Abstract
Intestinal health relies on the association between the mucosal immune system, intestinal barrier and gut microbiota. Bioactive components that affect the gut microbiota composition, epithelial physical barrier and intestinal morphology were previously studied. The current systematic review evaluated evidence of anthocyanin effects and the ability to improve gut microbiota composition, their metabolites and parameters of the physical barrier; this was conducted in order to answer the question: “Does food source or extract of anthocyanin promote changes on intestinal parameters?”. The data analysis was conducted following the PRISMA guidelines with the search performed at PubMed, Cochrane and Scopus databases for experimental studies, and the risk of bias was assessed by the SYRCLE tool. Twenty-seven studies performed in animal models were included, and evaluated for limitations in heterogeneity, methodologies, absence of information regarding allocation process and investigators’ blinding. The data were analyzed, and the anthocyanin supplementation demonstrated positive effects on intestinal health. The main results identified were an increase of Bacteroidetes and a decrease of Firmicutes, an increase of short chain fatty acids production, a decrease of intestinal pH and intestinal permeability, an increase of the number of goblet cells and tight junction proteins and villi improvement in length or height. Thus, the anthocyanin supplementation has a potential effect to improve the intestinal health. PROSPERO (CRD42020204835).
Keywords: microbiota, polyphenols, short chain fatty acids, intestinal barrier
1. Introduction
A healthy gut includes multiple positive aspects of the gastrointestinal (GI) tract, specifically, effective digestive and absorptive functions of the intestinal brush border membrane, and the absence of GI chronic conditions such as enzyme deficiencies, intestinal bower disease, coeliac disease, colorectal cancer and others. In addition, well-balanced intestinal microbiota was associated with an effective immune system function that is required to maintain the host homeostasis [1]. The intestinal microbiota consists of more than a trillion microorganisms that establish a symbiotic relationship with their host. These microorganisms prevent the colonization of potentially pathogenic microorganisms and regulate the mucosal immune system, and thus assist to maintain an intact intestinal barrier [2]. Thus, the physical barrier of epithelial cells and mucus layer provide the first line of defense by mechanisms such as microbial recognition, antibodies secretion, antimicrobial peptides and mucus production [1]. Therefore, impairments of the physical barrier may enhance the risk of infections, inflammatory intestinal diseases and other diseases occurring outside the intestine such as immune-related and metabolic disorders [1,3]. In this context, certain food and plant origin bioactive compounds were investigated to reduce the risk of the mentioned diseases by acting beneficially on the intestinal health.
Anthocyanins are bioactive water-soluble plant pigments that are responsible for bright colors, such as purple, red and blue, which are presented mainly as glycosides, with the basic structure consisting of an anthocyanidin core attached to sugars and organic acids [4]. The positive effects of anthocyanins and anthocyanin-rich foods are widely described in the literature. These effects are mainly associated with reduced risk of diseases associated with oxidative stress, such as cardiovascular disease [5] and inflammatory diseases such as diabetes mellitus [6], obesity [7] and insulin resistance [8]. In addition, the health-promoting effects attributed to anthocyanins were shown to be associated with the gut microbiota modulation [9].
Dietary anthocyanins undergo a specific metabolism with the absorption rate depending on their structure. Briefly, anthocyanins cross the gastric mucosa in their intact form. Thereafter, in the small intestine, mainly in the jejunum, they are absorbed by hydrolytic enzymes as phenolic aglycone. The unabsorbed anthocyanins reach the colon, and are metabolized by the colon microbiota, especially by genera and species that are equipped with enzymes such as β-glucosidase, which are necessary to catalyze the reaction. Intestinal bacteria such as Bifidobacterium spp. and Lactobacillus spp. possess these enzymes; thus, the anthocyanin metabolism by microbiota and/or their metabolites can modulate the growth of these specific bacteria [9,10,11]. In this sense, the modulation of gut microbiota by anthocyanin increases the short chain fatty acids (SCFA) producing bacteria, which acidifies the intestinal pH and inhibits the pathogenic bacteria proliferation, and SCFA as butyrate act as a fuel to provide energy for epithelial cells, thus improving the intestinal barrier to avoid the translocation of pathogens and antigens [12]. Thereby, it is suggested that the potential beneficial functions of anthocyanins could be indirectly attributed to the gut microbiota modulation and consequent production of metabolites due to bacterial fermentation activities, which improve several parameters related to the intestinal health [9,13].
Despite the several positive effects of anthocyanins, there is no consensus in the literature regarding their mechanisms of action on intestinal health in experimental studies. Recently, a systematic review (n = 6 studies: 3 in vitro, 2 animals and 1 human trial) verified the effects of anthocyanin supplementation on the gut microbiota composition, showing the proliferation of healthy anaerobic bacterial population and inhibition of pathogenic species [14]. However, a healthy gut is maintained by a set of parameters related to the metabolites of microbial bacteria, intestinal cells’ integrity and the physical barrier [1]. Therefore, the objective of the current systematic review was to investigate the effects of anthocyanins on several parameters of intestinal health in experimental studies, in order to understand the mechanism in which these parameters act in association. It is hypothesized that the supplementation of anthocyanin promotes beneficial changes to the gut microbiota with increased production of metabolites associated with intestinal barrier improvement, which contributes to a healthy gut.
2. Materials and Methods
2.1. Eligibility Criteria
The eligibility criteria were based on the PICOS (population, intervention, comparison, outcomes and study design) model strategy. Duplicate studies were excluded, and the search and screening for titles and abstracts were carried out independently by the authors according to the inclusion and exclusion criteria (Table 1).
Table 1.
PICOS criteria for inclusion and exclusion of studies.
| Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | In vivo animal studies | Clinical studies and in vitro studies |
| Intervention | Intake of foods’ source of anthocyanin or supplementation with extract of anthocyanin | Anthocyanin associated with other foods or not measured |
| Comparator | Negative control (without the intervention) | No control group |
| Outcomes | Changes in the gut microbiota composition, intraluminal pH, short chain fatty acids, histological parameters of small and large intestine, gene expression of tight junction’s proteins, gene expression of intestinal brush border membrane, integrity of intestinal barrier and intestinal permeability | |
| Study design | Experimental placebo-controlled studies | Review articles, clinical studies, theses, dissertations, book chapters, in vitro experiments and studies published in other languages than English. |
2.2. Information Source
Two researchers independently searched for original articles. PubMed, Cochrane and Scopus were used to search studies performed with animal models that evaluated the effects of anthocyanin on the intestinal health. No time restriction was used. The descriptors were identified based on Medical Subject Headings (MeSH).
2.3. Search Strategy
The following English search terms were used: (Anthocyanin OR Anthocyanidin OR Anthocyanidins OR Cyanidin OR Delphinidin OR Malvidin OR Peonidin OR Pelargonidin OR Petunidin) AND (intestinal OR gut). Only articles published in English were considered in this review. The last search was performed on 2 June 2020. The first selection of the studies was based on the title and abstract. We excluded review articles, clinical studies, theses, dissertations, book chapters, in vitro experiments and studies published in other languages than English. Further, we excluded studies in which the intake of anthocyanin was associated with other foods, or if the anthocyanin was not measured. Studies were eligible for inclusion if they fulfilled the following criteria: (a) studies conducted with animals; (b) the intervention was the intake of foods’ sources of anthocyanin or supplementation with an extract of anthocyanin; (c) the comparator was the negative control (without the intervention); (d) the outcomes searched were changes related to the intestinal health, mainly: changes in the gut microbiota composition, intraluminal pH, short chain fatty acids, histological parameters of small and large intestine, gene expression of tight junction’s proteins, gene expression of intestinal brush border membrane, integrity of intestinal barrier and intestinal permeability.
2.4. Selection, Data Collection Process and Data Items
After reading and reviewing the selected research articles in full, the data were compared to ensure integrity and reliability. Divergent decisions were resolved by consensus. The eligible outcomes evaluated were broadly categorized as follows:
-
-
Gut microbiota: short chain fatty acids (caecal, fecal or in the serum); intraluminal pH (ileal, caecal or feces); microbial quantification; secretory immunoglobulin A (sIgA);
-
-
Epithelial physical barrier: tight junction proteins; proteins of intestinal brush border membrane; intestinal permeability; plasm endotoxin;
-
-
Intestinal morphology: number of goblet cells; length, height and depth of villi and crypts; mucin secretion; antimicrobial peptides.
Any measure and methodology of these outcomes was eligible for inclusion.
Further, for each experimental study included, we reported relevant information related to the authors, publication year, country of publication and experimental model features such as animal model, age, sex, initial weight, number of groups and animal per group. To access the research methods, we extracted specific information related to the experimental groups such as type of food intervention, type of diet and control group. For the control test of food intake, we extracted information related to the method of administration that was used in the intervention, the duration of the intervention, the dosage of anthocyanin and main results (control x intervention).
For this review, data from the eligible studies are expressed in tables and figures. We provided a narrative synthesis of the results according to the main characteristics and results.
2.5. Study Risk-of-Bias Assessment
The methodological quality of the included studies was assessed, and the risk of bias was verified using the Systematic Review Centre for Laboratory Animal Experimentation Risk of Bias (SYRCLE RoB) tool [15], which is responsible for identifying study quality and measuring the bias in research involving animal studies [16]. The SYRCLE RoB toll considers ten entries that are related to six types of bias: selection bias, performance bias, detection bias, attrition bias, reporting bias and other. For each included study, the six bias types were classified as “high” (+), “low” (−) or “unclear” (?).
3. Results
3.1. Study Selection
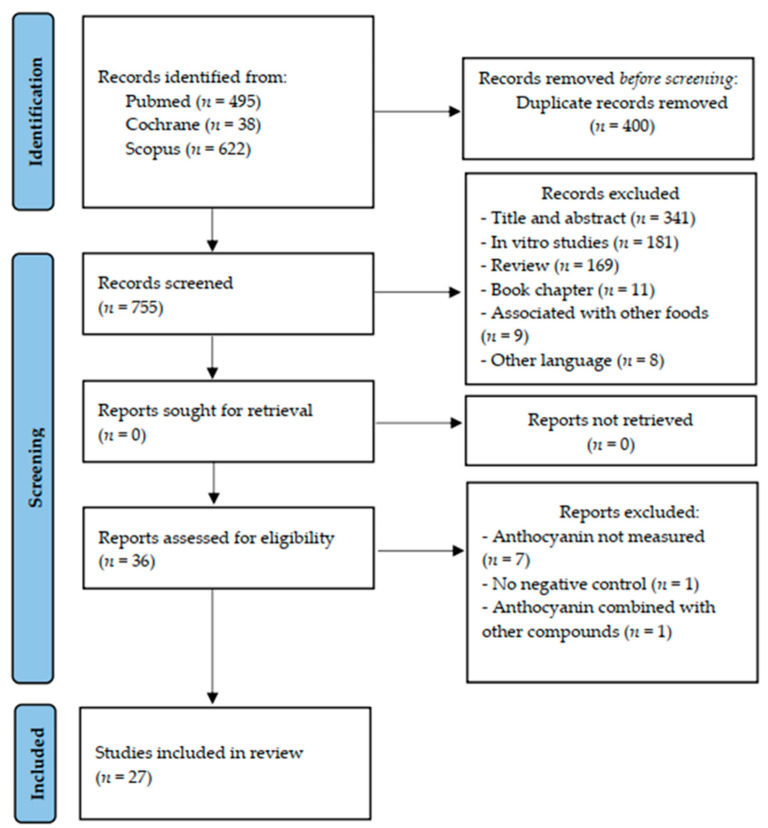
The flow diagram of the literature search and selection process was built in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (Figure 1). After the search in the selected databases, we identified 1155 articles (x = 495 Pubmed; x = 38 Cochrane and x = 622 Scopus). From these, 1117 were excluded for the following reasons: duplicate studies (n = 400), title and abstract not suited to the topic (n = 341), in vitro studies (n = 181), review articles (n = 169), book chapters (n = 11), supplementation of anthocyanin associated with other foods (n = 9), and other languages than English used (n = 8). After, 36 articles were read fully. From these, we excluded nine articles: total of anthocyanin not measured (n = 7); there was no negative control without intervention (n = 1); and anthocyanins were combined with other compounds (n = 1). Therefore, 27 studies were included in this systematic review.
Figure 1.
Flowchart of the search for articles included in the systematic review, according to PRISMA (2020) recommendation.
3.2. Study Characteristics
The included studies (n = 27) were performed in ten different countries. Most of them were conducted in China (n = 9) [17,18,19,20,21,22,23,24,25] or the United States of America (U.S.A.) (n = 5) [26,27,28,29,30]. Regarding the animal model used in the studies, 15 were performed with mice [17,20,21,22,24,25,26,27,28,29,30,31,32,33,34], 11 with rats [18,19,23,35,36,37,38,39,40,41,42] and only 1 with rabbits [43]. Most studies used male animals (n = 24) [17,18,20,21,22,23,24,25,26,27,28,29,31,33,34,35,36,37,38,39,40,41,42,43], but two used female animals [19,30], and only one study used both male and female animals [32]. Interesting, six studies did not describe animals’ initial weight [19,21,28,30,32,38]. The age of the animals ranged from 3 to 72 weeks, although five studies did not mention this information [17,27,35,36,39]. The studies’ main characteristics were chronologically organized by the publication year, starting with the first published (Table 2).
Table 2.
Characteristics of animal studies on the effects of anthocyanin on intestinal health.
| Author, Year | Country | Animal Model/Age | Sex | Initial Weight (g) | Nº of Groups | Nº of Animals/Groups |
|---|---|---|---|---|---|---|
| Jurgonski, Juskiewicz, Zdunczyk, 2008 [35] | Poland | Wistar rats/NS | Male | 161 ± 8 | 3 | 8 |
| Jurgonski, Juskiewicz, Zdunczyk, 2013 [36] | Poland | Wistar rats/NS | Male | 548 ± 36 | 3 | 8 |
| Espley, et al., 2014 [31] | New Zealand | Swiss mice/6–7 wk | Male | 30 | 3 | 10 |
| Jurgonski, et al., 2014 [43] | Poland | White rabbits/34 days | Male | 631 ± 26 | 4 | 5 |
| Paivarinta, et al., 2016 [32] | Finland | C57BL/6J Apcmim mice/5 wk | Male and female | NS | 3 | 5–6 male and 4–6 female |
| Overall, et al., 2017 [26] | U.S.A. | C57BL/6J mice/6 wk | Male | 20–30 | 8 | 12 or 8 |
| Tong, et al., 2017 [17] | China | Kunming mice/NS | Male | 22 ± 5 | 5 | 10 |
| Fernández, et al., 2018 [37] | Spain | Fischer 344 rats/5wk | Male | 200–270 | 3 | 10 |
| Jamar, et al., 2018 [38] | Brazil | Wistar rats/90 days | Male | NS | 3 | 7 |
| Lee, et al., 2018 [39] | Georgia | Wistar rats/NS | Male | 200–220 | 3 | 8 |
| Paturi, et al., 2018 [40] | New Zealand | Sprague-Dawley rats/3 wk | Male | 256–265 | 8 | 16 |
| Silva-Maia, et al., 2018 [41] | Brazil | Wistar rats/3 wks | Male | 0–100 | 3 | 5 or 8 |
| Van Hul, et al., 2018 [33] | France | C57BL/6J mice/9 wk | Male | 25–30 | 4 | 14 |
| Chen, et al., 2019 [18] | China | Wistar rats/13 wks | Male | 403 ± 4 | 5 | 8 |
| Cremonini, et al., 2019 [27] | U.S.A. | C57BL/6J mice/NS | Male | 20–25 | 4 | 10 |
| Gu, et al., 2019 [28] | U.S.A. | C57BL/6J mice/4 wk | Male | NS | 2 | 12 or 14 |
| Li, et al., 2019 [19] | China | SD rats/4 and 12 months | Female | NS | 6 | 10 |
| Liu, et al., 2019 [29] | U.S.A. | C57BL/6J mice/4–5 wk | Male | 18–21 | 3 | 9 or 10 |
| Luo, et al., 2019 [23] | China | Sprague-Dawley rats/4 wk | Male | 100–120 | 6 | 8 |
| Peng, et al., 2019 [20] | China | C57BL/6J mice/5 wk | Male | 21–24 | 4 | 10 |
| Su, et al., 2019 [21] | China | db/db mice C57BL/6J/6 wk | Male | NS | 2 | 12 |
| Tian, et al., 2019 [22] | China | C57BL/6J mice/4 wk | Male | 15–18 | 6 | 11 |
| Zary-Sikorska, et al., 2019 [42] | Poland | Wistar rats/13 wk | Male | 146 ± 1.051 | 5 | 8 |
| Cao, et al., 2020 [30] | U.S.A. | C57BL/6J mice/3–18 months | Female | NS | 4 | 3 |
| Peng, et al., 2020 [24] | China | C57BL/6J mice/5 wk | Male | 20–24 | 2 | 10 |
| Rodríguez-Daza, et al., 2020 [34] | Canada | C57BL/6J mice/6 wk | Male | 20–25 | 6 | 12 |
| Wang, et al., 2020 [25] | China | C57BL/6J mice/6 wk | Male | 19–20 | 5 | 12 |
NS: not specified; wk: weeks; U.S.A.: United States of America; Apc.: adenomatous polyposis coli.
The anthocyanin intervention varied by the source that was used. In 20 studies, diverse fruits were offered in the form of extract (n = 12) [17,19,25,30,32,33,34,35,36,40,41,43], or as a powder (n = 8) [22,26,28,31,37,38,39,42]. The fruits used as an anthocyanin source were chokeberry, Kamchatka berry, apple, blackcurrant, bilberry, blueberry, black raspberry, red cabbage, grape, berry juçara, jabuticaba, purple carrot, black gojy berry and black rice. In four studies, the intervention was with purified anthocyanin [20,23,24,27], and in three studies with monomeric anthocyanidins as cyanidin-3-O-glucoside [18], malvindin-3-glucoside [29] and pelargonidin-3-O-glucoside [21].
The form of anthocyanin administration was oral in all included studies, with the form of administration by addition in the diet (n = 20) [18,22,24,25,26,27,28,29,30,31,32,33,35,36,37,38,39,40,42,43], or via gavage (n = 5) [17,19,21,23,34] or drinking water (n = 2) [20,41]. The duration of interventions ranged widely from 1 to 20 weeks. Regarding the anthocyanin dosage, the doses observed varied from 12.9 mg/100 g diet [42] to 1280 mg/100 g diet [40], and 1.68 mg/kg body weight (BW) [34] to 200 mg/kg BW [20,24]. In addition, only one study offered a aqueous extract in the dose of 75 mg/L [41] (Table 3).
Table 3.
Main findings in animal studies on the effects of anthocyanin on intestinal health.
| Reference | Design (Intervention) | Control | Administration/Duration of Intervention (Weeks) | Method of Gut Microbiota Evaluation/Type of Sample | Anthocyanin Dosage (Total Anthocyanin) | Main Results (Intervention × Control) |
|---|---|---|---|---|---|---|
| Jurgonski, Juskiewicz, Zdunczyk, 2008 [35] | Chokeberry fruit extract (0.2%) + High fructose diet and streptozotocin | High fructose diet and streptozotocin | Oral (diet)/4 | NA | 80.9 mg/100 g diet | ↓ ileal pH; Mucosal disaccharidase activity: ↓ sucrase and maltase and ↑ lactase;  Total SCFA; Total SCFA;  cecum pH; cecum pH;  α- and β- glucosidase, α- and β- galactosidase and β-glucuronidase on cecum. α- and β- glucosidase, α- and β- galactosidase and β-glucuronidase on cecum. |
| Jurgonski, Juskiewicz, Zdunczyk, 2013 [36] | Kamchatka berry extract (2g/kg diet) + Diet with fructose replaced the corn starch | Diet with fructose replaced the corn starch | Oral (diet)/4 | NA | 65.4 mg/100 g diet | Mucosal disaccharidase activity:  sucrase, maltase and lactase; sucrase, maltase and lactase;  cecum pH; cecum pH; ↑ α- and β- glucosidase on cecum;  α- and β- galactosidase on cecum; α- and β- galactosidase on cecum;  ileal pH. ileal pH. |
| Espley, et al., 2014 [31] | Freeze-dried apple (20%) + Normal diet | Normal diet | Oral (diet)/3 | qPCR Colonic content |
397 µg/g diet * | ↑ Total bacteria; ↓ Lactobacillus spp.;  Bifidobacterium spp.;
Bifidobacterium spp.;  Bacteroides-Prevotella-Porphyromonas group.
Bacteroides-Prevotella-Porphyromonas group. |
| Jurgonski, et al., 2014 [43] | Blackcurrant pomace extract (1.5%) + HFD | HFD | Oral (diet)/4 | NA | 733.5 mg/100 g diet | ↓ Small intestine pH; Caecum pH; Caecum pH; ↓ β-glucuronidase;  α- and β- glucosidase, α- and β- galactosidase; α- and β- glucosidase, α- and β- galactosidase;  Total SCFA cecal. Total SCFA cecal. |
| Paivarinta, et al., 2016 [32] | Bilberry extract (10%) + HFD | HFD | Oral (diet)/10 | PCR-DGGE Cecum content |
553.2 mg/100 g diet | ↑ Bacterial diversity in cecal contents. |
| Overall, et al., 2017 [26] | Blueberry powder (400 µg/g total anth.) + HFD | HFD | Oral (diet)/12 | qPCR Fecal sample |
40 mg/100 g diet | ↑ Abundance of Bacteroidete and Actinobacteria. |
| Tong, et al., 2017 [17] | Anthocyanin from red cabbage extract (100mg/kg BW) + CPT-11 (to induce intestinal mucositis) | CPT-11 (to induce intestinal mucositis) | Oral (gavage)/1 | NA | 100 mg/kg BW | ↑ Goblet cell mucus; Preservation of the villi height and conserved epithelial cell surface in the ileum and colon. |
| Fernández, et al., 2018 [37] | Functional sausage (20g with 0.11% anth.) + AOM treatment (to induce CRC tumor) | AOM treatment + Control sausage (20g) | Oral (diet)/20 | NGS Caecal feces |
22 mg/20 g sausage | ↓ Hyperplastic payer patches in the small intestine mucosa; ↓ level of Desulfovibrionaceae and Enterobacteriaceae and ↑ of Clostridiaceae; ↓ Bilophila wadsworthia. |
| Jamar, et al., 2018 [38] | Juçara powder (0.25%) + HFD | HFD | Oral (diet)/1 | qPCR Colon content |
1.65 mg/kg/day | ↓ mRNA of TLR-4 in the colon;  mRNA ZO-1; mRNA ZO-1; ↑ DNA levels of Bifidobacterium spp. |
| Lee, et al., 2018 [39] | Blueberry powder (10%) + HFD | HFD | Oral (diet)/8 | NGS Caecal content |
213.4 mg/100 g diet | ↓ Bacteroidetes and Firmicutes abundance; ↑ Abundance of Proteobacteria and Fusobacteria; ↑ Bacilli and Lactobacillales; ↑ mRNA Muc2 ileal; ↑ ileal villus length and goblet cell number; ↑ serum acetate;  Serum propionate and butyrate; Serum propionate and butyrate; ↓ serum LBS (to assess LPS concentration);  mRNA antimicrobial peptide Defb2. mRNA antimicrobial peptide Defb2. |
| Paturi, et al., 2018 [40] | Blackcurrant extract (40g/kg) + Control diet | Control diet | Oral (diet)/6 | qPCR Caecal content |
1280 mg/100g diet | ↓ cecal acetic and butyric and ↑ of propionic acid; ↑ Bacteroides-Provotella-Porphyromonas group and Lactobacillus spp.; ↓ Bifidobacterium spp. and Clostridium perfringens;  crypt depth and goblet cells in the colon. crypt depth and goblet cells in the colon. |
| Silva-Maia, et al., 2018 [41] | Aqueous extract of berry (Plinia jaboticaba) peel (50g/L) + Normal diet | Normal diet | Oral (water)/7 | Colonies expressed as CFU Colon content |
75 mg/L | ↑ Enterobacteriaceae and Bifidobacterium, and  Lactobacillus;
Lactobacillus;  total SCFA. total SCFA. |
| Van Hul, et al., 2018 [33] | Grape pomace extract (8.2 g/kg diet) + HFD | HFD | Oral (diet)/8 | NGS Caecal content |
35.59 mg/100 g diet | ↑ Abundance of Bacteroidetes; ↓ Desulfovibrionaceae and Spreptoccaceae; ↑ Prevotellaceae and Erysipelotrichaceae;  mRNA of ZO-1, intectin, occludin, claudin3, Muc2, Reg3ϒ; mRNA of ZO-1, intectin, occludin, claudin3, Muc2, Reg3ϒ; ↑ mRNA Lyz1;  Total SCFA cecal. Total SCFA cecal. |
| Chen, et al., 2019 [18] | Purified cyanidin-3-O-glucoside (1000mg/kg) + 3-MCPD | 3-MCPD (to damage the intestinal mucosa) | Oral (diet)/8 | NGS Colonic content |
1000 mg/kg diet ** | ↓ Bacteroidetes levels and ↑ Proteobacteria and Actinobacteria; ↑ Villus height, and number of epithelial cells. |
| Cremonini, et al., 2019 [27] | Anthocyanin rich mix (40mg/kg) + HFD | HFD | Oral (diet)/14 | NGS Caecal content |
40 mg/kg BW | ↓ Intestinal permeability; ↓ Plasm endotoxin; ↓ ratio Firmicutes/Bacteroidetes; ↑ Romansia abundance; ↑ Protein expression of occludin, ZO-1 and claudin-1; ↑ Muc2 secretion. |
| Gu, et al., 2019 [28] | Black rasberry powder (10%) + Control diet | Control diet | Oral (diet)/6 | NGS Luminal content |
290 mg/100 g diet | ↓ Abundance of Firmicutes and ↑ of Bacteroidetes; ↓ Clostridium ↑ Barnessiella |
| Li, et al., 2019 [19] | Bilberry anthocyanin extract (20 mg/kg) + Old rats | Old rats | Oral (gavage)/10 | NGS Caecal content |
20 mg/kg BW | ↓ Abundance of Verrucomicrobia and Euryarchaeota;
↓ Ratio Firmicutes/Bacteroidetes; ↑ Species of Weissella confuse and Aspergillus oryzae; ↑ Lactobacillus and Bacteroides; ↑ Total SCFA in cecal content; ↓ β-glucosidade and α-galactosidade and  α-glucosidase, α-galactosidade, and β-glucoronidase; α-glucosidase, α-galactosidade, and β-glucoronidase; ↓ serum LPS. |
| Liu, et al., 2019 [29] | Malvindin 3-Glucoside (24mg/kg diet) + DSS | DSS | Oral (diet)/50 days | NGS Colon content |
24 mg/kg diet *** | ↓ Abundance of R. gnavus and ↑ Clostridium and Bacteroides ovatus; ↑ Firmicutes/Bacteroidetes ratio; ↑ crypt dilation. |
| Luo, et al., 2019 [23] | Purified anthocyanin from L. ruthenicum (200 mg/kg BW) + HFD + vit. D3 | HFD + vit. D3 (to induce atheroscherosis) | Oral (gavage)/6 | NGS Cecal content |
105.5 mg/kg BW | ↓ Abundance of Firmicutes and ↑ Bacteroidetes; ↑ Bifidobacterium and Lactobacillus; ↑ Abundance of Lria, Akkermansia and Lachnospiraceae; Improvement of structure and villi of the small intestine; |
| Peng, et al., 2019 [20] | Purified anthocyanin from L. ruthenicum (200 mg/kg BW) + DSS | DSS | Oral (water)/7 days | NGS Feces samples |
200 mg/kg BW | ↑ mRNA of ZO-1, occludin, claudin-1; ↑ total SCFA in cecal content and feces; ↑ goblet cells ; ↑ abundance of Actinobacteria;  Abundance of Firmicutes and Bacteroidete; Abundance of Firmicutes and Bacteroidete;  Firmicutes/Bacteroidetes ratio.
Firmicutes/Bacteroidetes ratio. |
| Su, et al., 2019 [21] | Pelargonidin-3-O-glucoside (150 mg/kg BW) from raspberry + Diabetic db/db | Diabetc db/db | Oral (gavage)/8 | NGS Caecal content |
150 mg/kg BW **** | ↓ Abundance of Firmicutes and ↑ Bacteroidetes; ↓ serum LPS; ↑ Bacteroidetes/Firmicutes ratio; ↑ Total SCFA fecal; ↑ mRNA of occludin e ZO-1, Muc 2, and  claudin; claudin; ↑ Pla2g2 and Lyz1 (antimicrobial peptides). |
| Tian, et al., 2019 [22] | L. ruthenicum dried (3%) + Normal diet | Normal diet | Oral (diet)/10 | NGS Fecal pellets |
104.2 mg/100 g diet | ↓ Abundance of Firmicutes; ↓ pH feces; ↓ Serum LPS; ↑ Serum and colon sIgA; ↑ Verrucomicrobia and Bacteroidetes; ↓ Proteobacteria and Deferribacteres; ↑ Total fecal SCFA; ↑ Ileal villus length and ratio of villus to crypt; ↑ mRNA of ZO-1, occludin, JAM-A and Muc2;  Colon crypt length. Colon crypt length. |
| Zary-Sikorska, et al., 2019 [42] | Purple carrot root (dried) (10%) | Control (without carrot) | Oral (diet)/4 | NA | 12.9 mg/100 g diet | ↓ Cecal pH; ↑ α- and β-Glucosidase; α- and β-Galactosidade; β-glucuronidase; ↑ Total cecal SCFA. |
| Cao, et al., 2020 [30] | Blackcurrant extract (1%) + Old rats | Old rats | Oral (diet)/16 | NGS Feces samples |
17.41 mg/100 g diet | ↓ Firmicutes/Bacteroidetes ratio; ↓ Abundance of Verrucomicrobia, ↑ Bacteroidetes and  Firmicutes and Proteobacteria.
Firmicutes and Proteobacteria. |
| Peng, et al., 2020 [24] | Anthocyanins from L. ruthenicum (200 mg/kg BW) | Control (without anth.) | Oral (diet)/12 | NGS Feces samples |
200 mg/kg BW | ↑ nº of intestinal villi, goblet cells and intestinal gland; ↑ mRNA of ZO-1, occludin, claudin and Muc1; ↑ total SCFA (cecal content and feces); ↑ Barnesiella, Alistipes, Eisenbergiella, Coprobacter and Odoribacter;  pH in feces and cecal sIgA. pH in feces and cecal sIgA. |
| Rodríguez-Daza, et al., 2020 [34] | Blueberry extract (200 mg/kg BW) + High fat and high sucrose diet | High fat and high sucrose diet | Oral (gavage)/8 | NGS Feces samples |
1.68 mg/kg BW | ↑ Mucus layer thickness (colon); ↑ Adlercreutzia equolifaciens;  Crypt depth and total goblet cells; Crypt depth and total goblet cells;  Firmicutes/Bacteroidetes ratio;
Firmicutes/Bacteroidetes ratio;  mRNA of ZO-1 and occludin. mRNA of ZO-1 and occludin. |
| Wang, et al., 2020 [25] | Black rice extract (0.48 g/kg diet) + High fat and cholesterol diet | High fat and cholesterol diet | Oral (diet)/12 | NGS Caecal content |
48 mg/100 g diet | ↓ Firmicutes/Bacteroidetes ratio; ↑ Abundance of Bifidobacterium and Lactobacillus; ↑ Cecal SCFA; ↑ Villus height (ileum and caecum); ↑ Goblet cell number per villus of the colon; ↑ mRNA of JAM-A, occludin and Muc-2. |
↓: reduced; ↑: increased;  : no change; * cyanidin galactoside; ** cyanidin-3-O-glucoside; *** Malvindin 3-Glucoside; **** Pelargonidin-3-O-glucoside; Abbreviations: BW: Body weight; HFD: High fat diet; CPT-11: irinotecan; AOM: azoxymethane; DSS: dextan sodium sulfate; ZO-1: zonula occludentes–1; 3-MCPD: 3-Chloro-1,2-propanediol; SCFA: short chain fatty acids; JAM-A: junctional adhesion molecule-A; L. ruthenicum: Lycium ruthenicum; CRC: colorectal cancer; Pla2g2: phospholipase A2 group-II; Lyz1: Lysosome-1; LPS: lipopolysaccharides; anth.: anthocyanin; TLR-4: toll like receptor 4; sIgA: secretory Immunoglobulin A; mRNA: messenger ribonucleic acid; Muc: mucin; Defb2: beta-defensin 2; LBS: LPS-binding protein; qPCR: quantitative polymerase chain reaction; CFU: colony forming unit; NA: not applicable; NGS: next generation sequencing; DGGE: denaturation gradient gel electrophoresis.
: no change; * cyanidin galactoside; ** cyanidin-3-O-glucoside; *** Malvindin 3-Glucoside; **** Pelargonidin-3-O-glucoside; Abbreviations: BW: Body weight; HFD: High fat diet; CPT-11: irinotecan; AOM: azoxymethane; DSS: dextan sodium sulfate; ZO-1: zonula occludentes–1; 3-MCPD: 3-Chloro-1,2-propanediol; SCFA: short chain fatty acids; JAM-A: junctional adhesion molecule-A; L. ruthenicum: Lycium ruthenicum; CRC: colorectal cancer; Pla2g2: phospholipase A2 group-II; Lyz1: Lysosome-1; LPS: lipopolysaccharides; anth.: anthocyanin; TLR-4: toll like receptor 4; sIgA: secretory Immunoglobulin A; mRNA: messenger ribonucleic acid; Muc: mucin; Defb2: beta-defensin 2; LBS: LPS-binding protein; qPCR: quantitative polymerase chain reaction; CFU: colony forming unit; NA: not applicable; NGS: next generation sequencing; DGGE: denaturation gradient gel electrophoresis.
3.3. Main Findings
The reviewed experimental studies demonstrated that the anthocyanin supplementation provided beneficial effects to intestinal health, and specific improvement in the intestinal microbiota population, short chain fatty acids production, goblet cell number, tight junction protein and villi improvement (Table 3).
Positive findings included the effects on the intestinal microbiome composition and function. In this context, the majority of the studies observed an increased abundance of Bacteroidetes [22,23,26,28,30,33]; two studies observed a reduction [18,39]; and one showed no changes on the abundance of Bacteroidetes [20]. On the other hand, the abundance of Firmicutes was reduced in five studies [21,22,23,28,39], and in two studies no changes were observed [20,30]. Further, a reduction in the Firmicutes/Bacteroidetes ratio (total of studies that evaluated = 7) was observed in four studies [19,25,27,30];in two other studies, no changes were observed [20,34], and only one study observed an increased ratio [29]. Further, an increase in Biffidobacterium spp. and Lactobacillus spp. populations were observed in some of the studies [19,23,25,38,40].
Out of all the studies included, 12 evaluated the production of short chain fatty acids (SCFA) by bacterial populations. This analysis indicated an increased total SCFA production in most of the studies (n = 7) [19,20,21,22,24,25,42]. Further, four studies reported on a reduction of the intestinal or cecal pH [22,35,42,43]. Moreover, regarding proteins that are related to intestinal permeability and function, most of the studies observed an increase in the gene expression of zonula occludents 1 (ZO-1), occludin, claudin-1 and Mucin (Muc) 2 [20,21,22,24,25,27,39], and three studies did not observe these effects [33,34,38]. The intestinal morphology was evaluated in some of the studies. From seven studies, which evaluated the number of goblet cells, five studies observed an increased number [17,20,24,25,39], and in the other two studies no changes were observed [34,40]. In addition, increased villi length or height was reported in many of the studies [17,18,22,23,25,39]. Four studies [19,21,22,39] observed a reduction in the serum lipopolysaccharides (LPS), and one study [27] showed a reduction in plasma endotoxin and intestinal permeability.
3.4. Risk of Bias
From all the studies that were included in the current systematic review (n = 27), the baseline characteristics, including sex, age and initial weight of animals, were complete in five studies [18,22,23,31,43]. In most of the studies, the allocation of animals was not described in detail, since there was no information about the randomization process. Six studies did not mention if the animal allocation to treatment groups was performed randomly [20,21,25,27,37,39]. Furthermore, none of the studies reported about blinding the investigators involved in the research. Four studies did not include all animals in the analysis, and the exclusion criteria were not reported [21,27,32,41] (Figure 2).
Figure 2.
Risk of bias of animal studies.
4. Discussion
In this systematic review, we evaluated the effects of anthocyanins or their extract on intestinal health parameters, in vivo. Therefore, this systematic review verified that food sources of anthocyanin or its extract are able to improve intestinal parameters changed by pathologic conditions or dietary patterns.
The animal models of the studies included were high fructose diet, high fat diet, intestinal mucositis, colorectal cancer, damage to the intestinal mucosa, diabetes and old animals. It is highlighted that all of these models promote intestinal changes, such as increased intestinal permeability, inflammation, altered morphology and changes in the intestinal microbiota composition such as dysbiosis. Despite different animal models being used in the studies, all of them except one [43] used rodents as the animal. Similarly as in humans, Bacteroidetes and Firmicutes are the two main phyla in rodents’ gastrointestinal tract. In this sense, microbiota composition in rodents is usually analyzed in interventions that study the casual role of gut microbiota in diet, health and disease interaction [44]. Since all animal models used in the studies of this review promote modification of the gut microbiota at some level, the choice of the best model depends on the main goal of the study [44]. Therefore, bioactive components with functional properties were investigated to verify their potential beneficial effects on intestinal health [7]. Anthocyanins are soluble components, from the class of flavonoids, with functional dietary properties that were previously associated with oxidative stress inhibition, antioxidant activity and intestinal microbiota modulation [45]. Further, the supplementation of anthocyanin cyanindin-3-O-glucoside for eight weeks in Wistar rats was able to restrain the gut microbial dysbiosis that was induced chemically, by suppressing the decrease of Rothia and Romboutsia and the increase of Clostridium verified in the disrupted gut microbiota [18].
In this review, the anthocyanin dietary intake induced increased abundance of Bacteroidetes and a reduction of Firmicutes. A reduction of the Firmicutes/Bacteroidetes ratio in the caecal content was observed in experimental in vivo models of high fat diet [25,27]. In recent years, research with animal models in samples of caecal content [23] and humans in fecal samples [46] demonstrated that obese organisms have a high abundance of Firmicutes and a low abundance of Bacteroidetes. These changes in the microbial composition result in an increased absorption of calories, reduced secretion of anorexigenic hormones and intestinal barrier damage [46]. Bacteroidetes and Firmicutes are the two main phylum that inhabit the large intestine, corresponding to 90% of total bacteria [47]. The Firmicutes phylum and gram-positive bacteria carry more enzymes that are required for carbohydrates metabolism, which contribute to their transport and energy absorption [48], besides higher fat deposition in adipocytes [49].
Following anthocyanin consumption, most are not absorbed in the upper gastrointestinal tract, and therefore reach the colon intact [11]. At the colonic level, anthocyanins are metabolized by the local microbiota, initially via deglycosylation, and is followed by a secondary degradation into phenolic acids, mainly protocatechuic, vanillic, syringic, gallic and p-coumaric [45]. The main bacterial populations that are able to metabolize anthocyanin are the Bifidobacterium spp. and Lactobacillus spp., which have probiotic effects, including the production of antimicrobial substances, competition with pathogens for adhesion to the epithelium and nutrients, immunomodulation and inhibition of bacterial toxin production [50]. In addition, these bacteria have enzymes such as β-glycosidase that are needed to catalyze reactions that release the glycose from the aglycon and provide the energy needed for bacterial populations to prosper [12]. In several studies included in this review, an increase of Bifidobacterium and Lactobacillus was documented [19,23,25,38,40]. In this context, 20 day intake of dealcoholized red wine in healthy adults increased the fecal concentration of Bifidobacterium, Enterococus and Eggerthella lenta. In addition, in this study, the produced metabolites associated with the increase of Bifidobacterium were those derived from anthocyanin degradation (4-hydroxybenzoic, syringic, p-coumaric, homovanillic ácidos) [51]. Bifidobacterium is related to pathogen inhibition by organic acids production, antimicrobial peptides and immune stimulation [52]. Besides this, among the acids produced by the microbial metabolism of anthocyanin, protocatechuic acid presents inhibitory effect on pathogenic bacteria growth [53], and gallic acid is effective in reversing changes in the microbiota caused by induced colitis in animals, by reducing Firmicutes and Proteobacteria and increasing Bacteroidetes [54]. Therefore, the beneficial effects that are associated with anthocyanin consumption may be achieved by its metabolites post-degradation.
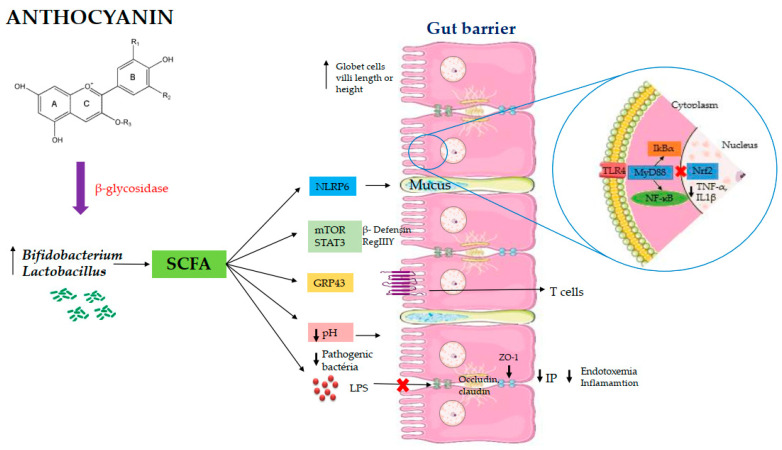
Some of the physiological properties of the gut microbiota are attributed to fermentation of non-digestible carbohydrates by anaerobic bacteria, producing short chain fatty acids (SCFA) [47]. In this review, the dietary supplementation of anthocyanin was able to increase the production of total SCFA (acetate, propionate and butyrate) in the majority of the studies. SCFA act as a fuel to intestinal cells by stimulating the cellular proliferation. The production of SCFA also reduces the intraluminal pH, which limits the growth of pathogenic bacteria due to the acidification [55,56]. Therefore, SCFA assist to maintain the intestinal epithelium integrity, and protect the host from potential immune and inflammatory diseases [56]. In a mice study, where diet included anthocyanin extract, SCFA production increased due to elevated microbial activity, specifically of Bifidobacterium and Lactobacillus [25]. Further, the increased abundance of Roseburia is associated with a higher production of SCFA in the intestine, and the abundance of Akkermansia was associated with propionic production [23]. SCFA also have an immunomodulatory effect by promoting the development of mucosal regulatory T cells (Tregs) through the interaction with the G protein-coupled receptor (GRP43) and the inhibition of histone deacetylases (HDACs) [57,58]. Furthermore, SCFA exert positive effects to the turnover and differentiation of colonic epithelial cells, and to stimulate the mucus production that prevents the pathogenic bacteria adherence [56]. It is suggested that these acids activate the mammalian target of rapamycin (mTOR) complex and the STAT3 (signal transducers and activator of transcription 3) in the intestinal epithelial cells, which promote the expression of antimicrobial peptides as β-defensin and RegIIIγ [59]. From the included studies in this systematic review, two observed an increase of Lysosome-1 (Lyz1) peptide, which acts to maintain the microbiota homeostasis and to eliminate commensal microorganisms [21,33]. Therefore, by these mechanisms, SCFA act to maintain the epithelial barrier integrity [60,61].
The intestinal epithelial barrier consists of a mucus layer and cells attached by a protein complex, including tight junctions, adherents junctions and desmosome [62]. The tight junctions complex is composed by proteins as claudins, occludin, junctional adhesion molecule (JAM-1) and zonula occludents (ZO-1), and the rupture in some of these proteins increases the paracellular permeability with permeation of pro-inflammatory molecules, immune activation and inflammation [63]. In this review, the majority of the included studies verified an increase in the gene expression of ZO-1, occludin and claudin 1. The anthocyanin has an anti-inflammatory effect through inhibition of the factor nuclear kappa B (NF-ĸB), and via regulation of I-Kappa-B-alpha (IĸBα) phosphorylation that decreases the gene expression of pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interferon gamma (IFNγ) and interleukins [64,65]. These cytokines are related to intestinal barrier damage by harming the tight junction protein expression [66]. The TNF-α promotes changes in the tight junctions via its receptor tumor necrosis factor receptor 1 (TNFR-1), so that anti-TNF strategies promoted tight junctions’ rearrangement with an improvement of occludin and ZO-1 [67]. Therefore, the anthocyanidin cyaniding-3-glucoside (C3G) was able to inhibit the IĸBα phosphorylation and the nuclear translocation of NF-ĸB (p65), and these effects were associated with the nuclear transcription factor Nrf2 (erythroid-2-related factor 2) that induces the expression of antioxidant enzymes [68]. Therefore, the anthocyanin can act directly or indirectly to improve tight junction’s integrity.
Moreover, the intestinal epithelial cells are covered by a mucus layer produced and secreted by the goblet cells. The mucus is composed of glycoproteins of mucins, mainly Muc2, forming a viscous layer that protects against pathogen invasion by preventing the sites of binding for bacteria [69,70]. The discontinuous mucus layer in the cecum of rats are considered hotspots for Salmonella; thus, the absence of the mucus layer can lead to infections’ development [71]. In addition, the increase in goblet cell number, mainly those classified as acids, promotes barrier function improvement by increasing the mucin secretion that prevents pathogen invasion and possible intestinal inflammation due to acidic mucin resistance to degradation by bacterial glycosidase and has higher viscosity [72]. SCFA also contribute to the intestinal cell proliferation since they stimulate the proliferation and differentiation of enterocytes [73]. Thus, there is evidence that the inflammasome nucleotide-oligomerization domain-like receptor 6 (NLRP6), expressed mainly on enterocytes, controls the mucin secretion and the mucosa renewal by the goblet cells [74]. A previous study with rats suggested that SCFA can activate the colonic NLRP6, thus protecting the intestinal barrier [75]. Considering this evidence, it is verified that the mulberry (50 mg anthocyanin/kg diet) supplementation in animals with induced colitis resulted in an increase in goblet cells and NLRP6 expression, therefore suggesting a link between mucin secretion and antimicrobial peptide production [76].
In order to optimize the digestion and absorption of nutrients, the gut (duodenum) morphology is unique and organized in villi [77]. It is observed that anthocyanins are related to an improvement of the absorptive function by increasing the villus length, villus length/crypt depth ratio and the total mucosa thickness [78]. These intestinal morphology changes result in a better intestinal digestion and absorption, since they assure a higher absorption surface, brush border enzyme expression and nutrient transport system [79].
Disruption of the intestinal barrier integrity can be occasioned by tight junction disruption and mucus layer depletion, thus allowing the paracellular translocation of bacteria and their components, such as lipopolysaccharides (LPS) [63]. Of the included studies in this systematic review, a few evaluated the endotoxemia; however, of these, the anthocyanin supplementation was able to reduce the intestinal permeability, endotoxemia and the levels of serum LPS [19,21,22,27,39]. LPS are cellular wall components of gram-negative bacteria that contain a pathogen-associated molecular pattern, Lipid A, able to interact with Toll-like Receptor 4 (TLR-4) via the Myeloid differentiation primary response 88 (MyD88) protein [80]. This interaction results in the activation of the pathway downstream and Nf-ĸB translocation, thus increasing the gene transcription of cytokines such as TNF-α, IL-1β e and IL-6 [80,81]. The analysis of monocyte from obese individuals supplemented with berry juçara (5 g/day; 131.2 mg total anthocyanins) for six weeks observed the reduction of mRNA (messenger ribonucleic acid), TLR4 and the protein expression of MyD88 [82]. Therefore, it is known that the inflammation mediated by LPS can exert local and systemic effects and be related to gastrointestinal diseases, such as Crohn disease [83], inflammatory bower disease [84] and metabolic disorders as diabetes mellitus type 2 [85] and obesity [86].
Animal experiments assist to design clinical studies in terms of doses, duration, type of intervention and other topics [15]. In this context, the positive changes observed at the gut microbiota following anthocyanin supplementation and consumption in the animal studies included in this review corroborate with results verified in several clinical studies. Cranberry consumption (30 g, with 83.7 mg anthocyanin) for five days by healthy adults resulted in increased abundance of Bacteroidetes and decreased abundance of Firmicutes [87]. Further, the intake of dealcoholized red wine for 20 d (9.72 mg anthocyanin) increased the fecal concentration of Bifidobacterium and Enterococus [51]. Thus, we highlighted the complexity of animal models of gut microbiota, which are able to tolerate the presence and effects of dietary components such as anthocyanins in a similar manner as described in human studies. Further, animal model studies related to anthocyanin supplementation are effective in demonstrating the safety and efficacy of their consumption. Hence, these aspects are relevant and important for the translation of results and adaptation to clinical studies, and in order to establish dietary guidelines for humans.
Finally, systematic reviews guarantee the gathering of evidence related to a specific topic; therefore, they obtain conclusions with greater scientific rigor. The evidence verified in this review, performed with 27 studies, demonstrates that the anthocyanin dietary intake is beneficial and improves specific parameters such as the gut microbiota composition, short chain fatty acids production and the intestinal physical barrier, such as the increase of tight junction protein and goblet cell number, and the reduction of intestinal permeability, that together promote intestinal health.
Dosage and Reporting Quality
This systematic review showed high heterogeneity among the studies, with several experimental models used, distinct methodologies in the intestinal parameters analyzed and high variation related to dose and time of anthocyanin supplementation. Probably, these variations were observed because of the large number of studies included. The anthocyanin supplementation dose ranged from 12.9 mg/100 g diet as dried purple carrot [42] to 1280 mg/100 g diet as extract [40]. The supplementation as an extract allows for the delivery of a higher dose of anthocyanins, since it concentrates the components, while the anthocyanin intake in its food source provides a lower dose, based on average daily intake of the animal. Hence, the safety of anthocyanin intake were tested and approved in animals, indicating no toxicity or any adverse effects to animal health, even at a high dosage [88]. In addition, studies showed that the anthocyanin supplementation exposure period varied from 1 week [17,38] to 20 weeks [37]. Analyzing the outcomes individually, the time of supplementation from 1 to 12 weeks promoted the increase of Bifidobacterium and Lactobacillus, the production of SCFA and an improvement of goblet cells and villi length or height; further, the increase of proteins related to intestinal permeability was verified from the time of 1 to 14 weeks of intervention. Thus, for the design of studies for new researches evaluating the effects of anthocyanin on intestinal health, this range of time should be considered in accordance with the goal of the research. Beneficial effects of anthocyanin supplementation were observed even in the lowest dosage [42] and the shortest exposure period [17,38]. Furthermore, most of the reviewed studies observed that anthocyanin was quantified as total anthocyanin; however, few studies showed its profile, as specific monomers of anthocyanidin such as cyanidin galactoside [31], cyanidin-3-O-glucoside [18], malvindin 3-glucoside [29] and pelargonidin-3-O-glucoside [21].
This systematic review evaluated the effects of dietary anthocyanin in the context of its potential intestinal health-promoting effects, such as beneficially changing the intestinal microbiota, increasing the short chain fatty acid production, reducing the intestinal permeability and improving parameters related to the intestinal physical barrier such as tight junctions protein and goblet cell number. The studies’ selection was based on methodologies that are recommended and approved for systematic review, thus allowing reliable conclusions. The risk of bias was evaluated according to the SYRCLE RoB tool [15], which establishes consistency and avoids discrepancies to evaluate the risk of bias from animal studies. Twenty-one (n = 21) studies did not show the animals’ completed baseline characteristics, and none of the studies showed information about whether researchers were blinded from knowledge of the intervention groups and/or the outcome assessor. In this sense, the absence of some baseline characteristics probably had no influence on the main conclusion of this review, since those characteristics were not comparable among studies. On the other hand, the risk of bias related to blind researchers and outcome assessors could have influenced the results of outcomes in each study. However, the conclusion of this review was performed with a large number of studies in association; thus, these biases may not represent a major impact on the main conclusions, considering the methodologic rigor that this review followed. Further, the risk of bias in analysis may represent a lack of information regarding the experimental design of animal studies, showing that progress is needed in this field. Therefore, we suggest that research performed with animals follows the SYRCLE protocol to avoid a lack of information in the studies.
5. Conclusions
The scientific evidence from the reviewed in vivo studies demonstrates that the supplementation of anthocyanin is effective to modulate the intestinal microbiota through the increase of Lactobacillus spp. and Bifidobacterium spp., and to increase the production of short chain fatty acids. In addition, the reviewed studies observed an improvement in the intestinal barrier by the increased expression of tight junction protein associated with an improvement of cells’ morphology and mucus production, which reduces the potential risk of inflammation (Figure 3). We highlighted that these intestinal changes in association may be the mechanisms by which upon anthocyanin supplementation that ranged from 1 to 14 weeks with the dosage that ranged from a 12.9 to 1280 mg/100 g diet exert beneficial effects on intestinal health. We consider that it is not adequate to establish a specific dose and a specific time to achieve all of these effects in association, since different animal models, methodologies and large ranges of time and dose of anthocyanin supplementation were observed. However, considering the methodological rigor that this review followed, the dose and time intervention ranges observed could be used as guidelines for future researchers. Despite the limitation of extrapolating animal results to human, with knowledge of all of the benefits observed, we consider that the daily intake of foods’ source of anthocyanin should be stimulated, with the population acting as a strategy to prevent health problems.
Figure 3.
Proposed mechanisms of action of anthocianins on intestinal health. Abbreviations: ZO-1: zonula occludentes–1; SCFA: short chain fatty acids; LPS: lipopolysaccharides; TLR-4: Toll like receptor 4; IP: intestinal permeability; mTOR: mammaliam target of rapamycin; STAT3: signal transducers and activator of transcription 3; NF-ĸB: factor nuclear kappa B; MyD88: Myeloid differentiation primary response 88; IkBα: I-Kappa-B-alpha; NLRP6: inflammasome nucleotide-oligomerization domain-like receptor 6; GRP43: G protein-coupled receptor; TNF-α: tumor necrosis factor alpha IL1β:interleukin 1 beta; Nrf2: erythroid-2-related factor.
Registration and Protocol
This systematic review was realized according to the protocol: Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement 2020 [89]. The review is registered in the PROSPERO under the number CRD42020204835 (Centre for Reviews and Dissemination, University of York). A systematic review was carried out to answer the question: “Does food source or extract of anthocyanin promote changes on intestinal parameters?”
Author Contributions
Conceptualization, T.A.V., H.S.D.M., M.C.D.P., and E.T.; methodology, T.A.V. and H.S.D.M.; writing—original drafting preparation, T.A.V.; writing—review and editing, T.A.V., H.S.D.M., M.C.D.P., and E.T.; supervision, H.S.D.M., M.C.D.P., and E.T.; project administration, H.S.D.M., M.C.D.P., and E.T. All authors have read and agreed to the published version of the manuscript.
Funding
The present work was performed with funding by Coordination for the Improvement of Higher Education Personnel (CAPES), Brazil, for the research scholarship support: sandwich doctoral (88887.569929/2020-00) and CAPES/PRINT—Senior Visiting Professor (88887.321642/2019-00) Program and the National Counsel of Technological and Scientific Development (CNPq, Brazil) for scholarship research support (310910/2020-0).
Institutional Review Board Statement
All animal protocols related to studies reviewed in this manuscript were conducted according to the guidelines of the Declaration of Helsinki.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data analyzed in this study are openly available in references number [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,40,41,42,43,44].
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bischoff S.C. “Gut health”: A new objective in medicine? BMC Med. 2011;9:1–14. doi: 10.1186/1741-7015-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kataoka K. The intestinal microbiota and its role in human health and disease. J. Med. Investig. 2016;63:27–37. doi: 10.2152/jmi.63.27. [DOI] [PubMed] [Google Scholar]
- 3.Natividad J.M.M., Verdu E.F. Modulation of intestinal barrier by intestinal microbiota: Pathological and therapeutic implications. Pharm. Res. 2013;69:42–51. doi: 10.1016/j.phrs.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Sui X., Zhang Y., Jiang L., Zhou W. Anthocyanins in food. Encycl. Food Chem. 2019;2:10–17. [Google Scholar]
- 5.Curtis P.J., van der Velpen V., Berends L., Jennings A., Feelisch M., Umpleby A.M., Evans M., Fernandez B.O., Meiss M.S., Minnion M., et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome—Results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019;109:1535–1545. doi: 10.1093/ajcn/nqy380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li D., Zhang Y., Liu Y., Sun R., Xia M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic. J. Nutr. Dis. 2015;145:742–748. doi: 10.3945/jn.114.205674. [DOI] [PubMed] [Google Scholar]
- 7.Jayarathne S., Stull A.J., Park O., Kim J.H., Thompson L., Moustaid-Moussa N. Protective effects of anthocyanins in obesity-associated inflammation and changes in gut microbiome. Mol. Nutr. Food Res. 2019;63:1–18. doi: 10.1002/mnfr.201900149. [DOI] [PubMed] [Google Scholar]
- 8.Park E., Edirisinghe I., Wei H., Vijayakumar L.P., Banaszewski K., Cappozzo J.C., Burton-Freeman B. A dose-response evaluation of freeze-dried strawberries independent of fiber content on metabolic indices in abdominally obese individuals with insulin resistance in a randomized, single-blinded, diet-controlled crossover trial. Mol. Nutr. Food Res. 2016;60:1099–1109. doi: 10.1002/mnfr.201500845. [DOI] [PubMed] [Google Scholar]
- 9.Tian L., Tan Y., Chen G., Wang G., Sun J., Ou S., Chen W., Bai W. Metabolism of anthocyanins and consequent effects on the gut microbiota. Crit. Rev. Food Sci. Nutr. 2018;59:982–991. doi: 10.1080/10408398.2018.1533517. [DOI] [PubMed] [Google Scholar]
- 10.Mcghie T.K., Walton M.C. Review The bioavailability and absorption of anthocyanins: Towards a better understanding. Mol. Nutr. Food Res. 2007;51:702–713. doi: 10.1002/mnfr.200700092. [DOI] [PubMed] [Google Scholar]
- 11.Fang J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014;46:508–520. doi: 10.3109/03602532.2014.978080. [DOI] [PubMed] [Google Scholar]
- 12.Morais C.A., Rosso V.V., Estadella D., Pisani L.P. Anthocyanins as inflammatory modulators and the role of the gut microbiota. J. Nutr. Biochem. 2016;33:1–7. doi: 10.1016/j.jnutbio.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Braga A.R.C., Murador D.C., Mesquita L.M.D.S., de Rosso V.V. Bioavailability of anthocyanins: Gaps in knowledge, challenges and future research. J. Food Compos. Anal. 2018;68:31–40. doi: 10.1016/j.jfca.2017.07.031. [DOI] [Google Scholar]
- 14.Igwe E.O., Charlton K.E., Probst Y.C., Kent K., Netzel M.E. A systematic literature review of the effect of anthocyanins on gut microbiota populations. J. Hum. Nutr. Diet. 2019;32:53–62. doi: 10.1111/jhn.12582. [DOI] [PubMed] [Google Scholar]
- 15.Hooijmans C.R., Rovers M.M., De Vries R.B.M., Leenaars M., Ritskes-Hoitinga M., Langendam M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014;14:1–9. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:1–9. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tong T., Niu Y.H., Yue Y., Wu S.-C., Ding H. Beneficial effects of anthocyanins from red cabbage (Brassica oleracea L. var. capitata L.) administration to prevent irinotecan-induced mucositis. J. Funct. Foods. 2017;32:9–17. doi: 10.1016/j.jff.2017.01.051. [DOI] [Google Scholar]
- 18.Chen G., Wang G., Zhu C., Jiang X., Sun J., Tian L., Bai W. Effects of cyanidin-3-O-glucoside on 3-chloro-1,2-propanediol induced intestinal microbiota dysbiosis in rats. Food Chem. Toxicol. 2019;133:1–9. doi: 10.1016/j.fct.2019.04.054. [DOI] [PubMed] [Google Scholar]
- 19.Li J., Wu T., Li N., Wang X., Chen G., Lyu X. Bilberry anthocyanin extract promotes intestinal barrier function and inhibits digestive enzyme activity by regulating the gut microbiota in aging rats. Food Funct. 2019;10:333–343. doi: 10.1039/C8FO01962B. [DOI] [PubMed] [Google Scholar]
- 20.Peng Y., Yan Y., Wan P., Chen D., Ding Y., Ran L., Mi J., Lu L., Zhang Z., Li X. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic. Biol. Med. 2019;136:96–108. doi: 10.1016/j.freeradbiomed.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Su H., Xie L., Xu Y., Ke H., Bao T., Li Y., Chen W. Pelargonidin-3- O-glucoside derived from wild raspberry exerts antihyperglycemic effect by inducing autophagy and modulating gut microbiota. J. Agric. Food Chem. 2019;68:13025–13037. doi: 10.1021/acs.jafc.9b03338. [DOI] [PubMed] [Google Scholar]
- 22.Tian B., Zhao J., An W., Zhang J., Cao X., Mi J. Lycium ruthenicum diet alters the gut microbiota and partially enhances gut barrier function in male C57BL/6 mice. J. Funct. Foods. 2019;52:516–528. doi: 10.1016/j.jff.2018.11.034. [DOI] [Google Scholar]
- 23.Luo Y., Fang J.L., Yuan K., Jin S.H., Guo Y. Ameliorative effect of purified anthocyanin from Lycium ruthenicum on atherosclerosis in rats through synergistic modulation of the gut microbiota and NF-κB/SREBP-2 pathways. J. Funct. Foods. 2019;59:223–233. doi: 10.1016/j.jff.2019.05.038. [DOI] [Google Scholar]
- 24.Peng Y., Yan Y., Wan P., Dong W., Huang K., Ran L., Mi J., Lu L., Zheng X., Cao Y. Effects of long-term intake of anthocyanins from Lycium ruthenicum Murray on the organism health and gut microbiota in vivo. Food Res. Int. 2020;130:1–11. doi: 10.1016/j.foodres.2019.108952. [DOI] [PubMed] [Google Scholar]
- 25.Wang H., Liu D., Ji Y., Liu Y., Xu L., Guo Y. Dietary supplementation of black rice anthocyanin extract regulates cholesterol metabolism and improves gut microbiota dysbiosis in C57BL/6J mice fed a high-fat and cholesterol diet. Mol. Nutr. Food Res. 2020;64:e1900876. doi: 10.1002/mnfr.201900876. [DOI] [PubMed] [Google Scholar]
- 26.Overall J., Bonney S.A., Wilson M., Beermann A., Grace M.H., Esposito D., Lila M.A., Komarntysky S. Metabolic effects of berries with structurally diverse anthocyanins. Int. J. Mol. Sci. 2017;18:422. doi: 10.3390/ijms18020422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cremonini E., Daveri E., Mastaloudis A., Adamo A.M., Mills D., Kalanetra K., Hester S.N., Wood S.M., Fraga C.G., Oteiza P.I. Anthocyanins protect the gastrointestinal tract from high fat diet-induced alterations in redox signaling, barrier integrity and dysbiosis. Redox. Biol. 2019;26:1–10. doi: 10.1016/j.redox.2019.101269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu J., Thomas-Ahner J.M., Riedl K.M., Bailey M.T., Vodovotz Y., Schwartz S.J., Clinton S.K. Dietary black raspberries impact the colonic microbiome and phytochemical metabolites in mice. Mol. Nutr. Food Res. 2019;63:1–9. doi: 10.1002/mnfr.201800636. [DOI] [PubMed] [Google Scholar]
- 29.Liu F., Wang T.T.Y., Tang Q., Xue C., Li R.W., Wu V.C.H. Malvidin 3-Glucoside modulated gut microbial dysbiosis and global metabolome disrupted in a murine colitis model induced by dextran sulfate sodium. Mol. Nutr. Food Res. 2019;63:1–14. doi: 10.1002/mnfr.201900455. [DOI] [PubMed] [Google Scholar]
- 30.Cao L., Lee S.G., Melough M.M., Sakaki J.R., Maas K.R., Koo S.I., Chun O.K. Long-term blackcurrant supplementation modified gut microbiome profiles in mice in an age- dependent manner: An exploratory study. Nutrients. 2020;12:290. doi: 10.3390/nu12020290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Espley R.V., Butts C.A., Laing W.A., Martell S., Smith H., McGhie T.K., Zhang J., Paturi G., Hedderley D., Bovy A., et al. Dietary flavonoids from modified apple reduce inflammation markers and modulate gut microbiota in mice. J. Nutr. 2014;144:146–154. doi: 10.3945/jn.113.182659. [DOI] [PubMed] [Google Scholar]
- 32.Päivärinta E., Niku M., Maukonen J., Storvik M., Heiman-Lindh A., Saarela M., Pajari A.M., Mutanen M. Changes in intestinal immunity, gut microbiota, and expression of energy metabolism–related genes explain adenoma growth in bilberry and cloudberry-fed ApcMin mice. Nutr. Res. 2016;36:1285–1297. doi: 10.1016/j.nutres.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Van Hul M., Geurts L., Plovier H., Druart C., Everard A., Ståhlman M., Rhimi M., Chira K., Teissedre P.L., Delzenne N.M., et al. Reduced obesity, diabetes, and steatosis upon cinnamon and grape pomace are associated with changes in gut microbiota and markers of gut barrier. Am. J. Physiol. Endocrinol. Metab. 2018;314:E334–E352. doi: 10.1152/ajpendo.00107.2017. [DOI] [PubMed] [Google Scholar]
- 34.Rodríguez-Daza M.C., Daoust L., Boutkrabt L., Pilon G., Varin T., Dudonné S., Levy E., Marette A., Roy D., Desjardins Y. Wild blueberry proanthocyanidins shape distinct gut microbiota profile and influence glucose homeostasis and intestinal phenotypes in high-fat high-sucrose fed mice. Sci. Rep. 2020;10:1–16. doi: 10.1038/s41598-020-58863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jurgoński A., Juśkiewicz J., Zduńczyk Z. Ingestion of black chokeberry fruit extract leads to intestinal and systemic changes in a rat model of prediabetes and hyperlipidemia. Plant. Foods Hum. Nutr. 2008;63:176–182. doi: 10.1007/s11130-008-0087-7. [DOI] [PubMed] [Google Scholar]
- 36.Jurgoński A., Juśkiewicz J., Zduńczyk Z. An anthocyanin-rich extract from Kamchatka honeysuckle increases enzymatic activity within the gut and ameliorates abnormal lipid and glucose metabolism in rats. Nutrition. 2013;29:898–902. doi: 10.1016/j.nut.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Fernández J., García L., Monte J., Villar C.J., Lombó F. Functional anthocyanin-rich sausages diminish colorectal cancer in an animal model and reduce pro-inflammatory bacteria in the intestinal microbiota. Genes. 2018;9:133. doi: 10.3390/genes9030133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jamar G., Santamarina A.B., Mennitti L.V., de Cesar H.C., Oyama L.M., de Rosso V.V., Pisani L.P. Bifidobacterium spp. reshaping in the gut microbiota by low dose of juçara supplementation and hypothalamic insulin resistance in Wistar rats. J Funct. Foods. 2018;46:212–219. doi: 10.1016/j.jff.2018.05.002. [DOI] [Google Scholar]
- 39.Lee S., Keirsey K.I., Kirkland R., Grunewald Z.I., Fischer J.G., de La Serre C.B. Blueberry supplementation influences the gut microbiota, inflammation, and insulin resistance in high-fat-diet-fed rats. J. Nutr. 2018;148:209–219. doi: 10.1093/jn/nxx027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paturi G., Butts C.A., Monro J.A., Hedderley D. Effects of blackcurrant and dietary fibers on large intestinal health biomarkers in rats. Plant. Foods Hum. Nutr. 2018;73:54–60. doi: 10.1007/s11130-018-0652-7. [DOI] [PubMed] [Google Scholar]
- 41.da Silva-Maia J.K., Batista A.G., Correa L.C., Lima G.C., Bogusz Junior S., Maróstica Junior M.R. Aqueous extract of berry (Plinia jaboticaba) byproduct modulates gut microbiota and maintains the balance on antioxidant defense system in rats. J. Food Biochem. 2018;43:1–11. doi: 10.1111/jfbc.12705. [DOI] [PubMed] [Google Scholar]
- 42.Żary-Sikorska E., Fotschki B., Fotschki J., Wiczkowski W., Juśkiewicz J. Preparations from purple carrots containing anthocyanins improved intestine microbial activity, serum lipid profile and antioxidant status in rats. J. Funct. Foods. 2019;60:103442. doi: 10.1016/j.jff.2019.103442. [DOI] [Google Scholar]
- 43.Jurgoński A., Juśkiewicz J., Zduńczyk Z., Matusevicius P., Kołodziejczyk K. Polyphenol-rich extract from blackcurrant pomace attenuates the intestinal tract and serum lipid changes induced by a high-fat diet in rabbits. Eur. J. Nutr. 2014;53:1603–1613. doi: 10.1007/s00394-014-0665-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hugenholtz F., Zhang J., O’Toole P.W., Smidt H. Studying the mammalian intestinal microbiome using animal models. In: Yates M.V., Nakatsu C.H., Miller R.V., Pillai S.D., editors. Manual of Environmental Microbiology. 4th ed. American Society for Microbiolog; Washington, DC, USA: 2015. [Google Scholar]
- 45.Faria A., Fernandes I., Norberto S., Mateus N., Calhau C. Interplay between anthocyanins and gut microbiota. J. Agric. Food Chem. 2014;62:6898–6902. doi: 10.1021/jf501808a. [DOI] [PubMed] [Google Scholar]
- 46.Crovesy L., Masterson D., Rosado E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur J Clin Nutr. 2020;74:1251–1262. doi: 10.1038/s41430-020-0607-6. [DOI] [PubMed] [Google Scholar]
- 47.Jandhyala S.M., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M., Reddy D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015;21:8787–8809. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ibrahim M., Anishetty S. A meta-metabolome network of carbohydrate metabolism: Interactions between gut microbiota and host. Biochem. Biophys. Res. Commun. 2012;428:278–284. doi: 10.1016/j.bbrc.2012.10.045. [DOI] [PubMed] [Google Scholar]
- 49.Gomes A.C., Hoffmann C., Mota J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut. Microbes. 2018;9:308–325. doi: 10.1080/19490976.2018.1465157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Markowiak P., Ślizewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9:1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boto-Ordóñez M., Urpi-Sarda M., Queipo-Ortuño M.I., Tulipani S., Tinahones F.J., Andres-Lacueva C. High levels of Bifidobacteria are associated with increased levels of anthocyanin microbial metabolites: A randomized clinical trial. Food Funct. 2014;5:1932–1938. doi: 10.1039/C4FO00029C. [DOI] [PubMed] [Google Scholar]
- 52.Hidalgo-Cantabrana C., Delgado S., Ruiz L., Ruas-Madiedo P., Sánchez B., Margolles A. Bifidobacteria and their health-promoting effects. Microbiol. Spectr. 2017;5:1–19. doi: 10.1128/microbiolspec.bad-0010-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ajiboye T.O., Habibu R.S., Saidu K., Haliru F.Z., Ajiboye H.O., Aliyu N.O., Ibitoye O.B., Uwazie J.N., Muritala H.F., Bello S.A., et al. Involvement of oxidative stress in protocatechuic acid-mediated bacterial lethality. Microbiologyopen. 2017;6:1–10. doi: 10.1002/mbo3.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y., Xie Z., Gao T., Li L., Chen Y., Xiao D., Liu W., Zou B., Lu B., Tian X., et al. A holistic view of gallic acid-induced attenuation in colitis based on microbiome-metabolomics analysis. Food Funct. 2019;10:4046–4061. doi: 10.1039/C9FO00213H. [DOI] [PubMed] [Google Scholar]
- 55.Wang C., Yang S., Gao L., Wang L., Cao L. Carboxymethyl pachyman (CMP) reduces intestinal mucositis and regulates the intestinal microflora in 5-fluorouracil-treated CT26 tumour-bearing mice. Food Funct. 2018;9:2695–2704. doi: 10.1039/C7FO01886J. [DOI] [PubMed] [Google Scholar]
- 56.Xiao S., Jiang S., Qian D., Duan J. Modulation of microbially derived short-chain fatty acids on intestinal homeostasis, metabolism, and neuropsychiatric disorder. Appl. Microbiol. Biotechnol. 2020;104:589–601. doi: 10.1007/s00253-019-10312-4. [DOI] [PubMed] [Google Scholar]
- 57.Luu M., Visekruna A. Short-chain fatty acids: Bacterial messengers modulating the immunometabolism of T cells. Eur. J. Immunol. 2019;49:842–848. doi: 10.1002/eji.201848009. [DOI] [PubMed] [Google Scholar]
- 58.Ratajczak W., Rył A., Mizerski A., Walczakiewicz K., Sipak O., Laszczyńska M. Immunomodulatory potential of gut microbiome-derived shortchain fatty acids (SCFAs) Acta. Biochim. Pol. 2019;66:1–12. doi: 10.18388/abp.2018_2648. [DOI] [PubMed] [Google Scholar]
- 59.Zhao Y., Chen F., Wu W., Sun M., Bilotta A.J., Yao S., Xiao Y., Huang X., Eaves-Pyles T.D., Golovko G., et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal. Immunol. 2018;11:752–762. doi: 10.1038/mi.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prasad K.N., Bondy S.C. Dietary fibers and their fermented short-chain fatty acids in prevention of human diseases. Bioact. Carbohydr. Diet. Fibre. 2019;17:100170. doi: 10.1016/j.bcdf.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Fachi J.L., de Felipe J.S., Pral L.P., da Silva B.K., Corrêa R.O., de Andrade M.C.P., da Fonseca D.M., Basso P.J., Camara N.O.S., de Sales E Souza E.L., et al. Butyrate protects mice from clostridium difficile-induced colitis through an HIF-1-dependent mechanism. Cell. Rep. 2019;27:750–761. doi: 10.1016/j.celrep.2019.03.054. [DOI] [PubMed] [Google Scholar]
- 62.Witten J., Samad T., Ribbeck K. Selective permeability of mucus barriers. Curr. Opin. Biotechnol. 2018;52:124–133. doi: 10.1016/j.copbio.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vendrame S., Klimis-Zacas D. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor- kB and mitogen-activated protein kinase signaling cascades. Nutr. Rev. 2015;73:348–358. doi: 10.1093/nutrit/nuu066. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Q., Luna-vital D., Gonzalez E., Mejia D. Anthocyanins from colored maize ameliorated the inflammatory paracrine interplay between macrophages and adipocytes through regulation of NF-κB and JNK-dependent MAPK pathways. J. Funct. Foods. 2019;54:175–186. doi: 10.1016/j.jff.2019.01.016. [DOI] [Google Scholar]
- 66.Xiao Y.T., Yan W.H., Cao Y., Yan J.K., Cai W. Neutralization of IL-6 and TNF-α ameliorates intestinal permeability in DSS-induced colitis. Cytokine. 2016;83:189–192. doi: 10.1016/j.cyto.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 67.Fries W., Muja C., Crisafulli C., Cuzzocrea S., Mazzon E. Dynamics of enterocyte tight junctions: Effect of experimental colitis and two different anti-TNF strategies. Am. J. Physiol. Gastrointest. Liver. Physiol. 2008;294:938–947. doi: 10.1152/ajpgi.00469.2007. [DOI] [PubMed] [Google Scholar]
- 68.Ferrari D., Speciale A., Cristani M., Fratantonio D., Molonia M.S., Ranaldi G., Cimino F. Cyanidin-3-O-glucoside inhibits NF-kB signalling in intestinal epithelial cells exposed to TNF-α and exerts protective effects via Nrf2 pathway activation. Toxicol. Lett. 2016;264:51–58. doi: 10.1016/j.toxlet.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 69.Tarabova L., Makova Z., Piesova E., Szaboova R., Faixova Z. Intestinal Mucus Layer and Mucins (A Review) Folia. Vet. 2016;60:21–25. doi: 10.1515/fv-2016-0003. [DOI] [Google Scholar]
- 70.Soderholm A.T., Pedicord V.A. Intestinal epithelial cells: At the interface of the microbiota and mucosal immunity. Immunology. 2019;158:267–280. doi: 10.1111/imm.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Furter M., Sellin M.E., Hansson G.C., Hardt W.D. Mucus architecture and near-surface swimming affect distinct Salmonella Typhimurium infection patterns along the murine intestinal tract. Cell. Rep. 2019;27:2665–2678. doi: 10.1016/j.celrep.2019.04.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghattamaneni N.K., Sharma A., Panchal S.K., Brown L. Pelargonidin 3-glucoside-enriched strawberry attenuates symptoms of DSS-induced inflammatory bowel disease and diet-induced metabolic syndrome in rats. Eur. J. Nutr. 2020;59:2905–2918. doi: 10.1007/s00394-019-02130-1. [DOI] [PubMed] [Google Scholar]
- 73.Park J.H., Kotani T., Konno T., Setiawan J., Kitamura Y., Imada S., Usui Y., Hatano N., Shinohara M., Saito Y., et al. Promotion of intestinal epithelial cell turnover by commensal bacteria: Role of short-chain fatty acids. PLoS ONE. 2016;11:e0156334. doi: 10.1371/journal.pone.0156334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wlodarska M., Thaiss C.A., Nowarski R., Henao-Mejia J., Zhang J.P., Brown E.M., Frankel G., Levy M., Katz M.N., Philbrick W.M., et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. 2014;156:1045–1059. doi: 10.1016/j.cell.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li J.M., Yu R., Zhang L.P., Wen S.Y., Wang S.J., Zhang X.Y., Xu Q., Kong L.D. Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: A benefit of short-chain fatty acids. Microbiome. 2019;7:1–14. doi: 10.1186/s40168-019-0713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y., Hatabu T. Mulberry juice freeze-dried powder attenuates the disease severity by the maintaining of colon mucosa in mice with DSS-induced acute colitis. Biosci. Biotechnol. Biochem. 2019;83:914–922. doi: 10.1080/09168451.2019.1580135. [DOI] [PubMed] [Google Scholar]
- 77.Crawley S.W., Mooseker M.S., Tyska M.J. Shaping the intestinal brush border. J. Cell. Biol. 2014;207:441–451. doi: 10.1083/jcb.201407015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Csernus B., Biró S., Babinszky L., Komlosi I., Jávor A., Stundl L., Remenyik J., Bai P., Olah J., Pesti-Asboth G., et al. Effect of carotenoids, oligosaccharides and anthocyanins on growth performance, immunological parameters and intestinal morphology in broiler chickens challenged with escherichia coli lipopolysaccharide. Animals. 2020;10:347. doi: 10.3390/ani10020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gessner D.K., Fiesel A., Most E., Dinges J., Wen G., Ringseis R., Eder K. Supplementation of a grape seed and grape marc meal extract decreases activities of the oxidative stress-responsive transcription factors NF-κB and Nrf2 in the duodenal mucosa of pigs. Acta. Vet. Scand. 2013;55:1–10. doi: 10.1186/1751-0147-55-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ghosh S.S., Wang J., Yannie P.J., Ghosh S. Intestinal barrier dysfunction, LPS translocation, and disease development. J. Endocr. Soc. 2020;4:1–15. doi: 10.1210/jendso/bvz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cochet F., Peri F. The role of carbohydrates in the lipopolysaccharide (LPS)/toll-like receptor 4 (TLR4) Signalling. Int. J. Mol. Sci. 2017;18:2318. doi: 10.3390/ijms18112318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Santamarina A.B., Jamar G., Mennitti L.V., de Cesar H.C., Vasconcelos J.R., Oyama L.M., de Rosso V.V., Pisani L.P. Obesity-related inflammatory modulation by juçara berry (Euterpe edulis Mart.) supplementation in Brazilian adults: A double-blind randomized controlled trial. Eur. J. Nutr. 2019;59:1693–1705. doi: 10.1007/s00394-019-02024-2. [DOI] [PubMed] [Google Scholar]
- 83.Magro D.O., Kotze P.G., Martinez C.A.R., Camargo M.G., Guadagnini D., Calixto A.R., Vasques A.C.J., de Lordes Setsuko Ayrizono M., Geloneze B., Pareja J.C., et al. Changes in serum levels of lipopolysaccharides and CD26 in patients with Crohn’s disease. Intest. Res. 2017;15:352–357. doi: 10.5217/ir.2017.15.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martini E., Krug S.M., Siegmund B., Neurath M.F., Becker C. The epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell. Mol. Gastroenterol. Hepatol. 2017;4:33–46. doi: 10.1016/j.jcmgh.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang X., Yan D., Xu M., Li F., Ren M., Zhang J., Wu M. Interactive association of lipopolysaccharide and free fatty acid with the prevalence of type 2 diabetes: A community-based cross-sectional study. J. Diabetes Investig. 2019;10:1438–1446. doi: 10.1111/jdi.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clemente-Postigo M., Oliva-Olivera W., Coin-Aragüez L., Ramos-Molina B., Giraldez-Perez R.M., Lhamyani S., Alcaide-Torres J., Perez-Martinez P., El Bekay R., Cardona F., et al. Metabolic endotoxemia promotes adipose dysfunction and inflammation in human obesity. Am. J. Physiol. Endocrinol. Metab. 2019;316:E319–E332. doi: 10.1152/ajpendo.00277.2018. [DOI] [PubMed] [Google Scholar]
- 87.Rodrigrez-Morató J., Matthan N.R., Liu J., Torre R., Chen C.Y.O. Cranberries attenuate animal-based diet-induced changes in microbiota composition and functionality: A randomized crossover controlled feeding trial. J. Nutr. Biochem. 2018;62:76–86. doi: 10.1016/j.jnutbio.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 88.Thounaojam M.C., Jadeja R.N., Sankhari J.M., Devkar R.V., Ramachandran A.V. Safety evaluations on ethanolic extract of red cabbage (Brassica oleracea L.) in Mice. J. Food Sci. 2011;76:T35–T39. doi: 10.1111/j.1750-3841.2010.01962.x. [DOI] [PubMed] [Google Scholar]
- 89.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;71:372. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed in this study are openly available in references number [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,40,41,42,43,44].