Pharmacokinetic data are now available for the absorption, plasma concentrations and renal excretion of vitamin C. With these new insights, it is worth reconsidering the clinical role of vitamin C in scurvy and subclinical deficiency, the amount of vitamin C required for good health and, in particular, the speculative use of high doses of vitamin C to treat cancer.
Scurvy, the dread of sailors past, conjures up images of perilous sea voyages and rough tattooed men laid low by ignorance of a vital amine, later designated vitamin C (which, as it happens, is not an amine). While scurvy devastated seafarers, it was endemic in the landbound, occurring widely wherever fruit and vegetables were in short supply. Military campaigns from the Crusades to the Napoleonic Wars, the American Civil War, and even World War I were stymied by widespread and often fatal scurvy among the troops. The antiscorbutic principle, identified and named ascorbic acid (vitamin C) in 1932, is a simple water-soluble sugar-like molecule. Early experiments, though perhaps flawed, showed that the consumption of as little as 10 mg of vitamin C a day would prevent signs of clinical scurvy.1 Although minute amounts will forestall death, the optimum requirements for good health are not known. Vitamin C is concentrated in many tissues, but these tissue stores are easily depleted. James Lind's Treatise on Scurvy, first published in 1753, reported the onset of the disease in sailors after a month and a half at sea and described lassitude as its early and invariable symptom.2 Depletion–repletion studies in volunteers using a diet free of vitamin C have shown that plasma vitamin C falls below 10 μmol/L in less than a month. At these concentrations, fatigue is invariably present,3 and physical signs appear soon after.1 A person who has been consuming 100 mg of vitamin C daily will not develop scurvy for a month, even if the intake of vitamin C is stopped altogether.3 Recently, on the advice of the Food and Nutrition Board of the US National Academy of Sciences, US and Canadian recommended dietary allowances were increased from 60 mg per day to 75 mg per day for women and 90 mg per day for men. Vitamin C intake is less than 60 mg in 20%–30% of US adults. It is even lower among many population subgroups, including children. Subclinical vitamin C deficiency is much more common than is generally recognized,4 especially because the first symptom of deficiency is fatigue, a nonspecific and common complaint.
As an electron donor, vitamin C acts as a cofactor for 8 enzymes involved in collagen hydroxylation, biosynthesis of carnitine and norepinephrine, tyrosine metabolism and amidation of peptide hormones.5 Vitamin C also has many nonenzymatic actions. It is a powerful water-soluble antioxidant and, at physiological concentrations, probably does not produce reactive intermediaries. It protects low-density lipoproteins from oxidation, reduces harmful oxidants in the stomach and promotes iron absorption. Its antioxidant role in vivo is, however, unclear. Plasma ascorbic acid concentrations may be low in chronic or acute oxidant states such as in diabetes, in smokers, or following acute pancreatitis or myocardial infarction. Ascorbic acid is easily oxidized to the unstable dehydroascorbic acid. Dehydroascorbic acid is not normally detectable in plasma but may occur transiently during oxidant stress. Ascorbic acid is transported into the cell by sodium-dependent vitamin C transporters SVCT1 and SVCT2, one or both of which are found in most tissues.6 Dehydroascorbic acid is transported by glucose transporters GLUT1 and GLUT3, and, in insulin-sensitive tissues, also by GLUT4. When exposed to bacteria, neutrophils oxidize extracellular ascorbic acid to form dehydroascorbic acid, which is transported into the neutrophil and rapidly reduced to ascorbic acid by the protein glutaredoxin (Fig. 1). As a result of this recycling of extracellular ascorbic acid, the neutrophil internal concentration of ascorbic acid increases 10-fold.7 Ascorbic acid may quench oxidants generated during phagocytosis and, thus, protect the neutrophil and surrounding tissues from oxidative damage. Brain, adrenal cortex, liver, spleen, pancreas and kidney tissues concentrate vitamin C for unknown reasons.
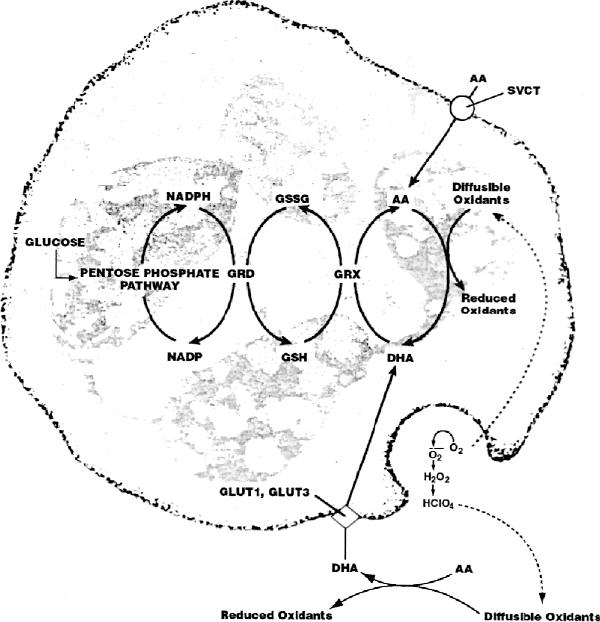
Fig. 1: Ascorbic acid (AA) transport, dehydroascorbic acid (DHA) transport and recycling in human neutrophils. AA, transported by sodium-dependent vitamin C transporters (SVCT), maintains mmol/L concentrations of AA inside neutrophils. Activated neutrophils secrete reactive oxygen species that oxidize extracellular AA to DHA. DHA is rapidly transported into the neutrophil by the glucose transporters GLUT1 and GLUT3 and immediately reduced to AA by glutaredoxin (GRX), producing a 10-fold increase in neutrophil internal AA concentration. Glutathione (GSH), used during DHA reduction, is regenerated from glutathione disulfide (GSSG) by glutathione reductase (GRD) and NADPH. NADPH is a product of glucose metabolism through the pentose phosphate pathway. As NADPH is oxidized to NADP, electrons are transferred to GRD so it can reduce GSSG to GSH. Modified and reproduced with permission of the Journal of Nutritional Biochemistry 1998;9:120,7 Elsevier Sciences Inc.
When given orally, ascorbic acid is well absorbed at lower doses, but absorption decreases as the dose increases. Thus, median bioavailability following an oral dose is 87% for 30 mg, 80% for 100 mg, 72% for 200 mg and 63% for 500 mg. Less than 50% of a 1250-mg dose is absorbed, and most of the absorbed dose is excreted in the urine.3,8 Ascorbic acid is not protein bound, so it is filtered and reabsorbed by the kidneys in healthy subjects but is lost in patients who have been hemodialyzed. Ascorbic acid begins to appear in urine at doses above 100 mg/day, corresponding to a plasma concentration of about 60 μmol/L, at which point plasma is 70% saturated and circulating white blood cells are fully saturated. Decreased bioavailability and renal excretion keep plasma vitamin C at less than 100 μmol/L, even with an oral dose of 1000 mg. In men at steady state, a 30-mg daily intake results in a mean plasma concentration of 9 μmol/L, 60 mg results in25 μmol/L, 100 mg in 56 μmol/L and 200 mg in 75 μmol/L. Thus, the dose–concentration relationship is sigmoidal, with the steep portion of the curve lying between 30 mg and 100 mg of oral vitamin C daily.3,8 Doses greater than 500 mg daily contribute little to plasma or tissue stores. Circulating white blood cells contain 10–30 times the plasma concentrations of vitamin C.
In addition to the physiological role of ascorbic acid, it may have unrelated pharmacological effects. When ascorbic acid is administered intravenously, the limiting absorptive mechanism is bypassed and very high plasma levels are attained. Following the administration of 1.25 g intravenously, a peak plasma level of 1000 μmol/L is reached, even though 100 μmol/L is not exceeded by oral dosing.8 When 5–10 g is given intravenously, the resulting plasma levels may be as high as 5000 μmol/L.9
This difference between the oral and intravenous administration of high doses was not adequately appreciated in studies of the treatment of cancer with vitamin C. In vitro, ascorbic acid is cytotoxic to many malignant cell lines10 at concentrations that can be achieved in plasma by intravenous, but not oral, administration. Whether similar effects would occur in vivo is not known. The unconventional studies of Cameron and Campbell,11 later joined by Linus Pauling, used high-dose intravenous vitamin C to treat terminal cancer. They reported clinical benefits and improved survival.12,13 Because these studies were not randomized or placebo controlled, Moertel and colleagues carried out 2 randomized placebo-controlled clinical trials at the Mayo Clinic to check these findings.14,15 Using high-dose oral vitamin C, they found no benefit. Given the recent appreciation of the differing plasma levels resulting from oral versus intravenous administration, it is difficult to compare the studies carried out by Moertel and coworkers with those of Cameron and his colleagues. The cause of cancer patients will be better served if advocates and sceptics concerning the efficacy of vitamin C re-examine these issues with both open minds and scientific rigour.
We now know that plasma vitamin C concentrations are tightly controlled and that the vitamin is concentrated by many tissues. The optimum intake of vitamin C, its function in various tissues and its antioxidant actions in vivo remain to be elucidated. In the meantime, we should rigorously explore the potential anticancer effects of vitamin C, when administered intravenously at high doses, in patients with well-documented cancer16 in whom other options have been exhausted. If these studies show promise, then randomized clinical trials should follow.
Footnotes
Clinicians who wish to submit cases for review can contact Drs. Padayatty and Levine for instructions at the address below.
This article has been peer reviewed.
Competing interests: None declared.
Correspondence to: Dr. Mark Levine, Molecular and Clinical Nutrition Section, Bldg. 10, Rm. 4D52–MSC 1372, National Institutes of Health, Bethesda MD 20892–1372; fax 301 402-6436; MarkL@intra.niddk.nih.gov
References
- 1.Hodges RE, Baker EM, Hood J, Sauberlich HE, March SC. Experimental scurvy in man. Am J Clin Nutr 1969;22:535-48. [DOI] [PubMed]
- 2.Lind J. Lind's Treatise on Scurvy. In: Stewart CP, Guthrie D, editors. Bicentenary Volume. Edinburgh: Edinburgh University Press; 1953. p. 69-112.
- 3.Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A 1996;93:3704-9. [DOI] [PMC free article] [PubMed]
- 4.Johnston CS, Thompson LL. Vitamin C status of an outpatient population. J Am Coll Nutr 1998;17:366-70. [DOI] [PubMed]
- 5.Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA 1999;281:1415-23. [DOI] [PubMed]
- 6.Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, et al. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature 1999;399:70-5. [DOI] [PubMed]
- 7.Rumsey SC, Levine M. Absorption, transport, and disposition of ascorbic acid in humans. J Nutr Biochem 1998;9:116-30.
- 8.Graumlich JF, Ludden TM, Conry-Cantilena C, Cantilena LR Jr, Wang Y, Levine M. Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharm Res 1997;14:1133-9. [DOI] [PubMed]
- 9.Riordan NH, Riordan HD, Meng X, Li Y, Jackson JA. Intravenous ascorbate as a tumor cytotoxic chemotherapeutic agent. Med Hypotheses 1995;44:207-13. [DOI] [PubMed]
- 10.Koh WS, Lee SJ, Lee H, Park C, Park MH, Kim WS, et al. Differential effects and transport kinetics of ascorbate derivatives in leukemic cell lines. Anticancer Res 1998;18:2487-93. [PubMed]
- 11.Cameron E, Campbell A. The orthomolecular treatment of cancer. II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chem Biol Interact 1974;9:285-315. [DOI] [PubMed]
- 12.Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A 1978;75:4538-42. [DOI] [PMC free article] [PubMed]
- 13.Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A 1976;73:3685-9. [DOI] [PMC free article] [PubMed]
- 14.Moertel CG, Fleming TR, Creagan ET, Rubin J, O'Connell MJ, Ames MM. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N Engl J Med 1985;312:137-41. [DOI] [PubMed]
- 15.Creagan ET, Moertel CG, O'Fallon JR, Schutt AJ, O'Connell MJ, Rubin J, et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med 1979;301:687-90. [DOI] [PubMed]
- 16.Hawkins MJ, Friedman MA. National Cancer Institute's evaluation of unconventional cancer treatments. J Natl Cancer Inst 1992;84:1699-702. Current version of Best Case Series Program is available: http://occam.nci.nih.gov (accessed 2000 Dec 21). [DOI] [PubMed]


