungunya virus. Appl. Microbiol. Biotechnol. 2019;103:881–891. doi: 10.1007/s00253-018-9488-1. [DOI] [PubMed] [Google Scholar]
- 109.Shen Y.H., Li R.T., Xiao W.L., Xu G., Lin Z.W., Zhao Q.S., Sun H.D. ent-Labdane diterpenoids from Andrographis paniculata. J. Nat. Prod. 2006;69:319–322. doi: 10.1021/np050160u. [DOI] [PubMed] [Google Scholar]
- 110.Rattanachaikunsopon P., Phumkhachorn P. Use of asiatic pennywort Centella asiatica aqueous extract as a bath treatment to control columnaris in Nile tilapia. J. Aquat. Anim. Health. 2010;22:14–20. doi: 10.1577/H09-021.1. [DOI] [PubMed] [Google Scholar]
- 111.Roy S., Yasmin S., Ghosh S., Bhattacharya S., Banerjee D. Anti-Infective Metabolites of a Newly Isolated Bacillus thuringiensis KL1 Associated with Kalmegh (Andrographis paniculata Nees.), a Traditional Medicinal Herb. Microbiol. Insights. 2016;9:1–7. doi: 10.4137/MBI.S33394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pfisterer P.H., Rollinger J.M., Schyschka L., Rudy A., Vollmar A.M., Stuppner H. Neoandrographolide from Andrographis paniculata as a potential natural chemosensitizer. Planta Med. 2010;76:1698–1700. doi: 10.1055/s-0030-1249876. [DOI] [PubMed] [Google Scholar]
- 113.Radhika P., Prasad Y.R., Sowjanya K. A new diterpene from the leaves of Andrographis paniculata Nees. Nat. Prod. Commun. 2012;7:485–486. doi: 10.1177/1934578X1200700417. [DOI] [PubMed] [Google Scholar]
- 114.Pholphana N., Rangkadilok N., Saehun J., Ritruechai S., Satayavivad J. Changes in the contents of four active diterpenoids at different growth stages in Andrographis paniculata (Burm.f.) Nees (Chuanxinlian) Chin. Med. 2013;8:2. doi: 10.1186/1749-8546-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Radhika P., Prasad Y.R., Lakshmi K.R. Flavones from the stem of Andrographis paniculata Nees. Nat. Prod. Commun. 2010;5:59–60. doi: 10.1177/1934578X1000500115. [DOI] [PubMed] [Google Scholar]
- 116.Pramanick S., Banerjee S., Achari B., Das B., Sen A.K., Mukhopadhyay S., Neuman A., Prange T. Andropanolide and isoandrographolide, minor diterpenoids from Andrographis paniculata: Structure and X-ray crystallographic analysis. J. Nat. Prod. 2006;69:403–405. doi: 10.1021/np050211n. [DOI] [PubMed] [Google Scholar]
- 117.Matsuda T., Kuroyanagi M., Sugiyama S., Umehara K., Ueno A., Nishi K. Cell differentiation-inducing diterpenes from Andrographis paniculata Nees. Chem. Pharm. Bull. 1994;42:1216–1225. doi: 10.1248/cpb.42.1216. [DOI] [PubMed] [Google Scholar]
- 118.Hossain M.M., Polash S.A., Takikawa M., Shubhra R.D., Saha T., Islam Z., Hossain S., Hasan M.A., Takeoka S., Sarker S.R. Investigation of the Antibacterial Activity and in vivo Cytotoxicity of Biogenic Silver Nanoparticles as Potent Therapeutics. Front. Bioeng. Biotechnol. 2019;7:239. doi: 10.3389/fbioe.2019.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Malahubban M., Alimon A.R., Sazili A.Q., Fakurazi S., Zakry F.A. Phytochemical analysis of Andrographis paniculata and Orthosiphon stamineus leaf extracts for their antibacterial and antioxidant potential. Trop. Biomed. 2013;30:467–480. [PubMed] [Google Scholar]
- 120.Li W., Xu X., Zhang H., Ma C., Fong H., van Breemen R., Fitzloff J. Secondary metabolites from Andrographis paniculata. Chem. Pharm. Bull. 2007;55:455–458. doi: 10.1248/cpb.55.455. [DOI] [PubMed] [Google Scholar]
- 121.Lee S., Yoon G., Jeong M., Lee M.J., Kang K., Cho J. Hierarchical surface atomic structure of a manganese-based spinel cathode for lithium-ion batteries. Angew. Chem. Int. Ed. Engl. 2015;54:1153–1158. doi: 10.1002/anie.201408853. [DOI] [PubMed] [Google Scholar]
- 122.Kuroyanagi M., Sato M., Ueno A., Nishi K. Flavonoids from Andrographis-Paniculata. Chem. Pharm. Bull. 1987;35:4429–4435. doi: 10.1248/cpb.35.4429. [DOI] [Google Scholar]
- 123.Hu X.Y., Wu R.H., Logue M., Blondel C., Lai L.Y.W., Stuart B., Flower A., Fei Y.T., Moore M., Shepherd J., et al. Andrographis paniculata (Chuan Xin Lian) for symptomatic relief of acute respiratory tract infections in adults and children: A systematic review and meta-analysis. PLoS ONE. 2017;12:e0181780. doi: 10.1371/journal.pone.0181780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jalal M.A.F., Overton K.H., Rycroft D.S. Formation of three new flavones by differentiating callus cultures of andrographis paniculata. Phytochemistry. 1979;18:149–151. doi: 10.1016/S0031-9422(00)90934-8. [DOI] [Google Scholar]
- 125.Geethangili M., Rao Y.K., Fang S.H., Tzeng Y.M. Cytotoxic constituents from Andrographis paniculata induce cell cycle arrest in jurkat cells. Phytother. Res. 2008;22:1336–1341. doi: 10.1002/ptr.2493. [DOI] [PubMed] [Google Scholar]
- 126.Das B., Khan M.I., Jayabalan R., Behera S.K., Yun S.I., Tripathy S.K., Mishra A. Understanding the Antifungal Mechanism of Ag@ZnO Core-shell Nanocomposites against Candida krusei. Sci. Rep. 2016;6:36403. doi: 10.1038/srep36403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hapuarachchi S.D., Ali Z., Abe N., Sugandhika S.T., Sandun S.T., Khan I.A. Andrographidine G, a new flavone glucoside from Andrographis paniculata. Nat. Prod. Commun. 2013;8:333–334. doi: 10.1177/1934578X1300800314. [DOI] [PubMed] [Google Scholar]
- 128.Hanh T.T.H., My N.T.T., Cham P.T., Quang T.H., Cuong N.X., Huong T.T., Nam N.H., Minh C.V. Diterpenoids and Flavonoids from Andrographis paniculata. Chem. Pharm. Bull. 2020;68:96–99. doi: 10.1248/cpb.c19-00662. [DOI] [PubMed] [Google Scholar]
- 129.Feng J., Leone J., Schweig S., Zhang Y. Evaluation of Natural and Botanical Medicines for Activity against Growing and Non-growing Forms of B. burgdorferi. Front. Med. 2020;7:6. doi: 10.3389/fmed.2020.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gupta K.K., Taneja S.C., Dhar K.L., Atal C.K. Flavonoids of Andrographis-Paniculata. Phytochemistry. 1983;22:314–315. doi: 10.1016/S0031-9422(00)80122-3. [DOI] [Google Scholar]
- 131.Dedhia J., Mukharjee E., Luke A.M., Mathew S., Pawar A.M. Efficacy of Andrographis paniculata compared to Azadirachta indica, Curcuma longa, and sodium hypochlorite when used as root canal irrigants against Candida albicans and Staphylococcus aureus: An in vitro antimicrobial study. J. Conserv. Dent. JCD. 2018;21:642–645. doi: 10.4103/JCD.JCD_118_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fujita T., Fujitani R., Takeda Y., Takaishi Y., Yamada T., Kido M., Miura I. On the Diterpenoids of Andrographis-Paniculata-X-Ray Crystallographic Analysis of Andrographolide and Structure Determination of New Minor Diterpenoids. Chem. Pharm. Bull. 1984;32:2117–2125. doi: 10.1248/cpb.32.2117. [DOI] [Google Scholar]
- 133.Chen L.X., Qiu F., Wei H., Qu G.X., Yao X.S. Nine new ent-labdane diterpenoids from the aerial parts of Andrographis paniculata. Helvetica Chim. Acta. 2006;89:2654–2664. doi: 10.1002/hlca.200690237. [DOI] [Google Scholar]
- 134.Chen L.X., He H., Xia G.Y., Zhou K.L., Qiu F. A new flavonoid from the aerial parts of Andrographis paniculata. Nat. Prod. Res. 2014;28:138–143. doi: 10.1080/14786419.2013.856907. [DOI] [PubMed] [Google Scholar]
- 135.Awang K., Abdullah N.H., Hadi A.H., Fong Y.S. Cardiovascular activity of labdane diterpenes from Andrographis paniculata in isolated rat hearts. J. Biomed. Biotechnol. 2012;2012:876458. doi: 10.1155/2012/876458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chao W.W., Kuo Y.H., Lin B.F. Anti-inflammatory activity of new compounds from Andrographis paniculata by NF-kappaB transactivation inhibition. J. Agric. Food Chem. 2010;58:2505–2512. doi: 10.1021/jf903629j. [DOI] [PubMed] [Google Scholar]
- 137.Burgos R.A., Alarcon P., Quiroga J., Manosalva C., Hancke J. Andrographolide, an Anti-Inflammatory Multitarget Drug: All Roads Lead to Cellular Metabolism. Molecules. 2020;26:5. doi: 10.3390/molecules26010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen L., Zhu H., Wang R., Zhou K., Jing Y., Qiu F. ent-Labdane diterpenoid lactone stereoisomers from Andrographis paniculata. J. Nat. Prod. 2008;71:852–855. doi: 10.1021/np0704452. [DOI] [PubMed] [Google Scholar]
- 139.Banerjee M., Parai D., Chattopadhyay S., Mukherjee S.K. Andrographolide: Antibacterial activity against common bacteria of human health concern and possible mechanism of action. Folia Microbiol. 2017;62:237–244. doi: 10.1007/s12223-017-0496-9. [DOI] [PubMed] [Google Scholar]
- 140.Zou Q.Y., Li N., Dan C., Deng W.L., Peng S.L., Ding L.S. A new ent-labdane diterpenoid from Andrographis paniculata. Chin. Chem. Lett. 2010;21:1091–1093. doi: 10.1016/j.cclet.2010.05.002. [DOI] [Google Scholar]
- 141.Xu C., Chou G.X., Wang C.H., Wang Z.T. Rare noriridoids from the roots of Andrographis paniculata. Phytochemistry. 2012;77:275–279. doi: 10.1016/j.phytochem.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 142.Xu C., Chou G.X., Wang Z.T. A new diterpene from the leaves of Andrographis paniculata Nees. Fitoterapia. 2010;81:610–613. doi: 10.1016/j.fitote.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 143.Wang G.Y., Wen T., Liu F.F., Tian H.Y., Chun-Lin F., Huang X.J., Ye W.C., Wang Y. Two new diterpenoid lactones isolated from Andrographis paniculata. Chin. J. Nat. Med. 2017;15:458–462. doi: 10.1016/S1875-5364(17)30068-7. [DOI] [PubMed] [Google Scholar]
- 144.Weiming C., Xiaotian L. Deoxyandrographolide-19β-D-Glucoside from the Leaves of Andrographis paniculata. Planta Med. 1982;45:245–246. doi: 10.1055/s-2007-971383. [DOI] [PubMed] [Google Scholar]
- 145.Tanwar A., Chawla R., Chakotiya A.S., Thakur P., Goel R., Basu M., Arora R., Khan H.A. Effect of Holarrhena antidysentrica (Ha) and Andrographis paniculata (Ap) on the biofilm formation and cell membrane integrity of opportunistic pathogen Salmonella typhimurium. Microb. Pathog. 2016;101:76–82. doi: 10.1016/j.micpath.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 146.Maity G.N., Maity P., Choudhuri I., Sahoo G.C., Maity N., Ghosh K., Bhattacharyya N., Dalai S., Mondal S. Green synthesis, characterization, antimicrobial and cytotoxic effect of silver nanoparticles using arabinoxylan isolated from Kalmegh. Int. J. Biol. Macromol. 2020;162:1025–1034. doi: 10.1016/j.ijbiomac.2020.06.215. [DOI] [PubMed] [Google Scholar]
- 147.Xu F.-F., Fu S.-J., Gu S.-P., Wang Z.-M., Wang Z.-Z., He X., Xiao W. Simultaneous determination of andrographolide, dehydroandrographolide and neoandrographolide in dog plasma by LC–MS/MS and its application to a dog pharmacokinetic study of Andrographis paniculata tablet. J. Chromatogr. B. 2015;990:125–131. doi: 10.1016/j.jchromb.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 148.Pholphana N., Panomvana D., Rangkadilok N., Suriyo T., Ungtrakul T., Pongpun W., Thaeopattha S., Satayavivad J. A Simple and Sensitive LC-MS/MS Method for Determination of Four Major Active Diterpenoids from Andrographis paniculata in Human Plasma and Its Application to a Pilot Study. Planta Med. 2016;82:113–120. doi: 10.1055/s-0035-1558115. [DOI] [PubMed] [Google Scholar]
- 149.Bera R., Ahmed S.K., Sarkar L., Sen T., Karmakar S. Pharmacokinetic analysis and tissue distribution of andrographolide in rat by a validated LC-MS/MS method. Pharm. Biol. 2014;52:321–329. doi: 10.3109/13880209.2013.836544. [DOI] [PubMed] [Google Scholar]
- 150.Tu Y.S., Sun D.M., Zhang J.J., Jiang Z.Q., Chen Y.X., Zeng X.H., Huang D.E., Yao N. Preparation and characterisation of andrographolide niosomes and its anti-hepatocellular carcinoma activity. J. Microencapsul. 2014;31:307–316. doi: 10.3109/02652048.2013.843727. [DOI] [PubMed] [Google Scholar]
- 151.Chen L., Yu A., Zhuang X., Zhang K., Wang X., Ding L., Zhang H. Determination of andrographolide and dehydroandrographolide in rabbit plasma by on-line solid phase extraction of high-performance liquid chromatography. Talanta. 2007;74:146–152. doi: 10.1016/j.talanta.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 152.Suo X.B., Zhang H., Wang Y.Q. HPLC determination of andrographolide in rat whole blood: Study on the pharmacokinetics of andrographolide incorporated in liposomes and tablets. Biomed. Chromatogr. 2007;21:730–734. doi: 10.1002/bmc.812. [DOI] [PubMed] [Google Scholar]
- 153.Gu Y., Ma J., Liu Y., Chen B., Yao S. Determination of andrographolide in human plasma by high-performance liquid chromatography/mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007;854:328–331. doi: 10.1016/j.jchromb.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 154.Ruengsitagoon W., Anuntakarun K., Aromdee C. Flow injection spectrophotometric determination of andrographolide from Andrographis paniculata. Talanta. 2006;69:900–905. doi: 10.1016/j.talanta.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 155.Akowuah G.A., Zhari I., Norhayati I., Mariam A. HPLC and HPTLC densitometric determination of andrographolides and antioxidant potential of Andrographis paniculata. J. Food Compos. Anal. 2006;19:118–126. doi: 10.1016/j.jfca.2005.04.007. [DOI] [Google Scholar]
- 156.Srivastava A., Misra H., Verma R.K., Gupta M.M. Chemical fingerprinting of Andrographis paniculata using HPLC, HPTLC and densitometry. Phytochem. Anal. 2004;15:280–285. doi: 10.1002/pca.779. [DOI] [PubMed] [Google Scholar]
- 157.Pholphana N., Rangkadilok N., Thongnest S., Ruchirawat S., Ruchirawat M., Satayavivad J. Determination and variation of three active diterpenoids in Andrographis paniculata (Burm.f.) Nees. Phytochem. Anal. 2004;15:365–371. doi: 10.1002/pca.789. [DOI] [PubMed] [Google Scholar]
- 158.Zhao J., Yang G., Liu H., Wang D., Song X., Chen Y. Determination of andrographolide, deoxyandrographolide and neoandrographolide in the Chinese herb Andrographis paniculata by micellar electrokinetic capillary chromatography. Phytochem. Anal. 2002;13:222–227. doi: 10.1002/pca.644. [DOI] [PubMed] [Google Scholar]
- 159.Cheung H.Y., Cheung C.S., Kong C.K. Determination of bioactive diterpenoids from Andrographis paniculata by micellar electrokinetic chromatography. J. Chromatogr. A. 2001;930:171–176. doi: 10.1016/S0021-9673(01)01160-8. [DOI] [PubMed] [Google Scholar]
- 160.Jain D.C., Gupta M.M., Saxena S., Kumar S. LC analysis of hepatoprotective diterpenoids from Andrographis paniculata. J. Pharm. Biomed. Anal. 2000;22:705–709. doi: 10.1016/S0731-7085(99)00297-6. [DOI] [PubMed] [Google Scholar]
- 161.Kumaran K.S., Thirugnanasambantham P., Viswanathan S., Murthy M.S.R. An HPLC method for the estimation of andrographolide in rabbit serum. Indian J. Pharmacol. 2003;35:109–112. [Google Scholar]
- 162.Li W., Fitzloff J.F. HPLC–PDA determination of bioactive diterpenoids from plant materials and commercial products of Andrographis paniculata. J. Liq. Chromatogr. Relat. Technol. 2004;27:2407–2420. doi: 10.1081/JLC-200028162. [DOI] [Google Scholar]
- 163.Wongkittipong R., Prat L., Damronglerd S., Gourdon C. Solid–liquid extraction of andrographolide from plants—experimental study, kinetic reaction and model. Sep. Purif. Technol. 2004;40:147–154. doi: 10.1016/j.seppur.2004.02.002. [DOI] [Google Scholar]
- 164.Cui Y., Wang Y., Ouyang X., Han Y., Zhu H., Chen Q. Fingerprint profile of active components for Andrographis paniculata Nees by HPLC-DAD. Sens. Instrum. Food Qual. Saf. 2009;3:165–179. doi: 10.1007/s11694-009-9082-4. [DOI] [Google Scholar]
- 165.Kavuri S.R., Mukkamala S.B., Ramesh P. Quantitative determination of two bioactive compounds in Andrographis paniculata (Burm. f) nees by ultra performance liquid chromatography. J. Pharm. Res. 2010;3:1682–1684. [Google Scholar]
- 166.Du Q.Z., Jerz G., Winterhalter P. Separation of andrographolide and neoandrographolide from the leaves of Andrographis paniculata using high-speed counter-current chromatography. J. Chromatogr. A. 2003;984:147–151. doi: 10.1016/S0021-9673(02)01831-9. [DOI] [PubMed] [Google Scholar]
- 167.Kulyal P., Tiwari U.K., Shukla A., Gaur A.K. Chemical constituents isolated from Andrographis paniculata. Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 2010;49:356–359. [Google Scholar]
- 168.Liu J., Wang Z.T., Ge B.X. Andrograpanin, isolated from Andrographis paniculata, exhibits anti-inflammatory property in lipopolysaccharide-induced macrophage cells through down-regulating the p38 MAPKs signaling pathways. Int. Immunopharmacol. 2008;8:951–958. doi: 10.1016/j.intimp.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 169.Aminah N.S., Tun K.N.W., Kristanti A.N., Aung H.T., Takaya Y., Choudhary M.I. Chemical constituents and their biological activities from Taunggyi (Shan state) medicinal plants. Heliyon. 2021;7:e06173. doi: 10.1016/j.heliyon.2021.e06173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xu Y., Marshall R.L., Mukkur T.K. An Investigation on the Antimicrobial Activity of Andrographis paniculata Extracts and Andrographolide in vitro. Asian J. Plant Sci. 2006;5:527–530. [Google Scholar]
- 171.Banerjee M., Moulick S., Bhattacharya K.K., Parai D., Chattopadhyay S., Mukherjee S.K. Attenuation of Pseudomonas aeruginosa quorum sensing, virulence and biofilm formation by extracts of Andrographis paniculata. Microb. Pathog. 2017;113:85–93. doi: 10.1016/j.micpath.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 172.Voravuthikunchai S.P., Limsuwan S. Medicinal Plant Extracts as AntiEscherichia coli O157: H7 Agents and Their Effects on Bacterial Cell Aggregation. J. Food Prot. 2006;69:2336–2341. doi: 10.4315/0362-028X-69.10.2336. [DOI] [PubMed] [Google Scholar]
- 173.Mishra U.S., Mishra A., Kumari R., Murthy P.N., Naik B.S. Antibacterial Activity of Ethanol Extract of Andrographis paniculata. Indian J. Pharm. Sci. 2009;71:436–438. doi: 10.4103/0250-474X.57294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ahmed Q.U., Samah O.A., Sule A. Andrographis paniculata (Burm.f) Wall. ex Ness: A Potent Antibacterial Plant. In: Bobbarala V., editor. Antimicrobial Agents. IntechOpen; London, UK: 2012. pp. 345–360. [DOI] [Google Scholar]
- 175.Sule A., Ahmed Q.U., Samah O.A., Omar M.N., Hassan N.M., Kamal L.Z.M., Yarmo M.A. Bioassay guided isolation of antibacterial compounds from Andrographis paniculata (Burm.f.) Wall. ex Nees (Hempedeu bumi) Am. J. Appl. Sci. 2011;8:525–534. doi: 10.3844/ajassp.2011.525.534. [DOI] [Google Scholar]
- 176.Majumdar M., Dubey A., Goswami R., Misra T.K., Roy D.N. In vitro and in silico studies on the structural and biochemical insight of anti-biofilm activity of andrograpanin from Andrographis paniculata against Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2020;36:143. doi: 10.1007/s11274-020-02919-x. [DOI] [PubMed] [Google Scholar]
- 177.Reddy V.L., Reddy S.M., Ravikanth V., Krishnaiah P., Goud T.V., Rao T.P., Ram T.S., Gonnade R.G., Bhadbhade M., Venkateswarlu Y. A new bis-andrographolide ether from Andrographis paniculata nees and evaluation of anti-HIV activity. Nat. Prod. Res. 2005;19:223–230. doi: 10.1080/14786410410001709197. [DOI] [PubMed] [Google Scholar]
- 178.Chang R.S., Ding L., Chen G.Q., Pan Q.C., Zhao Z.L., Smith K.M. Dehydroandrographolide succinic acid monoester as an inhibitor against the human immunodeficiency virus. Proc. Soc. Exp. Biol. Med. 1991;197:59–66. doi: 10.3181/00379727-197-43225. [DOI] [PubMed] [Google Scholar]
- 179.Xu H.X., Wan M., Loh B.N., Kon O.L., Chow P.W., Sim K.Y. Screening of traditional medicines for their inhibitory activity against HIV-1 protease. Phytother. Res. 1996;10:207–210. doi: 10.1002/(SICI)1099-1573(199605)10:3<207::AID-PTR812>3.0.CO;2-U. [DOI] [Google Scholar]
- 180.Chen J.X., Xue H.J., Ye W.C., Fang B.H., Liu Y.H., Yuan S.H., Yu P., Wang Y.Q. Activity of andrographolide and its derivatives against influenza virus in vivo and in vitro. Biol. Pharm. Bull. 2009;32:1385–1391. doi: 10.1248/bpb.32.1385. [DOI] [PubMed] [Google Scholar]
- 181.Aromdee C., Suebsasana S., Ekalaksananan T., Pientong C., Thongchai S. Stage of action of naturally occurring andrographolides and their semisynthetic analogues against herpes simplex virus type 1 in vitro. Planta Med. 2011;77:915–921. doi: 10.1055/s-0030-1250659. [DOI] [PubMed] [Google Scholar]
- 182.Tang L.I., Ling A.P., Koh R.Y., Chye S.M., Voon K.G. Screening of anti-dengue activity in methanolic extracts of medicinal plants. BMC Complement Altern. Med. 2012;12:3. doi: 10.1186/1472-6882-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sule A., Ahmed Q.U., Latip J., Samah O.A., Omar M.N., Umar A., Dogarai B.B. Antifungal activity of Andrographis paniculata extracts and active principles against skin pathogenic fungal strains in vitro. Pharm. Biol. 2012;50:850–856. doi: 10.3109/13880209.2011.641021. [DOI] [PubMed] [Google Scholar]
- 184.Misra P., Pal N., Guru P., Katiyar J., Srivastava V., Tandon J. Antimalarial activity of Andrographis paniculata (Kalmegh) against Plasmodium berghei NK 65 in Mastomys natalensis. Pharm. Biol. 1992;30:263–274. doi: 10.3109/13880209209054010. [DOI] [Google Scholar]
- 185.Dua V.K., Ojha V.P., Biswas S., Valecha N., Singh N., Sharma V.P. Antimalarial activity of different fractions isolated from the leaves of Andrographis paniculata. J. Med. Aromat. Plant Sci. 1999;21:1069–1073. [Google Scholar]
- 186.Chen H., Ma Y.B., Huang X.Y., Geng C.A., Zhao Y., Wang L.J., Guo R.H., Liang W.J., Zhang X.M., Chen J.J. Synthesis, structure-activity relationships and biological evaluation of dehydroandrographolide and andrographolide derivatives as novel anti-hepatitis B virus agents. Bioorganic Med. Chem. Lett. 2014;24:2353–2359. doi: 10.1016/j.bmcl.2014.03.060. [DOI] [PubMed] [Google Scholar]
- 187.Gan L., Zheng Y., Deng L., Sun P., Ye J., Wei X., Liu F., Yu L., Ye W., Fan C., et al. Diterpenoid Lactones with Anti-Inflammatory Effects from the Aerial Parts of Andrographis paniculata. Molecules. 2019;24:2726. doi: 10.3390/molecules24152726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sharma S.N., Sahu S., Jha Z., Sharma D.K. Evaluation of seasonal variation in relation to secondary metabolite and biomass production of Andrographis paniculata. J. Nat. Remedies. 2012;12:39–46. [Google Scholar]
- 189.Sharma M., Sharma R. Identification, purification and quantification of andrographolide from Andrographis paniculata (burm. F.) Nees by HPTLC at different stages of life cycle of crop. J. Curr. Chem. Pharm. Sci. 2013;3:23–32. [Google Scholar]
- 190.Dua V.K., Ojha V.P., Roy R., Joshi B.C., Valecha N., Devi C.U., Bhatnagar M.C., Sharma V.P., Subbarao S.K. Anti-malarial activity of some xanthones isolated from the roots of Andrographis paniculata. J. Ethnopharmacol. 2004;95:247–251. doi: 10.1016/j.jep.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 191.Dua V.K., Verma G., Dash A.P. In vitro antiprotozoal activity of some xanthones isolated from the roots of Andrographis paniculata. Phytother. Res. 2009;23:126–128. doi: 10.1002/ptr.2556. [DOI] [PubMed] [Google Scholar]
- 192.ChemFinder Structure Search. [(accessed on 25 November 2020)]; Available online: https://www.sigmaaldrich.com/catalog/search/substructure/SubstructureSearchPage.
- 193.ChemSpider Structure Search. [(accessed on 25 November 2020)]; Available online: https://www.chemspider.com/StructureSearch.aspx.
- 194.Lee J.C., Tseng C.K., Young K.C., Sun H.Y., Wang S.W., Chen W.C., Lin C.K., Wu Y.H. Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/Nrf2 pathway in human hepatoma cells. Br. J. Pharmacol. 2014;171:237–252. doi: 10.1111/bph.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Singha P.K., Roy S., Dey S. Antimicrobial activity of Andrographis paniculata. Fitoterapia. 2003;74:692–694. doi: 10.1016/S0367-326X(03)00159-X. [DOI] [PubMed] [Google Scholar]
- 196.Fabricant D.S., Farnsworth N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001;109:69–75. doi: 10.2307/3434847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Farnsworth N.R., Akerele O., Bingel A.S., Soejarto D.D., Guo Z. Medicinal plants in therapy. Bull. World Health Organ. 1985;63:965–981. doi: 10.1016/0378-8741(87)90016-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Deng W.L. Outline of current clinical and pharmacological research on Andrographis paniculata in China. Newsl. Chin. Herb. Med. 1978;10:27–31. [Google Scholar]
- 199.Parveen R., Parveen B., Parveen A., Ahmad S. Andrographis paniculata: From traditional to nano drug for cancer therapy. In: Husen A., Iqbal M., editors. Nanomaterials and Plant Potential. Springer; Cham, Switzerland: 2019. pp. 317–345. [Google Scholar]
- 200.Zaidan M.R., Noor Rain A., Badrul A.R., Adlin A., Norazah A., Zakiah I. In vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method. Trop. Biomed. 2005;22:165–170. [PubMed] [Google Scholar]
- 201.Sahalan A.Z., Sulaiman N., Mohammed N., Ambia K.M., Lian H.H. Antibacterial activity of Andrographis paniculata and Euphorbia hirta methanol extracts. J. Sains Kesihat. Malays. 2007;5:1–8. [Google Scholar]
- 202.Gupta S., Yadava J.N.S., Tandon J.S. Antisecretory (Antidiarrhoeal) Activity of Indian Medicinal Plants Against Escherichia Coli Enterotoxin-Induced Secretion in Rabbit and Guinea Pig Ileal Loop Models. Int. J. Pharmacogn. 2008;31:198–204. doi: 10.3109/13880209309082942. [DOI] [Google Scholar]
- 203.Roy S., Rao K., Bhuvaneswari C., Giri A., Mangamoori L.N. Phytochemical analysis of Andrographis paniculata extract and its antimicrobial activity. World J. Microbiol. Biotechnol. 2010;26:85–91. doi: 10.1007/s11274-009-0146-8. [DOI] [Google Scholar]
- 204.Wei L.S., Wee W., Siong J.Y.F., Syamsumir D.F. Characterizaion of antimicrobial, antioxidant, anticancer properties and chemical composition of Malaysian Andrographis paniculata leaf extract. Pharmacologyonline. 2011;2:996–1002. [Google Scholar]
- 205.Sule A., Ahmed Q.U., Samah O.A., Omar M.N. Screening for Antibacterial Activity of Andrographis paniculata Used in Malaysian Folkloric Medicine: A Possible Alternative for the Treatment of Skin Infections. Ethnobot. Leafl. 2010;14:445–456. [Google Scholar]
- 206.Radhika P., Sastry B.S., Madhu H.B. Antimicrobial screening of Andrographis paniculata (Acanthaceae) root extracts. Res. J. Biotechnol. 2008;3:62–63. [Google Scholar]
- 207.Sule A., Ahmed Q.U., Samah O.A., Omar M.N. Bacteriostatic and bactericidal activities of Andrographis paniculata extracts on skin disease causing pathogenic bacteria. J. Med. Plants Res. 2011;5:7–14. [Google Scholar]
- 208.Kataky A., Handique P.J. Antimicrobial activity and phytochemical estimation of micropropagated Andrographis paniculata (Burm.f) Nees. Asian J. Sci. Technol. 2010;5:091–094. [Google Scholar]
- 209.Monegro A.F., Muppidi V., Regunath H. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2020. Hospital Acquired Infections. [PubMed] [Google Scholar]
- 210.Taylor T.A., Unakal C.G. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2020. Staphylococcus aureus. [PubMed] [Google Scholar]
- 211.Wang W., Wang J., Dong S.F., Liu C.H., Italiani P., Sun S.H., Xu J., Boraschi D., Ma S.P., Qu D. Immunomodulatory activity of andrographolide on macrophage activation and specific antibody response. Acta Pharmacol. Sin. 2010;31:191–201. doi: 10.1038/aps.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang T., Zhu L., Li M., Hu Y., Zhang E., Jiang Q., Han G., Jin Y. Inhalable Andrographolide-beta-cyclodextrin Inclusion Complexes for Treatment of Staphylococcus aureus Pneumonia by Regulating Immune Responses. Mol. Pharm. 2017;14:1718–1725. doi: 10.1021/acs.molpharmaceut.6b01162. [DOI] [PubMed] [Google Scholar]
- 213.Hentzer M., Wu H., Andersen J.B., Riedel K., Rasmussen T.B., Bagge N., Kumar N., Schembri M.A., Song Z., Kristoffersen P., et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003;22:3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sakuragi Y., Kolter R. Quorum-sensing regulation of the biofilm matrix genes (pel) of Pseudomonas aeruginosa. J. Bacteriol. 2007;189:5383–5386. doi: 10.1128/JB.00137-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ma L., Liu X., Liang H., Che Y., Chen C., Dai H., Yu K., Liu M., Ma L., Yang C.H., et al. Effects of 14-alpha-lipoyl andrographolide on quorum sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2012;56:6088–6094. doi: 10.1128/AAC.01119-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wu C.M., Cao J.L., Zheng M.H., Ou Y., Zhang L., Zhu X.Q., Song J.X. Effect and mechanism of andrographolide on the recovery of Pseudomonas aeruginosa susceptibility to several antibiotics. J. Int. Med. Res. 2008;36:178–186. doi: 10.1177/147323000803600123. [DOI] [PubMed] [Google Scholar]
- 217.Guo X., Zhang L.Y., Wu S.C., Xia F., Fu Y.X., Wu Y.L., Leng C.Q., Yi P.F., Shen H.Q., Wei X.B., et al. Andrographolide interferes quorum sensing to reduce cell damage caused by avian pathogenic Escherichia coli. Vet. Microbiol. 2014;174:496–503. doi: 10.1016/j.vetmic.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 218.Bjarnsholt T. The role of bacterial biofilms in chronic infections. APMIS Suppl. 2013:1–51. doi: 10.1111/apm.12099. [DOI] [PubMed] [Google Scholar]
- 219.Bowler P., Murphy C., Wolcott R. Biofilm exacerbates antibiotic resistance: Is this a current oversight in antimicrobial stewardship? Antimicrob. Resist Infect. Control. 2020;9:162. doi: 10.1186/s13756-020-00830-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Li B., Webster T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018;36:22–32. doi: 10.1002/jor.23656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jiao Y., Cody G.D., Harding A.K., Wilmes P., Schrenk M., Wheeler K.E., Banfield J.F., Thelen M.P. Characterization of extracellular polymeric substances from acidophilic microbial biofilms. Appl. Environ. Microbiol. 2010;76:2916–2922. doi: 10.1128/AEM.02289-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Otake T., Mori H., Morimoto M., Ueba N., Sutardjo S., Kusumoto I.T., Hattori M., Namba T. Screening of Indonesian plant extracts for anti-human immunodeficiency virus—type 1 (HIV-1) activity. Phytother. Res. 1995;9:6–10. doi: 10.1002/ptr.2650090103. [DOI] [Google Scholar]
- 223.Yao X.J., Wainberg M.A., Parniak M.A. Mechanism of inhibition of HIV-1 infection in vitro by purified extract of Prunella vulgaris. Virology. 1992;187:56–62. doi: 10.1016/0042-6822(92)90294-Y. [DOI] [PubMed] [Google Scholar]
- 224.St-Pierre C., Ouellet M., Tremblay M.J., Sato S. Galectin-1 and HIV-1 Infection. In: Fukuda M., editor. Glycobiology. Volume 480. Academic Press; Cambridge, MA, USA: 2010. pp. 267–294. [DOI] [PubMed] [Google Scholar]
- 225.Basak A., Cooper S., Roberge A.G., Banik U.K., Chretien M., Seidah N.G. Inhibition of proprotein convertases-1, -7 and furin by diterpines of Andrographis paniculata and their succinoyl esters. Biochem. J. 1999;338:107–113. doi: 10.1042/bj3380107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ekalaksananan T., Sookmai W., Fangkham S., Pientong C., Aromdee C., Seubsasana S., Kongyingyoes B. Activity of Andrographolide and Its Derivatives on HPV16 Pseudovirus Infection and Viral Oncogene Expression in Cervical Carcinoma Cells. Nutr. Cancer. 2015;67:687–696. doi: 10.1080/01635581.2015.1019630. [DOI] [PubMed] [Google Scholar]
- 227.Gopalasatheeskumar K., Karthikeyen L., Anguraj M., Jerad S., Kalaichelvan V.K., Kumudhaveni B. Screening of Kabasura Kudineer Chooranam against COVID-19 through Targeting of Main Protease and RNA-Dependent RNA Polymerase of SARS-Cov-2 by Molecular Docking Studies. SSRN; Rochester, NY, USA: 2020. 38p. [DOI] [Google Scholar]
- 228.Suritra B., Omobolanle Abimbola A., Blessing Chinweotito O., Adeola Tawakalitu K.-M., Emmanuel Ifeanyi A., Lawrence E., Ankita K., Ravindran J., Niyi Samuel A. Polypharmacology of Some Medicinal Plant Metabolites Against SARS-CoV-2 and Host Targets: Molecular Dynamics Evaluation of NSP9 RNA Binding Protein. ChemRxiv. 2020 doi: 10.26434/chemrxiv.12945833.v1. [DOI] [Google Scholar]
- 229.Sukardiman M.E., Pratama M.R., Poerwono H., Siswodihardjo S. The coronavirus disease 2019 main protease inhibitor from Andrographis paniculata (Burm. f) Ness. J. Adv. Pharm. Technol. Res. 2020;11:157–162. doi: 10.4103/japtr.JAPTR_84_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Enmozhi S.K., Raja K., Sebastine I., Joseph J. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: An in silico approach. J. Biomol. Struct. Dyn. 2020:1–7. doi: 10.1080/07391102.2020.1760136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Khanal P., Dey Y.N., Patil R., Chikhale R., Wanjari M.M., Gurav S.S., Patil B.M., Srivastava B., Gaidhani S.N. Combination of system biology to probe the anti-viral activity of andrographolide and its derivative against COVID-19. RSC Adv. 2021;11:5065–5079. doi: 10.1039/D0RA10529E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wanchaitanawong P., Chaungwanit P., Poovarodom N., Nitisinprasert S. In vitro antifungal activity of Thai herb and spice extracts against food spoilage fungi. Kasetsart J. Nat. Sci. 2005;39:400–405. [Google Scholar]
- 233.Rehman A.N.N., Furuta T., Kojima S., Takane K., Ali Mohd M. Antimalarial activity of extracts of Malaysian medicinal plants. J. Ethnopharmacol. 1999;64:249–254. doi: 10.1016/S0378-8741(98)00135-4. [DOI] [PubMed] [Google Scholar]
- 234.Mishra K., Dash A.P., Dey N. Andrographolide: A Novel Antimalarial Diterpene Lactone Compound from Andrographis paniculata and Its Interaction with Curcumin and Artesunate. J. Trop. Med. 2011;2011:579518. doi: 10.1155/2011/579518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sachdeva M. Analysis of in-vitro antimalarial activity of Andrographolide and 5-hydroxy-7,8-dimethoxyflavone Isolated from andrographis paniculata against plasmodium Berghei parasite. Pharma. Sci. Monit. 2011;2:104–116. [Google Scholar]
- 236.Kaleysa R.R. Screening of indigenous plants for anthelmintic action against human Ascaris lumbricoides. Indian J. Physiol. Pharmacol. 1975;19:47–49. [PubMed] [Google Scholar]
- 237.Dutta A., Sukul N.C. Filaricidal properties of a wild herb, Andrographis paniculata. J. Helminthol. 1982;56:81–84. doi: 10.1017/S0022149X0003426X. [DOI] [PubMed] [Google Scholar]
- 238.Spasov A., Ostrovskij O., Chernikov M., Wikman G. Comparative controlled study of Andrographis paniculata fixed combination, Kan Jang® and an echinacea preparation as adjuvant, in the treatment of uncomplicated respiratory disease in children. Phytother. Res. 2004;18:47–53. doi: 10.1002/ptr.1359. [DOI] [PubMed] [Google Scholar]
- 239.Gabrielian E.S., Shukarian A.K., Goukasova G.I., Chandanian G.L., Panossian A.G., Wikman G., Wagner H. A double blind, placebo-controlled study of Andrographis paniculata fixed combination Kan Jang in the treatment of acute upper respiratory tract infections including sinusitis. Phytomedicine. 2002;9:589–597. doi: 10.1078/094471102321616391. [DOI] [PubMed] [Google Scholar]
- 240.Melchior J., Spasov A.A., Ostrovskij O.V., Bulanov A.E., Wikman G. Double-blind, placebo-controlled pilot and phase III study of activity of standardized Andrographis paniculata Herba Nees extract fixed combination (Kan jang) in the treatment of uncomplicated upper-respiratory tract infection. Phytomedicine. 2000;7:341–350. doi: 10.1016/S0944-7113(00)80053-7. [DOI] [PubMed] [Google Scholar]
- 241.Caceres D.D., Hancke J.L., Burgos R.A., Wikman G.K. Prevention of common colds with Andrographis paniculata dried extract. A Pilot double blind trial. Phytomedicine. 1997;4:101–104. doi: 10.1016/S0944-7113(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 242.Caceres D.D., Hancke J.L., Burgos R.A., Sandberg F., Wikman G.K. Use of visual analogue scale measurements (VAS) to asses the effectiveness of standardized Andrographis paniculata extract SHA-10 in reducing the symptoms of common cold. A randomized double blind-placebo study. Phytomedicine. 1999;6:217–223. doi: 10.1016/S0944-7113(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 243.Mkrtchyan A., Panosyan V., Panossian A., Wikman G., Wagner H. A phase I clinical study of Andrographis paniculata fixed combination Kan Jang versus ginseng and valerian on the semen quality of healthy male subjects. Phytomedicine. 2005;12:403–409. doi: 10.1016/j.phymed.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 244.Chen Q., Liu Y., Liu Y.M., Liu G.Y., Zhang M.Q., Jia J.Y., Lu C., Yu C. Pharmacokinetics and tolerance of dehydroandrographolide succinate injection after intravenous administration in healthy Chinese volunteers. Acta Pharmacol. Sin. 2012;33:1332–1336. doi: 10.1038/aps.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Burgos R.A., Hancke J.L., Bertoglio J.C., Aguirre V., Arriagada S., Calvo M., Caceres D.D. Efficacy of an Andrographis paniculata composition for the relief of rheumatoid arthritis symptoms: A prospective randomized placebo-controlled trial. Clin. Rheumatol. 2009;28:931–946. doi: 10.1007/s10067-009-1180-5. [DOI] [PubMed] [Google Scholar]
- 246.Amaryan G., Astvatsatryan V., Gabrielyan E., Panossian A., Panosyan V., Wikman G. Double-blind, placebo-controlled, randomized, pilot clinical trial of ImmunoGuard—A standardized fixed combination of Andrographis paniculata Nees, with Eleutherococcus senticosus Maxim, Schizandra chinensis Bail. and Glycyrrhiza glabra L. extracts in patients with Familial Mediterranean Fever. Phytomedicine. 2003;10:271–285. doi: 10.1078/094471103322004767. [DOI] [PubMed] [Google Scholar]
- 247.Ang L.P., Ng P.W., Lean Y.L., Kotra V., Kifli N., Goh H.P., Lee K.S., Sarker M.M.R., Al-Worafi Y.M., Ming L.C. Herbal Products Containing Aristolochic Acids: A Call to Revisit the Context of Safety. J. Herb. Med. 2021:100447. doi: 10.1016/j.hermed.2021.100447. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article or Supplementary Material.