Abstract
There is an urgent need to identify new therapies that prevent SARS-CoV-2 infection and improve the outcome of COVID-19 patients. This pandemic has thus spurred intensive research in most scientific areas and in a short period of time, several vaccines have been developed. But, while the race to find vaccines for COVID-19 has dominated the headlines, other types of therapeutic agents are being developed. In this mini-review, we report several databases and online tools that could assist the discovery of anti-SARS-CoV-2 small chemical compounds and peptides. We then give examples of studies that combined in silico and in vitro screening, either for drug repositioning purposes or to search for novel bioactive compounds. Finally, we question the overall lack of discussion and plan observed in academic research in many countries during this crisis and suggest that there is room for improvement.
1. Introduction
Since the occurrence of the Coronavirus Disease 2019 (COVID-19), a pathological process mediated by SARS-CoV-2, several millions of deaths worldwide (https://www.worldometers.info/coronavirus/) have been monitored. On top of this situation, we are witnessing a major crisis that is affecting the entire world economy and that is also devastating. SARS-CoV-2 is a coronavirus similar to SAR-CoV-1 (emerged in 2002) and MERS-CoV (emerged in 2012) that infect vertebrates. It is a large, enveloped, single-stranded positive-sense RNA virus [1]. Its genome comprises several open reading frames (ORFs), two-thirds of which encode nonstructural proteins (Nsp) that make up the replicase complex. The remaining encodes nine accessory proteins (ORF) and four structural proteins: Spike (S), Envelope (E), Membrane (M), and Nucleocapsid (N), of which the Spike protein mediates entry into host cells via binding to the angiotensin-converting enzyme 2 (ACE2) receptor [2], [3], [4], [5], [6]. The Spike, an oligomeric transmembrane protein, can be cleaved and further activated by several enzymes [7], [8], including the host surface serine protease, TMPRSS2 [3] (Fig. 1). The severity of the host response depends on numerous factors, including an innate response to viral recognition [9], [10]. If the antiviral response is delayed or inhibited, viral proliferation can lead to the large-scale recruitment of neutrophils and monocyte-macrophages to the lungs, creating a hyper-inflammatory environment [10]. It has been found, in some COVID-19 patients, that there is an intense release of pro-inflammatory cytokines, i.e., cytokine storm (CS) [11], [12], [13], [14], and in some patients, rapid progression to Acute Respiratory Distress Syndrome can occur [15], [16], [17].
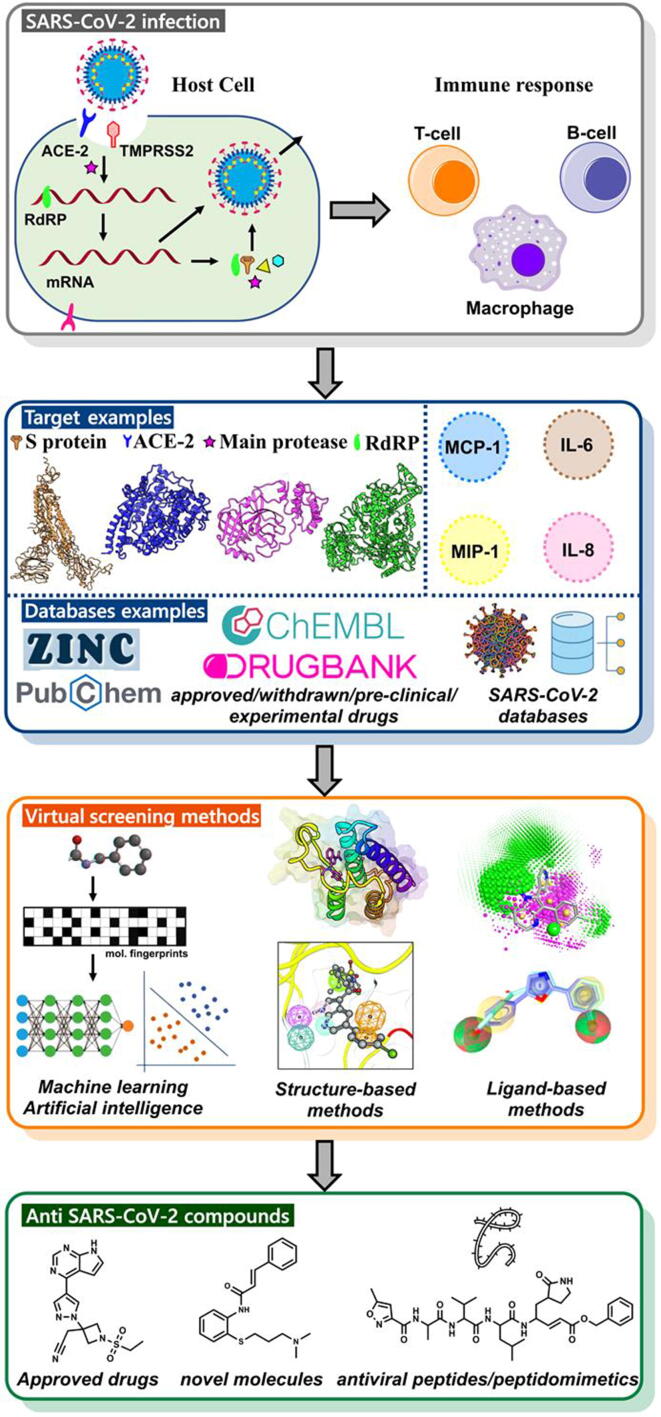
Fig. 1.
The virtual screening workflow for the identification and development of COVID-19 treatments using different drug discovery tools. This figure was inspired by the study reported here [215].
Many different strategies can be used to identify disease prevention drugs and/or treatments. In the present context, considering the virus life cycle, one may aim at drugs acting at different stages of the infection (e.g., entry, replication, and dissemination…) [7], [18], [19], [20], [21], [22], [23], [24]. The drugs could be small chemical compounds and (stapled) peptides [21], [25], [26], [27], [28], [29], [30], [31], [32], therapeutic proteins including antibodies or nanobodies [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], vaccines [46], [47] and cells [48], [49], [50]. These drugs could interfere with the functioning of viral macromolecules and/or with the host proteins and/or complex not fully understood molecular mechanisms and pathways (e.g., many cationic amphiphilic drugs act on specific targets but also on endocytosis [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65].
As in all drug discovery project involving small molecules (but also often for other types of therapeutic agents), there are many ways to prioritize targets depending on the goal (hit one target, several targets in a pathway…) [66], [67], [68], [69], [70], [71], [72], [73], [74]. Once some targets are selected, experimental high-throughput and in silico screening can be performed. If 3D structures are known or can be predicted by homology modeling [75], [76], [77], a critical step for target prioritization usually involves the identification of druggable or ligandable pockets (binding cavities, hot-spots, and cryptic sites) [78], [79], [80], [81], [82], [83], [84]. For SARS-CoV-2, major efforts have indeed been made in the field of structural biology (e.g., mainly X-ray and Cryo-EM considering the size of the macromolecules) [85] while homology modeling and related structural bioinformatics servers have in general launched a special service on “Covid-19” (i.e., pages dedicated to SARS-CoV-2 protein prediction and/or analysis). Further, knowledge about the SARS-CoV-2/human interactome is critical to assist the selection of targets and/or the discovery of drug candidates [86], [87], [88], [89]. Comparisons with other viruses can obviously give important insights about the molecular mechanisms at play and about valuable targets [90]. Taken together, about 20 proteins directly linked to the disease and involved at different stages of the SARS-CoV-2 virus life cycle could be druggable, including entry into the host cells (e.g., the viral Spike and the host ACE2 and TMPRSS2 proteins), RNA replication, and transcription (e.g., helicase and RNA-dependent RNA polymerase (RdRp)), and translation and proteolytic processing of viral proteins (e.g., viral main protease (Mpro), 3CLpro and the papain-like (PLpro) protease) (Fig. 1). Clearly, many other host proteins and protein–protein interactions can also be considered (e.g., possibly the CD147-Spike interaction and a few hundred others).
As mentioned above, developing (antiviral) drugs (from small molecules to vaccines) is extremely challenging [91], [92] and thus, selecting a strategy is obviously critical (e.g., designing a novel compound, a peptide, targeting the enzymes catalytic sites or exosites including allosteric sites…use approved drugs, virtual compounds, etc.). For example, while it might seem easier to identify small molecules inhibiting the catalytic site of viral enzymes as compared to finding inhibitors of protein–protein interactions, mutations in the viral genome can lead to amino acid changes in catalytic centers and possibly drug resistance, thereby making the compound totally or partially ineffective in a short period of time [93], [94]. This situation is less likely at protein–protein interaction (PPI) interfaces [94], [95], [96], [97]. Also, covalent binders [98] can be of interest, definitively to act on viral enzymes [99] but also to block PPIs as seen in the field of cancer [100]. Further and obviously, to find drugs, it is necessary to understand as much as possible the molecular mechanisms at play, and thus, important biochemical and biological studies have to be performed with associated efficient in vitro assays and animal models. These might be very difficult for complex diseases in general and definitively for this virus [101], [102]. Thus, each drug discovery strategy, each selection of targets or pathways, has strengths and weaknesses (e.g., in infectious diseases it could be interesting to target the host proteins to reduce resistance, in oncology the selection of the right therapeutic agents has to be consider early in the process if for instance the drug needs to penetrate some tumors, in such a case, some biologics are not appropriate). Along the same line of reasoning, it is well-known that time factor and cost have to be considered. The search for drug candidates can take years (e.g., the design of novel antiviral compounds starting from the screening of a large compound collection with various optimization steps including Absorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) properties) while other approaches are in general quicker such as drug repurposing or repositioning (e.g., [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114]). Vaccine and antibody development can also be a long and difficult process with potential additional problems for microorganisms with moderate and high mutation rates not even mentioning, manufacturing, shipping, and storage problems and overall public acceptance [115], [116] when it comes to administration to billions of people. What is also obvious from the above is that to design and find treatments, a large number of skills and methodologies are required. Altogether, to face a pandemic (or a global health) crisis, it would seem, at least in academia and at a national level if easier to implement at first, that all the interested scientists should be involved in the process, and that a roadmap should be collectively decided, avoiding as much as possible silo mentality, silo working and related devastating human behaviors commonly seen during a crisis.
In this mini-review, we are interested in computational methods that can assist the so-called early stages of drug discovery. We will focus on tools dedicated to the identification of low molecular weight drug candidates. First, we will briefly introduce some of the computational/data science approaches that can be used to gain insights to search for drug candidates. This first section will also present several online resources and databases that have been used or developed to study SARS-CoV-2. Readers specifically interested in computational approaches that facilitate the development of vaccines or antibodies can find many recent articles and reviews on the topic (e.g., [92], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126]. Then, we will give examples of studies that combined in silico and experimental approaches to identify putative chemical probes. Finally, we discuss the way research, essentially in academia, has been organized in several countries and suggest possible avenues for improvement.
2. Virtual screening methods and online resources to assist the study of SARS-CoV-2
In modern days, many different in silico approaches can be used to assist the design of a drug candidate or drug, from data (e.g., text and image) mining (e.g., annotated drug databases, antiviral peptide databases, electronic health patient records…), genome analysis, comparative genomics, multiple sequence alignments, visualization tools for epidemiological studies, analysis of macromolecular interaction networks, structural predictions (e.g., comparative modeling, protein folding…), antibody-drug conjugate, analysis of point mutations, protein docking, various types of molecular simulation engines (e.g., for proteins, peptides, small molecules, cell membrane, DNA, RNA, glycans, and interactions among these molecules…), binding pocket predictions, PROTACs (e.g., degradation of viral protein capsids), transcriptomic profile analysis, virtual screening (from small collections of approved drugs as in drug repositioning or repurposing projects to the screening of ultra-large virtual libraries), hit to lead optimization, drug combination, computational polypharmacology and compound profiling, ADMET prediction, multiparameter optimization methods associated with novel data visualization approaches, systems biology, systems pharmacology, with or without the use of machine learning and artificial intelligence (AI) algorithms depending on the type of methods, available data and the stage of the projects [19], [77], [80], [127], [128], [129], [130], [131], [132], [133], [134], [135], [136], [137], [138], [139], [140], [141], [142], [143], [144], [145], [146], [147], [148], [149], [150], [151], [152], [153], [154], [155], [156], [157], [158], [159], [160], [161], [162], [163], [164], [165], [166], [167], [168], [169], [170], [171], [172], [173], [174], [175], [176], [177], [178], [179], [180]. Clearly, at present, some computational approaches are more mature than others and some are not realistic [181], [182], [183].
With regard to the search for bioactive anti-SARS-CoV-2 molecules, a starting point could involve gathering information about SARS-CoV-1 and related viruses (e.g., molecular mechanisms, potential targets, structural predictions of SARS-CoV-2 proteins in 3D via homology modeling…) in parallel to the generation of new data, experimental or theoretical [184]. For example, numerous antiviral molecules are known [90] and could be used as starting points to explore the almost infinite chemical space [185]. These molecules act on different targets and on different types of molecular mechanisms. From such knowledge, different types of algorithms can be used, such as virtual screening (VS) approaches. Indeed, these methods are known to play a direct role in drug discovery by enabling the identification/optimization of hit or lead compounds that can exert therapeutic effect by binding to one or more targets (mainly proteins but other macromolecules are also considered, such as RNA and DNA) (e.g., [186], [187]). Virtual screening methods allow for the screening of large databases of compounds (e.g., real or virtual small chemical molecules, approved and investigational drugs, short peptides…) [134], [141], [188], [189], [190]. A short list of molecules selected after the VS computations are then validated experimentally, providing insights into the underlying mechanism of action and providing interesting starting points for further developments. The two prominent strategies used for VS are ligand-based virtual screening (LBVS) and structure-based virtual screening (SBVS). The main approaches for LBVS are similarity-based (shape or chemical fingerprint) approaches and pharmacophore-based methods [191], [192], [193], [194]. Quantitative structure–activity relationship (QSAR, employing various types of machine learning and artificial intelligence algorithms, but these approaches can also be used in docking, binding pocket prediction, scoring….) methods represent other types of powerful ligand-based techniques [107], [127], [132], [137], [138], [142], [195]. SBVS involves molecular docking of chemical libraries into one or several binding pockets, followed by qualitative and quantitative estimation of the docking poses [141], [196]. With such approaches, it is possible to target a catalytic site, an allosteric site, or a pocket present at macromolecular interfaces [80], [81], [151], [197]. The most appropriate pockets can be selected, either for docking or de novo compound generation taking into account properties of the cavity [198], [199], [200], [201], [202]. In most cases, flexibility of these putative targets/pockets have to be investigated [147], [148], [156]. The compound collections can be, like in LBVS studies, approved, investigational or experimental molecules [203], purchasable compounds and virtual compounds [204], covalent binders [130], [205] or small fragments [206]. Different types of scoring functions can then be used to select the molecules for experimental assays [155], [157], [160], [207], [208], [209], [210], [211]. LBVS and SBVS can be combined if the right data are available [134]. Then, as new knowledge is being reported, various types of data mining approaches and workflows can be developed and used [212]. It becomes possible to, for instance, develop statistical predictive models based on experimental screening data so as to predict the bioactivity of a novel compound [213] while, as the 3D structures of many potential targets are now known, SBVS can be performed on different binding pockets with, for instance, ultra-large compound collections [150].
During the Covid-19 pandemic, in wet and dry laboratories alike, numerous projects were initiated and supported by short-term research grants. In the field of computational and data sciences, a tremendous amount of work has been accomplished (e.g., online tools, open databases) in a short period of time [152]. These developments are of high interest as these approaches can help to design chemical probes, drug candidates, therapeutic peptides or proteins and generate new ideas [214]. Further, if these tools and databases are properly maintained (thus the question about short-term funding), they should be also essential for future research projects and for teaching purposes. What was also impressive, during this crisis, was the amount of computer power available. Most computer groups could have access to super computers and the difficulties were elsewhere, the lack of funding to employ scientists with a proper training in both, computer/data sciences and drug discovery (small molecules and/or biologics).
We have collected several of these online tools/databases, especially those dedicated to drug design (see Supplement Table 1). Yet, some of these are not directly concerned by drug design per se but can be used to gain knowledge about SARS-CoV-2, from genome analysis, visualization tools for epidemiological studies, approaches to study potential therapeutic targets, or to map variants on the 3D structures of the viral proteins (e.g., in binding pockets, in neutralizing antibody binding regions, to design vaccines that would still be effective against Covid-19 variants…). With regard to drug design, the online tools allow to run different types of virtual screening computations (e.g., already synthesized or virtual compounds, drug repositioning, peptide docking). At present, several annotated compound databases are available and can be used to develop predictive statistical models that should facilitate the selection or design of more active molecules. As new tools are reported basically every week, we also implement all the URLs on our website, https://www.vls3d.com/, Shortlist page, and all are flagged by the word “Covid-19” such that users can carry out a simple search using, for instance, the Google Chrome Find utility.
3. In silico and in vitro screening to search for novel anti-SARS-CoV-2 compounds
Many different types of computations have been carried out and combined with experimental approaches to search for anti-SARS-CoV-2 molecules. As thousands of articles have been reported, it is only possible to mention some here. For example, we will not cover the following studies to save space [216], [217], [218], [219], [220], [221], [222], [223], [224], [225], [226], [227].
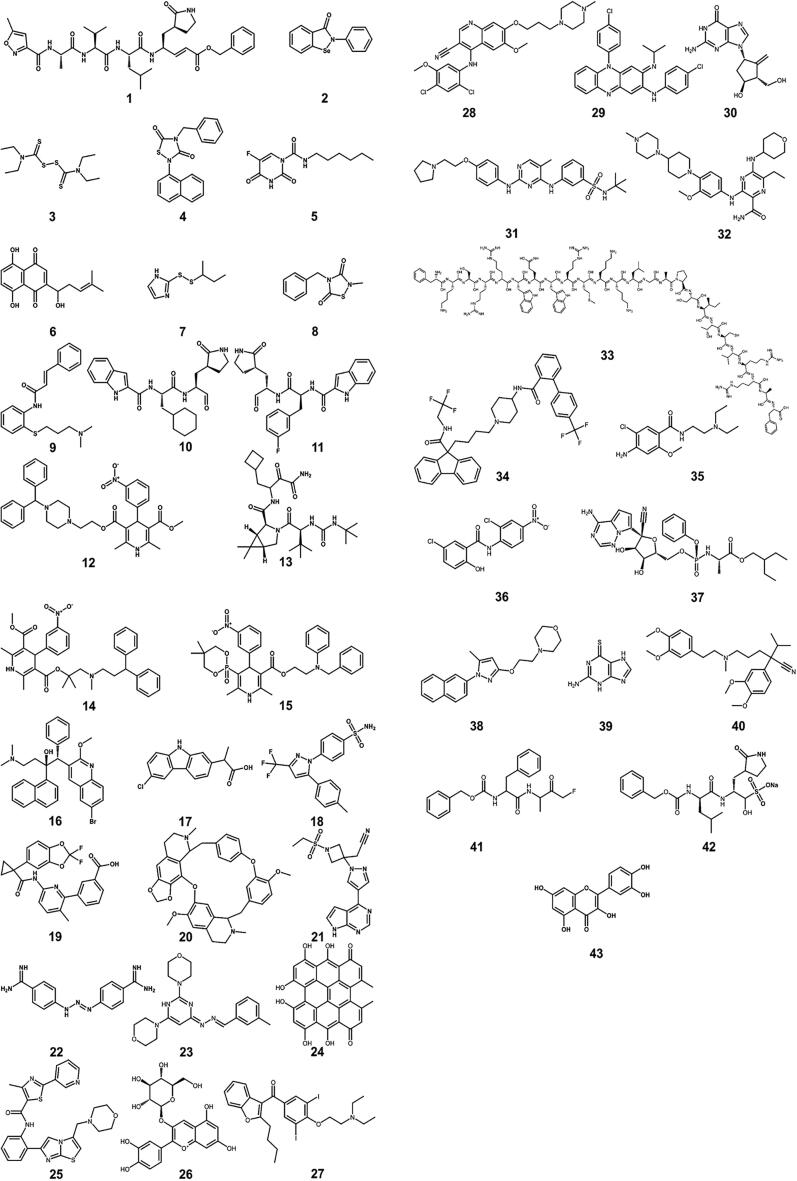
Jin et al. identified a mechanism-based peptidomimetic inhibitor N3 1 (Fig. 2) using computer-aided drug design (CADD) techniques [228]. They solved the crystal structure of the Mpro of SARS-CoV-2 in complex with this compound. The crystal structure revealed that N3 was covalently bonded to the catalytic cysteine (C145) of Mpro. Eight additional compounds, Ebselen 2, Disulfiram 3, Tideglusib 4, Carmofur 5, Shikonin 6, PX-12 7, TDZD-8 8, and Cinanserin 9 (Fig. 2) were identified through a combination of structure-based virtual and high-throughput screening of over 10,000 compounds, including approved drugs, drug candidates in clinical trials, and other pharmacologically active compounds. Out of eight compounds, 2–8 showed half-maximal inhibitory concentrations (IC50) ranging from 0.67 to 21.4 μM, whereas compound 9 (Cinanserin) has an IC50 value of 125 μM for Mpro. One of these compounds (Ebselen) also exhibited promising antiviral activity in cell-based assays. Yet, some of these molecules like Ebselen have to be considered with caution as suggested to be a nonspecific promiscuous protease inhibitor [229].
Fig. 2.
Chemical structures of small molecule inhibitors and peptides that are active against the SARS-CoV-2 virus. 1, N3, 2, Ebselen 3, Disulfiram 4, Tideglusib 5, Carmofur 6, Shikonin 7, PX-12 8, TDZD-8 9, Cinanserin 10, N-((S)-3-cyclohexyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)propan-2-yl)-1H-indole-2-carboxamide 11, N-((S)-3-(3-fluorophenyl)-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)propan-2-yl)-1H-indole-2-carboxamide 12, Manidipine 13, Boceprevir 14, Lercanidipine 15, Efonidipine 16, Bedaquiline 17, Carprofen 18, Celecoxib 19, Lumacaftor 20, Cepharanthine 21, Baricitinib 22, Diminazene 23, 4,4′-((Z)-6-(((E)-3-methylbenzylidene)hydrazono)-3,6-dihydropyrimidine-2,4-diyl)dimorpholine 24, Hypericin 25, Cyanidin-3-O-glucoside 26, SRT2104 27, Amiodarone 28, Bosutinib 29, Clofazimine 30, Entecavir 31, Fedratinib 32, Gilteritinib 33, Lactoferrin (the peptide segment expected to be important) 34, Lomitapide 35, Metoclopramide 36, Niclosamide 37, Remdesivir 38, S1RA 39, Thioguanine 40, Verapamil 41, Z-FA-FMK, 42, GC376 43, Quercetin. It is important to note that some of these molecules (e.g., Ebselen, Disulfiram, Carmofur, PX-12, Tideglusib, and Shikonin) could be nonspecific promiscuous compounds (see [229]).
Dai et al. designed two inhibitors, 10 and 11, by analyzing the substrate-binding pocket of SARS-CoV Mpro [230]. Both compounds strongly inhibited the activity of Mpro (IC50 values of 0.053 ± 0.005 μM and 0.040 ± 0.002 μM for 10 and 11, respectively) and showed good antiviral activity in cell culture. They also determined the crystal structures of SARS-CoV-2 Mpro in complex with these inhibitors, which revealed that the aldehyde groups of 10 and 11 are covalently attached to C145 of Mpro.
In another study [231], a consensus docking-based virtual screening protocol was applied to ~ 2000 approved drugs to find inhibitors of Mpro of SARS-CoV-2 using Glide SP, AutoDock Vina, and AutoDock 4.2. The predicted poses of the compounds were extensively scrutinized in terms of consensus docking score, intermolecular contacts, conformation, stability in molecular dynamics simulations, and potential for synthetic modification. Seventeen compounds were evaluated for Mpro inhibition, in which 14 compounds showed a reduction of Mpro activity at 100 μM concentration. Among these 14 compounds, five compounds, manidipine 12, boceprevir 13, lercanidipine 14, efonidipine 15, and bedaquiline 16, exhibited the IC50 values of 4.8, 5.4, 16.2, 38.5, and 18.7 μM, respectively.
Gimeno et al. performed docking-based virtual screening of two different libraries of approved drugs against the Mpro structure using Glide, FRED, and AutoDock Vina [232]. The study led to the identification of Carprofen 17 and Celecoxib 18 as weak noncovalent binders of Mpro, showing 3.97% and 11.90% inhibition at 50 μM concentration, respectively.
White et al. targeted the SARS-CoV2 helicase (Nsp13), which is critical for viral replication and the most conserved nonstructural protein within the coronavirus family [233]. By combining homology modeling and molecular dynamics approaches, they generated structural models of the SARS-CoV2 helicase in its apo- and ATP/RNA-bound conformations. The subsequent high-throughput virtual screening of ~970,000 chemical compounds against the ATP binding site of Nsp13 led to the identification of two drugs, Lumacaftor 19 and Cepharanthine 20, that displayed significant activity in inhibiting Nsp13 ATPase activity in purified recombinant SARS-CoV-2 helicase with estimated IC50 values of 0.3 and 0.4 mM, respectively.
Richardson et al., by applying artificial intelligence algorithms, identified Baricitinib (21) as a potential drug for COVID-19 infection [234], [235]. Baricitinib is a potent and selective inhibitor of the Janus kinases 1/2 (JAK1/JAK2) and is currently used in the therapy of rheumatoid arthritis. Based on the results of the BenevolentAI's knowledge graph tool, Baricitinib has been proposed to exert anti-cytokine effects as well as it was predicted to alter virus entry by inhibiting AP2-associated kinase 1 (AAK1) and cyclin G-associated kinase (GAK), which are likely involved in SARS-CoV-2 endocytosis. The BenevolentAI's knowledge graphical method utilizes machine learning to integrate the scientific information on the biological processes involved in viral infection with that on the mechanisms of action of available drugs in order to identify potential new therapeutic targets and agents [236]. Stebbing et al. validated the AI-prediction by performing the in vitro pharmacology of Baricitinib across relevant leukocyte subpopulations coupled to its in vivo pharmacokinetics, which revealed that Baricitinib inhibited signaling of cytokines implicated in COVID-19 [237], [238]. In addition, the inhibitory effects of baricitinib were also evaluated on human numb-associated kinase (hNAK) members, such as AAK1, BIKE, and GAK, for which it showed affinities in the nanomolar range (8.2 nM, 20 nM, and 120 nM, respectively). The NAK enzymes are believed to facilitate the propagation of coronavirus in epithelial cells [234], [239], [240]. Baricitinib triggered inhibition of NAKs led to reduced viral infectivity in human primary liver spheroids [235], [237]. Besides the use of AI in drug discovery, it was recently applied to improve the diagnosis or early warning signs of COVID-19 infection [241]. For instance, researchers at King’s College London, in collaboration with Massachusetts General Hospital, designed the COVID Symptom Tracker, developed an AI-based smartphone app that monitors viral transmission and symptoms [242]. This tool identified the importance of anosmia as an early warning symptom. The AI-powered NHSX contact tracing app warns users about viral exposure and so dramatically reduces transmission rates. Alerts are triggered when users self-report symptoms or test positive while respecting anonymity.
Wu et al. used the fact that the Spike protein of SARS-CoV-2 contains a furin cleavage site and proposed that furin could be a reason behind the high infectivity of SARS-CoV-2 [243]. By performing structure-based virtual ligand screening of a library of 4,000 compounds, including approved drugs and natural products, followed by in vitro enzyme-based assay, they discovered an anti-parasitic drug Diminazene 22, that showed competitive inhibition of furin, with an IC50 of 5.42 ± 0.11 μM.
Huang et al. applied a novel biological activity-based modeling (BABM) approach to discover a compound inhibiting the SARS-COV-2 [244]. The BABM method relies on the hypothesis that compounds with similar activity patterns tend to share similar targets or mechanisms of action. In the BABM method, compound activity profiles established on a massive scale across multiple assays were used as signatures to predict compound activity in a new assay or against a new target. They first trained and validated this approach by identifying new antiviral lead candidates for Zika and Ebola based on data from ~ 0.5 million compounds screened against ~ 2,000 assays. Subsequently, BABM models were then applied to predict ~ 300 compounds not previously reported to have activity for SARS-CoV-2, which were then tested in a live virus assay exhibiting high (>30%) hit rates. The most potent compound 23 showed an IC50 value of 0.5 μM.
Pitsillou et al. targeted the SARS-CoV-2 Mpro by performing a molecular docking screen of 300 small molecules [245], which included phenolic compounds and fatty acids from the OliveNet™ library [246], and an additional group of curated pharmacological and dietary compounds. The study led to the identification of three inhibitors, hypericin 24, cyanidin-3-O-glucoside 25, and SRT2104 26 that showed the IC50 values of 63.6 ± 5.7 μM, 65.1 ± 14.6 μM and 85.0 ± 16.8 μM, respectively, in the enzyme-linked immunosorbent assays. Also, molecular dynamics simulations further demonstrated that the lead compounds formed stable interactions with the Mpro active site.
Mirabelli et al., by using quantitative high-content morphological profiling and artificial intelligence-based machine learning strategy to classify features of cells for infection and stress, identified several efficacious single agents as well as combination therapies against SARS-CoV-2 [247]. This hybrid technique detected multiple antiviral mechanisms of action (MOA), including inhibition of viral entry, propagation, and modulation of host cellular responses. From a library of 1,441 FDA-approved compounds and clinical candidates, they identified 15 compounds with antiviral effects: Amiodarone 27 (EC50: 52 nM), Bosutinib 28 (20 nM), Clofazimine 29 (84 nM), Entecavir 30 (42 nM), Fedratinib 31 (24 nM), Gilteritinib 32 (224 nM), Lactoferrin 33 (308 nM), Lomitapide 34 (766 nM), Metoclopramide 35 (468 nM), Niclosamide 36 (142 nM), Remdesivir 37 (97 nM), S1RA 38 (222 nM), Thioguanine 39 (22 nM), Verapamil 40 (533 nM), and Z-FA-FMK 41 (107 nM).
Hung et al. reported GC376 42 as a potent inhibitor for the Mpro encoded by SARS-CoV-2, with an IC50 of 26.4 ± 1.1 nM, and inhibited SARS-CoV-2 replication with an EC50 of 0.91 ± 0.03 μM. Docking analysis revealed that the recognition and binding groups of GC376 were within the active site of SARS-CoV-2 Mpro [248].
Abian et al., by screening a small chemical library consisting of about 150 compounds, identified quercetin 43, a natural product, as a reasonably potent inhibitor of SARS-CoV-2 Mpro (Ki ~ 7 µM). Quercetin was shown to interact with Mpro using biophysical techniques, and molecular docking simulations were performed to clarify the interaction of quercetin with Mpro [249].
In a recent and innovative study, Cao et al. targeted the interaction between the SARS-CoV-2 spike protein and the human ACE2 using de novo design approaches [250]. Computer-generated scaffolds were built around an ACE2 helix that interacts with the spike receptor-binding domain (RBD) or docked against the RBD to identify new binding modes, and subsequently, their amino acid sequences were designed to optimize the target binding, folding, and stability. Ten designed miniprotein inhibitors were found to be binding to the RBD and exhibited affinities ranging from 100 pM to 10 nM. These molecules blocked SARS-CoV-2 infection of Vero E6 cells with the IC50 values between 24 pM and 35 nM (Table 1).
Table 1.
Biological activity values against the SARS-CoV-2 virus of some small molecules with the corresponding protein targets.
| Cpd. | Target | Biological activity | Ref. (PMID) |
|---|---|---|---|
| 1 (N3) | Mpro | not reported | [228] |
| 2 (Ebselen) | Mpro | 0.67 μM | [228] |
| 3 (Disulfiram) | Mpro | 9.35 μM | [228] |
| 4 (Tideglusib) | Mpro | 1.55 μM | [228] |
| 5 (Carmofur) | Mpro | 1.82 μM | [228] |
| 6 (Shikonin) | Mpro | 15.75 μM | [228] |
| 7 (PX-12) | Mpro | 21.39 μM | [228] |
| 8 (TDZD-8) | Mpro | 2.15 μM | [228] |
| 9 (Cinanserin) | Mpro | 125 μM | [228] |
| 10 | Mpro | 0.053 μM | [230] |
| 11 | Mpro | 0.04 μM | [230] |
| 12 (Manidipine) | Mpro | 4.8 μM | [231] |
| 13 (Boceprevir) | Mpro | 5.4 μM | [231] |
| 14 (Lercanidipine) | Mpro | 16.2 μM | [231] |
| 15 (Efonidipine) | Mpro | 38.5 μM | [231] |
| 16 (Bedaquiline) | Mpro | 18.7 μM | [231] |
| 17 (Carprofen) | Mpro | 3.97% | [232] |
| 18 (Celecoxib) | Mpro | 11.97% | [232] |
| 19 (Lumacaftor) | Nsp13 | 0.3 mM | [233] |
| 20 (Cepharanthine) | Nsp13 | 0.4 mM | [233] |
| 21 (Baricitinib) | AAK1 | 8.2 nM | [234], [235], [237] |
| 21 (Baricitinib) | BIKE | 20 nM | [234], [235], [237] |
| 21 (Baricitinib) | GAK | 120 nM | [234], [235], [237] |
| 22 (Diminazene) | Furin and Spike protein cleavage | 5.42 μM | [243] |
| 23 | Phenotypic screening | 0.5 μM | [244] |
| 24 (Hypericin) | Mpro | 63.6 μM | [245] |
| 25 (Cyanidin-3-O-glucoside) | Mpro | 65.1 μM | [245] |
| 26 (SRT2104) | Mpro | 85.0 μM | [245] |
| 27 (Amiodarone) | Phenotypic screen (SARS-CoV-2) | 52 nM | [247] |
| 28 (Bosutinib) | Phenotypic screen (SARS-CoV-2) | 20 nM | [247] |
| 29 (Clofazimine) | Phenotypic screen (SARS-CoV-2) | 84 nM | [247] |
| 30 (Entecavir) | Phenotypic screen (SARS-CoV-2) | 42 nM | [247] |
| 31 (Fedratinib) | Phenotypic screen (SARS-CoV-2) | 24 nM | [247] |
| 32 (Gilteritinib) | Phenotypic screen (SARS-CoV-2) | 224 nM | [247] |
| 33 (Lactoferrin) | Phenotypic screen (SARS-CoV-2) | 308 nM | [247] |
| 34 (Lomitapide) | Phenotypic screen (SARS-CoV-2) | 766 nM | [247] |
| 35 (Metoclopramide) | Phenotypic screen (SARS-CoV-2) | 468 nM | [247] |
| 36 (Niclosamide) | Phenotypic screen (SARS-CoV-2) | 142 nM | [247] |
| 37 (Remdesivir) | Phenotypic screen (SARS-CoV-2) | 97 nM | [247] |
| 38 (S1RA) | Phenotypic screen (SARS-CoV-2) | 222 nM | [247] |
| 39 (Thioguanine) | Phenotypic screen (SARS-CoV-2) | 22 nM | [247] |
| 40 (Verapamil) | Phenotypic screen (SARS-CoV-2) | 533 nM | [247] |
| 41 (Z-FA-FMK) | Phenotypic screen (SARS-CoV-2) | 107 nM | [247] |
| 42 (GC376) | Mpro | 26.4 nM | [248] |
| 43 (Quercetin) | Mpro | 7 μM | [249] |
4. Preparation for the next global health crises
Numerous problems about the present Covid-19 crisis have been reported by different groups [91], [251], [252], [253]. We add below some of our observations.
Working in silos. Since the beginning of this crisis, hundreds/thousands of scientists in academia (e.g., in Europe) have been puzzled by the lack of a roadmap with a clear, collectively planned definition of research priorities and distribution of tasks. As a result, many projects got started in uncoordinated ways, squandering scare resources. Many researchers, supposed to be working towards the same objective, often on the same campus, could not share information nor (precompetitive) data because nobody was clearly in charge. These, most likely, led to many missed opportunities. Along the same line of reasoning, if we take the specific case of drug repositioning, in several countries in Europe, no strategies were discussed among the teams and the combination of in silico and in vitro approaches insufficiently used (or not used at all). This would have been important as compounds missed in vitro could have been identified in silico and vice versa. Definitively the lack of discussions with decision makers and the fact in silico drug designer teams were essentially ignored during this crisis is frightening.
Pain but no gain and lost in the grant application jungles while the world was dying. Another point in many countries (not directly related to Covid-19 but) and at least in academic research, has been the realization that for over 20 years, financial supports for drug discovery have been very limited. Further, drug discovery skills are often not considered in academia and thus, the scientists working in this field have usually even less chance to move on with their careers [254]. During this crisis, it was surprising to have to spend so many precious hours in writing low success-rate grants while thousands of people were dying every day. Changes seem needed.
Education and information. Explanations about drug discovery to the general population, the strengths and weaknesses of each approach (silico, vitro, animal models) and about the therapeutic agents have been essentially lacking.
Rigidity versus agility and so many unclear entry points. Delays and unnecessary bureaucracy are well-known in academia, but scientists have been told, for several decades already, that processes were going to be improved (less bureaucracy, encouraging teamwork, self-organization…). Also, in many countries (e.g., in Europe), millions of euros were spent in a myriad of fragmented projects but definitively not enough were dedicated to the development of in vitro screening platforms (i.e., the cost of developing such platforms to quickly test hypotheses is negligible as compared to the social and economic turmoil caused by the pandemic). Animal model platforms were even less available to drug hunters, even more so for computer teams. The process, if any, to test a candidate molecule with the help of an experimental platform was thus very complex with no clear entry point. This suggests that many interesting compounds (or drug combinations) could not be explored in a timely manner. Along the same line, the procedure to initiate (or to build on the results of) clinical trials, at least for approved drugs, was also very frustrating and many interesting drugs or drug combinations could not be explored in some countries because of unnecessary bureaucracy.
Hope for the future. Definitively, we believe that these observations call for changes in the management of research in many countries. With regard to academic drug discovery, important efforts have been done, for example in the USA, with the creation of the Academic Drug Discovery Consortium or dynamic maps to develop small molecules or biologics proposed by the National Center for Advancing Translational Sciences [255], [256], [257], in UK [258], [259] or in Sweden [260], to mention only a few. Of course, we are all aware that academic drug discovery is challenging and difficult to organize [261]. Yet, instead of a fragmented and very costly academic drug discovery research as presently operated in many countries, it should be possible to develop true interdisciplinary (including computational groups and many other skills required for drug discovery endeavors), coordinated and agile drug discovery networks that recognize all actors along the value chain.
5. Concluding remarks
The COVID-19 pandemic has severely affected the everyday life of humans [262]. In this mini-review, we first reported some online in silico approaches and databases that can assist the study of SARS-CoV-2. Although the race to find vaccines has been dominating the headlines, it is important to note that the search for other types of anti-viral agents is also critical. Overall, computational approaches have assisted numerous projects and in several cases proposed interesting compounds that have been validated in vitro. When time is a critical factor, combined in silico and in vitro repurposing approaches can indeed be a possible avenue but efforts on the prediction of the potential benefit of drug combination will be needed. In parallel, screening large (and high quality) compound collections (including virtual small chemical compounds and peptides) can be extremely valuable but of course the process is more time consuming. Novel, more efficient tools to predict ADMET properties and to predict the best possible targets or pathways are still needed and so are strategies that can help selecting the best treatments for a given population. These challenges do not only apply to infectious diseases but to most disease conditions. Besides assisting the identification of putative drugs, computational approaches combined with molecular biology and medicine and with biophysical methods helped to improve our understanding of several mechanisms involved in the virus life cycle, in some cases, at the atomic level. Hit-to-lead optimization of the discovered chemotypes using various in silico including multi-parameter optimization strategies and medicinal chemistry skills are ongoing and may lead to superior ligands that could be brought to the clinics in a near future.
But to be prepared to face future global health challenges, we believe that establishing integrated academic in silico-in vitro-in vivo early drug discovery networks has to become a priority in many countries. At present, academic drug discovery research is often fragmented, while collaborations between the public and private sectors remain most of the time, in many countries, wishful thinking. Breaking silos is also going to be a major challenge for the years to come as so many skills and technologies/knowledge are needed to cost-effectively discover new drug candidates. Are we prepared for the next global health crisis (plans, structures and resources)? For the time being, the answer is no. The scientific community has done outstanding work during this crisis but the top-down management seen in many (nonprofit) organizations and in many countries has often stifled researchers’ creativity and destroyed engagement.
CRediT authorship contribution statement
Natesh Singh: Formal analysis, Writing - original draft. Bruno O. Villoutreix: Conceptualization, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We apologize for not being able to reference all the in silico studies in this review, this is not only due to space limitation but also to the fact that thousands of articles using in silico approaches have been reported these last several months and that it is impossible to review all in a mini-review.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.csbj.2021.04.059.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Viruses CSGotICoTo The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison A.G., Lin T., Wang P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020;41:1100–1115. doi: 10.1016/j.it.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. (2020) SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 181:271-80.e8. [DOI] [PMC free article] [PubMed]
- 4.Kim D, Lee JY, Yang JS, Kim JW, Kim VN, Chang H (2020) The Architecture of SARS-CoV-2 Transcriptome. Cell. 181:914-21.e10. [DOI] [PMC free article] [PubMed]
- 5.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet (London, England). 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gil C., Ginex T., Maestro I., Nozal V., Barrado-Gil L., Cuesta-Geijo M. COVID-19: drug targets and potential treatments. J Med Chem. 2020;63:12359–12386. doi: 10.1021/acs.jmedchem.0c00606. [DOI] [PubMed] [Google Scholar]
- 8.Tang T., Jaimes J.A., Bidon M.K., Straus M.R., Daniel S., Whittaker G.R. Proteolytic activation of SARS-CoV-2 spike at the S1/S2 boundary: potential role of proteases beyond furin. ACS Infect Dis. 2021;7:264–272. doi: 10.1021/acsinfecdis.0c00701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pazhouhandeh M., Sahraian M.A., Siadat S.D., Fateh A., Vaziri F., Tabrizi F. A systems medicine approach reveals disordered immune system and lipid metabolism in multiple sclerosis patients. Clin Exp Immunol. 2018;192:18–32. doi: 10.1111/cei.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 11.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England). 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheller J., Garbers C., Rose-John S. Interleukin-6: from basic biology to selective blockade of pro-inflammatory activities. Semin Immunol. 2014;26:2–12. doi: 10.1016/j.smim.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka T., Narazaki M., Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8:959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang Y., Wang M.L., Chien C.S., Yarmishyn A.A., Yang Y.P., Lai W.Y. Highlight of Immune Pathogenic Response and Hematopathologic Effect in SARS-CoV, MERS-CoV, and SARS-Cov-2 Infection. Front Immunol. 2020;11:1022. doi: 10.3389/fimmu.2020.01022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science (New York, NY). 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 18.Khalil B.A., Elemam N.M., Maghazachi A.A. Chemokines and chemokine receptors during COVID-19 infection. Comput Struct Biotechnol J. 2021;19:976–988. doi: 10.1016/j.csbj.2021.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Ortiz W., Zhou M.M. Could PROTACs Protect Us From COVID-19? Drug Discovery Today. 2020;25:1894–1896. doi: 10.1016/j.drudis.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh R.K., Yadav B.S., Mohapatra T.M. Molecular targets and system biology approaches for drug repurposing against SARS-CoV-2. Bull Natl Res Centre. 2020;44:193. doi: 10.1186/s42269-020-00444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su H, Zhou F, Huang Z, Ma X, Natarajan K, Zhang M, et al. (2020) Molecular Insights into Small-Molecule Drug Discovery for SARS-CoV-2. Angewandte Chemie (International ed in English). [DOI] [PubMed]
- 22.Wong N.A., Saier M.H., Jr. The SARS-coronavirus infection cycle: A survey of viral membrane proteins, their functional interactions and pathogenesis. Int J Mol Sci. 2021:22. doi: 10.3390/ijms22031308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muus C., Luecken M.D., Eraslan G., Sikkema L., Waghray A., Heimberg G. Single-cell meta-analysis of SARS-CoV-2 entry genes across tissues and demographics. Nat Med. 2021;27:546–559. doi: 10.1038/s41591-020-01227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jomah S., Asdaq S.M.B., Al-Yamani M.J. Clinical efficacy of antivirals against novel coronavirus (COVID-19): A review. J Infect Public Health. 2020;13:1187–1195. doi: 10.1016/j.jiph.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acar H., Ting J.M., Srivastava S., LaBelle J.L., Tirrell M.V. Molecular engineering solutions for therapeutic peptide delivery. Chem Soc Rev. 2017;46:6553–6569. doi: 10.1039/c7cs00536a. [DOI] [PubMed] [Google Scholar]
- 26.Di L. Strategic approaches to optimizing peptide ADME properties. AAPS J. 2015;17:134–143. doi: 10.1208/s12248-014-9687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fosgerau K., Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Henninot A., Collins J.C., Nuss J.M. The current state of peptide drug discovery: back to the future? J Med Chem. 2018;61:1382–1414. doi: 10.1021/acs.jmedchem.7b00318. [DOI] [PubMed] [Google Scholar]
- 29.Wang P., Zhou J. Proteolysis targeting chimera (PROTAC): A paradigm-shifting approach in small molecule drug discovery. Curr Top Med Chem. 2018;18:1354–1356. doi: 10.2174/1568026618666181010101922. [DOI] [PubMed] [Google Scholar]
- 30.Maas M.N., Hintzen J.C.J., Löffler P.M.G., Mecinović J. Chemical communications (Cambridge; England): 2021. Targeting SARS-CoV-2 spike protein by stapled hACE2 peptides. [DOI] [PubMed] [Google Scholar]
- 31.Ojha P.K., Kar S., Krishna J.G., Roy K., Leszczynski J. Therapeutics for COVID-19: from computation to practices-where we are, where we are heading to. Mol Diversity. 2021;25:625–659. doi: 10.1007/s11030-020-10134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan X.C., Sanders J.M., Gao Y.D., Tudor M., Haidle A.M., Klein D.J. Augmenting Hit Identification by Virtual Screening Techniques in Small Molecule Drug Discovery. J Chem Inf Model. 2020;60:4144–4152. doi: 10.1021/acs.jcim.0c00113. [DOI] [PubMed] [Google Scholar]
- 33.Bojkova D., Bechtel M., McLaughlin K.M., McGreig J.E., Klann K., Bellinghausen C. Aprotinin Inhibits SARS-CoV-2 Replication. Cells. 2020;9 doi: 10.3390/cells9112377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter P.J., Lazar G.A. Next generation antibody drugs: pursuit of the 'high-hanging fruit'. Nat Rev Drug Discovery. 2018;17:197–223. doi: 10.1038/nrd.2017.227. [DOI] [PubMed] [Google Scholar]
- 35.Papageorgiou A.C., Mohsin I. The SARS-CoV-2 Spike Glycoprotein as a Drug and Vaccine Target: Structural Insights into Its Complexes with ACE2 and Antibodies. Cells. 2020;9 doi: 10.3390/cells9112343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pecetta S., Finco O., Seubert A. Quantum leap of monoclonal antibody (mAb) discovery and development in the COVID-19 era. Semin Immunol. 2020;50 doi: 10.1016/j.smim.2020.101427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butreddy A., Janga K.Y., Ajjarapu S., Sarabu S., Dudhipala N. Instability of therapeutic proteins - An overview of stresses, stabilization mechanisms and analytical techniques involved in lyophilized proteins. Int J Biol Macromol. 2021;167:309–325. doi: 10.1016/j.ijbiomac.2020.11.188. [DOI] [PubMed] [Google Scholar]
- 38.Glasgow A., Glasgow J., Limonta D., Solomon P., Lui I., Zhang Y. Engineered ACE2 receptor traps potently neutralize SARS-CoV-2. PNAS. 2020;117:28046–28055. doi: 10.1073/pnas.2016093117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji P., Chen J., Golding A., Nikolov N.P., Saluja B., Ren Y.R. Immunomodulatory therapeutic proteins in COVID-19: current clinical development and clinical pharmacology considerations. J Clin Pharmacol. 2020;60:1275–1293. doi: 10.1002/jcph.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khodabakhsh F., Salimian M., Hedayati M.H., Ahangari Cohan R., Norouzian D. Challenges and advancements in the pharmacokinetic enhancement of therapeutic proteins. Prep Biochem Biotechnol. 2021:1–11. doi: 10.1080/10826068.2020.1839907. [DOI] [PubMed] [Google Scholar]
- 41.Schuster J., Koulov A., Mahler H.C., Detampel P., Huwyler J., Singh S. In vivo stability of therapeutic proteins. Pharm Res. 2020;37:23. doi: 10.1007/s11095-019-2689-1. [DOI] [PubMed] [Google Scholar]
- 42.Jing X., Hou Y., Hallett W., Sahajwalla C.G., Ji P. Key physicochemical characteristics influencing adme properties of therapeutic proteins. Adv Exp Med Biol. 2019;1148:115–129. doi: 10.1007/978-981-13-7709-9_6. [DOI] [PubMed] [Google Scholar]
- 43.Krause M.E., Sahin E. Chemical and physical instabilities in manufacturing and storage of therapeutic proteins. Curr Opin Biotechnol. 2019;60:159–167. doi: 10.1016/j.copbio.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 44.Sauna Z.E., Lagassé D., Pedras-Vasconcelos J., Golding B., Rosenberg A.S. Evaluating and mitigating the immunogenicity of therapeutic proteins. Trends Biotechnol. 2018;36:1068–1084. doi: 10.1016/j.tibtech.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Q., Qiu H. The mechanistic impact of N-glycosylation on stability, pharmacokinetics, and immunogenicity of therapeutic proteins. J Pharm Sci. 2019;108:1366–1377. doi: 10.1016/j.xphs.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 46.He L., Zhu J. Computational tools for epitope vaccine design and evaluation. Curr Opin Virol. 2015;11:103–112. doi: 10.1016/j.coviro.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Z., Bogdan P., Nazarian S. An in silico deep learning approach to multi-epitope vaccine design: a SARS-CoV-2 case study. Sci Rep. 2021;11:3238. doi: 10.1038/s41598-021-81749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fröhlich E. Therapeutic potential of mesenchymal stem cells and their products in lung diseases-intravenous administration versus inhalation. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, Kouroupis D, et al. (2021) Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem cells translational medicine. [DOI] [PMC free article] [PubMed]
- 50.Mahendiratta S, Bansal S, Sarma P, Kumar H, Choudhary G, Kumar S, et al. (2021) Stem cell therapy in COVID-19: Pooled evidence from SARS-CoV-2, SARS-CoV, MERS-CoV and ARDS: A systematic review. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 137:111300. [DOI] [PMC free article] [PubMed]
- 51.Gitahy Falcao Faria C., Weiner L., Petrignet J., Hingray C., De Pellon Ruiz, Santamaria Á. Antihistamine and cationic amphiphilic drugs, old molecules as new tools against the COVID-19? Med Hypotheses. 2021;148 doi: 10.1016/j.mehy.2021.110508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salata C., Calistri A., Parolin C., Baritussio A., Palù G. Antiviral activity of cationic amphiphilic drugs. Expert Rev Anti-Infect Ther. 2017;15:483–492. doi: 10.1080/14787210.2017.1305888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villoutreix B.O., Beaune P.H., Tamouza R., Krishnamoorthy R., Leboyer M. Prevention of COVID-19 by drug repurposing: rationale from drugs prescribed for mental disorders. Drug Discov Today. 2020;25:1287–1290. doi: 10.1016/j.drudis.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeouk I., Bekhti K., Lorenzo-Morales J. From Wuhan to COVID-19 pandemic: An up-to-date review of its pathogenesis, potential therapeutics, and recent advances. Microorganisms. 2020;8 doi: 10.3390/microorganisms8060850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blaess M, Kaiser L, Sommerfeld O, Rentschler S, Csuk R, Deigner H-P (2020) Rational drug repurposing: focus on lysosomotropism, targets in disease process, drug profile, and pulmonary tissue accumulation in SARS-CoV-2 infection/COVID-19. Frontiers in Pharmacology| www frontiersin org. 11. [DOI] [PMC free article] [PubMed]
- 56.Monpara J.D., Sodha S.J., Gupta P.K. COVID-19 associated complications and potential therapeutic targets. Eur J Pharmacol. 2020;886 doi: 10.1016/j.ejphar.2020.173548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pandey A., Nikam A.N., Shreya A.B., Mutalik S.P., Gopalan D., Kulkarni S. Potential therapeutic targets for combating SARS-CoV-2: Drug repurposing, clinical trials and recent advancements. Life Sci. 2020;256 doi: 10.1016/j.lfs.2020.117883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suganya S, Divya S, Parani M (2020) Severe acute respiratory syndrome-coronavirus-2: Current advances in therapeutic targets and drug development. Reviews in medical virology. [DOI] [PMC free article] [PubMed]
- 59.Wu Y., Li Z., Zhao Y.S., Huang Y.Y., Jiang M.Y., Luo H.B. Therapeutic targets and potential agents for the treatment of COVID-19. Med Res Rev. 2021 doi: 10.1002/med.21776. [DOI] [PubMed] [Google Scholar]
- 60.Fung T.S., Liu D.X. Human coronavirus: host-pathogen interaction. Annu Rev Microbiol. 2019;73:529–557. doi: 10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
- 61.Guy R.K., DiPaola R.S., Romanelli F., Dutch R.E. Rapid repurposing of drugs for COVID-19. Science (New York, NY). 2020;368:829–830. doi: 10.1126/science.abb9332. [DOI] [PubMed] [Google Scholar]
- 62.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 63.Xiu S., Dick A., Ju H., Mirzaie S., Abdi F., Cocklin S. Inhibitors of SARS-CoV-2 Entry: Current and Future Opportunities. J Med Chem. 2020;63:12256–12274. doi: 10.1021/acs.jmedchem.0c00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou H., Fang Y., Xu T., Ni W.J., Shen A.Z., Meng X.M. Potential therapeutic targets and promising drugs for combating SARS-CoV-2. Br J Pharmacol. 2020;177:3147–3161. doi: 10.1111/bph.15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Villoutreix B.O., Krishnamoorthy R., Tamouza R., Leboyer M., Beaune P. Chemoinformatic analysis of psychotropic and antihistaminic drugs in the light of experimental anti-SARS-CoV-2 activities. Advances and applications in bioinformatics and chemistry. AABC. 2021;14:71–85. doi: 10.2147/AABC.S304649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Emmerich C.H., Gamboa L.M., Hofmann M.C.J., Bonin-Andresen M., Arbach O., Schendel P. Improving target assessment in biomedical research: the GOT-IT recommendations. Nat Rev Drug Discov. 2021;20:64–81. doi: 10.1038/s41573-020-0087-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knowles J., Gromo G. A guide to drug discovery: Target selection in drug discovery. Nat Rev Drug Discov. 2003;2:63–69. doi: 10.1038/nrd986. [DOI] [PubMed] [Google Scholar]
- 68.Siramshetty V.B., Preissner R. Drugs as habitable planets in the space of dark chemical matter. Drug Discov Today. 2018;23:481–486. doi: 10.1016/j.drudis.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 69.Sosa E.J., Burguener G., Lanzarotti E., Defelipe L., Radusky L., Pardo A.M. Target-Pathogen: a structural bioinformatic approach to prioritize drug targets in pathogens. Nucleic Acids Res. 2018;46:D413–D418. doi: 10.1093/nar/gkx1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sydow D., Burggraaff L., Szengel A., van Vlijmen H.W.T., Ij A.P., van Westen G.J.P. Advances and challenges in computational target prediction. J Chem Inf Model. 2019;59:1728–1742. doi: 10.1021/acs.jcim.8b00832. [DOI] [PubMed] [Google Scholar]
- 71.Bergström F., Lindmark B. Accelerated drug discovery by rapid candidate drug identification. Drug Discov Today. 2019;24:1237–1241. doi: 10.1016/j.drudis.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 72.Canning P., Birchall K., Kettleborough C.A., Merritt A., Coombs P.J. Fragment-based target screening as an empirical approach to prioritising targets: a case study on antibacterials. Drug Discov Today. 2020 doi: 10.1016/j.drudis.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 73.Duarte Y., Márquez-Miranda V., Miossec M.J., González-Nilo F. Integration of target discovery, drug discovery and drug delivery: A review on computational strategies. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11 doi: 10.1002/wnan.1554. [DOI] [PubMed] [Google Scholar]
- 74.Wilkinson I.V.L., Terstappen G.C., Russell A.J. Combining experimental strategies for successful target deconvolution. Drug Discov Today. 2020 doi: 10.1016/j.drudis.2020.09.016. [DOI] [PubMed] [Google Scholar]
- 75.Cavasotto C.N., Phatak S.S. Homology modeling in drug discovery: current trends and applications. Drug Discov Today. 2009;14:676–683. doi: 10.1016/j.drudis.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 76.Muhammed M.T., Aki-Yalcin E. Homology modeling in drug discovery: Overview, current applications, and future perspectives. Chem Biol Drug Des. 2019;93:12–20. doi: 10.1111/cbdd.13388. [DOI] [PubMed] [Google Scholar]
- 77.Sliwoski G., Kothiwale S., Meiler J., Lowe E.W., Jr. Computational methods in drug discovery. Pharmacol Rev. 2014;66:334–395. doi: 10.1124/pr.112.007336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abrusán G., Marsh J.A. Ligands and receptors with broad binding capabilities have common structural characteristics: an antibiotic design perspective. J Med Chem. 2019;62:9357–9374. doi: 10.1021/acs.jmedchem.9b00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cavasotto C.N., Lamas M.S., Maggini J. Functional and druggability analysis of the SARS-CoV-2 proteome. Eur J Pharmacol. 2021;890 doi: 10.1016/j.ejphar.2020.173705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pérot S., Sperandio O., Miteva M.A., Camproux A.C., Villoutreix B.O. Druggable pockets and binding site centric chemical space: a paradigm shift in drug discovery. Drug Discov Today. 2010;15:656–667. doi: 10.1016/j.drudis.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 81.Stank A., Kokh D.B., Fuller J.C., Wade R.C. Protein binding pocket dynamics. Acc Chem Res. 2016;49:809–815. doi: 10.1021/acs.accounts.5b00516. [DOI] [PubMed] [Google Scholar]
- 82.Surade S., Blundell T.L. Structural biology and drug discovery of difficult targets: the limits of ligandability. Chem Biol. 2012;19:42–50. doi: 10.1016/j.chembiol.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 83.Kufareva I., Ilatovskiy A.V., Abagyan R. Pocketome: an encyclopedia of small-molecule binding sites in 4D. Nucleic Acids Res. 2012;40:D535–D540. doi: 10.1093/nar/gkr825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vajda S., Beglov D., Wakefield A.E., Egbert M., Whitty A. Cryptic binding sites on proteins: definition, detection, and druggability. Curr Opin Chem Biol. 2018;44:1–8. doi: 10.1016/j.cbpa.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arya R., Kumari S., Pandey B., Mistry H., Bihani S.C., Das A. Structural insights into SARS-CoV-2 proteins. J Mol Biol. 2021;433 doi: 10.1016/j.jmb.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gordon D.E., Hiatt J., Bouhaddou M., Rezelj V.V., Ulferts S., Braberg H. Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science (New York NY) 2020;370 doi: 10.1126/science.abe9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karunakaran K.B., Balakrishnan N., Ganapathiraju M.K. Interactome of SARS-CoV-2 / nCoV19 modulated host proteins with computationally predicted PPIs. Research Square. 2020 [Google Scholar]
- 89.Zhou N., Bao J., Ning Y. H2V: a database of human genes and proteins that respond to SARS-CoV-2, SARS-CoV, and MERS-CoV infection. BMC Bioinf. 2021;22:18. doi: 10.1186/s12859-020-03935-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ekins S., Mottin M., Ramos P., Sousa B.K.P., Neves B.J., Foil D.H. Déjà vu: Stimulating open drug discovery for SARS-CoV-2. Drug Discovery Today. 2020;25:928–941. doi: 10.1016/j.drudis.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adamson C.S., Chibale K., Goss R.J.M., Jaspars M., Newman D.J., Dorrington R.A. Antiviral drug discovery: preparing for the next pandemic. Chem Soc Rev. 2021 doi: 10.1039/d0cs01118e. [DOI] [PubMed] [Google Scholar]
- 92.Hwang W., Lei W., Katritsis N.M., MacMahon M., Chapman K., Han N. Current and prospective computational approaches and challenges for developing COVID-19 vaccines. Adv Drug Deliv Rev. 2021 doi: 10.1016/j.addr.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kuenemann M.A., Sperandio O., Labbé C.M., Lagorce D., Miteva M.A., Villoutreix B.O. In silico design of low molecular weight protein-protein interaction inhibitors: Overall concept and recent advances. Prog Biophys Mol Biol. 2015;119:20–32. doi: 10.1016/j.pbiomolbio.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 94.Villoutreix B.O., Kuenemann M.A., Poyet J.L., Bruzzoni-Giovanelli H., Labbé C., Lagorce D. Drug-like protein-protein interaction modulators: challenges and opportunities for drug discovery and chemical biology. Mol Inf. 2014;33:414–437. doi: 10.1002/minf.201400040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bosc N., Muller C., Hoffer L., Lagorce D., Bourg S., Derviaux C. Fr-PPIChem: An Academic Compound Library Dedicated to Protein-Protein Interactions. ACS Chem Biol. 2020;15:1566–1574. doi: 10.1021/acschembio.0c00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lu H., Zhou Q., He J., Jiang Z., Peng C., Tong R. Recent advances in the development of protein-protein interactions modulators: mechanisms and clinical trials. Sig Transd Target Ther. 2020;5:213. doi: 10.1038/s41392-020-00315-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Voter A.F., Keck J.L. Development of protein-protein interaction inhibitors for the treatment of infectious diseases. Adv Protein Chem Struct Biol. 2018;111:197–222. doi: 10.1016/bs.apcsb.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singh J., Petter R.C., Baillie T.A., Whitty A. The resurgence of covalent drugs. Nat Rev Drug Discov. 2011;10:307–317. doi: 10.1038/nrd3410. [DOI] [PubMed] [Google Scholar]
- 99.Douangamath A., Fearon D., Gehrtz P., Krojer T., Lukacik P., Owen C.D. Crystallographic and electrophilic fragment screening of the SARS-CoV-2 main protease. Nat Commun. 2020;11:5047. doi: 10.1038/s41467-020-18709-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheng S.S., Yang G.J., Wang W., Leung C.H., Ma D.L. The design and development of covalent protein-protein interaction inhibitors for cancer treatment. J Hematol Oncol. 2020;13:26. doi: 10.1186/s13045-020-00850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Datta P.K., Liu F., Fischer T., Rappaport J., Qin X. SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics. 2020;10:7448–7464. doi: 10.7150/thno.48076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Padron-Regalado E. Vaccines for SARS-CoV-2: Lessons from Other Coronavirus Strains. Infectious Diseases Therapy. 2020;9:1–20. doi: 10.1007/s40121-020-00300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dotolo S, Marabotti A, Facchiano A, Tagliaferri R (2020) A review on drug repurposing applicable to COVID-19. Briefings in bioinformatics. [DOI] [PMC free article] [PubMed]
- 104.Vela J.M. Repurposing sigma-1 receptor ligands for COVID-19 therapy? Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.582310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang J. Fast identification of possible drug treatment of coronavirus disease-19 (covid-19) through computational drug repurposing study. J Chem Inf Model. 2020;60:3277–3286. doi: 10.1021/acs.jcim.0c00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang X., Guan Y. COVID-19 drug repurposing: A review of computational screening methods, clinical trials, and protein interaction assays. Med Res Rev. 2021;41:5–28. doi: 10.1002/med.21728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou Y., Wang F., Tang J., Nussinov R., Cheng F. Artificial intelligence in COVID-19 drug repurposing. The Lancet Digital health. 2020;2:e667–e676. doi: 10.1016/S2589-7500(20)30192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abdulla A., Wang B., Qian F., Kee T., Blasiak A., Ong Y.H. Project IDentif.AI: harnessing artificial intelligence to rapidly optimize combination therapy development for infectious disease intervention. Adv Therap. 2020;2000034 doi: 10.1002/adtp.202000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Edwards A. What are the odds of finding a COVID-19 drug from a lab repurposing screen? J Chem Inf Model. 2020;60:5727–5729. doi: 10.1021/acs.jcim.0c00861. [DOI] [PubMed] [Google Scholar]
- 110.Ke Y.Y., Peng T.T., Yeh T.K., Huang W.Z., Chang S.E., Wu S.H. Artificial intelligence approach fighting COVID-19 with repurposing drugs. Biomedical journal. 2020;43:355–362. doi: 10.1016/j.bj.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brimacombe KR, Zhao T, Eastman RT, Hu X, Wang K, Backus M, et al. (2020) An OpenData portal to share COVID-19 drug repurposing data in real time. bioRxiv : the preprint server for biology.
- 112.Riva L., Yuan S., Yin X., Martin-Sancho L., Matsunaga N., Pache L. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. 2020;586:113–119. doi: 10.1038/s41586-020-2577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Touret F., Gilles M., Barral K., Nougairède A., van Helden J., Decroly E. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci Rep. 2020;10:13093. doi: 10.1038/s41598-020-70143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ellinger B, Bojkova D, Zaliani A, Cinatl J, Claussen C, Westhaus S, et al. (2020) Identification of inhibitors of SARS-CoV-2 in-vitro cellular toxicity in human (Caco-2) cells using a large scale drug repurposing collection.
- 115.Black S., Bloom D.E., Kaslow D.C., Pecetta S., Rappuoli R. Transforming vaccine development. Semin Immunol. 2020;50 doi: 10.1016/j.smim.2020.101413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Glassman P.M., Balthasar J.P. Physiologically-based modeling of monoclonal antibody pharmacokinetics in drug discovery and development. Drug Metab Pharmacokinet. 2019;34:3–13. doi: 10.1016/j.dmpk.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen WH, Strych U, Hotez PJ, Bottazzi ME (2020) The SARS-CoV-2 Vaccine Pipeline: an Overview. Current tropical medicine reports. 1-4. [DOI] [PMC free article] [PubMed]
- 118.Parker E.P.K., Shrotri M., Kampmann B. Keeping track of the SARS-CoV-2 vaccine pipeline. Nat Rev Immunol. 2020;20:650. doi: 10.1038/s41577-020-00455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yuan M., Liu H., Wu N.C., Wilson I.A. Recognition of the SARS-CoV-2 receptor binding domain by neutralizing antibodies. Biochem Biophys Res Commun. 2021;538:192–203. doi: 10.1016/j.bbrc.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang C., Wang Y., Zhu Y., Liu C., Gu C., Xu S. Development and structural basis of a two-MAb cocktail for treating SARS-CoV-2 infections. Nat Commun. 2021;12:264. doi: 10.1038/s41467-020-20465-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bourquard T, Musnier A, Puard V, Tahir S, Ayoub MA, Jullian Y, et al. (2018) MAbTope: A Method for Improved Epitope Mapping. Journal of immunology (Baltimore, Md : 1950). 201:3096-105. [DOI] [PubMed]
- 122.Luan B., Huynh T. In Silico Antibody Mutagenesis for Optimizing Its Binding to Spike Protein of Severe Acute Respiratory Syndrome Coronavirus 2. The journal of physical chemistry letters. 2020;11:9781–9787. doi: 10.1021/acs.jpclett.0c02706. [DOI] [PubMed] [Google Scholar]
- 123.Min Y.Q., Mo Q., Wang J., Deng F., Wang H., Ning Y.J. SARS-CoV-2 nsp1: Bioinformatics, Potential Structural and Functional Features, and Implications for Drug/Vaccine Designs. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.587317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ong E., Wong M.U., Huffman A., He Y. COVID-19 Coronavirus Vaccine Design Using Reverse Vaccinology and Machine Learning. Front Immunol. 2020;11:1581. doi: 10.3389/fimmu.2020.01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sohail M.S., Ahmed S.F., Quadeer A.A., McKay M.R. In silico T cell epitope identification for SARS-CoV-2: Progress and perspectives. Adv Drug Deliv Rev. 2021;171:29–47. doi: 10.1016/j.addr.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sultana J., Mazzaglia G., Luxi N., Cancellieri A., Capuano A., Ferrajolo C. Potential effects of vaccinations on the prevention of COVID-19: rationale, clinical evidence, risks, and public health considerations. Expert review of vaccines. 2020;19:919–936. doi: 10.1080/14760584.2020.1825951. [DOI] [PubMed] [Google Scholar]
- 127.Cavasotto C.N., Di Filippo J.I. Artificial intelligence in the early stages of drug discovery. Arch Biochem Biophys. 2021;698 doi: 10.1016/j.abb.2020.108730. [DOI] [PubMed] [Google Scholar]
- 128.Pereira N.L., Ahmad F., Byku M., Cummins N.W., Morris A.A., Owens A. COVID-19: Understanding Inter-Individual Variability and Implications for Precision Medicine. Mayo Clin Proc. 2021;96:446–463. doi: 10.1016/j.mayocp.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Platania C.B.M., Bucolo C. Molecular Dynamics Simulation Techniques as Tools in Drug Discovery and Pharmacology: A Focus on Allosteric Drugs. Methods in molecular biology (Clifton, NJ). 2021;2253:245–254. doi: 10.1007/978-1-0716-1154-8_14. [DOI] [PubMed] [Google Scholar]
- 130.Bianco G., Goodsell D.S., Forli S. Selective and Effective: Current Progress in Computational Structure-Based Drug Discovery of Targeted Covalent Inhibitors. Trends Pharmacol Sci. 2020;41:1038–1049. doi: 10.1016/j.tips.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen Y., Kirchmair J. Cheminformatics in Natural Product-based Drug Discovery. Mol Inf. 2020;39 doi: 10.1002/minf.202000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Musella S., Verna G., Fasano A., Di Micco S. New Perspectives of Machine Learning in Drug Discovery. Curr Med Chem. 2020 doi: 10.2174/0929867327666201111144048. [DOI] [PubMed] [Google Scholar]
- 133.Rivas-Barragan D., Mubeen S., Guim Bernat F., Hofmann-Apitius M., Domingo-Fernández D. Drug2ways: Reasoning over causal paths in biological networks for drug discovery. PLoS Comput Biol. 2020;16 doi: 10.1371/journal.pcbi.1008464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vázquez J., López M., Gibert E., Herrero E., Luque F.J. Merging Ligand-Based and Structure-Based Methods in Drug Discovery: An Overview of Combined Virtual Screening Approaches. Molecules (Basel. 2020;Switzerland). 25 doi: 10.3390/molecules25204723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gentile F., Agrawal V., Hsing M., Ton A.T., Ban F., Norinder U. Deep Docking: A Deep Learning Platform for Augmentation of Structure Based Drug Discovery. ACS Cent Sci. 2020;6:939–949. doi: 10.1021/acscentsci.0c00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Horvath D., Marcou G., Varnek A. Generative topographic mapping in drug design. Drug discovery today Technologies. 2019;32–33:99–107. doi: 10.1016/j.ddtec.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 137.Muratov E.N., Bajorath J., Sheridan R.P., Tetko I.V., Filimonov D., Poroikov V. QSAR without borders. Chem Soc Rev. 2020;49:3525–3564. doi: 10.1039/d0cs00098a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zabolotna Y., Lin A., Horvath D., Marcou G., Volochnyuk D.M., Varnek A. Chemography: Searching for Hidden Treasures. J Chem Inf Model. 2021;61:179–188. doi: 10.1021/acs.jcim.0c00936. [DOI] [PubMed] [Google Scholar]
- 139.Zhao L., Ciallella H.L., Aleksunes L.M., Zhu H. Advancing computer-aided drug discovery (CADD) by big data and data-driven machine learning modeling. Drug Discovery Today. 2020;25:1624–1638. doi: 10.1016/j.drudis.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.López-López E., Bajorath J., Medina-Franco J.L. Informatics for Chemistry, Biology, and Biomedical Sciences. J Chem Inf Model. 2021;61:26–35. doi: 10.1021/acs.jcim.0c01301. [DOI] [PubMed] [Google Scholar]
- 141.Rognan D. The impact of in silico screening in the discovery of novel and safer drug candidates. Pharmacol Ther. 2017;175:47–66. doi: 10.1016/j.pharmthera.2017.02.034. [DOI] [PubMed] [Google Scholar]
- 142.Yang X., Wang Y., Byrne R., Schneider G., Yang S. Concepts of Artificial Intelligence for Computer-Assisted Drug Discovery. Chem Rev. 2019;119:10520–10594. doi: 10.1021/acs.chemrev.8b00728. [DOI] [PubMed] [Google Scholar]
- 143.Hufsky F., Lamkiewicz K., Almeida A., Aouacheria A., Arighi C., Bateman A. Computational strategies to combat COVID-19: useful tools to accelerate SARS-CoV-2 and coronavirus research. Briefings in. 2020 doi: 10.1093/bib/bbaa232. bioinformatics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu B., Liu K., Zhang H., Zhang L., Bian Y., Huang L. CoV-Seq, a New Tool for SARS-CoV-2 Genome Analysis and Visualization: Development and Usability Study. Journal of medical Internet research. 2020;22 doi: 10.2196/22299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Martinez-Mayorga K., Madariaga-Mazon A., Medina-Franco J.L., Maggiora G. The impact of chemoinformatics on drug discovery in the pharmaceutical industry. Expert Opin Drug Discov. 2020;15:293–306. doi: 10.1080/17460441.2020.1696307. [DOI] [PubMed] [Google Scholar]
- 146.Amaro R.E., Mulholland A.J. Biomolecular Simulations in the Time of COVID19, and After. Comput Sci Eng. 2020;22:30–36. doi: 10.1109/MCSE.2020.3024155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ganesan A., Coote M.L., Barakat K. Molecular dynamics-driven drug discovery: leaping forward with confidence. Drug Discovery Today. 2017;22:249–269. doi: 10.1016/j.drudis.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 148.Liu X., Shi D., Zhou S., Liu H., Liu H., Yao X. Molecular dynamics simulations and novel drug discovery. Expert Opin Drug Discov. 2018;13:23–37. doi: 10.1080/17460441.2018.1403419. [DOI] [PubMed] [Google Scholar]
- 149.Xu J., Xue Y., Zhou R., Shi P.Y., Li H., Zhou J. Medicinal research reviews. 2020. Drug repurposing approach to combating coronavirus: Potential drugs and drug targets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gorgulla C., Padmanabha Das K.M., Leigh K.E., Cespugli M., Fischer P.D., Wang Z.F. A multi-pronged approach targeting SARS-CoV-2 proteins using ultra-large virtual screening. iScience. 2021;24(102021) doi: 10.1016/j.isci.2020.102021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Guterres H., Park S.J., Jiang W., Im W. Ligand-Binding-Site Refinement to Generate Reliable Holo Protein Structure Conformations from Apo Structures. J Chem Inf Model. 2021;61:535–546. doi: 10.1021/acs.jcim.0c01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kangabam R., Sahoo S., Ghosh A., Roy R., Silla Y., Misra N. Next-generation computational tools and resources for coronavirus research: From detection to vaccine discovery. Comput Biol Med. 2021;128 doi: 10.1016/j.compbiomed.2020.104158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rodrigues J., Barrera-Vilarmau S., J MCT, Sorokina M, Seckel E, Kastritis PL, Insights on cross-species transmission of SARS-CoV-2 from structural modeling. PLoS Comput Biol. 2020;16 doi: 10.1371/journal.pcbi.1008449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rosell M., Fernández-Recio J. Docking-based identification of small-molecule binding sites at protein-protein interfaces. Comput Struct Biotechnol J. 2020;18:3750–3761. doi: 10.1016/j.csbj.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ballester P.J. Selecting machine-learning scoring functions for structure-based virtual screening. Drug discovery today Technologies. 2019;32–33:81–87. doi: 10.1016/j.ddtec.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 156.Krieger J.M., Doruker P., Scott A.L., Perahia D., Bahar I. Towards gaining sight of multiscale events: utilizing network models and normal modes in hybrid methods. Curr Opin Struct Biol. 2020;64:34–41. doi: 10.1016/j.sbi.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shen C., Weng G., Zhang X., Leung E.L., Yao X., Pang J. Accuracy or novelty: what can we gain from target-specific machine-learning-based scoring functions in virtual screening? Briefings in. 2021 doi: 10.1093/bib/bbaa410. bioinformatics. [DOI] [PubMed] [Google Scholar]
- 158.Dubey A.K., Singh A., Prakash S., Kumar M., Singh A.K. Race to arsenal COVID-19 therapeutics: Current alarming status and future directions. Chem Biol Interact. 2020;332 doi: 10.1016/j.cbi.2020.109298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gyulkhandanyan A., Rezaie A.R., Roumenina L., Lagarde N., Fremeaux-Bacchi V., Miteva M.A. Analysis of protein missense alterations by combining sequence- and structure-based methods. Mol Genet Genomic Med. 2020;8 doi: 10.1002/mgg3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Singh N., Chaput L., Villoutreix B.O. Fast Rescoring Protocols to Improve the Performance of Structure-Based Virtual Screening Performed on Protein-Protein Interfaces. J Chem Inf Model. 2020;60:3910–3934. doi: 10.1021/acs.jcim.0c00545. [DOI] [PubMed] [Google Scholar]
- 161.Bietz S., Fährrolfes R., Rarey M. The Art of Compiling Protein Binding Site Ensembles. Mol Inf. 2016;35:593–598. doi: 10.1002/minf.201600043. [DOI] [PubMed] [Google Scholar]
- 162.Cereto-Massagué A., Ojeda M.J., Valls C., Mulero M., Pujadas G., Garcia-Vallve S. Tools for in silico target fishing. Methods (San Diego, Calif). 2015;71:98–103. doi: 10.1016/j.ymeth.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 163.Gleeson M.P., Modi S., Bender A., Robinson R.L., Kirchmair J., Promkatkaew M. The challenges involved in modeling toxicity data in silico: a review. Curr Pharm Des. 2012;18:1266–1291. doi: 10.2174/138161212799436359. [DOI] [PubMed] [Google Scholar]
- 164.Koutsoukas A., Simms B., Kirchmair J., Bond P.J., Whitmore A.V., Zimmer S. From in silico target prediction to multi-target drug design: current databases, methods and applications. J Proteomics. 2011;74:2554–2574. doi: 10.1016/j.jprot.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 165.Melo R., Lemos A., Preto A.J., Almeida J.G., Correia J.D.G., Sensoy O. Computational Approaches in Antibody-drug Conjugate Optimization for Targeted Cancer Therapy. Curr Top Med Chem. 2018;18:1091–1109. doi: 10.2174/1568026618666180731165222. [DOI] [PubMed] [Google Scholar]
- 166.Roel-Touris J., Bonvin A. Coarse-grained (hybrid) integrative modeling of biomolecular interactions. Comput Struct Biotechnol J. 2020;18:1182–1190. doi: 10.1016/j.csbj.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rosell M., Fernández-Recio J. Docking approaches for modeling multi-molecular assemblies. Curr Opin Struct Biol. 2020;64:59–65. doi: 10.1016/j.sbi.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cooper K., Baddeley C., French B., Gibson K., Golden J., Lee T. Novel Development of Predictive Feature Fingerprints to Identify Chemistry-Based Features for the Effective Drug Design of SARS-CoV-2 Target Antagonists and Inhibitors Using Machine Learning. ACS Omega. 2021;6:4857–4877. doi: 10.1021/acsomega.0c05303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Francés-Monerris A., Hognon C., Miclot T., García-Iriepa C., Iriepa I., Terenzi A. Molecular Basis of SARS-CoV-2 Infection and Rational Design of Potential Antiviral Agents: Modeling and Simulation Approaches. J Proteome Res. 2020;19:4291–4315. doi: 10.1021/acs.jproteome.0c00779. [DOI] [PubMed] [Google Scholar]
- 170.Singh T.U., Parida S., Lingaraju M.C., Kesavan M., Kumar D., Singh R.K. Drug repurposing approach to fight COVID-19. Pharmacological reports : PR. 2020;72:1479–1508. doi: 10.1007/s43440-020-00155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.El-Hachem N., Eid E., Nemer G., Dbaibo G., Abbas O., Rubeiz N. Integrative Transcriptome Analyses Empower the Anti-COVID-19 Drug Arsenal. iScience. 2020;23(101697) doi: 10.1016/j.isci.2020.101697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fagone P., Ciurleo R., Lombardo S.D., Iacobello C., Palermo C.I., Shoenfeld Y. Transcriptional landscape of SARS-CoV-2 infection dismantles pathogenic pathways activated by the virus, proposes unique sex-specific differences and predicts tailored therapeutic strategies. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Singh E., Khan R.J., Jha R.K., Amera G.M., Jain M., Singh R.P. A comprehensive review on promising anti-viral therapeutic candidates identified against main protease from SARS-CoV-2 through various computational methods. Journal, genetic engineering & biotechnology. 2020;18:69. doi: 10.1186/s43141-020-00085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Segall M. Advances in multiparameter optimization methods for de novo drug design. Expert Opin Drug Discov. 2014;9:803–817. doi: 10.1517/17460441.2014.913565. [DOI] [PubMed] [Google Scholar]
- 175.Segall M., Mansley T., Hunt P., Champness E. Capturing and applying knowledge to guide compound optimisation. Drug Discovery Today. 2019;24:1074–1080. doi: 10.1016/j.drudis.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 176.Villoutreix B, Krishnamoorthy R, Tamouza R, Leboyer M, Beaune P (2021) Chemoinformatic Analysis of Psychotropic and Antihistaminic Drugs in the Light of Experimental Anti-SARS-CoV-2 Activities. [DOI] [PMC free article] [PubMed]
- 177.Chaudhari R., Fong L.W., Tan Z., Huang B., Zhang S. An up-to-date overview of computational polypharmacology in modern drug discovery. Expert Opin Drug Discov. 2020;15:1025–1044. doi: 10.1080/17460441.2020.1767063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Das P., Delost M.D., Qureshi M.H., Smith D.T., Njardarson J.T. A Survey of the Structures of US FDA Approved Combination Drugs. J Med Chem. 2019;62:4265–4311. doi: 10.1021/acs.jmedchem.8b01610. [DOI] [PubMed] [Google Scholar]
- 179.Inizan T.J., Célerse F., Adjoua O., El Ahdab D., Jolly L.-H., Liu C. High-resolution mining of the SARS-CoV-2 main protease conformational space: supercomputer-driven unsupervised adaptive sampling. Chem Sci. 2021 doi: 10.1039/d1sc00145k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Smith MD, Smith JC Repurposing Therapeutics for COVID-19: Supercomputer-Based Docking to theSARS-CoV-2 Viral Spike Protein and Viral Spike Protein-Human ACE2 Interface.
- 181.Schneider P., Walters W.P., Plowright A.T., Sieroka N., Listgarten J., Goodnow R.A., Jr Rethinking drug design in the artificial intelligence era. Nat Rev Drug Discovery. 2020;19:353–364. doi: 10.1038/s41573-019-0050-3. [DOI] [PubMed] [Google Scholar]
- 182.Khan S.R., Al Rijjal D., Piro A., Wheeler M.B. Integration of AI and traditional medicine in drug discovery. Drug Discovery Today. 2021 doi: 10.1016/j.drudis.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 183.Bender A., Cortés-Ciriano I. Artificial intelligence in drug discovery: what is realistic, what are illusions? Part 1: Ways to make an impact, and why we are not there yet. Drug Discovery Today. 2021;26:511–524. doi: 10.1016/j.drudis.2020.12.009. [DOI] [PubMed] [Google Scholar]
- 184.Makarov V., Riabova O., Ekins S., Pluzhnikov N., Chepur S. The past, present and future of RNA respiratory viruses: influenza and coronaviruses. Pathogens and disease. 2020;78 doi: 10.1093/femspd/ftaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Awale M., Visini R., Probst D., Arús-Pous J., Reymond J.L. Chemical Space: Big Data Challenge for Molecular Diversity. Chimia. 2017;71:661–666. doi: 10.2533/chimia.2017.661. [DOI] [PubMed] [Google Scholar]
- 186.Panchal V., Brenk R. Riboswitches as Drug Targets for Antibiotics. Antibiotics (Basel. 2021;Switzerland). 10 doi: 10.3390/antibiotics10010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sheridan C (2021) Publisher Correction: First small-molecule drug targeting RNA gains momentum. Nature biotechnology. [DOI] [PubMed]
- 188.Maia E.H.B., Assis L.C., de Oliveira T.A., da Silva A.M., Taranto A.G. Structure-Based Virtual Screening: From Classical to Artificial Intelligence. Front Chem. 2020;8:343. doi: 10.3389/fchem.2020.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yang Z.Y., He J.H., Lu A.P., Hou T.J., Cao D.S. Application of Negative Design To Design a More Desirable Virtual Screening Library. J Med Chem. 2020;63:4411–4429. doi: 10.1021/acs.jmedchem.9b01476. [DOI] [PubMed] [Google Scholar]
- 190.Kardani K., Bolhassani A. Exploring novel and potent cell penetrating peptides in the proteome of SARS-COV-2 using bioinformatics approaches. PLoS ONE. 2021;16 doi: 10.1371/journal.pone.0247396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Leach A.R., Gillet V.J., Lewis R.A., Taylor R. Three-dimensional pharmacophore methods in drug discovery. J Med Chem. 2010;53:539–558. doi: 10.1021/jm900817u. [DOI] [PubMed] [Google Scholar]
- 192.Muthas D., Sabnis Y.A., Lundborg M., Karlén A. Is it possible to increase hit rates in structure-based virtual screening by pharmacophore filtering? An investigation of the advantages and pitfalls of post-filtering. J Mol Graph Model. 2008;26:1237–1251. doi: 10.1016/j.jmgm.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 193.Seidel T., Schuetz D.A., Garon A., Langer T. The Pharmacophore Concept and Its Applications in Computer-Aided Drug Design. Prog Chem Org Nat Prod. 2019;110:99–141. doi: 10.1007/978-3-030-14632-0_4. [DOI] [PubMed] [Google Scholar]
- 194.Willett P. Similarity searching using 2D structural fingerprints. Methods in molecular biology (Clifton, NJ). 2011;672:133–158. doi: 10.1007/978-1-60761-839-3_5. [DOI] [PubMed] [Google Scholar]
- 195.LeCun Y., Bengio Y., Hinton G. Deep learning. Nature. 2015;521:436–444. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- 196.Torres PHM, Sodero ACR, Jofily P, Silva-Jr FP (2019) Key Topics in Molecular Docking for Drug Design. International journal of molecular sciences. 20. [DOI] [PMC free article] [PubMed]
- 197.Macalino S.J.Y., Basith S., Clavio N.A.B., Chang H., Kang S., Choi S. Evolution of In Silico Strategies for Protein-Protein Interaction Drug Discovery. Molecules (Basel. 2018;Switzerland). 23 doi: 10.3390/molecules23081963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fischer T., Gazzola S., Riedl R. Approaching Target Selectivity by De Novo Drug Design. Expert Opin Drug Discov. 2019;14:791–803. doi: 10.1080/17460441.2019.1615435. [DOI] [PubMed] [Google Scholar]
- 199.Grisoni F., Schneider G. De novo Molecular Design with Generative Long Short-term Memory. Chimia. 2019;73:1006–1011. doi: 10.2533/chimia.2019.1006. [DOI] [PubMed] [Google Scholar]
- 200.Lin X., Li X., Lin X. A Review on Applications of Computational Methods in Drug Screening and Design. Molecules (Basel. 2020;Switzerland). 25 doi: 10.3390/molecules25061375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mouchlis VD, Afantitis A, Serra A, Fratello M, Papadiamantis AG, Aidinis V, et al. (2021) Advances in de Novo Drug Design: From Conventional to Machine Learning Methods. International journal of molecular sciences. 22. [DOI] [PMC free article] [PubMed]
- 202.Liang H, Zhao L, Gong X, Hu M, Wang H (2021) Virtual Screening FDA Approved Drugs against Multiple Targets of SARS-CoV-2. Clinical and translational science. [DOI] [PMC free article] [PubMed]
- 203.Wishart D.S., Wu A. Using DrugBank for In Silico Drug Exploration and Discovery. Current protocols in bioinformatics. 2016;54:14.4.1-.4.31 doi: 10.1002/cpbi.1. [DOI] [PubMed] [Google Scholar]
- 204.Irwin J.J., Tang K.G., Young J., Dandarchuluun C., Wong B.R., Khurelbaatar M. ZINC20-A Free Ultralarge-Scale Chemical Database for Ligand Discovery. J Chem Inf Model. 2020;60:6065–6073. doi: 10.1021/acs.jcim.0c00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Scarpino A., Ferenczy G.G., Keserű G.M. Covalent Docking in Drug Discovery: Scope and Limitations. Curr Pharm Des. 2020;26:5684–5699. doi: 10.2174/1381612824999201105164942. [DOI] [PubMed] [Google Scholar]
- 206.Bian Y., Xie X.S. Computational Fragment-Based Drug Design: Current Trends, Strategies, and Applications. The AAPS journal. 2018;20:59. doi: 10.1208/s12248-018-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Guedes I.A., Barreto A.M.S., Marinho D., Krempser E., Kuenemann M.A., Sperandio O. New machine learning and physics-based scoring functions for drug discovery. Sci Rep. 2021;11:3198. doi: 10.1038/s41598-021-82410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cavasotto C.N., Aucar M.G. High-Throughput Docking Using Quantum Mechanical Scoring. Front Chem. 2020;8:246. doi: 10.3389/fchem.2020.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cavasotto C.N., Di Filippo J.I. In silico Drug Repurposing for COVID-19: Targeting SARS-CoV-2 Proteins through Docking and Consensus Ranking. Mol Inf. 2021;40 doi: 10.1002/minf.202000115. [DOI] [PubMed] [Google Scholar]
- 210.Poli G., Granchi C., Rizzolio F., Tuccinardi T. Application of MM-PBSA Methods in Virtual Screening. Molecules (Basel. 2020;Switzerland). 25 doi: 10.3390/molecules25081971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang X., Shen C., Guo X., Wang Z., Weng G., Ye Q. ASFP (Artificial Intelligence based Scoring Function Platform): a web server for the development of customized scoring functions. J Cheminf. 2021;13:6. doi: 10.1186/s13321-021-00486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zahoránszky-Kőhalmi G, Siramshetty VB, Kumar P, Gurumurthy M, Grillo B, Mathew B, et al. (2020) A Workflow of Integrated Resources to Catalyze Network Pharmacology Driven COVID-19 Research. bioRxiv : the preprint server for biology. [DOI] [PMC free article] [PubMed]
- 213.Kc G, Bocci G, Verma S, Hassan M, Holmes J, Yang J, et al. (2020) REDIAL-2020: A Suite of Machine Learning Models to Estimate Anti-SARS-CoV-2 Activities. ChemRxiv : the preprint server for chemistry.
- 214.Singh N, Chaput L, Villoutreix BO (2020) Virtual screening web servers: designing chemical probes and drug candidates in the cyberspace. Briefings in bioinformatics. [DOI] [PMC free article] [PubMed]
- 215.Keshavarzi Arshadi A., Webb J., Salem M., Cruz E., Calad-Thomson S., Ghadirian N. Artificial intelligence for COVID-19 drug discovery and vaccine development. Front. Artif Intell. 2020;3:65. doi: 10.3389/frai.2020.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bimonte S., Crispo A., Amore A., Celentano E., Cuomo A., Cascella M. Potential Antiviral Drugs for SARS-Cov-2 Treatment: Preclinical Findings and Ongoing Clinical Research. vivo (Athens, Greece). 2020;34:1597–1602. doi: 10.21873/invivo.11949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Choudhary S., Silakari O. Scaffold morphing of arbidol (umifenovir) in search of multi-targeting therapy halting the interaction of SARS-CoV-2 with ACE2 and other proteases involved in COVID-19. Virus Res. 2020;289 doi: 10.1016/j.virusres.2020.198146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ngo S.T., Quynh Anh Pham N., Thi Le L., Pham D.H., Vu V.V. Computational Determination of Potential Inhibitors of SARS-CoV-2 Main Protease. J Chem Inf Model. 2020;60:5771–5780. doi: 10.1021/acs.jcim.0c00491. [DOI] [PubMed] [Google Scholar]
- 219.Singh N., Decroly E., Khatib A.M., Villoutreix B.O. Structure-based drug repositioning over the human TMPRSS2 protease domain: search for chemical probes able to repress SARS-CoV-2 Spike protein cleavages. Eur J Pharm. 2020;153 doi: 10.1016/j.ejps.2020.105495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang H., Yang Y., Li J., Wang M., Saravanan K.M., Wei J. A novel virtual screening procedure identifies Pralatrexate as inhibitor of SARS-CoV-2 RdRp and it reduces viral replication in vitro. PLoS Comput Biol. 2020;16 doi: 10.1371/journal.pcbi.1008489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bhowmik D., Nandi R., Jagadeesan R., Kumar N., Prakash A., Kumar D. Identification of potential inhibitors against SARS-CoV-2 by targeting proteins responsible for envelope formation and virion assembly using docking based virtual screening, and pharmacokinetics approaches. Infection, Genet Evol. 2020;84 doi: 10.1016/j.meegid.2020.104451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bocci G., Bradfute S.B., Ye C., Garcia M.J., Parvathareddy J., Reichard W. Virtual and In Vitro Antiviral Screening Revive Therapeutic Drugs for COVID-19. ACS Pharmacol Transl Sci. 2020;3:1278–1292. doi: 10.1021/acsptsci.0c00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Drożdżal S, Rosik J, Lechowicz K, Machaj F, Kotfis K, Ghavami S, et al. (2020) FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy. Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy. 53:100719. [DOI] [PMC free article] [PubMed]
- 224.Singh P., Tripathi M.K., Yasir M., Khare R., Tripathi M.K., Shrivastava R. Potential Inhibitors for SARS-CoV-2 and Functional Food Components as Nutritional Supplement for COVID-19: A Review. Plant Foods For Human Nutrition (Dordrecht, Netherlands). 2020;75:458–466. doi: 10.1007/s11130-020-00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Spinelli F.R., Conti F., Gadina M. HiJAKing SARS-CoV-2? The potential role of JAK inhibitors in the management of COVID-19. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abc5367. [DOI] [PubMed] [Google Scholar]
- 226.Alves V.M., Bobrowski T., Melo-Filho C.C., Korn D., Auerbach S., Schmitt C. QSAR Modeling of SARS-CoV M(pro) Inhibitors Identifies Sufugolix, Cenicriviroc, Proglumetacin, and other Drugs as Candidates for Repurposing against SARS-CoV-2. Mol Inf. 2021;40 doi: 10.1002/minf.202000113. [DOI] [PubMed] [Google Scholar]
- 227.Vatansever E.C., Yang K.S., Drelich A.K., Kratch K.C., Cho C.C., Kempaiah K.R. Bepridil is potent against SARS-CoV-2 in vitro. Proc Natl Acad Sci USA. 2021:118. doi: 10.1073/pnas.2012201118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 229.Ma C., Hu Y., Townsend J.A., Lagarias P.I., Marty M.T., Kolocouris A. Ebselen, Disulfiram, Carmofur, PX-12, Tideglusib, and Shikonin Are Nonspecific Promiscuous SARS-CoV-2 Main Protease Inhibitors. ACS Pharmacol Transl Sci. 2020;3:1265–1277. doi: 10.1021/acsptsci.0c00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Dai W., Zhang B., Jiang X.M., Su H., Li J., Zhao Y. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science (New York, NY). 2020;368:1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ghahremanpour MM, Tirado-Rives J, Deshmukh M, Ippolito JA, Zhang CH, de Vaca IC, et al. (2020) Identification of 14 Known Drugs as Inhibitors of the Main Protease of SARS-CoV-2. bioRxiv : the preprint server for biology. [DOI] [PMC free article] [PubMed]
- 232.Gimeno A, Mestres-Truyol J, Ojeda-Montes MJ, Macip G, Saldivar-Espinoza B, Cereto-Massagué A, et al. (2020) Prediction of Novel Inhibitors of the Main Protease (M-pro) of SARS-CoV-2 through Consensus Docking and Drug Reposition. International journal of molecular sciences. 21. [DOI] [PMC free article] [PubMed]
- 233.White M.A., Lin W., Cheng X. Discovery of COVID-19 Inhibitors Targeting the SARS-CoV-2 Nsp13 Helicase. The journal of physical chemistry letters. 2020;11:9144–9151. doi: 10.1021/acs.jpclett.0c02421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet (London, England). 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Schultz M.B., Vera D., Sinclair D.A. Can artificial intelligence identify effective COVID-19 therapies? EMBO Mol Med. 2020;12 doi: 10.15252/emmm.202012817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lisi L., Lacal P.M., Barbaccia M.L., Graziani G. Approaching coronavirus disease 2019: Mechanisms of action of repurposed drugs with potential activity against SARS-CoV-2. Biochem Pharmacol. 2020;180 doi: 10.1016/j.bcp.2020.114169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Stebbing J., Krishnan V., de Bono S., Ottaviani S., Casalini G., Richardson P.J. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol Med. 2020;12 doi: 10.15252/emmm.202012697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Stebbing J., Sánchez Nievas G., Falcone M., Youhanna S., Richardson P., Ottaviani S. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021;7 doi: 10.1126/sciadv.abe4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Bekerman E., Neveu G., Shulla A., Brannan J., Pu S.Y., Wang S. Anticancer kinase inhibitors impair intracellular viral trafficking and exert broad-spectrum antiviral effects. J Clin Investig. 2017;127:1338–1352. doi: 10.1172/JCI89857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Owczarek K., Szczepanski A., Milewska A., Baster Z., Rajfur Z., Sarna M. Early events during human coronavirus OC43 entry to the cell. Sci Rep. 2018;8:7124. doi: 10.1038/s41598-018-25640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ghose A., Roy S., Vasdev N., Olsburgh J., Dasgupta P. The Emerging Role of Artificial Intelligence in the Fight Against COVID-19. Eur Urol. 2020;78:775–776. doi: 10.1016/j.eururo.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Menni C., Valdes A.M., Freidin M.B., Sudre C.H., Nguyen L.H., Drew D.A. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26:1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wu C, Zheng M, Yang Y, Gu X, Yang K, Li M, et al. (2020) Furin: A Potential Therapeutic Target for COVID-19. iScience. 23:101642. [DOI] [PMC free article] [PubMed]
- 244.Huang R, Xu M, Zhu H, Chen CZ, Lee EM, He S, et al. (2020) Massive-scale biological activity-based modeling identifies novel antiviral leads against SARS-CoV-2. bioRxiv : the preprint server for biology. [DOI] [PMC free article] [PubMed]
- 245.Pitsillou E., Liang J., Karagiannis C., Ververis K., Darmawan K.K., Ng K. Interaction of small molecules with the SARS-CoV-2 main protease in silico and in vitro validation of potential lead compounds using an enzyme-linked immunosorbent assay. Comput Biol Chem. 2020;89 doi: 10.1016/j.compbiolchem.2020.107408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bonvino NP, Liang J, McCord ED, Zafiris E, Benetti N, Ray NB, et al. (2018) OliveNet™: a comprehensive library of compounds from Olea europaea. Database : the journal of biological databases and curation. 2018. [DOI] [PMC free article] [PubMed]
- 247.Mirabelli C, Wotring JW, Zhang CJ, McCarty SM, Fursmidt R, Frum T, et al. (2020) Morphological Cell Profiling of SARS-CoV-2 Infection Identifies Drug Repurposing Candidates for COVID-19. bioRxiv : the preprint server for biology. [DOI] [PMC free article] [PubMed]
- 248.Hung HC, Ke YY, Huang SY, Huang PN, Kung YA, Chang TY, et al. (2020) Discovery of M Protease Inhibitors Encoded by SARS-CoV-2. Antimicrobial agents and chemotherapy. 64. [DOI] [PMC free article] [PubMed]
- 249.Abian O., Ortega-Alarcon D., Jimenez-Alesanco A., Ceballos-Laita L., Vega S., Reyburn H.T. Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening. Int J Biol Macromol. 2020;164:1693–1703. doi: 10.1016/j.ijbiomac.2020.07.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Cao L., Goreshnik I., Coventry B., Case J.B., Miller L., Kozodoy L. De novo design of picomolar SARS-CoV-2 miniprotein inhibitors. Science (New York, NY). 2020;370:426–431. doi: 10.1126/science.abd9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Aghila Rani K.G., Hamad M.A., Zaher D.M., Sieburth S.M., Madani N., Al-Tel T.H. Drug development post COVID-19 pandemic: toward a better system to meet current and future global health challenges. Expert Opin Drug Discov. 2021;16:365–371. doi: 10.1080/17460441.2021.1854221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Chitalia V.C., Munawar A.H. A painful lesson from the COVID-19 pandemic: the need for broad-spectrum, host-directed antivirals. J Transl Med. 2020;18:390. doi: 10.1186/s12967-020-02476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lin M., Dong H.Y., Xie H.Z., Li Y.M., Jia L. Why do we lack a specific magic anti-COVID-19 drug? Analyses and solutions. Drug Discov Today. 2021;26:631–636. doi: 10.1016/j.drudis.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ogier R., Knecht W., Schwab M.E. Academic leadership: (with)holding the keys to translational medicine? Nat Med. 2019;25:1812–1813. doi: 10.1038/s41591-019-0670-5. [DOI] [PubMed] [Google Scholar]
- 255.Slusher B.S., Conn P.J., Frye S., Glicksman M., Arkin M. Bringing together the academic drug discovery community. Nat Rev Drug Discov. 2013;12:811–812. doi: 10.1038/nrd4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wagner J., Dahlem A.M., Hudson L.D., Terry S.F., Altman R.B., Gilliland C.T. A dynamic map for learning, communicating, navigating and improving therapeutic development. Nat Rev Drug Discov. 2018;17:150. doi: 10.1038/nrd.2017.217. [DOI] [PubMed] [Google Scholar]
- 257.Wagner J.A., Dahlem A.M., Hudson L.D., Terry S.F., Altman R.B., Gilliland C.T. Application of a dynamic map for learning, communicating, navigating, and improving therapeutic development. Clin Transl Sci. 2018;11:166–174. doi: 10.1111/cts.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Jordan A.M., Waddell I.D., Ogilvie D.J. Rethinking 'academic' drug discovery: the Manchester Institute perspective. Drug Discovery Today. 2015;20:525–535. doi: 10.1016/j.drudis.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 259.Shanks E., Ketteler R., Ebner D. Academic drug discovery within the United Kingdom: a reassessment. Nat Rev Drug Discovery. 2015;14:510. doi: 10.1038/nrd4661. [DOI] [PubMed] [Google Scholar]
- 260.Arvidsson P.I., Sandberg K., Sakariassen K.S. Institutional profile: the national Swedish academic drug discovery & development platform at SciLifeLab. Future Sci OA. 2017;3(Fso176) doi: 10.4155/fsoa-2017-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Roy A. Challenges with risk mitigation in academic drug discovery: finding the best solution. Expert Opin Drug Discov. 2019;14:95–100. doi: 10.1080/17460441.2019.1553952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Clark A., Jit M., Warren-Gash C., Guthrie B., Wang H.H.X., Mercer S.W. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. The Lancet Global health. 2020;8:e1003–e1017. doi: 10.1016/S2214-109X(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.