Abstract
Malignant melanoma is an aggressive cancer associated with a poor prognosis in patients with metastatic disease. As in many other cancers, the incidence of melanoma rises with age; and combined with the longer life expectancy, this led to an increasing prevalence of melanoma in the older population. Recently, immune checkpoint inhibitors significantly improved the treatment of melanoma given their efficacy and tolerability profile. Two major classes of agents include the anti-cytotoxic T lymphocyte-associated protein 4 (CTLA-4) inhibitors, such as ipilimumab, and the anti-programmed death-ligand 1 (PD-1) inhibitors, such as nivolumab and pembrolizumab. Treatment of metastatic disease with immune checkpoint inhibitors demonstrated improved efficacy and better safety profiles compared to cytotoxic drugs and appears to be an attractive treatment option. Nevertheless, there is a need for tools designed to better predict which older patients will benefit from its use and who will experience toxicities related to the treatment. Current data do not show a major increase in toxicity rates in older patients. However, patients above 75 are often under-represented and those who are included are not representative of the general population of older patients, thereby also stressing the need for real-life data. Ongoing research is aiming at maximizing the potential treatment efficacy and developing novel immune-targeting modalities. Future studies should include older patients and assess geriatric domains in these older patients to better guide decision-making. This review discusses published clinical trials and where known, the efficacy and toxicity in older patients. Moreover, the clinical implications and future perspectives are discussed, with current recommendations for older patients, management of toxicities, and a proposal for an initial approach to the treatment of older patients with metastatic melanoma.
Keywords: Melanoma, Older, Immunotherapy, Checkpoint inhibitors, Metastatic
1. Epidemiology – Melanoma in Older Patients
The global incidence for all stages of malignant melanoma is relevant, with reported numbers of 15 to 25 per 100,000 [1, 2]. Melanoma is associated with a poor prognosis in patients with metastatic disease. Prior to the advent of targeted therapies and immunotherapy, the median overall survival for advanced stage disease was <1 year [3,4]. The prevalence of melanoma in older adults is increasing owing to the rising incidence in the general population and to improved life expectancy.
Older age at melanoma diagnosis is associated with several poor disease-related prognostic factors, such as a higher Breslow thickness, the presence of tumour ulceration, and a higher mitotic rate [1]. In addition, older age itself is an independent negative prognostic factor [1], both in early stage and metastatic melanoma [5]. Differences in melanoma biology, complexity of treatment, socio-demographic factors (such as social support), delay in diagnosis, and under-treatment owing to comorbidities, organ dysfunction, and impaired functional status [6] may contribute to worse outcomes of older patients with melanoma [1]. The heterogeneity in the aging process may also further contribute to the complexity [7]. As merely chronologic age is often a poor descriptor in the aging process, a comprehensive geriatric assessment is needed to guide treatment decisions and identify patients at risk for adverse events [7]. The geriatric assessment should at least focus on determining or evaluating the functional capabilities, cognition, mental health and nutritional status of the older patient [1;7].
Melanoma is highly immunogenic and the efficacy of immunotherapy in this disease has been demonstrated in several studies in patients of different ages [8]. It was postulated in literature that immunosenescence, increased toxicities, and competing risks of death may negatively impact the efficacy of checkpoint inhibitors [9;10]. However, as will be discussed in the present review, older patients do seem to benefit from immunotherapies as much as their younger counterparts [6;11;12] even without increased toxicities [1;13].
2. Immunotherapy – An Introduction
Immunotherapy has long been investigated as an adjuvant treatment for high-risk loco-regional disease (interferon-α) or in unresectable melanoma due to its unique immunogenic properties, and it has served as a tumour model for immuno-oncology [14]. Some of these approaches include cytokines, adoptive cell immunotherapy, vaccines, and checkpoint inhibitors. Interleukin-2 (IL-2) and interferon-α (IFN-α) are examples of cytokine therapies, which are now infrequently used. The antitumor effects of IL-2 are mediated through T lymphocytes and natural killer (NK) cells [15]. Earlier studies evaluated the use of high-dose recombinant IL-2 in metastatic melanoma and demonstrated an objective response rate of 16%, and 6% of the patients achieved a complete response. However, the significant toxicities associated with IL-2 limit its use, especially in older adults [16]. IFN-α activates the immune response via several mechanisms including inhibition of tumour cell proliferation, anti-angiogenesis, and activation of immune cells [17]. IFN-α is usually considered for adjuvant treatment in melanoma patients at high risk of recurrence but it has now been largely replaced (or will become more common practice in the near future) by immune checkpoint inhibitors [18]. Similarly to IL-2, toxicities of IFN- α make it a challenging treatment option in older patients with melanoma.
Adoptive immunotherapy utilizes ex vivo expansion of human T-cells and adoptive transfer of these lymphocytes after lymphodepletion of the host immune system [19]. Although a number of studies demonstrated reasonable response rates in melanoma, the logistics and costs limit its use to specialized centres [20;21]. Multiple vaccines have also been studied and can be categorized into peptide-based, vector-based, dendritic cell-based, and tumour-based. An example of an in vivo vaccine approach that has been approved by the FDA is the oncolytic virus talimogene laherparepvec (T-VEC). T-VEC was the first oncolytic virus approved and has been shown to result in durable disease responses in patients with unresectable melanoma (especially in stage M1a) in a phase 3 trial [22].
Recently, immune checkpoint inhibitors (ICIs) have revolutionized the treatment of melanoma given their efficacy and tolerability profile. Two major classes of these agents include anti-cytotoxic T lymphocyte-associated protein 4 (CTLA-4) inhibitors, such as ipilimumab, and anti-programmed death-ligand 1 (PD-1) inhibitors, such as nivolumab and pembrolizumab. CTLA-4 is expressed on T-cells binding the antigen presenting cells and leads to inhibition of T-cell activation [23]. Anti CTLA-4 antibodies block such interactions and restore immune responses. Similarly, PD-1 is expressed on T-cells and interacts with PD-L1 ligands on tumour cells [23]. This interaction inhibits T-cell activation and provides an immune escape for cancer cells. Therefore, anti-PD-1 and anti-PD-L1 blocking antibodies restore antitumor immunity. Anti CTLA-4 and anti-PD-1 antibodies have been studied as monotherapies and combinations in melanoma patients in the adjuvant and metastatic setting [24;25]. These clinical trials are discussed in further detail below, along with evidence for older patients if available.
3. CTLA-4 Inhibitors – Ipilimumab
3.1. Advanced-Stage Setting
Ipilimumab is a fully human, IgG1B monoclonal antibody directed against the CTLA-4 receptor [26]. CTLA-4 inhibits T-cell-mediated immune responses through several mechanisms, including competitively binding to B7–1 and B7–2 on antigen presenting cells and preventing them to binding to CD28 on T cells, and limiting the cell-mediated immune response [27]. Consequently, CTLA-4 blockade amplifies T-cell activation and immune response and activates CD4+ and CD8+ cells [28]. Following a phase I study that documented response rates of 11.7% in patients with advanced-stage melanoma [4], a phase II study of incremental ipilimumab doses documented increased response rates in spite of higher toxicities [29]. Subsequently, a phase III trial comparing single-agent ipilimumab, a gp-100 peptide vaccine, and the combination of both in 676 pre-treated patients with advanced-stage melanoma demonstrated improved median overall survival (OS) in both the ipilimumab arms compared to the vaccine arm (10.0 versus 6.4 months, Hazard Ratio (HR): 0.68, p < .001). There was no significant difference between single-agent ipilimumab and the combination of ipilimumab and gp-100 peptide vaccine [24]. Another phase III study of 502 patients with treatment-naïve metastatic melanoma similarly showed improved OS with dacarbazine plus ipilimumab compared to dacarbazine plus placebo (11.2 versus 9.1 months), with a 3-year OS rate of 20.8% versus 12.2% (HR 0.72, p < .001). There was no difference in response rate (20%) [30]. Updated results showed a 5-year OS rate of 18% in the ipilimumab and dacarbazine arm compared to 9% in the dacarbazine and placebo arm [31]. In terms of toxicities, immune-related adverse events (IrAEs) occurred in 64.2% of patients on ipilimumab in various phase I-III trials and included enterocolitis, dermatitis, hepatitis, hypophysitis, and dysthyroidism. Approximately 20–30% of the adverse events were grade 3–4 [32]. A pooled analysis of ipilimumab studies documented a median OS of 11.4 months (95% Confidence Interval (CI) 10.7–12.1 months), a 3-year survival rate of 22% (95% CI 20–24%), and a Kaplan-Meier OS curve plateauing at 3 years and extending up to 10 years for some patients, regardless of prior therapy or ipilimumab dose [33]. The most frequent reason for discontinuation in the aforementioned studies was disease progression. Discontinuation due to drug-related adverse events was reported in a range from 36% up to 50% in the studies. Based on these encouraging results, ipilimumab was approved for use in the metastatic setting at 3 mg/kg by intravenous infusion given every three weeks for four doses.
3.2. Adjuvant setting
Ipilimumab was also found beneficial in the adjuvant setting (although this is not routine practice in all countries). At 5 years, ipilimumab improved recurrence-free survival (RFS) rates with 40.8% versus 38.9%, HR 0.76 (95% CI 0.64–0.89), distant metastasis–free survival (DMFS) rates with 48.3% versus 38.9%, HR 0.76 (95% CI 0.64–0.92), and OS rates with 65.4% versus 54.4%, HR 0.72 (95% CI 0.58–0.88) for high-risk (stage III) patients compared to placebo [34,35]. IrAEs were similar to those observed in the advanced stage setting, although overall toxicity was significant: 98.7% of patients treated with ipilimumab experienced adverse events and 54.1% were grade 3 or 4. Discontinuation rates due to adverse events were reported in more than half of the patients. Another phase III study is currently comparing two different doses of ipilimumab (10 mg/kg and the standard 3 mg/kg dose used in the metastatic setting) versus high-dose IFN-α with OS and RFS as co-primary endpoints (E-1609, NCT01274338). Preliminary data suggest a better toxicity profile for the 3 mg/kg dose without any RFS differences compared to the higher dose. The approved dose is 10 mg/kg every three weeks for four doses, with additional doses every 12 weeks for up to three years.
3.3. Older patients
Evidence regarding the use of ipilimumab in older patients is lacking. A meta-analysis including melanoma, lung, and kidney cancer patients suggested that patients over the age of 65–70 years benefit from anti-CTLA-4 in terms of OS [12]. For younger patients, the pooled HR for OS showed significant difference between the immune checkpoint inhibitors and a control regimen which did not include an immune checkpoint inhibitor with a HR of 0.75 (95% CI 0.68–0.82; p < .001). For older patients, an improved OS was also shown with a HR of 0.73 (95% CI 0.62–0.87; p < .001) in comparison with the control regimen. A consistent survival benefit was shown across different tumour type and treatment subgroups. The safety profile of ipilimumab in the older patients was also comparable to younger patients in terms of incidence of high-grade toxicity and unexpected adverse events. However, older patients included in these RCTs are likely not representative for the general older population due to the inclusion criteria. Another study also showed that the efficacy and toxicity of ipilimumab are similar in patients aged 70 years and older compared to their younger counterparts [36]. However, early ipilimumab discontinuation was common (31%) in a small series of older patients with melanoma from a population at the Memorial Sloan Kettering Cancer Center with 28% of them requiring systemic steroids [37].
4. PD-1/PD-L1 Inhibitors – Pembrolizumab And Nivolumab
4.1. Clinical Trials of Pembrolizumab
PD-1 is a cell surface receptor involved in down-regulating the immune response and promoting self-tolerance by suppressing T-cell inflammatory activity. PD-1 belongs to the CD28/CTLA4 receptor family [38–40]. PD-1 binds to two ligands PD-L1 (B7-H1) and PD-L2 (B7-DC) that are expressed in a variety of tissues [41;42]. PD-L1/PD-1 interactions inhibit CD8+ T-cell functions [39;40;43;44]. PD-L1 is expressed by a number of tumour types including melanoma [45] and anti-PD-1 monoclonal antibodies have demonstrated efficacy in melanoma in various clinical trials. Pembrolizumab is a humanized monoclonal antibody (IgG4/kappa isotype) blocking the interaction between PD-1 and its ligands PD-L1 and PD-L2. Pembrolizumab (formerly MK 3475 or lambrolizumab) was initially tested at three dose levels in the phase I KEYNOTE-001 study [46]. In the expansion cohort, patients with advanced stage melanoma were included (179 ipilimumab-naive and 115 previously treated with ipilimumab) and they received pembrolizumab 10 mg/kg (n = 183) or 2 mg/kg (n = 111) [47]. Results showed response rates of 40% in the ipilimumab-naïve group and 28% in the post-ipilimumab group with a median duration of response not reached (6–76+ weeks) and a median PFS at 5.5 months and 1-year OS at 69% [48]. Based on this, the FDA approved the use of pembrolizumab in September 2014 at the three-weekly dose of 2 mg/kg, initially in pre-treated patients and then in immunotherapy naive patients [49]. The phase II KEYNOTE-002 randomised controlled trial (RCT) of pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma documented improved PFS compared to chemotherapy in both the 2 mg/kg and 10 mg/kg arms [HR 0.57 (95%CI 0.45–0.73; p < .001) and HR 0.50 (95%CI 0.39–0.64; p < .01), respectively]. The response rates were also higher in the pembrolizumab arm compared to the chemotherapy arm (21% in the pembrolizumab 2 mg/kg arm and 25% in the pembrolizumab 10 mg/kg arm, respectively versus 4% in the chemotherapy arm, p < .001) [50].
Pembrolizumab was also evaluated in the phase III KEYNOTE-006 trial (10 mg/kg every two or every three weeks of pembrolizumab for four doses compared to ipilimumab at 3 mg/kg every three weeks for four doses). Objective response rate was 37% in patients on pembrolizumab every 2 weeks, 36% in patients on pembrolizumab every 3 weeks, and 13% in patients on ipilimumab [51]. Both of the pembrolizumab groups had superior OS compared to the ipilimumab group with a HR of 0.68 (95% CI 0.53–0.87; p = .0009] for the 2-week pembrolizumab schedule versus ipilimumab and a HR of 0.68 (95% CI 0.53–0.86; p = .0008) for the 3-week pembrolizumab schedule versus ipilimumab. PFS was longer with pembrolizumab than with ipilimumab with a HR of 0.61 (95% CI 0.50–0.75; p < .0001) for both the pembrolizumab schedules versus ipilimumab. Grade 3 to 5 adverse events were less frequent in the pembrolizumab groups (13.3% every two weeks and 10.1% every three weeks) compared to the ipilimumab group (19.9%) [51].
4.2. Clinical Trials of Nivolumab
Nivolumab is a fully human anti-PD-1 monoclonal antibody (IgG4) tested at different doses; the highest was 10 mg/kg every two weeks and no maximum tolerated dose was established. A phase I study in ipilimumab-naive patients with advanced disease documented a median OS of 17 months with all doses and 20 months with the 3 mg/kg dose. Median PFS was 3.7 months with all doses and 9.7 months with the 3 mg/kg dose [52]. FDA approval was granted to the 3 mg/kg dose given every two weeks based on the safety data and CheckMate-037 trial [53]. Nivolumab was the second PD-1 inhibitor approved in advanced melanoma progressing on ipilimumab and BRAF inhibitors in BRAF V600E-positive patients. The phase III CheckMate-037 trial evaluated nivolumab compared to investigator’s choice chemotherapy in BRAF V600E mutated metastatic melanoma patients after anti-CTLA-4 and BRAF inhibitors [53]. Objective response rate (ORR) was higher in the nivolumab group with 32% response rate compared to 11% in the chemotherapy group. However, there was no statistical difference in OS and PFS [53;54].
4.3. Older Patients
In a retrospective study by Betof et al., focusing on 254 patients with metastatic melanoma treated with anti PD-1 and/or anti PD-L1, there was no difference in OS across all age groups (age < 50: 22.9 months, age 50–64: 25.3 months, age 65–74: 22 months, age 75: 24.3 months). Similarly, PFS was not different across all age groups (age < 50: 4.1 months, age 50–64: 6.5 months, age 65–74: 5.4 months, age 75 and older: 7.9 months) [11]. European Cooperative Oncology Group Performance Status (ECOG PS) distribution was well balanced (mainly PS 0–1) and treatment was well tolerated across all age groups but the incidence of arthritis was significantly higher among patients aged 65–74 (10.8% p = .02). Patients over age 75 may experience more endocrine-related toxicities though this was not statistically significant. In another study of patients aged 80+, Friedman et al. observed a higher rate of grade 3 or higher toxicity in patients treated with the combination of ipilimumab and nivolumab who had at least 1 follow-up visit [37]. There were no treatment-related deaths reported in the abstract. In the patients who received the combination, more than a third (3/8 (37.5%)) required infliximab for diarrhoea and fatigue was reported for 4/8 (50%) of the patients. Median overall survival was 7.5 months (95% CI 6.0 13.7) in the ipilimumab patients, 14.2 months (95% CI 5.3-not reached (NR)) in the anti-PD1 patients and 23.5 months (95% CI 1.5-NR) in the patients who received the combination.
5. Combined Anti-CTLA-4 and Anti-PD-1 Immunotherapy
5.1. Clinical trials
Both anti CTLA-4 and PD-1 antibodies have been tested in combination in advanced and metastatic melanoma [55]. The CheckMate-069 phase II RCT compared nivolumab 1 mg/kg and ipilimumab 3 mg/kg versus ipilimumab 3 mg/kg and a placebo in 142 patients with stage III and IV melanoma [56]. After two years of follow-up, ORR was 59% (95% CI 48–69%) for the combined therapy and 11% (95%CI 3–23%; p < .001) for ipilimumab alone. The incidence of grade III adverse events was 44% in the combination arm and 17% in those who received ipilimumab alone. Most frequent adverse events in the combination arm were colitis (12%), increased liver enzymes (9%), and diarrhea (9%). Concomitantly, the CheckMate 067 phase III RCT was performed and included 945 patients with advanced melanoma [57]. The RCT compared nivolumab 3 mg/kg and ipilimumab 3 mg/kg versus ipilimumab 3 mg/kg alone versus nivolumab 3 mg/kg alone. An objective response was observed in 58% of patients in the combination arm (95% CI 53–65%), 44% in patients with nivolumab alone (95% CI 39–50%), and 19% in patients who received ipilimumab alone (95% CI 15–24%). The incidence of grade 3 or 4 events was 59% in the combination arm, 21% in the nivolumab alone arm, and 28% in the ipilimumab alone arm. The toxicity profile was confirmed in a pooled analysis of Checkmate-067, Checkmate-069, and the Phase Ib CA209–004 cohort 8, with the most common toxicity involving the gastrointestinal tract [58]. Other common adverse events included skin toxicities (pruritus, rash and vitiligo), endocrine toxicities (hypothyroidism and hypophysitis), and elevated liver enzymes.
5.2. Older Patients
With regard to the applicability of this evidence in older patients, little is known. The CheckMate-069 included patients with a median age of 65 (range 27–87), but no further age distribution or geriatric parameters were described. Most patients had a good performance status of 0 or 1, only two patients had a ECOG performance status of 2. Similarly, the CheckMate-067 included patients with a good performance status (ECOG 0 or 1), although almost half of the patients were aged 65 years or older. Subgroup analyses showed that the combination arm may be more effective in patients younger than 65 years (HR for PFS 0.73 in patients aged <65 years versus 0.90 in patients aged 65 years or older), although the statistical significance of these was not reported [57]. In addition, the high incidence of grade III adverse events, especially grade III diarrhea and colitis in the combination arm may affect quality of life and performance status in older patients who already have low physiologic reserves.
6. BRAF And MEK Inhibitors
6.1. Clinical Trials
Approximately 40–60% of advanced melanomas carry activating mutations in BRAF and 80–90% of these involve a substitution of glutamic acid for valine at amino-acid 600 (V600E mutation) [59]. A V600E or V600K mutation predicts effectiveness of BRAF or MEK inhibition. BRAF inhibitors such as vemurafenib, dabrafenib, and encorafenib, have shown considerable activity in phase III trials of patients with advanced stage BRAF-mutated melanoma [60]. These agents were initially found to be effective as a monotherapy [60–62], but the combination with MEK inhibitors, such as trametinib, cobimetinib, binimetinib, have now replaced single-agent BRAF inhibition. The phase III COMBI-d trial that included patients with metastatic melanoma who had either a V600E or V600K mutation demonstrated better median PFS [11.0 versus 8.8 months, HR 0.67 (95% CI 0.53–0.84)], OS [median 25.1 versus 18.7 months, HR 0.71 (95% CI 0.55–0.92)], and ORR (68% versus 55%) with dabrafenib plus trametinib compared to dabrafenib alone [63–65]. Skin toxicities (dry skin, pruritus, hyperkeratosis, hand-foot syndrome, alopecia, skin papilloma and squamous cell carcinoma [SCC]) were more frequent with dabrafenib alone, whereas the combination was associated with increased diarrhea, fever, and chills. Dabrafenib plus trametinib have also been found to be superior to vemurafenib alone in BRAF-mutated advanced stage disease (three-year OS 25% versus 11%; median PFS 11.4 versus 7.3 months, and ORR: 67% versus 53%) [66;67]. Response rate using this doublet regimen is relatively durable [68] especially in patients with normal LDH and less than three organs involved [69]. Moreover, vemurafenib plus cobimetinib showed superiority compared to vemurafenib alone in V600 mutated metastatic melanoma patients in the first line setting [median PFS 12.3 versus 7.2 months, HR 0.58, (95% CI 0.46–0.72) with an ORR of 70% versus 50% and a median OS 22.3 versus 17.4 months (HR 0.70 (95% CI 0.55–0.90)] [69;70]. The combination of encorafenib and binimetinib is currently being compared to vemurafenib alone and encorafenib alone (NCT01909453); first results showed improved outcomes for the combination in comparison to encorafenib alone [71].
The most common toxicities associated with BRAF inhibition as monotherapy include rash, hyperkeratosis, photosensitivity, arthralgia, fatigue, nausea, diarrhea and alopecia, reported in 15% of patients [13]. SCC, including keratoacanthomas, was observed in 19–26% of patients on vemurafenib or dabrafenib [72], although it is unclear whether they can increase the incidence of further primary melanomas [73]. QT interval prolongation, peripheral facial nerve palsy, and creatinine decrease are associated with vemurafenib, whereas dabrafenib may cause febrile reactions and hyperglycaemia. MEK inhibitors toxicities include dermatologic side effects, diarrhea, oedema, decreased cardiac ejection fraction and visual problems.
6.2. Older patients
Similarly, evidence is lacking for the use of BRAF and MEK inhibitors in older patients. In a safety analysis of vemurafenib [13], 8% of patients enrolled were aged above 75 and reported increase rates of serious adverse events and grade 3–4 toxicities mainly due to more frequent skin SCC, keratoacanthomas, QT interval prolongation and drug interactions. However, no differences in ORR and PFS were observed compared to their younger counterparts. In the subgroup analysis of the registration study for the combination of dabrafenib and trametinib, the OS benefit was maintained in older patients [66]. The benefit in PFS was also maintained across age groups in the trial testing the combination of vemurafenib and cobimetinib [69].
7. Clinical Implications & Future Perspectives
7.1. Current Recommendations
The last decade has brought an unprecedented amount of therapeutic advances and discoveries which have revolutionized the treatment of metastatic melanoma. However, there is still a paucity of data on the safety of immunotherapy and targeted therapies in older adults with melanoma, as well as on the optimal sequencing of treatment in this patient population. Most of the studies did not analyse the data based on age groups, and patients over the age of 75 are rarely included [6]. Therefore, the current therapeutic approach for older adults with metastatic melanoma closely resembles that of younger patients, albeit with less data from RCTs supporting it. Current recommendations take into account several patient and tumour-related factors when deciding the initial choice of treatment. These factors include the extent of metastatic disease, presence of brain metastases, patients’ performance status, and presence of BRAF V600 activating mutations [74]. A potentially useful way to determine which older adults with metastatic melanoma may be fit enough to undergo the various systemic treatments available is to perform a comprehensive geriatric assessment [75]. The International Society of Geriatric Oncology (SIOG) recommendations on geriatric assessment were updated in 2014 [7] with regards to the rationale for performing it, its ability to predict complications, the association with survival, its impact on treatment decisions and the selection of domains and tools and methods to implement them. Several geriatric domains are recommended to be evaluated as part of a comprehensive geriatric assessment including functional status, comorbidity, cognition, mental health status, fatigue, social status and support, nutrition, and presence of geriatric syndromes; however the expert panel could not recommend specific tools or models over another [7].
7.2. Proposed Approach to Treatment of Older Patients
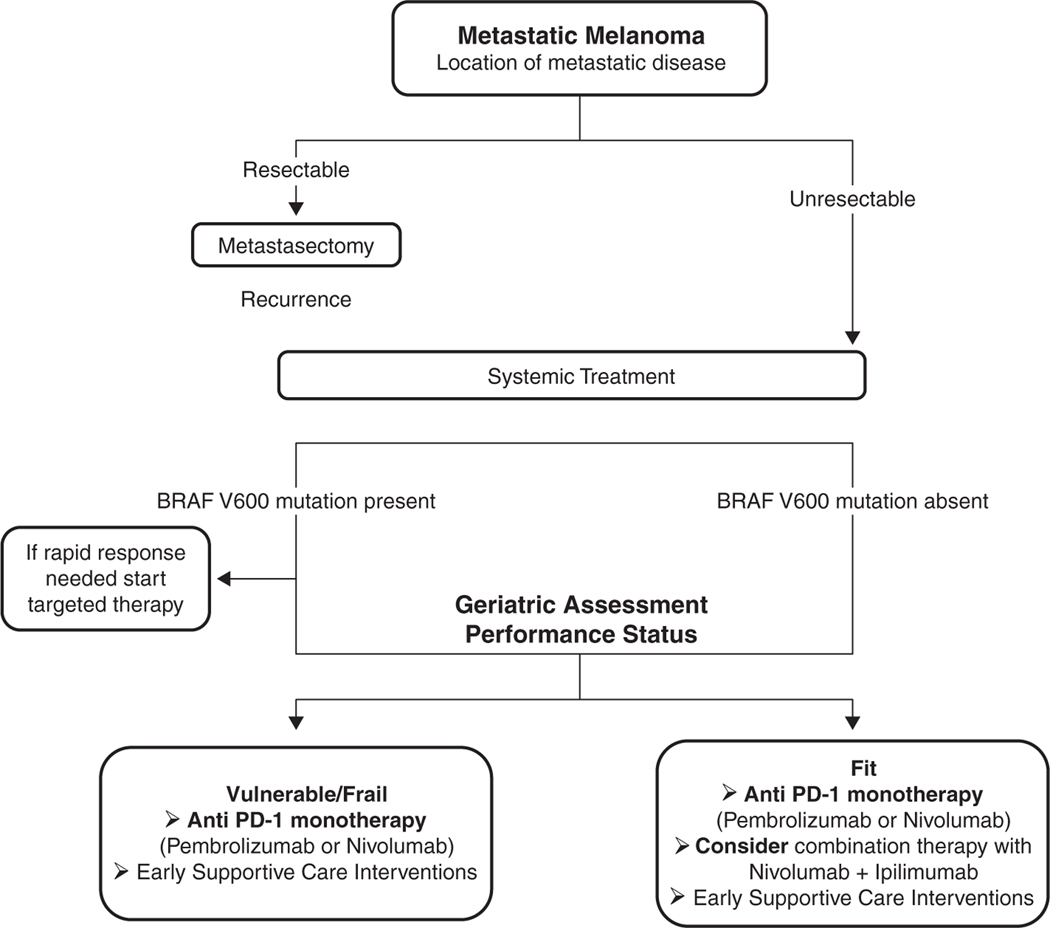
A suggested initial approach to the treatment of older patients with metastatic melanoma is shown in Fig. 1. For most patients (except those with BRAF mutation in which an early and fast response is needed to decrease tumour burden), the initial choice of treatment should consist of an anti PD-1 antibody (pembrolizumab or nivolumab) [74]. Although the combination of nivolumab and ipilimumab is approved for use in metastatic melanoma, its toxicity profile and the lack of evidence regarding improvements in OS over anti PD-1 monotherapy should discourage its use in the majority of older patients [57;74;76]. In patients progressing after an anti-PD-1 monotherapy, the preferred second-line therapy is ipilimumab [74]. Nevertheless, it must be emphasized that up to 50% of patients treated with this sequence may experience grade 3–5 toxicities, and therefore should be used cautiously in older patients, particularly those who are found to be vulnerable or frail on geriatric assessment [77]. Differences in adverse events might be attributable to differences in pharmaco-dynamic properties of the agents; an anti-CTLA-4 antibody bound to CTLA-4 might be rapidly internalised by the cell, while the anti-PD-1 antibody might occupy the PD-1 receptor for a prolonged period [77]. An alternative explanation might be that ipilimumab is more toxic than nivolumab; confirmed by studies showing that nivolumab and pembrolizumab had fewer high grade adverse events than ipilimumab monotherapy.
Fig. 1.
Proposed initial approach to the treatment of older patients with metastatic melanoma.
7.3. Management of Toxicities
Immune checkpoint–blockade toxicities can occur early and late in the treatment course. Most common toxicities include rash or dermatitis, diarrhea or colitis, hepatitis and endocrinopathies [78]. Progression of the underlying malignancy, infection, and other possible causes should also be ruled out along with identifying and treating a given toxicity. Their severity should be evaluated and their management should be individualised accordingly [78;79]. The primary treatment usually consists of supportive care interventions, high-dose steroids and/or treatment interruptions or discontinuation [80]. It is important to keep in mind that high-dose steroids may cause debilitating side effects in older adults such as cognitive impairment and worsening of preexisting diabetes. Studies to date do not suggest that age alone affects tolerance to immunotherapy. However, patients with prior immune disease (e.g. Crohn’s disease, rheumatoid arthritis, multiple sclerosis) are usually excluded from clinical trials and these diagnoses are more common in older adults. Also, hospitalization may have a different impact on older patients as compared to younger patients [81]. On the other hand, compared to other systemic treatment options, quality of life and functional status impairment is less frequent with checkpoint inhibitors [6].
7.4. Prediction Tools
Although the clinical effectiveness of immunotherapy in metastatic melanoma is proven, tools able to predict which older patients will benefit the most from its use and who will experience treatment toxicities are needed. In contrast with studies conducted in patients receiving chemotherapy [82;83], little is known about the use of geriatric assessment-based tools for the prediction of immunotherapy-related toxicities, and research on this topic is greatly needed in the field of geriatric oncology. An ongoing French study conducted within the MELBASE cohort (NCT03155217) is investigating outcomes and toxicities of older patients with melanoma treated with immunotherapy, and are correlating their outcomes with geriatric assessment variables. Although this study may shed some light into the predictors of immune-mediated toxicities in older adults, future prospective RCTs in metastatic melanoma should include more granular measures of various geriatric domains in order to better characterize the older patient population [84].
Another area of active interest is the use of predictive biomarkers in order to identify those patients more likely to benefit from immunotherapies. Examining both the biology and the genomics of the melanoma and the host immune system is critical to recognize potential biomarkers [85]. Baseline serum lactate dehydrogenase (LDH) was demonstrated to be a strong predictive factor; ipilimumab long-term benefit was unlikely in patients with a baseline serum LDH which was higher than twice the upper limit of normal [86]. A recent study found that a low fraction of partially exhausted cytotoxic T lymphocytes (peCTL) in pre-treatment melanoma samples (using flow cytometry) was predictive of response to combination immunotherapy across all age groups [87]. In this study with 102 melanoma patients, the peCTL responders had a higher peCTL fraction of 30.5% versus 15.4% in non-responders (p < .0001). Overall response rates (ORRs) to anti–PD-1 monotherapy or combination therapy were similar in high-peCTL (>20%) patients; 70% of patients treated with ipilimumab/nivolumab responded, and 66.7% of patients treated with anti–PD-1 monotherapy responded (p = .99). However, in the low peCTL population, 5.6% responded to anti–PD-1 monotherapy, whereas 35% responded to the combination (relative risk 6.3 (95% CI 1.2–37.7; p = .045). Additionally, a 15-gene pre-treatment classifier model was able to accurately identify patients with metastatic melanoma (median age 59, range 22–90) who were more likely to respond to CTLA-4 inhibitor tremelimumab [88]. In 210 patients, using correlated component regression analysis, a 15-gene classifier model (9 predictors and 6 non-predictive enhancer variables) was identified as optimal in terms of AUC for the discovery dataset. AUC was 0.86 (95% CI 0.81–0.91: p < .0001) for response and 0.60 (95% CI 0.54–0.67; p = .0066) for one-year survival in the discovery set. The model was validated; AUC in the validation set was 0.62 (95% CI 0.54–0.70; p = .0455) for response and 0.68 (95% CI 0.59–0.75; p = .0002) for one-year survival. Despite some publications of predictive markers, there is no definitive biomarker that could select or exclude patients for treatment with checkpoint inhibitors [85].
Better selection of patients is relevant not only because of its toxicity profile, but also because of the high costs. In several developed countries, such as the United States, Norway or the United Kingdom, new melanoma drugs (including the checkpoint inhibitors and BRAF/MEK inhibitors) have not been shown to be cost-effective compared to dacarbazine nor were cost-effective at what has normally been considered a reasonable willingness-to-pay in Norway [89;90]. In developing countries, where immunotherapy is frequently not covered by insurance, many patients will still receive cytotoxic chemotherapy given the financial burden associated with immunotherapy. Nevertheless, regardless of the treatment received, all patients with metastatic melanoma should be provided with early access to supportive care services in order to manage symptoms and treatment side effects. The Multinational Association of Supportive Care in Cancer (MASCC) [91] and the European Society of Medical Oncology [92] have recently published recommendations for the management of toxicities and supportive care of patients undergoing immunotherapy, and although these are not specific to older adults, they represent a useful resource for clinicians.
7.5. Conclusion and Future Perspectives
Treatment of metastatic disease with immune checkpoint inhibitors demonstrated improved efficacy and better safety profiles compared to cytotoxic drugs and appears to be an attractive treatment option [6]. Comparable efficacy in older as compared to younger patients has been shown with respect to overall survival [12]. However, treatment is associated with IrAEs, therefore it is recommended that all patients routinely have thyroid function studies, complete blood counts, liver function tests, and metabolic panels checked [6;93]. Current data do not show a major increase in toxicity rates in older patients. However, patients above 75 are often under-represented in the clinical trials. [3, 4, 17] In addition, older patients are frequently excluded from clinical trials due to a decreased general health or inadequate organ function, limiting the applicability of current evidence in this specific population [94]. It is however unlikely that this level of evidence will be achieved in older patients as it might not be feasible to repeat all RCTs for older patients [95]. Prospective observational studies or real-life data from registries may fill part of the gap in knowledge, especially in predicting toxicity for older patients.
The future of immune checkpoint inhibitors in melanoma will be aimed at maximizing the potential efficacy of immune checkpoint inhibitors, developing novel immune-targeting modalities, and focusing on the combination of the various immune checkpoint inhibitors, combinations of immune checkpoint inhibitors with established therapies including radiotherapy, chemotherapy and targeted therapy, and combinations of immune checkpoint inhibitors with novel forms of immunotherapy currently in development [96]. Although the role of immune checkpoint inhibitors in older patients has yet to be more thoroughly explored, this option remains highly recommended [6]. Future RCTs should be without upper age limit and include a geriatric assessment or screening for older patients to build a more solid amount of evidence to better guide decision-making. Given the increasing incidence of melanoma particularly among older patients, their participation in clinical trials is essential [1].
Acknowledgement
The authors would like to acknowledge Dr. M.A. Postow (Melanoma Service, Memorial Sloan Kettering Cancer Center, NY, USA) for his valuable comments on the manuscript.
Funding
Dr. Lichtman is supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. The other authors have no funding to report for this study.
Footnotes
Conflicts/Disclosures
No conflict of interest for all authors.
References
- [1].Rogiers A, van den Oord JJ, Garmyn M, Stas M, Kenis C, Wildiers H, et al. Novel therapies for metastatic melanoma: an update on their use in older patients. Drugs Aging 2015. October;32(10):821–34. [DOI] [PubMed] [Google Scholar]
- [2].Schadendorf D, Hauschild A. Melanoma in 2013: melanoma–the run of success continues. Nat Rev Clin Oncol 2014. February;11(2):75–6. [DOI] [PubMed] [Google Scholar]
- [3].Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 2008. February 1;26(4):527–34. [DOI] [PubMed] [Google Scholar]
- [4].Nashan D, Muller ML, Grabbe S, Wustlich S, Enk A. Systemic therapy of disseminated malignant melanoma: an evidence-based overview of the state-of-the-art in daily routine. J Eur Acad Dermatol Venereol 2007. November;21(10):1305–18. [DOI] [PubMed] [Google Scholar]
- [5].Kuk D, Shoushtari AN, Barker CA, Panageas KS, Munhoz RR, Momtaz P, et al. Prognosis of mucosal, uveal, acral, nonacral cutaneous, and unknown primary melanoma from the time of first metastasis. Oncologist 2016. July;21(7):848–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Elias R, Morales J, Rehman Y, Khurshid H. Immune checkpoint inhibitors in older adults. Curr Oncol Rep 2016. August;18(8):47. [DOI] [PubMed] [Google Scholar]
- [7].Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014. August 20;32(24):2595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Russo AE, Ferrau F, Antonelli G, Priolo D, McCubrey JA, Libra M. Malignant melanoma in elderly patients: biological, surgical and medical issues. Expert Rev Anticancer Ther 2015. January;15(1):101–8. [DOI] [PubMed] [Google Scholar]
- [9].Derhovanessian E, Solana R, Larbi A, Pawelec G. Immunity, ageing and cancer. Immun Age 2008. September 24;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chou JP, Effros RB. T cell replicative senescence in human aging. Curr Pharm Des 2013;19(9):1680–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Betof AS, Nipp RD, Giobbie-Hurder A, Johnpulle RAN, Rubin K, Rubinstein SM, et al. Impact of age on outcomes with immunotherapy for patients with melanoma. Oncologist 2017. August;22(8):963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nishijima TF, Muss HB, Shachar SS, Moschos SJ. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: a systematic review and meta-analysis. Cancer Treat Rev 2016. April;45:30–7. [DOI] [PubMed] [Google Scholar]
- [13].Larkin J, Del VM, Ascierto PA, Krajsova I, Schachter J, Neyns B, et al. Vemurafenib in patients with BRAF(V600) mutated metastatic melanoma: an open-label, multicentre, safety study. Lancet Oncol 2014. April;15(4):436–44. [DOI] [PubMed] [Google Scholar]
- [14].Zeuthen J, Dzhandzhugazyan K, Hansen MR, Kirkin AF. The immunogenic properties of human melanomas and melanoma-associated antigens recognized by cytotoxic T lymphocytes. Bratisl Lek Listy 1998. August;99(8–9):426–34. [PubMed] [Google Scholar]
- [15].Eklund JW, Kuzel TM. A review of recent findings involving interleukin-2-based cancer therapy. Curr Opin Oncol 2004. November;16(6):542–6. [DOI] [PubMed] [Google Scholar]
- [16].Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999. July;17(7): 2105–16. [DOI] [PubMed] [Google Scholar]
- [17].Hervas-Stubbs S, Perez-Gracia JL, Rouzaut A, Sanmamed MF, Le BA, Melero I. Direct effects of type I interferons on cells of the immune system. Clin Cancer Res 2011. May 1;17(9):2619–27. [DOI] [PubMed] [Google Scholar]
- [18].Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996. January;14(1):7–17. [DOI] [PubMed] [Google Scholar]
- [19].Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006. October 6;314(5796):126–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 2005. April 1;23(10):2346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008. November 10;26 (32):5233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].1alimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. 1–9-2017. Ref Type: Internet Communication. [DOI] [PubMed]
- [23].Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 2016. February;39(1):98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010. August 19;363(8):711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015. September 24;373(13):1270–1. [DOI] [PubMed] [Google Scholar]
- [26].Specenier P. Ipilimumab in melanoma. Expert Rev Anticancer Ther 2012. December;12 (12):1511–21. [DOI] [PubMed] [Google Scholar]
- [27].Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol 2006. April;18(2): 206–13. [DOI] [PubMed] [Google Scholar]
- [28].Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol 2005. December 1;175(11):7746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010. February;11 (2):155–64. [DOI] [PubMed] [Google Scholar]
- [30].Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011. June 30;364(26):2517–26. [DOI] [PubMed] [Google Scholar]
- [31].Maio M, Grob JJ, Aamdal S, Bondarenko I, Robert C, Thomas L, et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol 2015. April 1;33(10): 1191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012. July 20;30(21):2691–7. [DOI] [PubMed] [Google Scholar]
- [33].Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, et al. Pooled analysis of long-term survival data from phase II and phase III trials of Ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015. June 10;33(17):1889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015. May;16(5):522–30. [DOI] [PubMed] [Google Scholar]
- [35].Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Prolonged survival in stage III melanoma with Ipilimumab adjuvant therapy. N Engl J Med 2016. November 10;375(19):1845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chiarion S V, Pigozzo J, Ascierto PA, Grimaldi AM, Maio M, Di GL, et al. Efficacy and safety of ipilimumab in elderly patients with pretreated advanced melanoma treated at Italian centres through the expanded access programme. J Exp Clin Cancer Res 2014. April 4;33:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Friedman CF, Horvat TZ, Minehart J. Efficacy and safety of checkpoint blockade for treatment of advanced melanoma (mel) in patients (pts) age 80 and older (80+). American Society of Clinical Oncology annual meeting. 2016. [Ref type: Abstract]. [Google Scholar]
- [38].Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 2010. December;10(12):826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Okazaki T, Iwai Y, Honjo T. New regulatory co-receptors: inducible co-stimulator and PD-1. Curr Opin Immunol 2002. December;14(6):779–82. [DOI] [PubMed] [Google Scholar]
- [40].Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 2007. March;8(3):239–45. [DOI] [PubMed] [Google Scholar]
- [41].Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med 2001. April 2; 193(7):839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001. March;2(3): 261–8. [DOI] [PubMed] [Google Scholar]
- [43].Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000. October 2;192(7):1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dong H, Zhu G, Tamada K, Chen L. B7-H1, athird member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999. December;5(12):1365–9. [DOI] [PubMed] [Google Scholar]
- [45].Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, et al. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res 2004. February 1;64(3):1140–5. [DOI] [PubMed] [Google Scholar]
- [46].Patnaik A, Kang SP, Rasco D, Papadopoulos KP, Elassaiss-Schaap J, Beeram M, et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res 2015. October 1;21(19):4286–93. [DOI] [PubMed] [Google Scholar]
- [47].Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013. July 11;369(2):134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ribas A, Hodi S, Kefford R, Hamid O, Daud A, Wolchok JD, et al. Efficacy and safety of the anti-PD-1 monoclonal antibody MK-3475 in 411 patients (pts) with melanoma (MEL). ASCO meeting; 2014. [Ref Type: Abstract]. [Google Scholar]
- [49].Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014. September 20;384(9948):1109–17. [DOI] [PubMed] [Google Scholar]
- [50].Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015. August;16(8):908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017. August 16;390(10105):1853–62. [DOI] [PubMed] [Google Scholar]
- [52].Hodi FS, Lee S, McDermott DF, Rao UN, Butterfield LH, Tarhini AA, et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA 2014. November 5;312(17):1744–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015. April;16(4):375–84. [DOI] [PubMed] [Google Scholar]
- [54].Larkin J, Minor D, D’Angelo S, Neyns B, Smylie M, Miller WH Jr, et al. Overall survival in patients with advanced melanoma who received Nivolumab versus Investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol 2018. February 1;36(4):383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hao C, Tian J, Liu H, Li F, Niu H, Zhu B. Efficacy and safety of anti-PD-1 and anti-PD-1 combined with anti-CTLA-4 immunotherapy to advanced melanoma: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017. June;96(26):e7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015. May 21;372(21):2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined Nivolumab and Ipilimumab in advanced melanoma. N Engl J Med 2017. October 5;377(14):1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sznol M, Ferrucci PF, Hogg D, Atkins MB, Wolter P, Guidoboni M, et al. Pooled analysis safety profile of Nivolumab and Ipilimumab combination therapy in patients with advanced melanoma. J Clin Oncol 2017. September 15;35(34):3815–22 [JCO2016721167]. [DOI] [PubMed] [Google Scholar]
- [59].Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol 2011. April 1;29(10):1239–46. [DOI] [PubMed] [Google Scholar]
- [60].Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011. June 30;364(26):2507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].McArthur GA, Chapman PB, Robert C, Larkin J, Haanen JB, Dummer R, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): Extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 2014. March;15(3):323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012. July 28;380(9839):358–65. [DOI] [PubMed] [Google Scholar]
- [63].Long GV, Stroyakovskiy D, Gogas H, Levchenko E, De BF, Larkin J, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014. November 13;371(20):1877–88. [DOI] [PubMed] [Google Scholar]
- [64].Long GV, Stroyakovskiy D, Gogas H, Levchenko E, De BF, Larkin J, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet 2015. August 1; 386(9992):444–51. [DOI] [PubMed] [Google Scholar]
- [65].Long GV, Flaherty KT, Stroyakovskiy D, Gogas H, Levchenko E, De BF, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol 2017. July 1;28(7):1631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015. January 1;372(1):30–9. [DOI] [PubMed] [Google Scholar]
- [67].Robert C, Karaszewska B, Schacter J. Three-year estimate of overall survival in COMBI-v, a randomized phase 3 study evaluating first-line dabrafenib + trametinib in patients with unresectable or metastatic BRAF V600E/K-mutant cutaneous melanoma (abstract LBA40). European Society for Medical Oncology meeting; 2016. [Ref Type: Abstract]. [Google Scholar]
- [68].Long GV, Grob JJ, Nathan P, Ribas A, Robert C, Schadendorf D, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol 2016. December;17(12):1743–54. [DOI] [PubMed] [Google Scholar]
- [69].Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014. November 13;371(20):1867–76. [DOI] [PubMed] [Google Scholar]
- [70].Larkin J, Yan Y, McArthur GA. Update of progression-free survival (PFS) and correlative biomarker analysis from coBRIM: Phase III study of cobimetinib (cobi) plus vemurafenib (vem) in advanced BRAF-mutated melanoma. J Clin Oncol 2015;33 (suppl abstract 9006) [Ref Type: Abstract]. [Google Scholar]
- [71].Sullivan RY, Weber JS, Patel SP. A phase Ib/II study of BRAF inhibitor (BRAFi) encorafenib (ENCO) plus MEK inhibitor (MEKi) binimetinib (BINI) in cutaneous melanoma patients naive to BRAFi treatment. J Clin Oncol 2015;33(suppl abstract 9007) [Ref Type: Abstract]. [Google Scholar]
- [72].Lacouture ME, Duvic M, Hauschild A, Prieto VG, Robert C, Schadendorf D, et al. Analysis of dermatologic events in vemurafenib-treated patients with melanoma. Oncologist 2013;18(3):314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zimmer L, Haydu LE, Menzies AM, Scolyer RA, Kefford RF, Thompson JF, et al. Incidence of new primary melanomas after diagnosis of stage III and IV melanoma. J Clin Oncol 2014. March 10;32(8):816–23. [DOI] [PubMed] [Google Scholar]
- [74].National Comprehensive Cancer Network.Melanoma (Version 1.2018). 2017. Ref Type: Internet Communication.
- [75].Ferrat E, Paillaud E, Caillet P, Laurent M, Tournigand C, Lagrange JL, et al. Performance of four frailty classifications in older patients with cancer: prospective elderly cancer patients cohort study. J Clin Oncol 2017. March;35(7):766–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Gyawali B, Prasad V. Combining drugs and extending treatment - a PFS end point is not sufficient. Nat Rev Clin Oncol 2017. September;14(9):521–2. [DOI] [PubMed] [Google Scholar]
- [77].Weber JS, Gibney G, Sullivan RJ, Sosman JA, Slingluff CL Jr, Lawrence DP, et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol 2016. July;17(7):943–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Teply BA, Lipson EJ. Identification and management of toxicities from immune checkpoint-blocking drugs. Oncology (Williston Park) 2014. November;28(Suppl. 3): 30–8. [PubMed] [Google Scholar]
- [79].Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy. J Clin Oncol. American Society of Clinical Oncology Clinical Practice Guideline; 2018. February 14 [JCO2017776385]. [DOI] [PubMed] [Google Scholar]
- [80].Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist 2013. June;18(6):733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Shoushtari AN, Friedman CF, Navid-Azarbaijani P, Postow MA, Callahan MK, Momtaz P, et al. Measuring toxic effects and time to treatment failure for Nivolumab plus Ipilimumab in melanoma. JAMA Oncol 2018. January 1;4(1):98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011. September 1;29(25):3457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, Defelice J, et al. Predicting the risk of chemotherapy toxicity in older patients: the chemotherapy risk assessment scale for high-age patients (CRASH) score. Cancer 2012. July 1;118(13):3377–86. [DOI] [PubMed] [Google Scholar]
- [84].Soto-Perez-De-Celis E, Lichtman SM. Considerations for clinical trial design in older adults with cancer. Expert Opin Investig Drugs 2017. October;26(10):1099–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Maleki VS, Garrigos C, Duran I. Biomarkers of response to PD-1/PD-L1 inhibition. Crit Rev Oncol Hematol 2017. August;116:116–24. [DOI] [PubMed] [Google Scholar]
- [86].Kelderman S, Heemskerk B, Van TH, Van den Brom RR, Hospers GA, van den Eertwegh AJ, et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother 2014. May;63 (5):449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Loo K, Tsai KK, Mahuron K, Liu J, Pauli ML, Sandoval PM, et al. Partially exhausted tumor-infiltrating lymphocytes predict response to combination immunotherapy. JCI Insight 2017. July 20;2(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Friedlander P, Wassmann K, Christenfeld AM, Fisher D, Kyi C, Kirkwood JM, et al. Whole-blood RNA transcript-based models can predict clinical response in two large independent clinical studies of patients with advanced melanoma treated with the checkpoint inhibitor, tremelimumab. J Immunother Cancer 2017. August 15; 5(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Pike E, Hamidi V, Saeterdal I, Odgaard-Jensen J, Klemp M. Multiple treatment comparison of seven new drugs for patients with advanced malignant melanoma: a systematic review and health economic decision model in a Norwegian setting. BMJ Open 2017. August 21;7(8):e014880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Rubio-Rodriguez D, De Diego BS, Perez M, Rubio-Terres C. Cost-effectiveness of drug treatments for advanced melanoma: a systematic literature review. Pharmacoeconomics 2017. May 27;35(9):879–93. [DOI] [PubMed] [Google Scholar]
- [91].Rapoport BL, Van ER, Sibaud V, Epstein JB, Klastersky J, Aapro M, et al. Supportive care for patients undergoing immunotherapy. Support Care Cancer; 2017. July 13. [DOI] [PubMed] [Google Scholar]
- [92].Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017. July 1;28(suppl_4):iv119–42. [DOI] [PubMed] [Google Scholar]
- [93].Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of immunotherapy for the practitioner. J Clin Oncol 2015. June 20;33(18):2092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Battisti NML, Sehovic M, Extermann M. Assessment of the external validity of the National Comprehensive Cancer Network and European Society for Medical Oncology guidelines for non-small-cell lung cancer in a population of patients aged 80 years and older. Clin Lung Cancer 2017. September;18(5):460–71. [DOI] [PubMed] [Google Scholar]
- [95].de Glas NA, Kiderlen M, de Craen AJ, Hamaker ME, Portielje JE, van de Velde CJ, et al. Assessing treatment effects in older breast cancer patients: systematic review of observational research methods. Cancer Treat Rev 2015. March;41(3): 254–61. [DOI] [PubMed] [Google Scholar]
- [96].O’Reilly A, Larkin J. Checkpoint inhibitors in advanced melanoma: effect on the field of immunotherapy. Expert Rev Anticancer Ther 2017. July;17(7):647–55. [DOI] [PubMed] [Google Scholar]