Abstract
Ketamine has rapid-acting antidepressant properties but also potentially concerning transient dissociative side effects (SEs). Recent studies noted a positive correlation between treatment response to ketamine and general dissociative SEs, as well as “floating”, a depersonalization SE (a subtype of the dissociative SEs). This analysis sought to determine whether floating mediates treatment response to ketamine. Data were pooled from three double-blind, crossover, placebo-controlled ketamine clinical trials across which 82 participants with treatment-resistant depression (TRD) (44 with bipolar depression and 38 with major depressive disorder) received placebo and ketamine (0.5 mg/kg) infusions. SEs were actively solicited in a standardized fashion before and after ketamine infusion. The hypothesis that a post-infusion experience of floating would mediate antidepressant response to ketamine was assessed at 230 minutes post-infusion and at Day 1. Montgomery-Asberg Depression Rating Scale (MADRS) total score was the dependent variable in a linear mixed effects model. Ketamine significantly decreased MADRS scores (p<0.0001), but no relationship was detected between floating and MADRS score at either 230 minutes or Day 1 post-infusion. The hypothesized mediation effect of floating was also not detected at either 230 minutes or Day 1 post-infusion. Taken together, the findings do not support the hypothesis that ketamine’s antidepressant effects are mediated by the dissociative depersonalization subtype SE of floating.
Keywords: Ketamine, floating, dissociation, treatment-resistant depression, bipolar disorder
Introduction
Over the last two decades, multiple studies have demonstrated that subanesthetic-dose ketamine has rapid, robust, and sustained antidepressant effects in individuals with major depressive disorder (MDD) (Diazgranados et al., 2010a, Iadarola et al., 2015, Ibrahim et al., 2011, Zarate et al., 2006) and bipolar depression (BD) (Diazgranados et al., 2010b, Zarate et al., 2012). Commensurately, research efforts aimed at understanding this agent’s mechanism of action and therapeutic potential have grown exponentially, as have efforts to explore compounds with similar molecular activity. Over the last decade, intravenous racemic ketamine has also increasingly been used to treat a range of psychiatric disorders in a variety of clinical settings (Wilkinson et al., 2017). However, a key point of contention surrounding ketamine use in depression is its associated side effects (SEs), particularly psychotomimetic effects such as dissociation, depersonalization, altered perceptions, and hallucinations (Acevedo-Diaz et al., 2020, Zanos et al., 2018). These psychotomimetic SEs, which occur immediately during the infusion and resolve within hours, have also been well documented to occur in response to anesthetic doses of intravenous ketamine (Collier, 1972); in contrast, ketamine’s main antidepressant effects persist for several days (Zarate et al., 2006).
Ketamine’s SE profile is of particular interest given studies linking antidepressant response to ketamine in individuals with treatment-resistant depression (TRD) with the presence of dissociative symptoms. Such studies have typically measured ketamine’s acute psychoactive effects using the Clinician Administered Dissociative States Scale (CADSS). Luckenbaugh and colleagues observed a significant association between increased CADSS score at 40 minutes post-ketamine and percent improvement in depressive symptoms as measured by the Hamilton Depression Rating Scale (HAM-D) at 230 minutes and at Day 7 post-ketamine (Luckenbaugh et al., 2014). In 2018, Niciu and colleagues reported that changes in depersonalization items on the CADSS correlated with changes in depressive symptoms as measured by the HAM-D (Niciu et al., 2018). In contrast, Wilkinson and colleagues reported that changes in depressive symptoms, as assessed via the Montgomery-Asberg Depression Rating Scale (MADRS) over time, were the same regardless of whether or not peak CADSS score was included in the model (Wilkinson et al., 2018). Notably, opinions are mixed regarding the ability of the CADSS to fully capture dissociative symptoms linked to ketamine use (Neehoff and Glue, 2019, van Schalkwyk et al., 2018) as the scale was not validated for this purpose (Bremner et al., 1998).
More recently, Stocker and colleagues reported that, in 62 YouTube videos of depressed patients narrating their experience of receiving open-label ketamine, 27.4% of individuals self-reported a sense of “lightness” or “floating” that was associated with relief of their depressive symptoms (Stocker et al., 2019). Because self-reported floating experiences do not have associated psychometric validity concerns like those associated with the CADSS, they have the potential to narrow the scope of the general construct (e.g., “dissociation) to a single tangible experience. It should be noted that robust evidence supporting any putative association and/or mediating effect between the experience of floating and treatment response to ketamine could significantly impact the clinical use of ketamine and its enantiomers. This is particularly relevant given the recent U.S. Food and Drug Administration (FDA) approval of intranasal esketamine for the treatment of adult TRD. In particular, the experience of floating could be further explored and validated as a predictor of treatment response that could minimize the need to expose patients to repetitive dosing and the associated risks of ulcerative cystitis (Shahani et al., 2007), abuse (Bonnet, 2015, Sassano-Higgins et al., 2016, Strayer and Nelson, 2008), or neurotoxicity associated with high doses of ketamine in animal models (Olney et al., 1989).
This study sought to assess the association between treatment response and floating sensation in individuals with either MDD or BD who received a single intravenous subanesthetic dose of ketamine (0.5mg/kg) in a double-blind, crossover, placebo-controlled clinical trial. We hypothesized that the experience of floating during and after ketamine infusion would positively correlate with treatment response at approximately four hours as well as Day 1 post-ketamine infusion. We also hypothesized that the post-ketamine experience of floating would mediate antidepressant response to ketamine, with greater sensation of floating associated with increased response to ketamine.
Material and Methods
Participants
Data from 82 individuals with TRD (38 MDD and 44 BD; ages 18– 65) (see Table 1) who received a single subanesthetic-dose (0.5mg/kg) intravenous ketamine infusion over 40 minutes were pooled for retrospective analysis. All participants were studied as inpatients at the National Institute of Mental Health (NIMH), National Institutes of Health (NIH) Clinical Research Center in Bethesda, MD, USA and provided written informed consent after the nature of the procedures had been fully explained. The investigation was carried out in accordance with the latest version of the Declaration of Helsinki and was reviewed by the NIH Combined Neuroscience Institutional Review Board. Individuals participated in one of three trials conducted under the umbrella of a larger protocol, “The Investigation of the Rapid (Next Day) Antidepressant Effects of an NMDA Antagonist” (Clinical Trials Identifier: NCT00088699; NIH protocol 04-M-0222). For the purposes of this analysis, and in order to ensure the consistency of clinical measures, three substudies with identical designs were chosen; two examined the effect of a single ketamine infusion in individuals with treatment-resistant BD (KET-BD (Diazgranados et al., 2010b, Zarate et al., 2012)), and one explored ketamine’s mechanism of action in TRD (KETMOA (Nugent et al., 2019)). Individuals with unipolar MDD were tapered off psychotropic medications while individuals with BD were stabilized on a therapeutic dose of lithium or valproic acid prior to study participation. Further details for each specific study, including inclusion/exclusion criteria and demographic and clinical characteristics, can be found in Table 2 and in prior publications (Diazgranados et al., 2010b, Nugent et al., 2019, Zarate et al., 2012).
Table 1.
Demographic characteristics
| Variable | KET-BD* | KET-MOA |
|---|---|---|
| Subjects (N) | 41 | 41 |
| DSM IV Diagnosis | BD | MDD (38) & BD (3) |
| Age (yrs) | 47 (11.8) | 36.4 (10.7) |
| Male | 17 (41.5%) | 16 (39%) |
| Race – White | 36 (87.8%) | 33 (80.5%) |
Abbreviations: MDD: major depressive disorder; BD: bipolar disorder; KET-BD: ketamine in bipolar depression study (two BD substudies coded with the same study identifier); KET-MOA: Ketamine mechanism of action study
Table 2.
Substudies included in this report
| Substudy | References | Subjects receiving ketamine (N) | DSM IV Diagnosis | Dose (mg/kg) and duration of infusion | Route | Assessment Time Points Day of Infusion | Design |
|---|---|---|---|---|---|---|---|
| KET-BD | Diazgranados et al, 2010; Zarate et al, 2012 | 41 | BD | 0.5 over 40 min | IV | −60,40,80,120,230, Day1 | Double-blind, placebo-controlled crossover RCT |
| KET-MOA | Nugent et al, 2018 | 41 | MDD and BD | 0.5 over 40 min | IV | −60,40,80,120,230, Day1 | Double-blind, placebo-controlled crossover RCT |
Abbreviations: KET-BD: ketamine in bipolar depression study (two BD substudies coded with the same study identifier); KET-MOA: Ketamine mechanism of action study; RCT: randomized controlled trial; MDD: major depressive disorder; BD: bipolar disorder.
Instruments
Drug response was measured using the MADRS (Montgomery and Asberg, 1979) total score. One hundred and twenty SEs were assessed pre- and post-infusion (ketamine and placebo) at several time points via active solicitation by trained clinicians and recorded in a standardized fashion in the Data Safety and Monitoring Board (DSMB) form (subforms included the “Regular Adverse Side Effects” form and the “Specific Adverse Side Effects form”); these questionnaires were developed in-house (Acevedo-Diaz et al., 2020) for our clinical trials and sought to assess participants’ self-reported SEs over the course of a subanesthetic-dose (0.5mg/kg) intravenous ketamine infusion trial. SEs were recorded on a Likert scale, where 0 = none, 1 = mild, 2 = moderate, and 3 = severe. Both MADRS scores and information about SEs were collected via active solicitation at least 60 minutes prior to each infusion and at 40, 80, 120, and 230 minutes post-ketamine infusion, as well as on Days 1, 3, 7, 10, and 14 post-infusion.
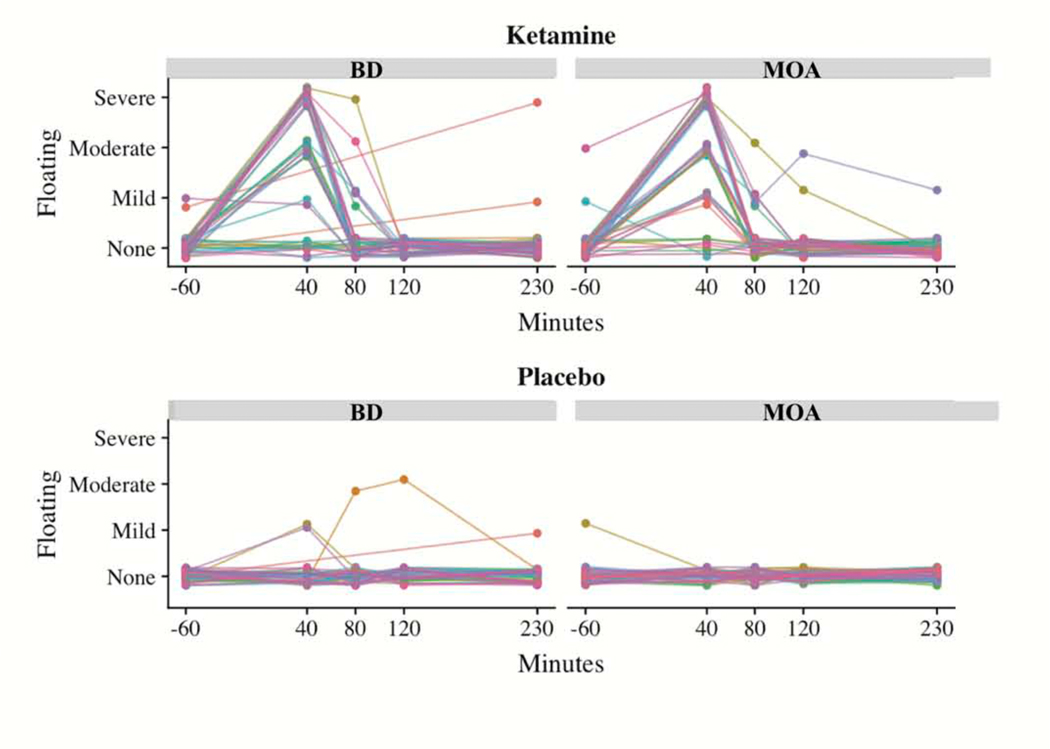
Data were also collected regarding the occurrence, timing, and severity of the floating SE, which is a specific dissociative depersonalization SE. When the experience of floating was reported during a ketamine infusion, it typically peaked at 40 minutes (at the end of the infusion) and rapidly declined to zero. However, only three of the 82 participants reported floating experiences post-placebo infusion (see Figure 1). Because of this lack of variation in post-placebo floating experiences, and because of minimal variation in post-ketamine floating patterns over time, we selected each person’s maximum floating experiences post-ketamine and post-placebo and took the difference to measure ketamine-induced floating (KI-floating). For the 11 participants who dropped out before their placebo infusion, we assumed zero post-placebo floating scores given that approximately 96% of participants (79 out of 82) reported no floating experiences post-placebo infusion.
Figure 1:
Effect of drug (ketamine versus placebo) on the experience of floating for up to four hours post-infusion. The figure illustrates the severity of floating experience as reported after a single subanesthetic-dose ketamine infusion (0.5 mg/kg) and placebo infusion for up to four hours post-infusion. Abbreviations: KET-BD: ketamine in bipolar depression study (two BD substudies coded with the same study identifier); KET-MOA: Ketamine mechanism of action study.
Statistical analyses
The rate of floating experiences within 24 hours post-ketamine infusion (number who reported floating at least once divided by 82) was calculated, as were rates for all other SEs in order to determine whether floating was among the most commonly reported SEs. To visually examine the temporal pattern of floating experiences, self-reported floating severity scores were plotted over time for each person in each study.
A linear mixed model was used to quantify and test the relationship between floating and MADRS scores as well as the mediating effect of floating on treatment response at 230 minutes post-infusion; a second, separate model was used to quantify and test these effects at Day 1 post-infusion. For each model, main effects of treatment (ketamine or placebo), KI-floating (entered as an ordinal variable), and treatment x KI-floating interaction (which was used to test the mediation hypothesis), were included. Period-specific baseline MADRS scores and study period were included as covariates; a random intercept per person was also included. Lastly, because data were pooled from different studies, study was included as a main (fixed) effect after determining that the interaction between study, treatment, and floating did not significantly contribute to either model. The two BD substudies were coded with the same study identifier due to their identical designs, comparable clinical characteristics, and the fact that they were conducted very close in time. ANOVAs were performed on each model, and F statistics and p values are reported for each fixed effect. Model assumptions were checked with visual inspection of residual histograms and quantile-quantile (q-q) plots. Due to missing treatment or baseline data, 78 participants were included in the model for response at 230 minutes, and 80 were included in the model for response at Day 1.
Results
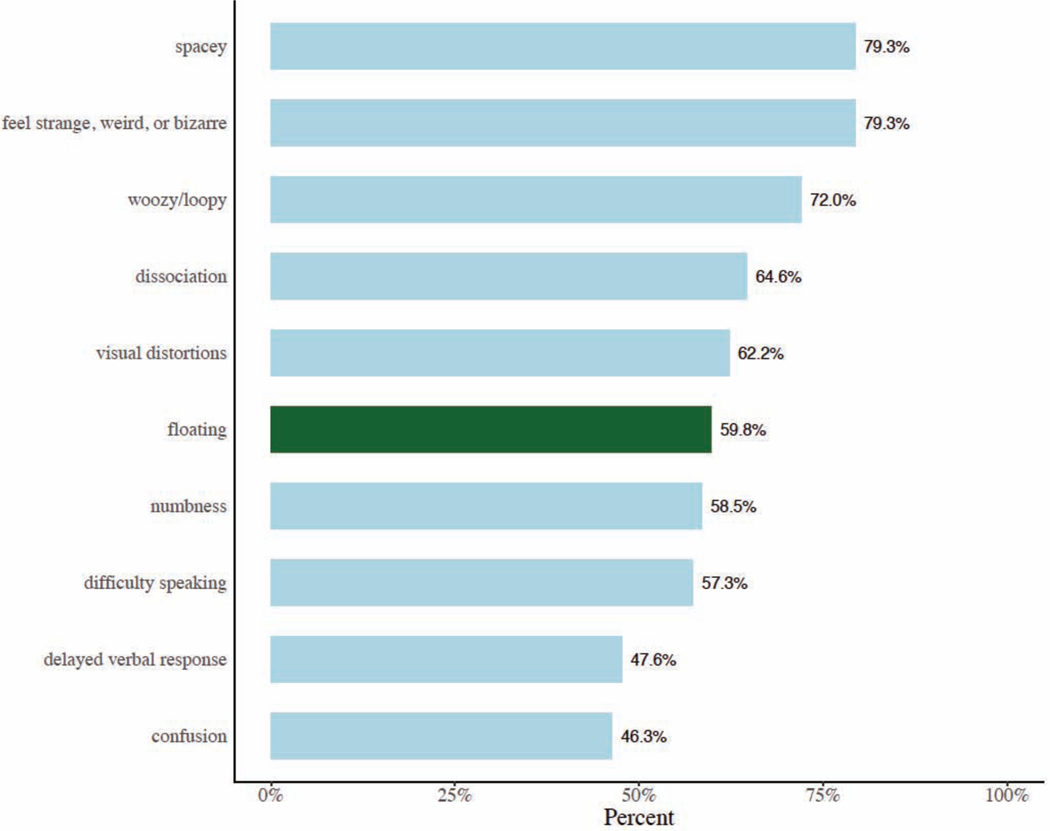
Floating was among the 10 most frequently reported SEs post-ketamine infusion, present in 60% of participants. For each of the studies included in this paper, floating peaked, for the most part, at 40 minutes post-infusion and was rarely reported post-placebo and/or at baseline (60 minutes before infusion) (Figure 2).
Figure 2:
The 10 most commonly reported side effects (SEs) in the pooled sample of 82 participants with treatment-resistant depression (TRD) (38 with major depressive disorder (MDD) and 44 with bipolar disorder (BD)).
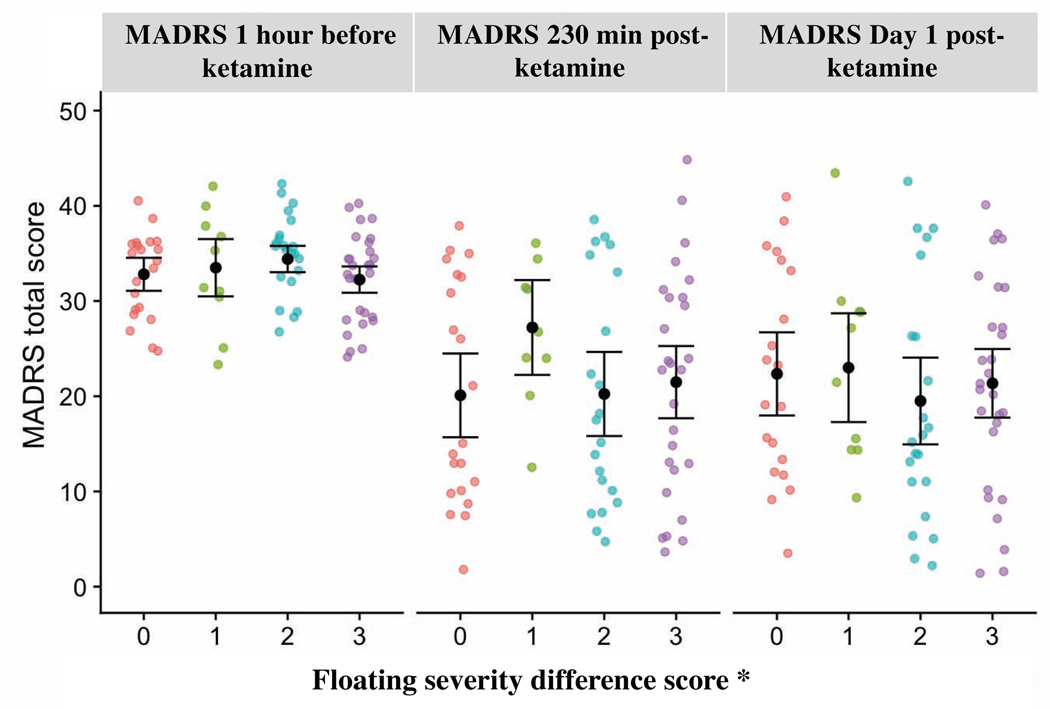
At 230 minutes post-infusion, KI-floating was not significantly associated with treatment response (F3, 73=0.82, p=0.48), nor did it significantly mediate ketamine’s effects on MADRS scores (F3, 61=1.02, p=0.39). However, at 230 minutes post-infusion, ketamine significantly reduced MADRS scores relative to placebo (F1, 61=53.8, p< 0.001), consistent with findings reported in the primary publications from which the current participants were drawn (Diazgranados et al., 2010b, Nugent et al., 2019, Zarate et al., 2012). The same pattern of results was evident for Day 1 (Table 3, Figure 3).
Table 3.
Results of linear mixed models
| F (df) | p-value | |
|---|---|---|
| 230 Minutes | ||
| Intercept | 5274.90 ( 1,73) | <.0001 |
| Infusion | 19.66 ( 1,61) | <.0001 |
| Baseline MADRS | 120.50 ( 1,61) | <.0001 |
| Drug | 53.84 ( 1,61) | <.0001 |
| KI-Floating | 0.83 ( 3,73) | 0.48 |
| Drug x KI-Floating | 1.02 ( 3,61) | 0.39 |
| Study | 13.14 ( 1,73) | 0.0005 |
| Day 1 | ||
| Intercept | 5010.83 ( 1,75) | <.0001 |
| Infusion | 10.02 ( 1,65) | 0.0024 |
| Baseline MADRS | 98.04 ( 1,65) | <.0001 |
| Drug | 61.26 ( 1,65) | <.0001 |
| KI-Floating | 0.30 ( 3,75) | 0.82 |
| Drug x KI-Floating | 0.66 ( 3,65) | 0.58 |
| Study | 2.91 ( 1,75) | 0.09 |
Abbreviations: df: degrees of freedom; MADRS: Montgomery-Asberg Depression Rating Scale; KI-floating: ketamine-induced floating (maximum floating experience post-ketamine minus maximum floating experience post-placebo).
Figure 3:
Maximum floating severity difference score* by Montgomery-Asberg Depression Rating Scale (MADRS) total score at different time points with means and 95% confidence intervals. *For each person, floating severity difference score was calculated by subtracting their maximum observed post-placebo floating score from their maximum observed post-ketamine floating score.
Discussion
In this study of 82 participants with TRD pooled from three crossover, placebo-controlled, ketamine clinical trials, floating was among the most commonly experienced dissociative SEs associated with a subanesthetic intravenous dose of ketamine. However, in contrast to previously reported findings (Stocker et al., 2019) and to our hypothesis, the results do not support an association between the dissociative depersonalization SE of floating and antidepressant response to ketamine in TRD participants diagnosed with either MDD or BD. In addition, floating did not appear to mediate antidepressant response to ketamine (see Figure 3). Notably, this finding is consistent with a previous notion that the mechanism of action underlying ketamine’s dissociative and psychotomimetic SEs is independent from—and may differ from—the mechanism of action underlying its antidepressant effects (Zanos et al., 2016).
In contrast to past reports linking either overall dissociative symptoms (Luckenbaugh et al., 2014) or depersonalization symptoms (Niciu et al., 2018) with ketamine’s antidepressant effects, this study found no relationship between the specific dissociative SE of floating and antidepressant response to ketamine. One possible reason for the discrepancy is that the present study examined only one specific SE, while the other studies (Luckenbaugh et al., 2014, Niciu et al., 2018) examined a general measure across a range of dissociative SEs (e.g., as measured by the CADSS) or a subgroup of dissociative symptoms (e.g., the CADSS depersonalization subscale). This study also does not support previous accounts from individuals’ qualitative reports (Stocker et al., 2019) that sensations of lightness or floating during ketamine infusion were associated with relief of depressive symptoms.
One of the major strengths of this study is the inclusion of data from both ketamine and placebo conditions. In addition, the studies’ crossover designs maximized power and permitted each participant with TRD to serve as their own control. Limitations of this study include its post-hoc design, which prevented a priori power analyses and increased the possibility of type 2 errors. In addition, a qualitative/subjective measure was being examined with a quantitative approach. Finally, differences between diagnostic groups (BD vs MDD) could not be investigated because diagnosis and study were confounding factors and because the data were treated as meta-analytic in nature; thus, no remaining variations in diagnosis could be examined.
Conclusion
The results of this study suggest that the dissociative SE of floating is not associated with antidepressant response to ketamine in individuals with TRD and therefore cannot be used as an indicator or predictor of treatment response. Identifying clinical markers or predictors of treatment response could minimize the need to expose individuals to the risks associated with ketamine (particularly with repetitive dosing) and continues to be a potentially fruitful area of research.
Supplementary Material
Highlights.
Antidepressant response to ketamine may be related to dissociative symptoms
Floating (or feeling of lightness) is a specific dissociative side effect
Patients (n=82) with treatment-resistant depression received placebo and ketamine
Ketamine’s antidepressant effects were not mediated by the symptom of floating
The specific dissociative symptom of floating does not predict response to ketamine
Acknowledgements
The authors gratefully acknowledge the support of the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIAMH002857; NCT00088699), and thank the 7SE Inpatient Mood and Anxiety Disorders Research Unit for their clinical support during the writing of this manuscript. Ioline Henter, M.A. (NIMH) provided invaluable editorial assistance.
Funding Information and Role of Funding Source
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIAMH002857; NCT00088699), by a NARSAD Independent Investigator Award to CAZ, and by the Brain & Behavior Mood Disorders Research Award to CAZ. These organizations had no further role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Acevedo-Diaz et al -- Can ‘Floating’ Predict Treatment Response to Ketamine? Data from Three Randomized Trials of Individuals with Treatment-Resistant Depression
Conflict of Interest
Dr. Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. All other authors have no conflict of interest to disclose, financial or otherwise.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo-Diaz EE, Cavanaugh GW, Greenstein D, Kraus C, Kadriu B, Zarate CAJ, et al. Comprehensive assesment of side effects associated with a single dose of ketamine in treatment-resistant depression. J Affect Disord. 2020;263:568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet U Long-term ketamine self-injections in major depressive disorder: focus on tolerance in ketamine’s antidepressant response and the development of ketamine addiction. J Psychoactive Drugs. 2015;47:276–85. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress. 1998;11:125–36. [DOI] [PubMed] [Google Scholar]
- Collier BB. Ketamine and the conscious mind. Anaesthesia. 1972;27:120–34. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010a;71:1605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010b;67:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadarola ND, Niciu MJ, Richards EM, Vande Voort JL, Ballard ED, Lundin NB, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6:97–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Luckenbaugh DA, Machado-Vieira R, Baumann J, Mallinger AG, et al. Rapid decrease in depressive symptoms with an N-methyl-d-aspartate antagonist in ECT-resistant major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, et al. Do the dissociative side effects of ketamine mediate its antidepressant effects? J Affect Disord. 2014;159:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. [DOI] [PubMed] [Google Scholar]
- Neehoff S, Glue P. Dissociation after ketamine dosing: Is the CADSS fit for purpose? J Affect Disord. 2019;244:239–40. [DOI] [PubMed] [Google Scholar]
- Niciu MJ, Shovestul BJ, Jaso BA, Farmer C, Luckenbaugh DA, Brutsche NE, et al. Features of dissociation differentially predict antidepressant response to ketamine in treatment-resistant depression. J Affect Disord. 2018;232:310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Ballard ED, Gould TD, Park LT, Moaddel R, Brutsche NE, et al. Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol Psychiatry. 2019;24:1040–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Labruyere J, Price MT. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science. 1989;244:1360–2. [DOI] [PubMed] [Google Scholar]
- Sassano-Higgins S, Baron D, Juarez G, Esmaili N, Gold M. A review of ketamine abuse and diversion. Depress Anxiety. 2016;33:718–27. [DOI] [PubMed] [Google Scholar]
- Shahani R, Streutker C, Dickson B, Stewart RJ. Ketamine-associated ulcerative cystitis: a new clinical entity. Urology. 2007;69:810–2. [DOI] [PubMed] [Google Scholar]
- Stocker K, Hasler G, Hartmann M. The altered-state-of-consciousness aspect of a feeling of lightness is reported to be associated with antidepressant benefits by depressed individuals receiving ketamine infusions: a systematic analysis of internet video testimonials. Psychother Psychosom. 2019;88:182–3. [DOI] [PubMed] [Google Scholar]
- Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med. 2008;26:985–1028. [DOI] [PubMed] [Google Scholar]
- van Schalkwyk GI, Wilkinson ST, Davidson L, Silverman WK, Sanacora G. Acute psychoactive effects of intravenous ketamine during treatment of mood disorders: Analysis of the Clinician Administered Dissociative State Scale. J Affect Disord. 2018;227:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson ST, Katz RB, Toprak M, Webler R, Ostroff RB, Sanacora G. Acute and longer-term outcomes using ketamine as a clinical treatment at the Yale Psychiatric Hospital. J Clin Psychiatry. 2018;79:pii:17m11731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson ST, Toprak M, Turner MS, Levine SP, Katz RB, Sanacora G. A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry. 2017;174:695–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70:621–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.