Abstract
Tumor‐derived cell‐free DNA (cfDNA) is an emerging biomarker for guiding the personalized treatment of patients with metastatic colorectal cancer (CRC). While patients with CRC liver metastases (CRC‐LM) have relatively high levels of plasma cfDNA, little is known about patients with CRC peritoneal metastases (CRC‐PM). This study evaluated the presence of tumor‐derived cfDNA in plasma and peritoneal fluid (i.e. ascites or peritoneal washing) in 20 patients with isolated CRC‐PM and in the plasma of 100 patients with isolated CRC‐LM. Among tumor tissue KRAS/BRAF mutation carriers, tumor‐derived cfDNA was detected by droplet digital polymerase chain reaction (ddPCR) in plasma of 93% of CRC‐LM and 20% of CRC‐PM patients and in peritoneal fluid in all CRC‐PM patients. Mutant allele fraction (MAF) and mutant copies per ml (MTc/ml) were lower in CRC‐PM plasma than in CRC‐LM plasma (median MAF = 0.28 versus 18.9%, p < 0.0001; median MTc/ml = 21 versus 1,758, p < 0.0001). Within patients with CRC‐PM, higher cfDNA levels were observed in peritoneal fluid than in plasma (median MAF = 16.4 versus 0.28%, p = 0.0019; median MTc/ml = 305 versus 21, p = 0.0034). These data imply that tumor‐derived cfDNA in plasma is a poor biomarker to monitor CRC‐PM. Instead, cfDNA detection in peritoneal fluid may offer an alternative to guide CRC‐PM treatment decisions.
Keywords: colorectal neoplasms, liquid biopsy, circulating tumor DNA, peritoneum, ascitic fluid, plasma, biomarkers
Introduction
The peritoneum is a common and underdiagnosed metastatic site of colorectal cancer (CRC) [1, 2, 3]. Although patients with CRC peritoneal metastases (CRC‐PM) have a poor prognosis [1, 2], those with limited disease could achieve long‐term survival or cure after peritoneal cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy [4]. However, due to the extent of the disease, the majority of patients with peritoneal metastases receive systemic treatment instead [5, 6]. When systemic treatment is offered, treatment response evaluation may be impeded by poor visibility of peritoneal metastases on radiological imaging [7]. Therefore, there is a clinical need to detect peritoneal spread earlier and monitor treatment response better in patients with CRC‐PM.
In general, tumor‐derived cell‐free DNA (cfDNA) in plasma has great potential for tumor detection and monitoring of response to (targeted) therapies [8]. Yet, clinical implementation requires thorough validation for each specific clinical need. While patients with CRC liver metastases (CRC‐LM) are known to have relatively high levels of tumor‐derived cfDNA in plasma, little is known about cfDNA levels in patients with CRC‐PM [9, 10]. Therefore, the present study compared plasma cfDNA levels between patients with extensive isolated CRC‐PM and patients with extensive isolated CRC‐LM. Moreover, proximal fluids derived from the extracellular surroundings of tissue have been shown to be a useful liquid biopsy source of cfDNA, e.g. cerebrospinal fluid for brain cancer [11] and cerebrospinal fluid, pleural effusion, and ascites for non‐small‐cell lung cancer and melanoma patients [12]. More recently, peritoneal fluid was reported as a suitable liquid biopsy source for the detection of tumor‐derived cfDNA for peritoneal surface malignancies [13]. These studies illustrate the feasibility and indicate the putative clinical potential of using peritoneal fluid or ascites as a liquid biopsy source to detect tumor‐derived cfDNA. However, direct comparison of the detection of tumor‐derived cfDNA in peritoneal fluid or ascites to plasma in patients with isolated CRC‐PM has not been reported. In the present study, tumor‐derived cfDNA levels were also compared between peritoneal fluid and plasma in patients with CRC‐PM to explore peritoneal fluid as a potential source of cfDNA in this patient group.
Materials and methods
Blood from patients with histologically proven CRC with isolated, initially unresectable liver metastases was collected in the multicenter CAIRO5 trial (NCT02162563) [14]. Blood and peritoneal fluid were obtained from patients with histologically proven isolated and unresectable CRC‐PM, participating in the CRC‐PIPAC trial (NCT03246321) [15]. Both trials were approved by a medical ethical committee, and all patients signed written informed consent for study participation, as well as liquid biopsy and tumor tissue collection for translational research. The liquid biopsies (i.e. blood and peritoneal fluid) were collected prior to study treatment, processed, and stored centrally (see supplementary material, Supplementary materials and methods). Peritoneal fluid (i.e. ascites or a peritoneal washing with saline if ascites was not present) in the CRC‐PIPAC trial was obtained during the initial laparoscopy. Mutation analysis of tumor tissue was performed in all enrolled patients (see supplementary material, Supplementary materials and methods). Patients were selected for cfDNA analysis when KRAS or BRAF mutations were found in their tumor tissue. In brief, cfDNA from plasma and peritoneal fluid was isolated using the QIAsymphony (Qiagen, Düsseldorf, Germany) and analyzed by droplet digital polymerase chain reaction (ddPCR; Bio‐Rad, Hercules, CA, USA). Levels of cfDNA were measured and presented as mutant allele fraction (MAF) and mutant copies per ml input (MTc/ml) and compared between groups using a Mann–Whitney U‐test with a two‐sided P‐value of 0.05 as a cut‐off for significance (see supplementary material, Supplementary materials and methods).
Results
To investigate tumor‐derived cfDNA using sensitive and validated PCR‐based assays, we focused on the detection of KRAS and BRAF mutations. Of 100 patients with isolated CRC‐LM (Table 1), 57 (57%) had a KRAS or BRAF tumor tissue mutation (see supplementary material, Table S1). Of these, 46 (81%) had synchronous metastases and 32 (56%) had an unresected primary tumor at the time of liquid biopsy collection. Of 20 patients with isolated CRC‐PM (Table 1), 11 (55%) had a KRAS or BRAF tumor tissue mutation and were selected for cfDNA analysis (see supplementary material, Table S2). Of these, seven (64%) had synchronous metastases with an unresected primary tumor at the time of liquid biopsy collection.
Table 1.
Summary of baseline characteristics of patients with isolated CRC‐LM enrolled in the CAIRO5 trial and patients with isolated CRC‐PM enrolled in the CRC‐PIPAC trial.
| Characteristic | CRC‐LM cohort (N = 100) | CRC‐PM cohort (N = 20) |
|---|---|---|
| Age at inclusion (years, mean ± SD) | 60 ± 10 | 63 ± 9.8 |
| Sex (N [%]) | ||
| Male | 64 (64) | 12 (60) |
| Female | 36 (36) | 8 (40) |
| Primary tumor (N [%]) | ||
| Resected | 45 (45) | 6 (30) |
| Unresected | 55 (55) | 14 (70) |
| Metastases (N [%]) | ||
| Synchronous | 82 (82) | 15 (75) |
| Metachronous | 18 (18) | 5 (25) |
| Source of tumor tissue mutation analysis (N [%]) | ||
| Primary tumor | 91 (91) | 5 (25) |
| Metastases | 9 (9) | 2 (10) |
| Both | 0 (0) | 13 (65) |
| Tumor tissue mutation (N [%]) | ||
| KRAS | 54 (54) | 9 (45) |
| BRAF | 3 (3) | 2 (10) |
| No mutation detected | 43 (43) | 9 (45) |
| Systemic therapy <6 months before study registration (N [%]) | ||
| Yes | 0 (0) | 11 (55) |
| No | 100 (100) | 9 (45) |
Among the patients with a KRAS/BRAF tumor tissue mutation, KRAS/BRAF mutations were detected in plasma cfDNA in 93% of patients with CRC‐LM compared to 20% of patients with CRC‐PM (p < 0.0001; Figure 1). In contrast, these mutations could be detected in all available peritoneal fluid samples from the same CRC‐PM patients (Figure 1). Both the MAF and MTc/ml plasma cfDNA values were lower in patients with CRC‐PM than in patients with CRC‐LM (MAF: 0.28% [0.12–0.92%] versus 18.9% [0.06–85.3%], p < 0.0001; MTC/ml: 21 [7–37] versus 1,758 [3–432.563], p < 0.0001; Figure 2). In patients with CRC‐PM, both the MAF and MTc/ml cfDNA values were higher in peritoneal fluid than in plasma (MAF: 16.4% [1.56–46.1%] versus 0.28 [0.12–0.92%], p < 0.0001; MTc/ml: 305 [36–8,947] versus 21 [7–37], p < 0.0001; Figure 2).
Figure 1.
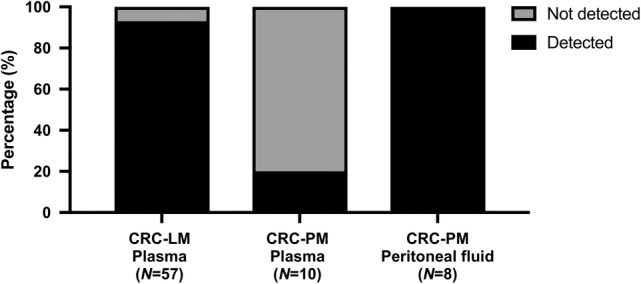
Among patients with a tumor tissue KRAS/BRAF mutation, the percentage of patients in whom a KRAS/BRAF mutation was also detected: (left) in plasma of patients with isolated CRC‐LM; (middle) in plasma of patients with isolated CRC‐PM; and (right) in peritoneal fluid of patients with isolated CRC‐PM.
Figure 2.

(A) MTc/ml plasma or peritoneal fluid and (B) MAF measured in plasma of patients with isolated CRC‐LM (N = 57), in plasma of patients with isolated CRC‐PM (N = 10), and in peritoneal fluid of patients with isolated CRC‐PM (N = 8). Red symbols: patients with a resected primary tumor at the time of blood and peritoneal fluid collection. Green symbols: patients with an unresected primary tumor at the time of blood and peritoneal fluid collection. ns, Not significant; ****p < 0.0001.
The presence of the primary tumor at the time of liquid biopsy collection likely affects cfDNA levels in plasma and peritoneal fluid. Indeed, compared to CRC‐LM patients with a resected primary tumor, CRC‐LM patients with an unresected primary tumor had higher MAF (p = 0.0019) and MTc/ml (p = 0.0034) plasma cfDNA levels. Interestingly, no such difference in the MAF or MTc/ml was found in patients with CRC‐PM between resected or unresected primary tumors, neither for plasma nor for peritoneal fluid (Figure 2). These data suggest that, in addition to the CRC‐PM, the primary tumors of patients with isolated CRC‐PM also have a low propensity to shed cfDNA into the circulation.
Discussion
The propensity of a tumor to shed cfDNA into the circulation varies among cancer types and is known to be relatively high for patients with metastatic CRC [16]. However, differences between CRC metastatic sites in general, and analyses of CRC‐PM in particular, have not been investigated extensively and require studies of carefully selected patient populations with isolated metastases. The present study demonstrates that plasma cfDNA is not a sensitive biomarker to detect isolated CRC‐PM, in sharp contrast to patients with isolated CRC‐LM.
The low levels of plasma cfDNA in CRC‐PM relative to CRC‐LM could be explained by several mechanisms. Peritoneal metastases and liver metastases originate from different dissemination patterns of the primary tumor. While dissemination to the liver mainly occurs hematogenously via the portal vein, peritoneal dissemination is characterized either by peritoneal penetration from the primary tumor or iatrogenic spread as a consequence of incomplete resection [17]. These intrinsic differences in dissemination pathways of the primary tumor may be associated with differences in the propensity to shed cfDNA into the circulation, which might explain why a confounding effect of the presence of the primary tumor on cfDNA plasma levels was not observed for patients with CRC‐PM but was present in patients with CRC‐LM (Figure 2). Moreover, the peritoneum–plasma barrier may restrict cfDNA release from peritoneal metastases into the systemic circulation [18]. The low levels of plasma cfDNA in patients with isolated CRC‐PM may indicate that peritoneal metastases are a locoregional rather than a systemic disease. This supports the general assumption that palliative systemic therapy is relatively less effective for isolated CRC‐PM than for isolated nonperitoneal colorectal metastases [19]. Therefore, plasma cfDNA does not appear to be a useful biomarker to detect CRC‐PM in an early operable stage or to monitor treatment response in this particular patient group. Theoretically, when tumor‐derived cfDNA is detected in the plasma of patients with presumably isolated CRC‐PM, this may be indicative of occult hematogenous metastases and future systemic disease progression. Consequently, patients who qualify for peritoneal cytoreductive surgery and who test positive for plasma cfDNA might benefit from perioperative systemic therapy, a hypothesis that is currently being tested in the multicenter CAIRO6 clinical trial [20].
The high detectability of cfDNA in peritoneal fluid of all patients with isolated CRC‐PM suggests that peritoneal fluid may serve as a more useful source of cfDNA than plasma for this patient group. As ascites is not (abundantly) present in all patients with peritoneal metastases and mostly manifests itself in a late stage of the disease, cfDNA analysis of peritoneal washes may offer an alternative to detect and monitor peritoneal spread in patients with CRC‐PM. Peritoneal washing could be a low‐risk minimally invasive method in patients with isolated CRC‐PM when laparoscopy is already part of clinical care, i.e. laparoscopy as part of follow‐up in patients with high‐risk (i.e. pT4a‐bN0‐2M0) CRC after resection [21]. Aspiration of ascites or peritoneal washing could be a method to obtain cfDNA for molecular profiling, response monitoring, and detection of chemotherapy resistance in patients with isolated CRC‐PM. This hypothesis is currently being tested in the multicenter INTERACT trial [22] and may also apply to other malignancies that frequently metastasize to the peritoneum, especially those that are difficult to access for histological biopsy, such as ovarian, pancreatic, appendiceal, and small bowel cancers.
This study has several limitations. First, the number of patients within the CRC‐PM cohort was relatively small. Second, cfDNA analyses were restricted to a subset of patients with KRAS/BRAF hot‐spot mutations. In addition, while the aim was to measure cfDNA derived from metastases, a subgroup of the patients still had an unresected primary tumor that may have contributed to the cfDNA signals measured. Finally, in the present study, some isolated CRC‐PM were previously treated with systemic therapy, whereas all isolated CRC‐LM were previously untreated at least 6 months before study registration. Despite these limitations, clear differences were observed between the CRC‐PM and CRC‐LM cohorts.
In conclusion, cfDNA in plasma is becoming a realistic approach to guide personalized treatment of patients with CRC‐LM, while this approach appears less suitable for patients with CRC‐PM, who instead may benefit from the detection of tumor‐derived cfDNA in peritoneal fluid. The observations in this study underscore the biological relevance of the differences in growth patterns between systemic and peritoneal metastases, which should be taken into account when considering the clinical utility of cfDNA.
Author contributions statement
IvE, OK and RJAF are responsible for the concept and design of the study. Funding was obtained by CJAP, IHJTdH and RJAF. Patient acquisition and material collection were performed by KPR, AC, KB, ECEW and RJL. IvE performed the analyses and drafted the manuscript under the supervision of GAM, CJAP, IHJTdH and RJAF. All authors were involved in reviewing the manuscript for important intellectual content.
Supporting information
Supplementary materials and methods
Table S1. Overview of individual patients with isolated CRC‐LM with a KRAS or BRAF mutation in tumor tissue
Table S2. Overview of individual patients with isolated CRC‐PM with a KRAS or BRAF mutation in tumor tissue
Acknowledgements
We thank Mirthe Lanfermeijer, Dorothé Linders, and Kalpana Ramkisoensing for laboratory assistance with the droplet digital PCR analyses. We thank Pien Delis‐van Diemen, Margriet Lemmens, Anne Bolijn, and Marianne Tijssen for laboratory assistance with the formalin‐fixed paraffin‐embedded (FFPE) DNA isolations. We acknowledge the NKI‐AVL Core Facility Molecular Pathology & Biobanking (CFMPB) for lab support. This work was funded by the ‘Stop Darmkanker Nederland’ Foundation and the Dutch Cancer Society (grant number 10438). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflicts of interest statement: IHJTdH received unrestricted research funds from QPS/RanD, ROCHE, and the Dutch Cancer Society (KWF). CJAP received a grant from Amgen for the CAIRO5 clinical trial. The funding received was not related to the current research and was paid to the institute. No other disclosures were reported.
References
- 1. van der Geest LG, Lam‐Boer J, Koopman M, et al. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis 2015; 32 : 457–465. [DOI] [PubMed] [Google Scholar]
- 2. van Gestel YR, de Hingh IH, van Herk‐Sukel MP, et al. Patterns of metachronous metastases after curative treatment of colorectal cancer. Cancer Epidemiol 2014; 38 : 448–454. [DOI] [PubMed] [Google Scholar]
- 3. Koppe MJ, Boerman OC, Oyen WJ, et al. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg 2006; 243 : 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goere D, Malka D, Tzanis D, et al. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg 2013; 257 : 1065–1071. [DOI] [PubMed] [Google Scholar]
- 5. van Oudheusden TR, Razenberg LG, van Gestel YR, et al. Systemic treatment of patients with metachronous peritoneal carcinomatosis of colorectal origin. Sci Rep 2015; 5 : 18632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Razenberg LG, Lemmens VE, Verwaal VJ, et al. Challenging the dogma of colorectal peritoneal metastases as an untreatable condition: results of a population‐based study. Eur J Cancer 2016; 65 : 113–120. [DOI] [PubMed] [Google Scholar]
- 7. de Bree E, Koops W, Kroger R, et al. Peritoneal carcinomatosis from colorectal or appendiceal origin: correlation of preoperative CT with intraoperative findings and evaluation of interobserver agreement. J Surg Oncol 2004; 86 : 64–73. [DOI] [PubMed] [Google Scholar]
- 8. Corcoran RB, Chabner BA. Application of cell‐free DNA analysis to cancer treatment. N Engl J Med 2018; 379 : 1754–1765. [DOI] [PubMed] [Google Scholar]
- 9. Vidal J, Muinelo L, Dalmases A, et al. Plasma ctDNA RAS mutation analysis for the diagnosis and treatment monitoring of metastatic colorectal cancer patients. Ann Oncol 2017; 28 : 1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baumgartner JM, Raymond VM, Lanman RB, et al. Preoperative circulating tumor DNA in patients with peritoneal carcinomatosis is an independent predictor of progression‐free survival. Ann Surg Oncol 2018; 25 : 2400–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Mattos‐Arruda L, Mayor R, Ng CKY, et al. Cerebrospinal fluid‐derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun 2015; 6 : 8839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Villatoro S, Mayo‐de‐Las‐Casas C, Jordana‐Ariza N, et al. Prospective detection of mutations in cerebrospinal fluid, pleural effusion, and ascites of advanced cancer patients to guide treatment decisions. Mol Oncol 2019; 13 : 2633–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leick KM, Kazarian AG, Rajput M, et al. Peritoneal cell‐free tumor DNA as biomarker for peritoneal surface malignancies. Ann Surg Oncol 2020; 27 : 5065–5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huiskens J, van Gulik TM, van Lienden KP, et al. Treatment strategies in colorectal cancer patients with initially unresectable liver‐only metastases, a study protocol of the randomised phase 3 CAIRO5 study of the Dutch Colorectal Cancer Group (DCCG). BMC Cancer 2015; 15 : 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rovers KP, Lurvink RJ, Wassenaar EC, et al. Repetitive electrostatic pressurised intraperitoneal aerosol chemotherapy (ePIPAC) with oxaliplatin as a palliative monotherapy for isolated unresectable colorectal peritoneal metastases: protocol of a Dutch, multicentre, open‐label, single‐arm, phase II study (CRC‐PIPAC). BMJ Open 2019; 9 : e030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early‐ and late‐stage human malignancies. Sci Transl Med 2014; 6 : 224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lemoine L, Sugarbaker P, Van der Speeten K. Pathophysiology of colorectal peritoneal carcinomatosis: role of the peritoneum. World J Gastroenterol 2016; 22 : 7692–7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hasovits C, Clarke S. Pharmacokinetics and pharmacodynamics of intraperitoneal cancer chemotherapeutics. Clin Pharmacokinet 2012; 51 : 203–224. [DOI] [PubMed] [Google Scholar]
- 19. Franko J, Shi Q, Goldman CD, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol 2012; 30 : 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rovers KP, Bakkers C, Simkens G, et al. Perioperative systemic therapy and cytoreductive surgery with HIPEC versus upfront cytoreductive surgery with HIPEC alone for isolated resectable colorectal peritoneal metastases: protocol of a multicentre, open‐label, parallel‐group, phase II‐III, randomised, superiority study (CAIRO6). BMC Cancer 2019; 19 : 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bastiaenen VP, Klaver CEL, Kok NFM, et al. Second and third look laparoscopy in pT4 colon cancer patients for early detection of peritoneal metastases; the COLOPEC 2 randomized multicentre trial. BMC Cancer 2019; 19 : 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Boer NL, Brandt‐Kerkhof ARM, Madsen EVE, et al. Concomitant intraperitoneal and systemic chemotherapy for extensive peritoneal metastases of colorectal origin: protocol of the multicentre, open‐label, phase I, dose‐escalation INTERACT trial. BMJ Open 2019; 9 : e034508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials and methods
Table S1. Overview of individual patients with isolated CRC‐LM with a KRAS or BRAF mutation in tumor tissue
Table S2. Overview of individual patients with isolated CRC‐PM with a KRAS or BRAF mutation in tumor tissue


