Abstract
With their ten peripheral substituents, pillar[5]arenes are attractive compact scaffolds for the construction of nanomaterials with a controlled number of functional groups distributed around the macrocyclic core. This review paper is focused on the functionalization of pillar[5]arene derivatives with small dendrons to generate dendrimer-like nanomaterials and bioactive compounds. Examples include non-viral gene vectors, bioactive glycoclusters, and liquid-crystalline materials.
Keywords: pillar[5]arene, dendrimers, liquid crystals, transfection, glycoclusters
1. Introduction
Dendrimers have been intensively investigated during the past decades and this field largely crossed the boundaries between chemistry and other disciplines such as physics and biology [1,2,3,4,5]. This paper being part of the special issue dedicated to Jean-Pierre Majoral, it must be mentioned that his group played a major role in dendrimer chemistry with the development of phosphorus dendrimers [6]. This beautiful chemistry has been used for the construction of a very large variety of functional nanomaterials and bioactive molecules [7,8,9,10,11,12,13,14]. As far as the synthesis of dendrimers is concerned, the convergent and divergent stepwise approaches used for their preparation are often efficient but the synthesis of high generation derivatives remains often difficult because of the large number of synthetic steps [1,2,3,4,5]. An alternative approach to construct large molecules in a rapid manner emerged in recent years and is based on the grafting of small dendrons to compact multifunctional molecular scaffolds to generate globular dendrimer-like compounds in a single step [15,16,17,18,19,20,21,22,23,24,25,26]. As part of this research, our group has intensively investigated fullerene hexa-adducts for this purpose [15,16,17,18]. The grafting of twelve peripheral groups onto the fullerene scaffold has been efficiently achieved by using click chemistry, thus giving rapid access to globular multifunctional nanomaterials [26,27,28,29,30,31,32,33,34,35]. Easy accessibility has been a clear advantage for their applicability in various fields. Examples include solar energy concentrators to improve the efficiency of photovoltaic cells [36], liquid-crystalline materials [37,38], bioactive glycoclusters [39,40,41,42,43,44,45], giant molecules with antiviral properties [46,47], and non-viral gene transfection vectors [48]. More recently, we have also shown that clickable pillar[5]arene scaffolds are versatile compact cores for the preparation of dendrimer-like compounds for various applications. These results are summarized in the present paper.
2. Clickable Pillar[5]Arene Building Blocks
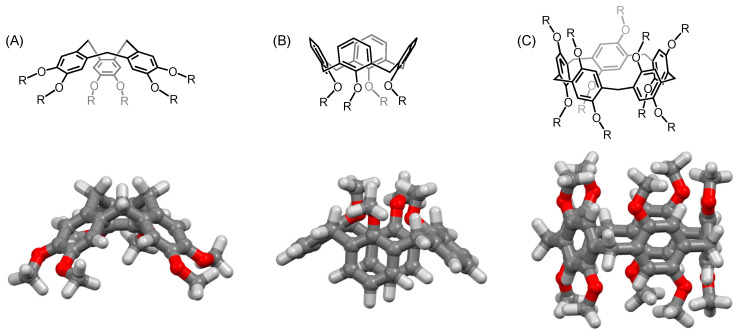
Pillar[5]arenes are macrocyclic compounds composed of hydroquinone units connected by methylene bridges [49,50,51,52] (Figure 1). In this respect, they are directly related to cyclotriveratrylenes (CTVs) and calix[n]arenes. These three classes of cyclophanes differ by the relative position of the methylene moieties bridging their aromatic subunits. Their shape is also very different. Whereas CTVs and calix[n]arenes generally adopt cone-shaped conformations, pillar[5]arenes are tubular-shaped [50,51,52]. As a result, both rims of the pillar[5]arenes are equivalent. With their ten peripheral alkoxy substituents, these compounds are therefore attractive compact scaffolds for the construction of nanomaterials with a controlled number of functional groups distributed around the macrocyclic core.
Figure 1.
(A) Cyclotriveratrylene; (B) calix[4]arene; (C) pillar[5]arene. The calculated structures of the methoxy-substituted derivatives highlight the different conformations adopted by these macrocycles: cone-shape in the case of the CTV and the calix[4]arene, and tubular in the case of the pillar[5]arene.
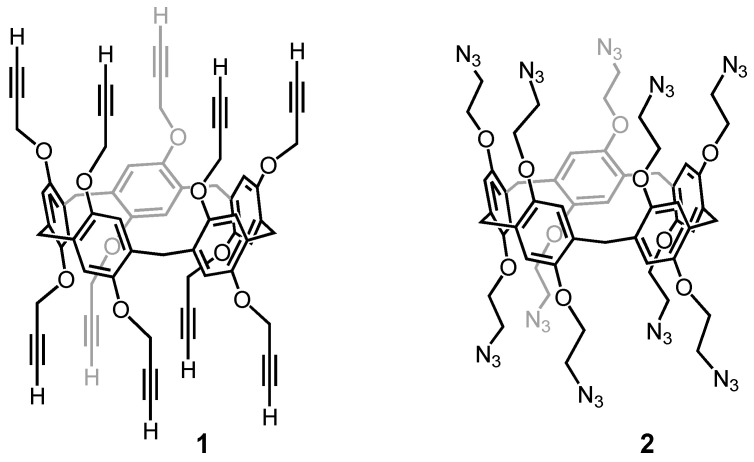
As far as their synthesis is concerned, pillar[5]arenes are conveniently prepared from 1,4-dialkoxybenzene and paraformaldehyde in the presence of a catalyst, typically BF3.Et2O [53]. The outcome of the reaction is sensitive to the solvent and CH2Cl2 or 1,2-dichloroethane favors the formation of the cyclopentamers [54]. In contrast, cyclohexameric macrocycles are preferentially formed in CHCl3 [55]. It is believed that the solvent molecules are somehow templating the cyclization step to preferentially generate a macrocyclic product with the appropriate size for inclusion of a solvent molecule in its cavity [54]. On the other hand, the yield of these cyclooligomerizations is particularly high owing to the reversibility of the Friedel–Crafts reaction [56]. The formation of pillar[5]arenes is indeed thermodynamically driven and their high yielding synthesis is explained by dynamic covalent bond formation [56]. Their preparation is, however, very sensitive to steric effects and hydroquinone monomers with large alkoxy substituents are not suited for the preparation of pillar[5]arenes. To solve this limitation, the most efficient approach is based on the post-functionalization of pillar[5]arene building block prepared in high yields from simple monomers. For this purpose, copper catalyzed alkyne-azide cycloaddition is particularly interesting [57]. This click reaction is effectively high yielding which is essential to achieve the efficient grafting of ten peripheral groups onto a single molecular scaffold [58]. Moreover, this chemistry is compatible with many functional groups thus allowing the grafting of a large variety of molecules. As typical examples, clickable pillar[5]arene scaffolds are depicted in Figure 2. These building blocks have been intensively used to prepare bioactive compounds and advanced materials [59,60,61,62,63,64,65]. They have been also functionalized with small dendrons to generate dendrimer-like nanostructures. These results are summarized in the next section.
Figure 2.
Typical examples of clickable pillar[5]arene derivatives used for the construction of multifunctional nanomaterials.
3. Bioactive Dendrimers with a Pillar[5]Arene Core
3.1. Dendritic Gene Delivery Vectors with a Pillar[5]Arene Core
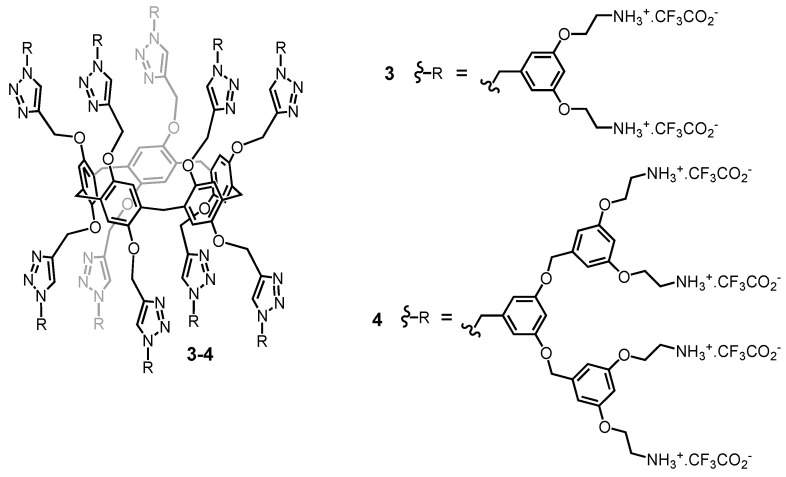
The first example of dendrimers with a pillar[5]arene core have been reported by our group [66]. Dendrons with peripheral Boc-protected amine functions have been grafted onto both rims of pillar[5]arene building block 1. Subsequent treatment with trifluoroacetic acid (TFA) gave deprotected dendrimers 3 and 4 (Figure 3).
Figure 3.
Dendronized pillar[5]arenes with peripheral ammonium functions (molecular weights (MW): 5787.41 (3); 11368.67 (4)). These polycationic derivatives have been used as non-viral gene vectors.
Dynamic light scattering (DLS) measurements revealed that both 3 and 4 form aggregates in water at concentrations higher than 3 nM. At concentrations lower than 1.5 nM, second-generation compound 4 no longer aggregates, whereas the first-generation analogue still forms nanoparticles with an average size of ca. 70 nm. Under these conditions, the hydrophobic interactions between the internal part of the dendrimers are vanished in the case of 4, thus suggesting that the compound may adopt a nearly globular conformation despite the low generation number of the dendrons grafted onto the pillar[5]arene core. The ability of 3 and 4 to bind DNA has been evidenced by electrophoresis, DLS measurements, and transmission electron microscopy (TEM) [66,67,68]. Polyplexes have been prepared from plasmid DNA (pCMV-Luc) and 3 or 4. Transfection efficiencies of the resulting self-assembled nanoparticles have been evaluated in vitro with HeLa cells (Figure 4). Practically useful levels of transfection have been obtained for both 3 and 4. At the same time, the high levels of total cellular proteins observed in these experiments revealed very low toxicity. Interestingly, the first-generation dendrimer already has optimum gene delivery capabilities. In this particular case, high efficiency is not associated to high generation numbers to ensure DNA compaction into stable polyplexes suited for transfection experiments as typically observed for dendrimers [69]. This has been explained by the bolaamphiphilic character of 3 allowing to increase the stability of the nanoparticles formed with DNA by the establishment of additional hydrophobic interactions.
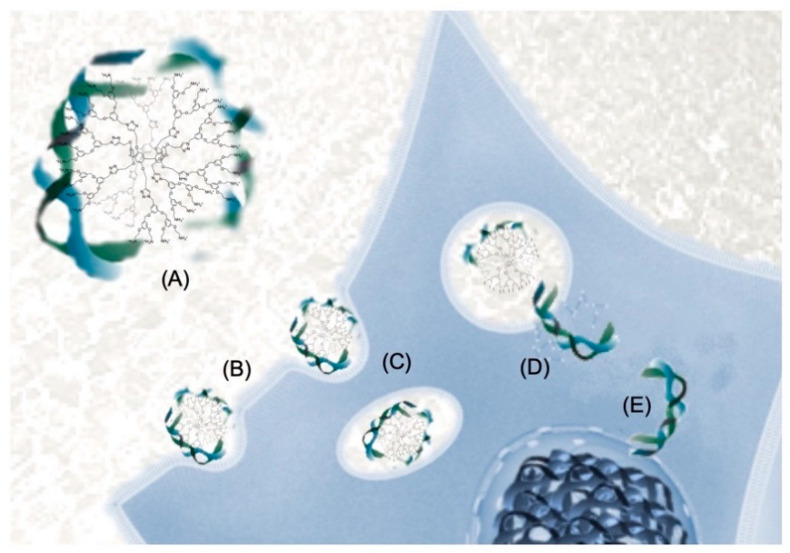
Figure 4.
Schematic representation of the main step of gene delivery into a cell: (A) complexation of DNA; (B) interaction with the cellular membrane; (C) entry into the cell; (D) release into the cytosol; (E) intracellular transport into the nucleus where the DNA is expressed.
3.2. Glycoclusters Constructed on a Pillar[5]Arene Scaffold
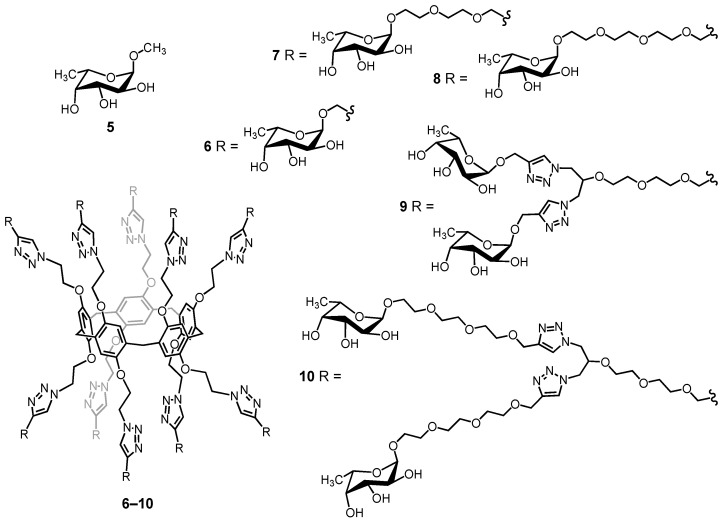
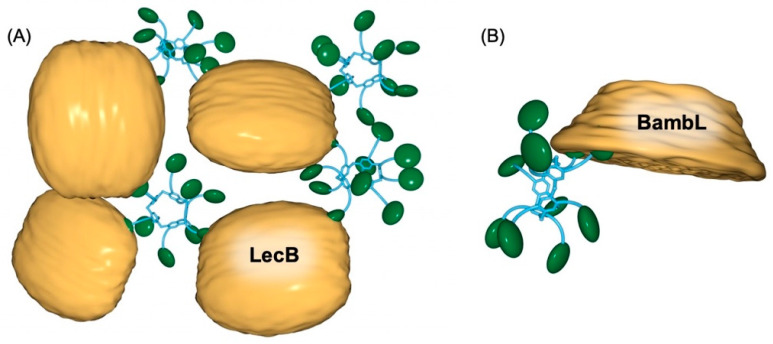
Pillar[5]arene scaffolds have been also used to prepare glycoclusters decorated with ten peripheral sugar residues [70,71,72,73,74]. These compounds have been assayed as multivalent ligands for various bacterial lectins and large binding enhancements have been evidenced through the well-established glycoside cluster effect [75,76,77,78,79]. To further increase the valency of the glycopillar[5]arene derivatives and hopefully their binding capabilities, first-generation glycodendrons have been grafted onto the pillar[5]arene core. A series of fucosylated compounds have been prepared from building block 2 and first-generation dendrons with an alkyne function at the focal point. Glycoclusters 9 and 10 with 20 peripheral fucose subunits are depicted in Figure 5 together with their related decavalent systems 6–8 and model compound 5. Compounds 5–10 have been assayed towards two fucolectins, namely LecB from Pseudomonas aeruginosa and BambL from Burkholderia ambifaria. These studies have been carried out by A. Imberty and co-workers [74]. The dissociation constants (KD) derived from isothermal titration microcalorimetry (ITC) experiments are summarized in Table 1. LecB is typically not very sensitive to the multivalent presentation of fucose residues [74]. This is explained by the topology of this lectin. The four binding pockets are actually quite far from each other, thus preventing the simultaneous binding of two fucose subunits of a multivalent ligand. As a result, binding enhancement resulting from chelate cooperativity is not possible in this particular case. As anticipated, there is no dramatic improvement when going from monovalent ligand 5 to the multivalent fucosylated pillar[5]arenes 6–10. Decavalent compound 6 is even a weaker ligand, most probably because of steric effects resulting from the short spacer that prevents optimal interactions of the fucose residue in the binding pocket of LecB. The small binding enhancement observed for 7–10 results exclusively from aggregation, as shown in Figure 6.
Figure 5.
Model monovalent ligand 5 and glycopillar[5]arenes with 10 and 20 peripheral fucose subunits (MW: 3323.31 (6); 4204.41 (7); 4644.94 (8); 8028.18 (9); 10671.86 (10)).
Table 1.
Isothermal titration microcalorimetry data for the interactions of glycopillar[5]arenes with LecB and RSL.
| Ligand | Valency | KD (nM) | β 1 |
|---|---|---|---|
| LecB from Pseudomonas aeruginosa 2 | |||
| 5 3 | 1 | 430 | 1 |
| 6 | 10 | 990 | 0.4 |
| 7 | 10 | 220 | 1.9 |
| 8 | 10 | 280 | 1.5 |
| 9 | 20 | 150 | 2.9 |
| 10 | 20 | 180 | 2.4 |
| BambL from Burkholderia ambifaria 2 | |||
| 5 4 | 1 | 960 | 1 |
| 6 | 10 | 60 | 16 |
| 7 | 10 | 19 | 50 |
| 8 | 10 | 57 | 17 |
| 9 | 20 | 17 | 56 |
| 10 | 20 | 27 | 36 |
Figure 6.
(A) Schematic representation of the clustering resulting from the association of a decavalent glycopillar[5]arene derivative with Lec A. (B) Schematic representation of the simultaneous binding of two fucose units of a decavalent glycopillar[5]arene derivative to BambL.
In this case, simultaneous binding of two fucose moieties of one glycopillar[5]arene occurs to two different LecB proteins within the same aggregate. This is beneficial from an enthalpic point of view. However, the positive enthalpic effect is largely counterbalanced by a strong entropic penalty. As a result, the overall enhancement is rather weak when going from 5 to 6–10.
The topology of BambL is very different. In this case, six binding pockets are located on the same face of the protein and the observed binding enhancement resulting from the multivalent presentation of fucose residues with 6–10 when compared to monovalent model 5 is likely mainly due to chelate cooperativity (Figure 6). The beneficial enthalpic contribution resulting from chelate cooperativity is, however, affected by an entropic penalty due to clustering. This is particularly true for ligands 9 and 10 with 20 peripheral fucose residues. Overall, the valency number plays an important role in the affinity of multivalent ligands 6–10 towards LecB and BambL, but the nature of the linker unit between the core and the peripheral fucose moieties is also important. Nonetheless, compounds 6–10 are amongst the most potent ligands for LecB and BambL reported to date, thus showing the potential of glycopillar[5]arenes for therapeutic applications based on an anti-adhesive strategy [75,76,77,78,79].
4. Dendritic Liquid-Crystalline Pillar[5]Arenes
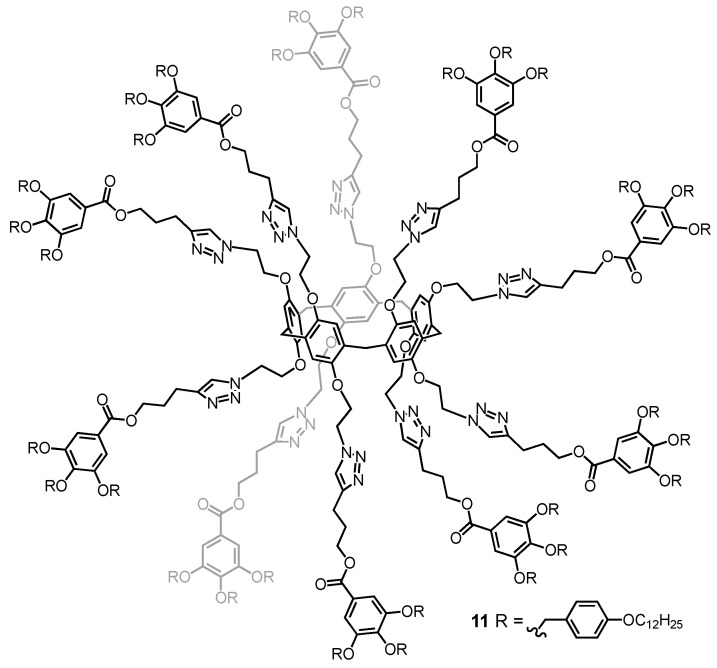
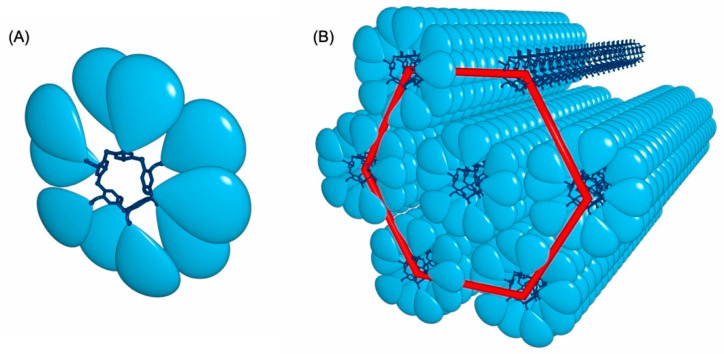
Pillar[5]arenes are attractive five-fold symmetrical hard-core units for the construction of original liquid-crystalline materials. The first examples have been constructed by grafting cyanobiphenyl-based mesogenic units onto the pillar[5]arene core [82,83]. The same design principle has been also used to design switchable liquid-crystalline derivatives by incorporating azobenzene-containing mesogenic moieties on both rims of the pillar[5]arene core [84]. In all the cases, smectic organization have been observed in the liquid-crystalline phase. In contrast, when the pillar[5]arene core has been functionalized with Percec-type poly(benzylether) dendrons [85], the peripheral subunits promote a totally different supramolecular organization [86,87]. A typical example of dendronized pillar[5]arene derivative is depicted in Figure 7. X-ray diffraction measurements revealed a supramolecular organization into a hexagonal columnar liquid-crystalline mesophase. The chemical information stored in the peripheral dendrons of 11 actually drives the conformational equilibrium towards the formation of a disc-like tertiary structure. As a result, the compound adopts a perfect shape, allowing the self-organization into columnar assemblies in which one molecule forms an entire disc, as schematically shown in Figure 8. Interestingly, the columnar assembly generates infinite self-assembled nanotubes despite the generated free volume. This work represents, therefore, a first step towards the preparation of a new class of organic nanotubes.
Figure 7.
Dendronized pillar[5]arene 11 (MW: 11896.72).
Figure 8.
(A) Schematic representation of compound 11. (B) Supramolecular arrangement of compound 11 into the hexagonal columnar phase, the dendrons have been omitted in one column (top right) to highlight the nanotubular arrangement of the pillar[5]arene cores.
5. Self-Assembled Dendrimers
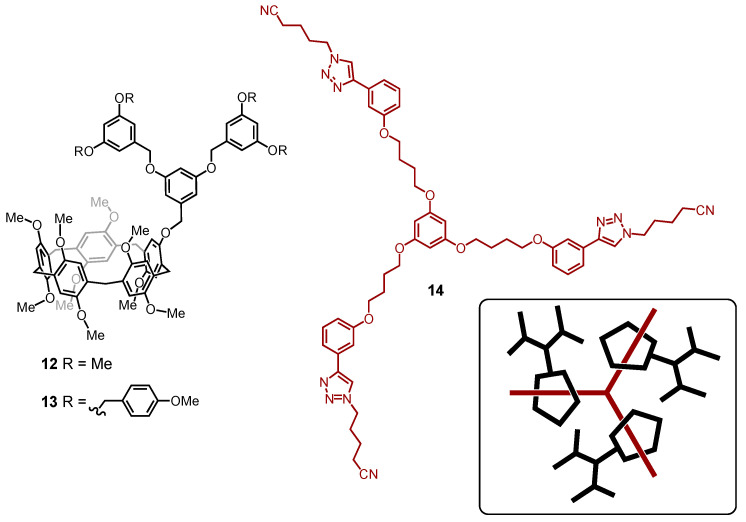
With their tubular structures, pillar[5]arene macrocycles are well-suited hosts for the formation of inclusion complexes with a large variety of guests [88]. Jia and Li used the complexation ability of pillar[5]arenes to self-assemble supramolecular dendrimers [89]. First and second-generation dendrons have been grafted onto a monohydroxylated pillar[5]arene derivative to generate compounds 12 and 13, respectively (Figure 9). Their self-assembly with a tritopic connector (14) has been investigated in CDCl3 solutions by 1H NMR binding studies. The 3:1 host-guest assemblies are rather stable under these conditions. The formation of the star-shaped dendritic trimers has been further confirmed by their diffusion coefficients estimated by diffusion-ordered NMR spectroscopy (DOSY).
Figure 9.
Dendronized pillar[5]arene derivatives 12 and 13, and tritopic ligand 14. The inset shows a schematic representation of the self-assembled dendrimer resulting from the binding of 14 with three pillar[5]arenes 13 (MW: 1159.34 (12); 1463.73 (13)).
Finally, one should also mention the outstanding work of Yang and co-workers on organometallic rotaxane dendrimers constructed with mechanically interlocked pillar[5]arene moieties [90,91,92,93,94,95,96]. These compounds are out of the scope of the present paper and this chemistry has been summarized in recent review articles [97].
6. Conclusions
Clickable pillar[5]arenes are versatile building blocks for the preparation of multifunctional nanomaterials. Whereas only a very few examples of dendronized derivatives have been reported so far, the easy access to dendritic-like structures with limited synthetic efforts is very attractive for future applications. Pillar[5]arene-containing dendrimers have already been used to generate non-viral gene delivery systems, bioactive glycoclusters, and liquid-crystalline materials. These results pave the way to new generations of more sophisticated advanced materials and bioactive compounds. Examples include heteroglycoclusters targeting several proteins and new gene vectors with additional functions such as sugars for cell targeting or fluorescent moieties for monitoring cell uptake. The host-guest properties of the pillar[5]arene macrocycle also open additional perspectives for the design of new supramolecular functional dendrimers for different applications.
Author Contributions
J.-F.N. and R.D. coordinated the preparation of this review through the contributions of all the authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the ANR (projects FastGiant ANR-17-CE07-0012-01 and Pillar ANR-19-CE06-0032), the International Center for Frontier Research in Chemistry, the LabEx “Chimie des Systèmes Complexes” and the Swiss National Science Foundation (grant number: 2000-178783).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable to this article.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References and Notes
- 1.Newkome G.R., Moorefield C.N., Vögtle F. Dendrimers and Dendrons: Concepts, Syntheses, Applications. Wiley-VCH; Weinheim, Germany: 2001. [Google Scholar]
- 2.Fréchet J.M.J., Tomalia D.A., editors. Dendrimers and Other Dendritic Polymers. John Wiley & Sons; Chichester, UK: 2001. [Google Scholar]
- 3.Vögtle F., Richardt G., Werner N. Dendrimer Chemistry: Concepts, Syntheses, Properties, Applications. Wiley-VCH; Weinheim, Germany: 2009. [Google Scholar]
- 4.Caminade A.-M., Turrin C.-O., Laurent R., Ouali A., Delavaux-Nicot B., editors. Dendrimers: Towards Catalytic, Material and Biomedical Uses. John Wiley & Sons; Chichester, UK: 2011. [Google Scholar]
- 5.Campagna S., Ceroni P., Puntoriero F., editors. Designing Dendrimers. John Wiley & Sons; Hoboken, NJ, USA: 2012. [Google Scholar]
- 6.Caminade A.-M., Turrin C.-O., Majoral J.-P., editors. Phosphorous Dendrimers in Biology and Nanomedicine: Syntheses, Characterization, and Properties. Pan Stanford Publishing; Singapore: 2018. [Google Scholar]
- 7.Majoral J.-P., Caminade A.-M. Dendrimers containing heteroatoms (Si, P, B, Ge, or Bi) Chem. Rev. 1999;99:845–880. doi: 10.1021/cr970414j. [DOI] [PubMed] [Google Scholar]
- 8.Majoral J.-P., Caminade A.-M. Nanomaterials based on phosphorus dendrimers. Acc. Chem. Res. 2004;37:341–348. doi: 10.1021/ar020077n. [DOI] [PubMed] [Google Scholar]
- 9.Caminade A.-M., Servin P., Laurent R., Majoral J.-P. Dendrimeric phosphines in asymmetric catalysis. Chem. Soc. Rev. 2008;37:56–67. doi: 10.1039/B606569B. [DOI] [PubMed] [Google Scholar]
- 10.Caminade A.-M., Majoral J.-P. Dendrimers and nanotubes: A fruitful association. Chem. Soc. Rev. 2010;39:2034–2047. doi: 10.1039/b926408f. [DOI] [PubMed] [Google Scholar]
- 11.Caminade A.-M., Ouali A., Keller M., Majoral J.-P. Organocatalysis with dendrimers. Chem. Soc. Rev. 2012;41:4113–4125. doi: 10.1039/c2cs35030k. [DOI] [PubMed] [Google Scholar]
- 12.Mignani S., El Kazzouli S., Bousmini M.M., Majoral J.-P. Dendrimer Space Exploration: An Assessment of Dendrimers/Dendritic Scaffolding as Inhibitors of Protein–Protein Interactions, a Potential New Area of Pharmaceutical Development. Chem. Rev. 2014;114:1327–1342. doi: 10.1021/cr400362r. [DOI] [PubMed] [Google Scholar]
- 13.Caminade A.-M., Ouali A., Laurent R., Turrin C.-O., Majoral J.-P. The dendritic effect illustrated with phosphorus dendrimers. Chem. Soc. Rev. 2015;44:3890–3899. doi: 10.1039/C4CS00261J. [DOI] [PubMed] [Google Scholar]
- 14.Mignani S., Rodrigues J., Tomas H., Zablocka M., Shi X., Caminade A.-M., Majoral J.-P. Dendrimers in combination with natural products and analogues as anti-cancer agents. Chem. Soc. Rev. 2018;47:514–532. doi: 10.1039/C7CS00550D. [DOI] [PubMed] [Google Scholar]
- 15.Nierengarten I., Nierengarten J.-F. The impact of the copper-catalyzed alkyne-azide 1,3-dipolar cycloaddition in fullerene chemistry. Chem. Rec. 2015;15:31–51. doi: 10.1002/tcr.201402081. [DOI] [PubMed] [Google Scholar]
- 16.Nierengarten I., Nierengarten J.-F. Fullerene Sugar Balls: A New Class of Biologically Active Fullerene Derivatives. Chem. Asian J. 2014;9:1436–1444. doi: 10.1002/asia.201400133. [DOI] [PubMed] [Google Scholar]
- 17.Ortiz Mellet C., Nierengarten J.-F., García Fernández J.M. Multivalency as an action principle in multimodal lectin recognition and glycosidase inhibition: A paradigm shift driven by carbon-based glyconanomaterials. J. Mater. Chem. B. 2017;5:6428–6436. doi: 10.1039/C7TB00860K. [DOI] [PubMed] [Google Scholar]
- 18.Nierengarten J.-F. Fullerene hexa-adduct scaffolding for the construction of giant molecules. Chem. Commun. 2017;53:11855–11868. doi: 10.1039/C7CC07479D. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y., Eguchi A., Kakehi K., Lee Y.C. Efficient Preparation of Glycoclusters from Silsesquioxanes. Org. Lett. 2004;6:3457–3460. doi: 10.1021/ol040043a. [DOI] [PubMed] [Google Scholar]
- 20.Ge Z., Wang D., Zhou Y., Liu H., Liu S. Synthesis of Organic/Inorganic Hybrid Quatrefoil-Shaped Star-Cyclic Polymer Containing a Polyhedral Oligomeric Silsesquioxane Core. Macromolecules. 2009;42:2903–2910. doi: 10.1021/ma802585k. [DOI] [Google Scholar]
- 21.Trastoy B., Pérez-Ojeda M.E., Sastre R., Chiara J.L. Octakis(3-azidopropyl)octasilsesquioxane: A Versatile Nanobuilding Block for the Efficient Preparation of Highly Functionalized Cube-Octameric Polyhedral Oligosilsesquioxane Frameworks Through Click Assembly. Chem. Eur. J. 2010;16:3833–3841. doi: 10.1002/chem.200902422. [DOI] [PubMed] [Google Scholar]
- 22.Ryu E.-H., Zhao Y. Efficient Synthesis of Water-Soluble Calixarenes Using Click Chemistry. Org. Lett. 2005;7:1035–1038. doi: 10.1021/ol047468h. [DOI] [PubMed] [Google Scholar]
- 23.Bew S.P., Brimage R.A., L’Hermite N., Sharma S.V. Upper Rim Appended Hybrid Calixarenes via Click Chemistry. Org. Lett. 2007;9:3713–3716. doi: 10.1021/ol071047t. [DOI] [PubMed] [Google Scholar]
- 24.Morales-Sanfrutos J., Ortega-Muñoz M., Lopez-Jaramillo J., Hernandez-Mateo F., Santoyo-Gonzalez F. Synthesis of Calixarene-Based Cavitands and Nanotubes by Click Chemistry. J. Org. Chem. 2008;73:7768–7771. doi: 10.1021/jo801325c. [DOI] [PubMed] [Google Scholar]
- 25.Cecioni S., Faure S., Darbost U., Bonnamour I., Parrot-Lopez H., Roy O., Taillefumier C., Wimmerová M., Praly J.-P., Imberty A., et al. Selectivity among Two Lectins: Probing the Effect of Topology, Multivalency and Flexibility of “Clicked” Multivalent Glycoclusters. Chem. Eur. J. 2011;17:2146–2159. doi: 10.1002/chem.201002635. [DOI] [PubMed] [Google Scholar]
- 26.Iehl J., Pereira de Freitas R., Delavaux-Nicot B., Nierengarten J.-F. Click chemistry for the efficient preparation of functionalized [60]fullerene hexakis-adducts. Chem. Commun. 2008;44:2450–2452. doi: 10.1039/b804393k. [DOI] [PubMed] [Google Scholar]
- 27.Iehl J., Nierengarten J.-F. A Click–Click Approach for the Preparation of Functionalized [5:1]-Hexaadducts of C60. Chem. Eur. J. 2009;15:7306–7309. doi: 10.1002/chem.200901291. [DOI] [PubMed] [Google Scholar]
- 28.Iehl J., Nierengarten J.-F. Sequential copper catalyzed alkyne–azide and thiol–ene click reactions for the multiple functionalization of fullerene hexaadducts. Chem. Commun. 2010;46:4160–4162. doi: 10.1039/c0cc00252f. [DOI] [PubMed] [Google Scholar]
- 29.Pierrat P., Vanderheiden S., Muller T., Bräse S. Functionalization of hexakis methanofullerene malonate crown-ethers: Promising octahedral building blocks for molecular networks. Chem. Commun. 2009:1748–1750. doi: 10.1039/b900367c. [DOI] [PubMed] [Google Scholar]
- 30.Pierrat P., Réthoré C., Muller T., Bräse S. Di- and Dodeca-Mitsunobu Reactions on C60 Derivatives: Post-Functionalization of Fullerene Mono- and Hexakis-Adducts. Chem. Eur. J. 2009;15:11458–11460. doi: 10.1002/chem.200902141. [DOI] [PubMed] [Google Scholar]
- 31.Sigwalt D., Caballero R., Holler M., Strub J.-M., Van Dorsselaer A., Nierengarten J.-F. Ultra-Fast Dendritic Growth Based on the Grafting of Fullerene Hexa-Adduct Macromonomers onto a Fullerene Core. Eur. J. Org. Chem. 2016:2882–2887. doi: 10.1002/ejoc.201600439. [DOI] [Google Scholar]
- 32.Fortgang P., Maisonhaute E., Amatore C., Delavaux-Nicot B., Iehl J., Nierengarten J.-F. Molecular Motion Inside an Adsorbed [5:1] Fullerene Hexaadduct Observed by Ultrafast Cyclic Voltammetry. Angew. Chem. Int. Ed. 2011;50:2364–2367. doi: 10.1002/anie.201007289. [DOI] [PubMed] [Google Scholar]
- 33.Iehl J., Holler M., Nierengarten J.-F., Yoosaf K., Malicka J.M., Armaroli N., Strub J.-M., Van Dorsselaer A., Delavaux-Nicot B. Photo-induced Energy Transfer in a Th-Symmetrical Hexakis-adduct of C60 Substituted with π-Conjugated Oligomers. Aust. J. Chem. 2011;64:153–159. doi: 10.1071/CH10319. [DOI] [Google Scholar]
- 34.Yoosaf K., Iehl J., Nierengarten I., Hmadeh M., Albrecht-Gary A.-M., Nierengarten J.-F., Armaroli N. A Supramolecular Photosynthetic Model Made of a Multiporphyrinic Array Constructed around a C60 Core and a C60-Imidazole Derivative. Chem. Eur. J. 2014;20:223–231. doi: 10.1002/chem.201303481. [DOI] [PubMed] [Google Scholar]
- 35.Gavat O., Trinh T.M.N., Moulin E., Ellis T., Maaloum M., Buhler E., Fleith G., Nierengarten J.-F., Giuseppone N. 3D supramolecular self-assembly of [60]fullerene hexaadducts decorated with triarylamine molecules. Chem. Commun. 2018;54:7657–7660. doi: 10.1039/C8CC04079F. [DOI] [PubMed] [Google Scholar]
- 36.Iehl J., Nierengarten J.-F., Harriman A., Bura T., Ziessel R. Artificial Light-Harvesting Arrays: Electronic Energy Migration and Trapping on a Sphere and between Spheres. J. Am. Chem. Soc. 2012;134:988–998. doi: 10.1021/ja206894z. [DOI] [PubMed] [Google Scholar]
- 37.Iehl J., Nguyen T.L.A., Frein S., Hahn U., Barberá J., Nierengarten J.-F., Deschenaux R. Designing liquid-crystalline dendronised hexa-adducts of [60]fullerene via click chemistry. Liq. Cryst. 2017;44:1852–1860. doi: 10.1080/02678292.2017.1336677. [DOI] [Google Scholar]
- 38.Guerra S., Iehl J., Holler M., Peterca M., Wilson D.A., Partridge B.E., Zhang S., Deschenaux R., Nierengarten J.-F., Percec V. Self-organisation of dodeca-dendronized fullerene into supramolecular discs and helical columns containing a nanowire-like core. Chem. Sci. 2015;6:3393–3401. doi: 10.1039/C5SC00449G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nierengarten J.-F., Iehl J., Oerthel V., Holler M., Illescas B.M., Muñoz A., Martín N., Rojo J., Sánchez-Navarro M., Cecioni S., et al. Fullerene sugar balls. Chem. Commun. 2010;46:3860–3862. doi: 10.1039/c0cc00034e. [DOI] [PubMed] [Google Scholar]
- 40.Durka M., Buffet K., Iehl J., Holler M., Nierengarten J.-F., Taganna J., Bouckaert J., Vincent S.P. The functional valency of dodecamannosylated fullerenes with Escherichia coli FimH—towards novel bacterial antiadhesives. Chem. Commun. 2011;47:1321–1323. doi: 10.1039/C0CC04468G. [DOI] [PubMed] [Google Scholar]
- 41.Cecioni S., Oerthel V., Iehl J., Holler M., Goyard D., Praly J.-P., Imberty A., Nierengarten J.-F., Vidal S. Synthesis of Dodecavalent Fullerene-Based Glycoclusters and Evaluation of Their Binding Properties towards a Bacterial Lectin. Chem. Eur. J. 2011;17:3252–3261. doi: 10.1002/chem.201003258. [DOI] [PubMed] [Google Scholar]
- 42.Compain P., Decroocq C., Iehl J., Holler M., Hazelard D., Mena Barragán T., Ortiz Mellet C., Nierengarten J.-F. Glycosidase Inhibition with Fullerene Iminosugar Balls: A Dramatic Multivalent Effect. Angew. Chem. Int. Ed. 2010;49:5753–5756. doi: 10.1002/anie.201002802. [DOI] [PubMed] [Google Scholar]
- 43.Sánchez-Navarro M., Muñoz A., Illescas B.M., Rojo J., Martín N. [60]Fullerene as Multivalent Scaffold: Efficient Molecular Recognition of Globular Glycofullerenes by Concanavalin-A. Chem. Eur. J. 2011;17:766–769. doi: 10.1002/chem.201002816. [DOI] [PubMed] [Google Scholar]
- 44.Buffet K., Gillon E., Holler M., Nierengarten J.-F., Imberty A., Vincent S.P. Fucofullerenes as tight ligands of RSL and LecB, two bacterial lectins. Org. Biomol. Chem. 2015;13:6482–6492. doi: 10.1039/C5OB00689A. [DOI] [PubMed] [Google Scholar]
- 45.Trinh T.M.N., Holler M., Schneider J.P., García Moreno M.I., García Fernández J.M., Bodlenner A., Compain P., Ortiz Mellet C., Nierengarten J.-F. Construction of giant glycosidase inhibitors from iminosugar-substituted fullerene macromonomers. J. Mater. Chem. B. 2017;5:6546–6556. doi: 10.1039/C7TB01052D. [DOI] [PubMed] [Google Scholar]
- 46.Luczkowiak J., Muñoz A., Sánchez-Navarro M., Ribeiro-Viana R., Ginieis A., Illescas B.M., Martín N., Delgado R., Rojo J. Glycofullerenes Inhibit Viral Infection. Biomacromol. 2013;14:431–437. doi: 10.1021/bm3016658. [DOI] [PubMed] [Google Scholar]
- 47.Muñoz A., Sigwalt D., Illescas B.M., Luczkowiak J., Rodríguez-Peréz L., Nierengarten I., Holler M., Remy J.-S., Buffet K., Vincent S.P., et al. Synthesis of giant globular multivalent glycofullerenes as potent inhibitors in a model of Ebola virus infection. Nat. Chem. 2016;8:50–57. doi: 10.1038/nchem.2387. [DOI] [PubMed] [Google Scholar]
- 48.Sigwalt D., Holler M., Iehl J., Nierengarten J.-F., Nothisen M., Morin E., Remy J.-S. Gene delivery with polycationic fullerene hexakis-adducts. Chem. Commun. 2011;47:4640–4642. doi: 10.1039/c0cc05783e. [DOI] [PubMed] [Google Scholar]
- 49.Ogoshi T., Kanai S., Fujinami S., Yamagishi T.-a., Nakamoto Y. para-Bridged Symmetrical Pillar[5]arenes: Their Lewis Acid Catalyzed Synthesis and Host–Guest Property. J. Am. Chem. Soc. 2008;130:5022–5023. doi: 10.1021/ja711260m. [DOI] [PubMed] [Google Scholar]
- 50.For a review on pillar[n]arenes, see: Cragg P.J., Sharma K. Pillar[5]arenes: Fascinating cyclophanes with a bright future. Chem. Soc. Rev. 2012;41:597–607. doi: 10.1039/C1CS15164A.
- 51.For a review on pillar[n]arenes, see: Strutt N.L., Zhang H., Schneebeli S.T., Stoddart J.F. Functionalizing Pillar[n]arenes. Acc. Chem. Res. 2014;47:2631–2642. doi: 10.1021/ar500177d.
- 52.For a review on pillar[n]arenes, see: Ogoshi T., Yamagishi T.-a., Nakamoto Y. Pillar-Shaped Macrocyclic Hosts Pillar[n]arenes: New Key Players for Supramolecular Chemistry. Chem. Rev. 2016;116:7937–8002. doi: 10.1021/acs.chemrev.5b00765.
- 53.Ogoshi T., Aoki T., Kitajima K., Fujinami S., Yamagishi T.-a., Nakamoto Y. Facile, Rapid, and High-Yield Synthesis of Pillar[5]arene from Commercially Available Reagents and Its X-ray Crystal Structure. J. Org. Chem. 2011;76:328–331. doi: 10.1021/jo1020823. [DOI] [PubMed] [Google Scholar]
- 54.Boinski T., Szumna A. A facile, moisture-insensitive method for synthesis of pillar[5]arenes—the solvent templation by halogen bonds. Tetrahedron. 2012;68:9419–9422. doi: 10.1016/j.tet.2012.09.006. [DOI] [Google Scholar]
- 55.Tao H.Q., Cao D.R., Liu L.Z., Kou Y.H., Wang L.Y., Meier H. Synthesis and host-guest properties of pillar[6]arenes. Sci. China Chem. 2012;55:223–228. doi: 10.1007/s11426-011-4427-3. [DOI] [Google Scholar]
- 56.Holler M., Allenbach N., Sonet J., Nierengarten J.-F. The high yielding synthesis of pillar[5]arenes under Friedel–Crafts conditions explained by dynamic covalent bond formation. Chem. Commun. 2012;48:2576–2578. doi: 10.1039/C2CC16795F. [DOI] [PubMed] [Google Scholar]
- 57.Kolb H.C., Finn M.G., Sharpless K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:112004::AID-ANIE20043.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 58.For a review, see: Kakuta T., Yamagashi T., Ogoshi T. Supramolecular chemistry of pillar[n]arenes functionalised by a copper(I)-catalysed alkyne–azide cycloaddition “click” reaction. Chem. Commun. 2017;53:5250–5266. doi: 10.1039/C7CC01833A.
- 59.Zhang H., Strutt N.L., Stoll R.S., Li H., Zhu Z., Stoddart J.F. Dynamic clicked surfaces based on functionalised pillar[5]arene. Chem. Commun. 2011;47:11420–11422. doi: 10.1039/c1cc14934b. [DOI] [PubMed] [Google Scholar]
- 60.Ogoshi T., Shiga R., Hashizume M., Yamagishi T.-A. “Clickable” pillar[5]arenes. Chem. Commun. 2011;47:6927–6929. doi: 10.1039/c1cc11864a. [DOI] [PubMed] [Google Scholar]
- 61.Yu G., Zhang Z., He J., Abliz Z., Huang F. Cavity-Extended Pillar[5]arenes: Syntheses and Host–Guest Complexation with Paraquat and Bispyridinium Derivatives. Eur. J. Org. Chem. 2012:5902–5907. doi: 10.1002/ejoc.201201003. [DOI] [Google Scholar]
- 62.Trinh T.M.N., Nierengarten I., Ben Aziza H., Meichsner E., Holler M., Chessé M., Abidi R., Bijani C., Coppel Y., Maisonhaute E., et al. Coordination-Driven Folding in Multi-ZnII-Porphyrin Arrays Constructed on a Pillar[5]arene Scaffold. Chem. Eur. J. 2017;23:11011–11021. doi: 10.1002/chem.201701622. [DOI] [PubMed] [Google Scholar]
- 63.Nierengarten J.-F. Weak Intramolecular Interactions to Stabilize Supramolecular Fullerene-Porphyrin Conjugates and to Control the Conformation of Multiporphyrinic Arrays. Eur. J. Inorg. Chem. 2019:4865–4878. doi: 10.1002/ejic.201900926. [DOI] [Google Scholar]
- 64.Steffenhagen M., Latus A., Trinh T.M.N., Nierengarten I., Lucas I.T., Joiret S., Landoulsi J., Delavaux-Nicot B., Nierengarten J.-F., Maisonhaute E. A Rotaxane Scaffold Bearing Multiple Redox Centers: Synthesis, Surface Modification and Electrochemical Properties. Chem. Eur. J. 2018;24:1701–1708. doi: 10.1002/chem.201705245. [DOI] [PubMed] [Google Scholar]
- 65.Delavaux-Nicot B., Ben Aziza H., Nierengarten I., Trinh T.M.N., Meichsner E., Chessé M., Holler M., Abidi R., Maisonhaute E., Nierengarten J.-F. A Rotaxane Scaffold for the Construction of Multiporphyrinic Light-Harvesting Devices. Chem. Eur. J. 2018;24:133–140. doi: 10.1002/chem.201704124. [DOI] [PubMed] [Google Scholar]
- 66.Nierengarten I., Nothisen M., Sigwalt D., Biellmann T., Holler M., Remy J.-S., Nierengarten J.-F. Polycationic Pillar[5]arene Derivatives: Interaction with DNA and Biological Applications. Chem. Eur. J. 2013;19:17552–17558. doi: 10.1002/chem.201303029. [DOI] [PubMed] [Google Scholar]
- 67.For another example of pillar[5]arene-based gene vector, see: Chang Y., Yang K., Wei P., Huang S., Pei Y., Zhao W., Pei Z. Cationic Vesicles Based on Amphiphilic Pillar[5]arene Capped with Ferrocenium: A Redox-Responsive System for Drug/siRNA Co-Delivery. Angew. Chem. Int. Ed. 2014;53:13126–13130. doi: 10.1002/anie.201407272.
- 68.For another example of pillar[5]arene-based gene vector, see: Yang K., Chang Y., Wen J., Lu Y., Pei Y., Cao S., Wang F., Pei Z. Supramolecular Vesicles Based on Complex of Trp-Modified Pillar[5]arene and Galactose Derivative for Synergistic and Targeted Drug Delivery. Chem. Mater. 2016;28:1990–1993. doi: 10.1021/acs.chemmater.6b00696.
- 69.Guillot-Nieckowski M., Eisler S., Diederich F. Dendritic vectors for gene transfection. New, J. Chem. 2007;31:1111–1127. doi: 10.1039/B614877H. [DOI] [Google Scholar]
- 70.For the first examples of glycopillar[5]arene derivatives, see: Nierengarten I., Buffet K., Holler M., Vincent S.P., Nierengarten J.-F. A mannosylated pillar[5]arene derivative: Chiral information transfer and antiadhesive properties against uropathogenic bacteria. Tetrahedron Lett. 2013;54:2398–2402. doi: 10.1016/j.tetlet.2013.02.100.
- 71.Yu G., Ma Y., Han C., Yao Y., Tang G., Mao Z., Gao C., Huang F. A Sugar-Functionalized Amphiphilic Pillar[5]arene: Synthesis, Self-Assembly in Water, and Application in Bacterial Cell Agglutination. J. Am. Chem. Soc. 2013;135:10310–10313. doi: 10.1021/ja405237q. [DOI] [PubMed] [Google Scholar]
- 72.Galanos N., Gillon E., Imberty A., Matthews S.E., Vidal S. Pentavalent pillar[5]arene-based glycoclusters and their multivalent binding to pathogenic bacterial lectins. Org. Biomol. Chem. 2016;14:3476–3481. doi: 10.1039/C6OB00220J. [DOI] [PubMed] [Google Scholar]
- 73.Li H., Chen Q., Schönbeck C., Han B.-H. Sugar-functionalized water-soluble pillar[5]arene and its host–guest interaction with fullerene. RSC Adv. 2015;5:19041–19047. doi: 10.1039/C4RA07523D. [DOI] [Google Scholar]
- 74.Buffet K., Nierengarten I., Galanos N., Gillon E., Holler M., Imberty A., Matthews S.E., Vidal S., Vincent S.P., Nierengarten J.-F. Pillar[5]arene-Based Glycoclusters: Synthesis and Multivalent Binding to Pathogenic Bacterial Lectins. Chem. Eur. J. 2016;22:2955–2963. doi: 10.1002/chem.201504921. [DOI] [PubMed] [Google Scholar]
- 75.Bernardi A., Jiménez-Barbero J., Casnati A., De Castro C., Darbre T., Fieschi F., Finne J., Funken H., Jaeger K.-E., Lahmann M., et al. Multivalent glycoconjugates as anti-pathogenic agents. Chem. Soc. Rev. 2013;42:4709–4727. doi: 10.1039/C2CS35408J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chabre Y.M., Roy R. Chapter 6—Design and Creativity in Synthesis of Multivalent Neoglycoconjugates. Adv. Carbohydr. Chem. Biochem. 2010;63:165–393. doi: 10.1016/S0065-2318(10)63006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Branson T.R., Turnbull W.B. Bacterial toxin inhibitors based on multivalent scaffolds. Chem. Soc. Rev. 2013;42:4613–4622. doi: 10.1039/C2CS35430F. [DOI] [PubMed] [Google Scholar]
- 78.Jiménez Blanco J.L., Ortiz Mellet C., García Fernández J.M. Multivalency in heterogeneous glycoenvironments: Hetero-glycoclusters, -glycopolymers and -glycoassemblies. Chem. Soc. Rev. 2013;42:4518–4531. doi: 10.1039/c3cs60089k. [DOI] [PubMed] [Google Scholar]
- 79.Wittman V., Pieters R.J. Bridging lectin binding sites by multivalent carbohydrates. Chem. Soc. Rev. 2013;42:4492–4503. doi: 10.1039/c3cs60089k. [DOI] [PubMed] [Google Scholar]
- 80.Sabin C., Mitchell E.P., Pokorná M., Gautier C., Utille J.-P., Wimmerová M., Imberty A. Binding of different monosaccharides by lectin PA-IIL from Pseudomonas aeruginosa: Thermodynamics data correlated with X-ray structures. FEBS Lett. 2006;580:982–987. doi: 10.1016/j.febslet.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 81.Audfray A., Claudinon J., Abounit S., Ruvoën-Clouet N., Larson G., Smith D.F., Wimmerová M., Le Pendu J., Römer W., Varrot A., et al. Fucose-binding Lectin from Opportunistic Pathogen Burkholderia ambifaria Binds to Both Plant and Human Oligosaccharidic Epitopes. J. Biol. Chem. 2012;287:4335–4347. doi: 10.1074/jbc.M111.314831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nierengarten I., Guerra S., Holler M., Nierengarten J.-F., Deschenaux R. Building liquid crystals from the 5-fold symmetrical pillar[5]arene core. Chem. Commun. 2012;48:8072–8074. doi: 10.1039/c2cc33746k. [DOI] [PubMed] [Google Scholar]
- 83.Nierengarten I., Guerra S., Holler M., Karmazin-Brelot L., Barberá J., Deschenaux R., Nierengarten J.-F. Macrocyclic Effects in the Mesomorphic Properties of Liquid-Crystalline Pillar[5]- and Pillar[6]arenes. Eur. J. Org. Chem. 2013:3675–3684. doi: 10.1002/ejoc.201300356. [DOI] [Google Scholar]
- 84.Pan S., Ni M., Mu B., Li Q., Hu X.-Y., Lin C., Chen D., Wang L. Well-Defined Pillararene-Based Azobenzene Liquid Crystalline Photoresponsive Materials and Their Thin Films with Photomodulated Surfaces. Adv. Funct. Mater. 2015;25:3571–3580. doi: 10.1002/adfm.201500942. [DOI] [Google Scholar]
- 85.Rosen B.M., Wilson C.J., Wilson D.A., Peterca M., Imam M.R., Percec V. Dendron-Mediated Self-Assembly, Disassembly, and Self-Organization of Complex Systems. Chem. Rev. 2009;109:6275–6540. doi: 10.1021/cr900157q. [DOI] [PubMed] [Google Scholar]
- 86.Nierengarten I., Guerra S., Ben Aziza H., Holler M., Abidi R., Barberá J., Deschenaux R., Nierengarten J.-F. Piling Up Pillar[5]arenes To Self-Assemble Nanotubes. Chem. Eur. J. 2016;22:6185–6189. doi: 10.1002/chem.201600688. [DOI] [PubMed] [Google Scholar]
- 87.For another example of liquid crystalline pillar[5]arene derivative, see: Concellón A., Romero P., Marcos M., Barberá J., Sánchez-Somolinos C., Mizobata M., Ogoshi T., Serano J.L., del Barrio J. Coumarin-Containing Pillar[5]arenes as Multifunctional Liquid Crystal Macrocycles. J. Org. Chem. 2020;85:8944–8951. doi: 10.1021/acs.joc.0c00852.
- 88.Ogoshi T., Yamagishi T. Pillar[5]- and pillar[6]arene-based supramolecular assemblies built by using their cavity-size-dependent host–guest interactions. Chem. Commun. 2014;50:4776–4787. doi: 10.1039/C4CC00738G. [DOI] [PubMed] [Google Scholar]
- 89.Li Q., Han K., Li J., Jia X., Li C. Synthesis of dendrimer-functionalized pillar[5]arenes and their self-assembly to dimeric and trimeric complexes. Tetrahedron Lett. 2015;56:3826–3829. doi: 10.1016/j.tetlet.2015.04.078. [DOI] [Google Scholar]
- 90.Wang W., Chen L.-J., Wang X.-Q., Sun B., Li X., Zhang Y., Shi J., Yu Y., Zhang L., Yang H.-B. Organometallic rotaxane dendrimers with fourth-generation mechanically interlocked branches. Proc. Natl. Acad. Sci. USA. 2015;112:5597–5601. doi: 10.1073/pnas.1500489112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu L., Yang H.-B. Our Expedition in Linear Neutral Platinum-Acetylide Complexes: The Preparation of Micro/nanostructure Materials, Complicated Topologies, and Dye-Sensitized Solar Cells. Chem. Rec. 2016;16:1274–1297. doi: 10.1002/tcr.201500271. [DOI] [PubMed] [Google Scholar]
- 92.Wang Y.-X., Zhou Q.-F., Chen L.-J., Xu L., Wang C.-H., Li X., Yang H.-B. Facile construction of organometallic rotaxane-terminated dendrimers using neutral platinum-acetylides as the main scaffold. Chem. Commun. 2018;54:2224–2227. doi: 10.1039/C7CC08729B. [DOI] [PubMed] [Google Scholar]
- 93.Wang X.-Q., Wang W., Li W.-J., Chen L.-J., Yao R., Yin G.-Q., Wang Y.-X., Zhang Y., Huang J.H., Tan H., et al. Dual stimuli-responsive rotaxane-branched dendrimers with reversible dimension modulation. Nat. Commun. 2018;9:3190. doi: 10.1038/s41467-018-05670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang X.-Q., Li W.-J., Wang W., Wen J., Zhang Y., Tan H., Yang H.-B. Construction of Type III-C Rotaxane-Branched Dendrimers and Their Anion-Induced Dimension Modulation Feature. J. Am. Chem. Soc. 2019;141:13923–13930. doi: 10.1021/jacs.9b06739. [DOI] [PubMed] [Google Scholar]
- 95.Li W.-J., Wang W., Wang X.-Q., Li M., Ke Y., Yao R., Wen J., Yin G.-Q., Jiang B., Li X., et al. Daisy Chain Dendrimers: Integrated Mechanically Interlocked Molecules with Stimuli-Induced Dimension Modulation Feature. J. Am. Chem. Soc. 2020;142:8473–8482. doi: 10.1021/jacs.0c02475. [DOI] [PubMed] [Google Scholar]
- 96.Li W.-J., Hu Z., Xu L., Wang X.-Q., Wang W., Yin G.-Q., Zhang D.-Y., Sun Z., Li X., Sun H., et al. Rotaxane-Branched Dendrimers with Enhanced Photosensitization. J. Am. Chem. Soc. 2020;142:16748–16756. doi: 10.1021/jacs.0c07292. [DOI] [PubMed] [Google Scholar]
- 97.Kwan C.-S., Leung K.C.-F. Development and advancement of rotaxane dendrimers as switchable macromolecular machines. Mater. Chem. Front. 2020;4:2825–2844. doi: 10.1039/D0QM00368A. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable to this article.