Figure 7.
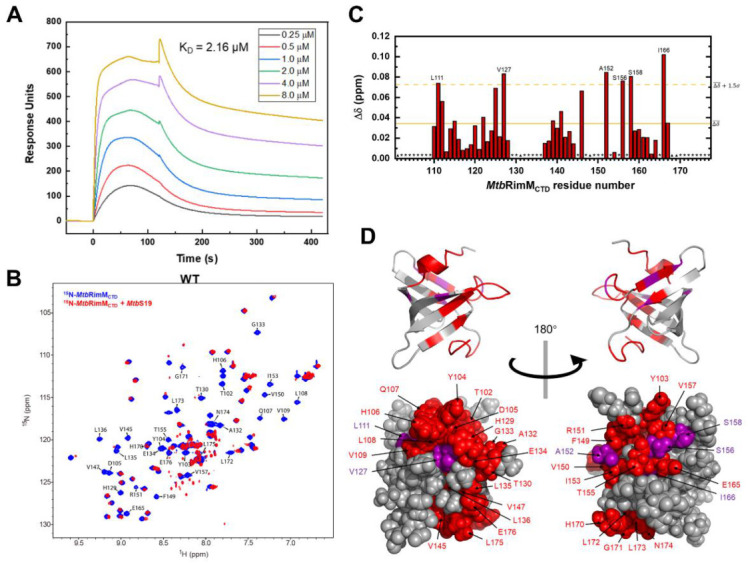
Interaction between MtbRimMCTD and MtbS19. (A) Surface plasmon resonance (SPR) affinity assay of MtbRimMCTD binding S19 at serial concentrations. Blank control had been deducted from the serial data. (B) Overlapped 1H-15N heteronuclear singular quantum correlation (HSQC) spectra of 15N-labeled MtbRimMCTD alone (blue) and in presence of equimolar MtbS19 (red) for NMR titration assay. Peaks experiencing broadening-induced disappearance are indicated. (C) Plot of chemical shift perturbations (CSPs, Δδ) of backbone amide groups. The mean value is indicated by a solid line, and the mean value plus 1.5 standard deviations by a dashed line. Asterisks indicate residues with disappear peaks at the titration destination, while triangles denote residues with invisible resonances before the titration, including D101, A131, and three prolines (residues 148, 168, and 169). (D) Mapping the binding surface to the 3D structure of MtbRimMCTD. Disappeared peaks are colored in red, and peaks with large CSPs (above the dashed line in (C)) in purple. Upper and lower panels are cartoon and sphere depictions of the structure, respectively.
