Abstract
Background:
Previous analyses of BREAK-2 and BREAK-3 showed that durable outcomes lasting ≥3 years are achievable with dabrafenib in some patients with BRAFV600-mutant metastatic melanoma (MM); however, additional follow-up is needed to fully characterise the long-term impact of dabrafenib in these patients.
Methods:
BREAK-2 was a single-arm phase 2 study evaluating dabrafenib in treatment-naive or previously treated BRAFV600E/K-mutant MM. BREAK-3, a randomised (3:1) phase 3 study, assessed dabrafenib vs dacarbazine in previously untreated unresectable or metastatic BRAFV600E-mutant melanoma. Five-year landmark analyses were performed.
Results:
All BREAK-2 patients (N=92 [V600E, n=76; V600K, n=16]) discontinued treatment by the data cutoff. Median follow-up was 13.0 months. In BRAFV600E patients, 5-year PFS and overall survival (OS) were 11% and 20%, respectively. Postprogression immunotherapy was received by 22% of patients. In BREAK-3, median follow-up was 17.0 and 12.0 months in the dabrafenib (n=187) and dacarbazine (n=63) arms, respectively. Thirty-seven patients (59%) receiving dacarbazine crossed over to dabrafenib following disease progression. Five-year PFS was 12% in the dabrafenib arm; all dacarbazine-arm patients progressed or were censored by 5 years. Dabrafenib improved PFS vs dacarbazine, regardless of baseline lactate dehydrogenase levels. Five-year OS rates were 24% and 22% in the dabrafenib and dacarbazine arms, respectively. Subsequent therapy in each arm included anti–CTLA-4 (dabrafenib [24%] and dacarbazine [24%]) and/or anti–PD-1 (8% and 2%) treatment. No new safety signals were observed.
Conclusions and Relevance:
These data, representing extended follow-up for BRAF inhibitor monotherapy, demonstrate that durable benefit lasting ≥5 years is achievable in a subset of patients.
Trial registration: ClinicalTrials.gov (BREAK-2, NCT01153763; BREAK-3, NCT01227889).
Keywords: melanoma, BRAF, dabrafenib, metastatic, long-term outcomes
INTRODUCTION
Targeted therapies and immune checkpoint inhibitors have significantly improved clinical outcomes in metastatic melanoma (MM); however, extended follow-up in randomised studies of these agents has been limited [1–9]. Optimisation of individualised treatment for BRAFV600-mutant MM will require a full understanding of the proportion and characteristics of patients most likely to achieve long-term benefit with current therapies.
Clinical activity and tolerability of the BRAF inhibitor (BRAFi) dabrafenib were initially demonstrated in patients with MM, including those with prior treatment, harbouring BRAFV600E or BRAFV600K mutations in the phase 2 trial BREAK-2 [10]. First-line dabrafenib significantly improved outcomes vs dacarbazine in patients with unresectable or metastatic BRAFV600E-mutant melanoma in the randomised phase 3 study BREAK-3 [11]. In both studies, dabrafenib had a manageable safety profile [10,11]. Results from the primary analyses of BREAK-2 and BREAK-3 were confirmed in extended follow-up analyses of these studies, including a 3-year analysis of BREAK-3, demonstrating durable clinical benefit of dabrafenib for ≥3 years in a substantial proportion of patients (3-year overall survival [OS], 31%) [3]. We report updated 5-year landmark efficacy and safety analyses for BREAK-2 and BREAK-3.
MATERIALS AND METHODS
In BREAK-2, patients with BRAFV600E/K-mutant (per central testing) stage IV melanoma received oral dabrafenib 150 mg twice daily. With the exception of BRAF/MEK inhibitors, eligible patients could have received systemic MM treatment prior to enrolment. The primary endpoint was objective response rate (ORR) in BRAFV600E patients; secondary endpoints included ORR in BRAFV600K patients, progression-free survival (PFS), OS, duration of response, and safety.
In BREAK-3, patients with previously untreated BRAFV600E-mutant (per central testing) unresectable or MM were randomised 3:1 to receive either oral dabrafenib 150 mg twice daily or intravenous dacarbazine 1000 mg/m2 every 3 weeks and were stratified according to tumour stage using American Joint Committee on Cancer seventh edition [11]. Patients on the dacarbazine arm with disease progression could cross over to receive dabrafenib. The primary endpoint was investigator-assessed PFS; secondary endpoints included PFS by independent central review, OS, ORR, PFS following crossover, duration of response, quality of life, and safety.
Additional details on study design and statistical analyses can be found in the supplementary material. Full study protocols for BREAK-2 and BREAK-3 were included in previous publications [10,11].
RESULTS
5-Year Efficacy Analysis: BREAK-2
Of 92 BREAK-2 patients (BRAFV600E, n=76; BRAFV600K, n=16), most (84%) had prior systemic MM therapy, including chemotherapy (80%), interleukin 2 (16%), and ipilimumab (14%), and 31% had elevated lactate dehydrogenase (LDH) (Supplementary Table 1; Supplementary Fig. 1A). All patients discontinued study treatment by the data cutoff (Supplementary Fig. 1A). Median patient follow-up was 13.0 months (interquartile range [IQR], 5.5–37.5 months).
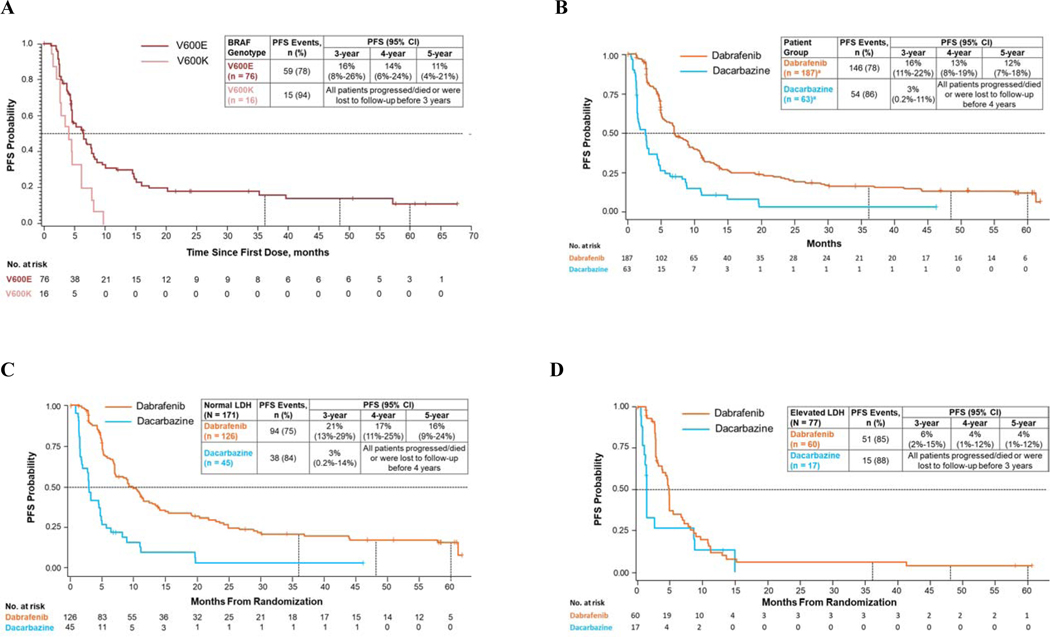
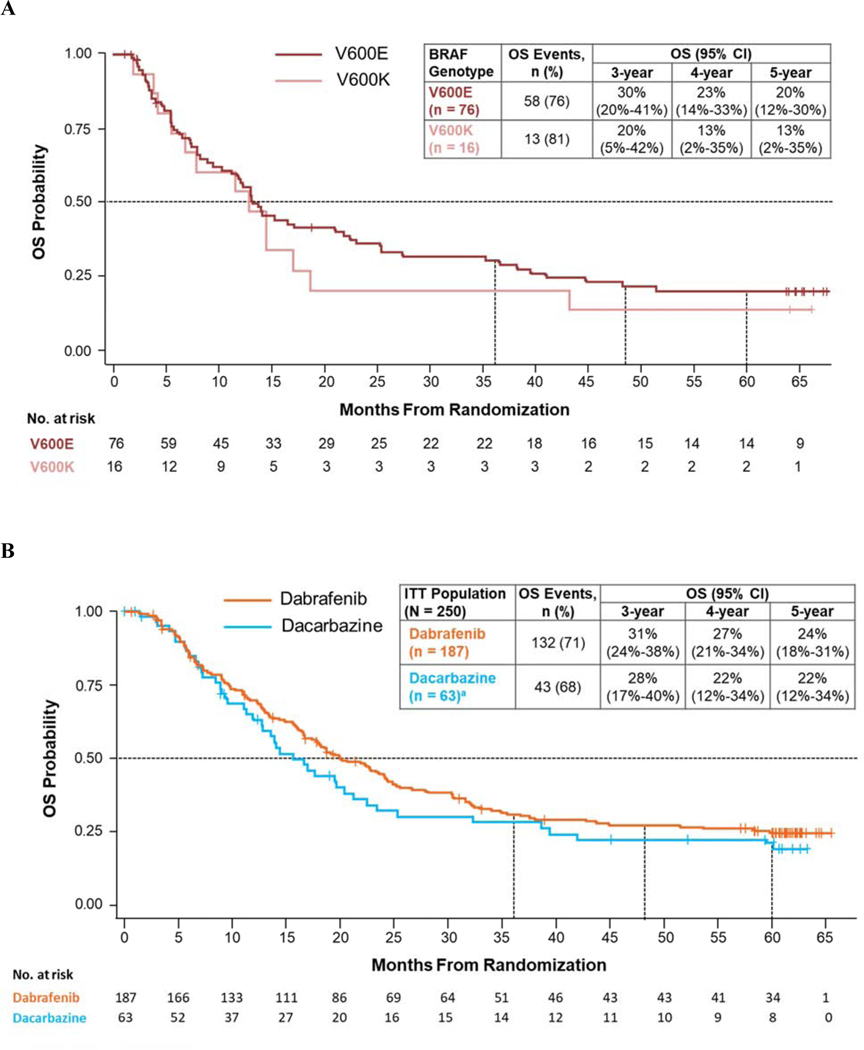
PFS events occurred in 59 BRAFV600E patients (78%) and 15 BRAFV600K patients (94%) (Fig. 1A). In BRAFV600E patients, 5-year PFS was 11%; all BRAFV600K patients progressed/died or were lost to follow-up before 3 years. Fifty-eight BRAFV600E patients (76%) and 13 BRAFV600K patients (81%) died, with 5-year OS rates of 20% and 13%, respectively (Fig. 2A). Across BRAF groups, 54 patients (59%) received subsequent therapies, including 22% who received checkpoint inhibitor immunotherapy (Table 1).
Fig. 1.
PFS in intention-to-treat patients in BREAK-2 (A) and BREAK-3 (B) and in patients with normal (C) and elevated (D) baseline LDH levels in BREAK-3. CI, confidence interval; LDH, lactate dehydrogenase; PFS, progression-free survival.
Fig. 2.
OS in intention-to-treat patients in BREAK-2 (A) and BREAK-3 (B). CI, confidence interval; ITT, intention-to-treat; OS, overall survival. a Thirty-seven patients (59%) receiving dacarbazine crossed over to receive dabrafenib; these patients were analysed for OS in the dacarbazine arm following ITT principle.
Table 1.
Postprogression systemic therapy.
| BREAK-2 | BREAK-3 | ||
|---|---|---|---|
| Postprogression Therapy, n (%) | Total (N=92) | Dabrafenib (n=187) | Dacarbazine (n=63) |
| Any subsequent anticancer therapya | 54 (59) | 129 (69) | 52 (83) |
| Chemotherapy | 22 (24) | 55 (29) | 18 (45) |
| Small-molecule–targeted therapy | 31 (34) | 30 (16) | 47 (75)b |
| Vemurafenib | 20 (22) | 12 (6) | 13 (21) |
| Dabrafenib | 10 (11) | 9 (5) | 39 (62) |
| Cobimetinib | 2 (2) | 1 (<1) | 0 |
| Checkpoint inhibitor immunotherapy | 20 (22) | 56 (30) | 15 (24) |
| Anti–CTLA-4 | 18 (20) | 45 (24) | 15 (24) |
| Anti–PD-1 | 5 (5) | 15 (8) | 1 (2) |
| Surgery | 16 (17) | 37 (20) | 15 (24) |
| Biologic therapy | 5 (5) | 20 (11) | 4 (6) |
| Other | 2 (2)c | 1 (1)d | 0 |
| Hormonal therapy | 0 | 1 (1) | 0 |
| Radiotherapy | 0 | 67 (36) | 27 (43) |
CTLA-4, cytotoxic T-lymphocyte–associated protein 4; PD-1, programmed cell death 1 protein.
Some patients received > 1 post-progression systemic therapy.
Crossover patients included.
Included axitinib (n = 1) and an unspecified investigational MEK inhibitor (n = 1).
Included lenvatinib mesylate (n = 1).
5-Year Efficacy Analysis: BREAK-3
Overall, 250 patients were randomised in BREAK-3. Baseline characteristics were balanced between patients in the dabrafenib arm (n=187) and those in the dacarbazine arm (n=63) (Supplementary Table 1; Supplementary Fig. 1B). Median patient follow-up was 17.0 months (IQR, 7.6–39.0 months) and 11.8 months (IQR, 5.7–24.5 months) in the dabrafenib and dacarbazine arms, respectively, with ≥60 months of follow-up from the time of randomisation to data cutoff. Thirty-seven dacarbazine-arm patients (59%) crossed over to receive dabrafenib (median time to crossover, 3.1 months [IQR, 1.9–5.3 months]; median time from treatment discontinuation to crossover, 1.0 month [IQR, 1.0–1.2 months]), with continued follow-up.
PFS events were observed in 146 dabrafenib-arm (78%) and 54 dacarbazine-arm (86%) patients. Five-year PFS was 12% in the dabrafenib arm (95% CI, 7%–18%; 6 patients at risk); in the dacarbazine arm, all patients progressed/died or were lost to follow-up before 4 years (Fig. 1B). PFS events occurred in 31 crossover patients (84%); all except 1 progressed/died or were lost to follow-up before 20 months. Median PFS was consistently longer in dabrafenib-arm vs dacarbazine-arm patients, regardless of baseline LDH levels. In the dabrafenib arm, 5-year PFS was 16% in patients with normal LDH and 4% in those with elevated LDH (Fig. 1C and 1D), whereas dacarbazine-arm patients in these subgroups progressed/died or were lost to follow-up before 4 and 3 years, respectively.
A total of 132 dabrafenib-arm (71%) and 43 dacarbazine-arm (68%) patients died, with 5-year OS rates of 24% and 22%, respectively (Fig. 2B). The result in the dacarbazine arm was likely confounded by patients who crossed over to receive dabrafenib. Of 8 patients in the dacarbazine arm alive at 5 years, 4 (50%) were patients who crossed over to receive dabrafenib. Following progression, 129 dabrafenib-arm (69%) and 52 dacarbazine-arm (83%) patients (including 37 of whom crossed over to receive dabrafenib) received subsequent anticancer therapies. Of these, a smaller proportion of patients in the dabrafenib arm received postprogression small molecule–targeted therapy compared with those in the dacarbazine arm; similar proportions received subsequent immunotherapy (Table 1).
Dabrafenib Safety Profile With Extended Follow-Up
The frequency and severity of common adverse events (AEs) in patients treated with dabrafenib were consistent across BREAK-2 and BREAK-3 (Supplementary Table 2), and no new safety signals were observed with extended follow-up. Approximately one-third of patients who received dabrafenib in each study experienced serious AEs (BREAK-2, 36%; BREAK-3, 34%). AEs led to permanent discontinuation in 5 patients (5%) in BREAK-2 and 13 (7%) in BREAK-3. No fatal AEs were observed with dabrafenib in BREAK-2; in BREAK-3, 2 deaths in the dabrafenib arm were attributed to serious AEs possibly related to study treatment.
DISCUSSION
These 5-year landmark analyses of BREAK-2 and BREAK-3 provide extended follow-up for BRAFis in MM. Across studies, 11% to 12% of patients who received first-line single-agent dabrafenib remained progression free at 5 years, with apparent plateaus for PFS and OS after 36 months. These findings are in contrast to previous views that almost all patients treated with BRAFis experience rapid deterioration related to development of secondary resistance [12]. Results from BREAK-3 indicate that previously identified predictors of outcomes with combination targeted therapy in MM (eg, LDH) [5,8] similarly impact the benefit achievable in patients treated with dabrafenib monotherapy (5-year PFS: normal LDH, 16%; elevated LDH, 4%). Thus, patients with favourable baseline clinical features such as normal LDH levels are more likely to derive long-term benefit from dabrafenib therapy. In a 5-year analysis of patients treated with dabrafenib plus trametinib, 5-year PFS and OS rates were 19% and 34%, respectively [13]. Similarly, favourable baseline characteristics were associated with long-term benefit.
The safety profile of dabrafenib with 5-year follow-up was similar to that reported in previous analyses, with no notable change in the frequency of key AEs [2,3]. Long-term treatment with dabrafenib appears to be well tolerated in patients benefiting from therapy, supporting the use of dabrafenib monotherapy in patients unable to tolerate combination therapy with dabrafenib plus trametinib.
The 95% CIs for 5-year PFS and OS rates were wide, and novel subsequent therapies may have contributed to survival outcomes; however, most patients (70%−78%) did not receive subsequent checkpoint inhibitor therapy. Postprogression treatment with BRAFis was allowed and may support long-term PFS and OS outcomes. Nevertheless, these results provide a robust long-term follow-up data set for BRAFi monotherapy.
Supplementary Material
Progression-free and overall survival with dabrafenib may plateau after 36 months
Dabrafenib demonstrates benefit ≥5 years in some patients with BRAF-mutant melanoma
Long-term treatment with dabrafenib monotherapy is well tolerated
ACKNOWLEDGEMENTS
Medical writing and editorial assistance was provided by William Fazzone, PhD (ArticulateScience LLC), and funded by Novartis Pharmaceuticals Corporation.
FUNDING
These studies were supported by GlaxoSmithKline; dabrafenib is an asset of Novartis Pharma AG as of March 2, 2015.
The sponsors, in collaboration with all authors, participated in the design and conduct of the study, as well as collection, management, analysis, and interpretation of the data. All authors participated in the preparation, review, or approval of the manuscript and were responsible for the decision to submit the manuscript for publication.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at [doi TBC by the journal].
Conflict of interest statement
Dr Hauschild reported receiving clinical trial support (grant to institution) from Amgen, Bristol-Myers Squibb, Merck Serono, Merck Sharp & Dohme/Merck, Philogen, Pierre Fabre, Provectus, Regeneron, Roche, Sanofi-Genzyme, and Novartis Pharma; personal fees for speaker honoraria from Amgen, Bristol-Myers Squibb, Merck Sharp & Dohme/Merck, Pierre Fabre, Provectus, Regeneron, Roche, Sanofi-Genzyme, and Novartis Pharma; and personal fees for consultancy from Amgen, Bristol-Myers Squibb, Merck Serono, Merck Sharp & Dohme/Merck, Philogen, Pierre Fabre, Provectus, Regeneron, Roche, OncoSec, Sanofi Genzyme, Sun Pharma, and Novartis Pharma. Dr Ascierto reported receiving consulting fees from Bristol-Myers Squibb, Roche/Genentech, Merck Sharp & Dohme, Novartis, Array BioPharma, Merck Serono, Pierre Fabre, NewLink Genetics, Genmab, Incyte, MedImmune, AstraZeneca, Syndax, Sun Pharma, Sanofi, Idera, Ultimovacs, Sandoz, and Immunocore; travel, accommodations, and expenses from Merck Sharp & Dohme; and research funding from Bristol-Myers Squibb, Roche/Genentech, and Array BioPharma. Dr Schadendorf reported receiving consulting or advisory role fees from Roche/Genentech, Novartis, Bristol-Myers Squibb, Merck Sharp & Dohme, Merck Serono, Amgen, Immunocore, Incyte, 4SC, Pierre Fabre, Mologen, and Sanofi/Regeneron; honoraria from Roche/Genentech, Novartis, Bristol-Myers Squibb, Merck Sharp & Dohme, Merck Serono, Amgen, Immunocore, Incyte, 4SC, Pierre Fabre, Sysmex, Grünenthal Group, Agenus, Array BioPharma, AstraZeneca, LEO Pharma, Pfizer, Philogen, Regeneron, and Mologen; travel, accommodations, and expenses from Roche/Genentech, Novartis, Bristol-Myers Squibb, Merck Serono, Amgen, and Merck; speakers bureau fees from Novartis, Bristol-Myers Squibb, Merck Sharp & Dohme, Amgen, Incyte, Pierre Fabre, and Roche; and research funding from Novartis and Bristol-Myers Squibb. Dr Grob reported receiving honoraria from Roche, Bristol-Myers Squibb, Novartis, Merck Sharp & Dohme, Amgen, Pierre Fabre, Sanofi, Merck, and Pfizer; consulting or advisory role fees from Roche, Bristol-Myers Squibb, Novartis, Merck Sharp & Dohme, Amgen, Pierre Fabre, Sanofi, Merck, and Pfizer; travel, accommodations, and expenses from Bristol-Myers Squibb, Novartis, Merck Sharp & Dohme, and Pierre Fabre; and speakers bureau fees from Novartis. Dr Ribas reported receiving consulting or advisory role fees from Merck, Amgen, Novartis, Sanofi, and Chugai Pharma and stock or other ownership interests in Compugen, FLX Bio, CytomX Therapeutics, Five Prime Therapeutics, Advaxis, Arcus Biosciences, Tango Therapeutics, PACT Pharma, Merus, Rgenix, ImaginAb, and Lutris. Dr Kiecker reported receiving consulting fees and honoraria from Novartis. Dr Dutriaux reported receiving consulting or advisory role fees from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, and Roche. Dr Lebbé reported receiving honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Amgen, Roche, Pierre Fabre, Pfizer, and Incyte; consulting fees from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Amgen, and Roche; speakers bureau fees from Bristol-Myers Squibb, Novartis, Amgen, and Roche; travel accommodations for meetings from Bristol-Myers Squibb and Merck Sharp & Dohme; advisory role fees from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, and Roche; and advisory board honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Amgen, Roche, and Avantis Medical Systems. Dr Rutkowski reported receiving honoraria for lectures from Novartis, Bristol-Myers Squibb, Roche, Merck Sharp & Dohme, GlaxoSmithKline, Amgen, Pfizer, and Pierre Fabre; and advisory board honoraria from Novartis, Bristol-Myers Squibb, Roche, Merck Sharp & Dohme, GlaxoSmithKline, Amgen, and Pierre Fabre. Dr Blank reported receiving research support from Bristol-Myers Squibb and Novartis and consulting fees from Bristol-Myers Squibb, Merck Sharp & Dohme, Roche, Novartis, Lilly, Pfizer, and GlaxoSmithKline. Dr Gutzmer reported receiving honoraria for lectures from Roche, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Pfizer, Amgen, Merck Serono, Almirall Hermal, SUN Pharma, Sanofi, and Pierre Fabre; honoraria for advice from Bristol-Myers Squibb, Roche, Novartis, Almirall Hermal, Merck Sharp & Dohme, Amgen, Incyte, 4SC, SUN Pharma, Sanofi, Merck-Serono, and Pierre Fabre; research funding from Novartis, Pfizer, Johnson & Johnson, Amgen, and Merck Serono; and travel/meeting support from Roche, Bristol-Myers Squibb, Pierre Fabre, and Merck Serono. Dr Millward reported receiving advisory board honoraria from Bristol-Myers Squibb, AstraZeneca, Merck Sharp & Dohme, Provectus Biopharmaceuticals, Roche, Amgen, Pfizer, and Novartis; travel/conference support from Roche, Boehringer Ingelheim, AstraZeneca, Merck Sharp & Dohme, and Bristol-Myers Squibb; and honoraria from Bristol-Myers Squibb, AstraZeneca, Merck Sharp & Dohme, Roche, and Novartis. Dr Kefford reported receiving consulting or advisory role fees (to institution) from Amgen and Teva and travel, accommodations, and expenses from Bristol-Myers Squibb and Amgen. Dr Haas reported employment, travel, accommodations, expenses, and stock and other ownership interests from Novartis. Dr D’Amelio Jr reported employment and stock options from Novartis and stock options from GlaxoSmithKline. Dr Mookerjee reported employment and stock options from Novartis and stock options from GlaxoSmithKline and AstraZeneca. Dr Chapman reported receiving consulting, advisory, or speaking compensation from Immunocore, Merck, Cell Medica, Takeda Millennium, and AstraZeneca; research funding from Pfizer; and stocks from Rgenix. All authors received support for third-party medical writing and editorial assistance provided by ArticulateScience LLC, funded by Novartis Pharmaceuticals Corporation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Ascierto PA, McArthur GA, Dréno B, Atkinson V, Liszkay G, Di Giacomo AM, et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol 2016;17(9):1248–60. [DOI] [PubMed] [Google Scholar]
- [2].Ascierto PA, Minor DR, Ribas A, Lebbe C, O’Hagan A, Swann RS, et al. Long-term safety and overall survival update for BREAK-2, a phase 2, single-arm, open-label study of dabrafenib in previously treated metastatic melanoma (NCT01153763). J Clin Oncol 2014;32(15 Suppl). Abstract 9034. [Google Scholar]
- [3].Grob JJ, Demidov LV, Jouary T, et al. Landmark analysis of 3-year overall survival and follow-on therapies in BREAK-3, a phase 3, randomized trial: dabrafenib vs dacarbazine in patients with BRAF V600E mutation-positive metastatic melanoma. Presented at the Society for Melanoma Research 11th International Congress; 13–17 November 2014; Zurich, Switzerland. [Google Scholar]
- [4].Long GV, Eroglu Z, Infante J, Patel S, Daud A, Johnson DB, et al. Long-term outcomes in patients with BRAF V600-mutant metastatic melanoma who received dabrafenib combined with trametinib. J Clin Oncol 2018;36(7):667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Long GV, Weber JS, Infante JR, Kim KB, Daud A, Gonzalez R, et al. Overall survival and durable responses in patients with BRAF V600–mutant metastatic melanoma receiving dabrafenib combined with trametinib. J Clin Oncol 2016;34(8):871–8. [DOI] [PubMed] [Google Scholar]
- [6].McArthur GA, Chapman PB, Robert C, Larkin J, Haanen JB, Dummer R, et al. Safety and efficacy of vemurafenib in BRAFV600E and BRAFV600K mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 2014;15(3):323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372(4):320–30. [DOI] [PubMed] [Google Scholar]
- [8].Schadendorf D, Long GV, Stroiakovski D, Karaszewska B, Hauschild A, Levchenko E, et al. Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur J Cancer 2017;82:45–55. [DOI] [PubMed] [Google Scholar]
- [9].Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377(14):1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ascierto PA, Minor D, Ribas A, Lebbe C, O’Hagan A, Arya N, et al. Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol 2013;31(26):3205–11. [DOI] [PubMed] [Google Scholar]
- [11].Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380(9839):358–65. [DOI] [PubMed] [Google Scholar]
- [12].Menzies AM, Long GV. Systemic treatment for BRAF-mutant melanoma: where do we go next? Lancet Oncol 2014;15(9):e371–81. [DOI] [PubMed] [Google Scholar]
- [13].Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 2019;381(7):626–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.