Abstract
Purpose:
In this study, we aimed to evaluate the relationship between macular hole closure types assessed by optical coherence tomography (OCT) and the preoperative prognostic factors.
Materials and methods:
In total, 183 patients who underwent pars plana vitrectomy and internal limiting membrane peeling for idiopathic macular hole between August 2014 and August 2019 were reviewed retrospectively. The preoperative measurements of the macular hole including minimum linear diameter (MLD), basal hole diameter (BHD) and hole height (HH) were measured on OCT images. The patients were divided into two closure types on the basis of postoperative OCT findings (type 1 closure: retinal edges were flat and there was no defect of the neurosensory retina on the fovea; type 2 closure: retinal edges were flat and there was a defect of the neurosensory retina on the fovea). The difference of prognostic factors such as age; duration of symptoms; preoperative best-corrected visual acuity (BCVA); preoperative macular hole measurements, including MLD, BHD and HH; and rate of reopening between two types were statistically analysed.
Results:
The mean age of patients was 66.33 ± 8.09 years (range: 48–88 years). According to OCT imaging, 117 eyes (63.9%) were classified into the type 1 closure group, and 66 eyes (36.1%) were classified into the type 2 closure group. There were no significant differences between two groups in age, duration of symptoms and preoperative BCVA (p = 0.694, p = 0.092 and p = 0.15). MLD and BHD were significantly larger, and reopening was significantly more common in type 2 group (p < 0.05, p = 0.04 and p < 0.005); however, there was no significant difference in HH between two groups (p = 0.239).
Conclusion:
Preoperative horizontal measurements of macular hole may help to determine postoperative visual expectations and anatomical success, and predict the possibility of reopening.
Keywords: macular hole, macular hole measurements, optic coherence tomography, pars plana vitrectomy
Introduction
The macular hole is a full thickness opening or dehiscence of the retinal tissue involving the fovea. The pathogenesis of macular hole formation is not clear; however, the tangential or anteroposterior vitreofoveal traction has been suggested as the most probable mechanism.1 Surgery for macular hole involves pars plana vitrectomy (PPV), core vitrectomy, removal of the posterior cortical hyaloid and obvious epiretinal membranes, and filling of the vitreous cavity with a tamponade since 1991.2,3
Since the development of optical coherence tomography (OCT), which can show high-resolution cross-sectional images of the retina, a lot of information such as the pathogenesis, classification and diagnosis of macular hole, measuring hole size and postoperative improvement, might be determined easily.4
Tornambe and colleagues5 categorized the macular hole closure types, in terms of the postoperative anatomical status of retinal edges as elevated and open, flat and open, or flat and closed. Kang and colleagues6 classified macular hole closure types according to postoperative OCT in two types: complete sealing of the macular hole without bare retinal pigment epithelium (RPE) and incomplete sealing of the macular hole with bare RPE.
In this study, we aimed to evaluate the relationship between macular hole closure types assessed by OCT and the preoperative prognostic factors like age, duration of symptoms, preoperative best-corrected visual acuity (BCVA), preoperative glaucoma and preoperative macular hole measurements, including minimum linear diameter (MLD), basal hole diameter (BHD) and hole height (HH).
Materials and methods
The records of the patients who underwent PPV for idiopathic macular hole in the retina department of Beyoğlu Eye Training and Research Hospital between August 2014 and August 2019 were reviewed retrospectively. The study was approved by Prof. Dr. Cemil Taşçıoğlu City Hospital Ethics Committee with number of 48670771-514.10, and written informed consent was obtained from each participant in accordance with the principles of the Declaration of Helsinki.
Patients with a history of trauma, more than 6 diopters of myopia, diabetes, age-related macular degeneration, previous vitreoretinal surgery and who were younger than 18 years were excluded from the study. All patients underwent detailed ophthalmological examination, including refraction, BCVA with a Snellen chart, slit-lamp biomicroscopy, intraocular pressure (IOP) measurements, fundus examination after dilatation and OCT imaging (SPECTRALIS; Heidelberg Engineering) at baseline and follow-up visits. The measurements of the macular hole including MLD, BHD and HH were measured on OCT images. We measured MLD as the minimum inner diameter between the macular hole edges, BHD as the diameter of the hole at the level of the RPE and HH as the distance from the RPE to the upper level of the retinal edges (Figure 1).
Figure 1.
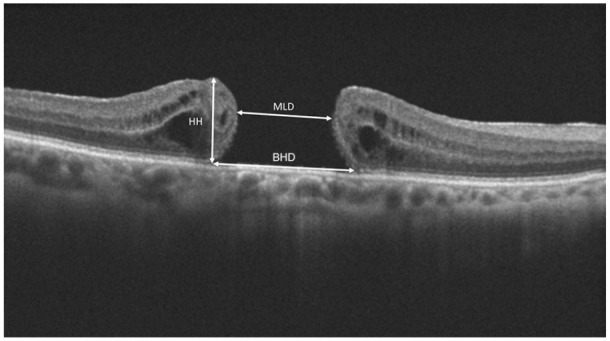
The measurements of the macular hole including MLD, BHD and HH were measured on OCT images. BHD, basal hole diameter; HH, hole height; MLD, minimum linear diameter; OCT, optical coherence tomography.
All patients underwent same surgical procedure. Surgical technique included a standard three-port PPV, then posterior adherent cortical vitreous was removed, internal limiting membrane (ILM) was peeled after staining with brilliant blue and the vitreous cavity was filled with 15% perfluoropropane (C3f8) gas tamponade. If patients had lens opacity resulting in poor visualization of fundus, phacoemulsification and intraocular lens implantation were combined with PPV. All of the patients took a facedown position for more than 10 days postoperatively. The anatomic and functional outcomes of surgery were evaluated at postoperative third month.
We classified the closure type on the basis of postoperative OCT findings. If retinal edges were flat and there was no postoperative defect of the neurosensory retina on the fovea, it was named as type 1 closure (Figure 2); if retinal edges were flat and there was a postoperative defect of the neurosensory retina on the fovea, it was named as type 2 closure (Figure 3).6 If retinal edges remained elevated postoperatively, it was evaluated as unsuccessful closure.
Figure 2.
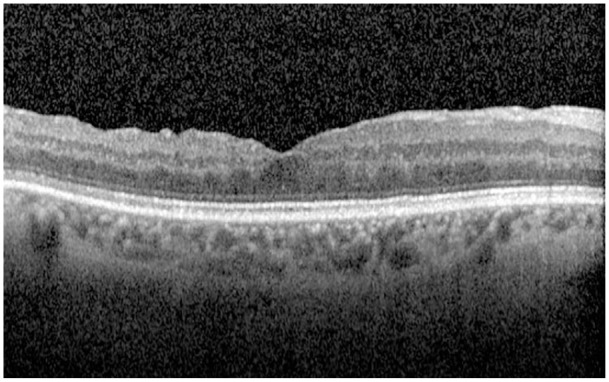
The OCT image of type 1 closure. OCT, optical coherence tomography.
Figure 3.
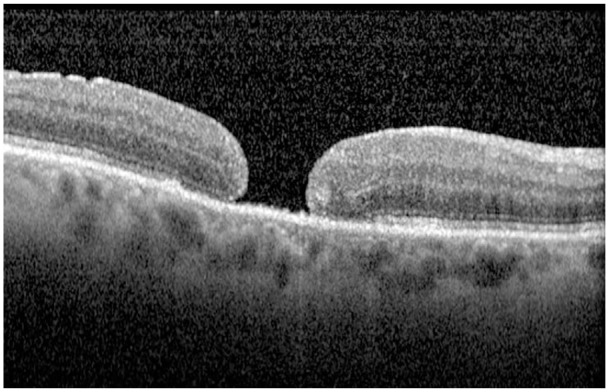
The OCT image of type 2 closure. OCT, optical coherence tomography.
The difference of possible prognostic factors such as age; duration of symptoms; preoperative BCVA; preoperative macular hole measurements, including MLD, BHD and HH; and rate of reopening between two types was statistically analysed.
Statistical analysis
All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS), version 20.0, for Windows. Descriptive statistics included mean values ± standard deviation (SD), percentage, minimum (min) and maximum (max) for normally distributed variables. Visual acuity values were converted to logarithm of the minimal angle of resolution (logMAR) units. Distribution of variables was measured by Kolmogorov–Smirnov test. For quantitative analysis, paired-sample t-test was used for normally distributed variables, and Wilcoxon signed-rank test was used when the measurements did not fit the normal distribution. The variables were compared between two study groups using independent t-test (parametric data) and Mann–Whitney test (nonparametric data). A p value <0.05 was considered significant. In correlation analysis, the Pearson correlation analysis was used for normally distributed variables, and the Spearman correlation analysis was used when the measurements did not fit the normal distribution.
Results
The study included 183 eyes of 183 participants [98 females (53.6%) 85 males (46.4%)], and the mean age of patients was 66.33 ± 8.09 years (range: 48–88 years); 99 cases (54.1%) were right eyes, and 84 cases (45.9%) were left eyes. The mean duration of symptoms was 1.71 ± 1.64 months (range: 1–11 months), and the mean postoperative follow-up period was 11.88 ± 5.37 months (range: 6–48 months). Ten cases (5.5%) had glaucoma preoperatively.
According to OCT imaging at postoperative third month, 117 eyes (63.9%) were classified into type 1 closure group, and 66 eyes (36.1%) were classified into type 2 closure group.
The mean age was 65.73 ± 8.11 years (range: 48–75 years) in type 1 group and 68.36 ± 7.89 years (range: 59–88 years) in type 2 group, and there was no significant difference between two groups (p = 0.694). The mean duration of symptoms was 1.8 ± 1.78 months (range: 1–11 months) in type 1 group, 1.4 ± 0.99 months (range: 1–5 months) in type 2 group, and the mean postoperative follow-up period was 11.60 ± 7.75 months (range: 6–48 months) in type 1 group, 12.88 ± 10.37 months (range: 6–39 months) in type 2 group (Table 1). There were no significant differences between two groups in duration of symptoms and postoperative follow-up period (p = 0.092 and p = 0.516, respectively).
Table 1.
Comparison of preoperative and postoperative variables between two closure types.
| Type 1 | Type 2 | p value | |
|---|---|---|---|
| The mean age | 65.73 ± 8.11 years (range: 48–75 years) | 68.36 ± 7.89 years (range: 59–88 years) | p = 0.694 |
| The mean duration of symptoms | 1.8 ± 1.78 months (range: 1–11 months) | 1.4 ± 0.99 months (range: 1–5 months) | p = 0.092 |
| Postoperative follow-up period | 11.60 ± 7.75 months (range: 6–48 months) | 12,88 ± 10,37 months (range: 6–39 months) | p = 0.516 |
| Phacoemulsification and intraocular lens application with PPV | 6 (5.1%) | 6 (9.1%) | p = 0.433 |
| Preoperative glaucoma | 6 (5.1%) | 4 (6.1%) | p = 0.599 |
| The mean preoperative BCVA (logMAR) | 0.94 ± 0.37 (range: 0.15–1.8) | 1.04 ± 0.43 (range: 0.30–1.8) | p = 0.15 |
| The mean postoperative third-month BCVA (logMAR) | 0.83 ± 0.36 (range: 0.10–1.30) | 0.98 ± 0.33 (range: 0.10–1.30) | p < 0.05 |
| The mean preoperative MLD | 384.24 ± 173.12 µm (range: 70–1226) | 573.24 ± 185.80 µm (range: 183–971) | p < 0.05 |
| The mean preoperative BHD | 924.72 ± 341.58 µm (range: 118–2148) | 1153.58 ± 399.86 µm (range: 401–2303) | p = 0.04 |
| The mean preoperative HH | 464.5 ± 92.86 µm (range: 276–934) | 506.67 ± 196.27 µm (range: 308–1506) | p = 0.239 |
| Reopening in postoperative period | 3 (2.6%) | 10 (15.2%) | p < 0.005 |
BCVA, best-corrected visual acuity; BHD, basal hole diameter; HH, hole height; logMAR, logarithm of the minimal angle of resolution; MLD, minimum linear diameter; PPV, pars plana vitrectomy.
Phacoemulsification and intraocular lens implantation combined with PPV were performed in six (5.1%) patients in type 1 group and six (9.1%) patients in type 2 group, and there was no significant difference between two groups (p = 0.433). Six (5.1%) patients in type 1 group and four (6.1%) patients in type 2 group had preoperative glaucoma, and there was no significant difference (p = 0.599; Table 1).
The mean preoperative BCVA was 0.94 ± 0.37 logMAR (range: 0.15–1.80) in type 1 group and 1.04 ± 0.43 logMAR (range: 0.3–1.80) in type 2 group, and there was no significant difference of preoperative BCVA between two groups (p = 0.15). The mean postoperative third-month BCVA was 0.83 ± 0.36 logMAR (range: 0.10–1.30) in type 1 group and 0.98 ± 0.33 logMAR (range: 0.10–1.30) in type 2 group, and postoperative BCVA was found to be higher in type 1 group than type 2 group (p < 0.05; Table 1). The postoperative BCVA changes were significant in both groups (p < 0.05). There was a moderate correlation between preoperative BCVA and postoperative BCVA in both groups (Table 2). However, there was no correlation between age and postoperative BCVA and between duration of symptoms and postoperative BCVA in both types (Table 2).
Table 2.
Correlations between continuous variables.
| Type 1 | Type 2 | |
|---|---|---|
| Preoperative BCVA–postoperative BCVA | ||
| r value | 0.397 | 0.345 |
| Age–postoperative BCVA | ||
| r value | 0.097 | 0.068 |
| Duration of symptoms–postoperative BCVA | ||
| r value | 0.089 | 0.121 |
BCVA, best-corrected visual acuity.
The mean preoperative MLD was 384.24 ± 173.12 µm (range: 70–1226), BHD was 924.72 ± 341.58 µm (range: 118–2148) and HH was 464.5 ± 92.86 µm (range: 276–934) in type 1 group, and the mean preoperative MLD was 573.24 ± 185.80 µm (range: 183–971), BHD was 1153.58 ± 399.86 µm (range: 401–2303) and HH was 506.67 ± 196.27 µm (range: 308–1506) in type 2 group. MLD and BHD were significantly larger in type 2 group (p < 0.05 and p = 0.04); however, there was no significant difference in HH between two groups (p = 0.239; Table 1).
No postoperative complication such as endophthalmitis, retinal detachment or epiretinal membrane was observed in either group.
Three (2.6%) cases of type 1 group and 10 (15.2%) cases of type 2 group reopened in postoperative period, and reopening was observed more common in type 2 group (p < 0.005; Table 1). After reoperation, four reopened cases in type 2 group showed type 1 closure, and in these four cases, the mean MLD was 278.5 ± 98.33 µm (range: 183–392), BHD was 630.25 ± 179.31 µm (range: 401–810) and HH was 113.67 ± 56.83 µm (range: 277–524). Six reopened cases in type 2 group showed type 2 closure again, and in these six cases, the mean MLD was 572.83 ± 136.83 µm (range: 507–820), BHD was 1456.83 ± 446.17 µm (range: 1125–1549) and HH was 543.17 ± 99.69 µm (range: 418–690). There was a significant difference in MLD and BHD between these groups (p = 0.03 and p = 0.04; Table 3).
Table 3.
Difference of mean preoperative minimum linear diameter, base diameter and hole height between secondary closure types in reopened cases in type 2 closure group.
| Type 1 | Type 2 | p value | |
|---|---|---|---|
| The mean preoperative MLD | 278.5 ± 98.33 µm (range: 183–392) | 572.83 ± 136.83 µm (range: 507–820) | p = 0.03 |
| The mean preoperative BHD | 630.25 ± 179.31 µm (range: 401–810) | 1456.83 ± 446.17 µm (range: 1125–1549) | p = 0.04 |
| The mean preoperative HH | 113.67 ± 56.83 µm (range: 277–524) | 543.17 ± 99.69 µm (range: 418–690) | p = 0.393 |
BD, base diameter; HH, hole height; MLD, minimum linear diameter.
Discussion
The anatomical closure rate of macular holes with vitrectomy has been reported as more than 90% in the literature.7–9 Macular hole closure types were first described by Tornambe as three types; however, one of them was regarded as unsuccessful closure.5 Imai and colleagues10 described the macular hole closure types into three types depending on postoperative OCT: normal foveal contour as U pattern, steep foveal contour as V pattern and foveal defect of neurosensory retina as W pattern. We evaluated postoperative hole closure type in two types, according to sensory retinal status on OCT imaging. Some studies consider the presence of a foveal defect as unsuccessful closure;11,12 however, we evaluated the cases as unsuccessful closure if the postoperative retinal edges remained elevated. We also considered the cases with flat retinal edges and a postoperative defect of the neurosensory retina on the fovea, as type 2 closure.
The postoperative visual function does not only depend on anatomical closure of macular hole, but it is also predicted by sensory retinal status.5 Imaging of sensory retinal status by OCT would help us to predict postoperative visual improvement. A number of possible prognostic factors on postoperative success such as the duration of symptoms, preoperative macular hole size, preoperative visual acuity, axial length, age and sex have been reported.13–15 However, there are different results in the literature. In this study, we aimed to analyse the difference of possible prognostic factors like age; duration of symptoms; preoperative BCVA; preoperative macular hole measurements, including MLD, BHD and HH; and rate of reopening between two types with a larger group of patients.
In this study, we found postoperative BCVA was better in type 1 closure group. Kang and colleagues6 reported that postoperative BCVA was correlated with the type of closure. Tornambe and colleagues5 reported that visual acuity was better in postoperative flat and closed macula status than flat and opened macula status. Imai and colleagues10 reported BCVA correlation with closure patterns as U > V > W. The U pattern and V pattern in their study correspond to type 1 closure in ours. It has been reported in the literature that restoration of outer retinal layers is important for visual improvement after macular hole surgery.16 This may explain the better visual acuity in type 1 group.
The correlation of preoperative macular hole size with anatomical success and visual improvement has been reported in many studies in the literature.13,17–20 There have been several studies with different types of macular hole measurements like macular hole index, hole form factor, diameter hole index, tractional hole index and macular hole closure index.13,21–23 We measured horizontal hole size as the MLD and BHD, and vertical hole size as HH. In our study, we found that MLD and BHD were smaller in type 1 closure group, and postoperative visual improvement was better with smaller MLD and BHD. Kang and colleagues6 reported that hole closure type depends on preoperative hole diameter stronger than other prognostic factors. Our results indicate that preoperative horizontal measurements of macular hole may help to determine postoperative visual expectations and anatomical success.
Reopening of macular hole after surgery has been reported between 2% and 10% in the literature.24 Ip and colleagues19 reported that reopening was seen in macular holes, which was larger than 400 µm. Kang and colleagues6 reported two reopened cases in type 2 closure group in postoperative second and fourth months. We found that reopening rate was significantly higher in type 2 closure group (2.6% in type 1 group and 15.2% in type 2 group; p < 0.005). Tornambe and colleagues5 reported that if the edges of macular hole were visible and separated (flat and open pattern), it may be caused by incomplete removal of epiretinal membrane, which may result in macular dehiscence. Closure of the macular hole has been reported to occur with termination of tangential vitreous traction, reattachment of the hole edges to the neurosensor retina and closure of the residual photoreceptor defect with glial proliferation.25–27 Histological examinations showed that photoreceptor defect varied between 16 and 250 µm after macular hole repair.25–27 There is one case in the literature without histologic glial proliferation sign.15 This might show that glial proliferation varies according to neurosensory retinal defect size, and it may not occur in some eyes after macular hole repair. Kumar and Yadav16 reported that ILM peeling may help ending tangential traction and reactive gliosis; however, it is not enough for large holes. This may indicate that wider neurosensory retinal defect may cause interruption of glial proliferation, which may cause reopening of repaired macular hole and that other surgical techniques such as inverted ILM flap technique may be needed in large holes. The restoration of foveal microstructure and the closure rate of macular hole have been reported higher in studies evaluating the inverted ILM flap technique in large macular holes.11,12
Rishi and colleagues28 reported two macular hole cases which underwent reoperation after type 2 closure and showed type 1 closure after reoperation. Kang and colleagues6 reported two cases in type 2 closure group which have reopened and showed subretinal fluid after PPV and ILM peeling. We observed reopening more common in type 2 closure group. Type 2 closure was observed more commonly in initial type 2 closure group after reoperation, and there was a significant difference between secondary closure types in preoperative MLD and BHD (p = 0.03 and p = 0.04). This result shows that initial MLD and BHD may predict the possibility of reopening and the success rate after reoperation.
Many studies reported that shorter duration of symptoms is correlated with better visual acuity.6,7,17–20 We found no correlation between visual acuity and the duration of symptoms in both types of closure. Kang and colleagues6 also reported no correlation between visual acuity and duration of symptoms in their study. We believe that this difference was caused by the fact that most of our elderly patients may not exactly determine when their symptoms started, and their admission to our tertiary clinic was delayed due to referrals from other hospitals.
There have been different results about the correlation between preoperative and postoperative visual acuity in macular hole cases in the literature.2,6,17 Kang and colleagues6 found no correlation between preoperative and postoperative visual acuity. In contrary, we found a moderate correlation between preoperative and postoperative visual acuity in both types (r = 0.397 in type 1 group, r = 0.345 in type 2 group). We also found no correlation between age and postoperative visual acuity in both types. Similar to our findings, Kang and colleagues6 also found no correlation between them.
The limitation of our study is that we classified the type of closure according to OCT in the postoperative third month, but it is known that recovery may take longer.
In conclusion, preoperative horizontal macular hole measurements such as MLD and BHD may provide foresight to the type of hole closure, the visual improvement, the possibility of reopening after surgery and the success of second surgery in reopened cases.
Footnotes
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Gülşah Gümüş  https://orcid.org/0000-0003-1954-2400
https://orcid.org/0000-0003-1954-2400
Gökhan Demir  https://orcid.org/0000-0002-3293-3396
https://orcid.org/0000-0002-3293-3396
Contributor Information
Gülşah Gümüş, Dr. Ersin Arslan Training and Research Hospital, Eyupoglu, Hurriyet Cd. No: 40, Sahinbey, 27010 Gaziantep, Turkey.
Gökhan Demir, University of Health Sciences, Fatih Sultan Mehmet Training and Research Hospital, Istanbul, Turkey.
Beril Tülü Aygün, University of Health Sciences, Beyoğlu Eye Training and Research Hospital, Istanbul, Turkey.
Ali Demircan, University of Health Sciences, Beyoğlu Eye Training and Research Hospital, Istanbul, Turkey.
Zeynep Alkın, Department of Ophthalmology, Dünyagöz Etiler Hospital, Istanbul, Turkey.
Onur Öztornacı, Biostatistics Department, Mersin University, Mersin, Turkey.
References
- 1. Gass JD. Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol 1988; 106: 629–639. [DOI] [PubMed] [Google Scholar]
- 2. Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes: results of a pilot study. Arch Ophthalmol 1991; 109: 654–659. [DOI] [PubMed] [Google Scholar]
- 3. Wendel RT, Patel AC, Kelly NE, et al. Vitreous surgery for macular holes. Ophthalmology 1993; 100: 1671–1676. [DOI] [PubMed] [Google Scholar]
- 4. Hee MR, Izatt JA, Swanson EA, et al. Optical coherence tomography of the human retina. Arch Ophthalmol 1995; 113: 325–332. [DOI] [PubMed] [Google Scholar]
- 5. Tornambe PE, Poliner LS, Cohen RG. Definition of macular hole surgery end points: elevated/open, flat/open, flat/closed. Retina 1998; 18: 286–287. [DOI] [PubMed] [Google Scholar]
- 6. Kang SW, Ahn K, Ham DI. Types of macular hole closure and their clinical implications. Br J Ophthalmol 2003; 87: 1015–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smiddy WE, Feuer W, Cordahi G. Internal limiting membrane peeling in macular hole surgery. Ophthalmology 2001; 108: 1471–1476. [DOI] [PubMed] [Google Scholar]
- 8. Min WK, Lee JH, Ham DI. Macular hole surgery in conjunction with endolaser photocoagulation. Am J Ophthalmol 1999; 127: 306–311. [DOI] [PubMed] [Google Scholar]
- 9. Brooks HL., Jr. Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology 2000; 107: 1939–1948. [DOI] [PubMed] [Google Scholar]
- 10. Imai M, Iijima H, Gotoh T, et al. Optical coherence tomography of successfully repaired idiopathic macular holes. Am J Ophthalmol 1999; 128: 621–627. [DOI] [PubMed] [Google Scholar]
- 11. Ramtohul P, Parrat E, Denis D, et al. Inverted internal limiting membrane flap technique versus complete internal limiting membrane peeling in large macular hole surgery: a comparative study. BMC Ophthalmol 2020; 20: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Silva N, Ferreira N, Pessoa B, et al. Inverted internal limiting membrane flap technique in the surgical treatment of macular holes: 8-year experience. Int Ophthalmol 2021; 41: 499–507. [DOI] [PubMed] [Google Scholar]
- 13. Ullrich S, Haritoglou C, Gass C, et al. Macular hole size as a prognostic factor in macular hole surgery. Br J Ophthalmol 2002; 86: 390–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mester U, Becker M. Prognostic factors in surgery of macular holes. Ophthalmologe 1998; 95: 158–162. [DOI] [PubMed] [Google Scholar]
- 15. Gander IC, Senn P, Luthi M, et al. Prognostic factors and results after surgical treatment of idiopathic macular holes, stage 2 and 3. Klin Monbl Augenheilkd 2000; 216: 272–277. [DOI] [PubMed] [Google Scholar]
- 16. Kumar V, Yadav B. Hole-door sign: a novel intraoperative optical coherence tomography feature predicting macular hole closure. Retina 2018; 38: 2045–2050. [DOI] [PubMed] [Google Scholar]
- 17. Kumagai K, Ogino N, Demizu S, et al. Variables that influence visual acuity after macular hole surgery. Jpn J Ophthalmol 2001; 45: 112. [DOI] [PubMed] [Google Scholar]
- 18. Kumagai K, Ogino N, Demizu S, et al. Factors related to initial success in macular hole surgery. Nippon Ganka Gakkai Zasshi 2000; 104: 792–796. [PubMed] [Google Scholar]
- 19. Ip MS, Baker BJ, Duker JS, et al. Anatomical outcomes of surgery for idiopathic macular hole as determined by optical coherence tomography. Arch Ophthalmol 2002; 120: 29–35. [DOI] [PubMed] [Google Scholar]
- 20. Kumagai K, Ogino N, Demizu S, et al. Clinical features of idiopathic macular holes. Nippon Ganka Gakkai Zasshi 2000; 104: 819–825. [PubMed] [Google Scholar]
- 21. Kusuhara S, Teraoka Escaño MF, Fujii S, et al. Prediction of postoperative visual outcome based on hole configuration by optical coherence tomography in eyes with idiopathic macular holes. Am J Ophthalmol 2004; 138: 709–716. [DOI] [PubMed] [Google Scholar]
- 22. Ruiz-Moreno JM, Staicu C, Piñero DP, et al. Optical coherence tomography predictive factors for macular hole surgery outcome. Br J Ophthalmol 2008; 92: 640–644. [DOI] [PubMed] [Google Scholar]
- 23. Liu P, Sun Y, Dong C, et al. A new method to predict anatomical outcome after idiopathic macular hole surgery. Graefes Arch Clin Exp Ophthalmol 2016; 254: 683–688. [DOI] [PubMed] [Google Scholar]
- 24. Benson WE, Cruickshanks KC, Fong DS, et al. Surgical management of macular holes: a report by the American Academy of Ophthalmology. Ophthalmology 2001; 108: 1328–1335. [DOI] [PubMed] [Google Scholar]
- 25. Funata M, Wendel RT, de la, Cruz Z, et al. Clinicopathologic study of bilateral macular holes treated with pars plana vitrectomy and gas tamponade. Retina 1992; 12: 289–298. [DOI] [PubMed] [Google Scholar]
- 26. Madreperla SA, Geiger GL, Funata M, et al. Clinicopathologic correlation of a macular hole treated by cortical vitreous peeling and gas tamponade. Ophthalmology 1994; 101: 682–686. [DOI] [PubMed] [Google Scholar]
- 27. Rosa RH, Jr, Glaser BM, de la Cruz Z, et al. Clinicopathologic correlation of an untreated macular hole and a macular hole treated by vitrectomy, transforming growth factor-beta 2, and gas tamponade. Am J Ophthalmol 1996; 122: 853–863. [DOI] [PubMed] [Google Scholar]
- 28. Rishi P, Reddy S, Rishi E. Delayed, spontaneous conversion of type 2 closure to type 1 closure following surgery for traumatic macular hole associated with submacular hemorrhage. Oman J Ophthalmol 2012; 5: 189–190. [DOI] [PMC free article] [PubMed] [Google Scholar]


