Abstract
OBJECTIVE:
The lymphatic system is a circulatory system that unidirectionally drains the interstitial tissue fluid back to blood circulation. Although lymph is utilized by leukocytes for immune surveillance, it remains inaccessible to platelets and erythrocytes. Activated cells release submicron extracellular vesicles (EV) that transport molecules from the donor cell. In rheumatoid arthritis, EV accumulate in the joint where they can interact with numerous cellular lineages. However, whether EV can exit the inflamed tissue to recirculate is unknown. Here, we investigated whether vascular leakage that occurs during inflammation could favor EV access to the lymphatic system.
APPROACH AND RESULTS:
Using an in vivo model of autoimmune inflammatory arthritis, we show that there is an influx of platelet EV, but not EV from erythrocytes or leukocytes, in joint-draining lymph. In contrast to blood platelet EV, lymph platelet EV lacked mitochondrial organelles and failed to promote coagulation. Platelet EV influx in lymph was consistent with joint vascular leakage and implicated the fibrinogen receptor α2bβ3 and platelet-derived serotonin.
CONCLUSIONS:
These findings show that platelets can disseminate their EV in fluid that is inaccessible to platelets and beyond the joint in this disease.
VISUAL OVERVIEW:
An online visual overview is available for this article.
Keywords: arthritis, extracellular vesicles, inflammation, leukocytes, lymphatic system, platelets
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease with a global prevalence of 0.5% to 1%.1,2 The best-known manifestations implicate the joints, although it is recognized that RA also affects the cardiovascular system. Hence, RA is considered a leading cause of disability and is associated with multiple complications such as cardiovascular diseases.1 Despite the emergence of several efficient therapies, a cure has not yet been achieved.1
Joint invasion by immune cells and activation of resident immune cells and fibroblast-like synoviocytes contribute to the disease.3 Inflammatory mediators drive progressive and irreversible disruption of the cartilage and bone.1 Leukocytes play a central role in joint destruction through the release of inflammatory mediators and enzymes like elastases and collagenases.4 The mechanism underlying disease initiation is not fully established, but autoantibodies such as rheumatoid factor and those directed against citrullinated proteins, and activation of FcR (Fc receptors) by immune complexes (IC), are suggested to drive the disease in seropositive RA.2 In mice, genetic ablation of the complete set of FcγR (FcR for IgG) protects from arthritis,5-7 further pointing to the critical role of autoantibodies in this disease.
Although platelets circulate in blood and maintain hemostasis, their role in the inflammatory response has also been recognized.8 Platelets are activated in RA patients,9,10 and their depletion in a mouse model of autoimmune inflammatory arthritis decreases arthritis severity.11,12 The genetic ablation of GPVI (glycoprotein VI), the platelet receptor for collagen, reduces arthritis,11 also pointing to platelet contribution to RA. Human platelets express FcγRIIA, a receptor for IC, which too could contribute to platelet activation in RA. However, murine platelets are completely devoid of any FcγR and thereby cannot respond to IC.13-15 In transgenic FcγRIIA mice (FcγRIIATGN) expressing FcγRIIA on myeloid cells including platelets, similarly to humans,15-17 circulating IC activate platelets.18-21 Antibody-mediated arthritis is also more severe in FcγRIIATGN mice.22,23 However, it remains unknown whether manifestations other than joint inflammation are affected by the expression of FcγRIIA by platelets in arthritis.
Extracellular vesicles (EV), such as exosomes and microvesicles, are small vesicles, respectively, released from cells through exocytosis of multivesicular bodies or plasma membrane budding.24 Upon activation, platelets release EV (PEV) containing molecules and organelles originating from the platelet25,26 and which can be transferred to cellular recipients if internalized.27-29 PEV levels increase in the blood of RA patients and correlate with disease severity.30,31 PEV accumulate in the synovial fluid of RA patients,11,32-34 where they amplify inflammation, in part through the activation of fibroblast-like synoviocytes by their IL (interleukin)-1 content.11 In RA PEV harbor autoantigens (eg, vimentin and citrullinated proteins) that are targeted by autoantibodies.32,33 Moreover, PEV are internalized by neutrophils in the arthritic joints28 leading to the transfer of platelet components, which profoundly modify the neutrophil transcriptome.28 Thus, PEV accumulate in the joint in RA where they can interact with numerous cellular lineages. Whether they can exit the inflamed tissue has never been examined.
Alongside the bloodstream, lymphatic circulation is a circulatory system that drains interstitial fluid from the tissue back into the blood via lymph nodes and the thoracic duct.35,36 Lymph transports immune cells, lipoproteins, soluble factors, and EV37 and is normally devoid of erythrocytes and platelets.38 Studies in mice indicate that lymphatic vessels are involved in the clearance of atherosclerotic lesions,37,39,40 and are enriched in EV from platelets and erythrocytes.37 How these EV reach lymph and whether this could also apply to other pathogeneses such as an autoimmune inflammatory disease is unknown.
During RA, enhanced permeability of the synovial microvasculature underlies tissue edema.41,42 Inflamed joints and neighboring tissues are drained by dense networks of lymphatics,43,44 which is suggested to contribute to edema resorption and the attenuation joint damage.45,46 Although the lymphatic system is mainly regarded as a route for immune cells to reach lymphoid organs, whether increased blood vessel permeability in inflammatory conditions can influence EV entry in lymphatics remains poorly documented.
Because lymphatic circulation connects the inflamed joints with lymph nodes and the blood circulation,44 we verified whether lymph could also drain EV of an inflamed joint during RA. Here, we show that after draining the inflamed articulation, lymph is enriched in PEV during RA. We provide evidence that PEV egress is tightly regulated by vascular permeability and implicates serotonin released by activated platelets following their activation by autoantibodies in arthritis.
MATERIALS AND METHODS
More information on methodologies and related citations are presented as Data Supplement. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Mice
FcγRIIATGN mice (Jackson Laboratory) were backcrossed to C57BL/6J for >10 generations. β3−/− and Tph1−/− mice were crossed with FcγRIIATGN mice to obtain FcγRIIATGN::β3−/− and FcγRIIATGN::Tph1−/− mice.19,47-49 FcγRIIATGN mice were also crossed with mice expressing RFP (red fluorescent protein) in mitochondria to generate FcγRIIATGN mice with fluorescent mitochondria, FcγRIIATGN::Mitochondria-DsRed (Discosoma Red) mice.50 Guidelines of the Canadian Council on Animal Care were followed in a protocol approved by the Animal Welfare Committee at Laval University (2017122-2).
EV Quantification
Samples (1 μL lymph) were labeled with 2 μg/mL of CD41-BV421 and 1 μM of CellTracker Deep Red for 30 minutes at room temperature in 100 μL PBS and diluted to 500 μL with PBS before flow cytometry analysis using BD Canto II Special Order Research Product, mounted with a forward scatter coupled to a photomultiplier tube, adapted to small particle quantification. PEV were identified as double-positive for both CD41 and CellTracker. A known quantity of fluorescent microspheres (2 μm diameter, Cy5-labeled, Nanocs, Inc, NY) was added to each tube.
Statistical Analysis
To avoid misinterpretations due to changes in laser performance or other confounders through the extended period that lasted this study, we only made comparisons between EV levels in lymph collected and processed the same day and analyzed the same day on the same instrument. Results are presented as mean±SEM. All statistical analyses were done using R software.51 Unpaired Student t test and Wilcoxon rank-sum test were used when applicable. One-way ANOVA followed with a post hoc Tukey honestly significant difference or 2-way ANOVA followed with a post hoc Holm-Šídák were used for multiple comparisons.
MicroRNA Data Analysis
For microRNA analysis, an exogenous synthetic control microRNA (Caenorhabditis elegans let-7 [lethal-7]-as mutated) was used, as described previously.52 The 30 most frequent microRNAs were selected, and their targets were predicted using the multiMiR R package,53 with the DIANA-microT database.54 Top 10 pathways potentially affected by the identified target genes were obtained using the clusterProfiler55 R package and the KEGG Pathway56 enrichment tool.
RESULTS
PEV Circulate in Lymph in Autoimmune Inflammatory Arthritis
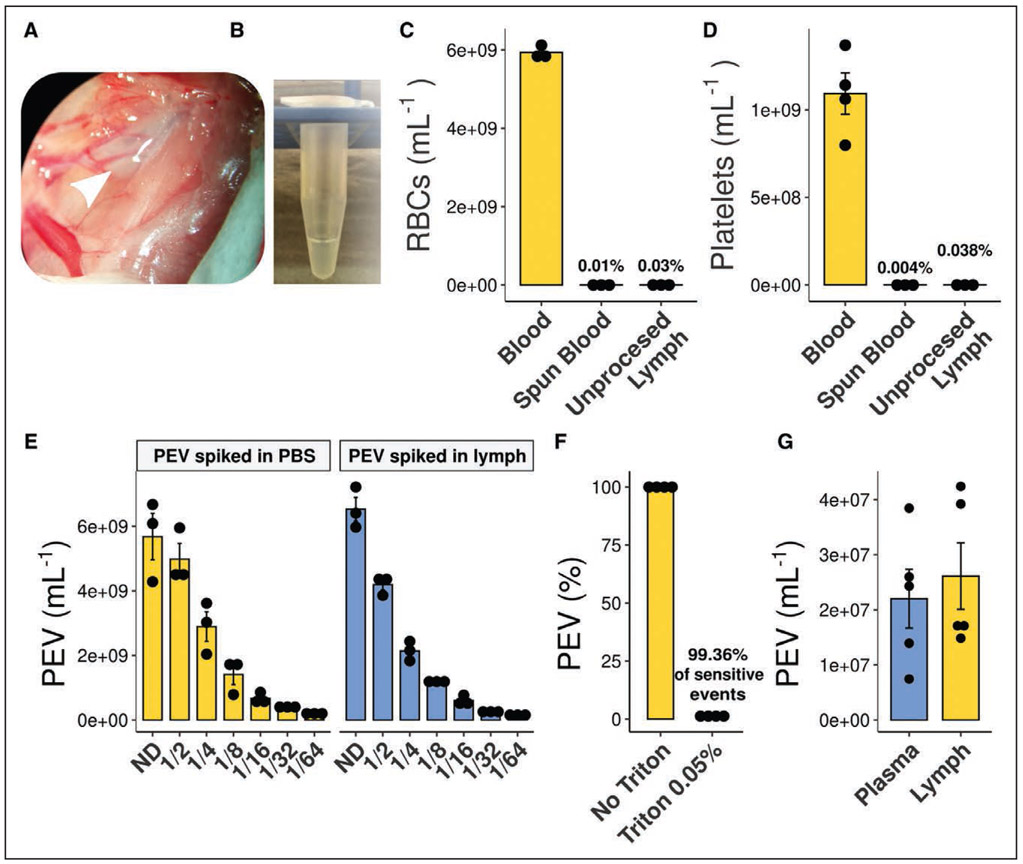
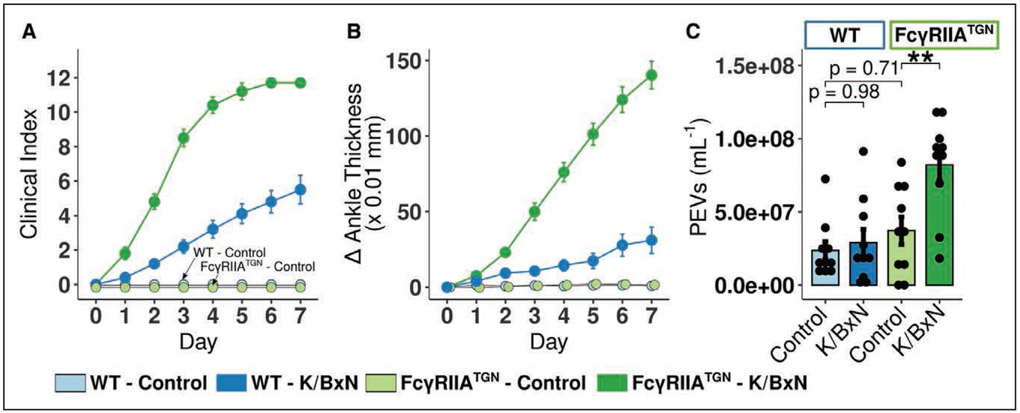
Lymph was collected at the peak of arthritis (day 7), and the absence of blood contamination was confirmed (Figure 1A through 1D). High-sensitivity flow cytometry analyses were optimized to specifically quantify lymph EV (Figure 1E and 1F). PEV concentrations in control lymph (2.5±0.6×107 /mL) and in plasma (1.1±0.1×107 /mL) were comparable (Figure 1G). Wild-type mice injected with K/BxN serum developed moderate arthritis (Figure 2A and 2B), and no increase in lymph PEV was detected in RA (Figure 2C).57
Figure 1. Lymph collection and lymph platelet extracellular vesicles (PEV) quantification.
A, Lymph is collected at the thoracic duct, between the transverse lumbar artery and the diaphragm in a collection tube coated with EDTA (0.1 M). B, Representative picture of translucent collected lymph. C and D, Red blood cells (RBCs; C) and platelet (D) counts in blood, spun blood (plasma), and unprocessed lymph (n=3). E, Efficient flow cytometry quantification of PEV in lymph was verified by spiking and serial-dilutions of in vitro-generated PEV in either lymph or PBS. Nondiluted (ND), n=3. F, Sensitivity to Triton 0.05% of the PEV quantified by flow cytometry (n=4). G, PEV were quantified in platelet-free plasma and in lymph in C57BL/6 wild-type mice (n=5).
Figure 2. Platelet extracellular vesicles (PEV) circulate in lymph in autoimmune inflammatory arthritis.
A and B, Clinical index (A) and delta ankle thickness (B) in wild-type (WT; blue) and FcγRIIATGN (green) mice injected with either 150 μL PBS (respectively light blue and light green) or 150 μL K/BxN serum (respectively dark blue and dark green) on day 0 and day 2 (n=10). C, Flow cytometry quantification of lymph PEV in WT and FcγRIIATGN mice at the peak of arthritis severity, day 7 (n=10). **P≤0.01, using a 1-way ANOVA, followed by post hoc Tukey honestly significant difference for multiple comparisons.
In the K/BxN serum transfer arthritis model, autoantibodies target glucose-6-phosphate isomerase and form pathogenic IC.58 Given the absence of FcγR on murine platelets,16 FcγRIIA-expressing mice (FcγRIIATGN) were used. No significant differences in the levels of lymph PEV due to the expression of the transgene were observed (Figure 2C and 2F, P=0.25). FcγRIIATGN mice injected with K/BxN serum displayed more severe RA (Figure 2D and 2E). Unlike wild-type mice, lymph PEV concentration increased during RA in FcγRIIATGN mice (Figure 2F). Lymph immunoblotting (Figure IA in the Data Supplement) showed a specific enrichment in CD41 fraction, whereas proteins expressed by EV and exosomes (TSG101) were not modulated, suggesting that PEV accumulation in lymph during RA had no significant impact on the lymph content of these exosomal proteins. Increased PEV levels in FcγRIIATGN mice peaked at day 7 (Figure IB in the Data Supplement). To obtain clues on whether PEV accumulate in lymph following drainage from the inflamed articulation, we assessed PEV in both thoracic and mesenteric lymph draining respectively the lower body including hind limbs and the digestive system. Only the thoracic lymph was enriched in PEV (Figure IC in the Data Supplement), suggesting that PEV influx in arthritic FcγRIIA-expressing mice may originate from the joint vasculature.
PEV Are a Predominant EV Population in FcγRIIA K/BxN Lymph
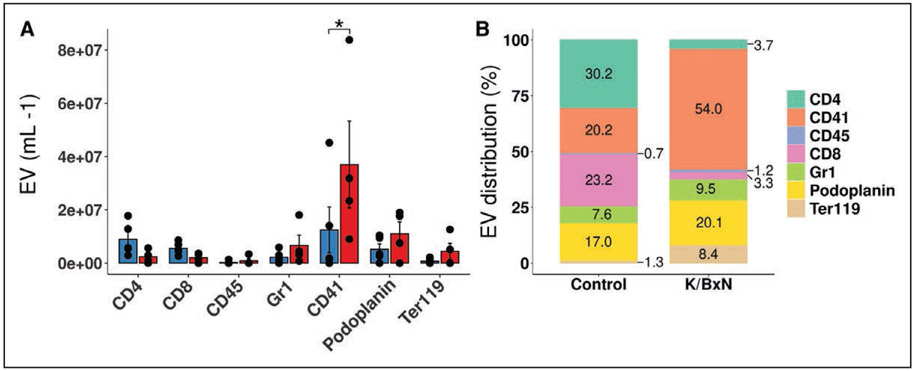
The majority of the circulating cells in lymph in healthy conditions were B and T-lymphocytes (Figure IIA in the Data Supplement). Concentrations of CD45-, CD3- or CD4-harboring cells significantly decreased in K/BxN lymph (Figure IIA in the Data Supplement), suggesting that these cells may be sequestered in other tissue locations or lymph nodes. This further highlights the specificity of PEV enrichment in RA lymph. Minor fractions of the B (3.3±0.7%) or T-lymphocytes (1.7±0.8%) harbored the platelet marker CD41 suggesting that PEV interaction with lymphocytes in lymph is a minor event (Figure IIB in the Data Supplement). Multiple EV populations were detected in lymph in addition to PEV: EV from leukocytes (CD45+), T-lymphocytes (CD4+, CD8+), granulocytes (Gr1+ [granulocyte marker 1]), lymphatic endothelial cells (podoplanin+), and erythrocytes (Ter119+ [terminal 119 antigen of glycophorin A]; Figure 3A). However, none of the EV derived from these cells were significantly modulated by K/BxN arthritis (Figure 3A). As PEV proportion increased to represent the main EV population we detected in lymph in arthritic conditions (Figure 3B), the data further highlight the preferential accumulation of PEV in lymph during RA.
Figure 3. Platelet extracellular vesicles (EV) are a predominant EV population in FcγRIIATGN K/BxN lymph.
A, Quantification of EV populations between control and K/BxN lymph, as assessed by flow cytometry. B, Assessment of EV populations proportion by flow cytometry in lymph from control and K/BxN mice. EV distribution are presented as a percentage based on the selected EV markers (CD4, CD41, CD45, CD8, Gr1 [granulocyte marker 1], Podoplanin, Ter119 [terminal 119 antigen of glycophorin A]). EV not presenting those markers were, therefore, not taken into account for the total EV population in this representation (n=4). Same samples were used for creating both A and B. *P≤0.05 using a 2-way ANOVA, followed by post hoc Holm-Šídák for multiple comparisons.
Characterization of EV in Lymph
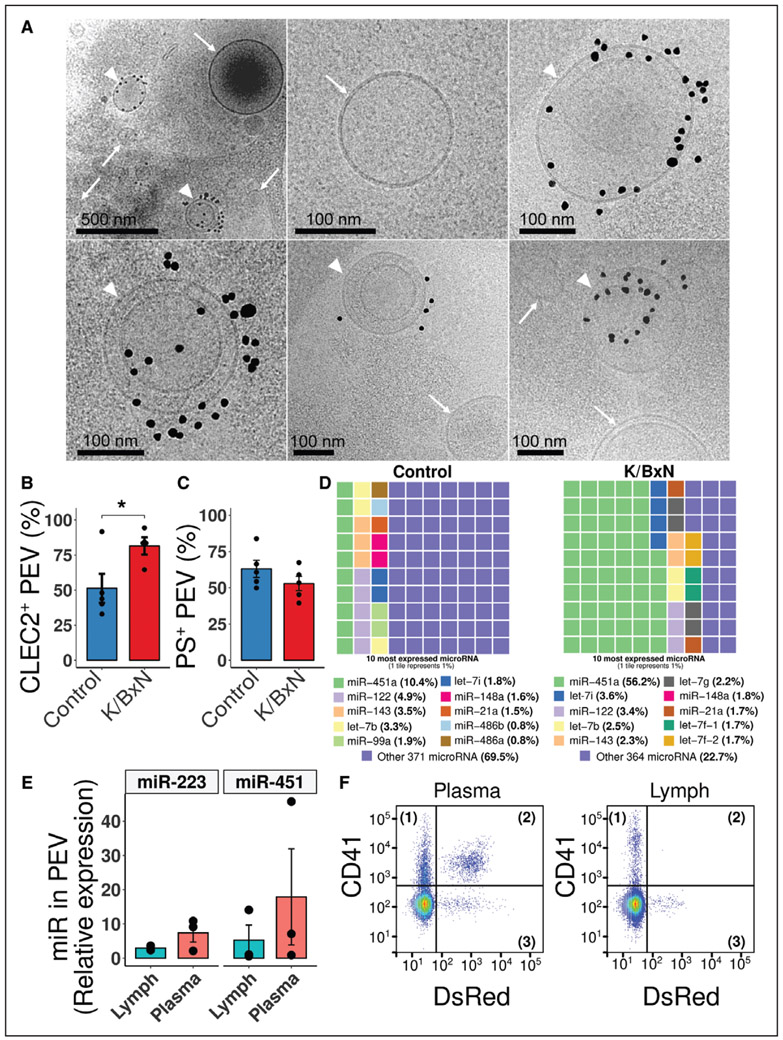
Cryo-transmission electron-microscopy permits the direct visualization of EV and the assessment of EV smaller in size than those distinguished by flow cytometry. The analysis of thoracic lymph from arthritic FcγRIIATGN mice revealed a wide variety of EV in lymph (Figure 4A). Approximately 5% of EV harbored CD41, although the majority were CD41 negative, consistent with the large heterogeneity of the EV present in lymph and potentially reflecting the absence of CD41 on certain PEV.59,60 Furthermore, vesicles located inside other vesicles, and vesicles presenting a tubular shape, were also observed (Figure 4A).
Figure 4. Characterization of extracellular vesicles (EV) in lymph.
A, Representative Cryo-electron-microscopy images of vesicles detected in FcγRIIATGN K/BxN lymph. Black dots identify CD41 antibody coupled to gold beads. Top left, the specificity of labeling, as all the black dots can be found in close proximity with EV. White arrowheads identify CD41+ EV, whereas white arrows identify CD41− EV (lymph pooled from 3 FcγRIIATGN K/BxN mice). B, CLEC-2 (C-type lectin domain family 1 member B) exposure and (C) phosphatidylserine (PS) exposure on platelet EV (PEV) in control and K/BxN mice (n=4 and n=5). D, Representations of the proportions of the 10 most expressed extracellular microRNA in Control (left) and K/BxN (right) FcγRIIATGN lymph. Each tile represents 1% (n=1, pooled from 3 mice per condition). E, Proportion of micro RNA (miR)-223 and miR-451 contained in K/BxN immunoprecipitated CD41+ PEV over total extracellular content in lymph vs plasma, as determined by real-time quantitative polymerase chain reaction (n=3). F, Representative gating illustration of plasma and lymph from K/BxN mice (n=2). Samples were labeled with an anti-CD41 (Pacific Blue), whereas mitochondria are endogenously fluorescent (Mitochondria-DsRed [Discosoma Red]). *P≤0.05, using an unpaired t test. let-7 indicates lethal-7.
EV from activated platelets in the blood of RA patients express CLEC-2 (C-type lectin-like receptor 2), a receptor for podoplanin61; however, it is unknown whether this receptor is found on PEV in lymph. In healthy mice, 50% of lymph PEV harbor CLEC-2, but this percentage increased to 80% in K/BxN lymph, confirming that PEV maintain the expression of CLEC-2 in arthritis, and pointing to enrichment of CLEC-2+ PEV in lymph during RA (Figure 4B). Moreover, half (58±4%) of the PEV in control and K/BxN mouse lymph exposed phosphatidylserine (Figure 4C), a percentage similar to what has been reported in blood (55%).62 Analysis of lymph microRNA content, undertaken because platelets63 and blood PEV25,29 contain gene-regulatory microRNA, showed profound alterations in the composition of the lymph extracellular microRNA during arthritis (Figure 4D) and changes in the pathways potentially targeted by these microRNA during arthritis (Figure III in the Data Supplement). Given the 5-fold enrichment of micro RNA (miR)-451, a microRNA known to be contained in PEV28 (Figure 4D), we utilized magnetic microspheres to specifically isolate PEV in lymph and confirmed their content in miR-451 and miR-223 (Figure 4E). Similar levels of these microRNA were quantified in PEV from plasma, suggesting that there is no enrichment in these microRNA species in lymph PEV. A proportion of PEV contain mitochondria and have been described in blood and the synovial fluid in RA.26,59 To determine if lymph PEV contain mitochondria, FcγRIIATGN mice were crossed with mice expressing RFP in the mitochondria.50 Alongside platelets, CD41+ EV containing or not mitochondria were detected in plasma from these mice but were absent in lymph (Figure 4F), which is consistent with lymphatic EV being of a small size (123.5±3.1 nm in control lymph versus 97.5±5.1 nm in K/BxN lymph; Figure IV in the Data Supplement). Taken together, these results reveal that lymph PEV, despite sharing common traits with blood PEV, can be distinguished from blood PEV by the absence of mitochondria.
PEV Do Not Contribute to Lymph Coagulation
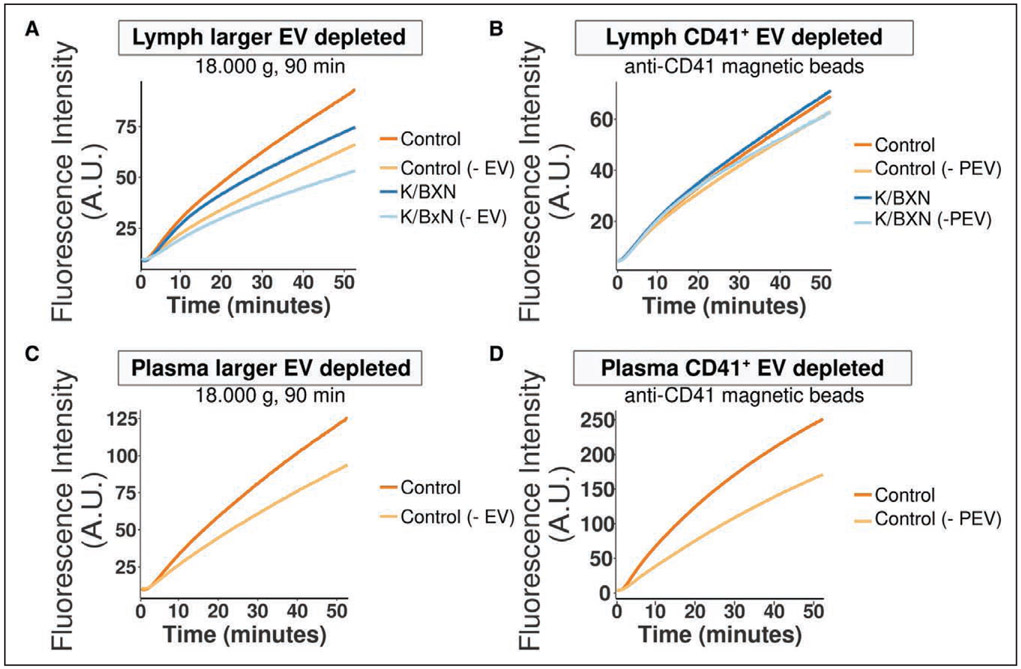
PEV circulating in blood can support the coagulation cascade.25,64 However, it is unknown whether lymph PEV can also participate in coagulation. Albeit at a lower level than plasma (Figure 5A and 5C), lymph was able to generate thrombin, thereby demonstrating that coagulation can occur in lymph, as previously reported.65 Thrombin generation in lymph of healthy versus arthritic mice was comparable, suggesting that the population of PEV enriched in FcγRIIATGN K/BxN mice does not augment lymph coagulation (Figure 5A). The complete removal of EV following high-speed centrifugation of acellular lymph and plasma reduced the coagulation potential of the fluids, suggesting that particulate material, likely EV from different cells, supports coagulation in lymph and in plasma (Figure 5A and 5C). However, the specific removal of CD41+ EV from lymph and plasma using magnetic beads coupled with anti-CD41 antibody effectively reduced thrombin generation in plasma, but not in lymph (Figure 5B and 5D), despite the efficient PEV depletion (Figure V in the Data Supplement). These data demonstrate that in contrast to PEV in blood, lymph PEV do not contribute to coagulation, pointing to other function(s) for PEV in lymph.
Figure 5. Platelet extracellular vesicles (PEV) do not contribute to lymph coagulation but interact with lymphatic endothelial cells.
A and B, Averaged curves showing fluorescence from cleavage of a fluorogenic substrate of thrombin in control (PBS) or FcγRIIATGN K/BxN lymph, either native or larger EV-depleted (centrifugation at 18,000 g for 90 min; A), or native and CD41+-EV-depleted (B; n=4). C and D, Averaged curves showing fluorescence from cleavage of a fluorogenic substrate of thrombin in control (PBS) or FcγRIIATGN K/BxN plasma, either native or larger EV-depleted (C), or native and CD41+-EV-depleted (D; n=3 and n=2). A.U. indicates arbitrary unit.
Role of FcγRIIA Signaling Through αIIbβ3 in Joint Vasculature Leakage
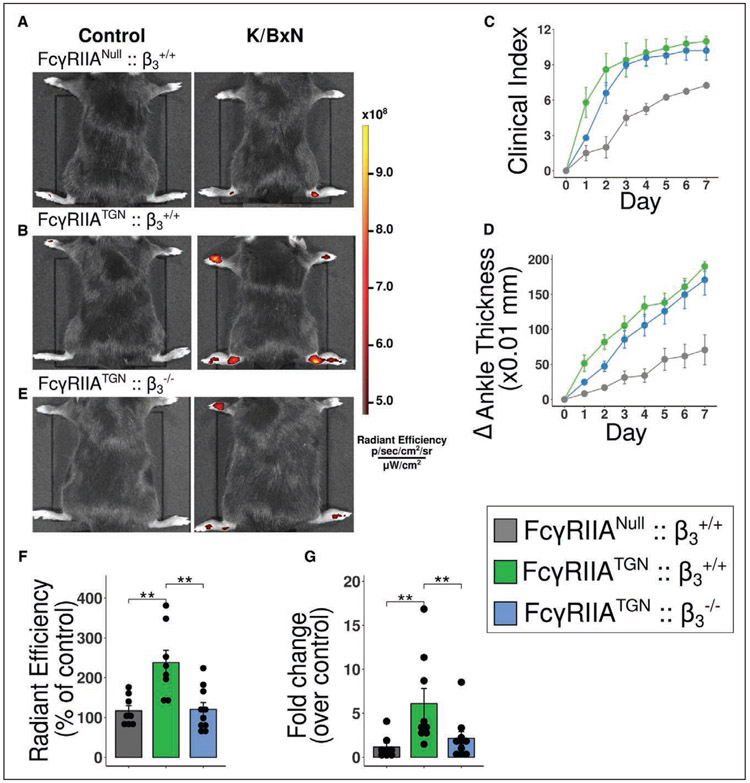
Blood vasculature leakage of the inflamed joint occurs during arthritis.41,66-68 Alterations to the blood vasculature permeability may be involved in the accumulation of PEV in lymph. Joint permeability was assessed by intravenous injection of fluorescent microspheres (510 nm) in the tail vein and quantification of their accumulation in joints.41 Unlike healthy control mice, vascular leakage was confirmed in arthritic mice and was significantly enhanced by the expression of FcγRIIA (Figure 6A and 6B). Since platelets FcγRIIA signaling requires fibrinogen binding to the αIIbβ3 receptor,69 mice lacking β3 subunit (FcγRIIATGN::β3−/−) were compared with control mice (FcγRIIATGN::β3+/+). Both groups developed similar arthritis (Figure 6C and 6D), revealing that β3 is dispensable in the promotion of joint inflammation. However, blood vessel leakage in joints was almost abrogated in the absence of β3 (Figure 6E and 6F), thereby pointing to critical αIIbβ3 signaling in platelets during the leakage process.
Figure 6. Role of FcγRIIA and its signaling through β3 in joint vasculature leakage.
A, B and E, FcγRIIANull::β3+/+ (A), FcγRIIATGN:: β3+/+ (B) and FcγRIIATGN:: β3−/− (E) K/BxN mice were injected intravenously with 0.51-μm diameter microspheres and visualized 2 minutes later using a Xenogen IVIS in vivo imaging system. All mice received the same concentration of fluorescent microspheres. C and D, Clinical index (C) and delta ankle thickness measured at the malleoli with the ankle in a fully flexed position (D). Comparison of arthritis severity for FcγRIIANull::β3+/+, FcγRIIATGN:: β3+/+ and FcγRIIATGN:: β3−/−. F, Radiant efficiency quantification of 0.51 μm diameter sky-blue-conjugated microspheres accumulation in arthritic joints 2 min after intravenous injection, in FcγRIIANull:: β3+/+, FcγRIIATGN:: β3+/+ and FcγRIIATGN:: β3−/−. Data are presented as a percentage of nonarthritic control mice injected with the same concentration of microspheres. G, Fold change of sky-blue fluorescence detected in the inguinal lymph nodes, 45 min after microsphere injection in FcγRIIANull:: β3+/+, FcγRIIATGN::β3+/+ vs FcγRIIATGN::β3−/− K/BxN mice relative to nonarthritic control mice injected with the same concentration of microspheres. **P≤0.01 using a one-way ANOVA, followed by post hoc Tukey honestly significant difference for multiple comparisons.
In vitro stimulation of platelet, FcγRIIA leads to PEV release, independently of β3 expression (Figure VI in the Data Supplement), suggesting that PEV may still be produced locally in joints during arthritis in mice lacking β3. However, it was impossible to assess lymph PEV levels in FcγRIIATGN::β3−/− mice due to unavoidable bleeding during lymph collection. To determine the potential role of β3 in PEV egress to lymph, the transit of fluorescent microspheres was thus monitored. Microspheres were injected in the tail vein and after their accumulation in the joints (45 minutes after injection), the microspheres were detected in hind paw-draining lymph nodes (inguinal lymph node), suggesting that the microspheres that could leave the blood circulation were drained from the extravascular bed to the lymphatic node (Figure 6G). Microsphere transit to the inguinal lymph node was markedly increased in FcγRIIATGN::β3+/+ compared with wild-type (FcγRIIANull::β3+/+) arthritic mice. In contrast, FcγRIIATGN::β3−/− mice showed a reduction in microsphere accumulation in inguinal lymph node (Figure 6G). Together, these data suggest that in arthritis, platelets are activated and mediate leakage of the joint blood vasculature in joints through a mechanism implicating FcγRIIA and αIIbβ3, a process that favors the egress of small particles through the lymphatic system.
Blood Vessel Permeability Induced by Platelet-Derived Serotonin Supports PEV Egress to the Lymphatic System
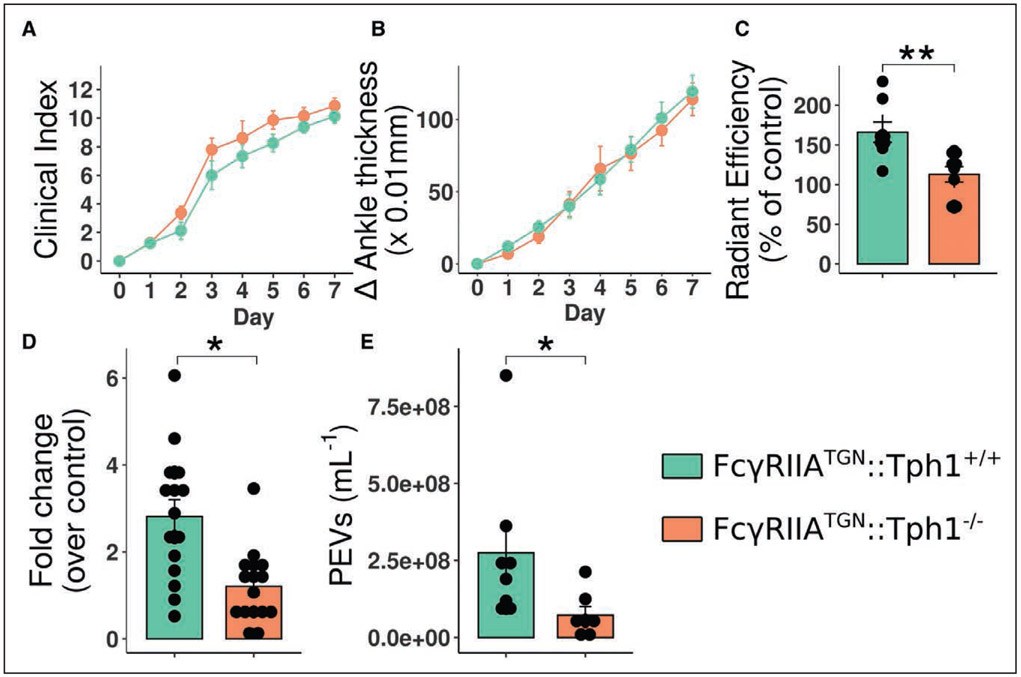
Serotonin and histamine are potent mediators capable of promoting blood vessel leakage.67,68,70 Outside the brain, platelet dense granules are the main reservoir of serotonin. The latter is, in fact, produced by TPH1 (tryptophan hydroxylase 1) in enterochromaffin cells in the intestine before their capture by platelets in blood.49 Given that FcγRIIA activation by IC and αIIbβ3 reportedly induces serotonin release,19 we examined the role of serotonin in PEV circulation in lymph using mice lacking peripheral serotonin (genetic ablation of the gene coding for TPH1)49 and expressing FcγRIIATGN. Arthritis severity was the same in both FcγRIIATGN::Tph1+/+ and FcγRIIATGN::Tph1−/− mice (Figure 7A and 7B), consistent with the reported sustained arthritis in mice lacking the serotonin transporter, the receptor that permits the capture of serotonin by platelets.41 FcγRIIATGN::Tph1−/− mice had a marked reduction in joint blood vessel permeability (Figure 7C). Fluorescent microspheres were injected in the tail vein, and their quantification in inguinal lymph node at later time points further highlighted that FcγRIIATGN::Tph1−/− mice showed a consistent reduction in microsphere transit from blood vessel to the lymphatic system (Figure 7D). It is important to note that despite sustained arthritis severity in FcγRIIATGN::Tph1−/− mice, PEV levels in lymph of these mice were greatly reduced, suggesting that PEV influx in the lymph and promotion of joint inflammation may implicate different pathways (Figure 7E). PEV reduction in lymph was not due to defective EV liberation by Tph1−/− platelets (Figure VII in the Data Supplement). Moreover, the quantification of lymphatic vessels (Lyve1+) in FcγRIIATGN::Tph1+/+ and FcγRIIATGN::Tph1−/− mice confirmed the absence of defects in lymphangiogenesis, as the lymphatic vessel number per mm2, lymphatic vessel area and total lymphatic vessel area per mm2 of tissue were the same in both groups (Figure VIIIA through VIIIE in the Data Supplement). In summary, the data confirm that following FcγRIIA activation by IC co-signaling by αIIbβ3, platelet-derived serotonin promotes PEV egress to the lymphatic system.
Figure 7. Blood vascular permeability induced by platelet-derived serotonin increases platelet extracellular vesicles (PEV) content in the lymphatic system.
A and B, Clinical index (A) and delta ankle thickness measured at the malleoli with the ankle in a fully flexed position (B). Comparison of arthritis severity for FcγRIIATGN:: Tph1+/+ and FcγRIIATGN:: Tph1−/− K/BxN mice. C, Radiant efficiency quantifications of 0.51-μm diameter sky-blue-conjugated microspheres accumulation in arthritic joints 2 min after intravenous injection, in FcγRIIATGN:: Tph1+/+ and FcγRIIATGN:: Tph1−/− K/BxN mice. Data are presented as a percentage of nonarthritic control mice injected with the same concentration of microspheres. D, Fold change of sky-blue fluorescence detected in the inguinal lymph nodes, 45 min after microsphere injection into FcγRIIATGN:: Tph1+/+ and FcγRIIATGN:: Tph1−/− K/BxN mice relative to nonarthritic control mice injected with the same concentration of microspheres. E, PEV quantification in FcγRIIATGN:: Tph1+/+ and FcγRIIATGN:: Tph1−/− thoracic lymph, in arthritic mice.
DISCUSSION
Platelets circulate in an activated state in the blood vessels of RA patients, and studies have confirmed the presence of PEV in the synovial fluid of RA patients where they can interact with other cellular lineages.11,32,34 The present study identifies PEV lymphatic influx as a consequence of articular inflammation and reveals that PEV can reach tissue locations beyond the inflamed tissue in RA. Moreover, the results show that platelets regulate the permeability of the blood vasculature to promote PEV influx into the lymphatic system in RA. As PEV transport platelet components, including inflammatory molecules and nucleic acids, this mechanism contributes to the dissemination of platelet molecules in a vasculature that is not usually accessible to platelets. As PEV influx in lymph also occurs in a mouse model of atherosclerosis,37 we further suggest that this concept may extend to other vascular inflammatory conditions in which platelets contribute.
Circulating leukocytes migrate into inflamed tissues. This process implicates both selectins and integrins, and studies suggest that platelets are critical to the efficient adhesion and migration of neutrophils in tissues. Activated platelets and neutrophils interact together through P-selectin or glycoprotein 1B on platelets, as well as P-selectin glycoprotein ligand and integrin CD11b/CD18 (Mac-1 [macrophage-1 antigen]) on neutrophils.71-74 Although platelets can use adhesion receptors, such as αIIbβ3, to scan the blood vessel wall and migrate,75 it is currently unclear whether their migratory activity is sufficient to reach the inflamed synovium in RA. Thus, platelets may potentially leave blood vessels through interactions with the migrating leukocytes or through an undefined intrinsic migratory activity. In RA, the egress of PEV from leaky blood vessels is suggested to be a function of their small dimensions.41,66 Thus, the generation of EV and the promotion of vascular leakage may represent another means for platelets to transfer their cargo to tissue locations outside blood vessels.
Human platelets express tyrosine-based activation motifs (ITAM) receptors: FcγRIIA, GPVI, and CLEC-2. In this model, FcγRIIA could play 2 roles in the promotion of PEV egress in lymph by (1) increasing PEV generation in response to IC and (2) inducing platelet degranulation and thus serotonin release. GPVI, a collagen receptor, was previously shown implicated in PEV release and promotion of arthritis in the K/BxN serum transfer model.11 Although GPVI was also shown involved in joint vascular leakage in this model,41 the present data suggest that its role is more modest in absence of FcγRIIA. We speculate that changes in K/BxN serum potency and animal facility environment may explain the much lower disease severity currently observed in our FcγRIIANull mice and may thereby mask the role of GPVI.76,77 Although enhanced arthritis severity in FcγRIIANull mice was achieved by the injection of higher volume of K/BxN serum, FcγRIIATGN mice still presented higher levels of PEV in lymph than their FcγRIIANull counterparts, and no positive correlation between arthritis severity and PEV concentration was detected (Figure IX in the Data Supplement). Although these data point to a critical involvement of FcγRIIA in PEV egress in lymph, the reduction PEV accumulation in lymph in the absence of αIIbβ3 or peripheral serotonin, despite full-blown arthritis in these mice, clearly illustrates the disconnect between arthritis severity and PEV recirculation. Given that FcγRIIA can amplify platelet activation in response to antibodies as well as in response to fibrinogen in mediating αIIbβ3 outside-in integrin signaling,78 we suggest that FcγRIIA also regulates PEV egress in lymph in other models of inflammation. Whether CLEC-2 is also involved in the release of EV by platelets or the promotion of joint leakage in RA was not investigated in the present study.
Although platelets are absent in lymph, their PEV are found in lymph under healthy conditions.37 They may be generated for instance by sustained platelet CLEC-2 activation at the separation of the blood and lymphatic systems or from megakaryocytes as they too express CD41 and CLEC-2.61,79 In immune thrombocytopenia, PEV can cross-express CD markers from other cells,80 pointing to interactions of PEV with EV from different cellular lineages. Such interactions were not investigated in the present study, but given the dominance of PEV in lymph in comparison to EV from other cells, these events would be expected to be rare. EV participate in intercellular communication25 and contain nucleic acid (eg,mRNA, microRNA, cytokines, and organelles).25,26,28,81 We could verify that lymph PEV also contain at least 2 microRNA species (miR-223 and miR-451). However, CD41+ PEV did not account for the majority of lymphatic microRNA (Figure 4E), suggesting that the profound change affecting microRNA lymphatic composition during RA could not be solely explained by the lymph PEV influx. The results hint that other vesicles, such as PEV lacking the expression of CD41, exosomes (from platelets or other cells), lipid vesicles, or Argonaute2-microRNA complexes,82 could contribute to the alteration of the microRNA repertoire. Future investigations are needed to better describe microRNA content of lymph PEV and their role in this tissue location.
In accordance with previous literature,65 lymph can clot but less efficiently than plasma or blood. It may be explained by altered levels of the clotting factors in lymph relative to plasma83 or potentially a reduced number of tissue factor exposing EV.84,85 Exosomes or other soluble mediators might also participate in lymph coagulation. Moreover, unlike in plasma, depletion of CD41+ EV had no impact on lymph coagulation, possibly due to the presence of other more potent populations of EV not harboring CD41. This suggests that lymph PEV may play roles other than in coagulation, such as the transmission of platelet-derived molecules to lymph nodes and cells that populate the lymphatics. Whether the drainage of PEV is a process that can reduce joint inflammation in RA could not be demonstrated in this present study, but others reported that interruption of lymphatic drainage due to lymphoproliferation in lymph nodes could enhance joint bone erosion and inflammation in mice.46,86 The drainage of inflammatory molecules (eg, cytokines, PEV) may thus reduce local inflammation in joints but may disseminate these molecules to other tissue locations and to cells that populate the lymphatics.
Lymphatic vessels draining the interstitial fluid are blind-ended by a mesh of pores, that selectively uptake smaller vesicles, up to 1 μm in diameter.87 The pore size possibly explains the size-filtration of EV entering the lymph, thereby favoring enrichment of smaller EV, possibly explaining the absence of mitochondria-containing PEV in lymph, in contrast to plasma. Another explanation is that certain populations of PEV may have preferentially interacted with other cells, such as endothelial cells, macrophages, and neutrophils,27-29 and even with the lymphatic endothelial cells which, we observed, can also internalize PEV (Figure X in the Data Supplement). Like EV, small molecules, such as cytokines, may thus be drained by the lymphatic vessels. By measuring 32 different cytokines and chemokines in mouse lymph, we found that solely G-CSF (granulocyte-colony stimulating factor) and IL-6 were increased during RA (Figure XI in the Data Supplement). However, with the exception of IL-6, serotonin deficiency did not hinder the presence of other cytokines or chemokines in arthritic lymph (Figure XII in the Data Supplement). Although PEV were able to stimulate the release of cytokines (eg, G-CSF, IL-1ra [interleukin-1 receptor antagonist]) by lymphatic endothelial cells, PEV failed to induce IL-6 release (Figure XIII and Figure XIV in the Data Supplement). Together, the data point to the specificity of serotonin-induced vascular permeability in the presence of PEV and IL-6 in lymph.
The lymphatic network is best known for its role in the transportation of immune cells to lymph nodes. Lymph is also utilized by metastatic lymphoma cells to reach the lung vasculature and propagate cancer.88,89 However, our work identifies platelets as cells capable of propagating their PEV through lymph in RA. This novel activity for platelets occurs downstream the inflamed site. Given that lymph unidirectionally converges at the thoracic duct near the heart in the left subclavian vein, PEV may also return to the blood circulation near this organ and may contribute to the enhanced risk of cardiovascular diseases.90 These observations are particularly relevant in RA, where inflammation affects the joints, but cardiovascular manifestations are one of the leading causes of death in patients.90 In sum, PEV have a privileged access to the lymphatic system relative to platelets, and their examination in lymph may reveal outstanding physiological and pathological roles played by PEV that cannot be performed by platelets themselves.
Supplementary Material
Highlights.
Platelet extracellular vesicles circulate in lymph, and their concentration increases during inflammatory arthritis.
Platelet extracellular vesicles transit to lymph implicates vascular permeability.
Vascular permeability modulating platelet extracellular vesicles transfer to lymph is regulated by platelet-derived serotonin.
Acknowledgments
We are grateful to the generous technical help provided by Dr Emmanuelle Rollet-Labelle throughout the study. We are also grateful for the generous donation of FcγRIIATGN::β3−/− mice by Dr Peter Newman.
Sources of Funding
This work was supported by a Foundation grant from the Canadian Institutes of Health Research (CIHR) and the Natural Sciences and Engineering Research Council of Canada (to E.B.); it was also supported, in part, by grant from the Institut Universitaire de France to A.R. Brisson. E. Boilard is recipient of a new investigator award from the CIHR and the Fonds de Recherche du Québec - Santé (FRQ-S). P.R. Fortin is recipient of a tier 1 Canada Research Chair on Systemic Autoimmune Rheumatic Diseases. A. Benmoussa, N. Tessandier, and I. Melki are recipients of fellowships from FRQ-S. N. Tessandier and I. Melki are recipients of fellowships from The Arthritis Society (TAS).
Nonstandard Abbreviations and Acronyms
- CLEC-2
C-type lectin-like receptor 2
- EV
extracellular vesicles
- FcR
Fc receptors
- FcγR
Fc receptors for IgG
- GPVI
glycoprotein VI
- IC
immune complexes
- IL
interleukin
- PEV
platelet EV
- RA
rheumatoid arthritis
- TPH1
tryptophan hydroxylase 1
Footnotes
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/ATVBAHA.119.313698.
Disclosures
None.
Contributor Information
Nicolas Tessandier, Centre de recherche du CHU de Québec, Canada; Département de microbiologie-infectiologie et d’immunologie, Université Laval, QC, Canada.
Imene Melki, Centre de recherche du CHU de Québec, Canada; Département de microbiologie-infectiologie et d’immunologie, Université Laval, QC, Canada.
Nathalie Cloutier, Centre de recherche du CHU de Québec, Canada; Département de microbiologie-infectiologie et d’immunologie, Université Laval, QC, Canada.
Isabelle Allaeys, Centre de recherche du CHU de Québec, Canada; Département de microbiologie-infectiologie et d’immunologie, Université Laval, QC, Canada.
Adam Miszta, Department of Pathology and Laboratory Medicine, University of North Carolina, Chapel Hill; Montreal Heart Institute, Quebec, Canada.
Sisareuth Tan, Extracellular Vesicles and Membrane Repair, UMR-5248-CBMN CNRS-University of Bordeaux-IPB, Allée Geoffroy Saint-Hilaire, Pessac, France.
Andreea Milasan, Department of Medicine, Faculty of Medicine, Université de Montréal, Quebec, Canada.
Sara Michel, Centre de recherche du CHU de Québec, Canada; Département de microbiologie-infectiologie et d’immunologie, Université Laval, QC, Canada.
Abderrahim Benmoussa, Department of Nutrition, CHU Sainte-Justine, Université de Montréal, Quebec, Canada.
Tania Lévesque, Centre de recherche du CHU de Québec, Canada; Département de microbiologie-infectiologie et d’immunologie, Université Laval, QC, Canada.
Francine Côté, Institut Imagine, Inserm U1163, Laboratoire Olivier Hermine, Paris, France.
Steven E. McKenzie, Cardeza Foundation for Hematological Research, Thomas Jefferson University, Philadelphia, PA
Caroline Gilbert, Centre de recherche du CHU de Québec, Canada; Département de microbiologie-infectiologie et d’immunologie, Université Laval, QC, Canada.
Patrick Provost, Centre de recherche du CHU de Québec, Canada; Département de microbiologie-infectiologie et d’immunologie, Université Laval, QC, Canada.
Alain R. Brisson, Extracellular Vesicles and Membrane Repair, UMR-5248-CBMN CNRS-University of Bordeaux-IPB, Allée Geoffroy Saint-Hilaire, Pessac, France
Alisa S. Wolberg, Department of Pathology and Laboratory Medicine, University of North Carolina, Chapel Hill
Paul R. Fortin, Centre de recherche du CHU de Quéebec, Canada; Département de microbiologie-infectiologie et d’immunologie, Université Laval, QC, Canada; Axe maladies infectieuses et inflammatoires, Centre de recherche du CHU de Québec–Université Laval, Québec, Canada
Catherine Martel, Department of Medicine, Faculty of Medicine, Université de Montréal, Quebec, Canada; Montreal Heart Institute, Quebec, Canada.
Éric Boilard, Centre de recherche du CHU de Québec, Canada; Département de microbiologie-infectiologie et d’immunologie, Université Laval, QC, Canada; Axe maladies infectieuses et inflammatoires, Centre de recherche du CHU de Québec–Université Laval, Québec, Canada.
REFERENCES
- 1.Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, Kavanaugh A, McInnes IB, Solomon DH, Strand V, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. doi: 10.1038/nrdp.2018.1 [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. The Lancet. 2016;6736:1–16. [DOI] [PubMed] [Google Scholar]
- 3.Lee DM, Kiener HP, Agarwal SK, Noss EH, Watts GF, Chisaka O, Takeichi M, Brenner MB. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315:1006–1010. doi: 10.1126/science.1137306 [DOI] [PubMed] [Google Scholar]
- 4.Wright HL, Moots RJ, Edwards SW. The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10:593–601. doi: 10.1038/nrrheum.2014.80 [DOI] [PubMed] [Google Scholar]
- 5.Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FM, Boackle SA, Takahashi K, Holers VM, Walport M, Gerard C, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16:157–168. doi: 10.1016/s1074-7613(02)00275-3 [DOI] [PubMed] [Google Scholar]
- 6.Mancardi DA, Jönsson F, Iannascoli B, Khun H, Van Rooijen N, Huerre M, Daäron M, Bruhns P. Cutting edge: the murine high-affinity IgG receptor FcγRIV is sufficient for autoantibody-induced arthritis. J Immunol. 2011;186:1899–1903. doi: 10.4049/jimmunol.1003642 [DOI] [PubMed] [Google Scholar]
- 7.Kyburz D, Carson DA, Corr M. The role of CD40 ligand and tumor necrosis factor alpha signaling in the transgenic K/BxN mouse model of rheumatoid arthritis. Arthritis Rheum. 2000;43:2571–2577. doi: [DOI] [PubMed] [Google Scholar]
- 8.Kapur R, Zufferey A, Boilard E, Semple JW. Nouvelle cuisine: platelets served with inflammation. J Immunol. 2015;194:5579–5587. doi: 10.4049/jimmunol.1500259 [DOI] [PubMed] [Google Scholar]
- 9.Wang F, Wang NS, Yan CG, Li JH, Tang LQ. The significance of platelet activation in rheumatoid arthritis. Clin Rheumatol. 2007;26:768–771. doi: 10.1007/s10067-007-0550-0 [DOI] [PubMed] [Google Scholar]
- 10.Mac Mullan PA, Peace AJ, Madigan AM, Tedesco AF, Kenny D, McCarthy GM. Platelet hyper-reactivity in active inflammatory arthritis is unique to the adenosine diphosphate pathway: a novel finding and potential therapeutic target. Rheumatology (Oxford). 2010;49:240–245. doi: 10.1093/rheumatology/kep377 [DOI] [PubMed] [Google Scholar]
- 11.Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O’Donnell E, Farndale RW, Ware J, et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–583. doi: 10.1126/science.1181928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mott PJ, Lazarus AH. CD44 antibodies and immune thrombocytopenia in the amelioration of murine inflammatory arthritis. PLoS One. 2013;8:e65805. doi: 10.1371/journal.pone.0065805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenfeld SI, Looney RJ, Leddy JP, Phipps DC, Abraham GN, Anderson CL. Human platelet Fc receptor for immunoglobulin G. Identification as a 40,000-molecular-weight membrane protein shared by monocytes. J Clin Invest. 1985;76:2317–2322. doi: 10.1172/JCI112242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson CL, Loone RJ. Human leukocyte IgG Fc receptors. Immunol Today. 1986;7:264–266. doi: 10.1016/0167-5699(86)90007-1 [DOI] [PubMed] [Google Scholar]
- 15.McKenzie SE, Taylor SM, Malladi P, Yuhan H, Cassel DL, Chien P, Schwartz E, Schreiber AD, Surrey S, Reilly MP. The role of the human Fc receptor FcRIIA in the immune clearance of platelets: a transgenic mouse model. J Immunol. 1999;162:4311–4318. [PubMed] [Google Scholar]
- 16.Bruhns P, Jönsson F. Mouse and human FcR effector functions. Immunol Rev. 2015;268:25–51. doi: 10.1111/imr.12350 [DOI] [PubMed] [Google Scholar]
- 17.Bournazos S, Ravetch JV. Fcγ receptor pathways during active and passive immunization. Immunol Rev. 2015;268:88–103. doi: 10.1111/imr.12343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robles-Carrillo L, Meyer T, Hatfield M, Desai H, Dávila M, Langer F, Amaya M, Garber E, Francis JL, Hsu YM, et al. Anti-CD40L immune complexes potently activate platelets in vitro and cause thrombosis in FCGR2A transgenic mice. J Immunol. 2010;185:1577–1583. doi: 10.4049/jimmunol.0903888 [DOI] [PubMed] [Google Scholar]
- 19.Cloutier N, Allaeys I, Marcoux G, Machlus KR, Mailhot B, Zufferey A, Levesque T, Becker Y, Tessandier N, Melki I, et al. Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration. Proc Natl Acad Sci U S A. 2018;115:E1550–E1559. doi: 10.1073/pnas.1720553115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beutier H, Hechler B, Godon O, Wang Y, Gillis CM, de Chaisemartin L, Gouel-Chéron A, Magnenat S, Macdonald LE, Murphy AJ, et al. Platelets expressing IgG receptor Fc/CD32A determine the severity of experimental anaphylaxis. Sci Immunol. 2018;3:eaan5997. doi: 10.1126/sciimmunol.aan5997 [DOI] [PubMed] [Google Scholar]
- 21.Amirkhosravi A, Boulaftali Y, Robles-Carrillo L, Meyer T, McKenzie SE, Francis JL, Bergmeier W. CalDAG-GEFI deficiency protects mice from FcγRIIa-mediated thrombotic thrombocytopenia induced by CD40L and β2GPI immune complexes. J Thromb Haemost. 2014;12:2113–2119. doi: 10.1111/jth.12748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van de Velde NC, Mottram PL, Powell MS, Lim B, Holmdahl R, Hogarth PM. Transgenic mice expressing human FcgammaRIIa have enhanced sensitivity to induced autoimmune arthritis as well as elevated Th17 cells. Immunol Lett. 2010;130:82–88. [DOI] [PubMed] [Google Scholar]
- 23.Tan Sardjono C, Mottram PL, van de Velde NC, Powell MS, Power D, Slocombe RF, Wicks IP, Campbell IK, McKenzie SE, Brooks M, et al. Development of spontaneous multisystem autoimmune disease and hypersensitivity to antibody-induced inflammation in Fcgamma receptor IIa-transgenic mice. Arthritis Rheum. 2005;52:3220–3229. doi: 10.1002/art.21344 [DOI] [PubMed] [Google Scholar]
- 24.Bobrie A, Colombo M, Krumeich S, Raposo G, Thery C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles. 2012;1. doi: 10.3402/jev.v1i0.18397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melki I, Tessandier N, Zufferey A, Boilard E. Platelet microvesicles in health and disease. Platelets. 2017;28:214–221. doi: 10.1080/09537104.2016.1265924 [DOI] [PubMed] [Google Scholar]
- 26.Boudreau LH, Duchez AC, Cloutier N, Soulet D, Martin N, Bollinger J, Paré A, Rousseau M, Naika GS, Lévesque T, et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014;124:2173–2183. doi: 10.1182/blood-2014-05-573543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laffont B, Corduan A, Rousseau M, Duchez AC, Lee CH, Boilard E, Provost P. Platelet microparticles reprogram macrophage gene expression and function. Thromb Haemost. 2016;115:311–323. doi: 10.1160/TH15-05-0389 [DOI] [PubMed] [Google Scholar]
- 28.Duchez AC, Boudreau LH, Naika GS, Bollinger J, Belleannée C, Cloutier N, Laffont B, Mendoza-Villarroel RE, Lévesque T, Rollet-Labelle E, et al. Platelet microparticles are internalized in neutrophils via the concerted activity of 12-lipoxygenase and secreted phospholipase A2-IIA. Proc Natl Acad Sci U S A. 2015;112:E3564–E3573. doi: 10.1073/pnas.1507905112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laffont B, Corduan A, Plé H, Duchez AC, Cloutier N, Boilard E, Provost P. Activated platelets can deliver mRNA regulatory Ago2•microRNA complexes to endothelial cells via microparticles. Blood. 2013;122:253–261. doi: 10.1182/blood-2013-03-492801 [DOI] [PubMed] [Google Scholar]
- 30.Knijff-Dutmer EA, Koerts J, Nieuwland R, Kalsbeek-Batenburg EM, van de Laar MA. Elevated levels of platelet microparticles are associated with disease activity in rheumatoid arthritis. Arthritis Rheum. 2002;46:1498–1503. doi: 10.1002/art.10312 [DOI] [PubMed] [Google Scholar]
- 31.Viñuela-Berni V, Doníz-Padilla L, Figueroa-Vega N, Portillo-Salazar H, Abud-Mendoza C, Baranda L, González-Amaro R. Proportions of several types of plasma and urine microparticles are increased in patients with rheumatoid arthritis with active disease. Clin Exp Immunol. 2015;180:442–451. doi: 10.1111/cei.12598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cloutier N, Tan S, Boudreau LH, Cramb C, Subbaiah R, Lahey L, Albert A, Shnayder R, Gobezie R, Nigrovic PA, et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes. EMBO Mol Med. 2013;5:235–249. doi: 10.1002/emmm.201201846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burbano C, Rojas M, Muñoz-Vahos C, Vanegas-García A, Correa LA, Vásquez G, Castaño D. Extracellular vesicles are associated with the systemic inflammation of patients with seropositive rheumatoid arthritis. Sci Rep. 2018;8:17917. doi: 10.1038/s41598-018-36335-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.György B, Szabó TG, Turiák L, Wright M, Herczeg P, Lédeczi Z, Kittel A, Polgár A, Tóth K, Dérfalvi B, et al. Improved flow cytometric assessment reveals distinct microvesicle (cell-derived microparticle) signatures in joint diseases. PLoS One. 2012;7:e49726. doi: 10.1371/journal.pone.0049726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabin FR. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. American Journal of Anatomy. 1902;1:367–389. [Google Scholar]
- 36.Oliver G, Alitalo K. The lymphatic vasculature: recent progress and paradigms. Annu Rev Cell Dev Biol. 2005;21:457–483. doi: 10.1146/annurev.cellbio.21.012704.132338 [DOI] [PubMed] [Google Scholar]
- 37.Milasan A, Tessandier N, Tan S, Brisson A, Boilard E, Martel C. Extracellular vesicles are present in mouse lymph and their level differs in atherosclerosis. J Extracell Vesicles. 2016;5:31427. doi: 10.3402/jev.v5.31427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.GOWANS JL. The recirculation of lymphocytes from blood to lymph in the rat. J Physiol. 1959;146:54–69. doi: 10.1113/jphysiol.1959.sp006177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milasan A, Smaani A, Martel C. Early rescue of lymphatic function limits atherosclerosis progression in Ldlr−/− mice. Atherosclerosis. 2019;283:106–119. doi: 10.1016/j.atherosclerosis.2019.01.031 [DOI] [PubMed] [Google Scholar]
- 40.Martel C, Li W, Fulp B, Platt AM, Gautier EL, Westerterp M, Bittman R, Tall AR, Chen SH, Thomas MJ, et al. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J Clin Invest. 2013;123:1571–1579. doi: 10.1172/JCI63685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cloutier N, Paré A, Farndale RW, Schumacher HR, Nigrovic PA, Lacroix S, Boilard E. Platelets can enhance vascular permeability. Blood. 2012;120:1334–1343. doi: 10.1182/blood-2012-02-413047 [DOI] [PubMed] [Google Scholar]
- 42.Schumacher HR Jr. Synovial membrane and fluid morphologic alterations in early rheumatoid arthritis: microvascular injury and virus-like particles. Ann N Y Acad Sci. 1975;256:39–64. doi: 10.1111/j.1749-6632.1975.tb36034.x [DOI] [PubMed] [Google Scholar]
- 43.Xu H, Edwards J, Banerji S, Prevo R, Jackson DG, Athanasou NA. Distribution of lymphatic vessels in normal and arthritic human synovial tissues. Ann Rheum Dis. 2003;62:1227–1229. doi: 10.1136/ard.2003.005876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olszewski WL, Pazdur J, Kubasiewicz E, Zaleska M, Cooke CJ, Miller NE. Lymph draining from foot joints in rheumatoid arthritis provides insight into local cytokine and chemokine production and transport to lymph nodes. Arthritis Rheum. 2001;44:541–549. doi: [DOI] [PubMed] [Google Scholar]
- 45.Zhou Q, Guo R, Wood R, Boyce BF, Liang Q, Wang YJ, Schwarz EM, Xing L. Vascular endothelial growth factor C attenuates joint damage in chronic inflammatory arthritis by accelerating local lymphatic drainage in mice. Arthritis Rheum. 2011;63:2318–2328. doi: 10.1002/art.30421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouta EM, Bell RD, Rahimi H, Xing L, Wood RW, Bingham CO 3rd, Ritchlin CT, Schwarz EM. Targeting lymphatic function as a novel therapeutic intervention for rheumatoid arthritis. Nat Rev Rheumatol. 2018;14:94–106. doi: 10.1038/nrrheum.2017.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Culleré M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhi H, Rauova L, Hayes V, Gao C, Boylan B, Newman DK, McKenzie SE, Cooley BC, Poncz M, Newman PJ. Cooperative integrin/ITAM signaling in platelets enhances thrombus formation in vitro and in vivo. Blood. 2013;121:1858–1867. doi: 10.1182/blood-2012-07-443325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Côté F, Thévenot E, Fligny C, Fromes Y, Darmon M, Ripoche MA, Bayard E, Hanoun N, Saurini F, Lechat P, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci U S A. 2003;100:13525–13530. doi: 10.1073/pnas.2233056100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hasuwa H, Muro Y, Ikawa M, Kato N, Tsujimoto Y, Okabe M. Transgenic mouse sperm that have green acrosome and red mitochondria allow visualization of sperm and their acrosome reaction in vivo. Exp Anim. 2010;59:105–107. doi: 10.1538/expanim.59.105 [DOI] [PubMed] [Google Scholar]
- 51.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.; 2019. Available at: http://www.R-project.org/ [Google Scholar]
- 52.Benmoussa A, Lee CH, Laffont B, Savard P, Laugier J, Boilard E, Gilbert C, Fliss I, Provost P. Commercial dairy cow milk microRNAs resist digestion under simulated gastrointestinal tract conditions. J Nutr. 2016;146:2206–2215. doi: 10.3945/jn.116.237651 [DOI] [PubMed] [Google Scholar]
- 53.Ru Y, Kechris KJ, Tabakoff B, Hoffman P, Radcliffe RA, Bowler R, Mahaffey S, Rossi S, Calin GA, Bemis L, et al. The multiMiR R package and database: integration of microRNA-target interactions along with their disease and drug associations. Nucleic Acids Res. 2014;42:e133. doi: 10.1093/nar/gku631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paraskevopoulou MD, Georgakilas G, Kostoulas N, Vlachos IS, Vergoulis T, Reczko M, Filippidis C, Dalamagas T, Hatzigeorgiou AG. DIANA-microT web server v5.0: service integration into miRNA functional analysis workflows. Nucleic Acids Res. 2013;41(Web Server issue):W169–W173. doi: 10.1093/nar/gkt393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44(D1):D457–D462. doi: 10.1093/nar/gkv1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monach P, Hattori K, Huang H, Hyatt E, Morse J, Nguyen L, Ortiz-Lopez A, Wu HJ, Mathis D, Benoist C. The K/BxN mouse model of inflammatory arthritis: theory and practice. Methods Mol Med. 2007;136:269–282. doi: 10.1007/978-1-59745-402-5_20 [DOI] [PubMed] [Google Scholar]
- 58.Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 1999;286:1732–1735. doi: 10.1126/science.286.5445.1732 [DOI] [PubMed] [Google Scholar]
- 59.Brisson AR, Tan S, Linares R, Gounou C, Arraud N. Extracellular vesicles from activated platelets: a semiquantitative cryo-electron microscopy and immuno-gold labeling study. Platelets. 2017;28:263–271. doi: 10.1080/09537104.2016.1268255 [DOI] [PubMed] [Google Scholar]
- 60.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 61.Gitz E, Pollitt AY, Gitz-Francois JJ, Alshehri O, Mori J, Montague S, Nash GB, Douglas MR, Gardiner EE, Andrews RK, et al. CLEC-2 expression is maintained on activated platelets and on platelet microparticles. Blood. 2014;124:2262–2270. doi: 10.1182/blood-2014-05-572818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arraud N, Gounou C, Turpin D, Brisson AR. Fluorescence triggering: a general strategy for enumerating and phenotyping extracellular vesicles by flow cytometry. Cytometry A. 2016;89:184–195. doi: 10.1002/cyto.a.22669 [DOI] [PubMed] [Google Scholar]
- 63.Plé H, Landry P, Benham A, Coarfa C, Gunaratne PH, Provost P. The repertoire and features of human platelet microRNAs. PLoS One. 2012;7:e50746. doi: 10.1371/journal.pone.0050746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x [DOI] [PubMed] [Google Scholar]
- 65.FANTL P, NELSON JF. Coagulation in lymph. J Physiol. 1953;122:33–37. doi: 10.1113/jphysiol.1953.sp004976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boilard E, Blanco P, Nigrovic PA. Platelets: active players in the pathogenesis of arthritis and SLE. Nat Rev Rheumatol. 2012;8:534–542. doi: 10.1038/nrrheum.2012.118 [DOI] [PubMed] [Google Scholar]
- 67.Levick JR. Permeability of rheumatoid and normal human synovium to specific plasma proteins. Arthritis Rheum. 1981;24:1550–1560. doi: 10.1002/art.1780241215 [DOI] [PubMed] [Google Scholar]
- 68.Binstadt BA, Patel PR, Alencar H, Nigrovic PA, Lee DM, Mahmood U, Weissleder R, Mathis D, Benoist C. Particularities of the vasculature can promote the organ specificity of autoimmune attack. Nat Immunol. 2006;7:284–292. doi: 10.1038/ni1306 [DOI] [PubMed] [Google Scholar]
- 69.Zhi H, Dai J, Liu J, Zhu J, Newman DK, Gao C, Newman PJ. Platelet activation and thrombus formation over IgG immune complexes requires integrin αIIbβ3 and lyn kinase. PLoS One. 2015;10:e0135738. doi: 10.1371/journal.pone.0135738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.MAJNO G, PALADE GE. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961;11:571–605. doi: 10.1083/jcb.11.3.571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399 [DOI] [PubMed] [Google Scholar]
- 72.Sreeramkumar V, Adrover JM, Ballesteros I, Cuartero MI, Rossaint J, Bilbao I, Nácher M, Pitaval C, Radovanovic I, Fukui Y, et al. Neutrophils scan for activated platelets to initiate inflammation. Science. 2014;346:1234–1238. doi: 10.1126/science.1256478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ehlers R, Ustinov V, Chen Z, Zhang X, Rao R, Luscinskas FW, Lopez J, Plow E, Simon DI. Targeting platelet-leukocyte interactions: identification of the integrin Mac-1 binding site for the platelet counter receptor glycoprotein Ibalpha. J Exp Med. 2003;198:1077–1088. doi: 10.1084/jem.20022181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ho-Tin-Noé B, Boulaftali Y, Camerer E. Platelets and vascular integrity: how platelets prevent bleeding in inflammation. Blood. 2018;131:277–288. doi: 10.1182/blood-2017-06-742676 [DOI] [PubMed] [Google Scholar]
- 75.Gaertner F, Ahmad Z, Rosenberger G, Fan S, Nicolai L, Busch B, Yavuz G, Luckner M, Ishikawa-Ankerhold H, Hennel R, et al. Migrating platelets are mechano-scavengers that collect and bundle bacteria. Cell. 2017;171:1368–1382.e23. doi: 10.1016/j.cell.2017.11.001 [DOI] [PubMed] [Google Scholar]
- 76.Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, Burzyn D, Ortiz-Lopez A, Lobera M, Yang J, et al. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells. Science. 2015;349:993–997. doi: 10.1126/science.aaa9420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boylan B, Gao C, Rathore V, Gill JC, Newman DK, Newman PJ. Identification of FcgammaRIIa as the ITAM-bearing receptor mediating alphaIIbbeta3 outside-in integrin signaling in human platelets. Blood. 2008;112:2780–2786. doi: 10.1182/blood-2008-02-142125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hess PR, Rawnsley DR, Jakus Z, Yang Y, Sweet DT, Fu J, Herzog B, Lu M, Nieswandt B, Oliver G, et al. Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life. J Clin Invest. 2014;124:273–284. doi: 10.1172/JCI70422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Semple JW, Milev Y, Cosgrave D, Mody M, Hornstein A, Blanchette V, Freedman J. Differences in serum cytokine levels in acute and chronic autoimmune thrombocytopenic purpura: relationship to platelet phenotype and antiplatelet T-cell reactivity. Blood. 1996;87:4245–4254. [PubMed] [Google Scholar]
- 81.Dieudé M, Bell C, Turgeon J, Beillevaire D, Pomerleau L, Yang B, Hamelin K, Qi S, Pallet N, Béland C, et al. The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci Transl Med. 2015;7:318ra200. doi: 10.1126/scitranslmed.aac9816 [DOI] [PubMed] [Google Scholar]
- 82.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miller GJ, Howarth DJ, Attfield JC, Cooke CJ, Nanjee MN, Olszewski WL, Morrissey JH, Miller NE. Haemostatic factors in human peripheral afferent lymph. Thromb Haemost. 2000;83:427–432. [PubMed] [Google Scholar]
- 84.Tripisciano C, Weiss R, Eichhorn T, Spittler A, Heuser T, Fischer MB, Weber V. Different potential of extracellular vesicles to support thrombin generation: contributions of phosphatidylserine, tissue factor, and cellular origin. Sci Rep. 2017;7:6522. doi: 10.1038/s41598-017-03262-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hisada Y, Mackman N. Measurement of tissue factor activity in extracellular vesicles from human plasma samples. Res Pract Thromb Haemost. 2019;3:44–48. doi: 10.1002/rth2.12165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li J, Ju Y, Bouta EM, Xing L, Wood RW, Kuzin I, Bottaro A, Ritchlin CT, Schwarz EM. Efficacy of B cell depletion therapy for murine joint arthritis flare is associated with increased lymphatic flow. Arthritis Rheum. 2013;65:130–138. doi: 10.1002/art.37709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Swartz MA. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50:3–20. doi: 10.1016/s0169-409x(01)00150-8 [DOI] [PubMed] [Google Scholar]
- 88.Ma Q, Dieterich LC, Ikenberg K, Bachmann SB, Mangana J, Proulx ST, Amann VC, Levesque MP, Dummer R, Baluk P, et al. Unexpected contribution of lymphatic vessels to promotion of distant metastatic tumor spread. Sci Adv. 2018;4:eaat4758. doi: 10.1126/sciadv.aat4758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Podgrabinska S, Skobe M. Role of lymphatic vasculature in regional and distant metastases. Microvasc Res. 2014;95:46–52. doi: 10.1016/j.mvr.2014.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nurmohamed MT, Heslinga M, Kitas GD. Cardiovascular comorbidity in rheumatic diseases. Nat Rev Rheumatol. 2015;11:693–704. doi: 10.1038/nrrheum.2015.112 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.