Abstract
Piper betle (L) is a popular medicinal plant in Asia. Plant leaves have been used as a traditional medicine to treat various health conditions. It is highly abundant and inexpensive, therefore promoting further research and industrialization development, including in the food and pharmaceutical industries. Articles published from 2010 to 2020 were reviewed in detail to show recent updates on the antibacterial and antifungal properties of betel leaves. This current review showed that betel leaves extract, essential oil, preparations, and isolates could inhibit microbial growth and kill various Gram-negative and Gram-positive bacteria as well as fungal species, including those that are multidrug-resistant and cause serious infectious diseases. P. betle leaves displayed high efficiency on Gram-negative bacteria such as Escherichia coli and Pseudomonas aeruginosa, Gram-positive bacteria such as Staphylococcus aureus, and Candida albicans. The ratio of MBC/MIC indicated bactericidal and bacteriostatic effects of P. betle leaves, while MFC/MIC values showed fungicidal and fungistatic effects. This review also provides a list of phytochemical compounds in betel leaves extracts and essential oils, safety profiles, and value-added products of betel leaves. Some studies also showed that the combination of betel leaves extract and essential oil with antibiotics (streptomycin, chloramphenicol and gentamicin) could provide potentiating antibacterial properties. Moreover, this review delivers a scientific resume for researchers in respected areas and manufacturers who want to develop betel leaves-based products.
Keywords: antibacterial, antifungal, betel leaves, Piper betle
1. Introduction
Piper betle (L) commonly known as betel vine belongs to the family Piperaceae. It is a popular medicinal plant in Asia. The leaf is the most widely used and studied part of the betel vine. There are chewing habit practices of betel leaves in many countries which are believed beneficial for avoiding bad breath, strengthening the gum, preserving the teeth, and stimulating the digestive system [1,2]. In traditional medicine practices, betel leaves are used for vaginal douching in Indonesia [3], as a gargle mouthwash in India and Thailand [4], and as a treatment for dental problems, headaches, arthritis, and joint pain in Malaysia [1]. In Srilanka, the betel leaf juice is used to treat skin ailments [5]. Additionally, its boiled leaves could be used as cough medicine, tonic, or astringent [2]. Traditional applications of betel leaves are related to their antibacterial and antifungal properties.
Over the past decades, antibacterial resistance has been threatening humans and has caused a global health crisis. Some bacterial strains are resistant to antibiotics such as vancomycin intermediate Staphylococcus aureus (VISA), vancomycin-resistant Enterococcus (VRE), methicillin-resistant S. aureus (MRSA), and extended spectrum β-lactamase (ESβL) enzyme producing Gram-negative bacteria, Pseudomonas aeruginosa, Streptococcus pneumoniae, S. aureus, and Mycobacterium tuberculosis, Enterococcus faecium, Klebsiella pneumoniae, Acinetobacter baumannii, and Enterobacter spp. [6,7]. Besides bacteria, fungi can also lead to infectious diseases. Approximately 300 fungal species on Earth are known to cause illnesses such as Candida spp. and dermatophytes [8,9]. Moreover, in the food industry, bacteria and fungi cause problems during product processing and storage. Food spoilage due to pathogen contamination is not only harmful to consumers but also brings heavy economic losses to manufacturers [10]. Therefore, research in this area continues to develop new safe and effective antimicrobial agents that could be applied in many related fields.
In this paper, a review of the literature was conducted to display recent studies (published in 2010–2020) on the antibacterial and antifungal properties of betel leaf extract (BLE), essential oil (BLEO), preparations, and isolates. In addition, the phytochemical constituents, safety profiles, and value-added products of betel leaves are also provided. Research on antibacterial and antifungal properties of betel leaves and their safety profiles have established their application as future active and additive ingredients in the pharmaceutical and food industries. Betel leaves are highly abundant and inexpensive, thus supporting their further development in manufacturing commercial products.
2. Phytochemicals in Betel Leaves
2.1. Betel Leaves Extract (BLE)
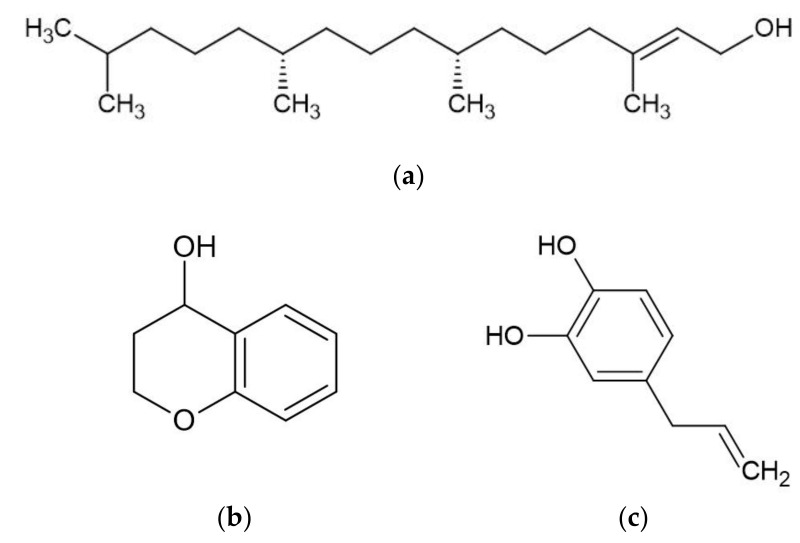
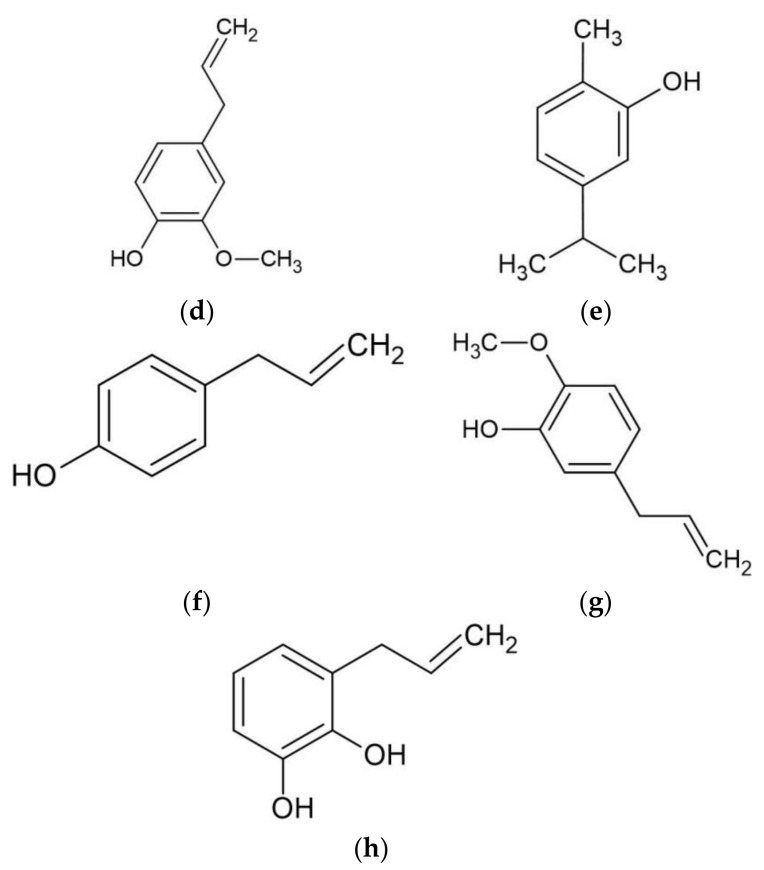
Piper betle contains numerous phytochemicals depending on its botanical origin and the solvent used for extraction. A preliminary phytochemical analysis of betel leaves from Malaysia showed that alkaloids, tannins, glycosides, reducing sugars, and saponins were found in the water extract of betel leaves [11]. Moreover, a study determined the total content of phenol, flavonoid, and tannin in water, ethanol, ethyl acetate, acetone, and dichloromethane extracts of betel leaves from Mauritius [12]. The highest total phenol, flavonoid, and tannin were found in the acetone, dichloromethane, and ethanol extracts, respectively. The sample of betel leaves collected from Tamilnadu, India is known to contain steroids, tannins, proteins, amino acids, flavonoids, terpenoids, mucilage, volatile oil, saponin, carbohydrates, and fixed oil, but an absence of alkaloids [13]. Furthermore, some studies have effectively isolated bioactive compounds from BLE (Figure 1) such as phytol, acyclic diterpene alcohol, 4-chromanol, hydroxychavicol or 4-allylpyrocatechol, and allylpyrocatechols 1 [14,15,16,17].
Figure 1.
Major bioactive compounds in betel leaves extracts and essential oil. (a) phytol; (b) 4-chromanol; (c) hydroxychavicol; (d) eugenol; (e) carvacrol; (f) chavicol; (g) chavibetol; (h) allylpyrocatechols 1.
2.2. Betel Leaves Essential Oil (BLEO)
Betel leaves contain 0.15% to 0.2% essential oil which are classified as monoterpenes, sesquiterpenes, phenylpropanoids, and aldehydes (Table 1). The constituents of BLEO are strongly dependent on its botanical origin, age of the plant, and harvesting time. Various compounds of BLEO may affect its aroma, taste, and bioactivity [18]. GC-MS analysis of BLEO from different places in India showed that phenylpropanoid groups such as acetyl eugenol, eugenol, chavicol, and safrole were the major components [19]. Interestingly, Indian BLEO obtained from the Sagar Bangla cultivar contained chavicol, but not from the Magahi cultivar. The study also revealed that BLEO contained eugenol (40%) and a combination of carvacrol and chavicol (up to 40%) with chavibetol as a marker compound as depicted in Figure 1. Meanwhile, another study found additional main compounds including estragole, linalool, α-copaene, anethole, and caryophyllene α-terpinene, p-cymene, 1,8-cineole, β-caryophyllene, α-humulene, allyl pyrocatechol, allylcatechol, methyl eugenol, estragol (methyl chavicol), chavibetol, chavibetol acetate, safrol, 4-allyl-2-methoxy-phenolacetate, and 3-allyl-6-methoxyphenol [18,20,21].
Table 1.
List of phytochemicals identified from betel leaf essential oil
| Classification | Compounds | Classification | Compounds |
|---|---|---|---|
| Monoterpenes | α-Thujene α-Pinene Camphene Sabinene Myrcene α-Terpinene β-Phellandrene 1,8-Cineole/Eucalyptol (E)-β-Ocimene γ-Terpinene Terpinolene Linalool Terpinen-4-ol α-Terpineol L-limonene Linalyl acetate |
Sesquiterpenes | δ-Elemene α-Copaene β-Elemene E-β-Caryophyllene β-Copaene γ-Elemene Aromadendrene α-Humulene γ-Muurolene Germacrene D Germacrene B β-Selinene α-Selinene Bicyclogermacrene α-Muurolene cis-β-Guaiene δ-Cadinene or δ-amorphene Palustrol Spathulenol Caryophyllene oxide Globulol Viridiflorol Cubenol α-Cadinol Ledene α-amorphene Cubebene |
| Phenylpropanoids | Estragole/Methyl chavicol Chavicol Anethole/Isoestragole Safrole Chavicol acetate Eugenol Methyl eugenol Acetyl eugenol Phenyl acetaldehyde |
Aldehydes | Undecanal Phenyl acetaldehyde |
3. Antibacterial Property of Betel Leaves
The extract, essential oil, preparation, and isolated compounds of betel leaves are effective against numerous Gram-negative (Table 2) and Gram-positive bacteria (Table 3). The bacteria tested included foodborne pathogens and other bacteria, including multidrug-resistant (MDR) bacteria that cause severe infectious diseases in humans. Most of the published research investigated the antibacterial activity of BLEs resulting from solvents with different polarities such as water, ethanol, ethyl acetate, acetone, and dichloromethane. Each extract contained diverse bioactive compounds which may affect their antibacterial activity [12,22]. The antibacterial tests of betel leaves were varied in methods and results, complicating the comparison between studies. Furthermore, the current review showed that the study of antibacterial activity of BLE was greater than that of BLEO.
Table 2.
Piper betle against Gram-negative bacteria.
| Extract/Preparation (Unit for Activities) | Method | Bacteria Species | Activities | Recalculated (%) | MBC/MIC | Inhibition Zone (mm) | Reference | ||
| MIC | MBC | MIC | MBC | ||||||
| Ethanol | Agar well diffusion | Pseudomenas aeruginosa | - | - | - | - | - | 6.7–7.2 | [11] |
| Escherichia coli | - | - | - | - | - | 8.9–11.0 | |||
| Water | Agar well diffusion | Pseudomenas aerugiaounosa | - | - | - | - | - | 7.2 | [11] |
| Escherichia coli | - | - | - | - | - | 8.5 | |||
| Ethanol ( µg/mL) | Disk diffusion | Escherichia coli ATCC 25922 | 625 | 625 | 0.0625 | 0.0625 | 1 * | 16 | [27] |
| Klebsiella pneumoniae ATCC BAA-1705 | 1250 | 1250 | 0.125 | 0.125 | 1 * | 17 | |||
| Pseudomenas aeruginosa ATCC 27853 | 625 | 625 | 0.0625 | 0.0625 | 1 * | 17 | |||
| MβL, Pseudomenas aeruginosa (CI) | 312 | 312 | 0.0312 | 0.0312 | 1 * | 17 | |||
| MβL, Acinetobacter baumannii (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 23 | |||
| ESβL, Escherichia coli (CI) | 312 | 625 | 0.0312 | 0.0625 | 2 * | 20 | |||
| ESβL, Klebsiella pneumoniae (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 20 | |||
| CRE, Klebsiella pneumoniae (CI) | 312 | 625 | 0.0312 | 0.0625 | 2 * | 21 | |||
| Ethyl acetate (µg/µL) | Micro dilution | Escherichia coli ATCC 25922 | 4.00 | - | 0.4 | - | - | - | [12] |
| Pseudomenas aeruginosa ATCC 27853 | 4.00 | - | 0.4 | - | - | - | |||
| Acetone (µg/µL) | Escherichia coli ATCC 25922 | 4.00 | - | 0.4 | - | - | - | [12] | |
| Pseudomenas aeruginosa ATCC 25922 | 4.00 | - | 0.4 | - | - | - | |||
| Ethanol (µg/mL) | Disc dilution & Broth microdilution | Escherichia coli ESβL(+) (CI) | 312 | 312 | 0.0312 | 0.0312 | 1 * | 20 | [29] |
| Klebsiella pneumoniae ESβL(+) (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 20 | |||
| Klebsiella pneumoniae CRE(+) 1 (CI) | 312 | 312 | 0.0312 | 0.0312 | 1 * | 21 | |||
| Klebsiella pneumoniae CRE(+) 2 (CI) | 312 | 312 | 0.0312 | 0.0312 | 1 * | 24 | |||
| Klebsiella pneumoniae CRE(+) 3 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 23 | |||
| Klebsiella pneumoniae CRE(+) 4 (CI) | 312 | 312 | 0.0312 | 0.0312 | 1 * | 23 | |||
| Serratia marcescens CRE(+) (CI) | 312 | 312 | 0.0312 | 0.0312 | 1 * | 20 | |||
| Pseudomonas aeruginosa MβL(+) 1 (CI) | 312 | 312 | 0.0312 | 0.0312 | 1 * | 17 | |||
| Pseudomonas aeruginosa MβL(+) 2 (CI) | 312 | 312 | 0.0312 | 0.0312 | 1 * | 19 | |||
| Pseudomonas aeruginosa MβL(+) 3 (CI) | 156 | 156 | 0.0156 | 0.0156 | 1* | 28 | |||
| Acinetobacter baumannii MβL(+) 1 (CI) | 625 | 625 | 0.0625 | 0.0625 | 2 * | 23 | |||
| Acinetobacter baumannii MβL(+) 2 (CI) | 156 | 312 | 0.0156 | 0.0312 | 2 * | 24 | |||
| Acinetobacter baumannii MβL(+) 3 (CI) | 312 | 312 | 0.0312 | 0.0312 | 1 * | 24 | |||
| Acinetobacter baumannii MβL(+) 4 (CI) | 312 | 312 | 0.0312 | 0.0312 | 1 * | 23 | |||
| Acinetobacter baumannii MβL(+) 5 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 26 | |||
| Methanol (µg/mL) | Disc dilution & Broth microdilution | Escherichia coli ESBL(+) (CI) | 312 | 312 | 0.0312 | 0.0312 | 1 * | 19 | [29] |
| Klebsiella pneumoniae ESβL(+) (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 19 | |||
| Klebsiella pneumoniae CRE(+) 1 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 21 | |||
| Klebsiella pneumoniae CRE(+) 2 (CI) | 312 | 312 | 0.0312 | 0.0312 | 1 * | 23 | |||
| Klebsiella pneumoniae CRE(+) 3 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 22 | |||
| Klebsiella pneumoniae CRE(+) 4 (CI) | 312 | 312 | 0.0312 | 0.0312 | 1 * | 22 | |||
| Serratia marcescens CRE(+) (CI) | 312 | 312 | 0.0312 | 0.0312 | 1 * | 19 | |||
| Pseudomonas aeruginosa MβL(+) 1 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 15 | |||
| Pseudomonas aeruginosa MβL(+) 2 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 18 | |||
| Pseudomonas aeruginosa MβL(+) 3 (CI) | 156 | 156 | 0.0156 | 0.0156 | 1 * | 27 | |||
| Acinetobacter baumannii MβL(+) 1 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 22 | |||
| Acinetobacter baumannii MβL(+) 2 (CI) | 625 | 1250 | 0.0625 | 0.125 | 2 * | 24 | |||
| Acinetobacter baumannii MβL(+) 3 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 23 | |||
| Acinetobacter baumannii MβL(+) 4 (CI) | 312 | 312 | 0.0312 | 0.0312 | 1 * | 22 | |||
| Acinetobacter baumannii MβL(+) 5 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 25 | |||
| SC-CO2 15MPa (µg/mL) | Disc dilution & Broth microdilution | Escherichia coli ESβL(+) (CI) | 625 | 1250 | 0.0625 | 0.125 | 2 * | 15 | [29] |
| Klebsiella pneumoniae ESβL(+) (CI) | 1250 | 1250 | 0.125 | 0.125 | 1 * | 15 | |||
| Klebsiella pneumoniae CRE(+) 1 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 15 | |||
| Klebsiella pneumoniae CRE(+) 2 (CI) | 625 | 1250 | 0.0625 | 0.125 | 2 * | 20 | |||
| Klebsiella pneumoniae CRE(+) 3 (CI) | 625 | 1250 | 0.0625 | 0.125 | 2 * | 16 | |||
| Klebsiella pneumoniae CRE(+) 4 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 16 | |||
| Serratia marcescens CRE(+) (CI) | 312 | 312 | 0.0312 | 0.0312 | 1 * | 18 | |||
| Pseudomonas aeruginosa MβL(+) 1 (CI) | 1250 | 1250 | 0.125 | 0.125 | 1 * | 11 | |||
| Pseudomonas aeruginosa MβL(+) 2 (CI) | 1250 | 1250 | 0.125 | 0.125 | 1 * | 14 | |||
| Pseudomonas aeruginosa MβL(+) 3 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 12 | |||
| Acinetobacter baumannii MβL(+) 1 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 20 | |||
| Acinetobacter baumannii MβL(+) 2 (CI) | 625 | 1250 | 0.0625 | 0.125 | 2 * | 20 | |||
| Acinetobacter baumannii MβL(+) 3 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 19 | |||
| Acinetobacter baumannii MβL(+) 4 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 18 | |||
| Acinetobacter baumannii MβL(+) 5 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 21 | |||
| SC-CO2 20MPa (µg/mL) | Disc dilution & Broth microdilution | Escherichia coli ESβL(+) (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 16 | [29] |
| Klebsiella pneumoniae ESβL(+) (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 16 | |||
| Klebsiella pneumoniae CRE(+) 1 (CI) | 312 | 312 | 0.0312 | 0.0312 | 1 * | 16 | |||
| Klebsiella pneumoniae CRE(+) 2 (CI) | 312 | 625 | 0.0312 | 0.0625 | 2 * | 20 | |||
| Klebsiella pneumoniae CRE(+) 3 (CI) | 625 | 625 | 0.0625 | 0.0625 | 2 * | 17 | |||
| Klebsiella pneumoniae CRE(+) 4 (CI) | 312 | 312 | 0.0312 | 0.0312 | 1 * | 17 | |||
| Serratia marcescens CRE(+) (CI) | 312 | 312 | 0.0312 | 0.0312 | 1 * | 18 | |||
| Pseudomonas aeruginosa MβL(+) 1 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 11 | |||
| Pseudomonas aeruginosa MβL(+) 2 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 15 | |||
| Pseudomonas aeruginosa MβL(+) 3 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 14 | |||
| Acinetobacter baumannii MβL(+) 1 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 22 | |||
| Acinetobacter baumannii MβL(+) 2 (CI) | 312 | 625 | 0.312 | 0.0625 | 2 * | 22 | |||
| Acinetobacter baumannii MβL(+) 3 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 22 | |||
| Acinetobacter baumannii MβL(+) 4 (CI) | 312 | 312 | 0.312 | 0.312 | 1 * | 21 | |||
| Acinetobacter baumannii MβL(+) 5 (CI) | 625 | 625 | 0.0625 | 0.0625 | 1 * | 24 | |||
| Ethanol (mg/mL) | Agar well diffusion & Broth microdilution | Aggregatibacter actino-mycetemcomitans ATCC 33384 | 1.04 | 2.08 | 0.104 | 0.208 | 2 * | ≥20 | [17] |
| Fusobacterium nucleatum ATCC 25586 | 1.30 | 2.08 | 0.13 | 0.208 | 1.6 * | ≥20 | |||
| Ethyl acetate | Broth dilution | Vibrio harveyi | 1600 | - | 0.16 | - | - | - | [32] |
| Extract-Ag nanoparticles | Kirby-Bauer’s Disc diffusion | Pseudomenas aeruginosa ATCC 27853 | - | - | - | - | - | 21.95 ± 0.45 | [25] |
| Salmonella typhi ATCC 14028 | - | - | - | - | - | 29.55 ± 0.45 | |||
| Escherichia coli ATCC 25922 | - | - | - | - | - | 27.12 ± 0.38 | |||
| Extract-CaO nanoparticles | Agar well diffusion | Escherichia coli ATCC 25922 | - | - | - | - | - | 18 | [28] |
| Pseudomonas aeruginosa ATCC 27853 | - | - | - | - | - | 13 | |||
| BLEO-nanoemulsion (µL/mL) | Microdilution plate | Escherichia coli MTCC 443 | 0.5–1 | 1–1.5 | 0.05–0.1 | 0.1–0.15 | 1–3 * | - | [26] |
| Klebsiella pneumoniae MTCC 432 | 1–1.25 | 2–2.5 | 0.1–0.125 | 0.2–0.25 | 1–2 * | - | |||
| Pseudomonas aeruginosa MTCC 424 | 0.5–0.75 | 1–1.5 µL/mL | 0.05–0.075 | 0.1–0.15 | 2 * | - | |||
| BLEO (mg/mL) | Micro-dilution broth & growth inhibitory assay | Acinetobacter baumannii (CI) | 8 | 8 | 0.8 | 0.8 | 1 * | - | [24] |
| Escherichia coli ATCC 25922 | 0.3 | 0.3 | 0.03 | 0.03 | 1 * | - | |||
| Escherichia coli (CI) | 2 | 2 | 0.2 | 0.2 | 1 * | - | |||
| Klebsiella pneumoniae (CI) | 4 | 4 | 0.4 | 0.4 | 1 * | - | |||
| Pseudomonas aeruginosa ATCC 27853 | 0.5 | 0.5 | 0.05 | 0.05 | 1 * | - | |||
| Pseudomonas aeruginosa (CI) | 2 | 2 | 0.2 | 0.2 | 1 * | - | |||
| Proteus vulgaris (CI) | 4 | 4 | 0.4 | 0.4 | 1 * | - | |||
| BLEO + Gentamicin (mg/mL) | Micro-dilution broth & growth inhibitory assay | Escherichia coli ATCC 25922 | 0.5-1 | - | 0.05–0.1 | - | - | - | [24] |
BLEO = betel leaves essential oil, ESβL = Extended spectrum β-lactamase, MRSA = Methicillin-resistant Staphylococcus aureus, MβL = metallo-β-lactam, - = data not available, * = bactericidal
Table 3.
Piper betle against Gram-positive bacteria.
| Extract/Preparation/Isolate (Unit for Activities) |
Method | BACTERIA SPECIES | Activitites | Recalculated (%) | MBC/MIC | Inhibition Zone (mm) | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | ||||||
| Ethanol | Agar well diffusion | Bacillus subtilis | - | - | - | - | - | 13.2–15.8 | [11] |
| Staphylococcus aureus | - | - | - | - | - | 9.7–18.0 | |||
| Micrococcus luteus | - | - | - | - | - | 5.0–5.4 | |||
| Water | Agar well diffusion | Bacillus subtilis | - | - | - | - | - | 4.9–6.8 | [11] |
| Staphylococcus aureus | - | - | - | - | - | 5.4–12.3 | |||
| Micrococcus luteus | - | - | - | - | – | 3.5–4.2 | |||
| Ethanol (µg/mL) | Disk diffusion | Staphylococcus aureus ATCC 29223 | 312 | 312 | 0.0312 | 0.0312 | 1 * | 30 | [27] |
| MRSA #1 (CI) | 156 | 312 | 0.0156 | 0.0312 | 2 * | 32 | |||
| MRSA #2 (CI) | 156 | 156 | 0.0156 | 0.0156 | 1 * | 34 | |||
| MRSA #3 (CI) | 156 | 156 | 0.0156 | 0.0156 | 1 * | 28 | |||
| MRSA #4 (CI) | 78 | 78 | 0.0078 | 0.0078 | 1 * | 34 | |||
| VRE | 19 | 19 | 0.0019 | 0.0019 | 1 * | 28 | |||
| Ethyl acetate (µg/µL) | Broth microdilution | Staphylococcus aureus ATCC 25923 | 0.50 | - | 0.0005 | - | - | - | [12] |
| Propionibacterium acnes ATCC 6919 | 2.00 | - | 0.002 | - | - | - | |||
| Staphylococcus epidermidis ATCC 12228 | 4.00 | - | 0.004 | - | - | - | |||
| Streptococcus pyogenes ATCC 19615 | 4.00 | - | 0.004 | - | - | - | |||
| Acetone (µg/µL) | Broth microdilution | Staphylococcus aureus ATCC 25923 | 0.25 | - | 0.00025 | - | - | - | [12] |
| Propionibacterium acnes ATCC 6919 | 2.00 | - | 0.002 | - | - | - | |||
| Staphylococcus epidermidis ATCC 12228 | 4.00 | - | 0.004 | - | - | - | |||
| Streptococcus pyogenes ATCC 19615 | 4.00 | - | 0.004 | - | - | - | |||
| Dichloromethane (µg/µL) | Broth microdilution | Staphylococcus aureus ATCC 25923 | 1.00 | - | 0.001 | - | - | - | [12] |
| Propionibacterium acnes ATCC 6919 | 4.00 | - | 0.004 | - | - | - | |||
| Staphylococcus epidermidis ATCC 12228 | 4.00 | - | 0.004 | - | - | - | |||
| Streptococcus pyogenes ATCC 19615 | 4.00 | - | 0.004 | - | - | - | |||
| Ethanol (µg/mL) | Disk diffusion | MRSA 1–7 | 78–156 | 78–312 | 0.0078–0.0156 | 0.0078–0.0312 | 1–2 * | 28–3833 | [29] |
| VRE 1–3 | 19–156 | 19–156 | 0.0019–0.0156 | 0.0019–0.0156 | 1 * | 25–3228 | |||
| Methanol (µg/mL) | Disk diffusion | MRSA 1–7 | 78–312 | 78–312 | 0.0078–0.0312 | 0.0078–0.0312 | 1–2 * | 28–3432 | [29] |
| VRE 1–3 | 19–156 µg/mL19µg/mL | 19–156 µg/mL19µg/mL | 0.0019–0.0156 | 0.0019–0.0156 | 1 * | 25–3226 | |||
| SC-CO2 15MPa (µg/mL) | Disk diffusion | MRSA 1–7 | 312–625 | 312–1250 | 0.0312–0.0625 | 0.0312–0.125 | 1 * | 21–3025 | [29] |
| VRE 1–3 | 19–156 | 19–156 | 0.0019–0.0156 | 0.0019–0.0156 | 1 * | 15–2820 | |||
| SC-CO2 20MPa (µg/mL) | Disk diffusion | MRSA 1–7 | 156–625 | 156–625 | 0.0156–0.0625 | 0.0156–0.0625 | 1 * | 22–3325 | [29] |
| VRE 1–3 | 19–156 | 19–156 | 0.0019–0.0156 | 0.0019–0.0156 | 1 * | 15–3124 | |||
| Ethanol (mg/mL) | Agar well diffusion & Broth microdilution | Enterobacter faecalis ATCC 19433 | 5.21 | 8.33 | 0.521 | 0.833 | 1.6 * | 10–20 | [17] |
| Lactobacillus fermentum ATCC 14931 | 4.17 | 8.33 | 0.417 | 0.833 | 2 * | 10–20 | |||
| Lactobacillus salivarius ATCC 11741 | 4.17 | 8.33 | 0.417 | 0.833 | 2 * | 10–20 | |||
| Streptococcus sobrinus ATCC 33478 | 1.56 | 3.17 | 0.156 | 0.317 | 2 * | ≥20 | |||
| Streptococcus mutans ATCC 25175 | 1.56 | 3.17 | 0.156 | 0.317 | 2 * | ≥20 | |||
| Hexane (µg/mL) | Disk diffusion | Streptococcus gordonii DMST 38731 | 1.00 | 2.00 | 0.0001 | 0.0002 | 2 * | 8.00 ± 0.00 | [30] |
| Streptococcus mutans DMST 18777 | 2.00 | 2.00 | 0.0002 | 0.0002 | 1 * | - | |||
| Ethyl acetate (µg/mL) | Streptococcus gordonii DMST 38731 | 0.50 | 2.00 | 0.00005 | 0.0002 | 4 ** | 12.50 ± 0.70 | [30] | |
| Streptococcus mutans DMST 18777 | 1.00 | 2.00 | 0.0001 | 0.0002 | 2 * | 11.00 ± 0.00 | |||
| Ethanol | Agar well diffusion | Staphylococcus aureus (CI) | - | - | - | - | - | 2..500–20.375 | [37] |
| Extract-Ag nanoparticles | Kirby-Bauer’s Disc diffusion | Staphylococcus aureus ATCC 25923 | - | - | - | - | - | 32.78 ± 0.64 | [25] |
| Extract-CaO nanoparticles | Agar well diffusion | Staphylococcus aureus ATCC 25923 | - | - | - | - | - | 13 | [28] |
| Streptococcus mutans MTCC 890 | - | - | - | - | - | 12 | |||
| BLEO-nanoemulsion (µL/mL) | Microdilution plate | Staphylococcus aureus MTCC 1144 | 0.5–0.75 | 1–1.5 | 0.05–0.075 | 0.1–0.15 | 2 * | [26] | |
| Bacillus cereus MTCC 1272 | 0.5–0.75 | 0.75–1.5 | 0.05–0.075 | 0.1–0.15 | 2 * | - | |||
| BLEO (mg/mL) | Micro-dilution broth & growth inhibitory assay | Escherichia faecalis (CI) | 4 | 4 | 0.4 | 0.4 | 1 * | [24] | |
| Propionibacterium acnes ATCC 6919 | 1 | 1 | 0.1 | 0.1 | 1 * | - | |||
| Staphylococcus aureus ATCC 25923 | 0.5 | 0.5 | 0.05 | 0.05 | 1 * | - | |||
| Staphylococcus epidermidis ATCC 12228 | 0.5 | 0.5 | 0.05 | 0.05 | 1 * | - | |||
| Streptococcus peroris (CI) | 2 | 2 | 0.2 | 0.2 | 1 * | - | |||
| MRSA (CI) | 8 | 8 | 0.8 | 0.8 | 1 * | - | |||
| BLEO+Gentamicin (mg/mL) | Micro-dilution broth & growth inhibitory assay | Staphylococcus epidermidis ATCC 12228 | 1-2 | - | 0.1–0.2 | - | - | [24] | |
| Allylpyrocatechols I (µg/mL) | Kirby–Bauer disk diffusion | Streptococcus sanguinis ATCC 10566 | 39.1 | 78.1 | 0.00391 | 0.00781 | 2 * | 11.85–25.15 | [15] |
| 4-allylpyrocatechol (µg/mL) | Broth microdilution | Streptococcus intermedius DMST 42700 | 200 | 500 | 0.02 | 0.05 | 2.5 * | - | [35] |
| Streptococcus mutans DMST 41283 | 200 | 500 | 0.02 | 0.05 | 2.5 * | - | |||
BLEO = betel leaves essential oil, CI = Clinical isolate, MRSA = Methicillin-resistant Staphylococcus aureus, VRE = vancomycin-resistant Enterococcus, - = data not available, * = bactericidal, ** = bacteriostatic.
A study showed that the ethanol extract of betel leaves was more effective than the water extract with greater inhibition zones. The ethanol extract at 50–100 µg/mL had the maximum inhibition zones (8.9–11.0 mm) on E. coli and moderate inhibition was observed on P. aeruginosa (<7.2 mm). Meanwhile, the water extract at 50 µg/mL did not actively inhibit bacterial growth [11]. Another investigation using the agar well diffusion method showed that the ethanol extract of betel leaves showed greater inhibition zones on Gram-negative than Gram-positive bacteria [17]. A study demonstrated the antibacterial effect of five types of BLE resulting from different polarities of solvents. Among these extracts, acetone and ethyl acetate extracts demonstrated the most remarkable activity against the six bacteria tested, with S. aureus being the most susceptible one. Moreover, the antibacterial property of BLEs was related to their phenol and flavonoid contents [12].
Other than the inhibition zone, the antibacterial activity was also presented as minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). MIC is defined as the lowest concentration of samples that inhibits microbial growth. Meanwhile, MBC is the lowest sample concentration at which 99.9% of the bacteria are killed [23]. For easier comparison, the MIC and MBC values from published articles were recalculated from μg/mL, mg/mL, and µg/µL to percentage (w/v or v/v).
The most frequently studied Gram-negative bacteria were laboratory strains of E. coli and P. aeruginosa with MIC range from 0.03 to 0.4% and 0.05–0.4%, respectively [12,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. Meanwhile, the lowest MIC (0.0156%) among Gram-negative bacteria was documented for clinical isolates of P. aeruginosa MβL(+) 3, A. baumannii MβL(+) 2, and P. aeruginosa MβL(+) 3 [29]. Additionally, S. aureus was the most commonly used Gram-negative bacteria to screen the antibacterial effect of betel leaves with MIC range from 0.00025 to 0.15% [12,24,25,26,27,28]. The lowest MIC among Gram-positive bacteria was recorded for an oral pathogen Streptococcus gordonii DMST 38731 (0.00005%) [30].
In this review, the MBC/MIC ratio was also measured to show the bacteriostatic and bactericidal effects of betel leaves. If the ratio is ≤2, the samples are considered to be bactericidal agents. The bacteriostatic mode of action is reflected when the ratio is ≥4 [31]. BLEO showed only a bactericidal effect and BLE was found to be bacteriostatic and bactericidal. The bactericidal action was reported against Gram-negative and Gram-positive bacteria, including those classified as MDR bacteria such as ESβL-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae (CRE), Metallo-β-lactamase (MβL)-producing P. aeruginosa and A. baumannii, MRSA, and VRE. On the other hand, a bacteriostatic effect was only observed against Gram-positive bacteria Streptococcus gordonii.
A previous study proved the promising antibacterial effect of BLE against oral pathogens including Gram-positive cariogenic bacteria and Gram-negative periodontal pathogenic bacteria. The study also found that 4-chromanol was the compound responsible for the antibacterial and antibiofilm properties of BLE [17]. Another study discovered the ability of BLE to control biofilm formation of Vibrio harveyi [32]. The antibacterial effect of BLE was dose-dependent. BLE was also found to be effective in reducing biofilm formation and extracellular polymeric substance production caused by P. aeruginosa and bacterial consortium without increasing the selective pressure for the growth of microorganisms [33]. Additionally, the ethyl acetate extract of betel leaves could act as antibiofilm agents against the nosocomial pathogen Serratia marcescens through the inhibition of quorum sensing mediated virulence factors production such as protease and lipase [16].
P. betle showed an outstanding antibacterial activity compared with other plants. The previous study compared the antibacterial activity of the ethanol extract of 12 plants from the Philippines, namely Cassia alata, Centella asiatica, Curcuma longa, Psidium guajava, Piper betle, Vitex negundo, Mitrephora lanotan, Moringa oleifera, Phyllanthus niruri, Tinospora rumphii, and Zingiber officinale, against clinical isolate of MRSA, VRE, ESβL-producing Enterobacteriaceae, CRE, and MβL-producing P. aeruginosa and A. baumannii. Piper betle was the only plant that showed potent bactericidal activity against all the bacteria tested with an MBC/MIC ratio between 1 to 2 [27]. Another investigation exhibited the higher antibacterial activity of ethanol extract from betel leaves compared to other medicinal plants such as Andrographis paniculata, Momordica charantia, Phyllantus emblica, Psidium guajava, and Sesbania grandiflora. The study also revealed that ethyl acetate fraction showed the strongest antimicrobial activity compared to hexane and ethanol fractions and crude ethanol extract. Further, the ethyl acetate fraction showed higher inhibition zones and MIC against Streptococcus gordonii than the positive control (chlorhexidine solution) [30].
It is noteworthy that natural products could provide additive antimicrobial activity and modify antibiotic resistance when combining with conventional antibiotics [34]. The synergistic effect was found in a combination of ethyl acetate or acetone extract of betel leaves and streptomycin and chloramphenicol against P. aeruginosa, S. aureus, Propionibacterium acnes, Staphylococcus epidermidis, and Streptococcus pyogenes. The highest synergy was observed when the acetone extract and chloramphenicol combination (70:30) was used against P. aeruginosa. However, there was no correlation between phytochemical content and the synergistic effect which indicated a different mechanism of action [12]. A study also revealed a potentiating effect of BLEO and gentamicin against Escherichia coli and S. epidermidis [24]. These results should be further confirmed to assure the effectiveness of betel leaves as an antibacterial potentiating agent.
Some research evaluated the antibacterial activity of the BLE or BLEO based preparation against different pathogens. The antibacterial activity of silver-BLE nanoparticles was found to be similar to standard drug (norfloxacin) against S. aureus. The nanoparticles also exhibited a bacteriostatic effect on Salmonella typhi, E. coli, and P. aeruginosa. Moreover, the previous study concluded that Gram-positive bacteria are more susceptible to silver-BLE nanoparticles rather than Gram-negative bacteria [25]. Another study also developed the green synthesis of CaO nanoparticles using the water extract of betel leaves. It showed maximum and minimum activity against E. coli and Streptococcus mutans, respectively [28]. Additionally, BLEO based nanoemulsion was observed to be effective against five strains of foodborne pathogens and can be used as a promising natural antibacterial agent in the food system [26].
The isolated phenolic compound of BLE, namely hydroxychavicol or allylpyrocatechols, were tested against Streptococcus sanguinis, a Gram-positive bacterium that contributes to caries [15]. The compound was a moderate antibacterial agent that functioned by blocking MurA that causes bacterial cell wall disruption. The result exhibited the potential of betel leaves as an alternative effective and efficient treatment for mechanical plaque removal through inhibition of bacterial growth. The isolate could also kill Streptococcus intermedius and S. mutans by a similar mechanism mentioned above. The study showed that the killing kinetic of 4-allylpyrocatechol was dose and pathogen dependent [35]. The overgrowth of these bacteria develops many serious oral infections and are the major cause of caries, gingivitis, and chronic periodontitis [36].
4. Antifungal Properties of Betel Leaves
Numerous methods have been applied to test the antifungal properties of betel leaves including solid dilution, broth dilution, micro-dilution, well diffusion, and solid diffusion assays, resulting in minimum inhibitory concentration (MIC), minimum fungicidal concentration (MFC), and inhibition zones (Table 4). Similar to antibacterial activity, recalculation of MIC and MFC, and measurement of MFC/MIC ratio to determine fungicidal and fungistatic effects, were also conducted. Candica albicans was the most screened fungal species with MIC ranging from 0.01% to 0.07% [2,24,30,35,38,39] The fungicidal effects of BLE and BLEO against various fungal species including Aspergillus flavus, Aspergillus fumigatus, Aspergillus niger, Aspergillus parasiticus, C. albicans, Candida glabrata, Candida krusei, Candida neoformans, Candida parapsilosis, Candida tropicalis, Epidermophyton floccosum, Trichophyton mentagrophytes, Trichophyton rubrum, Microsporum canis, and Microsporum gypseum [24,30,38,40]. Meanwhile, the fungistatic effect was only recorded from hexane and ethyl acetate extract of betel leaves against C. albicans [30], and its isolate, hyroxychavicol, against C. krusei [38]. A few of these species can contaminate food and spread aflatoxin, which is harmful to humans [18,41]. Other fungal species are clinically significant human pathogens that cause dental disorders and dermatophyte infections [2,14,35,40].
Table 4.
Piper betle against various fungal species.
| Extract/Preparation/Isolate (Unit for Activities) | Method | Fungal Species | Activities | Recalculated (%) | MFC/MIC | Inhibition Zone (mm) | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | ||||||
| Young leaves | [39] | ||||||||
| Ethanol (μg/mL) | Broth microdilution | Candida albicans (CI) | 500 | - | 0.05 | - | - | 8–15 | |
| Ethyl acetate (μg/mL) | Broth microdilution | Candida albicans (CI) | 250 | - | 0.025 | - | - | 10–22 | |
| Mature leaves | [39] | ||||||||
| Ethanol (μg/mL) | Broth microdilution | Candida albicans (CI) | 750 | - | 0.075 | - | - | 5–22 | |
| Ethyl acetate (μg/mL) | Broth microdilution | Candida albicans (CI) | 125 | - | 0.0125 | - | - | 17–26 | |
| Ethyl acetate | Well-diffusion | Aspergillus niger | - | - | - | - | - | 28 | [10] |
| Aspergillus sp. | - | - | - | - | - | 5 | |||
| Hexane | Well-diffusion | Aspergillus niger | - | - | - | - | - | 28 | [10] |
| Aspergillus sp. | - | - | - | - | - | 8 | |||
| Hexane (mg/mL) | Disk diffusion | Candida albicans DMST 8684 | 1.00 | 2.00 | 0.1 | 0.2 | 2 * | 21.00 ± 1.40 | [30] |
| Candida albicans DMST 5815 | 1.00 | 4.00 | 0.1 | 0.4 | 4 ** | 20.67 ± 0.58 | |||
| Ethyl acetate (mg/mL) | Disk diffusion | Candida albicans DMST 8684 | 0.50 | 2.00 | 0.05 | 0.2 | 4 ** | 23.00 ± 0.00 | [30] |
| Candida albicans DMST 5815 | 1.00 | 2.00 | 0.1 | 0.2 | 2 * | 24.33 ± 0.58 | |||
| BLEO (μL/mL) | Solid dilution | Alternaria alternate | 0.53 | - | 0.053 | - | - | - | [18] |
| Aspergillus candidus | 0.57 | - | 0.057 | - | - | - | |||
| Aspergillus flavus | 0.7 | - | 0.07 | - | - | - | |||
| Aspergillus fumigatus | 0.40 | - | 0.04 | - | - | - | |||
| Aspergillus niger | 0.73 | - | 0.073 | - | - | - | |||
| Aspergillus sydowi | 0.63 | - | 0.063 | - | - | - | |||
| Aspergillus terreus | 0.60 | - | 0.060 | - | - | - | |||
| Cladosporium cladosporoides | 0.67 | - | 0.067 | - | - | - | |||
| Culcularia lunata | 0.50 | - | 0.05 | - | - | - | |||
| Fusarium oxysporum | 0.50 | - | 0.05 | - | - | - | |||
| Mucor sp. | 0.37 | - | 0.037 | - | - | - | |||
| Mycelia sterilia | 0.30 | - | 0.03 | - | - | - | |||
| Nugrospora sp. | 0.53 | - | 0.053 | - | - | - | |||
| Penicillium italicum | 0.40 | - | 0.04 | - | - | - | |||
| BLEO (mg/mL) | Microdilution broth & growth inhibitory assay | Aspergillus niger ATCC 16404 | 2 | 2 | 0.2 | 0.2 | 1 * | - | [24] |
| Candida albicans ATCC 10231 | 1.5 | 1.5 | 0.15 | 0.15 | 1 * | - | |||
| Candida albicans (CI) | 2 | 2 | 0.2 | 0.2 | 1 * | - | |||
| Candida tropicalis ATCC 750 | 2 | 2 | 0.2 | 0.2 | 1 * | - | |||
| BLEO (μL/mL) | Broth microdilution | Trichophyton mentagrophytes (CI) | 0.2–0.4 | 0.4 | 0.00002–0.00004 | 0.00004 | 1–2 * | - | [40] |
| Trichophyton mentagrophytes DMST 19735 | 0.2–0.4 | 0.4 | 0.00002–0.00004 | 0.00004 | 1–2 * | - | |||
| Microsporum canis (CI) | 0.2–0.4 | 0.4 | 0.00002–0.00004 | 0.00004 | 1–2 * | - | |||
| Microsporum canis DMST 29297 | 0.2 | 0.4 | 0.00002–0.00004 | 0.00004 | 2 * | - | |||
| Microsporum gypseum (CI) | 0.4–0.8 | 0.8 | 0.00004–0.00008 | 0.00008 | 1–2 * | - | |||
| Microsporum gypseum DMST 21146 | 0.8 | 0.8 | 0.00008 | 0.00008 | 1 * | - | |||
| BLEO (%v/v) | Disk diffusion | Candida albicans ATCC 10231 | 0.078 | - | 0.078 | - | - | 33.83 + 0.76 | [2] |
| Candida glabrata ATCC 90030 | 0.039 | - | 0.039 | - | - | 33.83 + 0.76 | |||
| Candida krusei ATCC 6258 | 0.078 | - | 0.078 | - | - | 32.66 + 0.57 | |||
| Candida parapsilosis ATCC 22019 | 0.039 | - | 0.039 | - | - | 33.83 + 0.76 | |||
| Candida pseudotropicalis (CI) | 0.039 | - | 0.039 | - | - | 33.50+0.50 | |||
| Candida stellatoidia (CI) | 0.039 | - | 0.039 | - | - | 35.50+0.86 | |||
| Candida tropicalis (CI) | 0.078 | - | 0.078 | - | - | 30.83+0.28 | |||
| BLEO-microemulsion (μL/mL) | Broth dilution | Aspergillus flavus | - | 15 | - | 1.5 | - | - | [41] |
| Penicillium expansum | - | 15 | - | 1.5 | - | - | |||
| Hydroxychavicol (μg/mL) | Broth microdilution | Aspergillus flavus MTCC 1973, 2799 | 250 | 250 | 0.025 | 0.025 | 1 * | - | [38] |
| Aspergillus flavus (CI) | 125-500 | 125–500 | 0.0125–0.05 | 0.0125–0.05 | 1 * | ||||
| Aspergillus fumigatus MTCC 1811 | 250 | 250 | 0.025 | 0.025 | 1 * | - | |||
| Aspergillus niger ATCC 16404 | 125 | 125 | 0.0125 | 0.0125 | 1 * | - | |||
| Aspergillus niger (CI) | 125-250 | 125-250 | 0.0125–0.05 | 0.0125–0.05 | 1 * | ||||
| Aspergillus parasiticus MTCC 2796 | 250 | 250 | 0.025 | 0.025 | 1 * | - | |||
| Candida albicans ATCC 90028, 10231 | 250 | 250 | 0.025 | 0.025 | 1 * | - | |||
| Candida albicans (CI) | 125–500 | 250–500 | 0.0125–0.05 | 0.0125–0.05 | 1–2 * | ||||
| Candida glabrata ATCC 90030 | 31.25 | 31.25 | 0.003125 | 0.003125 | 1 * | - | |||
| Candida glabrata (CI) | 15.62–31.25 | 15.62–62.5 | 0.001562–0.003125 | 0.001562–0.00625 | 1–2 * | ||||
| Candida krusei ATCC 22019 | 15.62 | 62.5 | 0.001562 | 0.00625 | 4 ** | - | |||
| Candida krusei (CI) | 15.62–31.25 | 15.62–31.25 | 0.001562–0.003125 | 0.001562–0.003125 | 1 * | - | |||
| Candida neoformans ATCC 204092 | 62.5 | 62.5 | 0.00625 | 0.00625 | 1 * | - | |||
| Candida neoformans (CI) | 62.5 | 62.5 | 0.00625 | 0.00625 | 1 * | - | |||
| Candida parapsilosis ATCC 22019 | 31.25 | 31.25 | 0.003125 | 0.003125 | 1 * | - | |||
| Candida parapsilosis (CI) | 31,25–62.5 | 31,25–62.5 | 0.003125–0.00625 | 0.003125–0.00625 | 1 * | - | |||
| Candida tropicallis ATCC 750 | 250 | 250 | 0.025 | 0.025 | 1 * | - | |||
| Candida tropicallis (CI) | 125–500 | 250–500 | 0.0125–0.05 | 0.025–0.05 | 1–2 * | - | |||
| Epidermophyton floccosum MTCC 613 | 15.62 | 15.62 | 0.001562 | 0.001562 | 1 * | - | |||
| Epidermophyton floccosum (CI) | 15.62 | 31.25 | 0.001562 | 0.003125 | 2 * | ||||
| Microsporum canis MTCC 2820 | 15.62 | 31.25 | 0.001562 | 0.003125 | 2 * | - | |||
| Microsporum canis (CI) | 15.62 | 31.25 | 0.001562 | 0.003125 | 2 * | - | |||
| Micosporum gypsium MTCC 2819 | 15.62 | 31.25 | 0.001562 | 0.003125 | 2 * | - | |||
| Micosporum gypsium (CI) | 7.81–15.62 | 15.62–31.25 | 0.000781–0.001562 | 0.001562–0.003125 | 2 * | - | |||
| Trichophyton mentagrophytes ATCC 9533 | 15.62 | 15.62 | 0.001562 | 0.001562 | 1 * | - | |||
| Trichophyton mentagrophytes (CI) | 15.62–31.25 | 15.62–62.5 | 0.001562–0.003125 | 0.001562–0.00625 | 1–2 * | - | |||
| Trichophyton rubrum MTCC 296 | 31.25 | 31.25 | 0.003125 | 0.003125 | 1 * | - | |||
| Trichophyton rubrum (CI) | 15.62–62.5 | 31.25–62.5 | 0.001562–0.00625 | 0.003125–0.00625 | 1–2 * | - | |||
| 4-allylpyrocatechol (μg/mL) | Broth Microdilution | Candida albicans DMST 8684 | 400 | 500 | 0.04 | 0.05 | 1.25 * | - | [35] |
BLEO = betel leaves essential oil, CI = clinical isolate, MIC = minimum inhibitory concentration; MFC = minimum fungicidal concentration, - = Data not available, * = fungicidal, ** = fungistatic.
Ethanol and ethyl acetate extracts of betel leaves were found to be effective against C. albicans isolated from oral thrush patients. The ethyl acetate extract demonstrated the highest inhibition zone compared to extracts from another plant (Ocimum sanctum) and a standard drug (fluconazole) [39]. Other studies have also demonstrated the greater antifungal activity of ethyl acetate extract compared to hexane and ethanol extracts of betel leaves [10,30]. The killing kinetic study revealed that the fungistatic activity of the ethyl acetate extract was concentration-dependent. Furthermore, other research showed the anticandidal action of water extract from betel leaves. This effect was possibly related to its ability to reduce the cell surface hydrophobicity of several Candida species. Adhesion of fungal species and host tissues is crucial for fungal virulence, especially for successful colonization and infection. Hydrophobic domains in fungal surface proteins which consist of non-polar amino acids are a major factor involved in fungal adhesion. Thus a deviation in hydrophobic affinity produced by P. betle extract may influence the adherence mechanism of the fungal cell [42].
Some research investigated the antifungal activity of BLEO. A study showed that antifungal and aflatoxin suppressor actions of BLEO are related to its main components such as eugenol [18,40]. Eugenol contains a hydroxyl group that could form hydrogen bonds with the active site on fungal enzymes that are responsible for aflatoxin secretion and later causes denaturation [43]. Eugenol was also reported to induce fungal morphological abnormalities by changing or disrupting fungal cell wall structure, increasing cell membrane fluidity and permeability, and interfering with important regulator function [44]. Furthermore, docking simulation of eugenol acetate and chavicol acetate in BLEO showed strong interaction to amino acid constructing fungal protein structures, which is predicted to cause metabolic reduction and biomass breaking down, thus reducing fungal virulence [45].
The superior antifungal property of BLEO compared with essential oils from other Mauritius plants such as Psiadia argute, Psiadia terebinthina, Pimenta dioica, Salvia officinalis, Laurus nobilis, Rosmarinus officinalis, Cinnamomum zeylanicum, and Schinus terebinthifolius has been proven. The study revealed that BLEO was the strongest fungicidal agent with the lowest MIC against all the ATCC strains and clinical isolates fungi tested [24]. A formula of BLEO based microemulsion showed tremendous fungi toxic activity against a selected mold in raw apple juice at low concentration (<0.5 µL/mL). Meanwhile, spore inactivation of A. flavus and P. expansum by BLEO was found at a greater concentration (15 µL/mL) [41].
Hydroxychavicol or 4-allylpyrocatechol isolated from betel leaves was also reported to be effective against various fungi species. The compound could entirely kill C. albicans at a minimum concentration (400 μg/mL) [35]. The killing ability of hydroxychavicol against C. albicans and C. glabrata was dose-dependent. Hydrochavicol demonstrated fungicidal effects against other clinical isolates fungi, with the MICs ranging from 7.81 to 62.5 μg/mL for dermatophytes, 15.62 to 500 μg/mL for yeasts, and 125 to 500 μg/mL for Aspergillus species, while the MFCs were found to be equal or two-fold higher than the MICs [38]. Moreover, it could prevent biofilm formation and promote biofilm eradication [35,38]. The development of a biofilm, which is a network of microbial cells tightly adsorbed at the mucosal surface, is linked to a severe infection [46].
5. Safety Profiles of Betel Leaves
An acute toxicity study in both male and female ICR mice showed the safety of the methanol extract of betel leaves orally. The median lethal dose (LD50) of the extract was higher than 5000 mg/kg body weight [47]. There was also an evaluation of oral acute and sub-acute toxicity (28 days) and genotoxicity of an herbal formulation containing betel leaves alcoholic extract in rats and cellular models. This study revealed the absence of major adverse reactions [48]. Moreover, betel leaves were considered safe in terms of hematotoxicity, hepatotoxicity, genotoxicity, weights of organs, gross morphology, stress, or aversive behaviors in rats [49]. Another study discovered the nontoxicity of the ethanol extract of betel leaves on normal human dermal fibroblasts (HDFn) [29].
6. Commercial Application of Betel Leaves
There are some available commercial products containing betel leaves such as dietary supplements, mouthwash, medicinal products, and cosmetic and personal care goods including shampoo, soap, face cream, antiseptic lotions, toothpaste, and perfumes [50]. Current antimicrobial studies of betel leaves were focusing on oral pathogens, MDR Gram-negative and Gram-positive bacteria, and dermatophytes [17,29,30,38]. Thus, future development of medicinal products from betel leaves could be useful for preventing oral diseases, curing dermatophyte infections, and for the treatment and management of other infectious diseases. Additionally, a study has developed a simple, safe, cost-effective, and eco-friendly preparation of silver nanoparticles with polyaniline coating using water extract of betel leaves. The nanoparticles showed potential antibacterial properties and could be further studied in various applications such as medical devices and pharmaceutical and biomedical industries [25].
In the food industry, essential oil is a promising food additive to protect and enhance the shelf life of products during processing and storage. BLEO is an ideal food preservative agent due to its antifungal and antioxidant properties [18]. Many experiments have investigated the antimicrobial properties of BLEO against foodborne pathogens [18,26,41]. Moreover, BLEO is not only beneficial to prevent spoilage of food products but also guarantees their safety for consumer health especially due to the ability of BLEO to suppress aflatoxin production. Aflatoxin, a mycotoxin from A. flavus, is an example of fungal contamination in food products. The toxin is known to be hepatocarcinogenic, teratogenic, mutagenic, and immunosuppressive. An investigation revealed that BLEO in apple juice could deactivate spores or inhibit spore germination which is required to limit fungal infection and mycotoxin production [41]. Further research on the overall acceptability of sensory aspects of the essential oil-treated foodstuffs is necessary to avoid market failure of the product [51].
7. Conclusions and Outlook
The antibacterial and antifungal properties and safety profiles of betel leaves firmly support their application in the development of various products, especially in the food and pharmaceutical industries. The utilization of betel leaves in producing modern-commercial goods could increase the economy of local farmers, specifically in Asia. A good agricultural process should be applied to the farm to yield standardized raw material and should be followed by a good manufacturing process in industries to form high-quality final products. Additionally, clinical studies should be conducted to support the use of betel leaves in medical fields. Researcher, government, and manufacturer collaboration could facilitate this necessary task.
Acknowledgments
The authors would like to acknowledge the authorities of the Faculty of Pharmacy, Mahasaraswati University of Denpasar for the administrative and technical support.
Author Contributions
N.M.D.M.W.N.: Conceptualization, supervision, writing—original draft, writing—review and editing; M.M.V.S., D.A.S., P.E.S.K.Y., N.L.K.A.A.D., E.C.: Writing—original draft, project administration, R.H.: supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fazal F., Mane P.P., Rai M.P., Thilakchand K.R., Bhat H.P., Kamble P.S., Palatty P.L., Baliga M.S. The Phytochemistry, Traditional Uses and Pharmacology of Piper Betel. Linn (Betel Leaf): A Pan-Asiatic Medicinal Plant. Chin. J. Integr. Med. 2014 doi: 10.1007/s11655-013-1334-1. [DOI] [PubMed] [Google Scholar]
- 2.Kaypetch R., Thaweboon S. Antifungal Property of Piper Betle Leaf Oil against Oral Candida Species. Matec. Web Conf. 2018;242:01021. doi: 10.1051/matecconf/201824201021. [DOI] [Google Scholar]
- 3.Joesoef M.R., Sumampouw H., Linnan M., Schmid S., Idajadi A., St Louis M.E. Douching and Sexually Transmitted Diseases in Pregnant Women in Surabaya, Indonesia. Am. J. Obs. Gynecol. 1996;174:115–119. doi: 10.1016/S0002-9378(96)70382-4. [DOI] [PubMed] [Google Scholar]
- 4.Chowdhury U., Baruah P.K. Betelvine (Piper Betle L.): A Potential Source for Oral Care. Curr. Bot. 2020:87–92. doi: 10.25081/cb.2020.v11.6130. [DOI] [Google Scholar]
- 5.Arambewela L., Arawwawala M., Withanage D., Kulatunga S. Efficacy of Betel Cream on Skin Ailments. J. Complementary Integr. Med. 2010;7 doi: 10.2202/1553-3840.1391. [DOI] [Google Scholar]
- 6.Breijyeh Z., Jubeh B., Karaman R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules. 2020;25:1340. doi: 10.3390/molecules25061340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hafidh R.R., Abdulamir A.S., Vern L.S., Abu Bakar F., Abas F., Jahanshiri F., Sekawi Z. Inhibition of Growth of Highly Resistant Bacterial and Fungal Pathogens by a Natural Product. Open Microbiol. J. 2011;5:96–106. doi: 10.2174/1874285801105010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akpan A., Morgan R. Oral Candidiasis. Postgrad. Med. J. 2002;78:455–459. doi: 10.1136/pmj.78.922.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benedict K., Chiller T.M., Mody R.K. Invasive Fungal Infections Acquired from Contaminated Food or Nutritional Supplements: A Review of the Literature. Foodborne Pathog. Dis. 2016;13:343–349. doi: 10.1089/fpd.2015.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pawar S., Kalyankar V., Dhamangaonkar B., Dagade S., Waghmode S., Cukkemane A. Biochemical Profiling of Antifungal Activity of Betel Leaf (Piper Betle L.) Extract and Its Significance in Traditional Medicine. J. Adv. Res. Biotechnol. 2017;2:1–4. [Google Scholar]
- 11.Kaveti B., Tan L., Sarnnia , Kuan T.S., Baig M. Antibacterial Activity Of Piper Betel Leaves. Int. J. Pharm. Teach. Pract. 2011;2:129–132. [Google Scholar]
- 12.Taukoorah U., Lall N., Mahomoodally F. Piper Betle L. (Betel Quid) Shows Bacteriostatic, Additive, and Synergistic Antimicrobial Action When Combined with Conventional Antibiotics. S. Afr. J. Bot. 2016;105:133–140. doi: 10.1016/j.sajb.2016.01.006. [DOI] [Google Scholar]
- 13.Periyanayagam K., Jagadeesan M., Kavimani S., Vetriselvan T. Pharmacognostical and Phyto-Physicochemical Profile of the Leaves of Piper Betle L. Var Pachaikodi (Piperaceae)—Valuable Assessment of Its Quality—ScienceDirect. [(accessed on 22 February 2021)]; Available online: https://www.sciencedirect.com/science/article/abs/pii/S2221169112602627.
- 14.Ali A., Lim X.Y., Wahida P.F. The Fundamental Study of Antimicrobial Activity of Piper Betle Extract in Commercial Toothpastes. J. Herb. Med. 2018;14:29–34. doi: 10.1016/j.hermed.2018.08.001. [DOI] [Google Scholar]
- 15.Kurnia D., Hutabarat G.S., Windaryanti D., Herlina T., Herdiyati Y., Satari M.H. Potential Allylpyrocatechol Derivatives as Antibacterial Agent Against Oral Pathogen of S. Sanguinis ATCC 10,556 and as Inhibitor of MurA Enzymes: In Vitro and in Silico Study. Drug Des. Devel. 2020;14:2977–2985. doi: 10.2147/DDDT.S255269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srinivasan R., Devi K.R., Kannappan A., Pandian S.K., Ravi A.V. Piper Betle and Its Bioactive Metabolite Phytol Mitigates Quorum Sensing Mediated Virulence Factors and Biofilm of Nosocomial Pathogen Serratia Marcescens in Vitro. J. Ethnopharmacol. 2016;193:592–603. doi: 10.1016/j.jep.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Teanpaisan R., Kawsud P., Pahumunto N., Puripattanavong J. Screening for Antibacterial and Antibiofilm Activity in Thai Medicinal Plant Extracts against Oral Microorganisms. J. Tradit. Complementary Med. 2017;7:172–177. doi: 10.1016/j.jtcme.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prakash B., Shukla R., Singh P., Kumar A., Mishra P.K., Dubey N.K. Efficacy of Chemically Characterized Piper betle L. Essential Oil against Fungal and Aflatoxin Contamination of Some Edible Commodities and Its Antioxidant Activity. Int. J. Food Microbiol. 2010;142:114–119. doi: 10.1016/j.ijfoodmicro.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Karak S., Acharya J., Begum S., Mazumdar I., Kundu R., De B. Essential Oil of Piper Betle L. Leaves: Chemical Composition, Anti-Acetylcholinesterase, Anti-β-Glucuronidase and Cytotoxic Properties. J. Appl. Res. Med. Aromat. Plants. 2018;10:85–92. doi: 10.1016/j.jarmap.2018.06.006. [DOI] [Google Scholar]
- 20.Salehi B., Zakaria Z.A., Gyawali R., Ibrahim S.A., Rajkovic J., Shinwari Z.K., Khan T., Sharifi-Rad J., Ozleyen A., Turkdonmez E., et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules. 2019;24:1364. doi: 10.3390/molecules24071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madhumita M., Guha P., Nag A. Extraction of Betel Leaves (Piper Betle L.) Essential Oil and Its Bio-Actives Identification: Process Optimization, GC-MS Analysis and Anti-Microbial Activity. Ind. Crop. Prod. 2019;138:111578. doi: 10.1016/j.indcrop.2019.111578. [DOI] [Google Scholar]
- 22.Tan Y.P., Chan E.W.C. Antioxidant, Antityrosinase and Antibacterial Properties of Fresh and Processed Leaves of Anacardium Occidentale and Piper Betle. Food Biosci. 2014;6:17–23. doi: 10.1016/j.fbio.2014.03.001. [DOI] [Google Scholar]
- 23.Mogana R., Adhikari A., Tzar M.N., Ramliza R., Wiart C. Antibacterial Activities of the Extracts, Fractions and Isolated Compounds from Canarium Patentinervium Miq. against Bacterial Clinical Isolates. Bmc Complementary Med. Ther. 2020;20:55. doi: 10.1186/s12906-020-2837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aumeeruddy-Elalfi Z., Gurib-Fakim A., Mahomoodally F. Antimicrobial, Antibiotic Potentiating Activity and Phytochemical Profile of Essential Oils from Exotic and Endemic Medicinal Plants of Mauritius. Ind. Crop. Prod. 2015;71:197–204. doi: 10.1016/j.indcrop.2015.03.058. [DOI] [Google Scholar]
- 25.Rashida M., Islam I., Haque A., Rahman A., Hossain T., Hamid A. Antibacterial Activity of Polyaniline Coated Silver Nanoparticles Synthesized from Piper Betle Leaves Extract. Iran. J. Pharm. Res. 2016;15:591–597. [PMC free article] [PubMed] [Google Scholar]
- 26.Roy A., Guha P. Formulation and Characterization of Betel Leaf (Piper Betle L.) Essential Oil Based Nanoemulsion and Its in Vitro Antibacterial Efficacy against Selected Food Pathogens. J. Food Process. Preserv. 2018;42:e13617. doi: 10.1111/jfpp.13617. [DOI] [Google Scholar]
- 27.Valle D.L., Andrade J.I., Puzon J.J.M., Cabrera E.C., Rivera W.L. Antibacterial Activities of Ethanol Extracts of Philippine Medicinal Plants against Multidrug-Resistant Bacteria. Asian Pac. J. Trop. Biomed. 2015;5:532–540. doi: 10.1016/j.apjtb.2015.04.005. [DOI] [Google Scholar]
- 28.Yoonus J., Resmi. R., Beena B. Greener Nanoscience: Piper Betel Leaf Extract Mediated Synthesis of CaO Nanoparticles and Evaluation of Its Antibacterial and Anticancer Activity. Mater. Today Proc. 2020 doi: 10.1016/j.matpr.2020.05.246. [DOI] [Google Scholar]
- 29.Valle D.L., Cabrera E.C., Puzon J.J.M., Rivera W.L. Antimicrobial Activities of Methanol, Ethanol and Supercritical CO2 Extracts of Philippine Piper Betle L. on Clinical Isolates of Gram Positive and Gram Negative Bacteria with Transferable Multiple Drug Resistance. PLoS ONE. 2016;11:e0146349. doi: 10.1371/journal.pone.0146349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phumat P., Khongkhunthian S., Wanachantararak P., Okonogi S. Potential of Piper Betle Extracts on Inhibition of Oral Pathogens. Drug Discov. 2017;11:307–315. doi: 10.5582/ddt.2017.01061. [DOI] [PubMed] [Google Scholar]
- 31.Abdi R.D., Kerro Dego O. Antimicrobial Activity of Persicaria Pensylvanica Extract against Staphylococcus Aureus. Eur. J. Integr. Med. 2019;29:100921. doi: 10.1016/j.eujim.2019.05.007. [DOI] [Google Scholar]
- 32.Srinivasan R., Santhakumari S., Ravi A.V. In Vitro Antibiofilm Efficacy of Piper Betle against Quorum Sensing Mediated Biofilm Formation of Luminescent Vibrio Harveyi. Microb. Pathog. 2017;110:232–239. doi: 10.1016/j.micpath.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Siddiqui M.F., Sakinah M., Ismail A.F., Matsuura T., Zularisam A.W. The Anti-Biofouling Effect of Piper Betle Extract against Pseudomonas Aeruginosa and Bacterial Consortium. Desalination. 2012;288:24–30. doi: 10.1016/j.desal.2011.11.060. [DOI] [Google Scholar]
- 34.Barbieri R., Coppo E., Marchese A., Daglia M., Sobarzo-Sánchez E., Nabavi S.F., Nabavi S.M. Phytochemicals for Human Disease: An Update on Plant-Derived Compounds Antibacterial Activity. Microbiol. Res. 2017;196:44–68. doi: 10.1016/j.micres.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Phumat P., Khongkhunthian S., Wanachantararak P., Okonogi S. Comparative Inhibitory Effects of 4-Allylpyrocatechol Isolated from Piper Betle on Streptococcus Intermedius, Streptococcus Mutans, and Candida Albicans. Arch. Oral Biol. 2020;113:104690. doi: 10.1016/j.archoralbio.2020.104690. [DOI] [PubMed] [Google Scholar]
- 36.Faran Ali S.M., Tanwir F. Oral Microbial Habitat a Dynamic Entity. J. Oral Biol. Craniofac. Res. 2012;2:181–187. doi: 10.1016/j.jobcr.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lubis R.R., Marlisa , Wahyuni D.D. Antibacterial Activity of Betle Leaf (Piper Betle L.) Extract on Inhibiting Staphylococcus Aureus in Conjunctivitis Patient. Am. J. Clin. Exp. Immunol. 2020;9:1–5. [PMC free article] [PubMed] [Google Scholar]
- 38.Ali I., Khan F.G., Suri K.A., Gupta B.D., Satti N.K., Dutt P., Afrin F., Qazi G.N., Khan I.A. In Vitro Antifungal Activity of Hydroxychavicol Isolated from Piper Betle L. Ann. Clin. Microbiol. Antimicrob. 2010;9:7. doi: 10.1186/1476-0711-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sivareddy B., Reginald B.A., Sireesha D., Samatha M., Reddy K.H., Subrahamanyam G. Antifungal Activity of Solvent Extracts of Piper Betle and Ocimum Sanctum Linn on Candida Albicans: An in Vitro Comparative Study. J. Oral Maxillofac. Pathol. 2019;23:333–337. doi: 10.4103/jomfp.JOMFP_167_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aiemsaard J., Punareewattana K. Antifungal Activities of Essential Oils of Syzygium Aromaticum, Piper Betle, and Ocimum Sanctum against Clinical Isolates of Canine Dermatophytes. Sci. Asia. 2017;43:223. doi: 10.2306/scienceasia1513-1874.2017.43.223. [DOI] [Google Scholar]
- 41.Basak S., Guha P. Use of Predictive Model to Describe Sporicidal and Cell Viability Efficacy of Betel Leaf (Piper Betle L.) Essential Oil on Aspergillus Flavus and Penicillium Expansum and Its Antifungal Activity in Raw Apple Juice. LWT. 2017;80:510–516. doi: 10.1016/j.lwt.2017.03.024. [DOI] [Google Scholar]
- 42.Nordin M.-A.-F., Wan Harun W.H.A., Abdul Razak F. An in Vitro Study on the Anti-Adherence Effect of Brucea Javanica and Piper Betle Extracts towards Oral Candida. Arch. Oral Biol. 2013;58:1335–1342. doi: 10.1016/j.archoralbio.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Bluma R., Amaiden M.R., Etcheverry M. Screening of Argentine Plant Extracts: Impact on Growth Parameters and Aflatoxin B1 Accumulation by Aspergillus Section Flavi. Int. J. Food Microbiol. 2008;122:114–125. doi: 10.1016/j.ijfoodmicro.2007.11.050. [DOI] [PubMed] [Google Scholar]
- 44.de Oliveira Pereira F., Mendes J.M., de Oliveira Lima E. Investigation on Mechanism of Antifungal Activity of Eugenol against Trichophyton Rubrum. Med. Mycol. 2013;51:507–513. doi: 10.3109/13693786.2012.742966. [DOI] [PubMed] [Google Scholar]
- 45.Thuy B.T.P., Hieu L.T., My T.T.A., Hai N.T.T., Loan H.T.P., Thuy N.T.T., Triet N.T., Van Anh T.T., Dieu N.T.X., Quy P.T., et al. Screening for Streptococcus Pyogenes Antibacterial and Candida Albicans Antifungal Bioactivities of Organic Compounds in Natural Essential Oils of Piper Betle L., Cleistocalyx Operculatus L. and Ageratum Conyzoides L. Chem. Pap. 2020;75:1507–1519. doi: 10.1007/s11696-020-01404-x. [DOI] [Google Scholar]
- 46.Pozo J.L.D. Biofilm-Related Disease. Expert Rev. Anti-Infect. Ther. 2018;16:51–65. doi: 10.1080/14787210.2018.1417036. [DOI] [PubMed] [Google Scholar]
- 47.Al-Adhroey A.H., Nor Z.M., Al-Mekhlafi H.M., Amran A.A., Mahmud R. Antimalarial Activity of Methanolic Leaf Extract of Piper Betle L. Molecules. 2010;16:107–118. doi: 10.3390/molecules16010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sengupta K., Mishra A.T., Rao M.K., Sarma K.V., Krishnaraju A.V., Trimurtulu G. Efficacy of an Herbal Formulation LI10903F Containing Dolichos Biflorus and Piper Betle Extracts on Weight Management. Lipids Health Dis. 2012;11:176. doi: 10.1186/1476-511X-11-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arambewela L.S.R., Arawwawala L.D.A.M., Kumaratunga K.G., Dissanayake D.S., Ratnasooriya W.D., Kumarasingha S.P. Investigations on Piper Betle Grown in Sri Lanka. Pharm. Rev. 2011;5:159–163. doi: 10.4103/0973-7847.91111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madhumita M., Guha P., Nag A. Bio-Actives of Betel Leaf (Piper Betle L.): A Comprehensive Review on Extraction, Isolation, Characterization, and Biological Activity. Phytother. Res. 2020;34:2609–2627. doi: 10.1002/ptr.6715. [DOI] [PubMed] [Google Scholar]
- 51.Basak S. The Use of Fuzzy Logic to Determine the Concentration of Betel Leaf Essential Oil and Its Potency as a Juice Preservative. Food Chem. 2018;240:1113–1120. doi: 10.1016/j.foodchem.2017.08.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.