Abstract
Background
The lack of background disease incidence rates in sub-Saharan countries where the RTS,S/AS01E malaria vaccine is being implemented may hamper the assessment of vaccine safety and effectiveness. This study aimed to document baseline incidence rates of meningitis, malaria, mortality, and other health outcomes prior to vaccine introduction through the Malaria Vaccine Implementation Programme.
Methods
An ongoing disease surveillance study is combining prospective cohort event monitoring and hospital-based disease surveillance in three study sites in Ghana and Kenya. An interim analysis was performed on the prospective cohort in which children were enrolled in two age-groups (the 5 to 17 months or 6 to 12 weeks age-group), capturing data in the framework of routine medical practice before the introduction of the malaria vaccine. Incidence and mortality rates were computed with 95% confidential intervals (CI) using an exact method for a Poisson variable.
Results
This analysis includes 14,329 children; 7248 (50.6%) in the 6 to 12 weeks age-group and 7081 (49.4%) in the 5 to 17 months age-group. In the 5 to 17 months age-group (where the malaria vaccine was planned to be subsequently rolled out) the meningitis, malaria, severe malaria and cerebral malaria incidences were 92 (95% CI 25–236), 47,824 (95% CI 45,411–50,333), 1919 (95% CI 1461–2476) and 33 (95% CI 1–181) per 100,000 person-years, respectively. The all-cause mortality was 969 (95% CI 699–1310) per 100,000 person-years.
Conclusion
Incidence estimates of multiple health outcomes are being generated to allow before-after vaccine introduction comparisons that will further characterize the benefit-risk profile of the RTS,S/AS01E vaccine.
Trial registration: clinicaltrials.gov NCT02374450.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12936-021-03670-w.
Keywords: Malaria, Plasmodium falciparum, Adverse event, Meningitis, Mortality
Background
Despite remarkable progress in reducing the global burden of disease due to malaria, there were an estimated 228 million cases of malaria in 2018 and 405,000 malaria deaths globally, of which 380,000 occurred in the African region [1]. Plasmodium falciparum caused 99.7% of malaria cases, and children aged < 5 years were the most vulnerable age-group, accounting for 272,000 (67%) of global malaria deaths in 2018 [1]. Without additional tools such as vaccines, the World Health Organization (WHO) 2030 global targets of achieving at least a 90% reduction in malaria incidence and deaths compared to 2015 levels, may not be met [2].
RTS,S/AS01E is a pre-erythrocytic P. falciparum malaria vaccine developed for routine immunization of infants and children living in malaria-endemic countries, and is the first complementary tool to be implemented under the Global Technical Strategy for Malaria, 2016–2030. RTS,S/AS01E received a positive scientific opinion from the European Medicines Agency in 2015 [3]. Later that year the WHO recommended pilot implementation of routine RTS,S/AS01E vaccination in sub-Saharan Africa (SSA), administering three doses to children aged 5 to 9 months in areas of moderate-to-high malaria transmission, with a fourth dose 15 to 18 months later [4]. This pilot implementation will provide more clarity on uncertainties related to programmatic feasibility, impact and safety of the vaccine in routine use [4].
In 2019, RTS,S/AS01E was introduced in selected areas of Ghana, Kenya, and Malawi through national immunization programmes in the framework of the WHO-commissioned Malaria Vaccine Implementation Programme (MVIP) [5]. The MVIP is evaluating the vaccine’s public health impact in the context of routine use and will inform policy about its potential deployment on a broader scale. As part of this evaluation, GSK has committed to conduct a post-approval plan (PAP) in the pilot implementation areas to further assess vaccine safety, effectiveness and impact. Specific safety outcomes of interest included those identified during the Phase III clinical trial: an imbalance in meningitis and cerebral malaria cases between RTS,S/AS01E and control vaccine groups in children that started vaccination at 5 to 17 months of age [6], and across both age groups a higher mortality in girls vaccinated with RTS,S/AS01E versus girls vaccinated with control vaccines not explained by differences in risk factors, causes of death, or time to death. Detailed evaluation of these events and absence of a biological plausible explanation for a causal relationship to RTS,S/AS01E suggest that these observations were likely chance findings, possibly due to a low mortality rate in girls who received control vaccines, or low rates of meningitis in the control group [6].
Limited or absent healthcare and disease surveillance infrastructure, and the lack of robust disease incidence data in SSA before RTS,S/AS01E implementation [7], hamper the assessment of vaccine safety, effectiveness and impact. For this reason, the PAP design includes a before-after comparison in which data collected prior to (pre-vaccine introduction study; NCT02374450) and after (post-vaccine introduction study; NCT03855995) vaccine introduction are compared. Both studies are observational and follow a cohort study design to estimate incidence rates of meningitis, malaria, selected rare events (referred to here as adverse events of special interest [AESI]), other events that lead to hospitalization, and mortality in children aged less than 5 years. The present paper summarizes the interim results of this pre-vaccine introduction study that has been conducted so far in Ghana and Kenya in children not vaccinated with RTS,S/AS01E. The end of study results will be disclosed in a separate publication.
Methods
Study design and population
This pre-vaccine introduction study is a disease surveillance study with prospective cohort event monitoring including active surveillance of outpatient and inpatient visits by each enrolled study participant and scheduled home visits. In addition, hospital-based disease surveillance is organized in the entire study area for infants and young children not enrolled in the prospective cohort. The present interim results are limited to the analysis of data collected through the prospective cohort monitoring in which subjects are enrolled in two groups (either the 5 to 17 months or the 6 to 12 weeks age-groups) corresponding to the two age-groups evaluated in the RTS,S/AS01E Phase III study [8]. Those age-groups differ in the way their active surveillance is organized i.e., 10 home visits are organized according to the diphtheria-tetanus-pertussis-hepatitis B-Haemophilus influenzae (DTP-HepB-Hib) vaccination schedule for the 6 to 12 weeks age-group, or from 5 months of age (according to a schedule mimicking administration of RTS,S/AS01E, and referred to hereafter as the ‘virtual primary vaccination schedule’) for the 5 to 17 months age-group (Additional file 1). The children, distributed equally between the two age-groups, were recruited into the prospective cohort from three study sites (Kintampo and Navrongo in central and northern Ghana, respectively; Kombewa in Kenya) from January 2016 until June 2018.
Study objectives
The study objectives are to estimate the incidence of AESI (listed in Additional file 2), meningitis (aetiology-confirmed, probable, and clinically suspected meningitis), malaria (any, uncomplicated, severe, and cerebral malaria), other events leading to hospitalization, all-cause and malaria-attributable mortality rates (overall and by gender), and to describe causes of death (overall and by gender) in children prior to implementation of RTS,S/AS01E. Case definitions are described in Additional file 2. At-risk periods after DTP-HebB-Hib vaccination for the 6 to 12 weeks age group and virtual at-risk periods after a virtual vaccination mimicking RTS,S/AS01E administration for 5 to 17 months age group were defined for each study outcome (Additional file 3).
Study procedures
This study captures data generated in the framework of routine medical practice, which includes medical history, clinical diagnosis and results of locally conducted laboratory tests (referred to as first-line laboratory results). Training on pharmacovigilance and the diagnosis of AESI and meningitis was provided to all sites. Job aids (i.e., field guides with signs and symptoms of AESI and meningitis, and guidelines for diagnosis based on the case definition) were also provided, and laboratory testing capacities were enhanced to support case detection and ascertainment. For each suspected case of AESI and meningitis, a protocol-specified blood sample of 5 ml was sent to an external reference laboratory for testing (second-line laboratory). For meningitis and neurological AESI, when a lumbar puncture was conducted according to routine diagnostic practice, an aliquot of cerebrospinal fluid (CSF) was sent to the second-line laboratory if a sufficient quantity was available after first-line laboratory testing.
All cases of meningitis, cerebral malaria, and ambiguous cases of any other endpoint were reviewed by a panel of external experts (i.e., medical professionals with varied backgrounds who independently reviewed the cases). Cause of death was either established from the medical records when occurring at a healthcare facility, or through verbal autopsy for children who died in the community [9].
Statistical analysis
The total enrolled cohort of the interim analysis included all children enrolled in the prospective cohort who, by 05 October 2018, had reached the study home visit number five which is planned approximately 6 months after a 3-dose schedule of DTP-HepB-Hib for the 6 to 12 weeks age-group (primary vaccination schedule) or after a virtual 3-dose primary vaccination schedule mimicking RTS,S/AS01E administration for the 5 to 17 months age-group (virtual primary vaccination schedule). Study participants who reached home visit five after this date were excluded. The According-to-protocol (ATP) cohort included all children who met eligibility criteria (Additional file 4), complied with protocol-defined enrolment procedures, and who had no elimination criteria during the study.
Incidence rates were computed overall and by study site, and mortality rates were computed overall, by gender, and by study site using the data collected during a follow-up period of approximately 6 months after the (virtual) primary vaccination schedule. A 95% confidential interval (CI) was computed using an exact method for a Poisson variable. For the events with an at-risk period (Additional file 3), the incidence rate was computed by dividing the number of children reporting at least one event within the at-risk period after each (virtual) vaccinated dose by the accumulated at-risk period after (virtual) vaccination.
AEs (other than AESI, meningitis or malaria) leading to hospitalization and cause of death were coded according to the MedDRA preferred terms. Preferred terms of other AEs leading to hospitalization were grouped into medically relevant categories for the calculation of the incidence rates (Additional file 5).
Quality indicators of surveillance
To assess the operational conduct of the study, two quality surveillance indicators were monitored in health care facilities; abscess at the injection site during the 7-day period after each dose of the (virtual) primary vaccination schedule; and foot positional deformation, as defined in Additional file 6.
Ethics and consent
The study was conducted in accordance with Good Clinical Practice and Good Pharmacoepidemiology Practices, all applicable subject privacy requirements and the guiding principles of the Declaration of Helsinki. The study protocol and associated documents were approved by relevant Ethical Review Boards (Additional file 7). Written or witnessed and thumb written informed consent was obtained from the parent/ legal representative of each study participant prior to enrolment.
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Results
Study participants
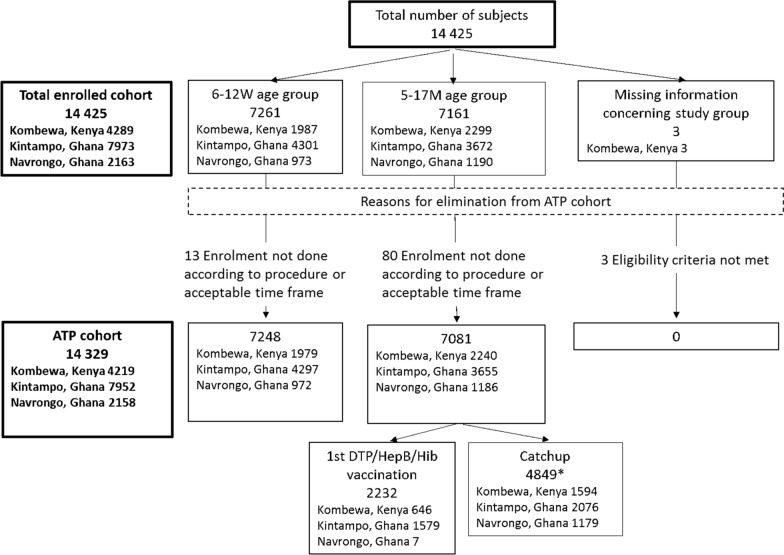
A total of 14,425 children were enrolled in the prospective cohort, among which 14,329 were part of the ATP analysis: 4219 (29.4%) in Kombewa (Kenya), 7952 (55.5%) in Kintampo (central Ghana), and 2158 (15.1%) in Navrongo (northern Ghana) (Fig. 1). There were 7248 (50.6%) participants in the 6 to 12 weeks age-group and 7081 (49.4%) in the 5 to 17 months age-group (Fig. 1, Table 1).
Fig. 1.
CONSORT diagram. KE, Kenya; GH, Ghana; ATP, According to protocol; M, Months; W, Weeks
Table 1.
Number of study participants by study site and by study group
| Study site | Study group | Number of participants (%) per study site |
|---|---|---|
| Kombewa, Kenya N = 4219 | 6 to 12 weeks | 1979 (46.9) |
| 5 to 17 months | 2240 (53.1) | |
| Kintampo, Ghana N = 7952 | 6 to 12 weeks | 4297 (54.0) |
| 5 to 17 months | 3655 (46.0) | |
| Navrongo, Ghana N = 2158 | 6 to 12 weeks | 972 (45.0) |
| 5 to 17 months | 1186 (55.0) |
According-to-protocol cohort
N, total number of study participants by study site
In the ATP cohort, the mean age of study participants at enrolment was 1.7 months (standard deviation [SD] 0.3) in the 6 to 12 weeks age-group and 7.7 months (SD 5.1) in the 5 to 17 months age-group (Table 2). Males and females were equally distributed in both age-groups. Overall, 9745 (68.0%) study participants resided in rural areas and 12,958 (90.4%) lived less than 5 km from a healthcare facility. Details per study site are provided in Additional file 8.
Table 2.
Demographic characteristics at study entry by study group
| 6 to 12 weeks age-group (N = 7248) | 5 to 17 months age-group (N = 7081) | Overall (N = 14,329) | |
|---|---|---|---|
| Age at informed consent (months) | |||
| Mean ± SD | 1.7 ± 0.3 | 7.7 ± 5.1 | 4.7 ± 4.7 |
| Range | 0.4–3.0 | 0.4–18.0 | 0.4–18.0 |
| Gender, n (%) | |||
| Female | 3585 (49.5) | 3484 (49.2) | 7069 (49.3) |
| Male | 3663 (50.5) | 3597 (50.8) | 7260 (50.7) |
| Neighbourhood of residence, n (%) | |||
| Urban | 1320 (18.2) | 920 (13.0) | 2240 (15.6) |
| Semi-rural | 1225 (16.9) | 1119 (15.8) | 2344 (16.4) |
| Rural | 4703 (64.9) | 5042 (71.2) | 9745 (68.0) |
| Distance to health care facility, n (%) | |||
| < 5 km | 6588 (90.9) | 6370 (90.0) | 12,958 (90.4) |
| 5– < 10 km | 543 (7.5) | 496 (7.0) | 1039 (7.3) |
| 10– < 20 km | 84 (1.2) | 149 (2.1) | 233 (1.6) |
| 20– < 30 km | 17 (0.2) | 42 (0.6) | 59 (0.4) |
| > 30 km | 16 (0.2) | 24 (0.3) | 40 (0.3) |
| Closest health care facility, n (%) | |||
| Primary health care facility | 5135 (70.9) | 5165 (72.9) | 10,300 (71.9) |
| Hospital | 2113 (29.2) | 1916 (27.1) | 4029 (28.1) |
According-to-protocol cohort
N, total number of study participants by study site; n, number of study participants in each category; SD: standard deviation
A total of 745 participants (5.2%) were hospitalized. Among the 5464 (38.1%) participants who had at least one outpatient visit, 32 (0.59%) were referred to hospital. The percentage of participants diagnosed for each endpoint is provided in Table 3.
Table 3.
Percentage of study participants with final diagnoses for the main interim analysis endpoints
| 6 to 12 weeks | Kombewa, Kenya | Kintampo, Ghana | Navrongo, Ghana | Overall | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 1979 | N = 4297 | N = 972 | N = 7248 | |||||||||
| e | n | % | e | n | % | e | n | % | e | n | % | |
| AESI | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 |
| Meningitis | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 |
| Malaria | 479 | 375 | 19.0 | 898 | 685 | 15.9 | 48 | 43 | 4.4 | 1425 | 1103 | 15.2 |
| Other AEa leading to hospitalization | 62 | 38 | 1.9 | 439 | 233 | 5.4 | 111 | 46 | 4.7 | 612 | 317 | 4.4 |
| All-cause hospitalization | 39 | 39 | 2.0 | 268 | 240 | 5.6 | 54 | 47 | 4.8 | 361 | 326 | 4.5 |
| 5 to 17 months | Kombewa, Kenya | Kintampo, Ghana | Navrongo, Ghana | Overall | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 2240 | N = 3655 | N = 1186 | N = 7081 | |||||||||
| e | n | % | e | n | % | e | n | % | e | n | % | |
| AESI | 1 | 1 | 0.04 | 1 | 1 | 0.0 | 0 | 0 | 0.0 | 2 | 2 | 0.0 |
| Meningitis | 3 | 3 | 0.1 | 1 | 1 | 0.0 | 0 | 0 | 0.0 | 4 | 4 | 0.1 |
| Malaria | 639 | 505 | 22.5 | 1363 | 938 | 22.7 | 127 | 118 | 10.0 | 2129 | 1561 | 22.0 |
| Other AEa leading to hospitalization | 119 | 69 | 3.1 | 495 | 254 | 6.7 | 188 | 78 | 6.6 | 802 | 392 | 5.5 |
| All-cause hospitalization | 85 | 79 | 3.5 | 310 | 262 | 7.2 | 92 | 78 | 6.6 | 487 | 419 | 5.9 |
According-to-protocol cohort
AESI: adverse event of special interest; AE: adverse event; N: total number of study participants by study group or by study site; e, number of events reported; n, number of study participants in each category; %, percentage of study participants who reported at least one event
The AESI in this study refer to a list of 15 diseases: Acute disseminated encephalomyelitis, Encephalitis, Guillain-Barré syndrome, Generalized convulsive seizure, Hypotonic hypo-responsive episode, Intussusception, Hepatic insufficiency, Renal insufficiency, Juvenile chronic arthritis, Stevens Johnson syndrome and toxic epidermal necrolysis, Henoch Schonlein purpura, Kawasaki disease, Diabetes mellitus type 1, Thrombocytopenia, Anaphylaxis
aAdverse events other than AESI, malaria or meningitis
In the framework of the MVIP, the RTS,S/AS01E primary vaccination schedule is currently administered to children aged 5 to 9 months. Therefore, the results of the ATP cohort presented below focus on the 5 to 17 months age-group. Results for the 6 to 12 weeks age-group are provided in Table 3 and Additional files 9–11.
Incidence rates of meningitis
Five meningitis cases were initially suspected in the 5 to 17 months age-group, among which two cases were classified as aetiology-confirmed meningitis and two as clinically-suspected meningitis. The fifth case was subsequently diagnosed as encephalomyelitis (Table 4). None of the cases were fatal. CSF was available for three cases. There were two cases of aetiology-confirmed meningitis (incidence 46 (95% CI 6–167) per 100,000 person-years): one due to Staphylococcus aureus, and the other (case of co-morbidity with cerebral malaria; see below) incorrectly classified as meningitis due to Parvovirus before being downgraded to probable meningitis following further review by the expert panel after the interim analysis, and clarification of laboratory testing results. The incidence rate of aetiology-confirmed, probable and/or clinically suspected meningitis was 92 (95% CI 25–236) per 100,000 person-years.
Table 4.
Incidence rate per 100,000 person-years of meningitis cases
| Incidence rate per 100,000 person-years | |||
|---|---|---|---|
| n | Person-years | Value (95% CI) | |
| Kombewa, Kenya (N = 2111) | |||
| Aetiology-confirmed meningitis | 1 | 1249 | 80 (2, 446) |
| Probable meningitis | 0 | 1249 | 0 (0, 295) |
| Clinically suspected meningitis | 2 | 1249 | 160 (19, 579) |
| Aetiology-confirmed, probable, and/or clinically suspected meningitis | 3 | 1248 | 240 (50, 703) |
| Suspected meningitis ruled out afterward | 1 | 1249 | 80 (2, 446) |
| Kintampo, Ghana (N = 3603) | |||
| Aetiology-confirmed meningitisa | 1 | 2330 | 43 (1, 239) |
| Probable meningitis | 0 | 2330 | 0 (0, 158) |
| Clinically suspected meningitis | 0 | 2330 | 0 (0, 158) |
| Aetiology-confirmed, probable, and/or clinically suspected meningitis | 1 | 2330 | 43 (1, 239) |
| Suspected meningitis ruled out afterward | 0 | 2330 | 0 (0, 158) |
| Navrongo, Ghana (N = 1183) | |||
| Aetiology-confirmed meningitis | 0 | 754 | 0 (0, 490) |
| Probable meningitis | 0 | 754 | 0 (0, 490) |
| Clinically suspected meningitis | 0 | 754 | 0 (0, 490) |
| Aetiology-confirmed, probable, and/or clinically suspected meningitis | 0 | 754 | 0 (0, 490) |
| Suspected meningitis ruled out afterward | 0 | 754 | 0 (0, 490) |
| Overall (N = 6897) | |||
| Aetiology-confirmed meningitisa | 2 | 4332 | 46 (6, 167) |
| Probable meningitis | 0 | 4332 | 0 (0, 85) |
| Clinically suspected meningitis | 2 | 4332 | 46 (6, 167) |
| Aetiology-confirmed, probable, and/or clinically suspected meningitis | 4 | 4331 | 92 (25, 236) |
| Suspected meningitis ruled out afterward | 1 | 4332 | 23 (1, 129) |
According-to-protocol cohort, 5 to 17 months age-group
N, number of study participants at risk during a follow-up period of approximately 6 months after each dose of the virtual primary vaccination schedule censored at the next dose; n, number of cases reported during that follow-up period; CI: confidence interval; M: Months
aOne case of aetiology-confirmed meningitis has been downgraded to probable meningitis after the interim analysis (see text for details)
Incidence rates of malaria
A total of 1561 participants (22%) experienced at least one episode of malaria (2129 episodes confirmed by rapid diagnostic test and/or microscopy) during an outpatient visit or a hospitalization. Uncomplicated malaria was diagnosed in 1512 children, which corresponds to 97% of the total number of malaria events (2059 out of 2129 episodes). The remaining 3% (70 episodes in 66 children) were severe and included two cases of cerebral malaria (fatal for one child, while the other child diagnosed with cerebral malaria and meningitis, recovered fully). Plasmodium falciparum was identified in 1747 cases (82%).
The malaria incidence rate was 47,824 (95% CI 45,411–50,333) per 100,000 person-years (Table 5). The incidence rates of uncomplicated and severe malaria were 45,905 (95% CI 43,541–48,364) and 1919 (95% CI 1461–2476) per 100,000 person-years, respectively. The cerebral malaria incidence rate was 33 (95% CI 1–181) per 100,000 person-years.
Table 5.
Incidence rates per 100,000 person-years of malaria cases
| Kombewa, Kenya | Kintampo, Ghana | Navrongo, Ghana | Overall | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 1743 | N = 3488 | N = 1143 | N = 6374 | |||||||||
| n | PY | Value (95% CI) | n | PY | Value (95% CI) | n | PY | Value (95% CI) | n | PY | Value (95% CI) | |
| Malaria | ||||||||||||
| Any | 381 | 810 | 47,048 (42,442, 52,018) | 1010 | 1714 | 58,928 (55,350, 62,678) | 79 | 550 | 14,364 (11,372, 17,902) | 1470 | 3074 | 47,824 (45,411, 50,333) |
| Uncomplicated | 364 | 810 | 44,949 (40,449, 49,812) | 972 | 1714 | 56,711 (53,202, 60,392) | 75 | 550 | 13,637 (10,726, 17,094) | 1411 | 3074 | 45,905 (43,541, 48,364) |
| Severe malaria | 17 | 810 | 2099 (1223, 3361) | 38 | 1714 | 2217 (1569, 3043) | 4 | 550 | 727 (198, 1862) | 59 | 3074 | 1919 (1461, 2476) |
| Cerebral malaria | 0 | 810 | 0 (0, 456) | 1 | 1714 | 58 (1, 325) | 0 | 550 | 0 (0, 671) | 1 | 3074 | 33 (1, 181) |
| P. falciparum | ||||||||||||
| Uncomplicated | 277 | 810 | 34,205 (30,296, 38,480) | 813 | 1714 | 47,434 (44,229, 50,810) | 74 | 550 | 13,455 (10,565, 16,891) | 1164 | 3074 | 37,869 (35,725, 40,109) |
| Severe | 15 | 810 | 1852 (1037, 3055) | 38 | 1714 | 2217 (1569, 3043) | 4 | 550 | 727 (198, 1862) | 57 | 3074 | 1854 (1405, 2403) |
According-to-protocol cohort, 5 to 17 months age-group N, Number of study participants at risk during a follow-up period of approximately 6 months after the 3rd virtual vaccine dose; n, number of cases reported during that follow-up period; PY: person-years; CI: confidence intervals
Malaria cases were confirmed by rapid diagnostic test and/or microscopy
Mortality rate
In total, 56 deaths (26 girls and 30 boys) were reported due to pneumonia (12 cases), malaria/P. falciparum infection (11 cases including one case of cerebral malaria), malnutrition (five cases), dehydration (four cases), gastroenteritis and thermal burn (three cases each), two cases each of asphyxia and herbal toxicity, one case each of acute chest syndrome, AIDS-related complication, anemia, bronchiolitis, HIV infection, infection, acute otitis media, pulmonary tuberculosis, renal insufficiency, sepsis, skin infection, sudden infant death syndrome, and two cases of death (cause unspecified). Approximately 29% of deaths occurred during hospitalization. The all-cause mortality rate per 100,000 person-years was 969 (95% CI 699–1310), with similar rates in girls (940, 95% CI 574–1451) and boys (998, 95% CI 626–1511) (Table 6). The rate of malaria-attributable deaths was 231 (95% CI 111–424) per 100,000 person-years (Table 6).
Table 6.
All-cause mortality rate per 100,000 person-years by gender
| Kombewa, Kenya | Kintampo, Ghana | Navrongo, Ghana | Overall | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | PY | Value (95% CI) | n | PY | Value (95% CI) | n | PY | Value (95% CI) | n | PY | Value (95% CI) | |
| Female | N = 1018 | N = 1786 | N = 584 | N = 3388 | ||||||||
| All causes | 7 | 600 | 1166 (469, 2402) | 12 | 1154 | 1040 (537, 1817) | 1 | 374 | 267 (7, 1488) | 20 | 2129 | 940 (574, 1451) |
| AESI | 0 | 600 | 0 (0, 614) | 0 | 1154 | 0 (0, 320) | 0 | 374 | 0 (0, 985) | 0 | 2129 | 0 (0, 173) |
| Meningitis | 0 | 600 | 0 (0, 614) | 0 | 1154 | 0 (0, 320) | 0 | 374 | 0 (0, 985) | 0 | 2129 | 0 (0, 173) |
| Malaria | 0 | 600 | 0 (0, 614) | 6 | 1154 | 520 (191, 1132) | 0 | 374 | 0 (0, 985) | 6 | 2129 | 282 (103, 614) |
| P. falciparum | 0 | 600 | 0 (0, 614) | 1 | 1154 | 87 (2, 483) | 0 | 374 | 0 (0, 985) | 1 | 2129 | 47 (1, 262) |
| Other AEs | 7 | 600 | 1166 (469, 2402) | 6 | 1154 | 520 (191, 1132) | 1 | 374 | 267 (7, 1488) | 14 | 2129 | 658 (360, 1104) |
| Male | N = 1093 | N = 1817 | N = 599 | N = 3509 | ||||||||
| All causes | 14 | 649 | 2157 (1179, 3620) | 7 | 1176 | 595 (239, 1227) | 1 | 379 | 264 (7, 1469) | 22 | 2204 | 998 (626, 1511) |
| AESI | 0 | 649 | 0 (0, 568) | 0 | 1176 | 0 (0, 314) | 0 | 379 | 0 (0, 973) | 0 | 2204 | 0 (0, 167) |
| Meningitis | 0 | 649 | 0 (0, 568) | 0 | 1176 | 0 (0, 314) | 0 | 379 | 0 (0, 973) | 0 | 2204 | 0 (0, 167) |
| Malaria | 3 | 649 | 462 (95, 1351) | 1 | 1176 | 85 (2, 474) | 0 | 379 | 0 (0, 973) | 4 | 2204 | 182 (49, 465) |
| P. falciparum | 2 | 649 | 308 (37, 1113) | 1 | 1176 | 85 (2, 474) | 0 | 379 | 0 (0, 973) | 3 | 2204 | 136 (28, 398) |
| Other AEs | 11 | 649 | 1695 (846, 3033) | 6 | 1176 | 510 (187, 1111) | 1 | 379 | 264 (7, 1469) | 18 | 2204 | 817 (484, 1291) |
| Total | N = 2111 | N = 3603 | N = 1183 | N = 6897 | ||||||||
| All causes | 21 | 1249 | 1681 (1041, 2569) | 19 | 2330 | 816 (491, 1274) | 2 | 754 | 265 (32, 959) | 42 | 4332 | 969 (699, 1310) |
| AESI | 0 | 1249 | 0 (0, 295) | 0 | 2330 | 0 (0, 158) | 0 | 754 | 0 (0, 490) | 0 | 4332 | 0 (0, 85) |
| Meningitis | 0 | 1249 | 0 (0, 295) | 0 | 2330 | 0 (0, 158) | 0 | 754 | 0 (0, 490) | 0 | 4332 | 0 (0, 85) |
| Malaria | 3 | 1249 | 240 (50, 702) | 7 | 2330 | 300 (121, 619) | 0 | 754 | 0 (0, 490) | 10 | 4332 | 231 (111, 424) |
| P. falciparum | 2 | 1249 | 160 (19, 578) | 2 | 2330 | 86 (10, 310) | 0 | 754 | 0 (0, 490) | 4 | 4332 | 92 (25, 236) |
| Other AEs | 18 | 1249 | 1441 (854, 2277) | 12 | 2330 | 515 (266, 900) | 2 | 754 | 265 (32, 959) | 32 | 4332 | 739 (505, 1043) |
According-to-protocol cohort, 5 to 17 months age-group N, Number of study participants at risk during a follow-up period of approximately 6 months after each dose of the virtual primary vaccination schedule censored at the next dose; n, number of cases reported during that follow-up period
CI: confidence interval; PY: person-years; AE: adverse event; AESI: adverse event of special interest
Incidence rates of AESI
Two AESI were diagnosed in participants in the 5 to 17 months age-group, one case of intussusception (144 per 100,000 person-years; 95% CI 4–800), and one case of encephalomyelitis occurring outside the at-risk period of 6 weeks (no incidence rate calculated). Both AESI led to hospitalization, neither was fatal.
Other AEs leading to hospitalization
In the 5 to 17 months age-group, 392 study participants (5.5%) experienced 802 other events (i.e., other than meningitis, malaria or AESI) that led to their hospitalization (Table 3). The most frequently reported other events were anaemia, gastroenteritis, and lower respiratory tract infection (Table 7 and Additional file 12).
Table 7.
Incidence rate per 100,000 person-year of other adverse events
| Overall (N = 6897) | Incidence rate per 100,000 person-years | |||
|---|---|---|---|---|
| n | Person-years | Value (95% CI) | ||
| Anemia | 69 | 1490 | 4632 | (3604, 5862) |
| Bacterial Infection | 1 | 1492 | 67 | (2, 373) |
| Burns | 2 | 1492 | 134 | (16, 484) |
| Conjunctivitis | 1 | 1492 | 67 | (2, 373) |
| Gastroenteritis | 41 | 1491 | 2751 | (1974, 3732) |
| Helminthic infection | 2 | 1492 | 134 | (16, 484) |
| Lower respiratory tract infection | 38 | 1491 | 2549 | (1804, 3499) |
| Malnutrition | 3 | 1492 | 201 | (41, 588) |
| Sepsis | 22 | 1491 | 1475 | (925, 2234) |
| Skin Infection | 3 | 1492 | 201 | (41, 588) |
| Upper respiratory tract infection | 19 | 1492 | 1274 | (767, 1989) |
| Urinary tract Infection | 0 | 1492 | 0 | (0, 247) |
According-to-protocol cohort, 5 to 17 months age-group. Other adverse events, AE other than AESI, meningitis or malaria that was diagnosed during hospitalization.
Preferred terms of other AEs leading to hospitalization were grouped into medically relevant categories (refer to Additional file 5 for more details) for the calculation on the incidence rates. N, Number of study participants at risk within 30 days after each dose of the virtual primary vaccination schedule censored at the next dose; n, number of cases reported during that follow-up period
CI: confidence interval; M: Months; ATP: According-to-protocol cohort
Quality indicators of surveillance
In the 6 to 12 weeks age-group, there were five cases of abscess at the injection site after a total of 21,149 DTP-HepB-Hib doses. No cases were reported in the 5 to 17 months age-group. No foot positional deformations were reported in either group.
Discussion
In the context of the MVIP, the RTS,S/AS01E malaria vaccine has recently been included in the Expanded Programmes on Immunization (EPI) of selected pilot implementation areas of Ghana, Kenya and Malawi. As RTS,S/AS01E has not been introduced as part of EPI anywhere else in the world, no post-authorization surveillance data has been generated so far. The MVIP includes an important vaccine evaluation component in which GSK is conducting a comprehensive PAP in collaboration with African scientific partners. Limited background data on the incidence of diseases of interest in the framework of this PAP are available in the SSA context. The present study enrolled children not vaccinated with RTS,S/AS01E in three study sites of moderate-to-high malaria transmission in Ghana and Kenya, countries where RTS,S/AS01E is being administered in selected areas since 2019. The study was designed to document baseline incidence data prior to RTS,S/AS01E implementation to allow a before-after vaccine introduction comparison, and more broadly to help interpretation of pharmacovigilance monitoring data after vaccine implementation. Preliminary study results pertaining to the 5 to 17 months age-group are discussed here.
Overall, the mortality rate per 100,000 person-years observed in the 5 to 17 months age-group was 969 (95% CI 699–1310) ranging from 265 (95% CI 32–959) to 1681 (95% CI 1041–2569) depending on the study site. Even though a formal comparison is not possible as age-groups and study period differ, those figures are not inconsistent with the INDEPTH Network mortality estimates for the period 2006 to 2012, ranging from 820 per 100,000 person-years in children aged 1 to 4 years in Navrongo, Ghana, to 7420 per 100,000 person-years in children aged 1 to 11 months in Kisumu, Kenya [10]. Overall, no marked difference in mortality rate between genders was observed.
Across the study sites, the incidence rate of meningitis (including aetiology-confirmed, probable and clinically suspected meningitis) was 92 (95% CI 25–236) per 100,000 person-years in the 5 to 17 months age-group. Of note, antibiotic therapy was initiated one to two days before CSF sample collection for two cases, which may have affected the CSF testing results and the final case classification. Though available data on meningitis incidence in SSA are scarce and clear case definitions are often lacking, this figure is in the range of incidence rates published for Burkina Faso (between 42 and 101 per 100,000 person-years in children aged less than 5 years for the 2011 to 2015 period) [11].
The incidence of malaria in the 5 to 17 months age-group was similar between Kintampo, Ghana and Kombewa, Kenya (as could be expected based on their similar malaria transmission intensity reported previously [12]) but fourfold lower in Navrongo, Ghana, emphasizing the need for generating local data when assessing vaccine effects. Incidence rates were estimated according to the level of disease severity, i.e., uncomplicated or severe (including cerebral) malaria, and following strict WHO case definitions. As clinical manifestations of malaria are highly age-dependent, especially in young children, final study results covering a longer follow-up period of enrolled children will provide key data that will be compared with post-approval data to assess both short and long-term vaccine effectiveness and impact.
Among the list of 15 defined AESI, only one case of intussusception (IS) occurred during the pre-defined risk period (14 days in the case of IS). It is not surprising to observe few AESIs at this stage given that these health outcomes are rare and that the current interim study results include a short subject follow-up period (6 months after the virtual primary vaccination schedule). The estimated incidence rate of IS was 144 (95% CI 4–800) per 100,000 person-years. Although the precision around this estimate is low, it is in the range of previously published data (mean incidence rate of 74 [range 9–328] per 100,000 person-years in children less than 1 year old between 1978 and 2012) [13].
The operational conduct of the study was assessed by two quality surveillance indicators. The rate of abscess at injection site observed for the 6 to 12 weeks age-group (24 events per 100,000 DTP-HepB-Hib doses) is within the expected range for this vaccine (22 to 108 cases per 100,000 doses) [14]. The absence of cases in the 5 to 17 months age-group was not unexpected as that assessment did not follow a real vaccine injection (i.e. virtual vaccination schedule mimicking RTS,S/AS01E administration). No foot positional deformations were reported in either group. As the study participants were around 6 weeks of age (corresponding to the 1st DTP-HepB-Hib vaccination dose) or older at time of enrolment, the choice of a congenital anomaly was not appropriate.
This study also generated incidence rates of more frequent conditions affecting children in SSA, such as anemia, gastroenteritis, respiratory infections, and sepsis, for which incidence estimates are often lacking [15, 16]. To a broader extent, the incidence rates reported here will support international efforts to estimate the global burden of diseases, especially in the SSA region.
This study has been designed to detect conditions that require access to specific diagnostic tools and expertise that is not systematically available in SSA. For this reason, multiple tools have been developed and put in place to enhance case detection/ascertainment, e.g., medical trainings, job aids, telemedicine support, second-line laboratory testing of CSF/serum samples, local laboratory support, home visits, and consultation of an external expert panel. Among other aspects, those tools have been pivotal in enhancing the routine practice of CSF collection to appropriately investigate cases of suspected meningitis, and to differentiate these with cerebral malaria, combining both first and second line laboratory results and medical judgement to reach the final diagnosis. Despite the strong capacity building and subject follow-up components of this study, data collection relies on routine practice and optimal case detection and ascertainment may not have been reached due to the complex setting. Additionally, shortages of medical supplies, limited radiological and laboratory investigational capacities and/or reduced availability of medical personnel during strikes and elections are events that may have influenced case detection and ascertainment. However, this should not affect future vaccine safety and effectiveness assessment as the post-introduction study is based on the same design and is conducted in the same conditions.
Conclusion
These preliminary results are key for several reasons: (1) they are the backbone of the RTS,S/AS01E PAP, generating background incidence figures around which the before-after vaccine introduction comparison will be articulated; (2) they provide baseline figures to support pharmacovigilance monitoring of the vaccine; (3) they demonstrate the technical and operational feasibility of conducting such a large scale prospective cohort study with limited pre-existing health care and surveillance infrastructures; (4) beyond the RTS,S/AS01E PAP, they provide incidence rate estimates of health outcomes for which only simulated/modelled figures are usually available. At the end of both pre- and post-vaccine introduction studies, all subjects enrolled in the prospective cohorts will have been followed up until 2 years after the fourth scheduled vaccination dose. This time-period will cover risks periods after each vaccine dose administration, as well as assessing vaccine effectiveness and impact after the primary vaccination schedule and the booster dose.
A plain language summary of the study is provided in Fig. 2.
Fig. 2.
Plain Language Summary
Supplementary Information
Additional file 1. Study age groups and home visits
Additional file 3. Pre-defined at-risk periods after the (virtual) primary vaccination schedule
Additional file 5. Grouping of other adverse events leading to hospitalization
Additional file 6. Quality surveillance indicators
Additional file 7. List of ethical review boards
Additional file 8. Demographic characteristics at study entry by study group and study site, According-to-protocol cohort
Additional file 9. Incidence rate per 100,000 person-years of malaria cases. According-to-protocol cohort, 6 to 12 weeks age-group
Additional file 10. All-cause mortality rate per 100,000 person-years by gender. According-to protocol-cohort, 6 to 12 weeks age-group
Additional file 11. Incidence rate per 100,000 person-years of other adverse events leading to hospitalization. ATP, 6 to 12 weeks age-group
Additional file 12. Incidence rate of other adverse events leading to hospitalization, by study site. ATP, 5 to 17 months age-group
Acknowledgements
The authors thank the following individuals or groups for their invaluable contribution to the study: GSK: Laurence Baril, and Edith Roset Bahmanihar. Kintampo Ghana: Elisha Adeniji, Oscar Agyei, David Dosoo and Kingsley Kayan. Navrongo, Ghana: Peter Wontuo. Kombewa, Kenya: Stellah K. Amoit, Consolata Appida, Dorothy Caroline Mabunde, Erick O. Otieno, Kennedy Otieno, Solomon Otieno, Nancy Sande, and Samuel O. Wangowe. All health facilities in the study areas, study participants and community leaders. PATH: Ishani Sheth and Madenwald Tamra. Expert Panel Members: Dr Annette Erhart, Pr. Philippa Musoke, Pr. Diederik Van de Beek, Pr. Daniel Weibel. The authors also acknowledge contributions to the study of “le Réseau en Afrique Francophone pour la Télémédecine” (RAFT) (represented by Professor Antoine Geissbuhler), “l’Agence de Médecine Préventive (AMP) Regional Office for Africa (represented by Dr. Tene-Alima Essoh) and the Clinical Laboratory Services (represented by Elongo Fritz). The authors also thank the Modis platform on behalf of GSK for writing assistance provided by Joanne Wolter and manuscript coordination provided by William Zonta.
Members of the RTS,S Epidemiology EPI-MAL-002 study group, in alphabetical order: Prince Darko Agyapong (Kintampo Health Research Centre, Kintampo, Ghana), Elaine Jacqueline Akite (GSK, Wavre, Belgium), Nana Akosua Ansah (Navrongo Health Research Centre, Navrongo, Ghana), Patrick Odum Ansah (Navrongo Health Research Centre, Navrongo, Ghana), Kwaku Poku Asante (Kintampo Health Research Centre, Kintampo, Ghana), Denis Azabra Awuni (Navrongo Health Research Centre, Navrongo, Ghana), Daniel K. Azongo (Navrongo Health Research Centre, Navrongo, Ghana), Owusu Boahen (Kintampo Health Research Centre, Kintampo, Ghana), Marie-Cecile Bozonnat (Clinics c/o GSK, Wavre, Belgium), Nathanial K. Copeland (KEMRI-Walter Reed Project, US Army Medical Research Directorate-Kenya, Kombewa, Kenya), Yolanda Guerra Mendoza (GSK, Wavre, Belgium), Valerie Haine (GSK, Wavre, Belgium), Samuel Bernard Ekow Harrison (Kintampo Health Research Centre, Kintampo, Ghana), Seyram Kaali (Kintampo Health Research Centre, Kintampo, Ghana), Michael Bandasua Kaburise (Navrongo Health Research Centre, Navrongo, Ghana), Abraham Oduro (Navrongo Health Research Centre, Navrongo, Ghana), Esther Oguk (KEMRI-Walter Reed Project, US Army Medical Research Directorate-Kenya, Kombewa, Kenya), Lucas Otieno (KEMRI-Walter Reed Project, US Army Medical Research Directorate-Kenya, Kombewa, Kenya), Walter Otieno (KEMRI-Walter Reed Project, US Army Medical Research Directorate-Kenya, Kombewa, Kenya), Seth Owusu-Agyei (Kintampo Health Research Centre, Kintampo, Ghana), Janet Oyieko (KEMRI-Walter Reed Project, US Army Medical Research Directorate-Kenya, Kombewa, Kenya), Jean-Yves Pirçon (GSK, Wavre, Belgium), Nicolas Praet (GSK, Wavre, Belgium), François Roman (GSK, Wavre, Belgium), Lode Schuerman (GSK, Wavre, Belgium), Valentine Sing’oei (KEMRI-Walter Reed Project, US Army Medical Research Directorate-Kenya, Kombewa, Kenya), Mathilda Tivura (Kintampo Health Research Centre, Kintampo, Ghana).
Abbreviations
- AE
Adverse event
- AESI
Adverse event of special interest
- ATP
According-to-protocol
- CI
Confidence interval
- CSF
Cerebrospinal fluid
- DTP-HepB-Hib
Diphtheria-tetanus-pertussis-hepatitis B-Haemophilus influenzae
- EPI
Expanded programme on immunization
- IS
Intussusception
- M
Months
- MVIP
Malaria vaccine implementation programme
- PAP
Post-approval plan
- SSA
Sub-Saharan Africa
- W
Weeks
Authors' contributions
POA, KPA, M-CB, YGM, VH, SK, AO, EO, WO, SO-A, JO, J-YP, NP, FR, LS and MT have been involved in the conception or the design of the study. PDA, NAA, POA, KPA, DAA, DKA, OB, M-CB, NKC, VH, SBEH, SK, MK, AO, EO, LO, WO, SO-A, JO, J-YP, NP, VS and MT collected the study data. PDA, EJA, NAA, KPA, DAA, DKA, M-CB, YGM, VH, SBEH, SK, MK, AO, LO, WO, SO-A, JO, J-YP, NP, FR, LS, VS and MT interpreted the data. All authors read and approved the final manuscript.
Funding
This work was supported and funded by GlaxoSmithKline Biologicals SA and PATH. GlaxoSmithKline Biologicals SA funded all costs associated with the development and the publishing of the present manuscript.
Availability of data and materials
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an enquiry via the website.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization Guideline for Good Clinical Practice and Good Epidemiological Practice, and with the approval of the ethical review boards (Additional file 7). Subjects’ whose parent(s)/legal representative provided written informed consent.
Consent for publication
Not applicable.
Competing interests
EJA and VH are employed by the GSK group of companies. YGM, J-YP, FR and LS are employed by the GSK group of companies and hold shares in the GSK group of companies. NP was employed by the GSK group of companies at the time of the work and hold shares in the GSK group of companies. NP is now employed by Janssen Pharmaceutica NV, Beerse, Belgium. M-CB is employed by 4Clinics on behalf of the GSK group of companies. DAA, DKA, PDA, NAA, POA, KPA, OB, NKC, SBEH, SK, MBK, AO, EO, LO, WO, SO-A, JO, VS, MT, via their institutions, received grants from the GSK group of companies for the conduct of this study/work and for the conduct of studies outside the submitted work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
The RTS,S Epidemiology EPI-MAL-002 Study Group, Email: valerie.x.haine@gsk.com.
The RTS,S Epidemiology EPI-MAL-002 Study Group:
Prince Darko Agyapong, Elaine Jacqueline Akite, Nana Akosua Ansah, Patrick Odum Ansah, Kwaku Poku Asante, Denis Azabra Awuni, Daniel K. Azongo, Owusu Boahen, Marie-Cecile Bozonnat, Nathanial K. Copeland, Yolanda Guerra Mendoza, Valerie Haine, Samuel Bernard Ekow Harrison, Seyram Kaali, Michael Bandasua Kaburise, Abraham Oduro, Esther Oguk, Lucas Otieno, Walter Otieno, Seth Owusu-Agyei, Janet Oyieko, Jean-Yves Pirçon, Nicolas Praet, François Roman, Lode Schuerman, Valentine Sing’oei, and Mathilda Tivura
References
- 1.WHO. World malaria report 2019. Geneva, World Health Organization, 2019. https://www.who.int/publications-detail/world-malaria-report-2019. Accessed 20 Jan 2020.
- 2.WHO. Global Technical Strategy for Malaria 2016–2030. Geneva, World Health Organization, 2015. https://www.who.int/malaria/areas/global_technical_strategy/en/. Accessed 19 March 2020
- 3.European Medicines Agency. Mosquirix H-W-2300. European Public Assessment Report. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/document_listing/document_listing_000395.jsp&mid= Accessed 19 March 2020.
- 4.Malaria vaccine: WHO position paper-January 2016. Wkly Epidemiol Rec. 2016;91:33–51. https://www.who.int/wer/2016/wer9104/en/ [PubMed]
- 5.WHO. Q&A on the malaria vaccine implementation programme (MVIP). Geneva, World Health Organization. https://www.who.int/malaria/media/malaria-vaccine-implementation-qa/en/ Accesssed 22 January 2020.
- 6.Guerra Mendoza Y, Garric E, Leach A, Lievens M, Ofori-Anyinam O, Pircon JY, et al. Safety profile of the RTS, S/AS01 malaria vaccine in infants and children: additional data from a phase III randomized controlled trial in sub-Saharan Africa. Hum Vaccin Immunother. 2019;15:2386–2398. doi: 10.1080/21645515.2019.1586040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guignard A, Praet N, Jusot V, Bakker M, Baril L. Introducing new vaccines in low- and middle-income countries: challenges and approaches. Expert Rev Vaccines. 2019;18:119–131. doi: 10.1080/14760584.2019.1574224. [DOI] [PubMed] [Google Scholar]
- 8.RTS,S Clinical Trials Partnership Efficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.INDEPTH Network. INDEPTH Standardized Verbal Autopsy questionnaire. http://www.indepth-network.org/index.php?option=com_content&task=view&id=1831. Accessed 26 Feb 2019.
- 10.Streatfield PK, Khan WA, Bhuiya A, Hanifi SM, Alam N, Ouattara M, et al. Cause-specific childhood mortality in Africa and Asia: evidence from INDEPTH health and demographic surveillance system sites. Glob Health Action. 2014;7:25363. doi: 10.3402/gha.v7.25363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diallo AO, Soeters HM, Yameogo I, Sawadogo G, Ake F, Lingani C, et al. Bacterial meningitis epidemiology and return of Neisseria meningitidis serogroup a cases in burkina faso in the five years following MenAfriVac mass vaccination campaign. PLoS ONE. 2017;12:e0187466. doi: 10.1371/journal.pone.0187466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12."RTS,S Epidemiology EPI-MAL-005 Study Group Estimating annual fluctuations in malaria transmission intensity and in the use of malaria control interventions in five Sub-Saharan African countries. Am J Trop Med Hyg. 2020;103:1883–1892. doi: 10.4269/ajtmh.19-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang J, Jiang B, Parashar U, Nguyen T, Bines J, Patel MM. Childhood intussusception: a literature review. PLoS ONE. 2013;8:e68482. doi: 10.1371/journal.pone.0068482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burton DC, Bigogo GM, Audi AO, Williamson J, Munge K, Wafula J, et al. Risk of injection-site abscess among infants receiving a preservative-free, two-dose vial formulation of pneumococcal conjugate vaccine in Kenya. PLoS ONE. 2015;10:e0141896. doi: 10.1371/journal.pone.0141896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gouda HN, Charlson F, Sorsdahl K, Ahmadzada S, Ferrari AJ, Erskine H, et al. Burden of non-communicable diseases in sub-Saharan Africa, 1990–2017: results from the Global Burden of Disease Study 2017. Lancet Glob Health. 2019;7:e1375–e1387. doi: 10.1016/S2214-109X(19)30374-2. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization Regional Office for Africa. The health of the people: what works–the African regional health report 2014. https://www.afro.who.int/publications/african-regional-health-report-2014-health-people-what-works Accessed 19 March 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Study age groups and home visits
Additional file 3. Pre-defined at-risk periods after the (virtual) primary vaccination schedule
Additional file 5. Grouping of other adverse events leading to hospitalization
Additional file 6. Quality surveillance indicators
Additional file 7. List of ethical review boards
Additional file 8. Demographic characteristics at study entry by study group and study site, According-to-protocol cohort
Additional file 9. Incidence rate per 100,000 person-years of malaria cases. According-to-protocol cohort, 6 to 12 weeks age-group
Additional file 10. All-cause mortality rate per 100,000 person-years by gender. According-to protocol-cohort, 6 to 12 weeks age-group
Additional file 11. Incidence rate per 100,000 person-years of other adverse events leading to hospitalization. ATP, 6 to 12 weeks age-group
Additional file 12. Incidence rate of other adverse events leading to hospitalization, by study site. ATP, 5 to 17 months age-group
Data Availability Statement
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an enquiry via the website.