Abstract
The available treatments for patients affected by Alzheimer’s disease (AD) are not curative. Numerous clinical trials have failed during the past decades. Therefore, scientists need to explore new avenues to tackle this disease. In the present review, we briefly summarize the pathological mechanisms of AD known so far, based on which different therapeutic tools have been designed. Then, we focus on a specific approach that is targeting astrocytes. Indeed, these non-neuronal brain cells respond to any insult, injury, or disease of the brain, including AD. The study of astrocytes is complicated by the fact that they exert a plethora of homeostatic functions, and their disease-induced changes could be context-, time-, and disease specific. However, this complex but fervent area of research has produced a large amount of data targeting different astrocytic functions using pharmacological approaches. Here, we review the most recent literature findings that have been published in the last five years to stimulate new hypotheses and ideas to work on, highlighting the peculiar ability of palmitoylethanolamide to modulate astrocytes according to their morpho-functional state, which ultimately suggests a possible potential disease-modifying therapeutic approach for AD.
Keywords: Alzheimer’s disease, astrocytes, astrogliosis, beta amyloid, neuroinflammation, neuroprotection, reactive gliosis, palmitoylethanolamide
1. Introduction
Aducanumab, a monoclonal antibody directed against the aggregated form of the beta-amyloid peptide (Aβ), was the last unfruitful attempt to treat Alzheimer’s disease (AD). At the beginning of November 2020, experts of the Peripheral and Central Nervous System Drugs Advisory Committee of the Food and Drug Administration expressed some concerns about the real efficacy of aducanumab, thus hindering its marketing claiming [1]. The AD field had high expectations for the aducanumab clinical trials, primarily because this human IgG1 monoclonal antibody was designed to selectively bind Aβ aggregates, including soluble oligomers and insoluble fibrils but not monomers [2], suggesting the possibility to overcome previously failed approaches of other anti-Aβ antibodies. Unfortunately, the aims were never met, despite they had been well demonstrated at the preclinical level and in the early stages of the clinical trial. This event reveals once again the limitations of both basic and medical research anxiously focused on counteracting Aβ in AD [3].
AD is the most common form of dementia in the elderly, affecting about 47 million people worldwide [4]. Most AD cases are sporadic, affecting people older than 65 years old, and aging represents the greatest risk factor [5]. As life expectancy increases, it is reasonable to foresee that the number of AD patients will grow in the next decades. However, other risk factors have been identified besides old age. Growing epidemiological data support the existence of a link between metabolic disorders and AD [6,7,8,9,10], and a correlation between head injury and future risk of dementia has also been suggested. The risk of developing AD or vascular dementia is increased in many pathological conditions of the heart and blood vessels, including heart failure, diabetes, stroke, high blood pressure, and high cholesterol level [11]. Family history and heredity are the most important risk factors for the genetic form of this disease, which affects about 1% of individuals with AD, and whose symptoms appear as early as 35 years old [12]. In contrast to heredity and aging, which are nonmodifiable factors, other risk factors could be controlled through general lifestyle improvement and effective management of unhealthy conditions. Indeed, healthy aging, which includes both physical and mental exercise, a balanced diet, staying socially active, and avoiding smoking, preserves both body and brain wellness and reduces the risk for developing dementia [13,14,15].
At a molecular level, the presence of two peculiar hallmarks characterizes the AD brain: (I) senile plaques, formed by the deposition of Aβ peptides in the extracellular space, and (II) neurofibrillary tangles (NFTs), due to the hyperphosphorylation of microtubule-associated tau proteins. A growing body of evidence indicates, however, that senile plaques and NFTs alone are not responsible for the cognitive impairments observed in AD [16]. Neuroinflammation and abnormal astrocytic and microglia responses exert a pivotal role in AD pathogenesis and progression, thus highlighting the complexity of this pathology [17,18,19].
AD can be considered as a continuum that spans decades [20], with brain modifications that begin 10–20 years before the clinical manifestations and change throughout the disease progression [21]. Various clinical stages have been classified, such as asymptomatic preclinical, prodromal, mild, moderate, and severe AD [22,23], also referred to as stage 1 to stage 6 [24,25]. Each stage is characterized by peculiar molecular changes that could represent possible targets for different therapeutic approaches [26,27,28].
AD is a neurodegenerative disease that impacts memory and cognition. In addition to the progressive impairment in mental abilities, other debilitating noncognitive symptoms usually appear, including sleep disturbances, loss of appetite, and neuropsychiatric conditions, including depression and/or apathy [29,30]. In the latest stages, symptoms worsen enough to interfere with daily activities such that people suffering from AD need continuous care. As a result, the economic burden of AD is impressive, mainly because currently approved drugs are not curative. Despite decades of intense research, no treatments are available to halt, slow, or cure AD, and the therapy still relies on cholinesterase inhibitors (donepezil, rivastigmine, and galantamine), and the N-methyl-D-aspartate (NMDA) antagonist memantine. Any of these drugs slightly help to manage behavioral symptoms, preserve mental skills, and slow down the disease progression. However, their effects are reversible and lessen over time due to the continued progression of the disease [31,32].
A final and confirmed diagnosis of AD can only be made through postmortem identification of histopathological hallmarks. Whenever a patient is suspected to have AD, he/she is already in a mild or moderate stage of the pathology, and substantial irreversible neuronal dysfunction and loss have already occurred. Nowadays, clinicians concur that intervening at the earliest stage of the disease could lead to a better outcome [22,33]. To do so, it will be necessary to identify biological markers allowing diagnosis in the asymptomatic (or, at most, prodromal) stage of the disease to recognize asymptomatic at-risk individuals and refer them to the use of disease-modifying drugs. This approach could be insidious and difficult to achieve since it falls into the field of preventive care. Despite the preclinical stage of AD could represent a temporal window in which it may be possible to reduce the incidence and progression of the disease [34], few preclinical data are available so far at this stage of the pathology [35]. To develop preventive therapeutic approaches for AD in the coming years, the key neurobiological mechanisms of AD need to be clarified.
In this review, we discuss the most recent findings on both old and new mechanisms implicated in AD, with a particular reference to the role played by glial cells. The brain homeostatic functions exerted by glia could represent a novel perspective in AD management, offering new strategies to treat this disease.
2. Old and New Pathophysiological Mechanisms in AD
2.1. The Amyloid Cascade Hypothesis
The correlation between Aβ deposition and dementia has extensively been studied during the past decades, and the amyloidogenic pathway has been widely investigated as a target for drug development [36]. Alois Alzheimer himself described the presence of plaques during the histological examination of his patient’s—named Augustine—brain [37,38]. Later after, such plaques were recognized to be protein deposits, mainly Aβ peptides [39,40]. Several forms of Aβ peptides have been found in AD brains [41,42]. Longitudinal PET studies demonstrated that proteins begin to deposit about two decades before first symptoms appear [43]; thus, plaque formation is a slow and prolonged process. Plaques accumulate extensively throughout the cortex, with the occipital and temporal lobes being the most affected [44].
Aβ peptides are generated by the cleavage of the type I transmembrane amyloid precursor protein (APP), a protein expressed ubiquitously, which biological functions remain unclear [45,46]. APP is particularly abundant in the brain, and evidence showed that it has trophic properties [47]. It plays a role in brain development by promoting neural stem cells (NSCs) proliferation, cell differentiation, and neuronal maturation [46,48]. APP seems necessary for synaptogenesis, synapse remodeling, and neurite outgrowth [49,50], as well as axonal outgrowth after injury in the adult brain [51]. A neuroregenerative role for brain APP has been hypothesized, even if the molecular mechanisms have not been elucidated yet. The production of APP increases in some physiological conditions, such as during neuronal maturation and differentiation, and in some pathological ones, including AD, brain trauma, and Down syndrome [52]. To complicate the picture, alternative transcriptional splicing could create 8 to 11 different APP isoforms [53].
The enzymatic processing of APP yields various peptides with distinct functions through three different proteolytic pathways, among which only one seems to be amyloidogenic. This process releases mainly two monomers of Aβ: about 90% is Aβ40, which is considered nontoxic because it does not self-aggregate much, and the remaining part is mainly constituted of longer Aβ peptides [54]. Being more hydrophobic and prone to aggregate than the shorter isoforms, the Aβ42 and Aβ43 could form oligomers and fibrils; thus, they are considered neurotoxic isoforms [36,55]. In addition to Aβ42 and Aβ43, some reports consider also the amyloid precursor protein intracellular domain to be involved in the pathophysiology of AD [52,56,57].
The nonamyloidogenic pathway is thought not to generate toxic Aβ and a third proteolytic pathway has been recently described, involving a η-secretase that cuts the APP extracellular domain releasing a soluble ectodomain. The biological functions of all peptides yielded through this newly described pathway are yet to be disclosed.
Although most of the research studies investigated the neurotoxicity of Aβ peptides, they also exert biological functions. They are not abundantly expressed, even in AD brains [58], and they execute trophic actions, including cell fate specification and proliferation. Exogenous application of soluble and fibrillary Aβ peptides (but not oligomeric forms) stimulates human embryonic stem cells (ESCs) proliferation [59]. Oligomeric Aβ peptides reduce the proliferative potential of human NSC, promoting their differentiation toward glial instead of neuronal cells [60]. The Aβ40 seems to preferably enhance neurogenesis, whereas the Aβ42 seems to promote gliogenesis [61,62]. Some authors have also observed neurogenesis induced by oligomers of Aβ42, and not Aβ40, in rat hippocampal NSCs [63]. Further studies are warranted since these contradictory results are probably due to the different forms of Aβ used.
The amyloid cascade hypothesis states that the progressive accumulation and oligomerization of Aβ42 creates diffuse plaques in the brain parenchyma, causing neuroinflammation and, later, neurofibrillary tangles, ultimately leading to synaptic dysfunction or loss, and neuronal death [36,64]. This hypothesis has been formulated after having identified the APP gene on chromosome 21, together with the observation that people affected by Down syndrome develop AD-like symptoms early in life. Several pathogenic coding mutations in the APP gene have been identified and linked to the onset of autosomal dominant AD [64,65,66]. This hypothesis is supported by the correlation between autosomal dominant mutations in both APP and genes coding for parts of the secretase, such as presenilin (PSEN) 1, PSEN2, with the incidence of AD [36,64,67].
However, reduced clearance of Aβ peptides could also account for their accumulation in the brain. A protein involved both in the clearance of Aβ and in its ability to aggregate and form fibrils is the apolipoprotein E (apoE) [68,69]. Homozygous carriers for the isoform ε4 have about a 12-fold higher risk to develop sporadic AD, while carriers of the less frequent ε2 isoform show a low risk for AD [70,71]. Despite all the findings that strongly support the amyloid cascade hypothesis, other data suggest instead that the accumulation of senile plaques in the brain does not correlate with cognitive impairment. Indeed, massive cerebral accumulation of Aβ plaques has also been observed in individuals without any cognitive impairment. Additionally, the reduction of Aβ load by immunotherapy does not improve cognition in AD patients [72]. Furthermore, all clinical trials carried out so far targeting either the production or the accumulation of Aβ have failed. The debate is fervent in the literature and undoubtfully more studies are needed to clarify the precise mechanism(s) by which Aβ deposits lead to tangle formation, and thus neurodegeneration [3].
2.2. Neurofibrillary Tangles
Neurofibrillary tangles are considered essential for the neuropathological diagnosis of AD [26]. They are intraneuronal bundles of filaments made of hyperphosphorylated microtubule-associated tau proteins [73]. Their accumulation causes a loss of cytoskeletal microtubules and tubulin-associated proteins, resulting in morphological modifications in neuronal dendrites and axons [74].
Since NFTs appearance in the brain seems to follow a pattern, in a seminal paper, Braak and Braak proposed to classify AD in six stages based on neuropathological findings [44,75].
At physiological conditions, the protein tau is mainly localized in the axon, and it is essential for the stabilization of microtubules [76]. Its phosphorylation is highly probable because tau has 85 potential sites of phosphorylation that are easily accessible because of the unfolded structure of the protein [77]. Tau has been found mislocalized (missorted) into the somatodendritic compartment at the early stages of AD. Since NFTs load correlates with cognitive decline and synapse loss [74], a role for tau missorting has been proposed in AD [78], which serves as diagnostic criteria and for the staging of disease progression [79]. Interestingly, abnormal phosphorylation of tau is detectable even before NFTs formation. In agreement, the reduction of tau has beneficial effects in preclinical AD models, whereas tau mislocalization from axons to dendrites has detrimental effects [80,81]. In general, the major modifications of tau found in AD are hyperphosphorylation, missorting, aggregation to oligomers and filaments forming paired helical filaments, dissociation from microtubules, and other post-translational modifications [78].
Mutations in genes encoding for tau have not been linked to AD. However, tau knockout mice show very mild neurite outgrowth changes and no microtubule-related defects [82,83]. Human findings showed that microtubule density is decreased in AD patients, but this reduction is surprisingly unrelated to tau abnormalities [84]. Consistent with the above, a simple loss of function of tau is not enough to explain the loss of microtubules observed in AD, and other mechanisms are probably involved.
Several tau-targeting therapies for AD have been proposed. These approaches are based mainly on (i) inhibition of kinases (responsible for aberrant tau phosphorylation), (ii) inhibition of tau aggregation, and (iii) stabilization of microtubules. Immunotherapies targeting tau in clinical trials have shown high toxicity and/or lack of efficacy and have been discontinued [85].
2.3. Unfolded Protein Response and Defective Proteostasis
AD is a neurological disease characterized by the ubiquitous association of misfolded and aggregated proteins, whose role in the pathogenesis and progression of the disease is still unclear. However, it is reasonable to hypothesize that a significant dysfunction in protein homeostasis (proteostasis) occurs. Proteostasis is complex since it requires proteins to be in a specific localization, aggregation, concentration, and conformation. Multiple events occurring in AD have been suggested to act as proteostasis perturbators, including NFTs [86], neuroinflammation [87], altered calcium signaling [88], mitochondrial energy imbalance [89], and oxidative stress [90]. Most of these have been linked to endoplasmic reticulum (ER) stress [91]. The ER is an essential organelle in eukaryotes responsible for the synthesis and folding of all secretory and membrane proteins [92]. Under physiological conditions, when aberrant proteins are synthesized, the ER exports them to the cytosol, where they are directed to the ubiquitin–proteasome system for degradation [93]. In AD, the massive accumulation of aberrant misfolded proteins at the ER engages the unfolded protein response (UPR), a complex signaling system stress response that orchestrates protein folding and initiates apoptosis, or autophagy, in irreversibly damaged cells [94]. Growing evidence indicates that ER stress responses may also affect metabolic pathways that generate Aβ, suggesting its direct role in AD etiology. For instance, it has been demonstrated that UPR signaling events increase BACE1 levels, causing Aβ overproduction and promoting the transcription of the PSEN gene [95].
2.4. Complement Cascade and Neuroinflammation
Inflammation has been recognized as a key component of AD pathology [96], likely contributing even to the progression of the disease [97,98]. Several transcription factors involved in the inflammatory responses have been found involved in AD. For example, the CCAAT/enhancer-binding protein (c/EBP) family of transcription factors is elevated in brains from AD patients, compared to healthy controls [99], and it was found to promote microglial neuroinflammatory response [100]. Another example is the NF-kB pathway that controls cytokine production and cell survival, which is strongly associated with AD neuroinflammation [101].
Both the classical and alternative complement pathways are induced in vitro by fibrillar Aβ [102] and NFTs [103]. Senile plaques colocalize with microglia and many proteins of the complement cascade in animal models of the disease and human AD [62,104,105,106]. Moreover, human AD brains show signs of activation of the complement in the same areas presenting senile plaques and NFTs [107]. Complement factors have been shown to be elevated during AD progression, likely as a general reaction to abnormal protein deposition and other cerebral injuries that occur in the AD brain [108,109,110]. This is not surprising, since the complement cascade is a fundamental effector of the innate immune system that favors the rapid clearance of pathogens, apoptotic cells, and their debris, as well as the extent and termination of the inflammatory immune response [111]. Some components of the complement cascade play a key role in synapse pruning. This process is active and fundamental during the development of the nervous system. However, it is scarcely seen in the adult brain when its occurrence is thought to be detrimental, as in AD brains. Indeed, evidence of excessive complement-mediated synapse pruning has been reported in AD and animal models of aging [112,113,114]. Regardless, some human evidence shows inconsistency between blood and cerebrospinal fluid (CSF) concentration of complement proteins [110], highlighting the heterogenicity of the pathology, which complicates the path to use complement proteins as diagnostic biomarkers. However, components of the complement could be also potential novel therapeutic targets [111,115]. In preclinical models of neurodegenerative disorders, the inhibition of specific complement proteins had beneficial effects [116,117]. Unfortunately, the blood–brain barrier (BBB) is not accessible to current complement-targeted therapeutics, making drug design challenging [117]. Additionally, the molecular mechanisms underlying the inflammatory process observed in AD have not been fully clarified yet. This could explain the failure of the clinical trials conducted so far using conventional anti-inflammatory drugs [118,119,120,121,122].
Neuroinflammation is a complex defensive process crucial for the preservation of brain homeostasis that becomes detrimental under certain circumstances, which is not fully understood. It is now accepted that any cerebral insult triggers the activation of glial cells in a defensive, preservative process aimed at restoring the lost homeostasis. Both morphological and functional modifications of mainly, but not exclusively, microglia and astrocytes occur accompanied by a pro-inflammatory environment [19]. Microglia cells, being the immune sentinels of the central nervous system (CNS), are the first cells responding with a potent inflammatory response, consequently leading to the activation of other glial cell types, including astrocytes [123,124]. If the stimuli that activate glial cells are very intense, and/or long lasting, and/or not counterbalanced by an interruption signal, reactive gliosis could be established and the normal brain functioning could be altered, leading even to neuronal death [125]. However, the exact timing and mechanisms that turn neuroinflammation from a physiological to a pathological process are still under study [126,127]. Therefore, the clarification of the underlying molecular and cellular mechanisms could allow scientists to develop and test new, and hopefully efficacious, pharmacological treatments. For example, a recent study identified a negative regulator of the transcription factor c/EBPb, responsible for microglia-mediated neuroinflammation, which could represent a novel AD therapeutic target [100]. Of note, c/EBPb is expressed also by astrocytes. Thus, additional studies should address the possibilities of targeting it in different cell types involved in the neuroinflammatory process.
2.5. The Neuroenergetic Hypothesis
Glucose is the main brain energy fuel, which crosses the BBB through GLUT1, a membrane-bound glucose transporter. Both aging and AD are associated with a reduction of GLUT1 [128,129]. Additionally, transgenic mouse models show a correlation between the decreased density of GLUT1 and Aβ peptide accumulation [129,130]. In aged humans, an association between glucose hypometabolism and apoE genotype has been made [131]. The main signaling that mediates the uptake of glucose inside cells is the interaction of the pancreatic hormone insulin with its receptor. AD demented patients show a high level of plasma insulin, while low levels of both CSF insulin and brain insulin receptors. In accordance, insulin resistance has been correlated with dementia, and patients with type-2 diabetes have a much higher risk to develop AD [132]. Indeed, glucose acts as a memory enhancer since the neuronal activity is tightly coupled to glucose utilization [133]. Using 5xFAD mice as an AD model, Andersen et al. showed that neuronal GABA synthesis in the brain is directly affected by glucose hypometabolism in astrocytes [134]. Under normal conditions, astrocytes produce ATP and lactate that are released to feed neighboring neurons, in a process known as the astrocyte–neuron lactate shuttle, that energetically supports neurons given their high-energy requirements, such as action potential firing [135,136,137]. This shuttle is necessary for long-term potentiation [135]. Berchtold et al. reported that many genes involved in mitochondrial bioenergetics were upregulated in aged individuals with mild cognitive impairment (MCI), relative to age-matched controls, but downregulated in full-blown AD patients [138]. All this evidence contributed to the so-called neuroenergetic hypothesis, which posits that the chronic progressing starving of brain cells could produce energy-deficiency stress. This reduces neuronal firing and induces a shift from pathways associated with physiological APP metabolism to pathological ones, related to Aβ/tau production [139], ultimately leading to AD.
3. Astrocytes as Targets for AD Therapeutics
In the beginning, the interest in glial cells in AD arose mainly from the role played by the microglia cells in the immune response [140]. Afterward, it became clear that all types of glial cells were probably involved in both the etiology and progression of the disease, as actors in the context of the immune response and key regulating elements involved in the molecular and cellular processes altered in AD [141]. Indeed, cell-type-specific transcriptomic changes in human AD brains have been associated with distinct molecular pathways [142].
Glial cells are a heterogeneous cell population exerting a plethora of different actions necessary for the correct functioning of the brain [143]. Glial cells are usually classified into microglia and macroglia. The latter have a neural origin and include astrocytes, oligodendrocytes, and NG-2 glia, also known as synantocytes [144].
Microglia are the main immunocompetent cells of the nervous system with a non-neural origin. Being macrophages, they fulfill primarily defensive functions [145]. These cells regularly scan the surrounding environment with their processes and adapt their morphology and functions depending on what they sense. Upon activation, microglia exert chemotactic and phagocytic properties, moving where needed and clearing waste products, cellular debris, and pathogens [146]. In addition to these crucial defensive functions, microglia exert many other key actions related to synapse formation, pruning, and functioning [147,148,149]. Microglia cells show various activation states and expression profiles in both human AD brains and murine AD models [150]. The pathway analysis of single-nucleus transcriptomic experiments revealed that microglial genes mostly related to the immune response were differentially expressed between human AD brains and control subjects [142]. Additionally, the mutation in TREM2, a cell surface protein selectively and highly expressed by microglia in the brain, has been associated with a three-fold higher risk to develop AD [151].
Oligodendrocytes originate from precursor cells (OPCs) mainly localized in the ventricular zones of the brain, from which they migrate during development, through which they become mature oligodendrocytes. This process starts during the third trimester of gestation and continues throughout life [152]. Oligodendrocyte’s main function is the creation of the myelin sheath, crucial for effective neuronal transmission of action potentials [153]. Under the myelin sheath, in the internodal periaxonal space, oligodendrocytes establish direct connections with axons via cytoplasmic-rich myelinic channels, in which a bidirectional movement of macromolecules occurs between the two cells [152,154,155]. Impairments in myelin formation and functions have implications in several neurodevelopmental and neuropsychiatric disorders [156,157,158,159,160], and the maturation of OPCs into oligodendrocytes is accelerated by the loss of myelin due to injuries, aging, or diseases, including AD [157].
Astrocytes maintain CNS homeostasis at molecular, cellular, organ, and system levels of organization [161]. Several morphologically distinct subtypes of astrocytes have been identified that likely correspond to specific functions [162]. Indeed, they are present both in the white and grey matter. Astrocytes are key components of the BBB, thus regulating the communication between the CNS and the periphery [163]. They control the CNS microenvironment in several ways, including by buffering extracellular ions and the pH, regulating blood flow through the release of vasoactive molecules, and clearing reactive oxygen species (ROS) [164]. Astrocytes are components of the so-called gliocrine system, releasing around 200 molecules, mainly neurotrophic factors, and energy substrates, fundamental for the maintenance of CNS homeostatic functions [165]. Astrocytes exert primary roles in synaptic transmission and information processing by neural circuits. It has been demonstrated the ability of a single astrocyte to be in contact with several neurons and to modulate synaptic transmission by tuning neurotransmitter levels in the synaptic cleft [162,163].
Originally classified as OPCs, synantocytes are stellate cells, with large process arborizations that specifically express a new type of chondroitin sulfate proteoglycan [166]. They are found both in white and grey matter and interact with other glial cell types and neurons. Synantocytes extend processes along myelin sheaths to contact also the paranodes and nodes of Ranvier. Moreover, they were found to take part in the synaptic cradle, but their specific function at synapses has not been clarified yet [167,168].
Given the essential and pleiotropic functions driven by glial cells, the interest in the involvement of these cells in the pathophysiology of several neurological and neuropsychiatric disorders has grown exponentially in the last years [169]. Additionally, different glial cell types can communicate and influence each other’s phenotype and functions. However, the mechanisms and implications of those cross-talks are only beginning to be elucidated [124,170,171]. Below we focus on the evidence supporting a role for impaired astrocyte functioning in AD, and the potential therapeutic benefit that approaches aimed at restoring them could have.
The role of astrocytes in AD is difficult to decipher, mainly for two reasons: firstly, astrocytes exert a huge plethora of different functions in the CNS that are not easy to tease apart, and secondly, astrocytes respond to any perturbation of CNS homeostasis, caused by either injuries or diseases, with a variety of changes at structural, transcriptional, and functional levels. Additionally, the alterations are specific to the astrocyte localization and the CNS insult, and even to the different stages of the disease [125,172,173,174]. Regarding AD, the evidence available so far suggests the presence of both glial reactivity and atrophy since the initial stages of AD [97]. Additionally, astrocytes close to amyloid plaques show greater transcriptional changes than those far from plaques [175]. To complicate the picture, recent human studies showed that postmortem AD brains contain a reduced proportion of neuroprotective astrocytes, which are associated with glutamate recycling and synaptic signaling, compared to controls [142]. Moreover, the notion that astrocytes are asthenic in the final stages of AD is gaining ground. Regardless, both reactive and asthenic astrocytes operate in an erratic manner, thus contributing differently to the worsening of the disease through neuronal impairment and death [176]. Therefore, the difficulty to develop a pharmacological approach targeting astrocytes increases, since a drug directed to hypertrophic astrocytes in a specific AD stage could be detrimental in another stage at which astrocytes are atrophic, and vice versa. Moreover, modulating astrocytes could affect the functioning of other glial cell types, besides neurons [177,178], altering the normal communication among brain cells. Another important challenge to overcome when designing a therapy directed to the brain is the necessity for it to cross the BBB. It has been reported that only 5% of about 7000 drugs screened in the Comprehensive Medical Chemistry database are actually able to enter the CNS passing the BBB [179,180].
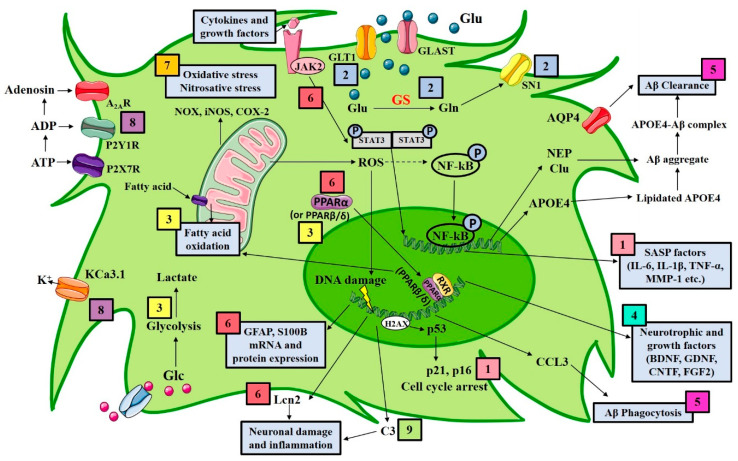
There is a growing number of reports looking at the role of astrocytes in AD, and several approaches targeting astrocytes have been proposed (Figure 1). The following sections review both in vitro and in vivo evidence that has been published in the last five years targeting astrocytes pharmacologically in models of AD (Table 1).
Figure 1.
Schematic representation summarizing different molecular mechanisms of astrocytes to be manipulated in AD. The figure shows the main astrocytic pharmacological targets for the treatment of AD: (1) astrocyte senescence; (2) glutamate transporters; (3) astrocytic metabolic system; (4) upregulation of neurotrophins and growth factors; (5) astrocytic amyloid clearance and phagocytosis; (6) astrocytic reactivity; (7) astrocytic oxidative stress; (8) astrocytic channels and receptors; (9) astrocytic complement cascade. A2AR, adenosine 2A receptor; Aβ, amyloid β; ADP, adenosine diphosphate; APOE4, apolipoprotein E4; ATP, adenosine triphosphate; AQP4, aquaporin 4; BDNF, brain-derived neurotrophic factor; CCL3, C-C motif chemokine ligand 3; Clu, clusterin; CNTF, ciliary neurotrophic factor; COX-2, cyclooxygenase-2; FGF2, fibroblast growth factor 2; GDNF, glial-derived neurotrophic factor; GFAP, glial fibrillary acidic protein; GLAST, glutamate aspartate transporter; Glc, glucose; Gln, glutamine; GLT-1, glutamate transporter-1; Glu, glutamate; GS, glutamine synthetase; H2AX, histone family member X; IL-1β, interleukin 1β; IL-6, interleukin 6; iNOS, inducible nitric oxide synthase; JAK2, janus kinase 2; KCa3.1, calcium-activated potassium channel 3.1; Lcn2, Lipocalin 2; MMP-1, matrix metalloproteinase-1; NEP, neprilysin; NF-kB, nuclear factor-kB; NOX, NADPH oxidase; PPARα, peroxisome proliferator-activated receptor α; PPARβ/δ, peroxisome proliferator-activated receptor β/δ; P2X7, purinergic receptor; P2Y1R, P2Y1 purinergic receptor; ROS, reactive oxygen species; RXR, retinoid X receptor; SASP, senescence-associated secretory phenotype; S100B, S100 calcium-binding protein B; SN1, N glutamine transporter 1; STAT3, signal transducer and activator of transcription 3; TNF-α, tumor necrosis factor α.
Table 1.
In vitro and in vivo approaches targeting astrocytes in Alzheimer’s disease.
| Astrocytic Target | Experimental Strategy | Results | References |
|---|---|---|---|
| Senescence | Removal of senescent cells in vivo by radiation treatment or by genetic ablation | Reduction in astrogliosis, tau hyperphosphorylation, neuronal degeneration; preservation of cognition | [181,182] |
| In vivo administration of the senolytic drug ABT263 (navitoclax) | Prevention from the upregulation of senescence-associated genes attenuated tau phosphorylation; cognitive improvements | [181,182] | |
| Overexpression of Δ133p53 in radiation-induced senescent astrocytes | Repression of the irradiation-induced SASP | [183] | |
| Ginsenoside F1 in vitro treatment | SASP suppression by downregulation of p38MAPK-dependent NF-κB pathway | [184] | |
| Glutamate transporters | Ceftriaxone administration in APP/PS1 mice | Raise in GLT1, GS and SN1 protein expression and cognitive performance improvements | [185] |
| Chronic oral administration of riluzole in 5xFAD mice | Prevention of senescent associated gene expression changes; reduction of Aβ oligomers and plaques | [186] | |
| Metabolism | PPARβ/δ agonist treatment of human AD astrocytes (PSEN1ΔE9) | Enhancement of AD-reduced fatty acid oxidation | [187] |
| Pantethine in vitro treatment of astrocytes obtained from 5xFAD mice | Reversal of the altered activity of several metabolic enzymes and of the induced IL-1β expression | [188] | |
| Hydroxytyrosol treatment of glioma cell cultures challenged with Aβ (25–35) | Proper glucose metabolism restoration by Akt activation | [189] | |
| GLP-1 in vitro treatment of Aβ-exposed astrocytes | Reversal of the Aβ-altered glycolysis by activation of the PI3K/Akt pathway | [190] | |
| Metformin in vitro treatment of astrocytes exposed to high glucose concentration | Inhibition of both the ER stress and inflammation induced by high glucose | [191] | |
| Neurotrophins and growth factors | HMF treatment of primary astrocytes and C6 glioma cell line | Raise in BDNF expression induced by both the activation of cAMP/ERK/CREB signaling and the inhibition of PDE4B and PDE4D | [192] |
| Primary neurons exposed to Aβ (25–35) incubated with quetiapine-treated astrocyte conditioned medium | High BDNF release by astrocytes treated with quetiapine promoted viability of primary neurons | [193] | |
| Overexpression of BDNF specifically in GFAP-positive astrocytes by genetic crossing in 5xFAD mice | The raise in BDNF levels that are reduced in 5xFAD mice improved synaptic plasticity and cognition | [194] | |
| In situ stem cell transplant in intrahippocampal Aβ42 infused mice | Reversal of the Aβ42-induced cognitive impairment by BDNF-TrKB pathway activation | [195] | |
| FGF2 treatment of primary astrocytes challenged with Aβ42 | Promotion of astrocyte proliferation through enhanced expression of c-Myc, Cyclin D1, Cyclin E | [196] | |
| Aβ clearance | HDL mimetic peptide in vitro treatment of primary human and murine astrocytes | Raise in apoE4 lipidation lowers its detrimental cellular accumulation | [197] |
| In vivo overexpression or downregulation of Clu specifically in GFAP-positive astrocytes in APP/PS1 mice | Clu overexpression is associated with a reduction in Aβ burden. The opposite phenomenon was found with Clu downregulation |
[198] | |
| In vivo overexpression of Clu specifically in GFAP-positive astrocytes in 5xFAD mice | Reduction in plaques number and sizes. Improvement in synaptic function | [199] | |
| EGCG treatment of Aβ40 challenged medium from cultured astrocytes | Elevation of the expression of NEP, an enzyme that degrades Aβ | [200] | |
| PUFAs oral administration in fat-1 transgenic mice and AQP4 knockout mice | PUFAs promoted Aβ clearance in fat-1 transgenic mice, but not in AQP4 knockout mice. PUFAs protected from Aβ-induced AQP4 polarization | [201] | |
| Complement cascade | Genetic ablation of C3 gene in APP/PS1 mice | Reduction of glia at plaques | [202] |
| Phagocytosis | Fingolimod oral administration in APP/PS1 mice infected by B. pertussis | Increase in astrocyte phagocytosis of Aβ; reduction of GFAP immunoreactivity | [203] |
| In vitro and in vivo downregulation of the Aβ-induced inflammasome, respectively in astrocytes and in 5xFAD mice | In vitro Aβ phagocytosis increase due to the release of the chemokine CCL3 and improved memory in vivo | [204] | |
| Cell reactivity | Iron chelators deferoxamine and deferiprone treatment in Aβ-challenged astrocytes | Inhibition of Aβ-induced Lcn2 | [205] |
| Glu-DAPPD chronic administration in APP/PS1 mice | Reduction of Aβ aggregates as well as GFAP and Iba1 immunostaining. Cognitive functions improvement | [206] | |
| Downregulation of the JAK2-STAT3 pathway in hippocampal astrocytes of transgenic APP mice | Reduction of Aβ deposits; mice spatial learning improvement; control of pro-inflammatory genes | [207] | |
| Downregulation of the JAK2-STAT3 pathway in hippocampal astrocytes of transgenic 3xTg-AD mice | Full reversal of early synaptic and LTP alterations; short-term memory and reduced anxiety behavior improvements | [176,207] | |
| In vitro treatment with PEA of Aβ42-challenged primary astrocytes and mixed astrocytes-neurons cultures | Prevention of Aβ-induced neuronal loss and reduction of neuronal viability | [208] | |
| In vitro treatment with PEA of Aβ42-challenged mixed astrocytes-neurons cultures isolated from 3xTg-AD mice | Prevention of Aβ-induced neuronal loss and reduction of neuronal viability | [209] | |
| In vitro treatment with PEA of primary cortical astrocytes and mixed astrocytes-neurons cultures isolated from 3xTg-AD mice | Reduction of astrogliosis and improvement of neuronal viability | [210] | |
| Um-PEA treatment in glioma and neuroblastoma cells challenged by lipopolisaccaride and interferon γ | Improvement of cell viability; reduction of protein expression of both iNOS and COX-2 | [211] | |
| Co-ultra PEALut administration for 14 days starting from the day that rats received a single intrahippocampal Aβ42 infusion | Prevention of Aβ-induced astrocyte hypertrophy, neuroinflammation; and BDNF and GDNF mRNA downregulation | [35] | |
| Oxidative stress | Electromagnetic fields exposure of human and rat primary astrocytes challenged with Aβ or H2O2 | Reduction of both ROS production and NADPH oxidase activity | [212] |
| In vivo pelargonidin administration in rats subjected to an intrahippocampal injection of Aβ(25–35) | Raise in acetylcholinesterase and catalase activities. Improvement in cognitive performance | [213] | |
| In vivo treatment of C. elegans with monascin | Reduction of Aβ-toxicity and activation of the expression of several anti-oxidative genes | [214] | |
| Channels and receptors | In vivo genetic ablation of the Ca2+-activated K+-channel KCa3.1 | Improvements in memory performance and insulin signaling.Reduction of glial hypertrophy and tau hyperphosphorylation | [215] |
| Chronic intracerebroventricular infusion of P2Y1R inhibitors in APP/PS1 mice | Reversal of structural and functional markers of astrocyte activation. Memory performance improvement |
[216] | |
| Inhibition of adenosine recycle by J4 hippocampal infusion in APP/PS1 mice | Improvement of memory deficits | [217] | |
| Oral administration of istradefylline, an A2A antagonist, to transgenic APP mice | Memory improvements | [218] | |
| Astrocyte modulation | Chronic um-PEA administration to 6-month-old 3xTg-AD mice | Reduction of cortical astrocyte hypertrophy and reactivity. Reduction in both cortical and hippocampal inflammation |
[210,219] |
| Chronic um-PEA administration to 12-month-old 3xTg-AD mice | Support for asthenic/atrophic astrocytes | [219] |
3.1. Targeting Astrocyte Senescence
Aging is considered one of the main risk factors for the development of neurodegenerative diseases, including AD [220]. Studies on cellular aging are attracting much attention as a fervent area of research [221,222], and recent evidence demonstrates that astrocytes senescence has a critical role in the pathogenesis of AD. As time goes by, astrocytes show peculiar cellular and molecular changes assuming the so-called senescence-associated secretory phenotype (SASP) [223]. This is accompanied by upregulation and release of proinflammatory cytokines, including interleukin(IL)-1β and IL-6, chemokines, and proteinases [175,184,224]. Overexpression of intermediate filament proteins glial fibrillary acidic protein (GFAP) and vimentin occurs, whereas neurotrophic growth factors result downregulated. The chromatin undergoes several modifications, and there is upregulation of p53, p21WAF1, and p16INK4A, leading to a permanent cell cycle arrest [225,226]. These features may not be specific senescence markers for astrocytes since they are postmitotic cells that do not usually divide in healthy tissues [126]. Regardless, one of the most common features of aging is the accumulation of senescent cells. Bussian et al. demonstrated that the presence of senescent astrocytes and microglia in a mouse model of aggressive tauopathy (the PS19 mice) promotes the formation of hyperphosphorylated tau aggregates. Removing p16INK4A-expressing senescent cells through a genetic approach prevented astrogliosis, hyperphosphorylation of tau, degeneration of cortical and hippocampal neurons, and it preserved transgenic mouse cognitive functions [182]. Comparable effects have been obtained by testing a senolytic agent, the orally active anticancer drug ABT263 (navitoclax), that acts as inhibiting Bcl-2. By this mechanism, this compound is able to induce apoptosis specifically in senescent cells [227]. The clearance of accumulated senescent astrocytes also rescued in vivo the radiation-induced impaired astrocytic neurovascular coupling and mice cognitive performance [181]. Another report showed that the overexpression of an inhibitory isoform of p53, the Δ133p53, which is downregulated in AD, repressed the SASP after its induction in astrocytes by exposure to radiation. Δ133p53 overexpression promoted also DNA repair and inhibited irradiated astrocyte-mediated neuroinflammation and neurotoxicity [183]. The antiprotozoal drug pentamidine upregulates p53 and increases the ratio BAX/Bcl2, ultimately promoting apoptosis in cultured astroglioma cells [228], and it exerts anti-inflammatory effects in mice receiving human Aβ42 into the hippocampus [229]. Finally, from the field of phytotherapy, an in vitro study showed that Ginsenoside F1 suppresses the SASP in astrocytes by downregulating the p38MAPK-dependent NF-κB activity [184], a pathway upregulated in AD.
3.2. Targeting Astrocyte Glutamate Transporters
Glutamate represents the major excitatory neurotransmitter of the CNS, whose neurotransmission is finely regulated by both neurons and glial cells [230]. Astrocytes, in particular, are responsible for glutamate reuptake from the synaptic cleft through excitatory amino acid transporters (EAATs). There are five subtypes of EAATs (EAAT1–EAAT5), but EAAT2 (glutamate transporter-1/GLT1) is responsible for more than 90% of glutamate reuptake [231]. Once inside the astrocyte, glutamate is converted mainly into glutamine by the glutamine synthetase (GS) and then shuttled back to the presynaptic neuron, which uses it to synthesize glutamate again. A portion of glutamate is converted to gamma-aminobutyric acid (GABA), which is usually catabolized. The glutamate–glutamine shuttle is crucial for glutamate homeostasis, and thereby for learning and memory. If the shuttle is dysfunctional, an abnormal glutamate stimulation could occur, which is neurotoxic [232]. Glutamate excitotoxicity has been observed in AD and correlated with cognitive decline [232,233]. In parallel, both accumulation of GABA, whose concentration is low in astrocytes under physiological circumstances [234], and its release from reactive astrocytes have been observed in transgenic animal models of AD (5xFAD and APP/PS1 mice), resulting in memory deficits [235,236]. However, astrocytic GABA content seems to follow a bell-shaped curve along aging and not relate to Aβ [237]. Human postmortem AD brains showed altered expression of several GABA transporters in cortical and hippocampal regions [238]. Therefore, counteracting dysfunctions in the content of neurotransmitters and the expression of their transporters could likely be beneficial in AD. Research targeting the modulation of astrocytic GABA is still not fully explored, and further studies are warranted. Instead, the enhancement of glutamate transporter function and expression has been tested using various activators in several neurological diseases [239]; however, few studies were carried out in AD models. β-lactam antibiotics are drugs that upregulate GLT1 gene transcription, in addition to having antibacterial effects [240,241]. Among them, ceftriaxone was found to ameliorate AD pathology by improving spatial learning and memory in APP/PS1 mice, upregulating the expression of both GS and the system N glutamine transporter 1 (SN1) [185]. Another drug already approved for human use is riluzole, which has been shown to improve memory performance in aged rats and in 5xFAD mice [186,242]. Riluzole is a neuroprotective agent able to increase Na+-dependent glutamate uptake in synaptosomes in a dose-dependent manner [243]. Riluzole chronic oral administration prevents age-related gene expression changes in rats’ hippocampi [244] and reduces the levels of Aβ42 and Aβ40 oligomers and neuritic plaques in 5xFAD mice [186]. Despite being so promising, these results have not been translated into the clinic yet.
3.3. Targeting the Astrocytic Metabolic System
As we mentioned before, failure of astrocytes in supporting neuronal energy needs could facilitate the progression from physiological to pathological brain aging. For instance, the metabolic products of fatty acid oxidation decrease during AD [245], making lipid metabolism a potential target for AD treatment. Recently, an in vitro study found that activation of the peroxisome proliferator-activated receptor proliferator-activated receptor (PPAR) beta/delta (PPARβ/δ) increases fatty acid oxidation [187]. Indeed, a rate-limiting enzyme of the fatty acid oxidation is the carnitine palmitoyltransferase 1A (CPT1A), which catalyzes the transfer of fatty acids into the mitochondria, where the β-oxidation occurs. Konttinen et al. tested the effects of GW0742, a synthetic PPARβ/δ agonist, in human astrocytes obtained from pluripotent stem cells (iPSCs) of AD patients carrying an amyloidogenic mutation of PSEN1 (PSEN1ΔE9). GW0742 enhanced the expression of CPT1a, increasing astrocyte fatty acid oxidation [187]. In primary astrocytes obtained by 5xFAD mice, which show an altered metabolic profile, administration of the vitamin B5 precursor pantethine reversed several metabolic alterations induced by Aβ challenge, including (i) altered activity of the glucose-6-phosphate dehydrogenase, the α-ketoglutarate dehydrogenase complex, and the succinate dehydrogenase; (ii) decreased ATP production; and (iii) altered expression of the hypoxia-inducible factor-1 alpha, known to protect against Aβ toxicity. Pantethine treatment showed some anti-inflammatory actions by downregulating IL-1β expression [188]. Similarly, treatment of the astroglioma cell line C6 with hydroxytyrosol, the major polyphenol contained in olives, ameliorated the metabolism of glucose, previously altered by Aβ(25–35) challenge, through activation of Akt [189]. Evidence demonstrates the ability of glucagon-like peptide-1 (GLP-1) to improve cognitive deficits in AD [246]. Zheng et al. just published that this effect is related to GLP-1 ability to restore in vitro the Aβ-induced glycolysis impairment in astrocytes, by activating the PI3K/Akt pathway [190]. A recent study by Wang et al. demonstrated that metformin, a hypoglycemic drug of clinical use, exerts anti-inflammatory and antioxidant effects in rat primary astrocytes treated with high glucose concentration [191], strengthening the link between altered metabolism and induction of inflammatory process.
3.4. Upregulation of Astrocytic Neurotrophins and Growth Factors
Neurotrophic factors imbalance and dysregulation are associated with neurodegenerative diseases, including AD [247]. The brain-derived neurotrophic factor (BDNF) is involved in cognition and memory formation, given its role in modulating synaptic plasticity. Astrocytes can release neurotrophic growth factors, including BDNF, exerting protective effects on neurons [248]. Thus, the increase in astrocyte neurotrophic factor expression and release could be a therapeutic approach for AD [249]. Sawamoto et al. found that the citrus flavonoid 3,5,6,7,8,30,40-heptamethoxyflavone (HMF) exerts neuroprotective effects by increasing the expression of BDNF in astrocytes within the hippocampus of mice and in the C6 glioma cell line. The BDNF increase was induced by the activation of cAMP/ERK/CREB signaling and inhibition of phosphodiesterase 4B and 4D [192]. Another molecule found to be able to upregulate BDNF expression in cultured astrocytes is quetiapine, a widely used atypical antipsychotic drug [193]. Recently, a paper in which transgene delivery in astrocytes was used to obtain the upregulation of BDNF in 5xFAD mice was published [194]. Specifically, 5xFAD mice were crossed with transgenic pGFAP-BDNF mice, expressing BDNF under the GFAP promoter. The resulting transgenic mice showed restored levels of BDNF, compared to 5xFAD mice, which have reduced levels of this neurotrophin compared to their wild-type counterparts. BDNF restoration also resulted in improvements in cognitive tasks and ameliorated synaptic plasticity [194].
Some studies have also explored the potential beneficial effects of neural stem cell transplantation in models of AD. An Indian group studied the lineage negative stem cells (Lin-ve) derived from human umbilical cord blood (hUCB) in an animal model of Aβ42-induced injury. They found that intrahippocampal transplant of these cells at specific dosage and timing shows potential to reverse hippocampal Aβ42-induced mouse cognitive impairment, measured by Morris water maze and passive avoidance, through a neuroprotective mechanism likely mediated by BDNF upregulation [195,250]. Blockade of the BDNF-TrkB pathway by systemic administration of a TrkB inhibitor nullified the benefit of Lin-ve cell transplant. Aβ42-challenged mice showed decreased BDNF and GFAP protein and gene expression, which were both reversed by Lin-ve cell transplant. Some less clear effects were detected also in the expression levels of both the glial-derived neurotrophic factor (GDNF) and the ciliary neurotrophic factor (CNTF), which deserve further studies [195].
AD pathogenesis is also affected by altered production of growth factors [251,252], including the fibroblast growth factor (FGF) 2 [247]. In particular, FGF2 is increased in reactive astrocytes around senile plaques [253]. Last year, Chen et al. demonstrated that FGF2 has protective effects against Aβ42-induced cytotoxicity in primary cultured cortical astrocytes. In their experiments, primary astrocytes challenged with Aβ42 were treated with either high or low molecular weight forms of FGF2. The low molecular isoform of FGF2 promoted astrocyte proliferation, enhancing the expression of c-Myc, Cyclin D1, Cyclin E [196].
3.5. Targeting Astrocytes-Driven Amyloid Aggregation and Clearance
Accumulation of Aβ could be the result of its increased synthesis or reduced clearance or a combination of both. Looking for AD treatment, an important area of investigation targets Aβ clearance, which depends, at least in part, on astrocytes. Indeed, astrocytes can take up Aβ and digest it in their lysosomes. However, the astrocytic degrading machine could get engulfed, leading to detrimental consequences [254]. Lysosome functions and gene expression for proteins involved in both autophagy and proteolysis were found altered in aging and AD [255,256]. Two of the apolipoproteins associated with high risk for developing sporadic AD are secreted by astrocytes and are involved in Aβ aggregation and clearance, the apoE4 and the apoJ (also known as clusterin) [69,257]. The apoE4, once secreted by astrocytes, binds to high-density lipoprotein (HDL)-like particles, and the level of its lipidation influences Aβ aggregation and clearance [258]. Chernick et al. demonstrated the ability of an HDL mimetic peptide, the 4F, to increase apoE4 lipidation in primary human and murine astrocytes. That counteracts the Aβ-induced accumulation of intracellular apoE4, mitigating Aβ detrimental effects on proper cellular trafficking and functionality of apoE [197]. Clusterin (Clu) is a ubiquitous protein whose functions are still not clear, but studies have shown its involvement in Aβ aggregation, toxicity, and clearance. Conflicting results have been published reporting both neuroprotective and detrimental properties of Clu [259,260]. Novel in vitro findings demonstrated a role for astrocytic Clu in promoting synapse formation and glutamatergic synaptic activity [199]. Wojtas et al. overexpressed Clu (>about 30%) selectively in GFAP-positive astrocytes of APP/PS1mice and noticed a reduction in Aβ accumulation and formation of fibrillary deposits in both cortex and hippocampus compared to control animals. In the same brain areas, the authors found that Clu overexpression was associated with a reduction of the number of cortical and hippocampal dystrophic neurites [198]. In accordance, the reduction (<about 50%) in Clu expression in GFAP-positive astrocytes of APP/PS1 mice leads to a worsening of the AD-like outcomes [198]. Novel in vivo findings demonstrated that Clu overexpression in astrocytes enhances excitatory neurotransmission and rescues the synaptic deficit in Clu knockout mice. Clu overexpression in GFAP-positive astrocytes of 5xFAD mice reduced plaque numbers and plaque size and rescued presynaptic dysfunction [199].
Another molecule that seems to promote Aβ clearance is the epigallocatechin gallate (EGCG), a member of the catechin family. In cultured astrocytes, ECGC elevates neprilysin (NEP) expression, one of the most important Aβ-degrading enzymes in the brain, involving also the activation of ERK and phosphoinositide 3-kinase [200].
Moreover, oral administration of fish oil, containing n-3 polyunsaturated fatty acids (PUFAs), was found effective in clearing Aβ from the brain of fat-1 transgenic mice [201], but not of aquaporin (AQP) 4 knockout mice, suggesting the involvement of AQP4 protein, expressed selectively in astrocytes, in Aβ clearance. Additionally, PUFAs administration protected from AQP4 polarization occurring after Aβ injection [201], a sign of astrocytic dysfunction [261].
3.6. Targeting Astrocytic Reactivity, Complement Cascade, Neuroinflammation, and Oxidative Stress
Neuroinflammation plays a pivotal role in the development and progression of AD. Indeed, Aβ plaques are surrounded by activated glial cells, and Aβ itself leads to the activation of astrocytes and microglia, together with the release of proinflammatory factors [97,262,263,264]. Brains of different transgenic mouse models of AD show activated astrocytes, even before the appearance of plaques and NFTs [265,266]. When astrogliosis occurs, reactive astrocytes produce inflammatory markers, such as tumor necrosis factor (TNF)-α, IL-1β, and IL-6, and calcineurin, a protein phosphatase that mediates inflammatory responses. This is associated with a wide number of cellular events, including the aforementioned activation of the complement cascade, the release of nitric oxide, and ROS. This phenomenon is normally engaged with the intent of defending the brain by removing injurious stimuli (e.g., Aβ fibrils phagocytosis). However, if prolonged beyond physiological limits, it would have detrimental effects. Therefore, targeting astrocyte reactivity and, consequently, the related activation of the complement cascade, the oxidative stress and the inflammatory response could represent an effective therapeutic strategy in AD. A compound that has shown such properties is cannabidiol, the main nonpsychoactive component of Cannabis Sativa [267]. Studies demonstrated cannabidiol effects in reducing both GFAP and S100B mRNA and protein expression, as well as neuroinflammatory parameters in different models of AD [268,269,270].
The complement component C3 is increased in human AD brains, and it is expressed by reactive astrocytes. Its increased expression is required for neurodegeneration to occur [271]; thus, its targeting could be beneficial. Indeed, Shi et al. compared aged plaque-rich transgenic APP/PS1 mice knockout (KO) for the C3 to transgenic APP/PS1 mice to evaluate Aβ plaque pathology, glial responses to plaques, and neuronal dysfunction in the brains. They found that C3 KO mice had less activation of glial cells at the center of Aβ plaques compared to control mice, suggesting that the downregulation of C3 controls astrocyte activation and neuroinflammation in AD [202].
Mc Manus et al. tested the effect of infection by Bordetella Pertussis in APP/PS1 mice and the potential benefit of fingolimod (FTY720) administration, an FDA-approved immunomodulatory drug for treating multiple sclerosis. Fingolimod reduced signs of infection-induced BBB increased permeability, GFAP immunoreactivity, and Aβ deposits, compared to control mice. Results of additional in vitro experiments in primary astrocytes suggested that the decreased Aβ accumulation was driven by the fingolimod-induced increase in the phagocytic capacity of astrocytes [203].
Since Aβ activates the astrocytic inflammasome promoting the release of IL-1β, Couturier et al. demonstrated that the downregulation of this Aβ-induced inflammatory process increases Aβ phagocytosis in astrocytes in vitro. That is due to the release of the chemokine CCL3, ultimately improving in vivo the memory deficits of 5xFAD mice [204]. Therefore, that phlogistic event represents a druggable therapeutic target, which still needs to be thoroughly investigated. Several molecules have been tested during the last years for their ability to dampen astrocyte reactivity in AD [269,270,272,273], but none have been translated to the clinic yet. Patients with MCI and vascular dementia show increased levels of Lipocalin 2 (Lcn2) in the CSF. In AD cases (stages V and VI), Lcn2 immunoreactivity increased in reactive astrocytes localized around plaques and in reactive microglia [274]. Astrocytes are the major producers of Lcn2 in the brain [275]. This protein is involved in several processes including inflammation, iron metabolism, cell death, and cell survival, modulating the cellular response to Aβ [275]. Staurenghi et al. demonstrated that increased levels of oxysterols observed in mild or severe AD brains, accompanied by increased levels of Lcn2, determine a clear morphological change in mouse primary astrocytes [276]. A recent study found that the iron chelators deferoxamine and deferiprone reduce Aβ-induced iron accumulation in astrocytes and inhibit Aβ-induced Lcn2, suggesting these molecules as promising therapeutic strategies against AD [205]. A novel synthesized compound, Glu-DAPPD, containing a glucose group linked to an anti-neuroinflammatory agent, the N,N′-diacetyl-p-phenylenediamine, showed in vivo to reduce Aβ aggregates and immunostaining for astrocytes and microglia, and to improve cognitive function in transgenic APP/PS1 mice being administered chronically for two months [206].
Recent studies identified the Janus kinase 2-signal transducer and activator of transcription 3 (JAK2-STAT3) pathway as a key pathway for the induction and maintenance of astrocyte reactivity. Using adenoviral delivery techniques, authors either downregulated or upregulated the JAK2-STAT3 pathway specifically in hippocampal astrocytes. They found that the JAK2-STAT3 pathway is necessary and sufficient to trigger astrocyte reactivity in the hippocampus of transgenic APP mice, controlling also for gene expression of a variety of genes, of which many involve the inflammatory process. The downregulation of this pathway reduced also Aβ deposits and improved mice spatial learning but not memory retrieval. On the other hand, the upregulation of the JAK2-STAT3 pathway resulted in opposite and deleterious results [207].
Astrocytes are involved in both the production and clearance of ROS, concurring to the oxidative stress found in AD, whose reduction has been tested as a potential therapeutic target. Interestingly, mobile phone radiofrequency electromagnetic fields (EMF) have been shown to reduce both Aβ and H2O2-induced ROS production in human and rat primary astrocytes, as well as the co-localization between the cytosolic (p47-phox) and membrane (gp91-phox) subunits of NADPH oxidase, indicating the suppression of its activity [212]. Other antioxidant anthocyanin compounds have recently been investigated [277]. Among them, pelargonidin, which acts as an estrogen receptor agonist, has been tested in rats that received an intrahippocampal injection of Aβ(25–35). Pelargonidin treatment resulted in improved Morris water maze test performance. Higher hippocampal catalase and acetylcholinesterase activities have been detected, accompanied by lower GFAP protein expression, but no change in inducible nitric oxide synthase (iNOS), compared to control animals [213].
Recently, the compound monascin has been found to activate the expression of several antioxidative genes such as SOD-1, SOD-2, SOD-3, and HSP16.2 and reduce Aβ-toxicity in C. elegans strain [214], suggesting its antioxidant potential. In addition, resveratrol [278], tocotrienol [279], epicatechins [280], H-1,2-dithiole-3-thione [281], curcumin, and epigallocatechin-3-gallate [282] have shown in vitro and in vivo anti/oxidant properties in several models of Aβ-mediated toxicity and AD.
As fundamental regulators of brain homeostasis, astrocytes also regulate the intracellular Ca2+ concentration through an intermediate conductance calcium-activated potassium channel, KCa3.1. This channel is actively involved in the phenotypic change of astrocytes during astrogliosis observed in AD. By using KCa3.1 knockout mice, memory deficits, neuronal loss, glial activation, tau phosphorylation, and insulin signaling deficits were ameliorated compared with control animals, making this channel an interesting pharmacological target in AD [215]. During the neuroinflammatory process, ATP and ADP are released around plaques, leading to the activation of the metabotropic P2Y1 purinoreceptors (P2Y1Rs) expressed by astrocytes, which increases the rate of spontaneous calcium events [283]. Chronic intracerebroventricular infusion of P2Y1R inhibitors resulted in structural and functional restoration of astrocytes and the preservation of memory deficits [216].
Since AD patients show increased levels of the Gs-coupled adenosine receptor A2A in astrocytes, Orr et al. studied in vivo the ablation of astrocytic A2A receptors demonstrating that it enhances long-term memory [284]. The adenosine tone on the astrocytic A2A receptors has also been modulated through a new BBB-permeable equilibrative nucleoside transporter (ENT) inhibitor, J4, tested in APP/PS1 mice. In particular, J4 inhibited the recycling of adenosine from the extracellular space performed by ENTs, resulting in the prevention of the decline in spatial memory, a common feature in AD patients [217]. Additionally, istradefylline, a selective antagonist of A2A receptors, enhanced the performance in behavioral tests in transgenic APP mice [218].
3.7. Modulation of Astrocytes According to Their Morphofunctional State: The Case of Palmitoylethanolamide
In AD, as in other neurodegenerative disorders, astrocytes undergo morphological, biochemical, metabolic, and transcriptional changes, as well as physiological remodeling. All these rearrangements could lead to either a gain or loss of one or more functions [126]. Thus, pathological changes of astrocytes should not just refer to hypertrophy. Indeed, also morphological atrophy could contribute to AD early synaptic failures and cognitive deficits [126,285]. For these reasons, molecules able to modulate astrocyte morphology and functions according to their reactive or atrophic status could be potentially valuable therapeutics. To the best of our knowledge, the only molecule that has so far shown some indications to exert such effects is palmitoylethanolamide (PEA).
PEA is a naturally occurring amide of ethanolamide and palmitic acid, firstly isolated from soy lecithin, egg yolk, and peanut meal. It acts as a lipid messenger that mimics several endocannabinoid-driven actions, even though it does not bind to cannabinoid receptors [286]. We and other groups have shown that PEA exerts anti-inflammatory and neuroprotective properties in several preclinical models of Aβ-induced toxicity and AD [287]. PEA in vitro attenuates Aβ-induced astrocyte expression of GFAP and S100B and the release of pro-inflammatory molecules [273,288]. In a surgical model of Aβ-neurotoxicity PEA treatment reduced astrocyte hypertrophy and markers of inflammation, including iNOS, cyclooxygenase (COX)-2, IL-1β, and TNF-α [289]. PEA also demonstrated the ability to protect Aβ-induced neuronal reduced viability and loss in vitro, ex vivo, and in vivo [208,209,286,289,290]. These results have been confirmed also in primary astrocytes derived from the prefrontal cortex of 3xTg-AD mice, in which PEA promoted neuronal viability [210]. All these reports concurred to demonstrate that PEA exerted these effects through the PPARα by the use of selective antagonists, corroborated by experiments in models where the receptor was genetically ablated [291,292,293]. However, studies showed that PEA effects could involve also the orphan G-protein coupled receptor 55 [294], and the transient receptor potential vanilloid type 1 channel [295]. Moreover, PEA is able to exert an indirect activation of cannabinoid receptors, via the so-called entourage effect [296], working as a false substrate for fatty acid amide hydrolase, an enzyme involved in the metabolism of the endocannabinoid anandamide (AEA) [297]. Indeed, due to the reduction of its catabolism, AEA levels rise. Thus, in turn, AEA could bind to cannabinoid receptors. One additional peculiar feature of PEA is its ability to act as an autacoid local injury antagonist, thus dampening mast cells that are now considered critical effectors during AD progression [298]. In this way, PEA contributes to protecting neurons from excitotoxicity [297]. Interestingly, the modulation of the cross talk between mast cells and glial cells is emerging as a valuable approach to treat several neuroinflammatory brain pathologies, including AD [299]. Some articles present an extensive review of PEA biological functions in the CNS [296,297,300].
Different formulations of PEA have been synthesized to improve its bioavailability and efficacy, including the ultramicronized (um-PEA) and PEA-oxazoline forms as well as the combination of PEA with luteolin (Lut), an antioxidant compound, ultramicronized together (co-ultra PEA/Lut). Pretreatment with um-PEA of rat hippocampal slices challenged acutely with Aβ42 significantly reduced iNOS and GFAP expression [301]. It also restored cell viability of glioma and neuroblastoma impaired by lipopolisaccaride and interferon-gamma treatment, reducing protein expression of both iNOS and COX-2 [211]. Um-PEA demonstrated oral bioavailability and its chronic administration reduced neuroinflammatory markers and showed neuroprotective effects in 3xTg-AD mice [210,219,302,303]. When comparing hippocampi of 6-month-old with 12-month-old 3xTg-AD mice, the younger animals did not show astrocyte hypertrophy (measured as an increase in GFAP immunoreactivity) but exhibited an ongoing intense neuroinflammatory process with high levels of iNOS, TNF-α, chemokines, and interleukins, whereas older mice showed significant astrocyte atrophy without elevation in neuroinflammatory markers. Chronic subcutaneous pretreatment with um-PEA for 3 months prevented the establishment of the phlogistic process in hippocampi of 6-month-old 3xTg-AD mice, compared to vehicle-treated ones. Um-PEA also prevented the altered performance in cognitive tasks and reduced Aβ formation and phosphorylation of tau protein in the hippocampus [219]. Astrocyte hypertrophy was detected in the cortices of vehicle-treated 6-month-old mice, and um-PEA chronic treatment decreased both GFAP mRNA and protein expression [210]. Interestingly, 3xTg-AD mice that received um-PEA subcutaneous administration for 3 months, before being tested at 12 months of age, showed restored astrocyte GFAP immunoreactivity to the level of non-Tg controls, also improving their outcome in behavioral assessment of short-term memory [219]. Collectively these reports show that um-PEA acted preventing either astrocyte hypertrophy either atrophy. This indicates that PEA behaved as a modulator of astrocyte morphology and cell reactivity state. This is in accordance with the current view seeing astrocyte reactivity as an evolving and reversible process caused by extrinsic triggers [126,304].
Another formulation that combines the aforementioned PEA effects with the antioxidant actions of Lut has been tested in preclinical AD models. Co-ultra PEA/Lut showed anti-inflammatory and antiapoptotic effects in Aβ42-challenged rat hippocampal slices and neuroblastoma cells [301]. In vivo, co-ultra PEA/Lut administration for two weeks in rats that received a single intrahippocampal infusion of Aβ42 prevented the Aβ-induced astrocyte hypertrophy, as well as the upregulation in gene expression of pro-inflammatory cytokines and enzymes found in rats treated with vehicle. Moreover, co-ultra PEA/Lut prevented the Aβ-mediated decrease in gene expression of both glial-derived and brain-derived neurotrophins [35]. Despite having these promising features, no studies have yet elucidated the synergic mechanisms of actions of the association of PEA with Lut. Regardless, since co-ultra PEA/Lut administration started the same day of the surgical infusion, to model the very first phase of Aβ42 accumulation as in the prodromal stage of AD, the above-reported study mimicked a potential therapeutic intervention at the earliest stage of the disease. The results support the thesis that targeting astrocytes at the beginning of the pathology could have a positive impact. Other very recent studies endorse this view. Reports from Dr. Escartin’s group modulated the activation of astrocytes in 9-month-old 3xTg-AD mice. The downregulation of the JAK2-STAT3 pathway fully restored mice early synaptic and long-term potentiation alterations [207], improved short-term memory, and reduced anxiety behavior [176], thus supporting the hypothesis that targeting astrocytes at the very early stages of AD could be beneficial.
The potential translational value of ultramicronized or co-micronized PEA as a preventive therapeutic strategy in AD is corroborated by its safety and tolerability, as it is already in the human and veterinary market as food for special medical purposes and complementary feed, respectively. Some single or few-cases human studies have been carried out showing favorable results in improving MCI and frontotemporal dementia [305,306], in recovering from stroke [307], and in managing neuropathic pain associated with neuroinflammation [308].
4. Conclusions
Despite the spasmodic basic and medical research and the existence of approved therapies, there is a huge unmet clinical need for effective therapies for AD, especially treatments that are intended to address the biological basis of the pathology to favorably modifying its long-term course. Currently approved drugs do not target the underlying pathology of AD since they only provide modest beneficial effects to a small subset of patients. Moreover, no treatments are available to counteract AD at its earliest stage, which could represent the best timepoint to start therapy. Indeed, Aβ deposition into amyloid plaques, followed by markers of neurodegeneration, tau pathology, and reduction of brain volume, initiates decades before the onset of observable clinical signs. Dysfunctions of astrocytes have been linked to the molecular alterations observed in AD, thus representing a promising target for disease management. However, morphofunctional changes occurring in astrocytes vary depending on the stage of the pathology. Therefore, molecules capable of correcting dysfunctions of astrocytes could represent a promising pharmacological strategy. Reviewing the literature findings, the only compound so far that seems to exert this effect is PEA. Our previous study indeed showed the ability of PEA to normalize the astrocyte alterations observed in an experimental model of AD, the 3xTg-AD mice, endowed with face, construct, and predictive validities, bringing them back to a homeostatic condition. That and other possibilities of new therapeutic approaches represent an important springboard for the development of therapies for a still incurable disease, such as AD.
Author Contributions
Conceptualization, C.S.; resources, C.S. and L.S.; writing—original draft preparation, R.F., M.V., and C.S.; figures and tables preparation, G.M., R.F., and M.V.; writing—review and editing, M.V., L.S., and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
Marta Valenza discloses a previous work-term contract (2019–2020) with Epitech Group S.p.A., which was already closed at the time of preparation of the present review article. The other authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.FDA Peripheral and Central Nervous System Drugs Advisory Committee (PCNS) Meeting. [(accessed on 6 November 2020)];2020 Available online: https://www.fda.gov/advisorycommittees/advisory-committee-calendar/november-6-2020-meeting-peripheral-and-central-nervous-system-drugs-advisory-committee-meeting.
- 2.Sevigny J., Chiao P., Bussiere T., Weinreb P.H., Williams L., Maier M., Dunstan R., Salloway S., Chen T., Ling Y., et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 3.Ayton S., Bush A.I. beta-amyloid: The known unknowns. Ageing Res. Rev. 2021;65:101212. doi: 10.1016/j.arr.2020.101212. [DOI] [PubMed] [Google Scholar]
- 4.2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020;16:391–460. doi: 10.1002/alz.12068. [DOI] [PubMed] [Google Scholar]
- 5.Reitz C., Rogaeva E., Beecham G.W. Late-onset vs nonmendelian early-onset Alzheimer disease: A distinction without a difference? Neurol. Genet. 2020;6:e512. doi: 10.1212/NXG.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuzaki T., Sasaki K., Tanizaki Y., Hata J., Fujimi K., Matsui Y., Sekita A., Suzuki S.O., Kanba S., Kiyohara Y., et al. Insulin resistance is associated with the pathology of Alzheimer disease: The Hisayama study. Neurology. 2010;75:764–770. doi: 10.1212/WNL.0b013e3181eee25f. [DOI] [PubMed] [Google Scholar]
- 7.Ott B.R., Lafleche G., Whelihan W.M., Buongiorno G.W., Albert M.S., Fogel B.S. Impaired awareness of deficits in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1996;10:68–76. doi: 10.1097/00002093-199601020-00003. [DOI] [PubMed] [Google Scholar]
- 8.Kivipelto M., Mangialasche F., Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 2018;14:653–666. doi: 10.1038/s41582-018-0070-3. [DOI] [PubMed] [Google Scholar]
- 9.Steen E., Terry B.M., Rivera E.J., Cannon J.L., Neely T.R., Tavares R., Xu X.J., Wands J.R., de la Monte S.M. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? J. Alzheimers Dis. 2005;7:63–80. doi: 10.3233/JAD-2005-7107. [DOI] [PubMed] [Google Scholar]
- 10.Crane P.K., Walker R., Hubbard R.A., Li G., Nathan D.M., Zheng H., Haneuse S., Craft S., Montine T.J., Kahn S.E., et al. Glucose levels and risk of dementia. N. Engl. J. Med. 2013;369:540–548. doi: 10.1056/NEJMoa1215740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tini G., Scagliola R., Monacelli F., La Malfa G., Porto I., Brunelli C., Rosa G.M. Alzheimer’s Disease and Cardiovascular Disease: A Particular Association. Cardiol. Res. Pract. 2020;2020:2617970. doi: 10.1155/2020/2617970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bateman R.J., Aisen P.S., De Strooper B., Fox N.C., Lemere C.A., Ringman J.M., Salloway S., Sperling R.A., Windisch M., Xiong C. Autosomal-dominant Alzheimer’s disease: A review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res. Ther. 2011;3:1. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng Q., Lin M.S., Tzeng I.S. Relationship Between Exercise and Alzheimer’s Disease: A Narrative Literature Review. Front. Neurosci. 2020;14:131. doi: 10.3389/fnins.2020.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsiao Y.H., Chang C.H., Gean P.W. Impact of social relationships on Alzheimer’s memory impairment: Mechanistic studies. J. Biomed. Sci. 2018;25:3. doi: 10.1186/s12929-018-0404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durazzo T.C., Mattsson N., Weiner M.W., Alzheimer’s Disease Neuroimaging I. Smoking and increased Alzheimer’s disease risk: A review of potential mechanisms. Alzheimers Dement. 2014;10:S122–S145. doi: 10.1016/j.jalz.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yiannopoulou K.G., Papageorgiou S.G. Current and future treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2013;6:19–33. doi: 10.1177/1756285612461679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., Cooper N.R., Eikelenboom P., Emmerling M., Fiebich B.L., et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez J.J., Olabarria M., Chvatal A., Verkhratsky A. Astroglia in dementia and Alzheimer’s disease. Cell Death Differ. 2009;16:378–385. doi: 10.1038/cdd.2008.172. [DOI] [PubMed] [Google Scholar]
- 19.Pekny M., Pekna M. Reactive gliosis in the pathogenesis of CNS diseases. Biochim. Biophys. Acta. 2016;1862:483–491. doi: 10.1016/j.bbadis.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Aisen P.S., Cummings J., Jack C.R., Jr., Morris J.C., Sperling R., Frolich L., Jones R.W., Dowsett S.A., Matthews B.R., Raskin J., et al. On the path to 2025: Understanding the Alzheimer’s disease continuum. Alzheimers Res. Ther. 2017;9:60. doi: 10.1186/s13195-017-0283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beason-Held L.L., Goh J.O., An Y., Kraut M.A., O’Brien R.J., Ferrucci L., Resnick S.M. Changes in brain function occur years before the onset of cognitive impairment. J. Neurosci. 2013;33:18008–18014. doi: 10.1523/JNEUROSCI.1402-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B., Holtzman D.M., Jagust W., Jessen F., Karlawish J., et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NIA . Alzheimer‘s Disease Diagnostic Guidelines. NIH National Institute on Aging; Bethesda, MD, USA: 2011. [Google Scholar]
- 24.Edgar C.J., Vradenburg G., Hassenstab J. The 2018 Revised FDA Guidance for Early Alzheimer’s Disease: Establishing the Meaningfulness of Treatment Effects. J. Prev. Alzheimers Dis. 2019;6:223–227. doi: 10.14283/jpad.2019.30. [DOI] [PubMed] [Google Scholar]
- 25.FDA Alzheimer’s Disease: Developing Drugs for Treatment Guidance for Industy. [(accessed on 10 April 2021)];2018 Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/alzheimers-disease-developing-drugs-treatment-guidance-industy.
- 26.Hyman B.T., Phelps C.H., Beach T.G., Bigio E.H., Cairns N.J., Carrillo M.C., Dickson D.W., Duyckaerts C., Frosch M.P., Masliah E., et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irwin K., Sexton C., Daniel T., Lawlor B., Naci L. Healthy Aging and Dementia: Two Roads Diverging in Midlife? Front. Aging Neurosci. 2018;10:275. doi: 10.3389/fnagi.2018.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan C.C., Yu J.T., Tan L. Biomarkers for preclinical Alzheimer’s disease. J. Alzheimers Dis. 2014;42:1051–1069. doi: 10.3233/JAD-140843. [DOI] [PubMed] [Google Scholar]
- 29.Ishii M., Iadecola C. Metabolic and Non-Cognitive Manifestations of Alzheimer’s Disease: The Hypothalamus as Both Culprit and Target of Pathology. Cell Metab. 2015;22:761–776. doi: 10.1016/j.cmet.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanctot K.L., Amatniek J., Ancoli-Israel S., Arnold S.E., Ballard C., Cohen-Mansfield J., Ismail Z., Lyketsos C., Miller D.S., Musiek E., et al. Neuropsychiatric signs and symptoms of Alzheimer’s disease: New treatment paradigms. Alzheimers Dement. 2017;3:440–449. doi: 10.1016/j.trci.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006:CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McShane R., Areosa Sastre A., Minakaran N. Memantine for dementia. Cochrane Database Syst. Rev. 2006:CD003154. doi: 10.1002/14651858.CD003154.pub5. [DOI] [PubMed] [Google Scholar]
- 33.Dubois B., Hampel H., Feldman H.H., Scheltens P., Aisen P., Andrieu S., Bakardjian H., Benali H., Bertram L., Blennow K., et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crous-Bou M., Minguillon C., Gramunt N., Molinuevo J.L. Alzheimer’s disease prevention: From risk factors to early intervention. Alzheimers Res. Ther. 2017;9:71. doi: 10.1186/s13195-017-0297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Facchinetti R., Valenza M., Bronzuoli M.R., Menegoni G., Ratano P., Steardo L., Campolongo P., Scuderi C. Looking for a Treatment for the Early Stage of Alzheimer’s Disease: Preclinical Evidence with Co-Ultramicronized Palmitoylethanolamide and Luteolin. Int. J. Mol. Sci. 2020;21:3802. doi: 10.3390/ijms21113802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alzheimer A. Über einen eigenartigen schweren Erkrankungsprozeβ der Hirnrincle. Neurol. Central. 1906;25:1134. [Google Scholar]
- 38.Alzheimer A., Stelzmann R.A., Schnitzlein H.N., Murtagh F.R. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin. Anat. 1995;8:429–431. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- 39.Glenner G.G., Wong C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984;120:885–890. doi: 10.1016/S0006-291X(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 40.Glenner G.G., Wong C.W., Quaranta V., Eanes E.D. The amyloid deposits in Alzheimer’s disease: Their nature and pathogenesis. Appl. Pathol. 1984;2:357–369. [PubMed] [Google Scholar]
- 41.Van Vickle G.D., Esh C.L., Kokjohn T.A., Patton R.L., Kalback W.M., Luehrs D.C., Beach T.G., Newel A.J., Lopera F., Ghetti B., et al. Presenilin-1 280Glu-->Ala mutation alters C-terminal APP processing yielding longer abeta peptides: Implications for Alzheimer’s disease. Mol. Med. 2008;14:184–194. doi: 10.2119/2007-00094.VanVickle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burnouf S., Gorsky M.K., Dols J., Gronke S., Partridge L. Abeta43 is neurotoxic and primes aggregation of Abeta40 in vivo. Acta Neuropathol. 2015;130:35–47. doi: 10.1007/s00401-015-1419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villemagne V.L., Burnham S., Bourgeat P., Brown B., Ellis K.A., Salvado O., Szoeke C., Macaulay S.L., Martins R., Maruff P., et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 44.Arnold S.E., Hyman B.T., Flory J., Damasio A.R., Van Hoesen G.W. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb. Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- 45.Puig K.L., Combs C.K. Expression and function of APP and its metabolites outside the central nervous system. Exp. Gerontol. 2013;48:608–611. doi: 10.1016/j.exger.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coronel R., Bernabeu-Zornoza A., Palmer C., Muniz-Moreno M., Zambrano A., Cano E., Liste I. Role of Amyloid Precursor Protein (APP) and Its Derivatives in the Biology and Cell Fate Specification of Neural Stem Cells. Mol. Neurobiol. 2018;55:7107–7117. doi: 10.1007/s12035-018-0914-2. [DOI] [PubMed] [Google Scholar]
- 47.Dawkins E., Small D.H. Insights into the physiological function of the beta-amyloid precursor protein: Beyond Alzheimer’s disease. J. Neurochem. 2014;129:756–769. doi: 10.1111/jnc.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caille I., Allinquant B., Dupont E., Bouillot C., Langer A., Muller U., Prochiantz A. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development. 2004;131:2173–2181. doi: 10.1242/dev.01103. [DOI] [PubMed] [Google Scholar]
- 49.Guo Q., Wang Z., Li H., Wiese M., Zheng H. APP physiological and pathophysiological functions: Insights from animal models. Cell Res. 2012;22:78–89. doi: 10.1038/cr.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng H., Koo E.H. The amyloid precursor protein: Beyond amyloid. Mol. Neurodegener. 2006;1:5. doi: 10.1186/1750-1326-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X., Huang T., Bu G., Xu H. Dysregulation of protein trafficking in neurodegeneration. Mol. Neurodegener. 2014;9:31. doi: 10.1186/1750-1326-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buoso E., Lanni C., Schettini G., Govoni S., Racchi M. beta-Amyloid precursor protein metabolism: Focus on the functions and degradation of its intracellular domain. Pharmacol. Res. 2010;62:308–317. doi: 10.1016/j.phrs.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Matsui T., Ingelsson M., Fukumoto H., Ramasamy K., Kowa H., Frosch M.P., Irizarry M.C., Hyman B.T. Expression of APP pathway mRNAs and proteins in Alzheimer’s disease. Brain Res. 2007;1161:116–123. doi: 10.1016/j.brainres.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 54.Hilbich C., Kisters-Woike B., Reed J., Masters C.L., Beyreuther K. Aggregation and secondary structure of synthetic amyloid beta A4 peptides of Alzheimer’s disease. J. Mol. Biol. 1991;218:149–163. doi: 10.1016/0022-2836(91)90881-6. [DOI] [PubMed] [Google Scholar]
- 55.McGowan E., Pickford F., Kim J., Onstead L., Eriksen J., Yu C., Skipper L., Murphy M.P., Beard J., Das P., et al. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Konietzko U. AICD nuclear signaling and its possible contribution to Alzheimer’s disease. Curr. Alzheimer. Res. 2012;9:200–216. doi: 10.2174/156720512799361673. [DOI] [PubMed] [Google Scholar]
- 57.Grimm M.O., Mett J., Stahlmann C.P., Haupenthal V.J., Zimmer V.C., Hartmann T. Neprilysin and Abeta Clearance: Impact of the APP Intracellular Domain in NEP Regulation and Implications in Alzheimer’s Disease. Front. Aging Neurosci. 2013;5:98. doi: 10.3389/fnagi.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts B.R., Lind M., Wagen A.Z., Rembach A., Frugier T., Li Q.X., Ryan T.M., McLean C.A., Doecke J.D., Rowe C.C., et al. Biochemically-defined pools of amyloid-beta in sporadic Alzheimer’s disease: Correlation with amyloid PET. Brain. 2017;140:1486–1498. doi: 10.1093/brain/awx057. [DOI] [PubMed] [Google Scholar]
- 59.Porayette P., Gallego M.J., Kaltcheva M.M., Bowen R.L., Vadakkadath Meethal S., Atwood C.S. Differential processing of amyloid-beta precursor protein directs human embryonic stem cell proliferation and differentiation into neuronal precursor cells. J. Biol. Chem. 2009;284:23806–23817. doi: 10.1074/jbc.M109.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee I.S., Jung K., Kim I.S., Park K.I. Amyloid-beta oligomers regulate the properties of human neural stem cells through GSK-3beta signaling. Exp. Mol. Med. 2013;45:60. doi: 10.1038/emm.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Y., Dong C. Abeta40 promotes neuronal cell fate in neural progenitor cells. Cell Death Differ. 2009;16:386–394. doi: 10.1038/cdd.2008.94. [DOI] [PubMed] [Google Scholar]
- 62.Fonseca M.B., Sola S., Xavier J.M., Dionisio P.A., Rodrigues C.M. Amyloid beta peptides promote autophagy-dependent differentiation of mouse neural stem cells: Abeta-mediated neural differentiation. Mol. Neurobiol. 2013;48:829–840. doi: 10.1007/s12035-013-8471-1. [DOI] [PubMed] [Google Scholar]
- 63.Lopez-Toledano M.A., Shelanski M.L. Neurogenic effect of beta-amyloid peptide in the development of neural stem cells. J. Neurosci. 2004;24:5439–5444. doi: 10.1523/JNEUROSCI.0974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karran E., Mercken M., De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug. Discov. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 65.Tomiyama T., Nagata T., Shimada H., Teraoka R., Fukushima A., Kanemitsu H., Takuma H., Kuwano R., Imagawa M., Ataka S., et al. A new amyloid beta variant favoring oligomerization in Alzheimer’s-type dementia. Ann. Neurol. 2008;63:377–387. doi: 10.1002/ana.21321. [DOI] [PubMed] [Google Scholar]
- 66.Maloney J.A., Bainbridge T., Gustafson A., Zhang S., Kyauk R., Steiner P., van der Brug M., Liu Y., Ernst J.A., Watts R.J., et al. Molecular mechanisms of Alzheimer disease protection by the A673T allele of amyloid precursor protein. J. Biol. Chem. 2014;289:30990–31000. doi: 10.1074/jbc.M114.589069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fernandez M.A., Klutkowski J.A., Freret T., Wolfe M.S. Alzheimer presenilin-1 mutations dramatically reduce trimming of long amyloid beta-peptides (Abeta) by gamma-secretase to increase 42-to-40-residue Abeta. J. Biol. Chem. 2014;289:31043–31052. doi: 10.1074/jbc.M114.581165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen Y., Strickland M.R., Soranno A., Holtzman D.M. Apolipoprotein E: Structural Insights and Links to Alzheimer Disease Pathogenesis. Neuron. 2021;109:205–221. doi: 10.1016/j.neuron.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castellano J.M., Kim J., Stewart F.R., Jiang H., DeMattos R.B., Patterson B.W., Fagan A.M., Morris J.C., Mawuenyega K.G., Cruchaga C., et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci. Transl. Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saunders A.M., Strittmatter W.J., Schmechel D., George-Hyslop P.H., Pericak-Vance M.A., Joo S.H., Rosi B.L., Gusella J.F., Crapper-MacLachlan D.R., Alberts M.J., et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/WNL.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 71.Corder E.H., Saunders A.M., Risch N.J., Strittmatter W.J., Schmechel D.E., Gaskell P.C., Jr., Rimmler J.B., Locke P.A., Conneally P.M., Schmader K.E., et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat. Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- 72.Holmes C., Boche D., Wilkinson D., Yadegarfar G., Hopkins V., Bayer A., Jones R.W., Bullock R., Love S., Neal J.W., et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: Follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 73.Serrano-Pozo A., Frosch M.P., Masliah E., Hyman B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Metaxas A., Kempf S.J. Neurofibrillary tangles in Alzheimer’s disease: Elucidation of the molecular mechanism by immunohistochemistry and tau protein phospho-proteomics. Neural. Regen. Res. 2016;11:1579–1581. doi: 10.4103/1673-5374.193234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 76.Hirokawa N., Funakoshi T., Sato-Harada R., Kanai Y. Selective stabilization of tau in axons and microtubule-associated protein 2C in cell bodies and dendrites contributes to polarized localization of cytoskeletal proteins in mature neurons. J. Cell Biol. 1996;132:667–679. doi: 10.1083/jcb.132.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanger D.P., Anderton B.H., Noble W. Tau phosphorylation: The therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 2009;15:112–119. doi: 10.1016/j.molmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Zempel H., Mandelkow E. Lost after translation: Missorting of Tau protein and consequences for Alzheimer disease. Trends Neurosci. 2014;37:721–732. doi: 10.1016/j.tins.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 79.Braak H., Alafuzoff I., Arzberger T., Kretzschmar H., Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ittner L.M., Ke Y.D., Delerue F., Bi M., Gladbach A., van Eersel J., Wolfing H., Chieng B.C., Christie M.J., Napier I.A., et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 81.Roberson E.D., Scearce-Levie K., Palop J.J., Yan F., Cheng I.H., Wu T., Gerstein H., Yu G.Q., Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 82.Dawson H.N., Ferreira A., Eyster M.V., Ghoshal N., Binder L.I., Vitek M.P. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J. Cell Sci. 2001;114:1179–1187. doi: 10.1242/jcs.114.6.1179. [DOI] [PubMed] [Google Scholar]
- 83.Harada A., Oguchi K., Okabe S., Kuno J., Terada S., Ohshima T., Sato-Yoshitake R., Takei Y., Noda T., Hirokawa N. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature. 1994;369:488–491. doi: 10.1038/369488a0. [DOI] [PubMed] [Google Scholar]
- 84.Cash A.D., Aliev G., Siedlak S.L., Nunomura A., Fujioka H., Zhu X., Raina A.K., Vinters H.V., Tabaton M., Johnson A.B., et al. Microtubule reduction in Alzheimer’s disease and aging is independent of tau filament formation. Am. J. Pathol. 2003;162:1623–1627. doi: 10.1016/S0002-9440(10)64296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Congdon E.E., Sigurdsson E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018;14:399–415. doi: 10.1038/s41582-018-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Selkoe D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 87.Weitz T.M., Town T. Microglia in Alzheimer’s Disease: It’s All About Context. Int. J. Alzheimers Dis. 2012;2012:314185. doi: 10.1155/2012/314185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Green K.N., LaFerla F.M. Linking calcium to Abeta and Alzheimer’s disease. Neuron. 2008;59:190–194. doi: 10.1016/j.neuron.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 89.Moreira P.I., Honda K., Liu Q., Santos M.S., Oliveira C.R., Aliev G., Nunomura A., Zhu X., Smith M.A., Perry G. Oxidative stress: The old enemy in Alzheimer’s disease pathophysiology. Curr. Alzheimer Res. 2005;2:403–408. doi: 10.2174/156720505774330537. [DOI] [PubMed] [Google Scholar]
- 90.Llanos-Gonzalez E., Henares-Chavarino A.A., Pedrero-Prieto C.M., Garcia-Carpintero S., Frontinan-Rubio J., Sancho-Bielsa F.J., Alcain F.J., Peinado J.R., Rabanal-Ruiz Y., Duran-Prado M. Interplay Between Mitochondrial Oxidative Disorders and Proteostasis in Alzheimer’s Disease. Front. Neurosci. 2019;13:1444. doi: 10.3389/fnins.2019.01444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cornejo V.H., Hetz C. The unfolded protein response in Alzheimer’s disease. Semin. Immunopathol. 2013;35:277–292. doi: 10.1007/s00281-013-0373-9. [DOI] [PubMed] [Google Scholar]
- 92.Schwarz D.S., Blower M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell Mol. Life Sci. 2016;73:79–94. doi: 10.1007/s00018-015-2052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lilienbaum A. Relationship between the proteasomal system and autophagy. Int. J. Biochem. Mol. Biol. 2013;4:1–26. [PMC free article] [PubMed] [Google Scholar]
- 94.Gerakis Y., Hetz C. Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer’s disease. FEBS J. 2018;285:995–1011. doi: 10.1111/febs.14332. [DOI] [PubMed] [Google Scholar]
- 95.O’Connor T., Sadleir K.R., Maus E., Velliquette R.A., Zhao J., Cole S.L., Eimer W.A., Hitt B., Bembinster L.A., Lammich S., et al. Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron. 2008;60:988–1009. doi: 10.1016/j.neuron.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Griffin W.S., Stanley L.C., Ling C., White L., MacLeod V., Perrot L.J., White C.L., 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tejera D., Mercan D., Sanchez-Caro J.M., Hanan M., Greenberg D., Soreq H., Latz E., Golenbock D., Heneka M.T. Systemic inflammation impairs microglial Abeta clearance through NLRP3 inflammasome. EMBO J. 2019;38:e101064. doi: 10.15252/embj.2018101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li R., Strohmeyer R., Liang Z., Lue L.F., Rogers J. CCAAT/enhancer binding protein delta (C/EBPdelta) expression and elevation in Alzheimer’s disease. Neurobiol. Aging. 2004;25:991–999. doi: 10.1016/j.neurobiolaging.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 100.Ndoja A., Reja R., Lee S.H., Webster J.D., Ngu H., Rose C.M., Kirkpatrick D.S., Modrusan Z., Chen Y.J., Dugger D.L., et al. Ubiquitin Ligase COP1 Suppresses Neuroinflammation by Degrading c/EBPbeta in Microglia. Cell. 2020;182:1156–1169.e12. doi: 10.1016/j.cell.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 101.Kaltschmidt B., Uherek M., Volk B., Baeuerle P.A., Kaltschmidt C. Transcription factor NF-kappaB is activated in primary neurons by amyloid beta peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1997;94:2642–2647. doi: 10.1073/pnas.94.6.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bradt B.M., Kolb W.P., Cooper N.R. Complement-dependent proinflammatory properties of the Alzheimer’s disease beta-peptide. J. Exp. Med. 1998;188:431–438. doi: 10.1084/jem.188.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shen Y., Lue L., Yang L., Roher A., Kuo Y., Strohmeyer R., Goux W.J., Lee V., Johnson G.V., Webster S.D., et al. Complement activation by neurofibrillary tangles in Alzheimer’s disease. Neurosci. Lett. 2001;305:165–168. doi: 10.1016/S0304-3940(01)01842-0. [DOI] [PubMed] [Google Scholar]
- 104.Eikelenboom P., Stam F.C. Immunoglobulins and complement factors in senile plaques. An immunoperoxidase study. Acta Neuropathol. 1982;57:239–242. doi: 10.1007/BF00685397. [DOI] [PubMed] [Google Scholar]
- 105.Zhou J., Fonseca M.I., Pisalyaput K., Tenner A.J. Complement C3 and C4 expression in C1q sufficient and deficient mouse models of Alzheimer’s disease. J. Neurochem. 2008;106:2080–2092. doi: 10.1111/j.1471-4159.2008.05558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Afagh A., Cummings B.J., Cribbs D.H., Cotman C.W., Tenner A.J. Localization and cell association of C1q in Alzheimer’s disease brain. Exp. Neurol. 1996;138:22–32. doi: 10.1006/exnr.1996.0043. [DOI] [PubMed] [Google Scholar]
- 107.Webster S., Lue L.F., Brachova L., Tenner A.J., McGeer P.L., Terai K., Walker D.G., Bradt B., Cooper N.R., Rogers J. Molecular and cellular characterization of the membrane attack complex, C5b-9, in Alzheimer’s disease. Neurobiol. Aging. 1997;18:415–421. doi: 10.1016/S0197-4580(97)00042-0. [DOI] [PubMed] [Google Scholar]
- 108.Cribbs D.H., Berchtold N.C., Perreau V., Coleman P.D., Rogers J., Tenner A.J., Cotman C.W. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: A microarray study. J. Neuroinflamm. 2012;9:179. doi: 10.1186/1742-2094-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tenner A.J. Complement-Mediated Events in Alzheimer’s Disease: Mechanisms and Potential Therapeutic Targets. J. Immunol. 2020;204:306–315. doi: 10.4049/jimmunol.1901068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Krance S.H., Wu C.Y., Zou Y., Mao H., Toufighi S., He X., Pakosh M., Swardfager W. The complement cascade in Alzheimer’s disease: A systematic review and meta-analysis. Mol. Psychiatry. :2019. doi: 10.1038/s41380-019-0536-8. [DOI] [PubMed] [Google Scholar]
- 111.Schartz N.D., Tenner A.J. The good, the bad, and the opportunities of the complement system in neurodegenerative disease. J. Neuroinflamm. 2020;17:354. doi: 10.1186/s12974-020-02024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hong S., Beja-Glasser V.F., Nfonoyim B.M., Frouin A., Li S., Ramakrishnan S., Merry K.M., Shi Q., Rosenthal A., Barres B.A., et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lui H., Zhang J., Makinson S.R., Cahill M.K., Kelley K.W., Huang H.Y., Shang Y., Oldham M.C., Martens L.H., Gao F., et al. Progranulin Deficiency Promotes Circuit-Specific Synaptic Pruning by Microglia via Complement Activation. Cell. 2016;165:921–935. doi: 10.1016/j.cell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vasek M.J., Garber C., Dorsey D., Durrant D.M., Bollman B., Soung A., Yu J., Perez-Torres C., Frouin A., Wilton D.K., et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016;534:538–543. doi: 10.1038/nature18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee J.D., Coulthard L.G., Woodruff T.M. Complement dysregulation in the central nervous system during development and disease. Semin. Immunol. 2019;45:101340. doi: 10.1016/j.smim.2019.101340. [DOI] [PubMed] [Google Scholar]
- 116.Fonseca M.I., Ager R.R., Chu S.H., Yazan O., Sanderson S.D., LaFerla F.M., Taylor S.M., Woodruff T.M., Tenner A.J. Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of Alzheimer’s disease. J. Immunol. 2009;183:1375–1383. doi: 10.4049/jimmunol.0901005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Carpanini S.M., Torvell M., Morgan B.P. Therapeutic Inhibition of the Complement System in Diseases of the Central Nervous System. Front. Immunol. 2019;10:362. doi: 10.3389/fimmu.2019.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aisen P.S., Davis K.L., Berg J.D., Schafer K., Campbell K., Thomas R.G., Weiner M.F., Farlow M.R., Sano M., Grundman M., et al. A randomized controlled trial of prednisone in Alzheimer’s disease. Alzheimer’s Disease Cooperative Study. Neurology. 2000;54:588–593. doi: 10.1212/WNL.54.3.588. [DOI] [PubMed] [Google Scholar]
- 119.Aisen P.S., Schafer K.A., Grundman M., Pfeiffer E., Sano M., Davis K.L., Farlow M.R., Jin S., Thomas R.G., Thal L.J., et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: A randomized controlled trial. JAMA. 2003;289:2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 120.Thal L.J., Ferris S.H., Kirby L., Block G.A., Lines C.R., Yuen E., Assaid C., Nessly M.L., Norman B.A., Baranak C.C., et al. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology. 2005;30:1204–1215. doi: 10.1038/sj.npp.1300690. [DOI] [PubMed] [Google Scholar]
- 121.Scharf S., Mander A., Ugoni A., Vajda F., Christophidis N. A double-blind, placebo-controlled trial of diclofenac/misoprostol in Alzheimer’s disease. Neurology. 1999;53:197–201. doi: 10.1212/WNL.53.1.197. [DOI] [PubMed] [Google Scholar]
- 122.Group A.D.C., Bentham P., Gray R., Sellwood E., Hills R., Crome P., Raftery J. Aspirin in Alzheimer’s disease (AD2000): A randomised open-label trial. Lancet Neurol. 2008;7:41–49. doi: 10.1016/S1474-4422(07)70293-4. [DOI] [PubMed] [Google Scholar]
- 123.Hemonnot A.L., Hua J., Ulmann L., Hirbec H. Microglia in Alzheimer Disease: Well-Known Targets and New Opportunities. Front Aging Neurosci. 2019;11:233. doi: 10.3389/fnagi.2019.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., Bennett M.L., Munch A.E., Chung W.S., Peterson T.C., et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sofroniew M.V. Astrogliosis. Cold Spring Harb. Perspect. Biol. 2014;7:a020420. doi: 10.1101/cshperspect.a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Escartin C., Galea E., Lakatos A., O’Callaghan J.P., Petzold G.C., Serrano-Pozo A., Steinhauser C., Volterra A., Carmignoto G., Agarwal A., et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021;24:312–325. doi: 10.1038/s41593-020-00783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bronzuoli M.R., Iacomino A., Steardo L., Scuderi C. Targeting neuroinflammation in Alzheimer’s disease. J. Inflamm. Res. 2016;9:199–208. doi: 10.2147/JIR.S86958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kalaria R.N., Harik S.I. Abnormalities of the glucose transporter at the blood-brain barrier and in brain in Alzheimer’s disease. Prog. Clin. Biol. Res. 1989;317:415–421. [PubMed] [Google Scholar]
- 129.Hooijmans C.R., Graven C., Dederen P.J., Tanila H., van Groen T., Kiliaan A.J. Amyloid beta deposition is related to decreased glucose transporter-1 levels and hippocampal atrophy in brains of aged APP/PS1 mice. Brain Res. 2007;1181:93–103. doi: 10.1016/j.brainres.2007.08.063. [DOI] [PubMed] [Google Scholar]
- 130.Winkler E.A., Nishida Y., Sagare A.P., Rege S.V., Bell R.D., Perlmutter D., Sengillo J.D., Hillman S., Kong P., Nelson A.R., et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat. Neurosci. 2015;18:521–530. doi: 10.1038/nn.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jagust W.J., Landau S.M., Alzheimer’s Disease Neuroimaging Initiative Apolipoprotein E, not fibrillar beta-amyloid, reduces cerebral glucose metabolism in normal aging. J. Neurosci. 2012;32:18227–18233. doi: 10.1523/JNEUROSCI.3266-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Griffith C.M., Eid T., Rose G.M., Patrylo P.R. Evidence for altered insulin receptor signaling in Alzheimer’s disease. Neuropharmacology. 2018;136:202–215. doi: 10.1016/j.neuropharm.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 133.Ragozzino M.E., Unick K.E., Gold P.E. Hippocampal acetylcholine release during memory testing in rats: Augmentation by glucose. Proc. Natl. Acad. Sci. USA. 1996;93:4693–4698. doi: 10.1073/pnas.93.10.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Andersen J.V., Christensen S.K., Westi E.W., Diaz-delCastillo M., Tanila H., Schousboe A., Aldana B.I., Waagepetersen H.S. Deficient astrocyte metabolism impairs glutamine synthesis and neurotransmitter homeostasis in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2021;148:105198. doi: 10.1016/j.nbd.2020.105198. [DOI] [PubMed] [Google Scholar]
- 135.Suzuki A., Stern S.A., Bozdagi O., Huntley G.W., Walker R.H., Magistretti P.J., Alberini C.M. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pellerin L., Magistretti P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rouach N., Koulakoff A., Abudara V., Willecke K., Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- 138.Berchtold N.C., Sabbagh M.N., Beach T.G., Kim R.C., Cribbs D.H., Cotman C.W. Brain gene expression patterns differentiate mild cognitive impairment from normal aged and Alzheimer’s disease. Neurobiol. Aging. 2014;35:1961–1972. doi: 10.1016/j.neurobiolaging.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Erickson M.A., Banks W.A. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2013;33:1500–1513. doi: 10.1038/jcbfm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hansen D.V., Hanson J.E., Sheng M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018;217:459–472. doi: 10.1083/jcb.201709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim Y.S., Jung H.M., Yoon B.E. Exploring glia to better understand Alzheimer’s disease. Anim. Cells Syst. 2018;22:213–218. doi: 10.1080/19768354.2018.1508498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lau S.F., Cao H., Fu A.K.Y., Ip N.Y. Single-nucleus transcriptome analysis reveals dysregulation of angiogenic endothelial cells and neuroprotective glia in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2020;117:25800–25809. doi: 10.1073/pnas.2008762117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jakel S., Dimou L. Glial Cells and Their Function in the Adult Brain: A Journey through the History of Their Ablation. Front. Cell Neurosci. 2017;11:24. doi: 10.3389/fncel.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Di Benedetto B., Rupprecht R. Targeting glia cells: Novel perspectives for the treatment of neuropsychiatric diseases. Curr. Neuropharmacol. 2013;11:171–185. doi: 10.2174/1570159X11311020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kettenmann H., Hanisch U.K., Noda M., Verkhratsky A. Physiology of microglia. Physiol. Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 146.Fan Y., Xie L., Chung C.Y. Signaling Pathways Controlling Microglia Chemotaxis. Mol. Cells. 2017;40:163–168. doi: 10.14348/molcells.2017.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Paolicelli R.C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., Giustetto M., Ferreira T.A., Guiducci E., Dumas L., et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 148.Schafer D.P., Lehrman E.K., Kautzman A.G., Koyama R., Mardinly A.R., Yamasaki R., Ransohoff R.M., Greenberg M.E., Barres B.A., Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Prinz M., Jung S., Priller J. Microglia Biology: One Century of Evolving Concepts. Cell. 2019;179:292–311. doi: 10.1016/j.cell.2019.08.053. [DOI] [PubMed] [Google Scholar]
- 150.Friedman B.A., Srinivasan K., Ayalon G., Meilandt W.J., Lin H., Huntley M.A., Cao Y., Lee S.H., Haddick P.C.G., Ngu H., et al. Diverse Brain Myeloid Expression Profiles Reveal Distinct Microglial Activation States and Aspects of Alzheimer’s Disease Not Evident in Mouse Models. Cell Rep. 2018;22:832–847. doi: 10.1016/j.celrep.2017.12.066. [DOI] [PubMed] [Google Scholar]
- 151.Guerreiro R., Wojtas A., Bras J., Carrasquillo M., Rogaeva E., Majounie E., Cruchaga C., Sassi C., Kauwe J.S., Younkin S., et al. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bergles D.E., Richardson W.D. Oligodendrocyte Development and Plasticity. Cold Spring Harb. Perspect. Biol. 2015;8:a020453. doi: 10.1101/cshperspect.a020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fields R.D. A new mechanism of nervous system plasticity: Activity-dependent myelination. Nat. Rev. Neurosci. 2015;16:756–767. doi: 10.1038/nrn4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lasiene J., Matsui A., Sawa Y., Wong F., Horner P.J. Age-related myelin dynamics revealed by increased oligodendrogenesis and short internodes. Aging Cell. 2009;8:201–213. doi: 10.1111/j.1474-9726.2009.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Simons M., Nave K.A. Oligodendrocytes: Myelination and Axonal Support. Cold Spring Harb. Perspect. Biol. 2015;8:a020479. doi: 10.1101/cshperspect.a020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bronzuoli M.R., Facchinetti R., Ingrassia D., Sarvadio M., Schiavi S., Steardo L., Verkhratsky A., Trezza V., Scuderi C. Neuroglia in the autistic brain: Evidence from a preclinical model. Mol. Autism. 2018;9:66. doi: 10.1186/s13229-018-0254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Papuc E., Rejdak K. The role of myelin damage in Alzheimer’s disease pathology. Arch. Med. Sci. 2020;16:345–351. doi: 10.5114/aoms.2018.76863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cartocci V., Tonini C., Di Pippo T., Vuono F., Schiavi S., Marino M., Trezza V., Pallottini V. Prenatal exposure to valproate induces sex-, age-, and tissue-dependent alterations of cholesterol metabolism: Potential implications on autism. J. Cell Physiol. 2019;234:4362–4374. doi: 10.1002/jcp.27218. [DOI] [PubMed] [Google Scholar]
- 159.Cainelli E., Arrigoni F., Vedovelli L. White matter injury and neurodevelopmental disabilities: A cross-disease (dis)connection. Prog. Neurobiol. 2020;193:101845. doi: 10.1016/j.pneurobio.2020.101845. [DOI] [PubMed] [Google Scholar]
- 160.Cartocci V., Catallo M., Tempestilli M., Segatto M., Pfrieger F.W., Bronzuoli M.R., Scuderi C., Servadio M., Trezza V., Pallottini V. Altered Brain Cholesterol/Isoprenoid Metabolism in a Rat Model of Autism Spectrum Disorders. Neuroscience. 2018;372:27–37. doi: 10.1016/j.neuroscience.2017.12.053. [DOI] [PubMed] [Google Scholar]
- 161.Arthur V.A.a.B. Glial Physiology and Pathophysiology. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2013. Astroglia; pp. 105–244. [DOI] [Google Scholar]
- 162.Verkhratsky A., Nedergaard M. Physiology of Astroglia. Physiol. Rev. 2018;98:239–389. doi: 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Verkhratsky A., Nedergaard M., Hertz L. Why are astrocytes important? Neurochem. Res. 2015;40:389–401. doi: 10.1007/s11064-014-1403-2. [DOI] [PubMed] [Google Scholar]
- 164.Parpura V., Verkhratsky A. The astrocyte excitability brief: From receptors to gliotransmission. Neurochem. Int. 2012;61:610–621. doi: 10.1016/j.neuint.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 165.Verkhratsky A., Parpura V., Vardjan N., Zorec R. Physiology of Astroglia. Adv. Exp. Med. Biol. 2019;1175:45–91. doi: 10.1007/978-981-13-9913-8_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Stallcup W.B. The NG2 proteoglycan: Past insights and future prospects. J. Neurocytol. 2002;31:423–435. doi: 10.1023/A:1025731428581. [DOI] [PubMed] [Google Scholar]
- 167.Butt A.M., Hamilton N., Hubbard P., Pugh M., Ibrahim M. Synantocytes: The fifth element. J. Anat. 2005;207:695–706. doi: 10.1111/j.1469-7580.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bergles D.E., Roberts J.D., Somogyi P., Jahr C.E. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- 169.Scuderi C., Verkhratsky A. The role of neuroglia in autism spectrum disorders. Prog. Mol. Biol. Transl. Sci. 2020;173:301–330. doi: 10.1016/bs.pmbts.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 170.Bernaus A., Blanco S., Sevilla A. Glia Crosstalk in Neuroinflammatory Diseases. Front. Cell Neurosci. 2020;14:209. doi: 10.3389/fncel.2020.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jha M.K., Kim J.H., Song G.J., Lee W.H., Lee I.K., Lee H.W., An S.S.A., Kim S., Suk K. Functional dissection of astrocyte-secreted proteins: Implications in brain health and diseases. Prog. Neurobiol. 2018;162:37–69. doi: 10.1016/j.pneurobio.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 172.Verkhratsky A., Parpura V., Rodriguez-Arellano J.J., Zorec R. Astroglia in Alzheimer’s Disease. Adv. Exp. Med. Biol. 2019;1175:273–324. doi: 10.1007/978-981-13-9913-8_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cassano T., Magini A., Giovagnoli S., Polchi A., Calcagnini S., Pace L., Lavecchia M.A., Scuderi C., Bronzuoli M.R., Ruggeri L., et al. Early intrathecal infusion of everolimus restores cognitive function and mood in a murine model of Alzheimer’s disease. Exp. Neurol. 2019;311:88–105. doi: 10.1016/j.expneurol.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 174.Maftei D., Ratano P., Fusco I., Marconi V., Squillace S., Negri L., Severini C., Balboni G., Steardo L., Bronzuoli M.R., et al. The prokineticin receptor antagonist PC1 rescues memory impairment induced by beta amyloid administration through the modulation of prokineticin system. Neuropharmacology. 2019;158:107739. doi: 10.1016/j.neuropharm.2019.107739. [DOI] [PubMed] [Google Scholar]
- 175.Orre M., Kamphuis W., Osborn L.M., Jansen A.H.P., Kooijman L., Bossers K., Hol E.M. Isolation of glia from Alzheimer’s mice reveals inflammation and dysfunction. Neurobiol. Aging. 2014;35:2746–2760. doi: 10.1016/j.neurobiolaging.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 176.Guillemaud O., Ceyzeriat K., Saint-Georges T., Cambon K., Petit F., Ben Haim L., Carrillo-de Sauvage M.A., Guillermier M., Bernier S., Herard A.S., et al. Complex roles for reactive astrocytes in the triple transgenic mouse model of Alzheimer disease. Neurobiol. Aging. 2020;90:135–146. doi: 10.1016/j.neurobiolaging.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 177.Matias I., Morgado J., Gomes F.C.A. Astrocyte Heterogeneity: Impact to Brain Aging and Disease. Front. Aging Neurosci. 2019;11:59. doi: 10.3389/fnagi.2019.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liddelow S.A., Barres B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity. 2017;46:957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 179.Cota-Coronado A., Diaz-Martinez N.F., Padilla-Camberos E., Diaz-Martinez N.E. Editing the Central Nervous System Through CRISPR/Cas9 Systems. Front. Mol. Neurosci. 2019;12:110. doi: 10.3389/fnmol.2019.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pardridge W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yabluchanskiy A., Tarantini S., Balasubramanian P., Kiss T., Csipo T., Fulop G.A., Lipecz A., Ahire C., DelFavero J., Nyul-Toth A., et al. Pharmacological or genetic depletion of senescent astrocytes prevents whole brain irradiation-induced impairment of neurovascular coupling responses protecting cognitive function in mice. Geroscience. 2020;42:409–428. doi: 10.1007/s11357-020-00154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bussian T.J., Aziz A., Meyer C.F., Swenson B.L., van Deursen J.M., Baker D.J. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 2018;562:578–582. doi: 10.1038/s41586-018-0543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Turnquist C., Beck J.A., Horikawa I., Obiorah I.E., Von Muhlinen N., Vojtesek B., Lane D.P., Grunseich C., Chahine J.J., Ames H.M., et al. Radiation-induced astrocyte senescence is rescued by Delta133p53. Neuro. Oncol. 2019;21:474–485. doi: 10.1093/neuonc/noz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hou J., Cui C., Kim S., Sung C., Choi C. Ginsenoside F1 suppresses astrocytic senescence-associated secretory phenotype. Chem. Biol. Interact. 2018;283:75–83. doi: 10.1016/j.cbi.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 185.Fan S., Xian X., Li L., Yao X., Hu Y., Zhang M., Li W. Ceftriaxone Improves Cognitive Function and Upregulates GLT-1-Related Glutamate-Glutamine Cycle in APP/PS1 Mice. J. Alzheimers Dis. 2018;66:1731–1743. doi: 10.3233/JAD-180708. [DOI] [PubMed] [Google Scholar]
- 186.Okamoto M., Gray J.D., Larson C.S., Kazim S.F., Soya H., McEwen B.S., Pereira A.C. Riluzole reduces amyloid beta pathology, improves memory, and restores gene expression changes in a transgenic mouse model of early-onset Alzheimer’s disease. Transl. Psychiatry. 2018;8:153. doi: 10.1038/s41398-018-0201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Konttinen H., Gureviciene I., Oksanen M., Grubman A., Loppi S., Huuskonen M.T., Korhonen P., Lampinen R., Keuters M., Belaya I., et al. PPARbeta/delta-agonist GW0742 ameliorates dysfunction in fatty acid oxidation in PSEN1DeltaE9 astrocytes. Glia. 2019;67:146–159. doi: 10.1002/glia.23534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.van Gijsel-Bonnello M., Baranger K., Benech P., Rivera S., Khrestchatisky M., de Reggi M., Gharib B. Metabolic changes and inflammation in cultured astrocytes from the 5xFAD mouse model of Alzheimer’s disease: Alleviation by pantethine. PLoS ONE. 2017;12:e0175369. doi: 10.1371/journal.pone.0175369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Crespo M.C., Tome-Carneiro J., Pintado C., Davalos A., Visioli F., Burgos-Ramos E. Hydroxytyrosol restores proper insulin signaling in an astrocytic model of Alzheimer’s disease. Biofactors. 2017;43:540–548. doi: 10.1002/biof.1356. [DOI] [PubMed] [Google Scholar]
- 190.Zheng J., Xie Y., Ren L., Qi L., Wu L., Pan X., Zhou J., Chen Z., Liu L. GLP-1 improves the supportive ability of astrocytes to neurons by promoting aerobic glycolysis in Alzheimer’s disease. Mol. Metab. 2021;47:101180. doi: 10.1016/j.molmet.2021.101180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang G., Cui W., Chen S., Shao Z., Li Y., Wang W., Mao L., Li J., Mei X. Metformin alleviates high glucose-induced ER stress and inflammation by inhibiting the interaction between caveolin1 and AMPKalpha in rat astrocytes. Biochem. Biophys. Res. Commun. 2021;534:908–913. doi: 10.1016/j.bbrc.2020.10.075. [DOI] [PubMed] [Google Scholar]
- 192.Sawamoto A., Okuyama S., Nakajima M., Furukawa Y. Citrus flavonoid 3,5,6,7,8,3′,4′-heptamethoxyflavone induces BDNF via cAMP/ERK/CREB signaling and reduces phosphodiesterase activity in C6 cells. Pharmacol. Rep. 2019;71:653–658. doi: 10.1016/j.pharep.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 193.Luo G., Huang Y., Jia B., Zhang X., Mo D., Ma N., Gao F., Song L., Wang B., Miao Z. Quetiapine prevents Abeta25-35-induced cell death in cultured neuron by enhancing brain-derived neurotrophic factor release from astrocyte. Neuroreport. 2018;29:92–98. doi: 10.1097/WNR.0000000000000911. [DOI] [PubMed] [Google Scholar]
- 194.de Pins B., Cifuentes-Diaz C., Farah A.T., Lopez-Molina L., Montalban E., Sancho-Balsells A., Lopez A., Gines S., Delgado-Garcia J.M., Alberch J., et al. Conditional BDNF Delivery from Astrocytes Rescues Memory Deficits, Spine Density, and Synaptic Properties in the 5xFAD Mouse Model of Alzheimer Disease. J. Neurosci. 2019;39:2441–2458. doi: 10.1523/JNEUROSCI.2121-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bali P., Banik A., Nehru B., Anand A. Neurotrophic Factors Mediated Activation of Astrocytes Ameliorate Memory Loss by Amyloid Clearance after Transplantation of Lineage Negative Stem Cells. Mol. Neurobiol. 2019;56:8420–8434. doi: 10.1007/s12035-019-01680-z. [DOI] [PubMed] [Google Scholar]
- 196.Chen X., Li Z., Cheng Y., Kardami E., Loh Y.P. Low and High Molecular Weight FGF-2 Have Differential Effects on Astrocyte Proliferation, but Are Both Protective Against Abeta-Induced Cytotoxicity. Front. Mol. Neurosci. 2019;12:328. doi: 10.3389/fnmol.2019.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chernick D., Ortiz-Valle S., Jeong A., Swaminathan S.K., Kandimalla K.K., Rebeck G.W., Li L. High-density lipoprotein mimetic peptide 4F mitigates amyloid-beta-induced inhibition of apolipoprotein E secretion and lipidation in primary astrocytes and microglia. J. Neurochem. 2018;147:647–662. doi: 10.1111/jnc.14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wojtas A.M., Sens J.P., Kang S.S., Baker K.E., Berry T.J., Kurti A., Daughrity L., Jansen-West K.R., Dickson D.W., Petrucelli L., et al. Astrocyte-derived clusterin suppresses amyloid formation in vivo. Mol. Neurodegener. 2020;15:71. doi: 10.1186/s13024-020-00416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chen F., Swartzlander D.B., Ghosh A., Fryer J.D., Wang B., Zheng H. Clusterin secreted from astrocyte promotes excitatory synaptic transmission and ameliorates Alzheimer’s disease neuropathology. Mol. Neurodegener. 2021;16:5. doi: 10.1186/s13024-021-00426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yamamoto N., Shibata M., Ishikuro R., Tanida M., Taniguchi Y., Ikeda-Matsuo Y., Sobue K. Epigallocatechin gallate induces extracellular degradation of amyloid beta-protein by increasing neprilysin secretion from astrocytes through activation of ERK and PI3K pathways. Neuroscience. 2017;362:70–78. doi: 10.1016/j.neuroscience.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 201.Ren H., Luo C., Feng Y., Yao X., Shi Z., Liang F., Kang J.X., Wan J.B., Pei Z., Su H. Omega-3 polyunsaturated fatty acids promote amyloid-beta clearance from the brain through mediating the function of the glymphatic system. FASEB J. 2017;31:282–293. doi: 10.1096/fj.201600896. [DOI] [PubMed] [Google Scholar]
- 202.Shi Q., Chowdhury S., Ma R., Le K.X., Hong S., Caldarone B.J., Stevens B., Lemere C.A. Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aaf6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.McManus R.M., Finucane O.M., Wilk M.M., Mills K.H.G., Lynch M.A. FTY720 Attenuates Infection-Induced Enhancement of Abeta Accumulation in APP/PS1 Mice by Modulating Astrocytic Activation. J. Neuroimmune Pharmacol. 2017;12:670–681. doi: 10.1007/s11481-017-9753-6. [DOI] [PubMed] [Google Scholar]
- 204.Couturier J., Stancu I.C., Schakman O., Pierrot N., Huaux F., Kienlen-Campard P., Dewachter I., Octave J.N. Activation of phagocytic activity in astrocytes by reduced expression of the inflammasome component ASC and its implication in a mouse model of Alzheimer disease. J. Neuroinflammation. 2016;13:20. doi: 10.1186/s12974-016-0477-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dekens D.W., De Deyn P.P., Sap F., Eisel U.L.M., Naude P.J.W. Iron chelators inhibit amyloid-beta-induced production of lipocalin 2 in cultured astrocytes. Neurochem. Int. 2020;132:104607. doi: 10.1016/j.neuint.2019.104607. [DOI] [PubMed] [Google Scholar]
- 206.Kim M., Park M.H., Nam G., Lee M., Kang J., Song I.S., Choi M.K., Jin H.K., Bae J.S., Lim M.H. A Glycosylated Prodrug to Attenuate Neuroinflammation and Improve Cognitive Deficits in Alzheimer’s Disease Transgenic Mice. Mol. Pharm. 2021;18:101–112. doi: 10.1021/acs.molpharmaceut.0c00677. [DOI] [PubMed] [Google Scholar]
- 207.Ceyzeriat K., Ben Haim L., Denizot A., Pommier D., Matos M., Guillemaud O., Palomares M.A., Abjean L., Petit F., Gipchtein P., et al. Modulation of astrocyte reactivity improves functional deficits in mouse models of Alzheimer’s disease. Acta Neuropathol. Commun. 2018;6:104. doi: 10.1186/s40478-018-0606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Beggiato S., Borelli A.C., Ferraro L., Tanganelli S., Antonelli T., Tomasini M.C. Palmitoylethanolamide Blunts Amyloid-beta42-Induced Astrocyte Activation and Improves Neuronal Survival in Primary Mouse Cortical Astrocyte-Neuron Co-Cultures. J. Alzheimers Dis. 2018;61:389–399. doi: 10.3233/JAD-170699. [DOI] [PubMed] [Google Scholar]
- 209.Beggiato S., Cassano T., Ferraro L., Tomasini M.C. Astrocytic palmitoylethanolamide pre-exposure exerts neuroprotective effects in astrocyte-neuron co-cultures from a triple transgenic mouse model of Alzheimer’s disease. Life Sci. 2020;257:118037. doi: 10.1016/j.lfs.2020.118037. [DOI] [PubMed] [Google Scholar]
- 210.Bronzuoli M.R., Facchinetti R., Steardo L., Jr., Romano A., Stecca C., Passarella S., Steardo L., Cassano T., Scuderi C. Palmitoylethanolamide Dampens Reactive Astrogliosis and Improves Neuronal Trophic Support in a Triple Transgenic Model of Alzheimer’s Disease: In Vitro and In Vivo Evidence. Oxid. Med. Cell Longev. 2018;2018:4720532. doi: 10.1155/2018/4720532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Casili G., Lanza M., Campolo M., Siracusa R., Paterniti I., Ardizzone A., Scuderi S.A., Cuzzocrea S., Esposito E. Synergic Therapeutic Potential of PEA-Um Treatment and NAAA Enzyme Silencing In the Management of Neuroinflammation. Int. J. Mol. Sci. 2020;21:7486. doi: 10.3390/ijms21207486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tsoy A., Saliev T., Abzhanova E., Turgambayeva A., Kaiyrlykyzy A., Akishev M., Saparbayev S., Umbayev B., Askarova S. The Effects of Mobile Phone Radiofrequency Electromagnetic Fields on beta-Amyloid-Induced Oxidative Stress in Human and Rat Primary Astrocytes. Neuroscience. 2019;408:46–57. doi: 10.1016/j.neuroscience.2019.03.058. [DOI] [PubMed] [Google Scholar]
- 213.Sohanaki H., Baluchnejadmojarad T., Nikbakht F., Roghani M. Pelargonidin improves memory deficit in amyloid beta25-35 rat model of Alzheimer’s disease by inhibition of glial activation, cholinesterase, and oxidative stress. Biomed. Pharmacother. 2016;83:85–91. doi: 10.1016/j.biopha.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 214.Shi Y.C., Pan T.M., Liao V.H. Monascin from Monascus-Fermented Products Reduces Oxidative Stress and Amyloid-beta Toxicity via DAF-16/FOXO in Caenorhabditis elegans. J. Agric. Food Chem. 2016;64:7114–7120. doi: 10.1021/acs.jafc.6b02779. [DOI] [PubMed] [Google Scholar]
- 215.Wei T., Wang Y., Xu W., Liu Y., Chen H., Yu Z. KCa3.1 deficiency attenuates neuroinflammation by regulating an astrocyte phenotype switch involving the PI3K/AKT/GSK3beta pathway. Neurobiol. Dis. 2019;132:104588. doi: 10.1016/j.nbd.2019.104588. [DOI] [PubMed] [Google Scholar]
- 216.Reichenbach N., Delekate A., Breithausen B., Keppler K., Poll S., Schulte T., Peter J., Plescher M., Hansen J.N., Blank N., et al. P2Y1 receptor blockade normalizes network dysfunction and cognition in an Alzheimer’s disease model. J. Exp. Med. 2018;215:1649–1663. doi: 10.1084/jem.20171487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lee C.C., Chang C.P., Lin C.J., Lai H.L., Kao Y.H., Cheng S.J., Chen H.M., Liao Y.P., Faivre E., Buee L., et al. Adenosine Augmentation Evoked by an ENT1 Inhibitor Improves Memory Impairment and Neuronal Plasticity in the APP/PS1 Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018;55:8936–8952. doi: 10.1007/s12035-018-1030-z. [DOI] [PubMed] [Google Scholar]
- 218.Orr A.G., Lo I., Schumacher H., Ho K., Gill M., Guo W., Kim D.H., Knox A., Saito T., Saido T.C., et al. Istradefylline reduces memory deficits in aging mice with amyloid pathology. Neurobiol. Dis. 2018;110:29–36. doi: 10.1016/j.nbd.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Scuderi C., Bronzuoli M.R., Facchinetti R., Pace L., Ferraro L., Broad K.D., Serviddio G., Bellanti F., Palombelli G., Carpinelli G., et al. Ultramicronized palmitoylethanolamide rescues learning and memory impairments in a triple transgenic mouse model of Alzheimer’s disease by exerting anti-inflammatory and neuroprotective effects. Transl. Psychiatry. 2018;8:32. doi: 10.1038/s41398-017-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kritsilis M., Rizou S.V., Koutsoudaki P.N., Evangelou K., Gorgoulis V.G., Papadopoulos D. Ageing, Cellular Senescence and Neurodegenerative Disease. Int. J. Mol. Sci. 2018;19:2937. doi: 10.3390/ijms19102937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Baker D.J., Childs B.G., Durik M., Wijers M.E., Sieben C.J., Zhong J., Saltness R.A., Jeganathan K.B., Verzosa G.C., Pezeshki A., et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bronzuoli M.R., Facchinetti R., Valenza M., Cassano T., Steardo L., Scuderi C. Astrocyte Function Is Affected by Aging and Not Alzheimer’s Disease: A Preliminary Investigation in Hippocampi of 3xTg-AD Mice. Front. Pharmacol. 2019;10:644. doi: 10.3389/fphar.2019.00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Han X., Tai H., Wang X., Wang Z., Zhou J., Wei X., Ding Y., Gong H., Mo C., Zhang J., et al. AMPK activation protects cells from oxidative stress-induced senescence via autophagic flux restoration and intracellular NAD(+) elevation. Aging Cell. 2016;15:416–427. doi: 10.1111/acel.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Crowe E.P., Tuzer F., Gregory B.D., Donahue G., Gosai S.J., Cohen J., Leung Y.Y., Yetkin E., Nativio R., Wang L.S., et al. Changes in the Transcriptome of Human Astrocytes Accompanying Oxidative Stress-Induced Senescence. Front. Aging Neurosci. 2016;8:208. doi: 10.3389/fnagi.2016.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chinta S.J., Woods G., Rane A., Demaria M., Campisi J., Andersen J.K. Cellular senescence and the aging brain. Exp Gerontol. 2015;68:3–7. doi: 10.1016/j.exger.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bitto A., Sell C., Crowe E., Lorenzini A., Malaguti M., Hrelia S., Torres C. Stress-induced senescence in human and rodent astrocytes. Exp. Cell Res. 2010;316:2961–2968. doi: 10.1016/j.yexcr.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 227.Zhu Y., Tchkonia T., Fuhrmann-Stroissnigg H., Dai H.M., Ling Y.Y., Stout M.B., Pirtskhalava T., Giorgadze N., Johnson K.O., Giles C.B., et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15:428–435. doi: 10.1111/acel.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Capoccia E., Cirillo C., Marchetto A., Tiberi S., Sawikr Y., Pesce M., D’Alessandro A., Scuderi C., Sarnelli G., Cuomo R., et al. S100B-p53 disengagement by pentamidine promotes apoptosis and inhibits cellular migration via aquaporin-4 and metalloproteinase-2 inhibition in C6 glioma cells. Oncol. Lett. 2015;9:2864–2870. doi: 10.3892/ol.2015.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Cirillo C., Capoccia E., Iuvone T., Cuomo R., Sarnelli G., Steardo L., Esposito G. S100B Inhibitor Pentamidine Attenuates Reactive Gliosis and Reduces Neuronal Loss in a Mouse Model of Alzheimer’s Disease. Biomed. Res. Int. 2015;2015:508342. doi: 10.1155/2015/508342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Maragakis N.J., Rothstein J.D. Mechanisms of Disease: Astrocytes in neurodegenerative disease. Nat. Clin. Pract. Neurol. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- 231.Pekny M., Pekna M. Astrocyte reactivity and reactive astrogliosis: Costs and benefits. Physiol. Rev. 2014;94:1077–1098. doi: 10.1152/physrev.00041.2013. [DOI] [PubMed] [Google Scholar]
- 232.Hynd M.R., Scott H.L., Dodd P.R. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2004;45:583–595. doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 233.Mookherjee P., Green P.S., Watson G.S., Marques M.A., Tanaka K., Meeker K.D., Meabon J.S., Li N., Zhu P., Olson V.G., et al. GLT-1 loss accelerates cognitive deficit onset in an Alzheimer’s disease animal model. J. Alzheimers Dis. 2011;26:447–455. doi: 10.3233/JAD-2011-110503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Garaschuk O., Verkhratsky A. GABAergic astrocytes in Alzheimer’s disease. Aging. 2019;11:1602–1604. doi: 10.18632/aging.101870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wu Z., Guo Z., Gearing M., Chen G. Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzheimer’s [corrected] disease model. Nat. Commun. 2014;5:4159. doi: 10.1038/ncomms5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jo S., Yarishkin O., Hwang Y.J., Chun Y.E., Park M., Woo D.H., Bae J.Y., Kim T., Lee J., Chun H., et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat. Med. 2014;20:886–896. doi: 10.1038/nm.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Brawek B., Chesters R., Klement D., Muller J., Lerdkrai C., Hermes M., Garaschuk O. A bell-shaped dependence between amyloidosis and GABA accumulation in astrocytes in a mouse model of Alzheimer’s disease. Neurobiol. Aging. 2018;61:187–197. doi: 10.1016/j.neurobiolaging.2017.09.028. [DOI] [PubMed] [Google Scholar]
- 238.Kwakowsky A., Calvo-Flores Guzman B., Pandya M., Turner C., Waldvogel H.J., Faull R.L. GABAA receptor subunit expression changes in the human Alzheimer’s disease hippocampus, subiculum, entorhinal cortex and superior temporal gyrus. J. Neurochem. 2018;145:374–392. doi: 10.1111/jnc.14325. [DOI] [PubMed] [Google Scholar]
- 239.Fontana A.C. Current approaches to enhance glutamate transporter function and expression. J. Neurochem. 2015;134:982–1007. doi: 10.1111/jnc.13200. [DOI] [PubMed] [Google Scholar]
- 240.Lee S.G., Su Z.Z., Emdad L., Gupta P., Sarkar D., Borjabad A., Volsky D.J., Fisher P.B. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J. Biol. Chem. 2008;283:13116–13123. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Rothstein J.D., Patel S., Regan M.R., Haenggeli C., Huang Y.H., Bergles D.E., Jin L., Dykes Hoberg M., Vidensky S., Chung D.S., et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 242.Pereira A.C., Lambert H.K., Grossman Y.S., Dumitriu D., Waldman R., Jannetty S.K., Calakos K., Janssen W.G., McEwen B.S., Morrison J.H. Glutamatergic regulation prevents hippocampal-dependent age-related cognitive decline through dendritic spine clustering. Proc. Natl. Acad. Sci. USA. 2014;111:18733–18738. doi: 10.1073/pnas.1421285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Fumagalli E., Funicello M., Rauen T., Gobbi M., Mennini T. Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. Eur. J. Pharmacol. 2008;578:171–176. doi: 10.1016/j.ejphar.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 244.Pereira A.C., Gray J.D., Kogan J.F., Davidson R.L., Rubin T.G., Okamoto M., Morrison J.H., McEwen B.S. Age and Alzheimer’s disease gene expression profiles reversed by the glutamate modulator riluzole. Mol. Psychiatry. 2017;22:296–305. doi: 10.1038/mp.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ciavardelli D., Piras F., Consalvo A., Rossi C., Zucchelli M., Di Ilio C., Frazzini V., Caltagirone C., Spalletta G., Sensi S.L. Medium-chain plasma acylcarnitines, ketone levels, cognition, and gray matter volumes in healthy elderly, mildly cognitively impaired, or Alzheimer’s disease subjects. Neurobiol. Aging. 2016;43:1–12. doi: 10.1016/j.neurobiolaging.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 246.Cai X.S., Tan Z.G., Li J.J., Gao W.H., Li S.J., Li J.L., Tang Y.M., Li H.W., Hui H.X. Glucagon-Like Peptide-1 (GLP-1) Treatment Ameliorates Cognitive Impairment by Attenuating Arc Expression in Type 2 Diabetic Rats. Med. Sci. Monit. 2017;23:4334–4342. doi: 10.12659/MSM.903252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Schindowski K., Belarbi K., Buee L. Neurotrophic factors in Alzheimer’s disease: Role of axonal transport. Genes Brain Behav. 2008;7:43–56. doi: 10.1111/j.1601-183X.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Huang E.J., Reichardt L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Allen S.J., Watson J.J., Shoemark D.K., Barua N.U., Patel N.K. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 2013;138:155–175. doi: 10.1016/j.pharmthera.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 250.Banik A., Prabhakar S., Kalra J., Anand A. Effect of human umbilical cord blood derived lineage negative stem cells transplanted in amyloid-beta induced cognitive impaired mice. Behav. Brain Res. 2015;291:46–59. doi: 10.1016/j.bbr.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 251.Taliyan R., Chandran S.K., Kakoty V. Therapeutic Approaches to Alzheimer’s Type of Dementia: A Focus on FGF21 Mediated Neuroprotection. Curr. Pharm. Des. 2019;25:2555–2568. doi: 10.2174/1381612825666190716101411. [DOI] [PubMed] [Google Scholar]
- 252.Bellucci C., Lilli C., Baroni T., Parnetti L., Sorbi S., Emiliani C., Lumare E., Calabresi P., Balloni S., Bodo M. Differences in extracellular matrix production and basic fibroblast growth factor response in skin fibroblasts from sporadic and familial Alzheimer’s disease. Mol. Med. 2007;13:542–550. doi: 10.2119/2007-00034.Bellucci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kato T., Sasaki H., Katagiri T., Sasaki H., Koiwai K., Youki H., Totsuka S., Ishii T. The binding of basic fibroblast growth factor to Alzheimer’s neurofibrillary tangles and senile plaques. Neurosci. Lett. 1991;122:33–36. doi: 10.1016/0304-3940(91)90186-W. [DOI] [PubMed] [Google Scholar]
- 254.Sollvander S., Nikitidou E., Brolin R., Soderberg L., Sehlin D., Lannfelt L., Erlandsson A. Accumulation of amyloid-beta by astrocytes result in enlarged endosomes and microvesicle-induced apoptosis of neurons. Mol. Neurodegener. 2016;11:38. doi: 10.1186/s13024-016-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Pan J., Ma N., Yu B., Zhang W., Wan J. Transcriptomic profiling of microglia and astrocytes throughout aging. J. Neuroinflamm. 2020;17:97. doi: 10.1186/s12974-020-01774-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Chen W.T., Lu A., Craessaerts K., Pavie B., Sala Frigerio C., Corthout N., Qian X., Lalakova J., Kuhnemund M., Voytyuk I., et al. Spatial Transcriptomics and In Situ Sequencing to Study Alzheimer’s Disease. Cell. 2020;182:976–991.e19. doi: 10.1016/j.cell.2020.06.038. [DOI] [PubMed] [Google Scholar]
- 257.Foster E.M., Dangla-Valls A., Lovestone S., Ribe E.M., Buckley N.J. Clusterin in Alzheimer’s Disease: Mechanisms, Genetics, and Lessons From Other Pathologies. Front. Neurosci. 2019;13:164. doi: 10.3389/fnins.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Fernandez C.G., Hamby M.E., McReynolds M.L., Ray W.J. The Role of APOE4 in Disrupting the Homeostatic Functions of Astrocytes and Microglia in Aging and Alzheimer’s Disease. Front. Aging Neurosci. 2019;11:14. doi: 10.3389/fnagi.2019.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.DeMattos R.B., O’Dell M.A., Parsadanian M., Taylor J.W., Harmony J.A.K., Bales K.R., Paul S.M., Aronow B.J., Holtzman D.M. Clusterin promotes amyloid plaque formation and is critical for neuritic toxicity in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2002;99:10843–10848. doi: 10.1073/pnas.162228299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Narayan P., Orte A., Clarke R.W., Bolognesi B., Hook S., Ganzinger K.A., Meehan S., Wilson M.R., Dobson C.M., Klenerman D. The extracellular chaperone clusterin sequesters oligomeric forms of the amyloid-beta(1-40) peptide. Nat. Struct. Mol. Biol. 2011;19:79–83. doi: 10.1038/nsmb.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Valenza M., Facchinetti R., Steardo L., Scuderi C. Altered Waste Disposal System in Aging and Alzheimer’s Disease: Focus on Astrocytic Aquaporin-4. Front. Pharmacol. 2020;10:1656. doi: 10.3389/fphar.2019.01656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Steardo L., Jr., Bronzuoli M.R., Iacomino A., Esposito G., Steardo L., Scuderi C. Does neuroinflammation turn on the flame in Alzheimer’s disease? Focus on astrocytes. Front. Neurosci. 2015;9:259. doi: 10.3389/fnins.2015.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Scuderi C., Stecca C., Iacomino A., Steardo L. Role of astrocytes in major neurological disorders: The evidence and implications. IUBMB Life. 2013;65:957–961. doi: 10.1002/iub.1223. [DOI] [PubMed] [Google Scholar]
- 264.Scuderi C., Facchinetti R., Steardo L., Valenza M. Neuroinflammation in Alzheimer’s Disease: Friend or Foe? FASEB J. 2020;34:1. doi: 10.1096/fasebj.2020.34.s1.00381. [DOI] [Google Scholar]
- 265.Scuderi C., Noda M., Verkhratsky A. Editorial: Neuroglia Molecular Mechanisms in Psychiatric Disorders. Front. Mol. Neurosci. 2018;11:407. doi: 10.3389/fnmol.2018.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Schwab C., Klegeris A., McGeer P.L. Inflammation in transgenic mouse models of neurodegenerative disorders. Biochim. Biophys. Acta. 2010;1802:889–902. doi: 10.1016/j.bbadis.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 267.Scuderi C., Filippis D.D., Iuvone T., Blasio A., Steardo A., Esposito G. Cannabidiol in medicine: A review of its therapeutic potential in CNS disorders. Phytother. Res. 2009;23:597–602. doi: 10.1002/ptr.2625. [DOI] [PubMed] [Google Scholar]
- 268.Scuderi C., Steardo L., Esposito G. Cannabidiol promotes amyloid precursor protein ubiquitination and reduction of beta amyloid expression in SHSY5YAPP+ cells through PPARgamma involvement. Phytother. Res. 2014;28:1007–1013. doi: 10.1002/ptr.5095. [DOI] [PubMed] [Google Scholar]
- 269.Esposito G., Scuderi C., Savani C., Steardo L., Jr., De Filippis D., Cottone P., Iuvone T., Cuomo V., Steardo L. Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression. Br. J. Pharmacol. 2007;151:1272–1279. doi: 10.1038/sj.bjp.0707337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Esposito G., Scuderi C., Valenza M., Togna G.I., Latina V., De Filippis D., Cipriano M., Carratu M.R., Iuvone T., Steardo L. Cannabidiol reduces Abeta-induced neuroinflammation and promotes hippocampal neurogenesis through PPARgamma involvement. PLoS ONE. 2011;6:e28668. doi: 10.1371/journal.pone.0028668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wu T., Dejanovic B., Gandham V.D., Gogineni A., Edmonds R., Schauer S., Srinivasan K., Huntley M.A., Wang Y., Wang T.M., et al. Complement C3 Is Activated in Human AD Brain and Is Required for Neurodegeneration in Mouse Models of Amyloidosis and Tauopathy. Cell Rep. 2019;28:2111–2123.e6. doi: 10.1016/j.celrep.2019.07.060. [DOI] [PubMed] [Google Scholar]
- 272.Scuderi C., Stecca C., Bronzuoli M.R., Rotili D., Valente S., Mai A., Steardo L. Sirtuin modulators control reactive gliosis in an in vitro model of Alzheimer’s disease. Front. Pharmacol. 2014;5:89. doi: 10.3389/fphar.2014.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Scuderi C., Esposito G., Blasio A., Valenza M., Arietti P., Steardo L., Jr., Carnuccio R., De Filippis D., Petrosino S., Iuvone T., et al. Palmitoylethanolamide counteracts reactive astrogliosis induced by beta-amyloid peptide. J. Cell Mol. Med. 2011;15:2664–2674. doi: 10.1111/j.1582-4934.2011.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Llorens F., Hermann P., Villar-Pique A., Diaz-Lucena D., Nagga K., Hansson O., Santana I., Schmitz M., Schmidt C., Varges D., et al. Cerebrospinal fluid lipocalin 2 as a novel biomarker for the differential diagnosis of vascular dementia. Nat. Commun. 2020;11:619. doi: 10.1038/s41467-020-14373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Mesquita S.D., Ferreira A.C., Falcao A.M., Sousa J.C., Oliveira T.G., Correia-Neves M., Sousa N., Marques F., Palha J.A. Lipocalin 2 modulates the cellular response to amyloid beta. Cell Death Differ. 2014;21:1588–1599. doi: 10.1038/cdd.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Staurenghi E., Cerrato V., Gamba P., Testa G., Giannelli S., Leoni V., Caccia C., Buffo A., Noble W., Perez-Nievas B.G., et al. Oxysterols present in Alzheimer’s disease brain induce synaptotoxicity by activating astrocytes: A major role for lipocalin-2. Redox. Biol. 2021;39:101837. doi: 10.1016/j.redox.2020.101837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Rehman S.U., Shah S.A., Ali T., Chung J.I., Kim M.O. Anthocyanins Reversed D-Galactose-Induced Oxidative Stress and Neuroinflammation Mediated Cognitive Impairment in Adult Rats. Mol. Neurobiol. 2017;54:255–271. doi: 10.1007/s12035-015-9604-5. [DOI] [PubMed] [Google Scholar]
- 278.Wang G., Chen L., Pan X., Chen J., Wang L., Wang W., Cheng R., Wu F., Feng X., Yu Y., et al. The effect of resveratrol on beta amyloid-induced memory impairment involves inhibition of phosphodiesterase-4 related signaling. Oncotarget. 2016;7:17380–17392. doi: 10.18632/oncotarget.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ibrahim N.F., Yanagisawa D., Durani L.W., Hamezah H.S., Damanhuri H.A., Wan Ngah W.Z., Tsuji M., Kiuchi Y., Ono K., Tooyama I. Tocotrienol-Rich Fraction Modulates Amyloid Pathology and Improves Cognitive Function in AbetaPP/PS1 Mice. J. Alzheimers Dis. 2017;55:597–612. doi: 10.3233/JAD-160685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Cuevas E., Limon D., Perez-Severiano F., Diaz A., Ortega L., Zenteno E., Guevara J. Antioxidant effects of epicatechin on the hippocampal toxicity caused by amyloid-beta 25-35 in rats. Eur. J. Pharmacol. 2009;616:122–127. doi: 10.1016/j.ejphar.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 281.Wang L., Wang M., Hu J., Shen W., Hu J., Yao Y., Wang X., Afzal C.M., Ma R., Li G. Protective effect of 3H-1, 2-dithiole-3-thione on cellular model of Alzheimer’s disease involves Nrf2/ARE signaling pathway. Eur. J. Pharmacol. 2017;795:115–123. doi: 10.1016/j.ejphar.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 282.Sharman M.J., Gyengesi E., Liang H., Chatterjee P., Karl T., Li Q.X., Wenk M.R., Halliwell B., Martins R.N., Munch G. Assessment of diets containing curcumin, epigallocatechin-3-gallate, docosahexaenoic acid and alpha-lipoic acid on amyloid load and inflammation in a male transgenic mouse model of Alzheimer’s disease: Are combinations more effective? Neurobiol. Dis. 2019;124:505–519. doi: 10.1016/j.nbd.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 283.Delekate A., Fuchtemeier M., Schumacher T., Ulbrich C., Foddis M., Petzold G.C. Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer’s disease mouse model. Nat. Commun. 2014;5:5422. doi: 10.1038/ncomms6422. [DOI] [PubMed] [Google Scholar]
- 284.Orr A.G., Hsiao E.C., Wang M.M., Ho K., Kim D.H., Wang X., Guo W., Kang J., Yu G.Q., Adame A., et al. Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat. Neurosci. 2015;18:423–434. doi: 10.1038/nn.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Verkhratsky A., Marutle A., Rodriguez-Arellano J.J., Nordberg A. Glial Asthenia and Functional Paralysis: A New Perspective on Neurodegeneration and Alzheimer’s Disease. Neuroscientist. 2015;21:552–568. doi: 10.1177/1073858414547132. [DOI] [PubMed] [Google Scholar]
- 286.Scuderi C., Steardo L. Neuroglial roots of neurodegenerative diseases: Therapeutic potential of palmitoylethanolamide in models of Alzheimer’s disease. CNS Neurol. Disord. Drug. Targets. 2013;12:62–69. doi: 10.2174/1871527311312010011. [DOI] [PubMed] [Google Scholar]
- 287.Beggiato S., Tomasini M.C., Ferraro L. Palmitoylethanolamide (PEA) as a Potential Therapeutic Agent in Alzheimer’s Disease. Front. Pharmacol. 2019;10:821. doi: 10.3389/fphar.2019.00821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Cipriano M., Esposito G., Negro L., Capoccia E., Sarnelli G., Scuderi C., De Filippis D., Steardo L., Iuvone T. Palmitoylethanolamide Regulates Production of Pro-Angiogenic Mediators in a Model of beta Amyloid-Induced Astrogliosis In Vitro. CNS Neurol. Disord. Drug Targets. 2015;14:828–837. doi: 10.2174/1871527314666150317224155. [DOI] [PubMed] [Google Scholar]
- 289.Scuderi C., Stecca C., Valenza M., Ratano P., Bronzuoli M.R., Bartoli S., Steardo L., Pompili E., Fumagalli L., Campolongo P., et al. Palmitoylethanolamide controls reactive gliosis and exerts neuroprotective functions in a rat model of Alzheimer’s disease. Cell Death Dis. 2014;5:e1419. doi: 10.1038/cddis.2014.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Scuderi C., Valenza M., Stecca C., Esposito G., Carratu M.R., Steardo L. Palmitoylethanolamide exerts neuroprotective effects in mixed neuroglial cultures and organotypic hippocampal slices via peroxisome proliferator-activated receptor-alpha. J. Neuroinflammation. 2012;9:49. doi: 10.1186/1742-2094-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Lo Verme J., Fu J., Astarita G., La Rana G., Russo R., Calignano A., Piomelli D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- 292.D’Agostino G., Russo R., Avagliano C., Cristiano C., Meli R., Calignano A. Palmitoylethanolamide protects against the amyloid-beta25-35-induced learning and memory impairment in mice, an experimental model of Alzheimer disease. Neuropsychopharmacology. 2012;37:1784–1792. doi: 10.1038/npp.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Esposito E., Impellizzeri D., Mazzon E., Paterniti I., Cuzzocrea S. Neuroprotective activities of palmitoylethanolamide in an animal model of Parkinson’s disease. PLoS ONE. 2012;7:e41880. doi: 10.1371/journal.pone.0041880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Pertwee R.G. GPR55: A new member of the cannabinoid receptor clan? Br. J. Pharmacol. 2007;152:984–986. doi: 10.1038/sj.bjp.0707464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zygmunt P.M., Ermund A., Movahed P., Andersson D.A., Simonsen C., Jonsson B.A., Blomgren A., Birnir B., Bevan S., Eschalier A., et al. Monoacylglycerols activate TRPV1—A link between phospholipase C and TRPV1. PLoS ONE. 2013;8:e81618. doi: 10.1371/journal.pone.0081618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Mattace Raso G., Russo R., Calignano A., Meli R. Palmitoylethanolamide in CNS health and disease. Pharmacol. Res. 2014;86:32–41. doi: 10.1016/j.phrs.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 297.Petrosino S., Di Marzo V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br. J. Pharmacol. 2017;174:1349–1365. doi: 10.1111/bph.13580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Harcha P.A., Garces P., Arredondo C., Fernandez G., Saez J.C., van Zundert B. Mast Cell and Astrocyte Hemichannels and Their Role in Alzheimer’s Disease, ALS, and Harmful Stress Conditions. Int. J. Mol. Sci. 2021;22:1924. doi: 10.3390/ijms22041924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Sandhu J.K., Kulka M. Decoding Mast Cell-Microglia Communication in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021;22:1093. doi: 10.3390/ijms22031093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Petrosino S., Schiano Moriello A. Palmitoylethanolamide: A Nutritional Approach to Keep Neuroinflammation within Physiological Boundaries-A Systematic Review. Int. J. Mol. Sci. 2020;21:9526. doi: 10.3390/ijms21249526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Paterniti I., Cordaro M., Campolo M., Siracusa R., Cornelius C., Navarra M., Cuzzocrea S., Esposito E. Neuroprotection by association of palmitoylethanolamide with luteolin in experimental Alzheimer’s disease models: The control of neuroinflammation. CNS Neurol. Disord. Drug Targets. 2014;13:1530–1541. doi: 10.2174/1871527313666140806124322. [DOI] [PubMed] [Google Scholar]
- 302.Beggiato S., Tomasini M.C., Cassano T., Ferraro L. Chronic Oral Palmitoylethanolamide Administration Rescues Cognitive Deficit and Reduces Neuroinflammation, Oxidative Stress, and Glutamate Levels in A Transgenic Murine Model of Alzheimer’s Disease. J. Clin. Med. 2020;9:428. doi: 10.3390/jcm9020428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Cassano T., Bellanti F., Bukke V.N., Archana M., Scuderi C., Ferraro L., Serviddio G., Cuomo V. Effects of ultramicronized palmitoylethanolamide treatment on the glutamatergic alterations and mitochondrial impairment in 3×tg-ad mice. Pharmadvances. 2021;3:87–88. doi: 10.36118/pharmadvances.03.2021.01. [DOI] [Google Scholar]
- 304.Scuderi C. Unraveling the complex glial response in aging and alzheimer’s disease. Pharmadvances. 2021;3 doi: 10.36118/pharmadvances.03.2021.01. [DOI] [Google Scholar]
- 305.Assogna M., Casula E.P., Borghi I., Bonni S., Sama D., Motta C., Di Lorenzo F., D’Acunto A., Porrazzini F., Minei M., et al. Effects of Palmitoylethanolamide Combined with Luteoline on Frontal Lobe Functions, High Frequency Oscillations, and GABAergic Transmission in Patients with Frontotemporal Dementia. J. Alzheimers Dis. 2020;76:1297–1308. doi: 10.3233/JAD-200426. [DOI] [PubMed] [Google Scholar]
- 306.Calabrò R.S., Naro A., De Luca R., Leonardi S., Russo M., Marra A., Bramanti P. PEALut efficacy in mild cognitive impairment: Evidence from a SPECT case study! Aging Clin. Exp. Res. 2016;28:1279–1282. doi: 10.1007/s40520-016-0533-6. [DOI] [PubMed] [Google Scholar]
- 307.Caltagirone C., Cisari C., Schievano C., Di Paola R., Cordaro M., Bruschetta G., Esposito E., Cuzzocrea S., Stroke Study G. Co-ultramicronized Palmitoylethanolamide/Luteolin in the Treatment of Cerebral Ischemia: From Rodent to Man. Transl. Stroke Res. 2016;7:54–69. doi: 10.1007/s12975-015-0440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Paladini A., Fusco M., Cenacchi T., Schievano C., Piroli A., Varrassi G. Palmitoylethanolamide, a Special Food for Medical Purposes, in the Treatment of Chronic Pain: A Pooled Data Meta-analysis. Pain Physician. 2016;19:11–24. [PubMed] [Google Scholar]