Summary
Cardiac catheterization is an integral part of medical management for pediatric patients with congenital heart disease. Owing to age and lack of cooperation in children who need this procedure, general anesthesia is typically required. These patients have increased anesthesia risk secondary to cardiac pathology. Furthermore, multiple catheterization procedures result in exposure to harmful ionizing radiation. Magnetic resonance imaging-guided right-heart catheterization (MRI-RHC) offers decreased radiation exposure and diagnostic imaging benefits over traditional fluoroscopy but potentially increases anesthetic complexity and risk. We describe our early experience with anesthetic techniques and challenges for pediatric MRI-RHC.
Keywords: Cardiac MRI, pediatric cardiac catheterization, congenital heart disease, pediatric cardiac anesthesia, anesthesia for cardiac catheterization, congenital cardiac imaging
Introduction
Cardiac catheterization remains the gold standard for diagnosis and management of many forms of congenital heart disease. Owing to patient age and lack of cooperation, general anesthesia is oftentimes required, and these patients have increased anesthesia risk secondary to their cardiac pathology.1 Some patients require multiple, prolonged procedures, which result in exposure to large amounts of harmful ionizing radiation.2 Standard cardiac catheterization measurements of cardiac output are poorly estimated by the Fick principle and inaccurate with thermodilution in the presence of congenital shunts.3 Noninvasive diagnostic tests such as echocardiography offer valuable information but have several limitations in the areas of anatomic visualization and diagnostic evaluation.4 Echocardiography can infer pressures from flow patterns but cannot perform pressure measurements. By contrast, cardiac magnetic resonance imaging (MRI) allows for accurate estimation of cardiac output and calculated vascular resistance irrespective of the underlying cardiac lesion, in addition to structural and functional assessment of the right ventricle.4,5 MRI-guided right-heart catheterization (MRI-RHC) offers decreased radiation exposure and diagnostic and imaging benefits over traditional fluoroscopy but increases anesthetic complexity and risk.6,7 We describe our early experience with anesthetic technique and challenges in pediatric MRI-RHC.
Potential Benefits of MRI-guided Right-Heart Catheterization
MRI-guided cardiac catheterization, an alternative to conventional x-ray–guided catheterization, has been successfully performed in the adult and pediatric population.8–11 In addition to standard pressure measurements, the MRI hybrid technique allows volumetric analysis of cardiac function and MRI flow measurements of stroke volume and cardiac output. Furthermore, this technique takes into account intracardiac shunts and, therefore, is more precise. Diagnostic cardiac MR imaging is considered the gold standard method of evaluation of right ventricular size, right ventricular function, and valvar regurgitation, which are paramount when making surgical and interventional decisions for children with these types of lesions. In addition, diagnostic cardiac MR imaging has shown promise in the ability to detect both fibrosis and edema, which can be used in in conjunction with clinical endpoints from invasive cardiac catheterization.
With integration of MRI and catheterization, pulmonary vascular resistance (PVR) can be derived more accurately.3,12 PVR calculations utilizing the Fick principle are known to be imprecise in the presence of high pulmonary blood flow and high oxygen concentrations. However, a prospective study in both children and adults demonstrated that phase-contrast MR was accurate under all conditions, including in patients with multiple sources of pulmonary blood flow, with oxygen therapy alone as well as with oxygen and nitric oxide therapy.12 These findings suggest a particularly useful role for MRI-RHC in guiding therapy of patients with pulmonary hypertension.
Furthermore, MRI offers the benefit of improved structural imaging in multiple planes when compared to conventional catheterization. MR angiography highlights cardiac abnormalities and allows for detailed soft tissue imaging, including assessment of areas of myocardial inflammation, fibrosis, and infarction.13–15 Importantly, this imaging is obtained without exposing patients or staff to ionizing radiation.
Anesthesia Workflow in MRI-RHC
At Children’s National Medical Center, a specialized interventional cardiovascular magnetic resonance suite is utilized in performing MRI-RHC. The suite consists of a 1.5 Tesla MR scanner suite that is connected via automatic doors to a separate catheterization suite that includes biplane fluoroscopy. The catheterization table (Miyabi, Siemens, Munich, Germany) moves along a track between the 2 suites to allow for smooth patient transfer (Figure 1).
Figure1.
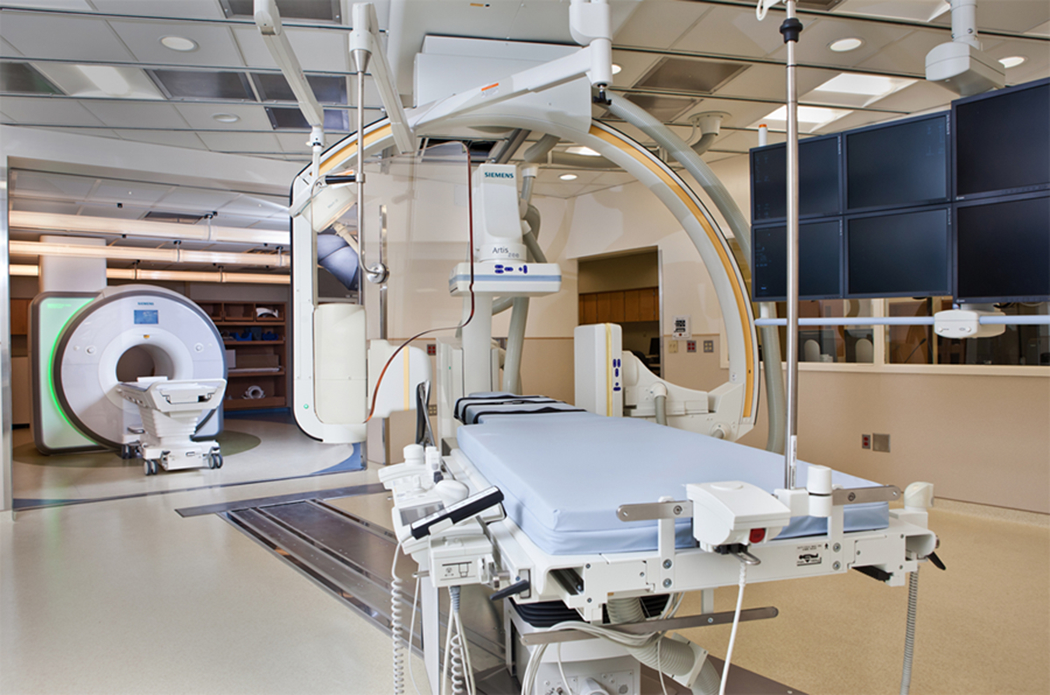
Combined cardiac catheterization and MRI suite at Children’s National Health System.
The team consisted of an imaging cardiologist, interventional cardiologist, anesthesiologist, cardiac catheterization nurse and technologist, MRI technologist, and cardiology nurse practitioner. Workflow, including troubleshooting of equipment placement and movement between suites, was discussed in detail. This was then simulated on two occasions with a volunteer “patient” to identify unforeseen complications. At this point, several logistical issues were discovered. For instance, it was determined that, with the exception of the MRI technologist, no one on the team knew how to emergently disengage the MRI bed from the machine in the case of a power failure. This information was then relayed to the entire team and specific roles were assigned to best care for the patient in various scenarios. In addition to these measures, quarterly evacuation drills, in which various patient emergencies are simulated, are conducted to this day to reinforce each provider’s role during an emergency as well as demonstrate how to most safely and quickly move to the MRI-free environment for further resuscitation. Based on these drills, the average evacuation time from the MRI scanner is 46.3 seconds.
MRI-RHC procedures are performed under general anesthesia or with deep sedation. Two MRI-safe anesthesia machines (GE Healthcare, Milwaukee, Wisconsin) are used, one located in the MRI suite and a second in the catheterization suite. Use of 2 machines facilitates patient transfer between the 2 suites and minimizes time without ventilation. MRI-compatible infusion pumps (IRadimed Corp., Winter Springs, Florida) are also available in the event that vasoactive or sedative infusions are needed. Throughout the procedure, including during transfers, a portable MRI-safe monitoring system (Invivo, Gainesville, Florida) is used and linked to the electronic anesthesia record. An anesthesia checklist is provided in Table 1.
Table 1.
Anesthesia Checklist
| ❒ Appropriately sized bag-valve mask |
| ❒ Circuit extensions on both anesthesia machines |
| ❒ Added extension tubing on intravenous lines |
| ❒ Additional intravenous fluids available in MRI suite |
| ❒ Emergency medications drawn at appropriate dilutions |
| ❒ Complete check of both anesthesia machines |
| ❒ MRI conditional infusion pumps and monitors available and functional |
| ❒ MRI compatable nitric oxide machine set up and checked (if pulmonary hypertension study planned) |
To address risks to patient and staff in the unique MRI environment, a multidisciplinary team developed process and safety measures for care delivery during MRI-RHC. Prior to procedure initiation, a full visual sweep of the catheterization suite is conducted to ensure that no MRI-incompatible objects (including non-safe IV poles, infusion pumps, or laryngoscopes) are present. Any such items are removed or tethered inside the lab as needed. If there is a question of whether equipment is MRI compatible, a hand-held ferrous metal screening wand is used to confirm that it is safe. After the environment is MRI safe, a full team huddle occurs so that each team member is present for case discussion and anticipated issues. Emergency procedures are reviewed during the huddle; each team member is assigned a specific task, and the location where the patient will be moved during an emergency (typically, the catheterization room) is confirmed (Figure 2). Following the final pre-procedural metal screening of the patient, the patient is brought into the catheterization suite.
Figure 2.
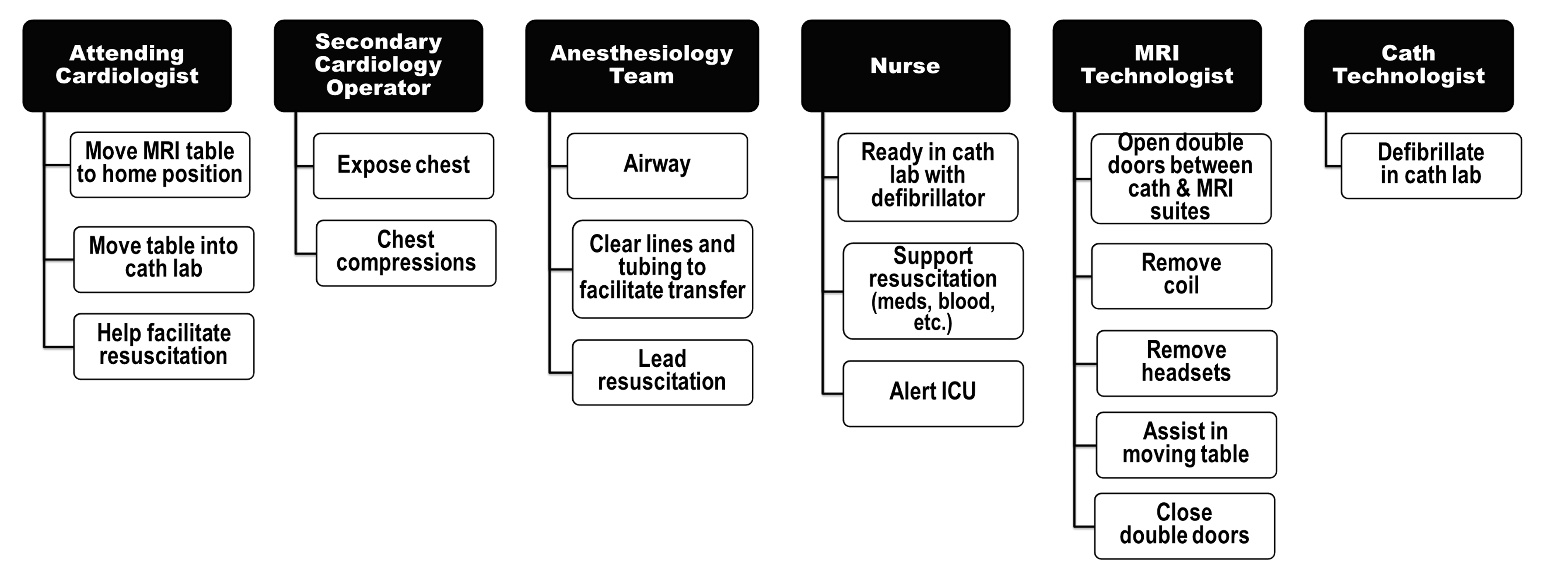
Provider roles in event of emergency evacuation from MRI suite to catheterization suite
Anesthesia induction occurs in the catheterization suite, with the doors to the MRI suite closed. Depending on the patient’s comorbidities, either inhalation or intravenous induction is performed with neuromuscular blockade, followed by endotracheal tube placement. Anesthesia is maintained with an inhalational agent (sevoflurane or isoflurane) per our standard of care. The interventional cardiologist then obtains, and sutures in place, access to the femoral artery and vein catheter. Invasive blood pressure monitoring via the femoral artery is used throughout the procedure. After an additional metal screen of the patient and environment is performed with a metal detector, the doors between the MRI and catheterization suites are opened. With sterility maintained, the movable table is used to transfer the patient into the MRI suite. The patient is continually ventilated on the anesthesia machine, which is outfitted with circuit tubing extensions. At the limit of the tubing, the patient is briefly (2-5 breaths) ventilated with a self-inflating Ambu bag until the circuit from the MRI suite’s anesthesia machine can be reached. The MRI-compatible wireless monitor that is linked to the electronic medical record detects patient vital signs as doors are opened. The anesthesia team directs the transfer to ensure monitoring and maintenance of all lines, tubes, and the sterile field. Only the anesthesia providers, after standard verbal screening by the MRI technician, are permitted to cross the threshold between catheterization suite and MRI suite. All other personnel must enter via standard entry doors in order to maintain scan room control and safety. Importantly, all medications that may be needed during the MRI procedure must be taken into the suite during patient transfer, including anesthetic and emergency medications and additional bags of crystalloid. Once the procedure is started, entry and introduction of additional equipment and medications into the suite are limited; therefore, planning for various emergency contingencies is crucial.
Once in the MRI suite, the patient is positioned and placed on FiO2 of 21% for baseline function and flow measurements and localizing scans. Obtaining these images, which are subsequently used for hemodynamic assessment, takes approximately 30–35 minutes. The team then performs the RHC using MRI guidance, which takes an average of 15 minutes. For better communication between team members inside the MRI scanner and control room, the interventional cardiologists and anesthesiologists wear open-microphone acoustic noise-cancelling headsets (IMROC, Optoacoustics, Tel Aviv, Israel). During pulmonary hypertension studies, MRI-compatible inhaled nitric oxide tanks (INOmax, Mallinckrodt Pharmaceuticals, Dublin, Ireland) are placed in line with the anesthesia machine to deliver nitric oxide. In the event that nitric oxide is needed prior to or following the MRI portion of the case, an MRI-safe tank is also set up in the catheterization suite.
When the MRI-RHC is complete, the patient is transferred back to the catheterization suite. If further interventions such as stenting or ballooning are required, they have thus far been performed using traditional fluoroscopic guidance. With future advances in equipment and increased operator experience, additional interventions within the MRI suite may be feasible, including MRI-compatible catheters and bioptomes. At the conclusion of the procedure, the patient is extubated and taken to the recovery area. There is no change in the recovery process, with the standard post-catheterization medications and procedures in place for patient management. Furthermore, there is no prolongation of hospital stay, as each patient remains in the Cardiac Procedure Recovery Unit (CPRU) for a minimum of six hours for all of our cardiac catheterization procedures, with or without MRI. A case workflow diagram is provided in Figure 3.
Figure 3.
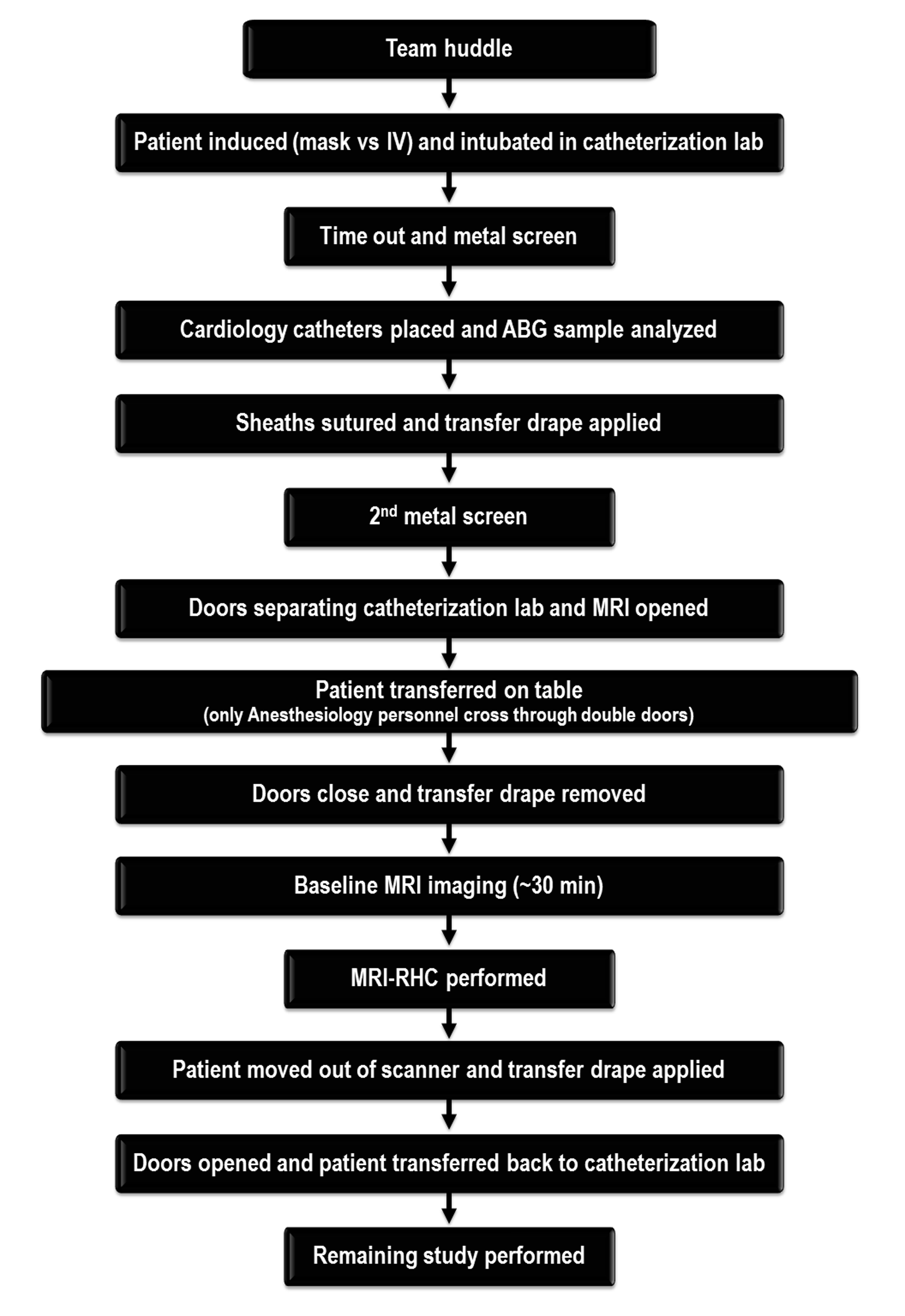
Diagram of case workflow
Early Anesthetic Outcomes at a Single Institution
Following IRB approval, informed consent was obtained for each patient as part of an ongoing study investigating the use of MRI-guided right heart catheterization that is supported by the National Institutes of Health (NIH) and Children’s National Medical Center (CNMC). We reviewed anesthetic records of the first 72 MRI-RHC procedures performed at Children’s National Medical Center to assess perioperative anesthetic management and complications. In order to establish a safety profile for the catheterization procedure, we progressively enrolled younger subjects in three groups: the first ten were limited to age 10 or older; the next ten, age 5 or older; and the next ten, age 2 or older. To date, 50 patients have undergone a total of 72 procedures: 38 had 1 MRI-RHC; 7 had 2 MRI-RHCs; 3 had 3; 1 had 4; and 1 individual has undergone the procedure 7 times. Biopsy for myocardial transplant was the most common indication for cardiac catheterization (Table 2). Patient demographics are presented in Table 3. Four patients are less than 5 years of age nine patients are age 6-10 years old, and the remainder are greater than 10 years old. We consented 54 patients for MRI-RHC procedures. Four patients did not undergo the procedure: 1 had an unknown abdominal foreign body found on screening x-ray, and 1 had femoral vein occlusion. Two patients underwent traditional catheterization rather than the planned MRI-RHC, secondary to hemodynamic instability within 20 minutes of anesthetic induction.
Table 2.
Number of Cases , Anesthesia Time, Fluoroscopy Time and Radiation Dosages by Procedure Type
| Procedure Type | # of Cases | Anesthesia Time (hr:min) (mean [range]) |
Fluoroscopy Time (min:sec) (mean [range]) |
Dose Area Product (μGym2) (mean [range]) |
|---|---|---|---|---|
| Transplant Biopsy (annual) | 21 | 3:21 (2:51-4:14) | 05:30 (02:00-14:19) | 1996.2 (30-11,398.6) |
| Transplant Biopsy (follow up) | 17 | 2:56 (2:16-3:30) | 01:54 (00:05-04:01) | 219.7 (8.7-1809.9) |
| Intracardiac Shunt | 12 | 3:53 (3:02-4:47) | 04:18 (01:00-12:01) | 648.9 (25-2927.2) |
| Cardiomyopathy | 9 | 3:19 (2:26-5:18) | 01:48 (00:00-07:08) | 915.3 (0-4348.6) |
| Pulmonary Hypertension | 10 | 3:23 (2:46-4:00) | 01:50 (00:00-08:09) | 112.8 (0-778) |
| Other | 3 | 4:16 (3:34-5:18) | 09:27 (02:07-19:06) | 5385.6 (1042.4-8072.3) |
Table 3.
Patient Demographics
| Characteristic | Finding |
|---|---|
| Age in years (mean [range]) | 12.2 (4–23) |
| Gender: Female (n [%]) | 25 (50%) |
| Gender: Male (n [%]) | 25 (50%) |
| Weight (kg) (mean [range]) | 47 (14.3–118) |
| Height (cm) (mean [range]) | 146 (97–191) |
Two patients (3 procedures) had intravenous sedation with a propofol infusion and oxygen via nasal cannula. All other patients underwent general anesthesia with inhalation or intravenous induction followed by neuromuscular blockade, intubation, and mechanical ventilation. Ten patients underwent pulmonary hypertension studies that required administration of inhaled nitric oxide during the MRI-RHC. One patient required vasoactive support with dopamine secondary to hypotension; this medication was discontinued at anesthetic end. The average anesthetic time (measured from time of induction until time that handoff to PACU is completed) for the procedures was 3 hours 24 minutes ± 38 minutes. While total anesthetic time varied depending on the procedure, average time from the start of MRI baseline scans to time of first catheter placement was 35.9 minutes (range 2-108) and average total RHC procedure time was 17.1 minutes (range 4-71). Approximated transfer time between catheterization suite and MRI was 2 minutes. Average anesthetic times for each procedure indication type are presented in Table 2. The average body temperature increased 0.3 ± 0.4 degrees Celsius. No complications from the anesthesia or the MRI-RHC procedure were reported. This included no reports of significant catheter-induced arrhythmias, defined as a rhythm change induced from the placement of the catheter that required either medical intervention or electrical cardioversion. Post-operatively, no complications, including increased post-operative nausea and vomiting, positional injuries, prolonged length of stay, or unplanned hospital admissions were reported. Fluoroscopy times and radiation dosages for procedure types are presented in Table 2. Of note, 7 of 10 patients with pulmonary hypertension and 5 of 9 cardiomyopathy patients received no radiation.
Discussion
Cardiac catheterization in patients with many forms of congenital heart disease results in exposure to large amounts of ionizing radiation. In 2009, the American Heart Association published a science advisory that attempted to quantitate radiation exposure in routine cardiology radiologic procedures.16 Using routine chest x-ray as comparison, they concluded that routine diagnostic coronary angiography was equivalent to 350 x-rays and that an interventional catheterization could be similar to the exposure of up to 4000 x-rays.16 In children, exposure could be even greater, as fluoroscopy times are 5–10 times longer than in adult procedures.17
Although the actual quantity of radiation exposure is somewhat case specific and controversial, even low-level exposure to ionizing radiation is thought to contribute to long-term malignancy risk in adults.2 Radiation exposure risk is an even greater concern in the pediatric population. Children are thought to have 3–4 times greater sensitivity to radiation, owing to rapidly dividing cells, and most pediatric patients will live longer to experience radiation toxicity.17 Several studies have demonstrated chromosomal damage in children exposed to catheterization-related radiation; consequently, practitioners have reported their concerted efforts to decrease radiation exposure as much as possible.18–23
Anesthetic risk is higher in pediatric cardiac patients than in the general pediatric population.1 Odegard and colleagues demonstrated a higher incidence of cardiac arrest in children undergoing cardiac catheterization than in those undergoing noncardiac surgery, with more than 50% of these arrests secondary to the sudden onset of dysrhythmias.24 Unlike adults, who typically undergo cardiac catheterization with sedation, pediatric patients often require general anesthesia. The combination of anesthesia and MRI places patients at risk for adverse events.25 These risks are amplified during MRI-guided cardiac catheterizations.
At Children’s National Medical Center, we have historically used general anesthesia in most MRI-RHC cases. Limited access to the patient caused by the physical confines of the MRI suite, as well as the need for multiple transfers, make neuromuscular blockade and endotracheal intubation appealing. Additionally, the time required to obtain localizing scans prolongs these cases beyond the time needed for a traditional catheterization, making general anesthesia more practical and comfortable for most pediatric patients. Our standard of care is to perform general anesthesia with endotracheal intubation in our patients undergoing standard fluoroscopic cardiac catheterization in a similar manner to that which has been used for MRI-RHC. However, many institutions perform these cases under sedation so that the introduction of MRI-guidance may necessitate more anesthetic interventions than is their current standard and thereby potentially increase the anesthetic risk even further. We have successfully performed MRI-RHC under deep sedation in mature, cooperative patients during shorter cases, and we continue to investigate the feasibility of this in more patients going forward. Furthermore, one cannot ignore that the addition of MRI-guidance does require a prolongation of the anesthetic time. The impact of this, including the potential for neurotoxicity in vulnerable populations, is yet to be determined but must be taken into account. We continue to hone the workflow with the goal of decreasing anesthesia time.
Despite the benefits to MRI-RHC, certain aspects of the environment and process incur increased risk over standard fluoroscopic RHC. Currently, an arterial line is placed in all patients for hemodynamic monitoring during MRI-RHC. While an arterial line would not be placed during a straightforward fluoroscopic-guided right-heart catheterization, it was felt that the ability to immediately recognize hemodynamic compromise was important to allow for a quicker intervention, especially in light of the limited patient access and the extra time that is needed to move to an MRI-safe environment in case of arrest. However, arterial line placement is associated with risks, including bleeding and the need for longer recovery immobility. As fluency with MRI-RHC increases, the need for arterial invasive monitoring will likely be case dependent. Also, the potential for increased body temperature exists secondary to continuous scanning with real-time imaging. However, we observed minimal temperature change.
As with all MRI environment anesthetics, vigilance and communication are critical to ensure patient safety. In addition to the standard risk of anesthesia in MRI, the use of RHC equipment within the MRI scan room introduces further complexity. Moreover, an MRI-RHC combined suite includes a catheterization lab. Strict non-ferromagnetic requirements are not normally maintained in this area, and suite and equipment inspection is required to ensure that potentially hazardous ferromagnetic objects are removed or tethered to the wall.
A large multidisciplinary team is needed to safely perform MRI-RHCs. As can be seen in the case workflow (Figure 3), multiple screening measures are in place to prevent adverse events. Importantly, a sound emergency evacuation plan must be developed and mastered in order to avoid morbidity and mortality. The plan must include processes to manage both equipment troubleshooting and patient issues, including hemodynamic instability and cardiac arrest. The anesthesiology team ensures that these cases run safely and efficiently.
In this single institutional experience investigating a new diagnostic modality, we have demonstrated that MRI-RHC is feasible in pediatric patients. Through a multi-disciplinary approach, we have developed a successful workflow with intrinsic safety features. However, important limitations to our findings exist. To date, we have performed MRI-RHC in a select, relatively small patient population. However, we intend to be more inclusive going forward, expanding both the indications for MRI-RHC as well as the patient population with respect to age and medical complexity. Furthermore, the described procedures require a highly specialized environment that may not be feasible in many institutions. As the technology progresses and expands to more institutions, this will be better determined.
Conclusion
MRI-RHC offers benefits over traditional fluoroscopy with less radiation and more in-depth measurements and imaging but at the cost of increased risk and complexity. Challenges that must be managed by the anesthesia team during MRI-RHC encompass patient, procedural, and environmental factors. Risk and complexity can be managed with thoughtful anesthetic and multidisciplinary team planning. Our initial experience demonstrates feasibility and safety of anesthesia for MRI-RHC both in a high-risk environment and in more complex patients. With decreased ionizing radiation exposure, superior multiplane soft-tissue images, and more accurate estimation of certain intracardiac indices such as pulmonary vascular resistance, MRI-RHC adds further possibilities to diagnostics and imaging in caring for cardiac patients. Consequently, multiple institutions are in the process of building combination MRI catheterization suites. Anesthesiologists should be aware of the complexity and risk involved in MRI-RHC procedures and patients and adequately versed in proper management. A well-developed workflow, anesthetic protocol, and emergency contingency plan are essential to the success of these cases.
Acknowledgment
The authors thank Tzipora Sofare, MA, ELS, for her fine editorial assistance in preparing this manuscript.
FUNDING: This work was supported by the National Heart Lung and Blood Institute, National Institutes of Health (Contract: HHSN268201500001C, to CNMC; Z01-HL005062 from the Division of Intramural Research, NHLBI).
Footnotes
ETHICS: Institutional Review Board approval was obtained at Children’s National Medical Center. IRB protocol ID: 00003860; Initial approval 6/9/2013; current approval 2/1/2018-1/31/2019.
DISCLOSURES: The authors have no conflicts of interest to report.
References
- 1.Ramamoorthy C, Haberkern C, Bhananker S, et al. Anesthesia-related cardiac arrest in children with heart disease: data from the Pediatric Perioperative Cardiac Arrest (POCA) registry. Anesth Analg 2010; 110: 1376–1382. [DOI] [PubMed] [Google Scholar]
- 2.Kleinerman RA. Cancer risks following diagnostic and therapeutic radiation exposure in children. Pediatr Radiol 2006; 36(Suppl 2): 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cigarroa RG, Lange RA, Williams RH, Bedotto JB, Hillis LD. Underestimation of cardiac output by thermodilution in patients with tricuspid regurgitation. Am J Med 1989; 86: 417–420. [DOI] [PubMed] [Google Scholar]
- 4.Pavlicek M, Wahl A, Rutz T, et al. Right ventricular systolic function assessment: rank of echocardiographic methods vs. cardiac magnetic resonance imaging. Eur J Echocardiogr 2011; 12: 871–880. [DOI] [PubMed] [Google Scholar]
- 5.Moledina S, Pandya B, Bartsota M, et al. Prognostic significance of cardiac magnetic resonance imaging in children with pulmonary hypertension. Circ Cardiovasc Imaging 2013; 6: 407–414. [DOI] [PubMed] [Google Scholar]
- 6.Rogers T, Ratnayaka K, Lederman RJ. MRI catheterization in cardiopulmonary disease. Chest 2014; 145: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stockton E, Hughes M, Broadhead M, Taylor A, McEwan A. A prospective audit of safety issues associated with general anesthesia for pediatric cardiac magnetic resonance imaging. Paediatr Anaesth 2012; 22: 1087–1093. [DOI] [PubMed] [Google Scholar]
- 8.Ratnayaka K, Faranesh AZ, Guttman MA, et al. Interventional cardiovascular magnetic resonance: still tantalizing. J Cardiovasc Magn Reson 2008; 10:62. doi: 10.1186/1532-429X-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratnayaka K, Faranesh AZ, Hansen MS, et al. Real-time MRI-guided right heart catheterization in adults using passive catheters. Eur Heart J 2013; 34: 380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratnayaka K, Kanter JP, Faranesh AZ, et al. Radiation-free CMR diagnostic heart catheterization in children. J Cardiovasc Magn Reson 2017; 19(1):65. doi: 10.1186/s12968-017-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers T, Ratnayaka K, Khan JM, et al. CMR fluoroscopy right heart catheterization for cardiac output and pulmonary vascular resistance: results in 102 patients. J Cardiovasc Magn Reson 2017; 19(1): 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muthurangu V, Atkinson D, Sermesant M, et al. Measurement of total pulmonary arterial compliance using invasive pressure monitoring and MR flow quantification during MR-guided cardiac catheterization. Am J Physiol Heart Circ Physiol 2005; 289: H1301–H1306. [DOI] [PubMed] [Google Scholar]
- 13.Gagliardi MG, Bevilacqua M, Di Renzi P, et al. Usefulness of magnetic resonance imaging for diagnosis of acute myocarditis in infants and children, and comparison with endomyocardial biopsy. Am J Cardiol 1991; 68: 1089–1091. [DOI] [PubMed] [Google Scholar]
- 14.Ugander M, Oki AJ, Hsu LY, et al. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J 2012; 33: 1268–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdel-Aty H, Zagrosek A, Schulz-Menger J, et al. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation 2004; 109: 2411–2416. [DOI] [PubMed] [Google Scholar]
- 16.Gerber TC, Carr JJ, Arai AE, et al. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation 2009; 119: 1056–1065. [DOI] [PubMed] [Google Scholar]
- 17.Ait-Ali L, Andreassi MG, Foffa I, Spadoni I, Vano E, Picano E. Cumulative patient effective dose and acute radiation-induced chromosomal DNA damage in children with congenital heart disease. Heart 2010; 96: 269–274. [DOI] [PubMed] [Google Scholar]
- 18.Abu Hazeem AA, Dori Y, Whitehead KK, et al. X-ray magnetic resonance fusion modality may reduce radiation exposure and contrast dose in diagnostic cardiac catheterization of congenital heart disease. Catheter Cardiovasc Interv 2014; 84: 795–800. [DOI] [PubMed] [Google Scholar]
- 19.Glatz AC, Purrington KS, Klinger A, King AR, et al. Cumulative exposure to medical radiation for children requiring surgery for congenital heart disease. J Pediatr 2014; 164: 789–794. e10. [DOI] [PubMed] [Google Scholar]
- 20.Goske MJ, Frush DP, Brink JA, et al. Curbing potential radiation-induced cancer risks in oncologic imaging: perspectives from the “image gently” and “image wisely” campaigns. Oncology (Williston Park) 2014; 28: 232–238. [PubMed] [Google Scholar]
- 21.Johnson JN, Hornik CP, Li JS, et al. Cumulative radiation exposure and cancer risk estimation in children with heart disease. Circulation 2014; 130(2): 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andressai MG, Ait-Ali L, Botto N, Manfredi S, Mottola G, Picano E. Cardiac catheterization and long-term chromosomal damage in children with congenital heart disease. Eur Heart J 2006; 27: 2703–2708. [DOI] [PubMed] [Google Scholar]
- 23.Beels L, Bacher K, De Wolf D, Werbrouck J, Thierens H. gamma-H2AX foci as a biomarker for patient x-ray exposure in pediatric cardiac catheterization: are we underestimating radiation risks? Circulation 2009; 120: 1903–1909. [DOI] [PubMed] [Google Scholar]
- 24.Odegard KC, Bergersen L, Thiagarajan R, et al. The frequency of cardiac arrests in patients with congenital heart disease undergoing cardiac catheterization. Anesth Analg 2014; 118: 175–182. [DOI] [PubMed] [Google Scholar]
- 25.Malviya S, Voepel-Lewis T, Eldevik OP, Rockwell DT, Wong JH, Tait AR. Sedation and general anaesthesia in children undergoing MRI and CT: adverse events and outcomes. Br J Anaesth 2000; 84: 743–748. [DOI] [PubMed] [Google Scholar]


