Abstract
Thyroid associated ophthalmopathy (TAO) is an organ-specific autoimmune disease occurring in patients with thyroid disease. Patients with TAO-related proptosis is largely due to excessive orbital adipose tissue Adipocyte phospholipase A2 (AdPLA) is one of the most important regulatory factors in adipocyte lipolysis, which may be associated with TAO-related proptosis. Thus, silencing AdPLA by RNA interference may be beneficial for the treatment of TAO. In this study, we sought to evaluate the efficiency of two types of microneedles to deliver siRNAs for silencing AdPLA. Our results showed that AdPLA mRNA was up-regulated in the orbit adipose tissues from TAO patients. Silence of AdPLA by siRNA can reduce lipid accumulation in both human and mouse adipocyte cell lines. Moreover, silence effects of silicon microneedle array patch-based and injectable microneedle device-based siRNA administration were examined at the belly site of the mice, and injectable microneedle device showed higher knockdown efficiency than silicon microneedle array patch. This study sets the stage not only for future treatment of TAO-related proptosis using AdPLA siRNA, but also provides the foundation for targeted siRNA delivery by using microneedles.
Keywords: TAO, AdPLA, RNA interference, adipocytes, microneedles
Introduction
Thyroid associated ophthalmopathy (TAO), also known as Graves’ orbitopathy, is an organ-specific autoimmune disease occurring in patients with thyroid disease. Approximately 52% of patients with TAO-related proptosis is due to excessive orbital adipose tissue. The production of adipose tissue in patients with thyroid-related eye disease is often accelerated, however, it is unclear whether adipose hydrolysis is reduced. In clinical practice, the main treatments for TAO are immunosuppressants, retrobulbar radiotherapy, or surgical resection1–3. However, there is still a lack of safe and effective treatments for TAO-related eyeball protrusion. Recently, accumulating studies are focusing on adipose hydrolysis strategy for reducing excessive orbital adipose tissue. Adipocyte phospholipase A2 (AdPLA) is one of the most important regulatory factors in adipocyte lipolysis. Human AdPLA is encoded by Pla2g16 gene4 and regulates adipocyte lipolysis and release of fatty acids through a G-protein coupled pathway. It has been reported that AdPLA knock-out mice have higher rate of lipolysis, higher energy expenditure and remain lean, indicating the critical role of AdPLA in obesity development5. Thus, suppressing AdPLA may be beneficial for TAO-related proptosis treatment.
RNA interference (RNAi) is induced by double-stranded RNA (dsRNA), which causes degradation of specific homologous messenger RNA (mRNA), thus repressing gene expression. RNAi therapy can be widely used for the treatment of genetic diseases. Chemically synthesized small interfering RNA (siRNA) has an efficient inhibitory effect and is degradable in cells6,7. In 2018, the U.S. Food and Drug Administration approved the first drug to harness RNAi, indicating this type of therapeutics may represent the third major platform of drug development8.
Microneedle transdermal delivery technology represents a new type of drug delivery method based on the research of micro-electronic mechanical system (MEMS) processing technology and percutaneous penetration promotion technology. Microneedle transdermal drug delivery uses a microneedle array of micrometer size to act on the skin surface and puncturing the stratum corneum produces hundreds of thousands of tiny holes, thereby promoting the percutaneous penetration of drugs. It has the advantages of precise control of penetration position and depth, dose sparing and painless puncture9. Previously, we evaluated the capability of a silicon microneedle array for delivering siRNA across the skin in vivo and the efficacy of an injectable microneedle device for local deliver of siRNA to the mouse xenograft, and both studies showed promising results10,11. In this study, we for the first time demonstrated the up-regulation of AdPLA in orbit adipose tissues of TAO patients. Moreover, the silencing efficacy of specific AdPLA siRNA was determined in both human and mouse adipocyte cell lines. The effects of AdPLA siRNA on adipose accumulation were also detected. Subsequently, silicon solid microneedle array-based and injectable microneedle device-based delivery siRNA targeting AdPLA at the belly site of the mice were performed. The knockdown effects in adipose tissue were further determined.
Materials and Methods
Sample Collection
A total of 45 participants were recruited between December 2016 and December 2017 in Shenzhen Eye Hospital, Shenzhen, China. Among these participants, 16 participants were TAO patients and 29 participants were controls. The TAO orbit adipose tissues were collected during orbital decompression surgery. The diagnosis of TAO was based on the Bartley criteria12. Systematic examination and orbital computed tomography or magnetic resonance imaging scans were performed to verify the enlarged extraocular muscles. Inclusion criteria in this study were as follows: (1) TAO grade III and above; (2) never used or have stopped glucocorticoids; (3) age between 18 and 60 years old; (4) body mass index (BMI) > 18.5 and < 24.99. Exclusion criteria in this study were as follows: (1) high myopia, orbital tumors, trauma, and etc; (2) age or BMI was not in the range from the inclusion criteria; (3) using glucocorticoids. For age and BMI matched control samples, they were collected during cosmetic operations, such as ptosis surgery, eye pouch plastic operation, or subconjunctival orbital fat prolapses resection. The study was approved by the Institutional Review Board of Shenzhen Eye Hospital and informed consent was signed and obtained from each participant prior to the surgery.
Quantitative Real-Time PCR (pRT-PCR)
For mRNA quantification, it was performed according to the manufacturer’s protocols and the MIQE guidelines as previously described13. Briefly, total RNA was extracted from adipose tissue or cells by using RNAiso Plus (Cat. No. 9109, TaKaRa, China) and dissolved in RNase free water (Cat. No. 1768385, Invitrogen). The concentration and quality of RNA were determined by using NanoDrop 2000 (ThermoFisher). The RNA with 260/280 ratio of around 2 was chosen for further use. Then 100 ng RNA was reverse-transcribed to cDNA using PrimeScriptTM RT Master Mix (Cat. No. RR036A, TaKaRa, China). In detail, 100 ng RNA was diluted with RNase-free water, until the total volume up to 8 μl. Then 2 µl PrimeScript RT Master Mix was added into the reaction solution. Reaction conditions were as follows: 37°C for 15 min, 85°C for 5 min, and 4°C for constant temperature. The cDNA was kept in the –80°C refrigerator. Transcript expression was determined by SYBR Green real-time PCR on an ABI 7900 system (Applied Biosystem, USA). The PCR mixture for each well included 10 µl SYBR Green Premix Ex Taq (Tli RnaseH Plus) (2X), 0.4 µl PCR forward primer, 0.4 µl PCR reverse primer, 0.4 µl ROX reference Dye II (50X), 2 µl template cDNA, 6.8 µl sterile purified water, total 20μl. The qRT-PCR program was as follows: Stage 1: Initial denaturation. 95°C, 30 s, Cycle: 1; Stage 2: PCR. 95°C, 5 s, 60°C, 30 s, Cycle: 40; Stage 3: Dissociation stage. 95°C, 15 s, 60°C, 60 s, 95°C, 15 s, Cycle:1. The PCR measurements were done in triplicates. GAPDH was used as the internal control and the relative expression of AdPLA was normalized by GAPDH. The primer sequences were as follows: Human AdPLA forward: 5′-TCAAGAAACAAGCGACAAA-3′, reverse: 5′-TCCACAAACCAAACCCCAAACTCTC-3′; human GAPDH forward: 5′-GGCATGGACTGTGGTCATGAG-3′, reverse: 5′-TGCACCACCAACTGCTTAGC-3′; mouse AdPLA forward: 5′-TGACCAAGCTGCTACTGA-3′, reverse: 5′-GGAAATCCACTGTCCCTA-3′; mouse GAPDH forward: 5′-CATCACTGCCACCCAGAAGACTG-3′, reverse: 5′-ATGCCAGTGAGCTTCCCGTTCAG-3′.
Cell Culture and Differentiation
Human preadipocyte (HPA-s) was purchased from ScienCell Research Laboratories (Cat. No. 7220; Carlsbad, CA, USA,) and mouse fibroblast 3T3-L cell lines was obtained from ATCC (Cat. No. ATCC® CL-173™; Manassas, USA,) and cultured in corresponding full medium in a humidified incubator at 37°C with 5% CO2.
Adipogenic differentiation of HPA-s was carried out according to previous methods14. HPA-s were cultured for 3 days followed by stimulating with differentiation medium for 5 days. The differentiation medium consists of 500 ml of PADM basal medium (Cat. No. 7221, ScienCell), 25 ml of fetal bovine serum (FBS, Cat. No. 0025, ScienCell), 5 ml of preadipocyte differentiation supplement (PAdDS, Cat. No. 7232, ScienCell), 5 ml of penicillin/streptomycin solution (P/S, Cat. No. 0503, ScienCell). The medium was replaced every two days.
3T3-L1 preadipocytes were cultured for 2 days, then 3T3-L1 cells were stimulated with differentiation medium for 3 days. The differentiation medium consists of DMEM (Cat. No.11965092, ThermoFisher) with 10% FBS and MDI (0.5 mM 1-methyl-3-isobutylxanthine (Cat. No. I5879, Sigma-Aldrich), 1 μM dexamethasone (Cat. No.D4902, Sigma-Aldrich), and 10 μg/mL insulin, Cat. No. 12585014, Sigma-Aldrich). Cells were cultured with DMEM containing 10% FBS and 10 μg/mL insulin after three days. The medium was replaced every two days.
Cell Transfections
The AdPLA siRNAs and nonsense siRNAs were purchased from Guangzhou RiboBio Co., LTD (China). Cell transfection was performed by using the Lipofectamine 2000 reagent (Cat. No. 11668019, ThermoFisher). For each well, 6 pmol siRNA and 0.3 µl Lipofectamine 2000 reagent were diluted in 5 µl Opti-MEM (Cat. No. 31985088, ThermoFisher) respectively, then mixed gently and incubated at room temperature for 5 mins. Diluted siRNA was added to diluted Lipofectamine 2000 reagent and incubated for 20 mins. Subsequently, 10 µl siRNA-lipid mixture was added to each well. The nonsense siRNAs were served as negative control. The siRNA sequences were as follows: mouse AdPLA siRNA sense 5′- CCCACACAAUAUUCAUAAUdTdT-3′, antisense 5′- AUUAUGAAUAUUGUGUGGGdTdT-3′; human AdPLA siRNA sense 5′- GCCGUGACAUACAUCUUGUdTdT-3′, antisense 5′-ACAAGAUGUAUGUCACGGCdTdT-3′. The transfection was done in triplicates.
Western Blotting
Equal amounts of proteins were separated by 12% SDS-PAGE and transferred to PVDF membranes (Amersham; GE Healthcare, Buckinghamshire, England). After blocking with 1.5% skimmed milk at room temperature for 1 h, the membranes were incubated with rabbit anti-human/mouse AdPLA antibody (Cat. No. 10337, Cayman chemical, 1:1000) and β-actin antibody (Santa Cruz Biotechnology, Paso Robles, CA, 1:1000) at 4°C for overnight. The membrane was washed three times and incubated with horseradish peroxidase-conjugated secondary antibodies (1:4000; Santa Cruz Biotechnology,) at room temperature for 1 h, respectively. The western blot bands were detected by ECL kit (Thermo Fisher Scientific). β-actin was used as the internal control.
Oil red O for Lipid Staining
The adipocytes were fixed with 4% paraformaldehyde for 30 min and washed three times with phosphate buffered saline (PBS). Next, the adipocytes were stained with 0.5% Oil Red O for 30 min, followed by washing with PBS. Images were captured at 100 or 200 × magnification under a light microscope. For quantitative analysis, isopropyl alcohol was added to dissolve the Oil Red O, and the optical density value was measured by spectrophotometer at 520 nm. The experiment was repeated for 6 times.
Delivery of AdPLA siRNA using the Microneedles in vivo
All experiments were performed under license from the Government of the Hong Kong SAR and endorsed by the Animal Experimentation Ethics Committee of the Chinese University of Hong Kong. Male C57 mice (6 weeks old) were used in the study. Mice were anesthetized by intraperitoneal injection of ketamine (75 mg/kg) and xylazine (10 mg/kg). As previously described10, the solid microneedle array patch was placed in contact with 5 µl of mouse AdPLA siRNA solution (10 μg/μl) on the skin at the belly site, pressed six times and left unmoved for 5 minutes. For injectable microneedle, 5 µl of the siRNA solution was directly injected with microneedle into the belly site of the mice10. The mice were killed and adipose tissues at the injection site were collected 24 h after injection. The silence effects of the siRNA were detected by qRT-PCR. Each group had 5 mice.
Statistical Analysis
Numeric data in each group was expressed as mean ± standard deviation and analyzed using GraphPad Prism 5.0 software (GraphPad Inc., San Diego, CA, USA) from at least three independent experiments. Significant difference of the mean between different group was assessed using unpaired Student’s t-test. The categorical data was analyzed using Chi-square test. Statistical significance is set at P < .05.
Results
AdPLA was Upregulated in the Adipose Tissue of TAO Patients
The average age of 16 TAO patients was 36.9 ± 9.3 years old. There were 6 male and 10 female participants. For the control subjects, the average age was 37.1 ± 12.7 years old, and there were 10 male and 19 female participants. No gender difference was detected between control and TAO group (P = .084).The average exophthalmos of TAO patients and control subjects were 20.406 ± 1.369 and 14.207 ± 1.146 mm, respectively, and exophthalmos was significantly higher in the TAO patients than that in the control subjects (P < .05). In addition, adipose tissues in orbit of TAO patients were 32.162 ± 1.923 ml, and that in the control group was 24.279 ± 1.070 ml, and the amount of adipose tissues in the TAO group was higher than that in the control group (P < .05) (Table 1).
Table 1.
Clinical Characteristics of TAO Patients and Control Subjects.
| TAO (n = 16) | Control (n = 29) | P value | |
|---|---|---|---|
| Age (years) | 36.9 ± 9.3 | 37.1 ± 12.7 | >.05 |
| Male | 6 | 10 | .084 |
| Female | 10 | 19 | |
| Exophthalmos (mm) | 20.406 ± 1.369 | 14.207 ± 1.146 | <.05 |
| Adipose tissue (ml) | 32.162 ± 1.923 | 24.279 ± 1.070 | <.05 |
As AdPLA is a regulator of adipocyte lipolysis, we first examined AdPLA expression level in orbit adipose tissue of TAO patients and control subjects. AdPLA mRNA expression was significantly up-regulated in the TAO group when comparing with control group (Fig. 1).
Figure 1.
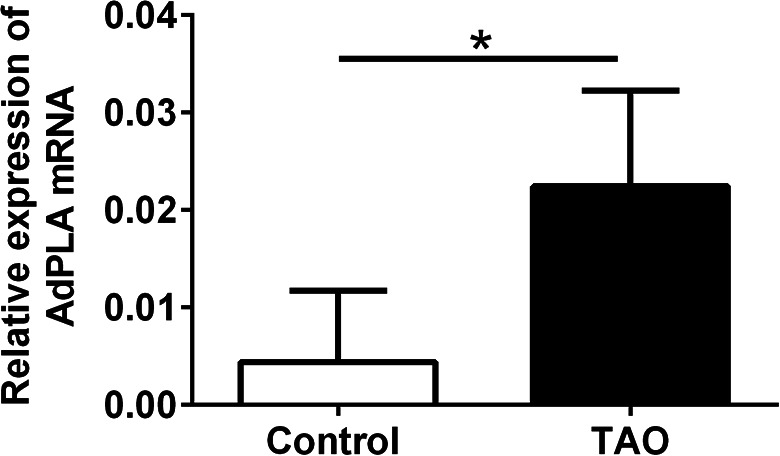
The AdPLA mRNA expression was up-regualted in the eye orbit adipose tissue of TAO patients. The mRNA expression levels in the eye orbit adipose tissue of TAO patients (n = 16) and control subjects (n = 29) were determined by qRT-PCR. Data were presented as mean ± standard deviation. *P < 0.05 (unpaired Student’s t-test).
Silence of AdPLA Was Achieved by Specific siRNA
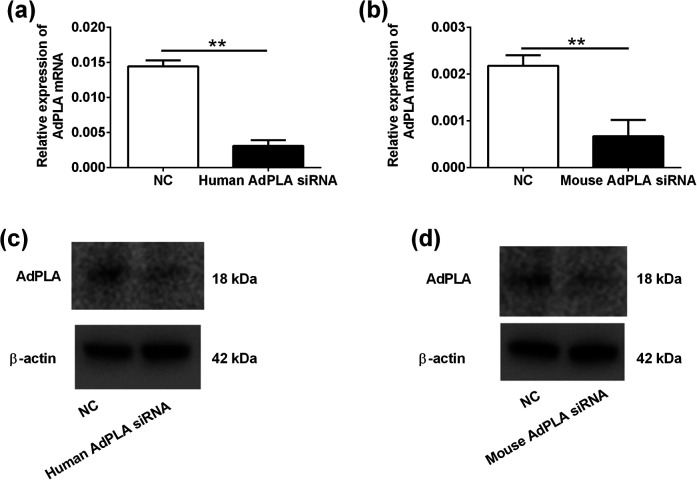
The silencing effects of both human and mouse AdPLA siRNAs were determined in HPA-s and 3T3-L1 cells. After 24 h transfection, significant knockdown effect was observed in the cells. For HPA-s, human AdPLA siRNA transfection significantly repressed the mRNA and protein expression levels of AdPLA in HPA-s (Fig. 2a, c). Consistently, mouse AdPLA siRNA transfection also remarkably suppressed the mRNA and protein expression levels of AdPLA in 3T3-L1 cells (Fig. 2b, d).
Figure 2.
The effects of AdPLA siRNA transfection on the expression of AdPLA in the HPA-s and 3T3-L1 cells. HPA-s cells and 3T3-L1 cells were transfected with human and mouse AdPLA siRNAs, respectively, or the corresponding scrambled siRNAs, at 24 h after transfection, (a and b) the mRNA expression levels of AdPLA in HPA-s and 3T3-L1 cells were determined by qRT-PCR, and (c, d) the protein expression levels of AdPLA in HPA-s and 3T3-L1 cells were determined by western blot. Data were presented as mean ± standard deviation; n = 3. *P < .05 (unpaired Student’s t-test).
AdPLA Silence Reduced Lipid Accumulation
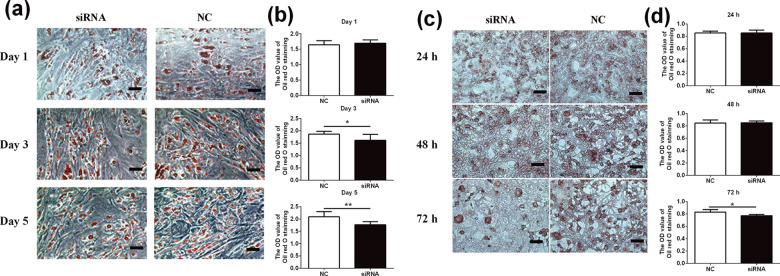
To explore the regulatory role of AdPLA in lipid accumulation, we performed Oil red O staining in HPA-s and 3T3-L1 cells after AdPLA siRNA transfection. The HPA-s cells were transfected with human AdPLA siRNA one day before differentiation, and the Oil red O staining was performed at day 1, day 3, and day 5 after transfection, respectively. As shown in Fig. 3a, b, the intensity of Oil red O staining in the silencing group was remarkably less than that in the control group at day 3 and 5 after AdPLA siRNA transfection. No difference was observed at day 1. Similarly, the 3T3-L1 cells were treated with mouse AdPLA siRNA during the late differentiation period (DMEM + insulin culture). The Oil red O staining analysis was performed at 24, 48, and 72 h after transfection, respectively. The intensity of Oil red O staining in AdPLA siRNA silencing group was significantly reduced at 72 h after transfection when compared to that in the NC group. No difference was observed at 24 or 48 h (Fig. 3c, d).
Figure 3.
The effects of AdPLA knockdown on the Oil red O staining intensity in HPA-s and 3T3-L1 cells. (a) Oil red O staining in HPA-s cell lines. (b) The average OD value of Oil red O staining in HPA-s cell lines. (c) Oil red O staining in 3T3-L1 cell lines at different time points. (d) The average OD value of Oil red O staining in 3T3-L1 cell lines at different time points. Data were presented as mean ± standard deviation; n = 6. *P < .05 (unpaired Student’s t-test).
The in vivo Knockdown Effect of AdPLA siRNA Delivered by the Microneedles
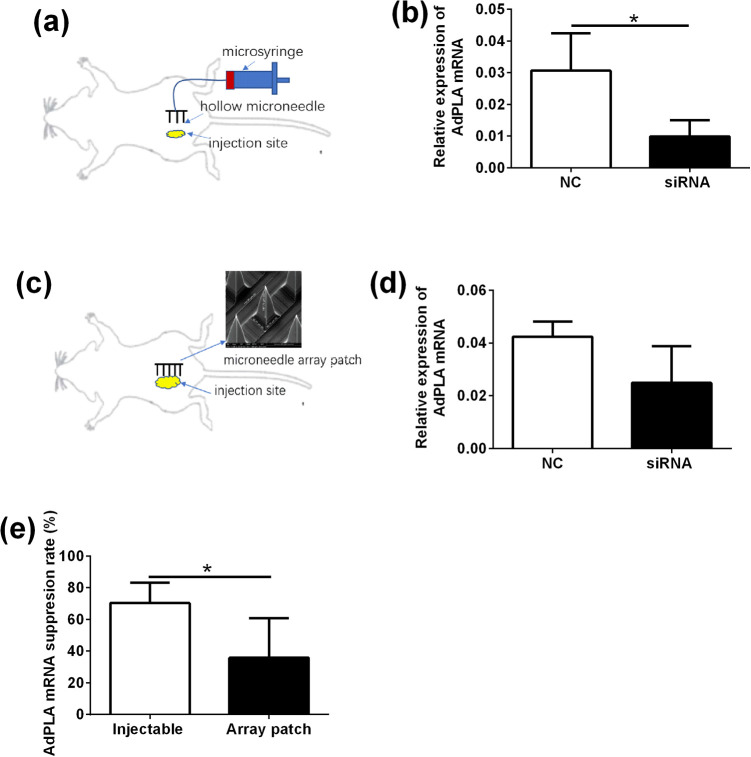
Previously, we have evaluated the capability of a silicon microneedle array and an injectable microneedle device for delivering siRNA across the skin in vivo10,11. Here, we compared the silence effects of AdPLA siRNAs delivered by these microneedles. The injectable microneedles were connected to a micro-syringe. After transdermal injection, a significant reduction of AdPLA expression was detected in adipose tissue at the injection site (Fig. 4a, b). The microneedle patch administration failed to significantly reduce AdPLA expression level (Fig. 4d). When comparing the suppression rate between two types of microneedles, the injectable microneedle had an average of 70% suppression rate and the microneedle patch showed around 50% suppression rate. The suppression rate in the injection microneedles group was significantly higher than in that in the microneedle patch group (Fig. 4e).
Figure 4.
The in vivo knockdown effect of AdPLA siRNA delivered by the microneedles. (a) Method of siRNA delivery using injectable microneedle device. (b) The AdPLA expression in mice adipose tissue administrated with injectable microneedle device (c) Method of siRNA delivery using solid microneedle patch. (d) The AdPLA expression in mice adipose tissue administrated with solid microneedle patch. (e) Comparation of AdPLA suppression rate between injectable microneedle device group and solid microneedle patch group. Data were presented as mean ± standard deviation; n = 5. *P < .05 (unpaired Student’s t-test).
Discussion
Transdermal administration is not only a local administration method, but also a systemic administration method that can be performed through the blood circulation. Compared with oral administration, transdermal administration can effectively avoid liver first-pass effect and degradation in the gastrointestinal tract. When comparing with intravenous injection, it shows convenient administration, less toxic and side effects, and painless15. However, the human skin has the function of a natural barrier, and maintains the balance of various functions of the tissue itself. Thus, how to overcome this barrier and improve the transdermal efficiency is a challenge for the delivery of those drugs.
Gene therapy is to use nucleic acids (RNA or DNA) as drugs to treat diseases by silencing or overexpressing genes in patients’ cells. The transdermal delivery of nucleic acids is beneficial for a wide range of diseases. One way to overcome skin barriers and promote the transdermal delivery of nucleic acids is to adopt microneedle technology. At present, there have been many studies on the use of microneedles for nucleic acids delivery. Pearton et al. coated the plasmid DNA onto the microneedles, and found that the pDNA-coated microneedles promoted gene expression in the human skin16. Pamornpathomkul et al. studied the effects of different types of microneedles and nanocarriers for promoting skin penetration and in vivo immunity of ovalbumin-encoding plasmid DNA (pOVA), and discovered that hollow microparticles have excellent enhancing effects on pOVA skin penetration17. Another study prepared siRNA-loaded PVA soluble microneedles, and this kind of microneedle-mediated siRNA could effectively silence gene expression. In addition, after delivering plasmid DNA encoding luciferase for 24 h, mice bioluminescence was detected in the skin of the ears, back and soles18. In the previous study, we used a solid silicon microneedle array to form a microporous channel in the mouse ear to deliver cholesterol-modified siRNA, and the siRNA successfully down-regulated the expression of Gapdh gene after passing through the skin, and did not accumulate in the main organs, demonstrating that the array can effectively deliver siRNA to the corresponding site10. The injectable microneedle device for local deliver of siRNA to the mouse xenograft was also assessed, and local administration of siRNA could effectively deliver siRNA into the tumor region, inhibiting tumor progression without major adverse effects11.
The adipose tissue accumulation is one of the most crucial cause of TAO-related proptosis and the current main treatment is surgery. Patients who underwent surgeries were annoyed by pain, long recovery period, and economic loss due to hospital bills for the surgical treatment and work delay. Thus, we are wondering whether TAO-related proptosis is associated with some genes and can be cured by gene therapy. AdPLA has been reported to be a major regulator of adipocyte lipolysis and is important for the development of obesity. AdPLA regulates adipocyte lipolysis and release of fatty acids through a G-protein coupled pathway, which involves prostaglandin and EP34. Based on gene expression result, AdPLA mRNA expression level in TAO patients is significantly higher than that in the control group, which indicates that AdPLA may be related to TAO-related proptosis. Furthermore, specific siRNAs were used to target AdPLA in in human and mouse adipocytes efficiently. Additionally, knockdown of AdPLA reduced the number of adipocytes in vitro. Therefore, silence of AdPLA may be an effective strategy for TAO treatment.
In this study, we sought to compare the capability of two types of microneedles to deliver siRNA to silence AdPLA. Since the microneedle array patch surface is rough and layer upon layer, which has a relative large surface area, it can increase the capacity for siRNA loading10. Comparing with solid microneedle array patch, the hollow injectable microneedles show efficient delivery of liquid medication and can make the dose accurate11. In this study, injectable microneedle device showed higher AdPLA suppression rate. The siRNA distribution and safety issue of this kind of microneedle were addressed in previous study, thus, no safety experiments were involved in this study. However, there are some limitations in the study. Firstly, there was not enough clinical samples for measuring AdPLA protein expression levels and future studies should collect more clinical samples to assess the difference in the AdPLA protein expression levels between TAO patients and control subjects. Secondly, the in vivo knockdown effects of AdPLA siRNA were only examined in the normal mice, and future studies may consider to determine the silencing efficiency of AdPLA siRNA in the TAO mouse model. Thirdly, we only used male mice for proving the concept, and future studies may use mixed genders of mice to confirm our findings.
In summary, this study was a proof-of-concept study. The present study demonstrated that AdPLA was up-regulated in orbit adipose tissues of TAO patients. Silence of AdPLA by siRNA can reduce the amount of adipocytes. Moreover, silicon microneedle array-based and injectable microneedle device-based delivery siRNA successfully silenced AdPLA at the belly site, and injectable microneedle device showed higher knockdown efficiency. This study sets the stage not only for future treatment of TAO-related proptosis using AdPLA siRNA, but also provides the foundation for targeted siRNA delivery by using microneedles.
Footnotes
Author Contributions: Conceptualization, G.L. and T.T.; methodology, Y.D. and Y.S.; formal analysis, Y.D., Y.S., and Z, S; data curation, Y.S. (Yi Song) and Y.S. (Yi Sui); writing—original draft preparation, Y.D., J.C., and Y.S. writing—review and editing, Y.S. and H.L.; supervision, G.L. and T.T.; funding acquisition, G.L and Y.S. All authors have read and agreed to the published version of the manuscript.
Guiqin Liu, Yan Deng, Yi Song, Yi Sui are authors equally contributed to this work.
Ethical Approval: The study was approved by the Institutional Review Board of Shenzhen Eye Hospital and informed consent was signed and obtained from each participant prior to the surgery.
Statement of Human and Animal Rights: All experiments were performed under license from the Government of the Hong Kong SAR and endorsed by the Animal Experimentation Ethics Committee of the Chinese University of Hong Kong.
Statement of Informed Consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by Shenzhen Science and Technology Innovation Project (JCYJ20140414114853648), the Basic Research for 2015 Shenzhen Municipal Science and Technology Programme (JCYJ20150630165236963) and National Natural Science Foundation of China (81660150).
ORCID iD: Tao Tang  https://orcid.org/0000-0003-0653-7987
https://orcid.org/0000-0003-0653-7987
References
- 1. Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362(8):726–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Subekti I, Pramono LA. Current diagnosis and management of graves’ disease. Acta Med Indones. 2018;50(2):177–1182. [PubMed] [Google Scholar]
- 3. Bartalena L. Diagnosis and management of Graves disease: a global overview. Nat Rev Endocrinol. 2013;9(12):724–734.24126481 [Google Scholar]
- 4. Duncan RE, Sarkadi-Nagy E, Jaworski K, Ahmadian M, Sul HS. Identification and functional characterization of adipose-specific phospholipase A2 (AdPLA). J Biol Chem. 2008;283(37):25428–25436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jaworski K, Ahmadian M, Duncan RE, Sarkadi-Nagy E, Varady KA, Hellerstein MK, Lee HY, Samuel VT, Shulman GI, Kim KH, de Val S, et al. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat Med. 2009;15(2):159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–498. [DOI] [PubMed] [Google Scholar]
- 7. Robb GB, Brown KM, Khurana J, Rana TM. Specific and potent RNAi in the nucleus of human cells. Nat Struct Mol Biol. 2005;12(2):133–137. [DOI] [PubMed] [Google Scholar]
- 8. Mullard A. FDA approves landmark RNAi drug. Nat Rev Drug Discov. 2018;17(9):613. [DOI] [PubMed] [Google Scholar]
- 9. Waghule T, Singhvi G, Dubey SK, Pandey MM, Gupta G, Singh M, Dua K. Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–1258. [DOI] [PubMed] [Google Scholar]
- 10. Deng Y, Chen J, Zhao Y, Yan X, Zhang L, Choy K, Hu J, Sant HJ, Gale BK, Tang T. Transdermal delivery of siRNA through microneedle array. Sci Rep. 2016;6:21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang T, Deng Y, Chen J, Zhao Y, Yue R, Choy KW, Wang CC, Du Q, Xu Y, Han L, et al. Local administration of siRNA through microneedle: optimization, bio-distribution, tumor suppression and toxicity. Sci Rep. 2016;6:30430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bartley GB, Gorman CA. Diagnostic criteria for graves’ ophthalmopathy. Am J Ophthalmol. 1995;119(6):792–795. [DOI] [PubMed] [Google Scholar]
- 13. Chen J, Deng Y, Ao L, Song Y, Xu Y, Wang CC, Choy KW, Tony Chung KH, Du Q, Sui Y, Yang T, et al. The high-risk HPV oncogene E7 upregulates miR-182 expression through the TGF-β/Smad pathway in cervical cancer. Cancer Lett. 2019;460:75–85. [DOI] [PubMed] [Google Scholar]
- 14. Gu N, You L, Shi C, Yang L, Pang L, Cui X, Ji C, Zheng W, Guo X. Expression of miR-199a-3p in human adipocytes is regulated by free fatty acids and adipokines. Mol Med Rep. 2016;14(2):1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hao Y, Li W, Zhou X, Yang F, Qian Z. Microneedles-based transdermal drug delivery systems: a review. J Biomed Nanotechnol. 2017;13(12):1581–1597. [DOI] [PubMed] [Google Scholar]
- 16. Pearton M, Saller V, Coulman SA, Gateley C, Anstey AV, Zarnitsyn V, Birchall JC. Microneedle delivery of plasmid DNA to living human skin: formulation coating, skin insertion and gene expression. J Control Release. 2012;160(3):561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pamornpathomkul B, Wongkajornsilp A, Laiwattanapaisal W, Rojanarata T, Opanasopit P, Ngawhirunpat T. A combined approach of hollow microneedles and nanocarriers for skin immunization with plasmid DNA encoding ovalbumin. Int J Nanomedicine. 2017;12:885–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gonzalez-Gonzalez E, Speaker TJ, Hickerson RP, Spitler R, Flores MA, Leake D, Contag CH, Kaspar RL. Silencing of reporter gene expression in skin using siRNAs and expression of plasmid DNA delivered by a soluble protrusion array device (PAD). Mol Ther. 2010;18(9):1667–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]