Abstract
Host preference of root endophytes of the three European tree species of Norway spruce (Picea abies), common ash (Fraxinus excelsior), and sycamore maple (Acer pseudoplatanus) were investigated in two forest stands near Zurich, Switzerland. The focus was placed on members of the Phialocephala fortinii s.l. (sensu lato)—Acephala applanata species complex (PAC), as well as other dark septate endopyhtes (DSE). PAC species were identified based on 13 microsatellite loci. Eleven PAC species were found, with Phialocephala helvetica, P. europaea being the most frequent. All but cryptic species 12 (CSP12) preferred Norway spruce as a host. Though very rare in general, CSP12 was most frequently isolated from maple roots. Regarding the abundant PAC species, P. helvetica and P. europaea, the preference of spruce as a host was least pronounced in P. europaea, as it was also often isolated from ash and maple. It is the first record of PAC found on common ash (Fraxinus excelsior). Cadophora orchidicola, a close relative of PAC, has frequently been isolated from ash. Various species of the Nectriaceae (Cylindrocarpon spp.) have often been isolated, particularly from maple roots. By comparison, Pezicula spp. (Cryptosporiopsis spp.) was found to be abundant on all three hosts. Phomopsis phaseoli exhibits a clear preference for spruce.
Keywords: Picea abies, Fraxinus excelsior, Acer pseudoplatanus, Ilyonectria, Neonectria, Mycosphaerellaceae, microsatellite genotyping
1. Introduction
Mycorrhizal fungi tend to be associated with roots of certain tree species. Many ectomycorrhizal fungi are associated with multiple tree species [1,2], whereas representatives of the genera Suillus and Rhizopogon are Pinaceae-specific [3]. In cases when a fungal taxon occurs exclusively on one host, the term “host-specificity” is used [4]. The relationships between fungi and host are also known to be less specific. In such cases, “host preference” is more appropriate, meaning that the fungus prefers one host over another [5]. For mycorrhizal fungi, strong host preference was apparent for many species [6,7], but the contrary is also true for many other species [8,9].
In addition to mycorrhizal fungi, woody roots are colonized by a plethora of endophytic fungi. In contrast to mycorrhizal fungi, which colonize only primary roots, endophytic fungi occur throughout the entire root system. Nevertheless, host specificity and host preference of endophytic fungi have not been as well studied as those of mycorrhizal fungi. Dark septate endophytes (DSE), i.e., species that form dark, septated mycelia in culture, and Non-DSE, i.e., species without darkly pigmented hyphae, manifest as root endophytes [10,11,12,13]. The DSE are mainly Cadophora species and representatives of the the Phialocephala fortinii s.l. (sensu lato)—Acepahala applanata species complex (PAC) [14]. The Non-DSE mostly belong to the genera Gibberella (Fusarium), Ilyonectria, Neonectria (Cylindrocapron) species, and some are representatives of the Sebacinales. In addition, Pezicula (Cryptosporiopsis) species are common and are classified as DSE or Non-DSE depending on the species and the age of the mycelium. Depending on the plant family, either DSE or Non-DSE dominate. According to current knowledge, DSE tend to dominate on Pinaceae, while, on deciduous woods (numerous families) Non-DSE dominate (see Figure 38.1 in Sieber and Grünig [15]).
DSE have been identified in the roots of over 600 different plant species [12], ranging from subtropical to arctic environments [10,16,17,18,19]. Their broad host range and substantial abundance suggest that these fungi play an important, though as yet largely unknown, role in various ecosystems [12,20]. For example, it has been demonstrated that DSE have the potential to facilitate nutrient absorption in their host plants, as well as increase their resistance to toxic environmental conditions [21,22,23,24].
Probably the best-known endophytes present in woody roots are the representatives of the PAC, which have mainly been colonizing the roots of conifers and ericaceous plants all across the Northern hemisphere [13,25,26,27,28,29]. So far, 21 morphologically indistinguishable cryptic species (CSP) have been identified, of which eight have been formally described: Phialocephala turicensis (CSP1), P. letzii (CSP2), P. europaea (CSP3), P. helvetica (CSP4), P. uotilensis (CSP5), P. subalpina (CSP6), P. fortinii s.s., and Acephala applanata [14,30,31,32,33]. There is evidence of the discovery of a 22nd CSP found in the Pfynwald—a forest in Switzerland dominated by Scots pine (Pinus sylvestris) [34]. PAC communities are highly diverse and the factors governing community assembly remain unknown [28,35,36,37], as do their modes of reproduction and dispersal. Despite evidence for sexual reproduction, a sexual state has never been witnessed [16,38], leaving long distance transfer with colonized plant material as one possible explanation for local diversity [39,40].
Very few studies on DSE involve non-coniferous plants, but DSE (including PAC) have also been detected in the roots of deciduous trees such as beech (Fagus sylvatica), sessile oak (Quercus petraea), and common oak (Quercus robur) [11,41]. However, the dominant DSE in deciduous trees appear to be Pezicula and Cadophora species rather than PAC. In oak roots (Q. robur and Q. petraea), Pezicula melanigena, P. radicicola and Cadophora fastigiata dominate the root endophyte community [41]. Likewise, P. radiciola was the most frequently presenting endophyte in roots of European beech (Fagus sylvatica) [11]. Accordingly, data pertaining to Non-DSE in deciduous trees are also scarce. In oak roots (Q. petraea and Q. robur) only Ilyonectria radicicola and one species of Cystodendron were pervasive [41]. The Non-DSE Cylindrocarpon didymum was the second most present endophyte in F. sylvatica roots [11]. Zheng [42] reported the detection of a Neonectria species in roots of Ginkgo (Gingko biloba). Even if G. biloba resembles a deciduous tree in its habitus, it is classified as a gymnosperm. Phylogenetically, G. biloba is positioned between conifers and deciduous trees, and one would expect it to be colonized by both conifer-specific and deciduous-tree-specific endophytes. However, this requires more extensive research. Non-DSE, i.e., Fusarium and Neonectria species, have also been detected in roots of wild cherry (Prunus avium) [43].
In the present study, we set out to investigate the degree to which roots of deciduous tree species growing in forest stands with a high rooting density of Norway spruce (Picea abies) are colonized by PAC, Non-PAC DSE and Non-DSE fungi. Mixed forest stands with Norway spruce-dominated canopies and understories dominated by deciduous tree species, in particular common ash (Fraxinus excelsior) and sycamore maple (Acer pseudoplatanus) were chosen as locations for the study.
2. Materials and Methods
2.1. Study Site
Two Norway spruce (Picea abies L. Karst) forest stands were selected in the vicinity of Zurich, Switzerland (Site 1: N 47°22′55′′, E 8°28′1.9′′, 532 m MSL.; Site 2: N 47°23′31.9′′ E 8°33′55′′, 628 m MSL), both showing dense undergrowth of common ash (Fraxinus excelsior L.) and sycamore maple (Acer pseudoplatanus L.).
2.2. Sampling Design
A sampling grid consisting of nine grid points (6 m × 6 m; distance of 3 m between grid points) was established at each study site. Sampling of Norway spruce, common ash and sycamore maple took place within a 1 m radius of each grid point. Within each circle, 5 small ash trees and 5 small maple trees (~20 cm in height) were carefully excavated. Additionally, root complexes of mature Norway spruce trees were collected at 5 sampling points within each circle. All plant material was placed in plastic bags and stored at 4 °C in the refrigerator until further processing within the following 2–3 days.
2.3. Isolation of Fungal Cultures
The root systems of the collected ash and maple, as well as the spruce root pieces, were carefully washed under running tap water to remove all soil particles. Afterwards, 10 root segments (approx. 5 cm in length) with varying diameters (approx. 1–5 mm) were removed per tree or root complex (spruce), respectively, and surfaces were sterilized in accordance with Ahlich and Sieber [11]. In brief: immersion of root segments in 99% (v/v) ethanol for 1 min, followed by 5 min in 35% (v/v) hydrogen peroxide and, finally, 30 s in 99% (v/v) ethanol. A smaller root segment of 0.5 cm was excised aseptically from the midpoint of each surface sterilized root, placed on terramycin malt agar (TMA; 20 g/L malt extract, 16 g/L agar, 50 mg/L terramycin (active ingredient: oxytetracycline, Pfizer Ltd., Hyderabad, Telangana, India)), and incubated at 20 °C in the dark for approximately 2 weeks [11].
2.4. Classification of Fungal Cultures
All emerging fungal cultures were first visually divided into DSE and Non-DSE fungi based on the presence or absence of dark, melanized mycelium. DSE fungi were further divided into PAC and Non-PAC isolates based on microsatellite genotyping (described below). All the Non-DSEs were visually grouped into 4 main categories based on culture morphology: Cylindrocarpon spp., Cryptosporiopsis spp., Phomopsis spp., and others. The classification of Pezicula (Cryptosporiopsis) species as either DSE or Non-DSE poses a problem. Many species initially grow in culture with a colorless white mycelium. In some species, however, the colonies become darker with age. Since the assignment of the cultures to morphotypes occurred when the colonies were still relatively young, the Pezicula species were considered to be Non-DSE.
2.5. Microsatellite Genotyping of DSE Isolates
In order to clearly identify members of the PAC within the large number of DSE isolates, single-hyphal tip (SHT) cultures were prepared from all DSE emerging from the root segments and incubated in the dark for a further 2 weeks. Afterwards, DNA was extracted from each culture using the NucleoSpin® 96 Plant II kit by Macherey-Nagel (Macherey-Nagel, Düren, Germany). Multiplex PCR and fragment analysis of 14 microsatellite loci relevant for CSP assignment was performed, as described in Queloz et al. [44].
The obtained microsatellite data were analyzed using the software GeneMapper® (v. 4.0, Applied Biosystems, Waltham, MA, USA). Samples showing two peaks at any of the loci were considered diploid, and therefore removed from the dataset. The software GeneClass2 (v. 2.0, INRA/CIRAD 2003, Paris, France, [45]) was used for assignment tests for CSP recognition. Since GeneClass2 only uses a subset of allele data for species assignment, all samples classified with a probability below 95% were manually compared to our PAC database containing approximately 5000 records.
2.6. Sequencing of Non-PAC DSE and Non-DSE Cultures
The internal transcribed spacer (ITS) region of the nuclear rDNA of all Non-PAC DSE and a selection of representative isolates of Non-DSE fungi were sequenced [46]. A detailed protocol of all steps can be found in [47]. The sequences were BLASTed against GenBank.
2.7. Statistical Analyses
Differences between the three tree species with respect to endophytic root colonization were tested pairwise for statistical significance using the Wilcoxon–Mann–Whitney test [48,49]. All statistical tests were performed using the software R Version 3.1.3 (R Development Core Team, Vienna, Austria, 2017).
3. Results
A total of 1350 root segments were obtained at each sampling site, resulting in a total of 2700 root segments being plated for fungal growth. Of these 2700 root segments, 651 (24%) showed no fungal growth and were considered sterile. Amongst the remaining 2049 (76%) segments, DSE grew from 615 segments. With the remaining 1434 segments, we observed Non-DSE fungi emerge (Table 1).
Table 1.
The number and the percentage of isolates of the morphotypes Dark septate endophytes (DSE), Cylindrocarpon, Cryptosporiopsis, Phomopsis, others, and the number and percentage of sterile root segments (no fungal growth) for each host tree species at each site. The percentages refer to the 450 root segments (=100%) examined per host and site.
| Host | Sterile | DSE 1 | Cylindrocarpon spp. | Cryptosporiopsis spp. | Phomopsis spp. | Others | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | ||
| Site 1 | Spruce | 49 | 10.9 | 268 | 59.6 | 43 | 9.6 | 12 | 2.7 | 42 | 9.3 | 38 | 8.4 |
| Ash | 164 | 36.4 | 63 | 14.0 | 44 | 9.8 | 44 | 9.8 | 4 | 0.9 | 131 | 29.1 | |
| Maple | 82 | 18.2 | 51 | 11.3 | 151 | 33.6 | 13 | 2.9 | 1 | 0.2 | 156 | 34.7 | |
| Total | 295 | 21.9 | 382 | 28.3 | 238 | 17.6 | 69 | 5.1 | 47 | 3.5 | 325 | 24.1 | |
| Site 2 | Spruce | 58 | 12.9 | 210 | 46.7 | 41 | 9.1 | 47 | 10.4 | 20 | 4.4 | 78 | 17.3 |
| Ash | 201 | 44.7 | 19 | 4.2 | 66 | 14.7 | 16 | 3.6 | 0 | 0.0 | 155 | 34.4 | |
| Maple | 97 | 21.6 | 4 | 0.9 | 116 | 25.8 | 27 | 6.0 | 0 | 0.0 | 209 | 46.4 | |
| Total | 356 | 26.4 | 233 | 17.3 | 223 | 16.5 | 90 | 6.7 | 20 | 1.5 | 442 | 32.7 | |
| Total | all | 651 | 24.1 | 615 | 22.8 | 461 | 17.1 | 159 | 5.9 | 67 | 2.5 | 767 | 28.4 |
| TOTAL: 2720 2 (100.7%) | |||||||||||||
1 Dark septate endophytes including PAC; 2 Total 2720 > 2700 root segments: few occasions with more than one isolate growing from a single root segment.
3.1. PAC and Non-PAC DSE
3.1.1. PAC
The PAC-specific microsatellites were successfully amplified in 501 of the 615 DSE isolates (Table 2). PAC was most frequently isolated from spruce (426; 47.3%). Only few isolates were obtained from maple (38; 4.2%) and ash (37; 4.1%). The PAC isolates belonged to eleven different cryptic species (CSP). Clearly, Phialocephala helvetica was found to be the most abundant CSP, followed by Phialocephala europaea (Table 2). Five CSPs (Phialocephala subalpina, Phialocephala fortinii s.s. (senso stricto), CSP8, CSP14, and Acephala applanata) were only found once, and, thus, only on one particular tree species. CSP13 was found twice on spruce. An additional 32 isolates were also PAC, but not assignable to any CSP because too few loci amplified successfully, and eight isolates were diploid. For 74 DSE isolates, the microsatellite method did not work at all, and, therefore, they were considered Non-PAC DSE.
Table 2.
The number and the percentage of isolates per PAC species isolated from spruce, maple, and ash, as well as the total number and the percentage of isolates for both sites. Further, the sum of isolates and species for each tree species. (n = 450 root segments per host and site).
| Spruce | Ash | Maple | Totals | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAC 1 Species | Site 1 | Site 2 | Total | Site 1 | Site 2 | Total | Site 1 | Site 2 | Total | Total Isolates | ||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| P. turicensis (CSP11) | 9 | 2 | 6 | 1.3 | 15 | 1.7 | 0 | 0 | 1 | 0.2 | 1 | 0.1 | 2 | 0.4 | 0 | 0 | 2 | 0.2 | 18 | 0.67 |
| P. letzii (CSP2) | 3 | 0.7 | 13 | 2.9 | 16 | 1.8 | 0 | 0 | 1 | 0.2 | 1 | 0.1 | 1 | 0.2 | 0 | 0 | 1 | 0.1 | 18 | 0.67 |
| P. europaea (CSP3) | 80 | 17.8 | 21 | 4.7 | 101 | 11.2 | 18 | 4 | 1 | 0.2 | 19 | 2.1 | 21 | 4.7 | 0 | 0 | 21 | 2.3 | 141 | 5.22 |
| P. helvetica (CSP4) | 146 | 32.4 | 143 | 31.8 | 289 | 32.1 | 6 | 1.3 | 8 | 1.8 | 14 | 1.6 | 0 | 0 | 3 | 0.7 | 3 | 0.3 | 306 | 11.33 |
| P. subalpina (CSP6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.2 | 0 | 0 | 1 | 0.1 | 1 | 0.04 |
| P. fortinii s.s. (CSP7) | 1 | 0.2 | 0 | 0 | 1 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.04 |
| CSP8 | 0 | 0 | 1 | 0.2 | 1 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.04 |
| CSP12 | 1 | 0.2 | 0 | 0 | 1 | 0.1 | 1 | 0.2 | 0 | 0 | 1 | 0.1 | 9 | 2 | 0 | 0 | 9 | 1 | 11 | 0.41 |
| CSP13 | 2 | 0.4 | 0 | 0 | 2 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.07 |
| CSP14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.2 | 0 | 0 | 1 | 0.1 | 1 | 0.04 |
| A. applanata | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.2 | 0 | 0 | 1 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.04 |
| Sum isolates | 242 | 53.8 | 184 | 40.9 | 426 | 47.3 | 26 | 5.8 | 11 | 2.4 | 37 | 4.1 | 35 | 7.8 | 3 | 0.7 | 38 | 4.2 | 501 | 18.56 |
| Sum species | 7 | 5 | 8 | 4 | 4 | 6 | 6 | 1 | 7 | 11 | ||||||||||
1 Phialocephala fortinii s.l. (sensu lato)—Acephala applanata species complex.
PAC colonization rate was 1.5 times higher at site one (22% of the root segments colonized) than at site two (15%). Phialocephala turicensis, Phialocephala letzii, P. europaea, and P. helvetica were found at both sites and on all three tree species. CSP12 was isolated from all tree species, but solely at site one. P. subalpina and CSP 14 were only found at site one and solely on maple. P. fortinii s.s., and CSP13 only occurred on spruce at site one. CSP8 also occurred only on spruce at site two. A. applanata was only found on ash and only at site one. A total of 10 CSPs were found at site one, whereas only 5 were found at site two (Table 2).
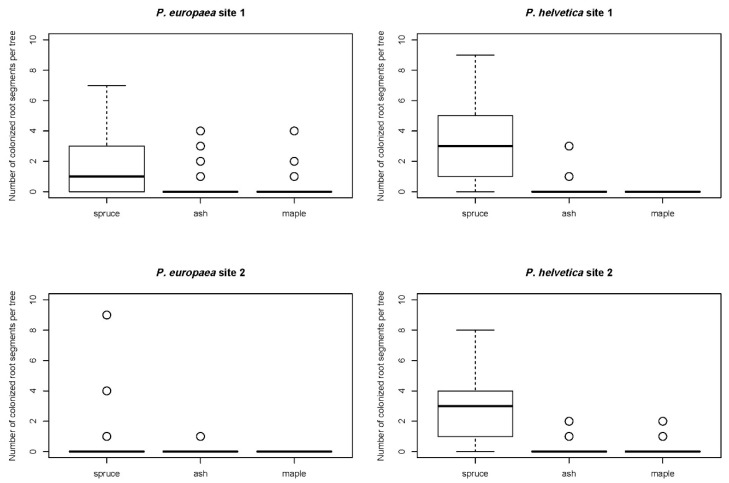
The number of colonized root segments per tree was investigated for the two most abundant CSPs, P. europaea and P. helvetica (Figure 1). Regarding both P. europaea and P. helvetica, spruce colonization was significantly higher than that of maple and ash, whereas maple and ash were in line with each other (Figure 1, Table 2, Table 3 and Table 4).
Figure 1.
Number of root segments colonized by Phialocephala europaea (left) and Phialocephala helvetica (right) per tree for spruce, ash, and maple at sites 1 and 2 (n = 10 per tree).
Table 3.
Wilcoxon–Mann–Whitney test results (p-values) for the pairwise comparisons of the three hosts in regard to the frequency of colonization by Phialocephala europaea. The values for site 1 are in the upper-right half of the table, those for site 2 are in the lower-left half of the table.
| Spruce | Ash | Maple | |
|---|---|---|---|
| Spruce | . | 0.0008 ** | 0.002 * |
| Ash | 0.0039 * | . | 0.7476 |
| Maple | 0.0009 ** | 0.3282 | . |
Signif. codes: ** 0.001 < p ≤ 0.01; * 0.01 < p ≤ 0.05; 0.05 < p ≤ 0.1.
Table 4.
Wilcoxon–Mann–Whitney test results (p-values) for the pairwise comparisons of the three hosts in regard to the frequency of colonization by Phialocephala helvetica. The values for site 1 are in the upper-right half of the table, those for site 2 are in the lower-left half of the table.
| Spruce | Ash | Maple | |
|---|---|---|---|
| Spruce | . | <0.0001 *** | <0.0001 *** |
| Ash | <0.0001 *** | . | 0.1596 |
| Maple | <0.0001 *** | 0.1478 | . |
Signif. codes: *** p ≤ 0.001; 0.05 < p ≤ 0.1.
3.1.2. Non-PAC DSE
Sequencing of the ITS regions was successful for 64 (of 74), DSEs for which microsatellite analysis did not work at all and which were thus considered Non-PAC DSE. Of these 64 DSE, only one (Phoma sp.) was from spruce, 49 from ash and 14 from maple. The similarity of the 10 isolate sequences with existing ones deposited in GenBank was below 93 % and, as a result, these isolates could not be identified. The similarity of the remaining 54 isolates exceeded 98 %, with at least one sequence found in GenBank, and could at least be identified at the genus level. The most frequent Non-PAC DSE was a species of Phoma, an anamorphic form of a Mycosphaerellaceae, followed by Cadophora orchidicola (Table 5, Table S1).
Table 5.
DSE colonization of ash and maple at site 1 and site 2. Data on PAC colonization see Table 2. Data for spruce not shown.
| Taxon | Site 1 | Site 2 | ||
|---|---|---|---|---|
| Ash | Maple | Ash | Maple | |
| PAC | 26 | 35 | 11 | 3 |
| C. orchidicola | 13 | - | 6 | 1 |
| Phoma sp. | 27 | 6 | 1 | 7 |
| Rhexocercosporidium sp. | 2 | - | - | - |
C. orchidicola and Phoma sp. were more frequently isolated from ash than from maple. At site one, C. orchidicola could not be detected on maple, solely on ash (Table 5). Another species of Cadophora was rarely observed on ash and maple and two isolates of a Rhexocercosporidium species occurred on ash (Table S1).
3.2. Non-DSE
Almost half of the Non-DSE isolates belonged to one of the following three taxa: Cylindrocarpon spp. (461 isolates), Cryptosporiopsis spp. (149 isolates), and Phomopsis spp. (67 isolates) (Table 1). The culture morphology of the other half (767 isolates) was highly diverse and no larger group of isolates with identical culture morphology could be recognized. This suggests that these isolates belong to many different species. However, since the main aim of the study was to focus on the most abundant endopyhtes, no further attempt was made to identify all these species, and they were assigned to the taxon “Others” (Table 1). Colonization by Cylindrocarpon spp. was significantly higher on maple than on ash or spruce (p < 0.0001), and that of spruce was slightly lower, yet still of significance, than that of ash (p = 0.049). Cryptosporiopsis spp. occurred equally frequently on both spruce and ash (p = 0.924), whereas the colonization rate of maple was lower (p = 0.041). Interestingly, the colonization of ash was distinctly higher at site one than at site two, whereas the opposite was true for the colonization of spruce (Table 1). Phomopsis spp. occurred almost exclusively on spruce at both sites (p < 0.0001) (Table 1).
With a few exceptions, the ITS sequences of the isolates selected from each of the three most abundant morphotypes, Cylindrocarpon spp., Cryptosporiopsis spp. and Phomopsis spp., showed that the isolates had been assigned to the correct morphotype based on culture morphology. Isolates of morphotype “Cylindrocarpon spp.” belonged to at least four different species: Cylindrocarpon sp., Ilyonectria destructans, Ilyonectria sp. and Neonectria sp. (Table S1). Isolates of morphotype “Cryptosporiopsis spp.” belonged to at least three different species: Pezicula ericae, P. melanigena and P. radicicola. In contrast, all the isolates selected for sequencing from Phomopsis spp. belonged to the same species: Phomopsis phaseoli (Table S1).
4. Discussion
4.1. PAC and Non-PAC DSE
4.1.1. PAC
Our results indicate a clear preference of PAC for Norway spruce as a host. Ash and maple were only very scarcely colonized. As far as we know, this is the first report of PAC in roots of common ash. Despite intensive sampling, Menkis [50] never found Phialocephala spp. in Fraxinus excelsior. Possibly, maple and ash are only colonized by PAC due to the density of Norway-spruce roots heavily colonized by PAC being very high, making the transfer of PAC through root contact highly probable. It has been experimentally shown that PAC is transferred preferentially via root contact [51]. Ash and maple roots from conifer-free stands must be studied in more detail to prove this hypothesis.
Looking at the individual PAC species, it is noticeable that P. helvetica has a clear preference for Norway spruce. In contrast, P. europaea is only the second most frequent PAC species in spruce roots, but the most frequent PAC species in the roots of the two deciduous hosts, though at a much lower frequency. Thus, P. europaea is certainly the least host-specific PAC species in this study. A very similar picture was found in another study comparing PAC in pine (Pinus sylvestris) and oak (Quercus pubescens) roots. The dominant species was again P. Helvetica, with a distinct preference for Pinus sylvestris. The second most common species was P. europaea, which was more common on pine, but the difference between pine and oak root colonization was far less pronounced than that for P. helvetica [34]. Halmschlager and Kowalski [41] have demonstrated that PAC (P. fortinii s.l.) were quite rare in oak roots (Quercus robur and Q. petraea). In their study, the majority of endopyhtes in oak roots were species of Cadophora, Cylindrocarpon (Ilyonectria, Neonectria), Cryptosporiopsis (Pezicula) and Cystodendron (Mollisia) [41], which is similar to the observations made within the scope of this study for ash and maple.
Phialocephala subalpina is considered non-host-specific since it has been isolated from 16 different plant species thus far [15]. P. subalpina is likely the most common PAC species in the world. Interestingly, it was found only once on maple in this study, and also very rarely in the study of Landolt et al. [34]. Some patterns of host preference also emerged from three undisturbed, subalpine forest sites in Switzerland, where the roots of Vaccinium spp. and Picea abies had been examined [38,52]. Acephala applanata was almost exclusively isolated from P. abies, whereas P. subalpina showed a preference for Vaccinium spp. but also occurred frequently on P. abies. Again, host preference of P. europaea was less pronounced. Over 70% of the P. europaea isolates originated from P. abies located at one of the three sites, whereas the repartition between Vaccinium spp. and P. abies was about 50:50 at the other two sites [38,52]. The reasons for this disparity in repartition can be attributed to climatic and edaphic differences or on-habitat occupation by competitors.
Evaluation of host preference/specificity of PAC species based on field observations is potentially hampered by confounding factors such as environmental parameters (climatic and edaphic conditions) and the presence–absence of competitors, including other PAC species. Thus, one can only speculate as to the relative effects of host, environment, and competitors on PAC host preference. Moreover, PAC do not spread through the air. Thus, if a PAC species is not present, potential hosts remain clean of the species. For example, P. subalpina is very rare in the region of Zurich, where many of our studies were conducted [35,37]. Consequently, it is absent or very rare regardless of the presence of the host. The name “subalpina” was assigned to this species because it was mainly found in subalpine regions of Switzerland [14]. However, more recent results show that the species is also highly abundant at lower elevations (600–700 m a.s.l.), e.g., in the Allgäu, Germany [40]. Thus, the absence of P. subalpina in the region of Zurich is presumably not due to low altitude, but, rather, that both sites were deforested at the end of the 19th century and only reafforested thereafter, probably with trees whose roots had not been colonized by P. subalpina.
The relative influence of host and environmental factors (climatic and edaphic conditions) on ectomycorrhizal (ECM) communities has been widely discussed. Kennedy et al. [2] found 39 out of a total of 56 ectomycorrhizal fungi to be host-specific on either Pseudotsuga menziesii or Lithocarpus densiflora. Similarly, the combination of molecular identification of plants and ectomycorrhizal fungi in the Yasuni National Park (Ecuador) revealed a significant degree of host preference within the most common fungal species [53]. In contrast, only 8 of the 205 ectomycorrhizal species proved to be strictly host-specific in one of eight tree species in a study conducted by Ishida et al. [6]. Dickie [54] concluded that ECM are not definitively host-specific since there are no physiological or anatomical obstacles. The question as to whether host preference reflects, rather, the influence of environmental factors, can only be answered by in vitro experiments under controlled environmental conditions.
A comparison of data gathered for PAC with those available for ECM would be worthwhile, as PAC have been reported as co-inhabitants of ECM [27] (Summerbell, 2005), or even forming ECM themselves [55], as does Acephala macrosclerotiorum, a close relative of PAC, on P. sylvestris [56]. However, in contrast to mycorrhizal fungi, PAC do not only colonize primary roots but can be found throughout the root system.
4.1.2. Non-PAC DSE
With the exception of two Rhexocercosporidium species on ash, all Non-PAC DSE were either Phoma sp. or Cadophora orchidicola. Together with PAC and C. orchidicola, Phoma sp. was one of the most common endophytes found in the roots of ash and maple in this study. It would certainly be interesting to learn more about the biology of this species. In our study, C. orchidicola displayed a preference for ash and was not present on spruce. The scarcity of PAC in ash roots could be partly due to the fungicidal activity of C. orchidicola (see below). Conflicting this hypothesis is the fact that PAC is also rarely detected on sycamore maple, although C. orchidicola was found only once in maple roots.
Cadophora orchidicola was originally described as a root endophyte of the orchid Platanthera hyperborean [57]. However, the fungus has also been found as an endophyte in the roots of various other plants from various plant families, such as Littorella uniflora (Plantaginaceae), a plant found on the sandy shores of freshwater lakes [58], Kalimeris indica (Asteraceae) [59], Colobanthus quitensis (Caryophyllaceae), and Deschampsia antarctica (Poaceae) in Antarctica [60]. The effects of C. orchidicola on their hosts are not fully understood. While the roots of Salix glauca (Salicaceae) became significantly longer through inoculation with C. orchidicola [61], no comparable effect was observed for roots of Saussurea involucrata (Asteraceae) [62]. However, S. involucrata inoculated with the fungus grew to be significantly larger and had a greater biomass. The metabolite cercosporamide, produced by C. orchidicola, was shown to possess fungicidal properties against Pestalotia diospyri, Botrytis cinerea, Fusarium oxysporum, Sclerotium rolfsii, and Penicillium digitatum [59].
4.2. Non-DSE
The taxon Cylindrocarpon spp. included at least three different species: Ilyonectria destructans (anamorphic form: Cylindrocarpon destructans), Ilyonectria sp., and Neonectria sp. (see Table S1 for sequence information). I. destructans is often more common in the roots of deciduous trees than conifer roots [11]. This finding is also true for sycamore maple and spruce in the present study. However, if we include ash, we see that the infestation level of ash is about the same as that of spruce (Table 1). This means that, within the parameters of this study, I. destructans is generally not more common in roots of deciduous trees than conifers. According to the literature, I. destructans is often found on and in the roots of a large number of plant species, and is also prevalent in soil [63]. I. destructans is usually associated with root dieback, but information on the virulence of this species in the existing literature is contradictory. Pathogenicity tests were often carried out on young seedlings [64,65], and virulence depended strongly on the isolate [66,67]. Similarly, isolates of I. destructans demonstrated a high incidence of sequence divergence, suggesting the existence of several phylogenetic species in this complex [68]. I. destructans was one of the most frequent endophytes in roots of Quercus petraea and Q. robur but it occurred 1.5 times more frequently in the living roots of declining trees than in those of healthy trees [41]. Consequently, Halmschlager and Kowalski [41] considered I. destructans a weak pathogen. Interestingly, the occurrence of I. destructans was inversely proportional to that of PAC (Phialocephala fortinii s.l.), possibly because of antagonistic behaviour between the two.
Cryptosporiopsis spp. are anamorphic forms of the Pezicula species. We found the following three species: Pezicula ericae, P. melanigena and P. radiciola. P. melanigena was the most common. As far as we know, P. radiciola and P. melanigena have, so far, only been witnessed in oak roots [41,69,70]. Spruce, ash, and sycamore maple would therefore be three newly discovered hosts for these two species. The same may be true for P. ericae, which has been uncovered in the roots of various ericaceous plant species [71]. We have identified these species, once each on ash and sycamore maple (Table S1). All three species grow initially as white colorless mycelia which, however, become dark with age. Thus, these three species could also be considered as DSE. However, it would be interesting to know whether older endophytic mycelium of these species also appears dark in the roots. For PAC, we know that the endophytic mycelium is always melanized [72].
Phomopsis phaseoli was almost exclusively found in spruce roots, where it likely supports PAC in the control of root pathogens [73]. P. phaseoli (Diaporthe phaseolorum) is actually known to be a causal agent of pod and stem blight of soybean. With one exception, the ITS sequences of our P. phaseoli isolates are, however, in sound agreement (>98.2% similarity) with those of an isolate from leaves of an Espeletia (Asteraceae) collected at 3250 m above sea level in the Colombian Andes [74]. These P. phaseoli isolates from Espeletia spp. were shown to strongly inhibit growth of Phytophthora infestans, the late blight pathogen affecting potato plants, in dual cultures [74].
It will be exciting to study the host preference of PAC in other forest communities. The choice of sites would also have to take into account conifer-free deciduous forests.
Acknowledgments
We wish to thank Fabienne Santschi for conducting a preliminary study at the two study sites, Anja Gall for excellent technical assistance, Christine Syrad for linguistic and stylistic corrections, and the Genetic Diversity Centre (GDC) at ETH Zurich for providing the necessary laboratory facilities for microsatellite genotyping and sequencing.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jof7040317/s1, Table S1: Strains of which the DNA ITS region was sequenced, with closest matching sequence in GenBank and GenBank accession numbers of the new sequence.
Author Contributions
Conceptualization, S.S. and T.N.S.; methodology, S.S. and V.D.; software, S.S., validation, V.D. and I.V.; formal analysis, S.S. and V.D.; resources, I.V.; data curation, I.V.; writing—original draft preparation, S.S. and T.N.S.; writing—review and editing, S.S., V.D., I.V. and T.N.S.; visualization, S.S.; supervision, T.N.S. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Horton T.R., Bruns T.D. Multiple-Host Fungi Are the Most Frequent and Abundant Ectomycorrhizal Types in a Mixed Stand of Douglas Fir (Pseudotsuga menziesii) and Bishop Pine (Pinus muricata) New Phytol. 1998;139:331–339. doi: 10.1046/j.1469-8137.1998.00185.x. [DOI] [Google Scholar]
- 2.Kennedy P.G., Izzo A.D., Bruns T.D. There Is High Potential for the Formation of Common Mycorrhizal Networks between Understorey and Canopy Trees in a Mixed Evergreen Forest: Mycorrhizal Networks in Mixed Evergreen Forest. J. Ecol. 2003;91:1071–1080. doi: 10.1046/j.1365-2745.2003.00829.x. [DOI] [Google Scholar]
- 3.Molina R., Trappe J.M. Patterns of Ectomycorrhizal Host Specificity and Potential among Pacific Northwest Conifers and Fungi. For. Sci. 1982;28:423–458. [Google Scholar]
- 4.Holliday P. A Dictionary of Plant. Pathology. 2nd ed. Cambridge University Press; Cambridge, UK: New York, NY, USA: 1998. [Google Scholar]
- 5.Zhou D., Hyde K.D. Host-Specificity, Host-Exclusivity, and Host-Recurrence in Saprobic Fungi. Mycol. Res. 2001;105:1449–1457. doi: 10.1017/S0953756201004713. [DOI] [Google Scholar]
- 6.Ishida T.A., Nara K., Hogetsu T. Host Effects on Ectomycorrhizal Fungal Communities: Insight from Eight Host Species in Mixed Conifer–Broadleaf Forests. New Phytol. 2007;174:430–440. doi: 10.1111/j.1469-8137.2007.02016.x. [DOI] [PubMed] [Google Scholar]
- 7.Tedersoo L., Jairus T., Horton B.M., Abarenkov K., Suvi T., Saar I., Kõljalg U. Strong Host Preference of Ectomycorrhizal Fungi in a Tasmanian Wet Sclerophyll Forest as Revealed by DNA Barcoding and Taxon-Specific Primers. New Phytol. 2008;180:479–490. doi: 10.1111/j.1469-8137.2008.02561.x. [DOI] [PubMed] [Google Scholar]
- 8.Rasmussen A.L., Busby R.R., Hoeksema J.D. Host Preference of Ectomycorrhizal Fungi in Mixed Pine–Oak Woodlands. Can. J. For. Res. 2018;48:153–159. doi: 10.1139/cjfr-2017-0227. [DOI] [Google Scholar]
- 9.Roy-Bolduc A., Laliberte E., Hijri M. High Richness of Ectomycorrhizal Fungi and Low Host Specificity in a Coastal Sand Dune Ecosystem Revealed by Network Analysis. Ecol. Evol. 2015;6:349–362. doi: 10.1002/ece3.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoyke G., Currah R.S. Endophytic Fungi from the Mycorrhizae of Alpine Ericoid Plants. Can. J. Bot. 1991;69:347–352. doi: 10.1139/b91-047. [DOI] [Google Scholar]
- 11.Ahlich K., Sieber T.N. The Profusion of Dark Septate Endophytic Fungi in Non-Ectomycorrhizal Fine Roots of Forest Trees and Shrubs. New Phytol. 1996;132:259–270. doi: 10.1111/j.1469-8137.1996.tb01845.x. [DOI] [Google Scholar]
- 12.Jumpponen A., Trappe J.M. Dark Septate Endophytes: A Review of Facultative Biotrophic Root-Colonizing Fungi. New Phytol. 1998;140:295–310. doi: 10.1046/j.1469-8137.1998.00265.x. [DOI] [PubMed] [Google Scholar]
- 13.Grünig C.R., Queloz V., Sieber T.N., Holdenrieder O. Dark Septate Endophytes (DSE) of the Phialocephala fortinii s.l.–Acephala applanata Species Complex in Tree Roots: Classification, Population Biology, and Ecology. Botany. 2008;86:1355–1369. doi: 10.1139/B08-108. [DOI] [Google Scholar]
- 14.Grünig C.R., Duò A., Sieber T.N., Holdenrieder O. Assignment of Species Rank to Six Reproductively Isolated Cryptic Species of the Phialocephala fortinii s.l.- Acephala applanata Species Complex. Mycologia. 2008;100:47–67. doi: 10.1080/15572536.2008.11832498. [DOI] [PubMed] [Google Scholar]
- 15.Sieber T., Grünig C.R. Fungal Root Endophytes. In: Eshel A., Beeckman T., editors. Plant Roots-The Hidden Half. CRC Press; Taylor and Francis Group; Boca Raton, FL, USA: 2013. pp. 38.31–38.49. [Google Scholar]
- 16.Grünig C.R., McDonald B.A., Sieber T.N., Rogers S.O., Holdenrieder O. Evidence for Subdivision of the Root-Endophyte Phialocephala fortinii into Cryptic Species and Recombination within Species. Fungal Genet. Biol. 2004;41:676–687. doi: 10.1016/j.fgb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Jumpponen A. Spatial Distribution of Discrete RAPD Phenotypes of a Root Endophytic fungus, Phialocephala fortinii, at a Primary Successional Site on a Glacier Forefront. New Phytol. 1999;141:333–344. doi: 10.1046/j.1469-8137.1999.00344.x. [DOI] [PubMed] [Google Scholar]
- 18.Addy H.D., Hambleton S., Currah R.S. Distribution and Molecular Characterization of the Root Endophyte Phialocephala fortinii along an Environmental Gradient in the Boreal Forest of Alberta. Mycol. Res. 2000;104:1213–1221. doi: 10.1017/S0953756200002896. [DOI] [Google Scholar]
- 19.Ruotsalainen A.L. Dark Septate Endophytes (DSE) in Boreal and Subarctic Forests. In: Pirttilä A.M., Frank A.C., editors. Endophytes of Forest Trees: Biology and Applications. Springer International Publishing; Cham, Switzerland: 2018. pp. 105–117. [Google Scholar]
- 20.Mandyam K., Jumpponen A. Seeking the Elusive Function of the Root-Colonising Dark Septate Endophytic Fungi. Stud. Mycol. 2005;53:173–189. doi: 10.3114/sim.53.1.173. [DOI] [Google Scholar]
- 21.Spagnoletti F.N., Tobar N.E., Fernández Di Pardo A., Chiocchio V.M., Lavado R.S. Dark Septate Endophytes Present Different Potential to Solubilize Calcium, Iron and Aluminum Phosphates. Appl. Soil Ecol. 2017;111:25–32. doi: 10.1016/j.apsoil.2016.11.010. [DOI] [Google Scholar]
- 22.Spagnoletti F.N., Chiocchio V.M. Tolerance of Dark Septate Endophytic Fungi (DSE) to Agrochemicals in Vitro. Rev. Argent. Microbiol. 2020;52:43–49. doi: 10.1016/j.ram.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Vergara C., Araujo K.E.C., Alves L.S., de Souza S.R., Santos L.A., Santa-Catarina C., da Silva K., Pereira G.M.D., Xavier G.R., Zilli J.É. Contribution of Dark Septate Fungi to the Nutrient Uptake and Growth of Rice Plants. Braz. J. Microbiol. 2018;49:67–78. doi: 10.1016/j.bjm.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J., Li T., Liu G., Smith J.M., Zhao Z. Unraveling the Role of Dark Septate Endophyte (DSE) Colonizing Maize (Zea Mays) under Cadmium Stress: Physiological, Cytological and Genic Aspects. Sci. Rep. 2016;6:22028. doi: 10.1038/srep22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C.J.K., Wilcox H.E. New Species of Ectendomycorrhizal and Pseudomycorrhizal Fungi: Phialophora finlandia, Chloridium paucisporum, and Phialocephala fortinii. Mycologia. 1985;77:951–958. doi: 10.1080/00275514.1985.12025185. [DOI] [Google Scholar]
- 26.Grünig C.R., Sieber T.N., Rogers S.O., Holdenrieder O. Spatial Distribution of Dark Septate Endophytes in a Confined Forest Plot. Mycol. Res. 2002;106:832–840. doi: 10.1017/S0953756202005968. [DOI] [Google Scholar]
- 27.Summerbell R.C. Root Endophyte and Mycorrhizosphere Fungi of Black Spruce, Picea Mariana, in a Boreal Forest Habitat: Influence of Site Factors on Fungal Distributions. Stud. Mycol. 2005;53:121–145. doi: 10.3114/sim.53.1.121. [DOI] [Google Scholar]
- 28.Sieber T.N., Grünig C.R. Biodiversity of Fungal Root-Endophyte Communities and Populations, in Particular of the Dark Septate Endophyte Phialocephala fortinii s. l. In: Schulz B.J.E., Boyle C.J.C., Sieber T.N., editors. Microbial Root Endophytes. Volume 9. Springer; Berlin/Heidelberg, Germany: 2006. pp. 107–132. [Google Scholar]
- 29.Read D.J., Haselwandter K. Observations on the Mycorrhizal Status of Some Alpine Plant Communities. New Phytol. 1981;88:341–352. doi: 10.1111/j.1469-8137.1981.tb01729.x. [DOI] [Google Scholar]
- 30.Grünig C.R., Linde C.C., Sieber T.N., Rogers S.O. Development of Single-Copy RFLP Markers for Population Genetic Studies of Phialocephala fortinii and Closely Related Taxa. Mycol. Res. 2003;107:1332–1341. doi: 10.1017/S0953756203008669. [DOI] [PubMed] [Google Scholar]
- 31.Grünig C.R., Sieber T.N. Molecular and Phenotypic Description of the Widespread Root Symbiont Acephala Applanata Gen. et Sp. Nov., Formerly Known as Dark-Septate Endophyte Type 1. Mycologia. 2005;97:628–640. doi: 10.1080/15572536.2006.11832794. [DOI] [PubMed] [Google Scholar]
- 32.Grünig C.R., Brunner P.C., Duò A., Sieber T.N. Suitability of Methods for Species Recognition in the Phialocephala fortinii–Acephala applanata Species Complex Using DNA Analysis. Fungal Genet. Biol. 2007;44:773–788. doi: 10.1016/j.fgb.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Queloz V., Duò A., Grünig C.R. Isolation and Characterization of Microsatellite Markers for the Tree-Root Endophytes Phialocephala subalpina and Phialocephala fortinii s.s. Mol. Ecol. Resour. 2008;8:1322–1325. doi: 10.1111/j.1755-0998.2008.02279.x. [DOI] [PubMed] [Google Scholar]
- 34.Landolt M., Stroheker S., Queloz V., Gall A., Sieber T.N. Does Water Availability Influence the Abundance of Species of the Phialocephala fortinii s.l.–Acephala applanata Complex (PAC) in Roots of Pubescent Oak (Quercus Pubescens) and Scots Pine (Pinus Sylvestris)? Fungal Ecol. 2020;44:100904. doi: 10.1016/j.funeco.2019.100904. [DOI] [Google Scholar]
- 35.Queloz V., Grunig C.R., Sieber T.N., Holdenrieder O. Monitoring the Spatial and Temporal Dynamics of a Community of the Tree-Root Endophyte Phialocephala fortinii s.l. New Phytol. 2005;168:651–660. doi: 10.1111/j.1469-8137.2005.01529.x. [DOI] [PubMed] [Google Scholar]
- 36.Queloz V., Sieber T.N., Holdenrieder O., McDonald B.A., Grünig C.R. No Biogeographical Pattern for a Root-Associated Fungal Species Complex: Biogeography of a Fungal Species Complex. Glob. Ecol. Biogeogr. 2011;20:160–169. doi: 10.1111/j.1466-8238.2010.00589.x. [DOI] [Google Scholar]
- 37.Stroheker S., Queloz V., Sieber T.N. Spatial and Temporal Dynamics in the Phialocephala fortinii s.l.–Acephala applanata Species Complex (PAC) Plant Soil. 2016;407:231–241. doi: 10.1007/s11104-015-2790-0. [DOI] [Google Scholar]
- 38.Grünig C.R., Duò A., Sieber T.N. Population Genetic Analysis of Phialocephala fortinii s.l. and Acephala applanata in Two Undisturbed Forests in Switzerland and Evidence for New Cryptic Species. Fungal Genet. Biol. 2006;43:410–421. doi: 10.1016/j.fgb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Brenn N., Menkis A., Grünig C.R., Sieber T.N., Holdenrieder O. Community Structure of Phialocephala fortinii s. Lat. in European Tree Nurseries, and Assessment of the Potential of the Seedlings as Dissemination Vehicles. Mycol. Res. 2008;112:650–662. doi: 10.1016/j.mycres.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Stroheker S., Dubach V., Queloz V., Sieber T.N. Resilience of Phialocephala fortinii s.l.–Acephala applanata Communities–Effects of Disturbance and Strain Introduction. Fungal Ecol. 2018;31:19–28. doi: 10.1016/j.funeco.2017.10.006. [DOI] [Google Scholar]
- 41.Halmschlager E., Kowalski T. The Mycobiota in Nonmycorrhizal Roots of Healthy and Declining Oaks. Can. J. Bot. 2004;82:1446–1458. doi: 10.1139/b04-101. [DOI] [Google Scholar]
- 42.Zheng J.-H., Meng Z.-B., Kang J.-C., Lei B.-X., Li Q.-R., Wen T.C. Diversity of Endophytic Fungi Associated with Ginkgo Biloba. Mycosystema. 2013;32:671–681. [Google Scholar]
- 43.Haddadderafshi N., Halasz K., Posa T., Peter G., Gaspar L., Lukacs N. Diversity of Endophytic Fungi Isolated from Cherry (Prunus Avium) J. Hortic. For. Biotechnol. 2011;15:1–6. [Google Scholar]
- 44.Queloz V., Duò A., Sieber T.N., Grünig C.R. Microsatellite Size Homoplasies and Null Alleles Do Not Affect Species Diagnosis and Population Genetic Analysis in a Fungal Species Complex. Mol. Ecol. Resour. 2010;10:348–367. doi: 10.1111/j.1755-0998.2009.02757.x. [DOI] [PubMed] [Google Scholar]
- 45.Piry S., Alapetite A., Cornuet J.-M., Paetkau D., Baudouin L., Estoup A. GENECLASS2: A Software for Genetic Assignment and First-Generation Migrant Detection. J. Hered. 2004;95:536–539. doi: 10.1093/jhered/esh074. [DOI] [PubMed] [Google Scholar]
- 46.White T.J., Bruns T.D., Lee S., Taylor S. PCR-Protocols and Applications-A Laboratory Manual. Academic Press, Inc.; New York, NY, USA: 1990. Amplification and direct sequencing of fungal ribosomal RNA Genes for phylogenetics; pp. 315–322. [Google Scholar]
- 47.Queloz V., Grünig C.R., Berndt R., Kowalski T., Sieber T.N., Holdenrieder O. Cryptic Speciation in Hymenoscyphus Albidus: Speciation in Hymenoscyphus Albidus. For. Pathol. 2011;41:133–142. doi: 10.1111/j.1439-0329.2010.00645.x. [DOI] [Google Scholar]
- 48.Mann H.B., Whitney D.R. On a Test of Whether One of Two Random Variables Is Stochastically Larger than the Other. Ann. Math. Statist. 1947;18:50–60. doi: 10.1214/aoms/1177730491. [DOI] [Google Scholar]
- 49.Wilcoxon F. Individual Comparisons by Ranking Methods. Biom. Bull. 1945;1:80. doi: 10.2307/3001968. [DOI] [Google Scholar]
- 50.Menkis A., Allmer J., Vasiliauskas R., Lygis V., Stenlid J., Finlay R. Ecology and Molecular Characterization of Dark Septate Fungi from Roots, Living Stems, Coarse and Fine Woody Debris. Mycol. Res. 2004;108:965–973. doi: 10.1017/S0953756204000668. [DOI] [PubMed] [Google Scholar]
- 51.Stroheker S. Ph.D. Thesis. ETH Zurich; Zürich, Switzerland: 2017. Community Dynamics and Transmission within the Phialocephala fortinii s.l.–Acephala applanata Species Complex. [Google Scholar]
- 52.Queloz V. La face cachée du Creux du Van. Société Jura. d’Émulation 2007. 2008;11:47–68. [Google Scholar]
- 53.Tedersoo L., Sadam A., Zambrano M., Valencia R., Bahram M. Low Diversity and High Host Preference of Ectomycorrhizal Fungi in Western Amazonia, a Neotropical Biodiversity Hotspot. ISME J. 2010;4:465–471. doi: 10.1038/ismej.2009.131. [DOI] [PubMed] [Google Scholar]
- 54.Dickie I.A. Host Preference, Niches and Fungal Diversity. New Phytol. 2007;174:230–233. doi: 10.1111/j.1469-8137.2007.02055.x. [DOI] [PubMed] [Google Scholar]
- 55.Otgonsuren B., Lee M.-J. Pinus Sylvestris Can Form Ectomycorrhiza with Phialocephala fortinii. Taiwan J. For. Sci. 2012;27:265–281. [Google Scholar]
- 56.Münzenberger B., Bubner B., Wöllecke J., Sieber T.N., Bauer R., Fladung M., Hüttl R.F. The Ectomycorrhizal Morphotype Pinirhiza Sclerotia Is Formed by Acephala macrosclerotiorum Sp. Nov., a Close Relative of Phialocephala fortinii. Mycorrhiza. 2009;19:481–492. doi: 10.1007/s00572-009-0239-0. [DOI] [PubMed] [Google Scholar]
- 57.Currah R.S., Sigler L., Hambleton S. New Records and New Taxa of Fungi from the Mycorrhizae of Terrestrial Orchids of Alberta. Can. J. Bot. 1987;65:2473–2482. doi: 10.1139/b87-336. [DOI] [Google Scholar]
- 58.Kohout P., Sýkorová Z., Čtvrtlíková M., Rydlová J., Suda J., Vohník M., Sudová R. Surprising Spectra of Root-Associated Fungi in Submerged Aquatic Plants. FEMS Microbiol. Ecol. 2012;80:216–235. doi: 10.1111/j.1574-6941.2011.01291.x. [DOI] [PubMed] [Google Scholar]
- 59.Wang L., Shen J., Xu L., Gao J., Zhang C., Wang Y., Chen F. A Metabolite of Endophytic Fungus Cadophora Orchidicola from Kalimeris Indica Serves as a Potential Fungicide and TLR 4 Agonist. J. Appl. Microbiol. 2019;126:1383–1390. doi: 10.1111/jam.14239. [DOI] [PubMed] [Google Scholar]
- 60.Upson R., Newsham K.K., Bridge P.D., Pearce D.A., Read D.J. Taxonomic Affinities of Dark Septate Root Endophytes of Colobanthus Quitensis and Deschampsia Antarctica, the Two Native Antarctic Vascular Plant Species. Fungal Ecol. 2009;2:184–196. doi: 10.1016/j.funeco.2009.02.004. [DOI] [Google Scholar]
- 61.Fernando A., Currah R.S. Leptodontidium Orchidicola (Mycelium-Radicis-Atrovirens Complex)–Aspects of Its Conidiogenesis and Ecology. Mycotaxon. 1995;54:287–294. [Google Scholar]
- 62.Wu L., Guo S. Interaction between an Isolate of Dark-Septate Fungi and Its Host Plant Saussurea Involucrata. Mycorrhiza. 2008;18:79–85. doi: 10.1007/s00572-007-0159-9. [DOI] [PubMed] [Google Scholar]
- 63.Domsch K., Gams W., Anderson T.-H. Compendium of Soil Fungi. 2nd ed. IHW; Eching, Germany: 2007. [Google Scholar]
- 64.Kowalski S. Role of Mycorrhiza and Soil Fungi in Natural Regeneration of Fir (Abies Alba Mill.) in Polish Carpathians and Sudetes. For. Pathol. 1982;12:107–112. doi: 10.1111/j.1439-0329.1982.tb01380.x. [DOI] [Google Scholar]
- 65.Unestam T., Beyer-Ericson L., Strand M. Involvement of Cylindrocarpon Destructans in Root Death of Pinus Sylvestris Seedlings: Pathogenic Behaviour and Predisposing Factors. Scand. J. For. Res. 1989;4:521–535. doi: 10.1080/02827588909382585. [DOI] [Google Scholar]
- 66.Booth C. The Genus Cylindrocarpon. Mycol. Pap. 1966;104:1–56. [Google Scholar]
- 67.Lyr H., Kluge E. Zusammenhänge Zwischen Pathogenität, Enzym- Und Toxinproduktion Bei Cylindrocarpon Radicicola. J. Phytopathol. 1968;62:220–231. doi: 10.1111/j.1439-0434.1968.tb03036.x. [DOI] [Google Scholar]
- 68.Seifert K.A., McMullen C.R., Yee D., Reeleder R.D., Dobinson K.F. Molecular Differentiation and Detection of Ginseng-Adapted Isolates of the Root Rot Fungus Cylindrocarpon Destructans. Phytopathology. 2003;93:1533–1542. doi: 10.1094/PHYTO.2003.93.12.1533. [DOI] [PubMed] [Google Scholar]
- 69.Kowalski T., Bartnik C. Cryptosporiopsis radicicola Sp. Nov. from Roots of Quercus Robur. Mycol. Res. 1995;99:663–666. doi: 10.1016/S0953-7562(09)80524-8. [DOI] [Google Scholar]
- 70.Kowalski T., Halmschlager E., Schrader K. Cryptosporiopsis melanigena Sp. Nov., a Root-Inhabiting Fungus of Quercus Robur and Q. Petraea. Mycol. Res. 1998;102:347–354. doi: 10.1017/S0953756297004991. [DOI] [Google Scholar]
- 71.Sigler L., Allan T., Lim S.R., Berch S., Berbee M. Two New Cryptosporiopsis Species from Roots of Ericaceous Hosts in Western North America. Stud. Mycol. 2005;53:53–62. doi: 10.3114/sim.53.1.53. [DOI] [Google Scholar]
- 72.Sieber T.N. Fungal Root Endophytes. In: Waisel Y., Eshel A., Kafkafi U., editors. Plant Roots: The Hidden Half. Marcel Dekker; New York, NY, USA: Basel, Switzerland: 2002. [Google Scholar]
- 73.Tellenbach C., Sieber T.N. Do Colonization by Dark Septate Endophytes and Elevated Temperature Affect Pathogenicity of Oomycetes? FEMS Microbiol. Ecol. 2012;82:157–168. doi: 10.1111/j.1574-6941.2012.01415.x. [DOI] [PubMed] [Google Scholar]
- 74.Miles L.A., Lopera C.A., Gonzalez S., Cepero de Garcìa M., Franco A., Restrepo S. Exploring the Biocontrol Potential of Fungal Endophytes from an Andean Colombian Paramo Ecosystem. BioControl. 2012;57:697–710. doi: 10.1007/s10526-012-9442-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.