Abstract
Diet is a modifiable factor that ensures optimal growth, biochemical performance, improved mood and mental functioning. Lack of nutrients, notably vitamin B, has an impact on human health and wellbeing. The United Arab Emirates is facing a serious problem of micronutrient deficiencies because of the growing trend for bariatric surgery, including Roux-en-Y gastric bypass and sleeve gastrectomy. People undergoing bariatric surgery are at high risk of developing neurological, cognitive, and mental disabilities and cardiovascular disease due to deficiency in vitamin B. Vitamin B is involved in neurotransmitter synthesis, including γ-aminobutyric acid, serotonin, dopamine, and noradrenaline. Deficiency of vitamin B increases the risk of depression, anxiety, dementia and Alzheimer’s disease. In addition, vitamin B deficiency can disrupt the methylation of homocysteine, leading to hyperhomocysteinemia. Elevated homocysteine levels are detrimental to human health. Vitamin B deficiency also suppresses immune function, increases the production of pro-inflammatory cytokines and upregulates NF-κB. Considering the important functions of vitamin B and the severe consequences associated with its deficiency following bariatric surgery, proper dietary intervention and administration of adequate supplements should be considered to prevent negative clinical outcomes.
Keywords: vitamin B, serotonin, dopamine, homocysteine, bariatric surgery, pro-inflammatory cytokines
1. Introduction
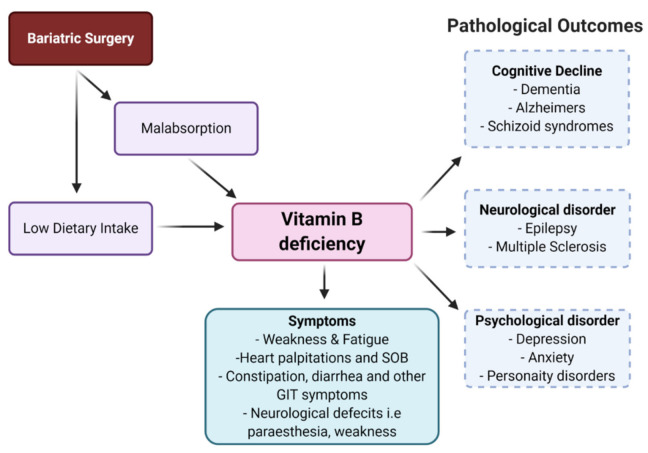
Over the past three decades, the world has undergone substantial demographic, economic, political, socio-cultural, and environmental changes that are affecting diet, nutrition, and health more broadly. Due to these nutritional transitions, undernutrition coexisting with overnutrition is widely prevalent in many parts of the world [1], with estimates that micronutrient deficiencies affect more than 2 billion people globally [2]. Additionally, there is an increase in worldwide obesity due to changes in eating pattern and lifestyle. To prevent and manage obesity, bariatric surgery is often recommended to sustain weight loss [3]. Whilst helpful, bariatric surgery has some side effects, including decreased absorption of various essential nutrients such as B complex vitamins, vitamins A, D, K, iron, selenium, zinc, and copper [4,5]. There are three major types of bariatric surgery: (1) laparoscopic sleeve gastrectomy (LSG); (2) laparoscopic adjustable gastric banding; and (3) Roux-en-Y gastric bypass (RYGB). RYGB alters the gastrointestinal tract, bypassing the duodenum and jejunum, reducing nutrient absorption and metabolism [5,6]. Given that the duodenum, jejunum, and ileum are involved in vitamin B absorption, bariatric surgery could induce intestinal malabsorption of the vitamin B complex (Table 1) [4,7]. Vitamin B complex is a group of eight related vitamin Bs (vitamin B1,2,3,5,6,7,9,12), and deficiency in any of these is associated with a wide range of disorders. The B complex vitamins are integral to the synthesis of neurotransmitters and proper functioning of the central nervous system, and play a key role in the methylation and decarboxylation reactions necessary for the integrity and synthesis of DNA, proteins, phospholipids, monoamine, and catecholamine neurotransmitters [8,9,10]. Further, vitamin B regulates immune response by decreasing the production of pro-inflammatory cytokines, NF-κB, and nerve growth factor [11]. In addition, B vitamins, particularly folic acid (B9), pyridoxine (B6), and cobalamin (B12), are involved in the re-methylation and metabolism of homocysteine. High homocysteine levels contribute to neurodegenerative disorders, psychiatric disorders, and cardiovascular disease [12]. It has been shown that vitamin B deficiency leads to declines in cognitive function and causes several other mental disorders such as depression, anxiety, dementia, and Alzheimer’s disease (Figure 1) [9,11,13,14,15,16].
Figure 1.
Symptoms and outcomes of vitamin B deficiency.
This narrative review aims to explore the nutritional deficiencies of vitamin B following bariatric surgery and its clinical outcomes, such as mental and cognitive problems. This paper also identifies critical strategies for managing and preventing vitamin B deficiency in bariatric surgery patients.
2. Methodology
Literature searches were conducted in ‘PubMed’ and ‘Google Scholar’ databases. Search terms included ‘bariatric surgery’ OR ‘gastric banding’ OR ‘laparoscopic sleeve gastrectomy’ OR ‘Roux-en-Y gastric bypass’ AND ‘micronutrients deficiency’ OR ‘vitamin B deficiency’ OR ‘vitamin B complex’ OR ‘vitamin B1’ OR ‘thiamine’ OR ‘vitamin B2’ OR ‘riboflavin’ OR ‘vitamin B3’ OR ‘niacin’ OR ‘vitamin B5’ OR ‘pantothenic acid’ OR ‘vitamin B6’ OR ‘pyridoxine’ OR ‘vitamin B9’ OR ‘folic acid’ OR ‘folate’ OR ‘vitamin B12’ OR ‘cobalamin’ AND ‘psychological disorder’ OR ‘depression’ OR ‘anxiety’ OR ‘bipolar’ AND ‘cognitive disorder’ OR ‘Alzheimer’ OR ‘dementia’ AND ‘neurological disorders’ OR ‘Wernicke encephalopathy’ OR ‘peripheral neuropathy’, OR ‘hyperhomocysteinemia’, with filters identifying studies published between 2000 to 2021. Irrelevant studies were excluded after examination of the title and the abstract. A total of 133 relevant studies, mainly clinical trials on bariatric surgery, have been included.
3. Role of Vitamin B in Human Health and the Immune System
Vitamin B1 (thiamine) acts as a coenzyme in the pentose phosphate pathway, which is essential for the production of fatty acids, steroids, nucleic acids, and aromatic amino acid precursors, neurotransmitters, and other bioactive compounds that are necessary for brain function [17]. Vitamin B1 deficiency causes overexpression of pro-inflammatory cytokines such as interleukin (IL)-1, IL-6, and tumor necrosis factor-alpha (TNF-α) as well as increased expression of CD40 and CD40 ligand by microglial cells and astrocytes, which eventually leads to the death of neuron cells [18,19]. Vitamin B2 (riboflavin) is derived from two flavoprotein coenzymes: flavin adenine mononucleotide and flavin adenine dinucleotide, which are important rate-limiting factors in cellular enzymatic processes [20]. Interestingly, the flavoproteins (a derivative of riboflavin) are known cofactors in the metabolism of essential fatty acids of brain lipids [21], as well as being a neuroactive compound with immunomodulatory effects. Additionally, B2 deficiency leads to pro-inflammatory patterns of gene expression [22] and leads to negative consequences for brain function. Further, Vitamin B3 (niacin) is derived from nucleotides such as nicotinamide adenine dinucleotide and nicotinamide adenine dinucleotide phosphate, which are involved in a number of body processes and enzymatic reactions [21]. Dietary niacin is primarily absorbed in the small intestine; however, a small amount can also be absorbed in the stomach [23]. Niacin is involved in the DNA metabolism and repair, cellular signaling events, cell migration [24], and decreases the expression of the pro-inflammatory cytokines, IL-1, IL-6, and TNF-α by the macrophages [25]. Low concentrations of niacin impair nicotinamide adenine dinucleotide-dependent nuclear and mitochondrial functioning, resulting in age-associated neurological disorders [26,27]. Vitamin B5 (pantothenic acid) is incorporated into coenzyme A that is central to a number of vital metabolic processes. Coenzyme A is needed for acetylation, an important part of a number of physiological chemical reactions, most notably in the metabolism of energy [28].
Vitamin B6 (pyridoxine) is a cofactor involved in carbohydrate, fat, and amino acid metabolism [29]. The phosphorylated form of B6 (pyridoxal phosphate) is first hydrolyzed, then absorbed and transported through carrier-mediated sodium dependent transporters [30]. B6 deficiency influences both the innate and adaptive immune systems, reducing the number, activity and proliferation of immune cells, impairing the growth and maturation of lymphocytes, affecting the production of antibodies by B cells, and reducing the size of the thymus gland [22]. Vitamin B9 (folate) is involved in the biosynthesis of nucleic acids, protein, blood cells, and the nervous system tissues [31], while B12 (cobalamin) is involved in DNA synthesis as well as fatty acid and amino acid metabolism [32]. Dietary vitamin B12 is bound to protein in food, and its absorption follows after stomach acid hydrolysis. It is then bound by the gastric R binder protein secreted in both saliva and gastric juices and passes into the duodenum. In the small intestine, detached from the R binder protein, the free B12 binds to intrinsic factor from the stomach’s parietal cells, allowing for absorption in the terminal ileum [33]. Folate and B12 deficiencies can cause T cell proliferation and influence the production of pro-inflammatory cytokines [34]. B12 enhances TNF-α and nerve growth factor secretion and reduces the levels of epidermal growth factor and IL-6 [35]. High levels of TNF-α and nerve growth factor can damage myelin and reduce epidermal growth factor, thus decreasing their myelinotrophic effects [36,37]. B12 deficiency can also adversely affect the methylation reaction, increasing inflammatory responses [38], and reducing CD8+ and natural killer cell activity [39]. The functions of vitamin B and its deficiency related outcomes are indicated in Table 1.
Table 1.
The functions of B vitamins, site of absorption, and deficiency related outcomes.
| B Vitamins | Functions | Absorption Site | Deficiency Related Outcomes |
|---|---|---|---|
| B1 (Thiamine) [9,22] | Acetylcholine production, action potential generation, structure and function of cellular membranes | Duodenum Jejunum |
Reduces enzymatic activity and energy production, alters mitochondrial activity |
| B2 (Riboflavin) [40] | Maintains the integrity of mucous membranes, skin, eyes, and the nervous system | Duodenum Jejunum |
Mitochondrial dysfunction, effects one-carbon metabolism |
| B3 (Niacin) [41,42] | Acts as an antioxidant, produces energy, protects against axonal damage, neuroprotective role | Duodenum Jejunum |
Increases oxidative stress and inflammatory cytokines, mitochondrial dysfunction |
| B5 (Pantothenic acid) [43] | Regulates iron by transporting oxygen to the brain, synthesizes neurotransmitters, helps in the synthesis and regeneration of myelin | Jejunum | Increased cell stress and translocation of NF-κB, altered fatty acid metabolism |
| B6 (Pyridoxine) [22,28] | Assists in the synthesis of hemoglobin, neurotransmitters, DNA methylation, and homocysteine metabolism | Jejunum | Altered tryptophan and one-carbon metabolism |
| B9 (Folate) [44] | Synthesizes norepinephrine, dopamine, and serotonin. Involved in methylation of homocysteine to methionine | Duodenum Jejunum Ileum |
Disrupts DNA methylation and alters nitric-oxide balance in the blood |
| B12 (Cobalamin) [45,46,47] | Synthesizes new cells, involved in nerve cells maintenance, assists in breaking fatty acids and amino acids | Ileum (terminal only) | Effects on DNA synthesis, adverse effects on brain function |
4. Bariatric Surgery and Vitamin B Deficiency
4.1. Bariatric Surgery
Nutritional interventions, medication, and exercise have limited effectiveness in weight loss. Therefore, obese people with body mass index (BMI) ≥ 40 kg/m2 have been advised to undergo bariatric surgery [48]. Bariatric surgery is a metabolic surgery associated with long-term weight loss and remission of weight-related comorbidities [49]. Some 634,897 surgical bariatric/metabolic interventions were performed worldwide in 2016 [50]. Laparoscopic sleeve gastrectomy (LSG), Roux-en-Y gastric bypass (RYGB), and gastric banding dominate the field [51]. Sleeve gastrectomy is a procedure in which 70–85% of the stomach is removed, resulting in a reduction in gastric reservoir size [51,52] and accelerated nutrient transit time, thus decreasing the absorption of nutrients [52]. In RYGB, a 30-milliliter pouch is created from the proximal stomach. The jejunum is divided, with one part attached to the artificially created pouch and the other to the duodenum to allow the pancreatic and biliary secretions to enter the intestine. The changes in the gut hormone affect eating behavior and appetite [53]. Gastric bypass affects hormones that control the body weight and eating behavior, such as ghrelin and glucagon-like peptide, while sleeve gastrectomy affects ghrelin hormone [53]. In gastric banding, a band is placed around the proximal stomach to create a small pouch to minimize the food intake without affecting the absorption [5]. Although these approaches have superior long-term weight loss results, patients are at high risk of vitamin B malabsorption following bariatric surgery (Table 2). Nevertheless, vitamin B deficiency could also exist in pre-operative stages of obesity. Therefore, vitamin B supplementation is crucial to prevent the deficiency in both pre- and post-operative stages. However, the composition of multivitamins is extremely variable. For example, vitamin B12 contained in multivitamin can vary from half of the recommended daily allocation (RDA) (1.2 µg/day) to 24 µg/day (10× the RDA). Furthermore, some multivitamins are designed specifically for bariatric surgery (with 250 µg–350 µg B12 per tablet, B1 (4.2 mg), B2 (4.8 mg), B6 (6 mg) [54]. Gasteyger et al. observed patients after two years of bariatric surgery, who were systematically taking multivitamins containing 2.4 µg of vitamin B12, that 80% of their patients had a deficit and had to be supplemented [54]. In the longer term, in a series of 75 patients followed for 83.4 ± 14.3 months (7 years) and not supplemented, 61.8% of patients had a low vitamin B12 level [54,55]. In another study, at 5 years, it was noted there was vitamin B12 deficiency in 70% of patients [55]. After restrictive surgery (gastric band), vitamin B12 deficiency is not uncommon and can affect 10% of patients [56], but is less harmful when patients take multivitamins. A case of vitamin B12 deficiency with neurological complications has been reported after gastroplasty [57].
Table 2.
Percentage of vitamin B deficiency in bariatric surgery.
| Number of Participants | Duration and Stage | Percentage (%) of Vitamin B Deficiency |
|---|---|---|
| 232 bariatric surgery participants [60] | Post-operative | Folate (3.4%), B12 (18.1%), B3 (5.6%), B6 (2.2%) |
| 169 RYGB patients [61] | Pre-operative, 1,2,3, years’ post-operative | Pre-operative B12 deficient (12.3%), Postoperative B12 after 1, 2, 3 years (19%, 28%, 29%) |
| 149 bariatric surgery participants [62] | Post-operative | B12 (11%) |
| 30 patients underwent laparoscopic RYGB [63] | 6-months preoperative and 3-year post-operative | B12 at 2 years (33.3%) and 3 years (27.2%). No folic acid deficiency |
| 98 participants underwent RYGB and LSG [64] | 1-year pre-operative and 1-year post-operative | B12 deficient one-year post-operative elevated from 6.4–25.5% in the RYGB group |
| 468 patients underwent RYGB and LSG [65] | Pre-operative and post-operative and after one year | Pre-operative B1 deficiency in LSG (8.1%) and RYGB (1.7%) Post-operative B1 deficient in LSG (10.5%) and RYGB (13.7%). One-year B1 deficient in LSG (7.2%) and in RYGB (5.9%). |
| 95 participants underwent RYGB and SG [66] | Post-operative | Low level of vitamin B12 in RYGB (42.1%) and LSG (5%). Folate deficiency in RYGB (20%) and LSG (18.4%). |
| 74 Gastric bypass participants [67] | >1 year | Folate (38%) |
| 253 RYGB and 142 SG participants [68] | 1–2 years post-operative | The serum concentration of vitamin B12 was significantly higher in the group who had undergone SG as compared to RYBG at 2 years |
| 37 patients with severe obesity undergoing bariatric surgery [69] | 3 months and 1 year post-operatively | During the year following operation, vitamin B6 level enhanced |
| 60 bariatric surgery patients (gastric bypass, duodenal switch) All patients received multivitamin, and gastric bypass patients received B12 substitute [70] | 6 months pre-operative, and 1 year post-operative | Duodenal switch patients showed thiamine deficiency after surgery. The level of riboflavin and vitamin B6 did not change after surgery |
| 1160 subject with RYGB, 883 received, and 258 did not receive, specialized multivitamin supplements [71] | 3 years post-operative | Participants who received specialized multivitamin supplements were less deficient in vitamin B12, vitamin D, folic acid, and ferritin as compared to other group receiving no supplements |
| 45 Bariatric patients treated with intramuscular hydroxocobalamin injections, while 45 did not receive [72] | Post-operative | The treated group reported significantly increased vitamin B12 and showed fewer clinical complaints |
| 1538 patients’ micronutrient status assessed prior to bariatric surgery [73] | Pre-operative | Vitamin B12 deficiency was 16%, and various other micronutrient deficiencies pre-exit High level of vitamin B6 by 24% found before surgery |
| 103 morbidly obese women before bariatric surgery [74] | Pre-operative | 10.6% of participants had B12 deficiency, No folic acid deficiency Deficiency of other micronutrients (iron, zinc, calcium, phosphorus) |
| 1732 patients with morbid obesity wishing to undergo bariatric surgery [75] | Pre-operative | 63.2% of participants had a folic acid deficiency and various other micronutrient deficiencies |
| 2008 morbid obese participants wanted bariatric surgery [76] | Pre-operative | Participants deficient in vitamin D, vitamin B12, iron, and hemoglobin by 53.6%, 34.4%, 10.2%, and 16.6%, respectively, prior to bariatric surgery |
| 114 patients assigned for bariatric surgery [77] | Pre-operative | Participants deficient in iron, folic acid, ferritin, vitamin B12, and calcium by 35%, 24%, 24%, 3.6%, and 0.9%, respectively, prior to bariatric surgery |
| 200 patients with SG [78] | Pre- and Post-operative | Participants deficient in B1, B6, B12, folic acid, vitamin D by 5.5%, 3%, 11.5%, 24%, and 81, respectively, prior to surgery and deficient after surgery in B1, B6, B12, and vitamin D by 9%, 4%, 11.5%, and 36%, respectively |
Abbreviations: LSG, Laparoscopic sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy.
In a comparative study between RYGB and sleeve gastrectomy (SG), the risk of vitamin B12 deficiency was 3.55 times higher after RYGB than after SG (95% CI, 1.26–10.01; p < 0.001) however, this difference disappeared when GB patients followed routine supplementation [56]. Six studies with a small number of subjects (between 9 and 60) evaluated the risk of vitamin B12 deficiency in between 0 and 19.6% of deficient patients, after a maximum follow-up of 36 months (reviewed in [58]). To date, no study with a follow-up of more than three years, with reliable data and a sufficient number of patients, is available to assess this long-term risk [59].
4.2. Mechanisms of Vitamin B Deficiency Following Bariatric Surgery
The degree to which bariatric surgery can cause vitamin B deficiencies depends mainly on the particular type of operation performed [52,53]. Therefore, assessing bariatric surgery patients’ nutritional consequences should be based on the surgical procedure type [53].
The gastrointestinal tract’s physiological and anatomical changes accompanying gastric bypass mainly result in vitamin B9 and B12 malabsorption [79]. Therefore, vitamin B12 deficiency is more commonly associated with the RYGB procedure [51,64,80], however, it is present after both procedures [81]. Evaluation of the long-term impact of RYGB on B12 status showed an increase in B12 deficiency from 2.3% at the baseline to 6.5% at 12 months following the surgery [82]. Likewise, B12 was significantly lower in the RYGB group compared to the LSG group [82,83]. B12 deficiency onsets rapidly, with changes in absorption present as little as two months after the surgery, with associated increases in homocysteine levels [81]. Absorption of B12 depends on the intrinsic factor, which is almost in the gastric bypass population [84]. 35% of RYGB patients in the Lakhani study experienced bacterial growth syndrome, an important factor that can lead to B12 deficiency [83]. Notably, approximately 12% of bariatric surgery candidates are already B12 deficient before their operation, likely worsening deficiency following the operation [61]. Oher factors that lead to B12 deficiency are intolerance of dietary meat intake, which is the primary source of vitamin B12, and a reduction of intrinsic factor in the terminal ileum, which is essential for B12 absorption [79]. A systematic review assessing the relationship between bariatric surgery and diet quality noted that those receiving gastric banding were more likely to experience gastrointestinal symptoms and food intolerances than sleeve gastrectomy and RYGB populations during the first year. Besides, the SG population had a better food intolerance than RYGB [85]. Additionally, the alteration in the stomach acid and pepsin enzyme secretion that accompanies gastric bypass interferes with cobalamin absorption [86].
Some gastric banding patients may experience recurrent vomiting due to the banding [62], and prolonged and aggressive vomiting occurring following bariatric surgery can lead to thiamine deficiency in this population [87,88,89]. Further, 35% to 65% of patients experience hyperemesis following the operation due to feelings of fullness or digestive tract plugging, which exacerbates B1 deficiency in bariatric surgery patients [53]. The sleeve gastrectomy patients are more likely to experience thiamin deficiency [51,64,80]. As opposed to the narrow zone of absorption of B12, folate absorption occurs along the whole length of the small intestine [80]; therefore, folate deficiency in this population was primarily attributed to non-adherence to supplementation rather than malabsorption. Patients who adhered to an 800 µg of folic acid daily did not experience folate deficiency [5]. Indeed, RYGB patients are more likely to experience vitamin B12, while SG and gastric banding patients are more likely to experience thiamine deficiency.
Non adherence to supplements contributes to worsening the micronutrient deficiency among bariatric surgery patients [90]. The deficiency of micronutrients is higher in non-adherence bariatric surgery patients than adherence bariatric surgery patients [91,92]. Bariatric surgery-related factors include vomiting, which is a common postoperative complication seen in 30% of SG patients [53], and the disturbed eating habit leads to difficulty in supplement adherence. Likewise, difficulty swallowing drugs and forgetting to take supplements are barriers to supplement adherence among bariatric surgery patients [93]. The high cost of particular multivitamins for bariatric surgery patients contributes to long-term non-adherence to the supplement [94].
4.3. Bariatric Surgery and Hyperhomocysteinemia
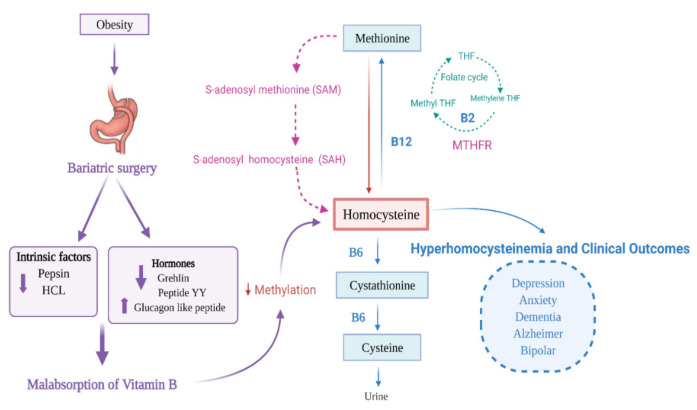
Bariatric patients with vitamin B deficiency are at risk of secondary neuropsychological disorders and CVD [91]. The malabsorption of vitamin B 9 and B12 following bariatric surgery affects the re-methylation pathway of homocysteine, leading to hyper-homocysteinemia [83]. Increased levels of homocysteine beyond 15 µmol/L are expected among bariatric surgery patients [86]. Elevated homocysteine level has been detected in 29% of bariatric surgery patients [62]. A high homocysteine level is found in the bypass group (10.4 µmol/L), compared to (9.2 µmol/L) in control [95]. Likewise, a study showed a higher homocysteine level in patients after the surgery (14.6 µmol/L) compared to (11.6 µmol/L) at the baseline values before the operation [79]. Multivitamin supplements lower the homocysteine level [95]. If methylation reactions are limited, it can lead to a range of problems such as anxiety, depression, bipolar disorder, Alzheimer’s disease (AD), schizophrenia, and sleep-cycle disturbance [96,97] (Figure 2).
Figure 2.
Bariatric surgery, hyper-homocysteinemia, and importance of vitamin B.
4.4. Depression and Anxiety in Patients Following Bariatric Surgery
Vitamins B has an essential role in synthesizing neurotransmitters and factors such as serotonin that affect mood and other brain functions. Vitamin B deficiencies, particularly B1, B6, B9, and B12, are known causes of psychiatric disorders, including depression and dementia [98]. Patients undergoing bariatric surgery commonly experience thiamin deficiency, crucial for Thiamin-dependent enzyme function [99]. Thiamin-dependent enzymes play a critical role in glucose metabolism, which is essential to ensure optimal brain function. Further, the brain is susceptible to thiamin availability, and it has been shown that glucose levels are diminished in 20–30% of brain regions in those living with Alzheimer’s [99].
Clinically, mental issues are common in severely obese adults seeking bariatric surgery [100,101,102,103,104,105], and depression is the most crucial issue in this population [102]. Depression exists in 45.2% of bariatric surgery candidates and 2.7% show severe depression [101]. In this study, the Beck score improved at six months compared with the baseline score, and the improvement continues during the first year. A longitudinal assessment of bariatric surgery, including 2148 patients, showed an improvement in depression symptoms during the first year following bariatric surgery; then it deteriorated during 1–3 years following bariatric surgery [100].
Furthermore, bariatric surgery procedure affects the hospitalization rate for depression. When assessing this comparing RYGB and LAGB, 1.2% of RYGB reported hospitalization for depression compared with 0% of LAGB. Further, at three years from surgery, the rate of RYGB admitted for depression increased to 2.1% compared with 0.6%. They attributed this deterioration of mental health to the BMI change [100]. Further, the severity of depression symptoms before surgery predicts the postoperative BDI score. A longitudinal assessment of bariatric surgery study revealed patients with moderate, severe depression symptoms at baseline had 7.8 higher odds of having a moderate-severe symptom of depression. In contrast, those with minimal symptoms had 6.77 higher odds of experiencing mild depression symptoms. 35.3% of bariatric surgery candidates reported taking at least one antidepressant medication, and serotonin reuptake inhibitors (SSRI) are the most common antidepressant [100]. Serotonin reuptake inhibitor level dropped in RYGB patients after one month, and 54% of patients relapsed after one month of the surgery [106]. The diminished intestinal surface that reduces the drug’s exposure to the absorption area as in RYGB negatively affects bariatric surgery patients’ drug disposition [106].
Likewise, after bariatric surgery, psychological outcomes showed an improvement in depression symptoms at 6, 12, 24 months post-operatively, and after 24 months, it returned to the baseline levels [107]. Further, bariatric surgery patients scored higher in healthy quality of life post-operatively and scored lower after the weight-stability phase [53]. In a case report, a 38-year-old male patient who had undergone bariatric surgery developed episodes of psychotic depression, which was attributed to vitamin B12 deficiency. Supplementing vitamin B12 caused remission of the patient’s clinical symptoms of depression [108]. Conversely, one hundred RYGB participants using the Beck Depression inventory reported a worsening mood in 3.7% of participants during the 6 to 12 months following the operation [109]. Given the well-known physiological consequences of vitamin B deficiency and its common presentation in those who have undergone bariatric surgery, it is a plausible mechanism underlying the onset of depression and anxiety in these patients.
4.5. Neurological Complications in Patients Following Bariatric Surgery
About, 4.6–16% of bariatric surgery patients experience postoperative neurological complications [87]. Thiamin deficiency is common following bariatric surgery [110] due to the aggressive vomiting attacks in this population [87], commonly seen in so called ‘bariatric beriberi’ [89]. Thiamine deficiency commonly manifests as neurological complications, including Wernicke encephalopathy (WE) [88,89]. A study of 100 cases of Wernicke Encephalopathy (WE) following bariatric surgery found that onset of the WE symptoms varied according the surgery type, and RYGB is associated more with neurological complication [89]. The most consistent factor among WE patients was persistent vomiting [89,111]. Bariatric surgery with high BMI seems to have less severe WE symptoms, explained by “preferential intracellular thiamin cycling”, suggesting rapid weight loss and depleted thiamin store, resulting in WE symptoms [89].
Peripheral neuropathy is a common neurological complication in this population [88,112]. The rapid weight loss accompanying bariatric surgery leads to neuropathy compression by exposing the nerve to subcutaneous tissue loss [87,113] Intravenous thiamin replacement therapy contributes to reverse neurological symptoms at early stages. 93.3% of bariatric surgery patients recovered fully from neurological complications [87]. The neurological disorder was reversed if they were diagnosed early [4]. A study assessing the neurological clinical manifestation noted that B1, B2, B6 and B12 deficiencies were common amongst RYGB and SG who showed neurological clinical manifestations including paresthesia muscle weakness and abnormal gait [4].
5. Effectiveness of Vitamin B Supplementation in Bariatric Surgery Patients
Vitamin B supplements are a key means of meeting the body’s vitamin B needs in individuals following bariatric surgery [4,83]. Currently, the B12 doses in over-the-counter multivitamin formulations are insufficient to meet post-operative patient needs [5,83,114], and as such tailored doses and administration of B supplementation are crucial considerations for these patients [5,71,83,114]. The dose of B supplementation depends predominantly on the surgery type [83]. High doses of oral cyanocobalamin are ideal for RYGB patients, while lower doses of vitamin B12 should be enough for those receiving LSG and gastric banding [80,83]. The recommended dose of B12 by the British Obesity and Metabolic Surgery Society is inadequate in RYGB due to the high prevalence of B vitamin deficiencies in this group [83]. Therefore, they require a higher dose than the RDA to meet the increased demand for vitamin B12. Vitamin B12 (350 μg/day) should be the minimum dose after gastric bypass and can be administered orally or parentally [5]. 1000 μg, and 2000 μg doses of vitamin B12 have been suggested for optimal absorptive capacity [71].
Oral, intramuscular, intranasal, intravenous, and enteral parental are the suggested routes to administrate vitamin B. A systematic review of randomized controlled trials assessed the efficacy of oral B12 and intramuscular B12 injection. A high serum level of B12 was observed in an oral group when given in high doses compared with the intramuscular group at 2 and 4 months follow up [115]. Likewise, a systematic review showed a B12 of dose <15 µg is insufficient to correct B12 serum level in RYGB patients, and 10,000 µg showed superior results and increased B12 serum level hence, preventing B12 deficiency in this cohort [114]. A meta-analysis of studies comparing oral and parenteral routes showed in 108 gastrectomized or achlorhydric patients deficient in vitamin B12 that the oral route was as effective, or even faster, than the intramuscular route on the condition of 1000 to 2000 µg/day of vitamin B12 during the first weeks, then weekly and monthly [116]. Pharmacokinetic studies show that approximately 1% of a dose >25 µg of crystalline form is absorbed passively, or 10 µg for a dose of 1000 µg [117], yet the RDA for this vitamin is 2.4 µg/j. Further, the crystalline form of B12 showed its efficacy for absorption in the absence of intrinsic factor when given in high doses [118]. In contrast, a study showed that an oral supplement is not enough to correct the serum level of B12 in gastric bypass patients [119]. The British Obesity and Metabolic society recommend intramuscular injection of vitamin B12 should be taken every three weeks in RYGB and BPD/DS patients, since the deficiency of B12 still exists in the presence of higher doses of the oral supplement [120]. The intramuscular route is used for those who have severe B12 deficiency symptoms, have gastrointestinal intolerance, and are not compliant with their oral supplementation, or when oral supplementation does not maintain B12 level. Intravenous B12 is associated with anaphylactic shock, while nasal and sublingual routes are under evaluation for their efficiency [83].
Following GB, the doses to be prescribed are not clearly defined. In some studies, a dose of 350 µg/day is adequate to maintain plasma levels [118]. A dose of 1000 µg/week seems sufficient, a fraction of this contribution being able to be absorbed by the intrinsic factor [116,118,121]. This dose must sometimes be doubled and recourse to the intramuscular route should be preferred only when patients are not very observant. This strategy has been validated in randomized study [122], but most of the studies recommend supplements through the oral route [123,124,125,126,127,128,129].
6. Strategies to Prevent Vitamin B Deficiency in Bariatric Surgery Patients
Given B deficiency is caused by the anatomical changes accompanying bariatric surgery, vitamin B status provision is imperative. Managing vitamin B status among BS patients has been divided into pre-operative, postoperative phase (<5 days), and postoperative phase (>5 day) [116].
Vitamin B deficiency could exist in the pre-operative stage; 20–30% of BS candidates have micronutrient deficiencies before surgery [88]. Early detection is important to identify vitamin B deficiency. Therefore, vitamin B supplementation is very important to prevent the deficiency in both pre- and post-operative stage. The pre-operative period is critical to emphasize the importance of vitamin adherence [83,130], and inform the patients about the side effects of vitamin B deficiency. Assessing B12 status in bariatric surgery patients requires a reliable test that reflects the B12 status. Homocysteine and methylmalonic acid (MMA) are the two reliable tests that reflect B12 status [131]. Since B12 deficiency results in MMA accumulation before B12 reduction is seen in the serum [132], methylmalonic acid is a better indicator and preferred marker reflecting B12 status before B12 deficiency appears [120].
Patients may experience nausea, vomiting, and dumping syndrome; therefore, administrating, and replenishing BS patients with vitamin B supplementation immediately after the operation is crucial to avoid thiamin deficiency [109]. As some deficiencies (particularly vitamin B12) may take years to present clinical symptoms, the long-term management of vitamin B deficiency is crucial. Constant follow-up and lifelong mineral and multivitamin supplementation are recommended [5,53,133].
7. Conclusions
There is a strong relationship between nutritional deficiencies and disease. Patients who receive bariatric surgery are at high risk of developing mental, cognitive, and neurological complications resulting from micronutrient deficiencies. Recognition of the clinical presentations of vitamin B deficiency is vital, enabling early intervention and minimizing long-term adverse effects. A primary clinical concern that needs to be addressed is the relationship between vitamin B deficiency and the development of depression, anxiety, and other neurological complications. Vitamin B supplements lessen the impact of these conditions and quality of life. Further studies are needed to determine optimal vitamin B supplements in patients following bariatric surgery to minimize adverse clinical outcomes. Providing awareness regarding healthy eating habits and lifestyle changes to reduce obesity are needed. There is also a need for ongoing monitoring of these patients to avoid bariatric surgery’s unwanted side effects. Early dietary and lifestyle intervention should be implemented to reduce obesity and avoid post-operative deficiency and its associated side-effects. This will lead to a decrease in the growing prevalence of vitamin B deficiency while improving patients’ outcomes post-bariatric surgery.
Acknowledgments
H.S., A.A.M., H.I.A., L.S., and A.S.A.D. would like to acknowledge the Department of Nutrition and Health, United Arab Emirates University for their ongoing support. L.C.I. thanks College of Health Sciences and RIMHS University of Sharjah for their support. J.F. would like to acknowledge the Australian Government for the support for an RTP training scholarship and University of Melbourne PhD Stipend. J.F. and V.A. would like to thank the Immunology and Translational Research Group within the Institute for Health and Sport, Victoria University Australia for their support.
Author Contributions
Conceptualization, L.S., A.A.M., H.S.; writing—original draft preparation, A.A.M., H.S., J.F.; writing—review and editing, L.S., V.A., H.I.A., A.S.A.D., L.C.I., M.B.; visualization, H.S., A.A.M., J.F.; supervision, L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Min J., Zhao Y., Slivka L., Wang Y. Double burden of diseases worldwide: Coexistence of undernutrition and overnutrition-related non-communicable chronic diseases. Obes. Rev. 2018;19:49–61. doi: 10.1111/obr.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center for Disease Control and Prevention . Micronutrient Malnutrition: Reducing Nutritional Deficiencies Globally. National Center for Chronic Disease Prevention and Health Promotion; Atlanta, GA, USA: 2016. [Google Scholar]
- 3.Aminian A., Zelisko A., Kirwan J.P., Brethauer S.A., Schauer P.R. Exploring the impact of bariatric surgery on high density lipoprotein. Surg. Obes. Relat. Dis. 2015;11:238–247. doi: 10.1016/j.soard.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Punchai S., Hanipah Z.N., Meister K.M., Schauer P.R., Brethauer S.A., Aminian A. Neurologic manifestations of vitamin B deficiency after bariatric surgery. Obes. Surg. 2017;27:2079–2082. doi: 10.1007/s11695-017-2607-8. [DOI] [PubMed] [Google Scholar]
- 5.Shankar P., Boylan M., Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition. 2010;26:1031–1037. doi: 10.1016/j.nut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Parkes E. Nutritional management of patients after bariatric surgery. Am. J. Med. Sci. 2006;331:207–213. doi: 10.1097/00000441-200604000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Szmulewicz A., Wanis K.N., Gripper A., Angriman F., Hawel J., Elnahas A., Alkhamesi N.A., Schlachta C.M. Mental health quality of life after bariatric surgery: A systematic review and meta-analysis of randomized clinical trials. Clin. Obes. 2019;9:e12290. doi: 10.1111/cob.12290. [DOI] [PubMed] [Google Scholar]
- 8.Mattson M.P., Shea T.B. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003;26:137–146. doi: 10.1016/S0166-2236(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 9.Mikkelsen K., Stojanovska L., Prakash M., Apostolopoulos V. The effects of vitamin B on the immune/cytokine network and their involvement in depression. Maturitas. 2017;96:58–71. doi: 10.1016/j.maturitas.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Hughes C.F., Ward M., Tracey F., Hoey L., Molloy A.M., Pentieva K., McNulty H. B-vitamin intake and biomarker status in relation to cognitive decline in healthy older adults in a 4-year follow-up study. Nutrients. 2017;9:53. doi: 10.3390/nu9010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duthie S.J., Whalley L.J., Collins A.R., Leaper S., Berger K., Deary I.J. Homocysteine, B vitamin status, and cognitive function in the elderly. Am. J. Clin. Nutr. 2002;75:908–913. doi: 10.1093/ajcn/75.5.908. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy D.O., Veasey R., Watson A., Dodd F., Jones E., Maggini S., Haskell C.F. Effects of high-dose B vitamin complex with vitamin C and minerals on subjective mood and performance in healthy males. Psychopharmacology. 2010;211:55–68. doi: 10.1007/s00213-010-1870-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis J.E., Tiozzo E., Melillo A.B., Leonard S., Chen L., Mendez A., Woolger J.M., Konefal J. The effect of methylated vitamin B complex on depressive and anxiety symptoms and quality of life in adults with depression. Int. Sch. Res. Not. 2013;2013:1–7. doi: 10.1155/2013/621453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker J.G., Mackinnon A.J., Batterham P., Jorm A.F., Hickie I., McCarthy A., Fenech M., Christensen H. Mental health literacy, folic acid and vitamin B 12, and physical activity for the prevention of depression in older adults: Randomised controlled trial. Br. J. Psychiatry. 2010;197:45–54. doi: 10.1192/bjp.bp.109.075291. [DOI] [PubMed] [Google Scholar]
- 15.Nijst T.Q., Wevers R.A., Schoonderwaldt H.C., Hommes O.R., De Haan A.F. Vitamin B12 and folate concentrations in serum and cerebrospinal fluid of neurological patients with special reference to multiple sclerosis and dementia. J. Neurol. Neurosurg. Psychiatry. 1990;53:951–954. doi: 10.1136/jnnp.53.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds E.H. Schizophrenia-like psychoses of epilepsy and disturbances of folate and vitamin B 12 metabolism induced by anticonvulsant drugs. Br. J. Psychiatry. 1967;113:911–919. doi: 10.1192/bjp.113.501.911. [DOI] [PubMed] [Google Scholar]
- 17.Kerns J.C., Arundel C., Chawla L.S. Thiamin deficiency in people with obesity. Adv. Nutr. 2015;6:147–153. doi: 10.3945/an.114.007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scalabrino G. Vitamin-regulated cytokines and growth factors in the CNS and elsewhere. J. Neurochem. 2009;111:1309–1326. doi: 10.1111/j.1471-4159.2009.06417.x. [DOI] [PubMed] [Google Scholar]
- 19.Mikkelsen K., Prakash M.D., Kuol N., Nurgali K., Stojanovska L., Apostolopoulos V. Anti-Tumor Effects of Vitamin B2, B6 and B9 in Promonocytic Lymphoma Cells. Int. J. Mol. Sci. 2019;20:3763. doi: 10.3390/ijms20153763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alam M.M., Iqbal S., Naseem I. Ameliorative effect of riboflavin on hyperglycemia, oxidative stress and DNA damage in type-2 diabetic mice: Mechanistic and therapeutic strategies. Arch. Biochem. Biophys. 2015;584:10–19. doi: 10.1016/j.abb.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy D.O. B vitamins and the brain: Mechanisms, dose and efficacy—A review. Nutrients. 2016;8:68. doi: 10.3390/nu8020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spinas E., Saggini A., Kritas S.K., Cerulli G., Caraffa A., Antinolfi P., Pantalone A., Frydas A., Tei M., Speziali A. Crosstalk between vitamin B and immunity. J. Biol. Regul. Homeost. Agents. 2015;29:283–288. [PubMed] [Google Scholar]
- 23.Pitkin R.M., Allen L.H., Bailey L.B., Bernfield M. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin and Choline. National Academy Press; Washington, DC, USA: 2000. [PubMed] [Google Scholar]
- 24.Ferreira R.G., Matsui T.C., Gomides L.F., Godin A.M., Menezes G.B., de Matos Coelho M., Klein A. Niacin inhibits carrageenan-induced neutrophil migration in mice. Naunyn Schmiedeberg Arch. Pharmacol. 2013;386:533–540. doi: 10.1007/s00210-013-0854-3. [DOI] [PubMed] [Google Scholar]
- 25.Bialonska D., Ramnani P., Kasimsetty S.G., Muntha K.R., Gibson G.R., Ferreira D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol. 2010;140:175–182. doi: 10.1016/j.ijfoodmicro.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 26.Imai S.-I., Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24:464–471. doi: 10.1016/j.tcb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson S., Imai S. NAD+ biosynthesis, aging, and disease. F1000Research. 2018;7:132. doi: 10.12688/f1000research.12120.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maqbool M.A., Aslam M., Akbar W., Iqbal Z. Biological importance of vitamins for human health: A review. J. Agric. Basic Sci. 2018;2 [Google Scholar]
- 29.Said H.M., Mohammed Z.M. Intestinal absorption of water-soluble vitamins: An update. Curr. Opin. Gastroenterol. 2006;22:140–146. doi: 10.1097/01.mog.0000203870.22706.52. [DOI] [PubMed] [Google Scholar]
- 30.Said H.M., Ortiz A., Ma T.Y. A carrier-mediated mechanism for pyridoxine uptake by human intestinal epithelial Caco-2 cells: Regulation by a PKA-mediated pathway. Am. J. Physiol. Cell Physiol. 2003;285:C1219–C1225. doi: 10.1152/ajpcell.00204.2003. [DOI] [PubMed] [Google Scholar]
- 31.Stover P.J. Physiology of folate and vitamin B 12 in health and disease. Nutr. Rev. 2004;62:S3–S12. doi: 10.1111/j.1753-4887.2004.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 32.Fang H., Kang J., Zhang D. Microbial production of vitamin B 12: A review and future perspectives. Microb. Cell Factories. 2017;16:15. doi: 10.1186/s12934-017-0631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Leary F., Samman S. Vitamin B12 in health and disease. Nutrients. 2010;2:299–316. doi: 10.3390/nu2030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kishimoto K., Kobayashi R., Sano H., Suzuki D., Maruoka H., Yasuda K., Chida N., Yamada M., Kobayashi K. Impact of folate therapy on combined immunodeficiency secondary to hereditary folate malabsorption. Clin. Immunol. 2014;153:17–22. doi: 10.1016/j.clim.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Scalabrino G., Veber D. Cobalamin and normal prions: A new horizon for cobalamin neurotrophism. Biochimie. 2013;95:1041–1046. doi: 10.1016/j.biochi.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Karadag S., Gursu M., Sakin A., Atalay E., Basinoglu F., Aydin Z., Uzun S., Sumnu A., Cebeci E., Koldas M. The Relationship between Soluble Tumor Necrosis Factor-like Weak Inducer of Apoptosis Levels and Cardiac Functions in Peritoneal Dialysis Patients. Eur. J. Inflamm. 2014;12:429–437. doi: 10.1177/1721727X1401200304. [DOI] [Google Scholar]
- 37.Kritas S.K., Caraffa A., Antinolfi P., Saggini A., Pantalone A., Rosati M., Tei M., Speziali A., Saggini R., Pandolfi F. Nerve Growth Factor Interactions with Mast Cells. SAGE Publications Sage UK; London, UK: 2014. [DOI] [PubMed] [Google Scholar]
- 38.Seemungal T.A.R., Lun J.C.F., Davis G., Neblett C., Chinyepi N., Dookhan C., Drakes S., Mandeville E., Nana F., Setlhake S. Plasma homocysteine is elevated in COPD patients and is related to COPD severity. Int. J. Chronic Obstr. Pulm. Dis. 2007;2:313. doi: 10.2147/COPD.S2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamura J., Kubota K., Murakami H., Sawamura M., Matsushima T., Tamura T., Saitoh T., Kurabayshi H., Naruse T. Immunomodulation by vitamin B12: Augmentation of CD8+ T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin. Exp. Immunol. 1999;116:28–32. doi: 10.1046/j.1365-2249.1999.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thakur K., Tomar S.K., Singh A.K., Mandal S., Arora S. Riboflavin and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2017;57:3650–3660. doi: 10.1080/10408398.2016.1145104. [DOI] [PubMed] [Google Scholar]
- 41.Kaneko S., Wang J., Kaneko M., Yiu G., Hurrell J.M., Chitnis T., Khoury S.J., He Z. Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models. J. Neurosci. 2006;26:9794–9804. doi: 10.1523/JNEUROSCI.2116-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chai J.T., Digby J.E., Ruparelia N., Jefferson A., Handa A., Choudhury R.P. Nicotinic acid receptor GPR109A is down-regulated in human macrophage-derived foam cells. PLoS ONE. 2013;8:e62934. doi: 10.1371/journal.pone.0062934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He W., Hu S., Du X., Wen Q., Zhong X.-P., Zhou X., Zhou C., Xiong W., Gao Y., Zhang S. Vitamin B5 reduces bacterial growth via regulating innate immunity and adaptive immunity in mice infected with Mycobacterium tuberculosis. Front. Immunol. 2018;9:365. doi: 10.3389/fimmu.2018.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tolmunen T., Voutilainen S., Hintikka J., Rissanen T., Tanskanen A., Viinamäki H., Kaplan G.A., Salonen J.T. Dietary folate and depressive symptoms are associated in middle-aged Finnish men. J. Nutr. 2003;133:3233–3236. doi: 10.1093/jn/133.10.3233. [DOI] [PubMed] [Google Scholar]
- 45.Kuroishi T., Endo Y., Muramoto K., Sugawara S. Biotin deficiency up-regulates TNF-α production in murine macrophages. J. Leukoc. Biol. 2008;83:912–920. doi: 10.1189/jlb.0607428. [DOI] [PubMed] [Google Scholar]
- 46.Scalabrino G., Corsi M.M., Veber D., Buccellato F.R., Pravettoni G., Manfridi A., Magni P. Cobalamin (vitamin B12) positively regulates interleukin-6 levels in rat cerebrospinal fluid. J. Neuroimmunol. 2002;127:37–43. doi: 10.1016/S0165-5728(02)00095-4. [DOI] [PubMed] [Google Scholar]
- 47.Vogiatzoglou A., Refsum H., Johnston C., Smith S.M., Bradley K.M., De Jager C., Budge M.M., Smith A.D. Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology. 2008;71:826–832. doi: 10.1212/01.wnl.0000325581.26991.f2. [DOI] [PubMed] [Google Scholar]
- 48.Switzer N.J., Karmali S., Gill R.S., Sherman V. Revisional bariatric surgery. Surg. Clin. 2016;96:827–842. doi: 10.1016/j.suc.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Quercia I., Dutia R., Laferrere B., Kotler D.P., Belsley S. Gastrointestinal changes after bariatric surgery. Diabetes Metab. 2014;40:87–94. doi: 10.1016/j.diabet.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Angrisani L., Santonicola A., Iovino P., Vitiello A., Higa K., Himpens J., Buchwald H., Scopinaro N. IFSO worldwide survey 2016: Primary, endoluminal, and revisional procedures. Obes. Surg. 2018;28:3783–3794. doi: 10.1007/s11695-018-3450-2. [DOI] [PubMed] [Google Scholar]
- 51.Strang B.J., McGinnis S.L. Nutritional Concerns in Bariatric Surgery: Thiamin Deficiency after Sleeve Gastrectomy. Support. Line. 2016;38:9–14. [Google Scholar]
- 52.Goodman J.C. Neurological complications of bariatric surgery. Curr. Neurol. Neurosci. Rep. 2015;15:79. doi: 10.1007/s11910-015-0597-2. [DOI] [PubMed] [Google Scholar]
- 53.Ziegler O., Sirveaux M.A., Brunaud L., Reibel N., Quilliot D. Medical follow up after bariatric surgery: Nutritional and drug issues General recommendations for the prevention and treatment of nutritional deficiencies. Diabetes Metab. 2009;35:544–557. doi: 10.1016/S1262-3636(09)73464-0. [DOI] [PubMed] [Google Scholar]
- 54.Gasteyger C., Suter M., Gaillard R.C., Giusti V. Nutritional deficiencies after Roux-en-Y gastric bypass for morbid obesity often cannot be prevented by standard multivitamin supplementation. Am. J. Clin. Nutr. 2008;87:1128–1133. doi: 10.1093/ajcn/87.5.1128. [DOI] [PubMed] [Google Scholar]
- 55.Halverson J.D. Metabolic risk of obesity surgery and long-term follow-up. Am. J. Clin. Nutr. 1992;55:602S–605S. doi: 10.1093/ajcn/55.2.602s. [DOI] [PubMed] [Google Scholar]
- 56.Kwon Y., Kim H.J., Lo Menzo E., Park S., Szomstein S., Rosenthal R.J. Anemia, iron and vitamin B12 deficiencies after sleeve gastrectomy compared to Roux-en-Y gastric bypass: A meta-analysis. Surg. Obes. Relat. Dis. 2014;10:589–597. doi: 10.1016/j.soard.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Moschos M., Droutsas D. A man who lost weight and his sight. Lancet. 1998;351:1174. doi: 10.1016/S0140-6736(97)11074-1. [DOI] [PubMed] [Google Scholar]
- 58.Eltweri A.M., Bowrey D.J., Sutton C.D., Graham L., Williams R.N. An audit to determine if vitamin b12 supplementation is necessary after sleeve gastrectomy. SpringerPlus. 2013;2:1–4. doi: 10.1186/2193-1801-2-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gagner M., Deitel M., Erickson A.L., Crosby R.D. Survey on laparoscopic sleeve gastrectomy (LSG) at the Fourth International Consensus Summit on Sleeve Gastrectomy. Obes. Surg. 2013;23 doi: 10.1007/s11695-013-1040-x. [DOI] [PubMed] [Google Scholar]
- 60.Ernst B., Thurnheer M., Schmid S.M., Schultes B. Evidence for the necessity to systematically assess micronutrient status prior to bariatric surgery. Obes. Surg. 2009;19:66–73. doi: 10.1007/s11695-008-9545-4. [DOI] [PubMed] [Google Scholar]
- 61.Arias P.M., Domeniconi E.A., García M., Esquivel C.M., Martínez Lascano F., Foscarini J.M. Micronutrient Deficiencies After Roux-en-Y Gastric Bypass: Long-Term Results. Obes. Surg. 2020;30:169–173. doi: 10.1007/s11695-019-04167-x. [DOI] [PubMed] [Google Scholar]
- 62.Toh S.Y., Zarshenas N., Jorgensen J. Prevalence of nutrient deficiencies in bariatric patients. Nutrition. 2009;25:1150–1156. doi: 10.1016/j.nut.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 63.Vargas-Ruiz A.G., Hernández-Rivera G., Herrera M.F. Prevalence of iron, folate, and vitamin B12 deficiency anemia after laparoscopic Roux-en-Y gastric bypass. Obes. Surg. 2008;18:288–293. doi: 10.1007/s11695-007-9310-0. [DOI] [PubMed] [Google Scholar]
- 64.Antoniewicz A., Kalinowski P., Kotulecka K.J., Kocoń P., Paluszkiewicz R., Remiszewski P., Zieniewicz K. Nutritional deficiencies in patients after Roux-en-Y gastric bypass and sleeve gastrectomy during 12-month follow-up. Obes. Surg. 2019;29:3277–3284. doi: 10.1007/s11695-019-03985-3. [DOI] [PubMed] [Google Scholar]
- 65.Johnson L.M., Ikramuddin S., Leslie D.B., Slusarek B., Killeen A.A. Analysis of vitamin levels and deficiencies in bariatric surgery patients: A single-institutional analysis. Surg. Obes. Relat. Dis. 2019;15:1146–1152. doi: 10.1016/j.soard.2019.04.028. [DOI] [PubMed] [Google Scholar]
- 66.Brotto M., Johnson M.L. Endocrine crosstalk between muscle and bone. Curr. Osteoporos. Rep. 2014;12:135–141. doi: 10.1007/s11914-014-0209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Halverson J.D. Micronutrient deficiencies after gastric bypass for morbid obesity. Am. Surg. 1986;52:594–598. [PubMed] [Google Scholar]
- 68.Javanainen M., Pekkarinen T., Mustonen H., Scheinin T., Leivonen M. Two-Year Nutrition Data in Terms of Vitamin D, Vitamin B12, and Albumin After Bariatric Surgery and Long-term Fracture Data Compared with Conservatively Treated Obese Patients: A Retrospective Cohort Study. Obes. Surg. 2018;28:2968–2975. doi: 10.1007/s11695-018-3336-3. [DOI] [PubMed] [Google Scholar]
- 69.Christensen M.H.E., Fadnes D.J., Røst T.H., Pedersen E.R., Andersen J.R., Våge V., Ulvik A., Midttun Ø., Ueland P.M., Nygård O.K. Inflammatory markers, the tryptophan-kynurenine pathway, and vitamin B status after bariatric surgery. PLoS ONE. 2018;13:e0192169. doi: 10.1371/journal.pone.0192169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aasheim E.T., Björkman S., Søvik T.T., Engström M., Hanvold S.E., Mala T., Olbers T., Bøhmer T. Vitamin status after bariatric surgery: A randomized study of gastric bypass and duodenal switch. Am. J. Clin. Nutr. 2009;90:15–22. doi: 10.3945/ajcn.2009.27583. [DOI] [PubMed] [Google Scholar]
- 71.Schijns W., Schuurman L.T., Melse-Boonstra A., van Laarhoven C.J.H.M., Berends F.J., Aarts E.O. Do specialized bariatric multivitamins lower deficiencies after RYGB? Surg. Obes. Relat. Dis. 2018;14:1005–1012. doi: 10.1016/j.soard.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 72.Smelt H.J.M., Smulders J.F., Said M., Nienhuijs S.W., Boer A.K. Improving Bariatric Patient Aftercare Outcome by Improved Detection of a Functional Vitamin B12 Deficiency. Obes. Surg. 2016;26:1500–1504. doi: 10.1007/s11695-015-1952-8. [DOI] [PubMed] [Google Scholar]
- 73.Al-Mutawa A., Anderson A.K., Alsabah S., Al-Mutawa M. Nutritional Status of Bariatric Surgery Candidates. Nutrients. 2018;10:67. doi: 10.3390/nu10010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sánchez A., Rojas P., Basfi-fer K., Carrasco F., Inostroza J., Codoceo J., Valencia A., Papapietro K., Csendes A., Ruz M. Micronutrient Deficiencies in Morbidly Obese Women Prior to Bariatric Surgery. Obes. Surg. 2016;26:361–368. doi: 10.1007/s11695-015-1773-9. [DOI] [PubMed] [Google Scholar]
- 75.Krzizek E.-C., Brix J.M., Herz C.T., Kopp H.P., Schernthaner G.-H., Schernthaner G., Ludvik B. Prevalence of micronutrient deficiency in patients with morbid obesity before bariatric surgery. Obes. Surg. 2018;28:643–648. doi: 10.1007/s11695-017-2902-4. [DOI] [PubMed] [Google Scholar]
- 76.Asghari G., Khalaj A., Ghadimi M., Mahdavi M., Farhadnejad H., Valizadeh M., Azizi F., Barzin M., Hosseinpanah F. Prevalence of Micronutrient Deficiencies Prior to Bariatric Surgery: Tehran Obesity Treatment Study (TOTS) Obes. Surg. 2018;28:2465–2472. doi: 10.1007/s11695-018-3187-y. [DOI] [PubMed] [Google Scholar]
- 77.Schweiger C., Weiss R., Berry E., Keidar A. Nutritional deficiencies in bariatric surgery candidates. Obes. Surg. 2010;20:193–197. doi: 10.1007/s11695-009-0008-3. [DOI] [PubMed] [Google Scholar]
- 78.Van Rutte P.W.J., Aarts E.O., Smulders J.F., Nienhuijs S.W. Nutrient Deficiencies Before and After Sleeve Gastrectomy. Obes. Surg. 2014;24:1639–1646. doi: 10.1007/s11695-014-1225-y. [DOI] [PubMed] [Google Scholar]
- 79.Wilhelm J., Müller A., Gruner-Labitzke K., Lichtinghagen R., Hillemacher T., Bleich S., Frieling H., Köhler H. Homocysteine and Cognition in Bariatric Surgery. Bariatr. Surg. Pract. Patient Care. 2017;12:190–196. doi: 10.1089/bari.2017.0008. [DOI] [Google Scholar]
- 80.Moore C.E., Sherman V. Effectiveness of B Vitamin Supplementation Following Bariatric Surgery: Rapid Increases of Serum Vitamin B 12. Obes. Surg. 2015;25:694–699. doi: 10.1007/s11695-014-1441-5. [DOI] [PubMed] [Google Scholar]
- 81.Kornerup L.S., Hvas C.L., Abild C.B., Richelsen B., Nexo E. Early changes in vitamin B12 uptake and biomarker status following Roux-en-Y gastric bypass and sleeve gastrectomy. Clin. Nutr. 2019;38:906–911. doi: 10.1016/j.clnu.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 82.Weng T.C., Chang C.H., Dong Y.H., Chang Y.C., Chuang L.M. Anaemia and related nutrient deficiencies after Roux-en-Y gastric bypass surgery: A systematic review and meta-analysis. BMJ Open. 2015;5:e006964. doi: 10.1136/bmjopen-2014-006964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Majumder S., Soriano J., Louie Cruz A., Dasanu C.A. Vitamin B12 deficiency in patients undergoing bariatric surgery: Preventive strategies and key recommendations. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2013;9:1013–1019. doi: 10.1016/j.soard.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 84.Marcuard S.P., Sinar D.R., Swanson M.S., Silverman J.F., Levine J.S. Absence of luminal intrinsic factor after gastric bypass surgery for morbid obesity. Dig. Dis. Sci. 1989;34:1238–1242. doi: 10.1007/BF01537272. [DOI] [PubMed] [Google Scholar]
- 85.Zarshenas N., Tapsell L.C., Neale E.P., Batterham M., Talbot M.L. The relationship between bariatric surgery and diet quality: A systematic review. Obes. Surg. 2020;30:1–25. doi: 10.1007/s11695-020-04392-9. [DOI] [PubMed] [Google Scholar]
- 86.Komorniak N., Szczuko M., Kowalewski B., Stachowska E. Nutritional Deficiencies, Bariatric Surgery, and Serum Homocysteine Level: Review of Current Literature. Obes. Surg. 2019;29:3735–3742. doi: 10.1007/s11695-019-04100-2. [DOI] [PubMed] [Google Scholar]
- 87.Algahtani H.A., Khan A.S., Khan M.A., Aldarmahi A.A., Lodhi Y. Neurological complications of bariatric surgery. Neurosciences. 2016;21:241–245. doi: 10.17712/nsj.2016.3.20160039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Landais A. Neurological complications of bariatric surgery. Obes. Surg. 2014;24:1800–1807. doi: 10.1007/s11695-014-1376-x. [DOI] [PubMed] [Google Scholar]
- 89.Oudman E., Wijnia J.W., Van Dam M., Biter L.U., Postma A. Preventing Wernicke Encephalopathy After Bariatric Surgery. Obes. Surg. 2018:1–9. doi: 10.1007/s11695-018-3262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smelt H.J.M., Pouwels S., Smulders J.F. Different supplementation regimes to treat perioperative vitamin B12 deficiencies in bariatric surgery: A systematic review. Obes. Surg. 2017;27:254–262. doi: 10.1007/s11695-016-2449-9. [DOI] [PubMed] [Google Scholar]
- 91.Ledoux S., Msika S., Moussa F., Larger E., Boudou P., Salomon L., Roy C., Clerici C. Comparison of nutritional consequences of conventional therapy of obesity, adjustable gastric banding, and gastric bypass. Obes. Surg. 2006;16:1041–1049. doi: 10.1381/096089206778026415. [DOI] [PubMed] [Google Scholar]
- 92.Ledoux S., Coupaye M., Bogard C., Clerici C., Msika S. Determinants of hyperhomocysteinemia after gastric bypass surgery in obese subjects. Obes. Surg. 2011;21:78–86. doi: 10.1007/s11695-010-0269-x. [DOI] [PubMed] [Google Scholar]
- 93.Smelt H.J.M., Pouwels S., Smulders J.F., Hazebroek E.J. Patient adherence to multivitamin supplementation after bariatric surgery: A narrative review. J. Nutr. Sci. 2020;9:9. doi: 10.1017/jns.2020.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Homan J., Schijns W., Janssen I.M.C., Berends F.J., Aarts E.O. Adequate multivitamin supplementation after Roux-En-Y gastric bypass results in a decrease of national health care costs: A cost-effectiveness analysis. Obes. Surg. 2019;29:1638–1643. doi: 10.1007/s11695-019-03750-6. [DOI] [PubMed] [Google Scholar]
- 95.Dixon J.B., Dixon M.E., O’Brien P.E. Elevated homocysteine levels with weight loss after Lap-Band® surgery: Higher folate and vitamin B 12 levels required to maintain homocysteine level. Int. J. Obes. 2001;25:219–227. doi: 10.1038/sj.ijo.0801474. [DOI] [PubMed] [Google Scholar]
- 96.De Jager C.A. Critical levels of brain atrophy associated with homocysteine and cognitive decline. Neurobiol. Aging. 2014;35:S35–S39. doi: 10.1016/j.neurobiolaging.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 97.Mikkelsen K., Stojanovska L., Tangalakis K., Bosevski M., Apostolopoulos V. Cognitive decline: A vitamin B perspective. Maturitas. 2016;93:108–113. doi: 10.1016/j.maturitas.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 98.Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006;5:949–960. doi: 10.1016/S1474-4422(06)70598-1. [DOI] [PubMed] [Google Scholar]
- 99.Gibson G.E., Hirsch J.A., Fonzetti P., Jordon B.D., Cirio R.T., Elder J. Vitamin B1 (thiamine) and dementia. Ann. N. Y. Acad. Sci. 2016;1367:21. doi: 10.1111/nyas.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mitchell J.E., King W.C., Chen J.Y., Devlin M.J., Flum D., Garcia L., Inabet W., Pender J.R., Kalarchian M.A., Khandelwal S. Course of depressive symptoms and treatment in the longitudinal assessment of bariatric surgery (LABS-2) study. Obesity. 2014;22:1799–1806. doi: 10.1002/oby.20738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alabi F., Guilbert L., Villalobos G., Mendoza K., Hinojosa R., Melgarejo J.C., Espinosa O., Sepúlveda E.M., Zerrweck C. Depression before and after bariatric surgery in low-income patients: The utility of the beck depression inventory. Obes. Surg. 2018;28:3492–3498. doi: 10.1007/s11695-018-3371-0. [DOI] [PubMed] [Google Scholar]
- 102.Dawes A.J., Maggard-Gibbons M., Maher A.R., Booth M.J., Miake-Lye I., Beroes J.M., Shekelle P.G. Mental health conditions among patients seeking and undergoing bariatric surgery: A meta-analysis. JAMA. 2016;315:150–163. doi: 10.1001/jama.2015.18118. [DOI] [PubMed] [Google Scholar]
- 103.Fisher D., Coleman K.J., Arterburn D.E., Fischer H., Yamamoto A., Young D.R., Sherwood N.E., Trinacty C.M., Lewis K.H. Mental illness in bariatric surgery: A cohort study from the PORTAL network. Obesity. 2017;25:850–856. doi: 10.1002/oby.21814. [DOI] [PubMed] [Google Scholar]
- 104.Castaneda D., Popov V.B., Wander P., Thompson C.C. Risk of suicide and self-harm is increased after bariatric surgery—a systematic review and meta-analysis. Obes. Surg. 2019;29:322–333. doi: 10.1007/s11695-018-3493-4. [DOI] [PubMed] [Google Scholar]
- 105.Morledge M.D., Pories W.J. Mental Health in Bariatric Surgery: Selection, Access, and Outcomes. Obesity. 2020;28:689–695. doi: 10.1002/oby.22752. [DOI] [PubMed] [Google Scholar]
- 106.Hamad G.G., Helsel J.C., Kozak G.M., McShea M.C., Hughes C., Confer A.L., McCloskey C.A., Sit D.K., Perel J.M., Wisner K.L.P.U.o.P.P.P.A.U.S. P-35 The effect of gastric bypass on the pharmacokinetics of serotonin reuptake inhibitors. Surg. Obes. Relat. Dis. 2011;7:384. doi: 10.1016/j.soard.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Spirou D.R., Raman J., Smith E. Psychological outcomes following surgical and endoscopic bariatric procedures: A systematic review. Obes. Rev. 2020;21:e12998. doi: 10.1111/obr.12998. [DOI] [PubMed] [Google Scholar]
- 108.Sozer K., Gorgulu Y., Sonmez M.B., Kose Cinar R. Psychotic depression after obesity surgery and recovery with vitamin B12 replacement. Dusunen Adam J. Psychiatry Neurol. Sci. 2019;32:65. doi: 10.14744/DAJPNS.2019.00009. [DOI] [Google Scholar]
- 109.Ivezaj V., Grilo C.M. When Mood Worsens after Gastric Bypass Surgery: Characterization of Bariatric Patients with Increases in Depressive Symptoms Following Surgery. Obes. Surger. 2015;25:423–429. doi: 10.1007/s11695-014-1402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lewis C.-A., de Jersey S., Hopkins G., Hickman I., Osland E. Does bariatric surgery cause vitamin A, B1, C or E deficiency? A systematic review. Obes. Surg. 2018;28:3640–3657. doi: 10.1007/s11695-018-3392-8. [DOI] [PubMed] [Google Scholar]
- 111.Milone M., Di Minno M.N.D., Lupoli R., Maietta P., Bianco P., Pisapia A., Gaudioso D., Taffuri C., Milone F., Musella M. Wernicke encephalopathy in subjects undergoing restrictive weight loss surgery: A systematic review of literature data. Eur. Eat. Disord. Rev. 2014;22:223–229. doi: 10.1002/erv.2292. [DOI] [PubMed] [Google Scholar]
- 112.Philippi N., Vinzio S., Collongues N., Vix M., Boehm N., Tranchant C., Echaniz-Laguna A. Peripheral neuropathies after bariatric surgery. Rev. Neurol. 2011;167:607–614. doi: 10.1016/j.neurol.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 113.Thaisetthawatkul P., Collazo-Clavell M.L., Sarr M.G., Norell J.E., Dyck P.J.B. A controlled study of peripheral neuropathy after bariatric surgery. Neurology. 2004;63:1462–1470. doi: 10.1212/01.WNL.0000142038.43946.06. [DOI] [PubMed] [Google Scholar]
- 114.Mahawar K.K., Reid A., Graham Y., Callejas-Diaz L., Parmar C., Carr W.R.J., Jennings N., Singhal R., Small P.K. Oral vitamin B 12 supplementation after Roux-en-Y gastric bypass: A systematic review. Obes. Surg. 2018;28:1916–1923. doi: 10.1007/s11695-017-3102-y. [DOI] [PubMed] [Google Scholar]
- 115.Butler C.C., Vidal-Alaball J., Cannings-John R., McCaddon A., Hood K., Papaioannou A., McDowell I., Goringe A. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: A systematic review of randomized controlled trials. Fam. Pract. 2006;23:279–285. doi: 10.1093/fampra/cml008. [DOI] [PubMed] [Google Scholar]
- 116.Quilliot D., Coupaye M., Ciangura C., Czernichow S., Sallé A., Gaborit B., Alligier M., Nguyen-Thi P.L., Dargent J., Msika S. Recommendations for nutritional care after bariatric surgery: Recommendations for best practice and SOFFCO-MM/AFERO/SFNCM/expert consensus. J. Visc. Surg. 2021;158:51–61. doi: 10.1016/j.jviscsurg.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 117.Allen L.H. How common is vitamin B-12 deficiency? Am. J. Clin. Nutr. 2009;89:693S–696S. doi: 10.3945/ajcn.2008.26947A. [DOI] [PubMed] [Google Scholar]
- 118.Rhode B.M., Arseneau P., Cooper B.A., Katz M., Gilfix B.M., MacLean L.D. Vitamin B-12 deficiency after gastric surgery for obesity. Am. J. Clin. Nutr. 1996;63:103–109. doi: 10.1093/ajcn/63.1.103. [DOI] [PubMed] [Google Scholar]
- 119.Provenzale D., Reinhold R.B., Golner B., Irwin V., Dallal G.E., Papathanasopoulos N., Sahyoun N., Samloff I.M., Russell R.M. Evidence for diminished B12 absorption after gastric bypass: Oral supplementation does not prevent low plasma B12 levels in bypass patients. J. Am. Coll. Nutr. 1992;11:29–35. doi: 10.1080/07315724.1992.10718193. [DOI] [PubMed] [Google Scholar]
- 120.O’Kane M., Parretti H.M., Pinkney J., Welbourn R., Hughes C.A., Mok J., Walker N., Thomas D., Devin J., Coulman K.D. British Obesity and Metabolic Surgery Society Guidelines on perioperative and postoperative biochemical monitoring and micronutrient replacement for patients undergoing bariatric surgery—2020 update. Obes. Rev. 2020;21:e13087. doi: 10.1111/obr.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nuzzo A., Czernichow S., Hertig A., Ledoux S., Poghosyan T., Quilliot D., Le Gall M., Bado A., Joly F. Prevention and treatment of nutritional complications after bariatric surgery. Lancet Gastroenterol. Hepatol. 2021;6:238–251. doi: 10.1016/S2468-1253(20)30331-9. [DOI] [PubMed] [Google Scholar]
- 122.Schijns W., Homan J., van der Meer L., Janssen I.M., van Laarhoven C.J., Berends F.J., Aarts E.O. Efficacy of oral compared with intramuscular vitamin B-12 supplementation after Roux-en-Y gastric bypass: A randomized controlled trial. Am. J. Clin. Nutr. 2018;108:6–12. doi: 10.1093/ajcn/nqy072. [DOI] [PubMed] [Google Scholar]
- 123.Kumari A., Nigam A. Bariatric surgery in women: A boon needs special care during pregnancy. J. Clin. Diagn. Res. JCDR. 2015;9:QE01. doi: 10.7860/JCDR/2015/14258.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wax J.R., Pinette M.G., Cartin A., Blackstone J. Female reproductive issues following bariatric surgery. Obstet. Gynecol. Surv. 2007;62:595–604. doi: 10.1097/01.ogx.0000279291.86611.46. [DOI] [PubMed] [Google Scholar]
- 125.Kaska L., Kobiela J., Abacjew-Chmylko A., Chmylko L., Wojanowska-Pindel M., Kobiela P., Walerzak A., Makarewicz W., Proczko-Markuszewska M., Stefaniak T. Nutrition and pregnancy after bariatric surgery. ISRB Obes. 2013;2013:1–6. doi: 10.1155/2013/492060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fullmer M.A., Abrams S.H., Hrovat K., Mooney L., Scheimann A.O., Hillman J.B., Suskind D.L. Nutritional strategy for adolescents undergoing bariatric surgery: Report of a working group of the Nutrition Committee of NASPGHAN/NACHRI. J. Pediatric Gastroenterol. Nutr. 2012;54:125–135. doi: 10.1097/MPG.0b013e318231db79. [DOI] [PubMed] [Google Scholar]
- 127.Kominiarek M.A. Preparing for and managing a pregnancy after bariatric surgery. Semin. Perinatol. 2011;35:356–361. doi: 10.1053/j.semperi.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Harris A.A., Barger M.K. Specialized care for women pregnant after bariatric surgery. J. Midwifery Women Health. 2010;55:529–539. doi: 10.1016/j.jmwh.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 129.Woodard C.B. Pregnancy following bariatric surgery. J. Perinat. Neonatal Nurs. 2004;18:329–340. doi: 10.1097/00005237-200410000-00003. [DOI] [PubMed] [Google Scholar]
- 130.Becker D.A.B., Laura J., Galetta S.L. The Neurological Complications of Nutritional Deficiency following Bariatric Surgery. J. Obes. 2012;2012:608534. doi: 10.1155/2012/608534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schroder T.H., Tan A., Mattman A., Sinclair G., Barr S.I., Vallance H.D., Lamers Y. Reference intervals for serum total vitamin B12 and holotranscobalamin concentrations and their change points with methylmalonic acid concentration to assess vitamin B12 status during early and mid-pregnancy. Clin. Chem. Lab. Med. CCLM. 2019;57:1790–1798. doi: 10.1515/cclm-2018-1337. [DOI] [PubMed] [Google Scholar]
- 132.Klee G.G. Cobalamin and folate evaluation: Measurement of methylmalonic acid and homocysteine vs. vitamin B12 and folate. Clin. Chem. 2000;46:1277–1283. doi: 10.1093/clinchem/46.8.1277. [DOI] [PubMed] [Google Scholar]
- 133.Simoens C., Verbiest A., Brenninkmeijer K., Moyson C., Matthys C., Meulemans A., Lannoo M., Van der Schueren B., Mertens A. Nonsurgical complications after bariatric surgery. Proc. Nutr. Soc. 2020;79 doi: 10.1017/S0029665120006308. [DOI] [Google Scholar]