Abstract
Purpose:
To study the dosimetric risk factors for radiation-induced proximal bronchial tree (PBT) toxicity in patients treated with radiotherapy for non-small cell lung cancer (NSCLC).
Methods and Materials:
Patients with medically inoperable/unresectable NSCLC treated with conventionally fractionated 3D conformal radiotherapy (3DCRT) in prospective clinical trials were eligible for this study. PBT and PBT wall (PBTW) were contoured consistently per RTOG 1106 OAR-Atlas. The dose-volume histograms (DVHs) of physical prescription dose (DVHp) and biological effective dose (α/β=2.5, DVH2.5) were generated respectively. The primary endpoint was PBT toxicities, defined by CTCAE 4.0 under the terminology of bronchial stricture/atelectasis.
Results:
Of a total of 100 patients enrolled, with a median follow-up of 64 (95% CI, 50–78) months, 73% received 70 Gy or above, and 17% developed PBT toxicity (Grade 1, 8%; Grade 2, 6%; Grade 3, 0% and Grade 4, 3%). The median time interval between RT initiation and onset of PBT toxicity was 8.4 (95% CI, 4.7–44.1) months. The combined DVHs showed that no patient with a PBT maximum physical dose <65 Gy developed any PBT toxicity. Cox proportional hazards analysis and receiver operating characteristic analysis demonstrated that V75 of PBT was the most significant dosimetric parameter for both Grade 1+ (P=0.035) and Grade 2+ (P=0.037) PBT toxicities. The dosimetric thresholds for V75 of PBT were 6.8% and 11.9% for Grade 1+ and Grade 2+ PBT toxicity, respectively.
Conclusions:
V75 of PBT appeared be the most significant dosimetric parameter for PBT toxicity after conventionally fractionated thoracic 3DCRT. Constraining V75 of PBT may limit clinically significant PBT toxicity.
Keywords: Proximal bronchial tree, Toxicity, Dose-volume histogram, Lung cancer, Radiotherapy
INTRODUCTION
Lung cancer, predominantly non-small cell lung cancer (NSCLC), is the leading cause of cancer-related death worldwide, and radiotherapy (RT) plays a major role in lung cancer treatment.1,2 For locally advanced NSCLC, conventionally fractionated 3-dimensional conformal RT (3DCRT) with or without concurrent chemotherapy is the standard of care.3,4 For early stage NSCLC, stereotactic body radiation therapy (SBRT) has become the treatment of choice for medically inoperable peripheral disease, with excellent local control and low toxicity,5 but conventional 3DCRT remains a viable modality for high-risk centrally-located tumors owing to the risk of toxicity of SBRT to central organs. Higher doses of 3DCRT may improve local/regional tumor control; however, it is accompanied by the increased risk of RT-induced toxicities in thoracic organs at risk (OARs).3,6 Among these OARs toxicities, radiation-induced central airway toxicity, i.e. proximal bronchial tree (PBT) damage, is a concerning long-term toxicity issue.
Acute bronchitis during thoracic RT usually presents as slight or mild respiratory symptoms, while long-term radiation-induced damage of bronchi can lead to bronchial fibrosis, stenosis, and partial or complete lung collapse.7 RT-induced PBT toxicity can be clinically unnoticeable, severe, or even life-threatening, depending on the extent of damage and patient-specific issues, including pulmonary function and general performance status. However, due to the lower rate of incidence and longer emerging period, studies on PBT toxicity in conventional thoracic 3DCRT are limited and the safe dose constraints to central airway remain unknown.8 To guide RT treatment planning in clinical practice and to minimize severe long-term central airway toxicities, evidence on radiation dose-tolerance to PBT is needed.
The aim of the present study was to assess the relationship between dosimetric parameters and PBT toxicities with consideration of clinical factors. We hypothesized that clinical or dose-volume histogram (DVH) parameters can predict radiation-induced PBT toxicity. Specifically, this study aimed to investigate whether: 1) clinical factors and/or dosimetric parameters from DVHs of PBT or PBT wall (PBTW) can predict PBT toxicity; and 2) dosimetric constraints of PBT can be identified to limit clinically significant toxicities.
METHODS AND MATERIALS
Patients’ characteristics
This study included patients with stage I-III NSCLC enrolled in prospective clinical trials approved by institutional review boards (IRBs). Written informed consent was obtained from all patients. Eligible patients received definitive thoracic 3DCRT with or without concurrent chemotherapy with a total prescription dose at least 60 Gy and had computed tomography (CT) scans, treatment plans, volumes of interest, and dose distributions available for this analysis. Details of these prospective clinical trials were summarized in supplementary Table S1.
Radiation treatment
CT scans were acquired in the treatment position with patients immobilized in the supine position with arms above their heads. Scan covered the entire thorax, with a minimum of 3-mm slice thickness. Simulation CT scans of the chest were performed either under natural breath, three phases with breath held at the end of voluntary inhale, at the end of voluntary exhale, and while breathing freely, or under multiple phases of 4D scans. When 4D-CT was used, average scans were applied for PBT/PBTW contouring. When three phase scans were performed, scans at the end of natural exhale were used, as it was considered to be the most representative scan since 75% of the breathing time in human beings is during exhalation. Intravenous contrast was applied in most of the patients for target delineation. Radiotherapy was delivered using a 3D conformal technique. A total dose of 60–85.5 Gy was delivered with daily fractions of 1.8–3.8 Gy given over 6–7 weeks using 6 or 16 MV photons. Equivalent doses in 2 Gy fractions (EQD2) for the total dose were computed for those received doses other than 2 Gy daily, using an alpha/beta ratio of 10. Gross tumor volume (GTV) was contoured and edited according to the treating radiation oncologist. The total dose delivered to the planning target volume (PTV) was adjusted when necessary based on the tolerance of critical organs at risk (OARs): the normal lung was limited to a mean dose of <20 Gy or the normal tissue complication probability (NTCP) for lung less than 17–20%; maximum spinal cord dose <50 Gy; maximum brachial plexus dose <66 Gy; the whole, 2/3, and 1/3 of heart received <40 Gy, <45 Gy and <60 Gy, respectively. Of note, no constraint to PBT was applied in these clinical trials.
Dosimetric analyses of PBT and PBTW
The PBT and PBTW were retrospectively contoured on each axial scan of the planning CT according to the atlas of RTOG 1106 recommendations (http://www.rtog.org/CoreLab/ContouringAtlases/LungAtlas.aspx).9 The contours were drawn by the first physician author and checked by the senior author. The PBT included the distal 2 cm of the trachea, the carina, the right and left mainstem bronchi, the right and left upper lobe bronchi, the bronchus intermedius, the right middle lobe bronchus, the lingular bronchus, and the right and left lower lobe bronchi. In the treatment planning system, the PBT was contoured by auto-segmenting the airspace of the central airway with 3 mm expansion to include the wall. The PBTW was contoured by subtracting the airspace of the central airway from the PBT. The cumulative DVHs of physical dose (DVHp) of PBT and PBTW were generated from the summation of treatment plans. To consider the biological effect caused by different dose fractionation, the biological effective dose DVHs (DVH2.5) for PBT and PBTW were generated using the Linear Quadratic model with alpha/beta ratio of 2.5. Dosimetric parameters including mean dose, maximum point doses computed in 0.03 cc, 0.5 cc and 1.0 cc and volumes receiving doses greater than 5–100 Gy (V5-V100) were extracted from DVHp and DVH2.5, respectively. To evaluate the potential effect of primary tumor location on PBT toxicity, we graded the tumor-PBT relationship based on tumor location as following: Group I, the tumor is out of main bronchus; Group II, the tumor is within the main bronchus, but >2 cm from the carina; Group III, the tumor is within the main bronchus, and <2m from the carina but not involving it; Group IV, the tumor involves the tracheal carina.
DVH atlas analysis
As recommended by the Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) group,10,11 DVH atlases were mapped for the risk of PBT toxicity using Microsoft Excel spreadsheet. Each cell within the atlas described a small range of dose and volume (5 Gy in dose and 10% in PBT volume). The number in percentage in each cell presents the percentile of patients with PBT toxicity within the patients whose DVH fell within the range of dose and volume of this cell.
Follow-up and PBT toxicity evaluations
Patients were followed in clinic and chest CT scans were performed at 3, 6, 9, 12, 18, and 24 months after RT, and yearly thereafter. Additional CT or PET/CT scans were performed as clinically indicated. Toxicity was assessed using the National Cancer Institute Common Terminology Criteria for adverse events (CTECAE) version 4.0 for bronchial stricture or atelectasis. Briefly, Grade 1: Asymptomatic, clinical or diagnostic observations only, intervention not indicated; Grade 2: symptomatic, general medicine/clinical procedure indicated; Grade 3: severe symptom, specialized/surgical intervention indicated; Grade 4: life-threatening respiratory or hemodynamic compromise; intubation or urgent intervention indicated; Grade 5: death.
Statistical analysis
The primary endpoint was PBT toxicity greater than or equal to grade 2 (Grade 2+); however, we also analyzed PBT toxicity greater than or equal to grade 1 (Grade 1+). The time frame for developing PBT toxicity was calculated from the beginning of thoracic RT to the date of reported PBT toxicity. Patients without PBT toxicity were censored at death or last follow-up. Cox proportional hazards model was used to assess the correlations between the variables (clinical factors and dosimetric parameters) and the PBT toxicity, by estimating the hazard ratios (HR) with 95% confidence interval (95% CI). Any variables with P<0.05 in univariate analysis were selected as co-variants for adjustment in multivariate analysis. The areas under the curve (AUC) of receiver operating characteristic (ROC) curves was used to quantify the ability of positive factors to discriminate patients who did versus did not develop PBT toxicity. Optimal cutoff values for statistically significant variables were determined by the maximum Youden index (sensitivity + specificity – 1) plus calculation of positive predict value (PPV) and negative predict value (NPV). Analyses were carried out using SPSS, version 22.0 (IBM, Armonk, NY) software. Differences were considered significant if P<0.05 (2-sided).
RESULTS
Patients’ characteristics and PBT toxicity
A total of 106 patients were enrolled between 2004 to 2012 in prospective radiotherapy trials in which PBT toxicity data was recorded prospectively. Six patients were excluded due either to palliative dose (<60 Gy, n=4) or lost follow-up (off-study, n=2). All patients were treated with 3DCRT. Eighty-three patients (83%) had stage III NSCLC; 88 patients (88%) received concurrent chemo-radiation therapy. A total of 17 patients (17%) developed PBT toxicity (8% Grade 1, 6% Grade 2, 0% Grade 3, and 3% Grade 4) at a median follow-up of 64 (95% CI, 50–78) months. The median time interval between treatment initiation and onset of PBT toxicity was 8.4 (95% CI, 4.7–44.1) months. Univariate analysis showed that none of the clinical factors, including age, gender, smoking history, tumor location, tumor-PBT relationship, histology, clinical stage, Karnofsky performance status (KPS), chronic obstructive pulmonary disease (COPD), radiation dose (EQD2) and chemotherapy, correlated significantly with the risk of Grade 1+ or Grade 2+ PBT toxicity (all P-values>0.05). Specifically, the PBT toxicity had a trend of increase with the increase of grade of the tumor-PBT relationship, but did not reach statistical significance (Table 1).
Table 1.
Comparison of PBT toxicity based on clinical characteristics of patients
| Clinical factors | Patients n (%) |
PBT Grade 1+ | PBT Grade 2+ | |||||
|---|---|---|---|---|---|---|---|---|
| n (%) | P* | HR (95% CI) | n (%) | P* | HR (95% CI) | |||
| Age (years) | 0.217 | 0.719 | ||||||
| ≤66 | 50 (50) | 8 (16) | 1.00 (ref.) | 4 (8) | 1.00 (ref.) | |||
| >66 | 50 (50) | 9 (18) | 1.03 (0.98–1.08) | 5 (10) | 1.01 (0.95–1.08) | |||
| Gender | 0.763 | 0.369 | ||||||
| Male | 82 (82) | 13 (16) | 1.00 | 6 (7) | 1.00 (ref.) | |||
| Female | 18 (18) | 4 (22) | 0.92 (0.52–1.61) | 3 (17) | 0.73 (0.36–1.46) | |||
| Smoking | 0.753 | 0.661 | ||||||
| No | 4 (4) | 1 (25) | 1.00 (ref.) | 0 (0) | - | |||
| Yes | 96 (96) | 16 (17) | 0.72 (0.10–5.47) | 9 (9) | - | |||
| Tumor location | ||||||||
| Right upper lobe | 45 (45) | 7 (16) | 1.00 (ref.) | 4 (9) | 1.00 (ref.) | |||
| Right lower lobe | 16 (16) | 4 (25) | 0.472 | 0.76 (0.36–1.60) | 2 (13) | 0.718 | 0.83 (0.30–2.29) | |
| Left upper lobe | 28 (28) | 4 (14) | 0.703 | 1.18 (0.50–2.83) | 1 (4) | 0.800 | 1.17 (0.34–4.00) | |
| Left lower lobe | 11 (11) | 2 (18) | 0.557 | 0.77 (0.32–1.84) | 2 (18) | 0.230 | 0.38 (0.08–1.84) | |
| Tumor-PBT relationship# | ||||||||
| I | 39 (39) | 6 (15) | 1.00 (ref.) | 3 (8) | 1.00 (ref.) | |||
| II | 23 (23) | 4 (17) | 0.698 | 1.29 (0.36–4.58) | 1 (4) | 0.669 | 0.61 (0.06–5.87) | |
| III | 24 (24) | 4 (17) | 0.801 | 1.18 (0.33–4.17) | 3 (13) | 0.468 | 1.81 (0.37–8.98) | |
| IV | 14 (14) | 3 (21) | 0.472 | 1.66 (0.42–6.66) | 2 (14) | 0.394 | 2.18 (0.36–13.05) | |
| Histology | ||||||||
| Adenocarcinoma | 25 (25) | 5 (20) | 1.00 (ref.) | 3 (12) | 1.00 (ref.) | |||
| Squamous cell | 32 (32) | 6 (19) | 0.854 | 1.12 (0.34–3.67) | 4 (13) | 0.966 | 7.35 (−) | |
| NOS | 43 (43) | 6 (14) | 0.631 | 0.75 (0.23–2.45) | 2 (5) | 0.970 | 0.005 (−) | |
| Clinical stage | ||||||||
| I | 9 (9) | 1 (11) | 1.00 (ref.) | 0 (0) | - | |||
| II | 8 (8) | 0 (0) | 0.983 | 0 (0) | 0 (0) | - | - | |
| III | 83 (83) | 16 (19) | 0.356 | 2.60 (0.34–19.84) | 9 (11) | 0.483 | - | |
| KPS | 0.168 | 0.782 | ||||||
| ≤80 | 13 (13) | 3 (23) | 1.00 (ref.) | 1 (8) | 1.00 (ref.) | |||
| >80 | 87 (87) | 14 (16) | 0.41 (0.12–1.45) | 8 (9) | 0.75 (0.09–6.02) | |||
| COPD | 0.271 | 0.742 | ||||||
| No | 56 (56) | 6 (11) | 1.00 (ref.) | 4 (7) | 1.00 (ref.) | |||
| Yes | 44 (44) | 11 (25) | 1.75 (0.65–4.75) | 5 (11) | 0.90 (0.46–1.73) | |||
| EQD2 (Gy) | 0.600 | 0.322 | ||||||
| <70 | 51 (51) | 7 (14) | 1.00 (ref.) | 3 (6) | 1.00 (ref.) | |||
| ≥70 | 49 (49) | 10 (20) | 1.01 (0.97–1.06) | 6 (12) | 1.03 (0.97–1.10) | |||
| Chemotherapy | 0.431 | 0.472 | ||||||
| No | 12 (12) | 1 (8) | 1.00 (ref.) | 0 (0) | - | |||
| Yes | 88 (88) | 16 (18) | 2.25 (0.30–16.98) | 9 (10) | - | |||
Abbreviations: PBT, proximal bronchial tree; COPD, chronic obstructive pulmonary disease; EQD2, the 2 Gy-per-fraction equivalent dose; KPS, Karnofsky performance status; NOS, non-otherwise specified.
Tumor-PBT relationship: I, the tumor is out of main bronchus; II, the tumor is within main, but >2cm to carina; III, the tumor is within main, and <2m to carina but not involve; IV, the tumor involves carina.
P values were assessed by Cox hazard univariate analysis. Age, KPS and EQD2 were analyzed as continuous variables.
Of note, 3 patients (3%) developed Grade 4 PBT toxicity at 5.5, 14.0, and 14.5 months after thoracic RT. The three patients presented with symptoms including dyspnea, hypoxia, cough, or pneumonia due to bronchial stenosis. PBT damage was verified for these 3 patients by bronchoscopy with biopsy showing no evidence of tumor progression. Figure S1 showed a patient with post-RT distal tracheal stenosis and bronchomalacia/tracheomalacia had undergone tracheal stent placement for the complication.
Combined DVHs of PBT/PBTW for 100 patients
Figure 1 shows combined DVHp of PBT (Figure 1A) and PBTW (Figure 1B) for all 100 patients. Although distributions of DVHp were heterogeneous, the DVHp in patients with Grade 2+ PBT toxicity (the red curves) tended to fall in the high dose region (V75~V100) compared to those patients without PBT toxicity (the blue curves). The combined DVHp of PBTW showed similar results, and so did the combined DVH2.5 for both PBT and PBTW (Figure S2). Because all patients with PBT toxicity had V65 ≥0, we selected V65-V100, together with mean doses and maximum point doses of PBT and PBTW, for further Cox proportional hazards analysis.
Figure 1.
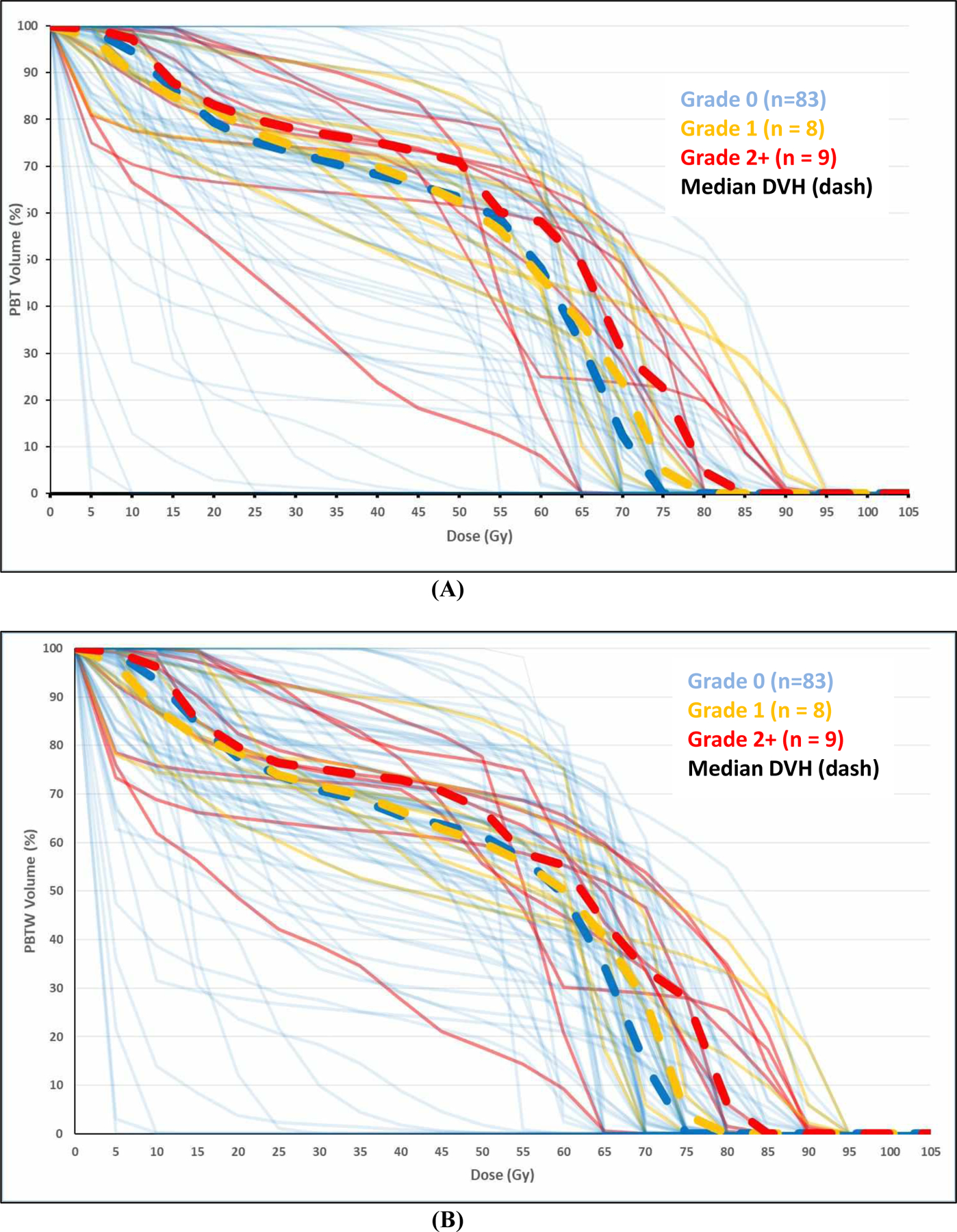
Combined Dose-Volume Histograms (DVHs) of proximal bronchial tree (A) and proximal bronchial tree wall (B) for 100 patients.
DVH atlas for PBT
Mapping DVH atlases for Grade 1+ (Figure 2A) Grade 2+ (Figure 2B) PBT toxicity demonstrated that areas of DVHs exhibiting higher rates of Grade 1+ or 2+ toxicity lay in the bottom right corner of the plots. In this area, the rates of Grade 1+ and Grade 2+ toxicity reached 40%–75% and 20%–75% when 20%–50% of PBT volume received radiation doses of 70–90 Gy. Although at 45 Gy there was a small area exhibiting rates of 50% for 20% volume for both toxicity grades, the majority of high risk toxicity fell in the high dose region (70–90 Gy).
Figure 2.
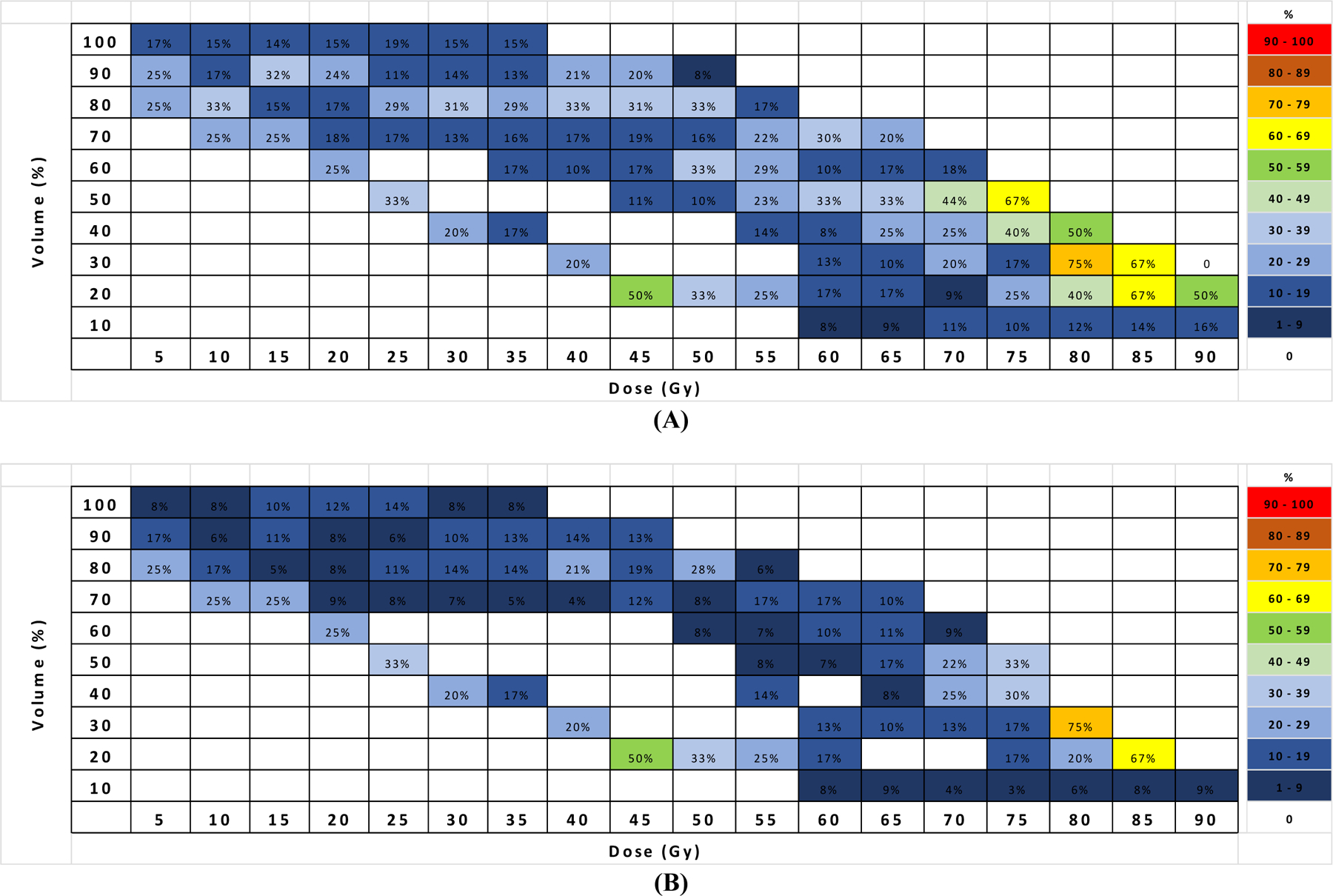
Dose-Volume Histograms (DVHs) atlas plots for (A) Grade 1+ and (B) Grade 2+ PBT toxicity.
DVH parameters for PBT/PBTW and PBT toxicity
None of maximal point doses or mean doses from PBT/PBTW DVH correlated significantly with either Grade 1+ or Grade 2+ PBT toxicities (P>0.05). Cox proportional hazards analysis showed that several volumetric parameters from DVHp or DVH2.5 were significantly associated with PBT toxicity (Table 2). By ROC analysis, V80_PBTW (AUC=0.684) and V75_PBT (AUC=0.729) had the best capacity for predicting Grade 1 and Grade 2 PBT toxicity, respectively (Table 2, Figure 3A and 3B).
Table 2.
Significant DVH parameters correlate PBT toxicity.
| DVH parameters | Mean | HR* | 95% CI | P* | AUC | |
|---|---|---|---|---|---|---|
| PBT Grade 1+ | Grade 0 (N=83) | Grade ≥ 1 (N=17) | ||||
| V75_PBT | 7.8 | 17.9 | 1.03 | 1.00–1.05 | 0.035 | 0.657 |
| V75_PBTW | 9.3 | 18.8 | 1.03 | 1.00–1.05 | 0.047 | 0.642 |
| V80_PBTW | 4.3 | 12.4 | 1.03 | 1.00–1.06 | 0.035 | 0.684 |
| V85_PBTW | 1.9 | 7.6 | 1.04 | 1.00–1.08 | 0.032 | 0.651 |
| V95_PBTW2.5 | 3.4 | 9.7 | 1.04 | 1.00–1.07 | 0.033 | 0.639 |
| V100_PBTW2.5 | 1.6 | 5.8 | 1.05 | 1.00–1.09 | 0.029 | 0.602 |
| PBT Grade 2+ | Grade 0–1 (N=91) | Grade ≥ 2 (N=9) | ||||
| V75_PBT | 8.4 | 21.3 | 1.04 | 1.00–1.07 | 0.037 | 0.729 |
| V75_PBTW | 9.7 | 23.3 | 1.04 | 1.00–1.07 | 0.030 | 0.719 |
| V80_PBT2.5 | 10.3 | 26.4 | 1.03 | 1.00–1.06 | 0.043 | 0.723 |
| V75_PBTW2.5 | 15.8 | 33.6 | 1.03 | 1.00–1.06 | 0.048 | 0.697 |
| V80_PBTW2.5 | 10.9 | 27.0 | 1.03 | 1.00–1.06 | 0.044 | 0.724 |
Abbreviations: PBT, proximal bronchial tree; DVH, dose-volume histogram; AUC, The areas under the receiver operating characteristic (ROC) curves.
From Cox proportional hazards univariate analysis, DVH parameters were analyzed as continuous variables.
Figure 3.
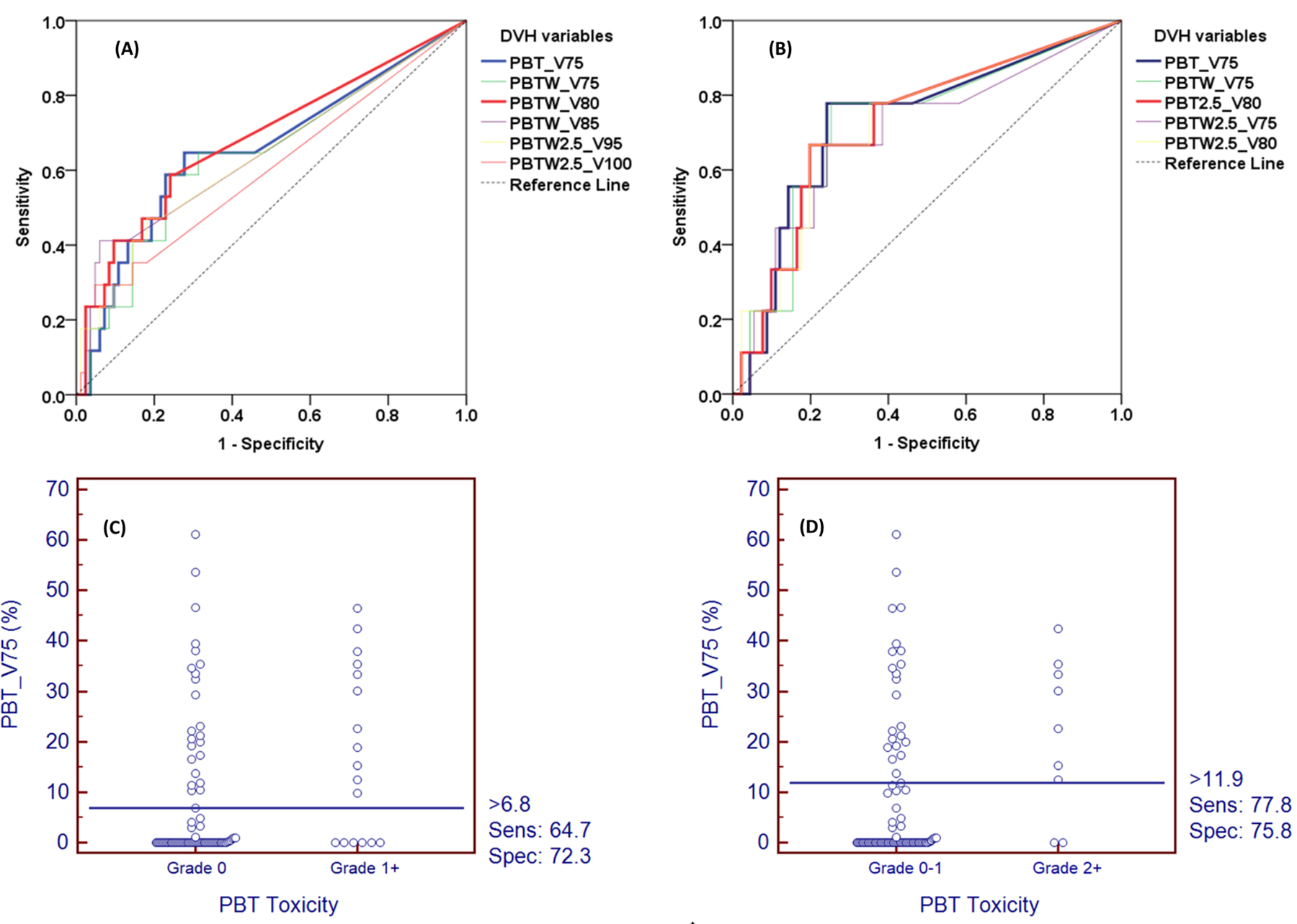
Receiver operating characteristic (ROC) analysis of Dose-Volume Histograms (DVHs) parameters for PBT toxicity Grade 1+ (A, C) and Grade 2+ (B, D).
Predictive accuracy and cut-offs recommendation
As it had a higher AUC for clinically significant PBT toxicity (Grade 2+) compared to that of other volumetric parameters, V75_PBT was selected as the dosimetric constraint to test predicting accuracy, such that an optimal cutoff for dose-limitation could be elucidated. To maximize the Youden index, the optimal cutoff points of V75 of PBT to predict toxicity were 6.8% for Grade 1 toxicity (sensitivity 65%, specificity 72%, PPV 32%, NPV 91%) and 11.9% for Grade 2 toxicity (sensitivity 78%, specificity 76%, PPV 24%, NPV 97%) (Figure 3C and 3D).
Kaplan-Meier Log Rank test validated that the hazards of PBT toxicity were significantly decreased by limiting V75_PBT ≤ 6.8% for Grade 1 (32% vs. 9%, P = 0.026) and V75_PBT or ≤ 11.9% Grade 2 (24% vs. 3%, P = 0.003), respectively (Figure 4A and 4B).
Figure 4.
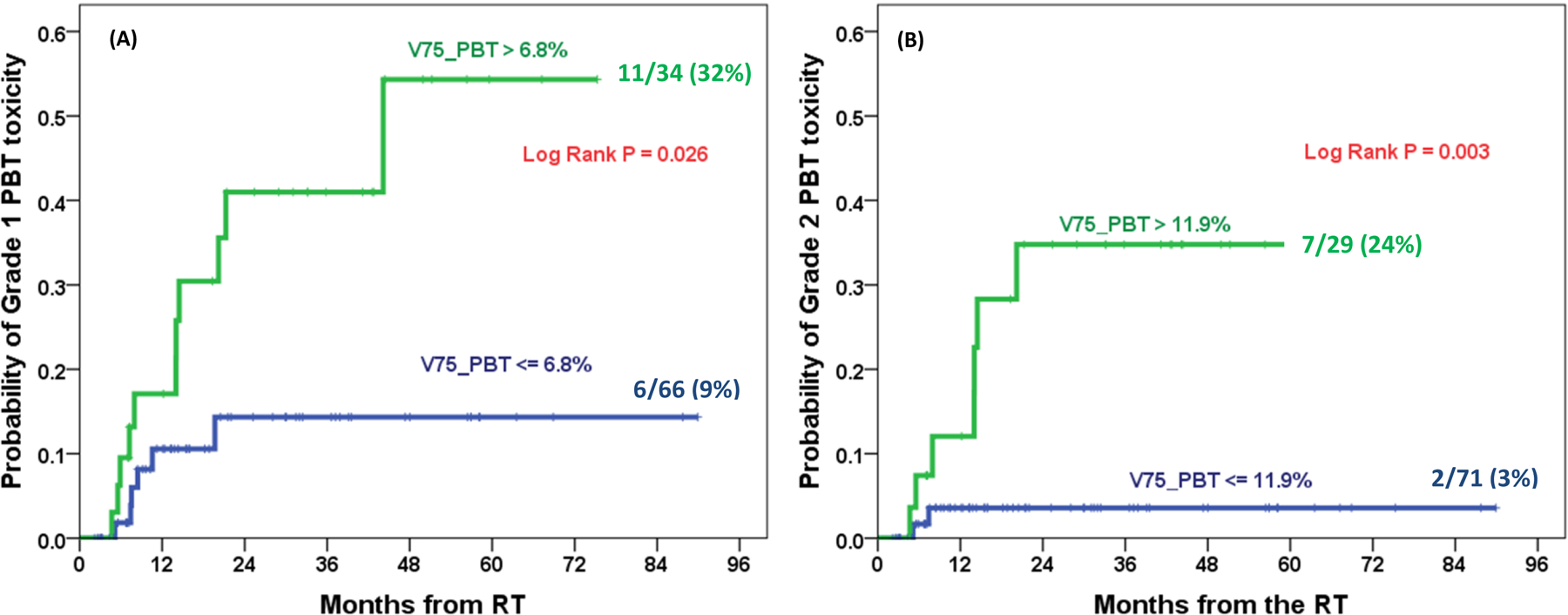
Kaplan-Meier cumulative incidence plots for Grade 1+ (A) and Grade 2+ (B) PBT toxicity with optimal cutoff values of V75 of PBT.
PBT, proximal bronchial tree; V75, volumes receiving doses greater than 75 Gy.
DISCUSSION
This study demonstrates in patients treated with daily fractionated thoracic 3DCRT that: (1) high dose volumes rather than maximal point doses or tumor prescription doses (EQD2) were significantly correlated with radiation-induced central airway toxicity; (2) dosimetric parameters from PBT or PBT wall were comparable in their correlation with the toxicity; (3) none of the patients with PBT receiving less than 65 Gy physical dose developed any PBT toxicity; (4) V75 of PBT was the most significant dosimetric parameter for Grade 2+ PBT toxicity, and dosimetric constraint of V75 of PBT ≤ 11.9% may be used to limit clinical significant Grade 2+ PBT toxicity. These results are the first in the human literature support our hypothesis that DVH parameters can predict radiation-induced PBT toxicity, and that PBT toxicity can be limited by limiting the volume to 75 Gy.
Radiation-induced central airway damage may be an underreported complication in lung cancer patients treated with thoracic RT, due to its mostly unnoticeable nature and to censoring by short post-treatment patient survival.8 PBT was not even listed as a critical structure in some 3DCRT3,12 and intensity-modulated RT (IMRT)13 clinical trials although PBT adverse events did occur in these studies. Our findings that 9% had Grade 2+ toxicity after median RT dose 78.4 Gy (range, 66–85.5 Gy) at a median interval of onset of PBT toxicity of 8.4 months, with a wide range of 4.7–44.1 months, agree with and extend findings of Kelsey et al., who reported a narrowing of the mainstem bronchi as early as 3 months after thoracic RT.7 These numbers indicate that PBT toxicity is a typical late adverse event (defined as >3 months after treatment). Our data also showed that the incidence rates of Grade 1+ and Grade 2+ PBT toxicity were 17% and 9%, respectively. Most studies in the current literature reported sparse rates of late toxicity after conventional external beam thoracic RT.14,15 Miller et al. reported that bronchial stenosis reached 38% after 4 years follow up in 103 NSCLC patients treated with definitive thoracic RT with prescription doses of 70.8–74.5 Gy with unknown PBT dosimetry. The rate of PBT toxicity in that study appeared to be significantly higher than our study. The likely explanation is that, although the total prescription doses were similar to the current study, they used twice-daily fractionation.8 This disparity suggests that a more conservative dose constraint to PBT should be considered when using a non-conventional RT modality in treatment planning. The DVH parameters with the cutoffs we provide in this study should be considered to be references only for daily fractionated 3DCRT treatment planning.
As there was no other comprehensive dosimetric study performed previously on PBT toxicity after 3DCRT, particularly like this one with clearly defined PBT OAR structure, this study may be the first of its kind to appear in the literature. Current practice used maximal point doses in various volumes for PBT dose constraints, for clinical trials of SBRT,16,17 largely based on assumption of series organ in biological functions. However, our data support volumetric dose parameters as more important than maximum point dose parameters in predicting radiation-induced PBT toxicity after 3DCRT. Of note, by retrospectively analyzing 74 patients treated with SBRT, Karlsson et al. reported a significant dose-response relationship between the incidence of atelectasis and the maximal dose of the bronchi (defined by 0.1 cc).18 This difference in the dosimetric predictor for PBT toxicity - specifically, the difference between 3DCRT and SBRT - suggests that hypofractionated radiation possesses different biologic effects on normal tissues, namely PBT.
It is worth highlighting that parameters from biologically-corrected dose DVHs (DVH2.5) were not superior to those from physical dose DVHs in PBT toxicity prediction based on AUC values (Table 2) although we analyzed all important parameters from both DVHs. A likely explanation is that most patients received radiotherapy with conventional dose fractionations (2 Gy/fraction). In addition, parameters from DVHs of PBT wall (excluding air cavity) were also not significantly superior to those from DVHs of PBT in toxicity prediction. This finding suggests that including versus excluding the air in the bronchial lumen during PBT contouring planning has minor influence on the DVH calculation, the common way of contouring per RTOG1106 trial is clinically reasonable.
Interestingly, none of the clinical factors significantly correlated with any grade of PBT toxicity. Furthermore, no patients with a PBT maximum dose <65 Gy developed any grade of PBT toxicity in this study. Hence, development of clinically noticeable PBT damage requires that a significant part of PBT volume be located in the high dose region (≥65 Gy). Thus, high dose-volume is the key factor for radiation-induced PBT toxicity, a finding further supported by the DVH atlas analysis. The high incidence of PBT toxicity region (>40%) mostly lay in the high dose area (70–90 Gy) with essential PBT volumes of 20%−50%. Most importantly, by comparing prediction accuracy, we found that V75 of PBT may be the most optimal dosimetric parameter for the prediction of PBT toxicity, a finding that will require independent validation. Furthermore, by maximizing Youden index, a commonly used method of threshold determination, we identified the optimal cutoff values for V75 of PBT (<11.9%) to predict Grade 2+ PBT toxicity.
We recognize that this study is limited in several aspects. Firstly, a sample size of 100 is small with a limited number of PBT toxicity events (especially for Grade 2+ 9% only), which may have underpowered the significance of testing in clinical variables. Secondly, similar to other OARs, chemotherapy could affect the rate and severity of PBT toxicity. This study was not powered to detect the effect of chemotherapy on PBT toxicity, as only 12 of our 100 patients did not receive chemotherapy, nor was it the primary aim of the current study. Rather, this study will be the first of its kind to perform comprehensive dosimetric modeling for PBT. Nonetheless, additional validation studies with larger sample sizes are desired to increase the clinical significance of the data.
CONCLUSIONS
This study demonstrates that the PBT volumes receiving high dose may be more important than the traditionally believed maximal point dose parameters to predict radiation-induced central airway toxicity following 3DCRT. Patients with PBT receiving dose less than 65 Gy have minimal risk of developing PBT toxicity after thoracic 3DCRT. V75 of PBT may be the most predictive dosimetric parameter in predicting clinically significant PBT toxicity in thoracic radiotherapy. Dosimetric constraint of V75 of PBT ≤ 11.9% may be used to limit clinical significant Grade 2+ PBT toxicity.
Supplementary Material
【Funding Statement】
This project was funded in parts by the National Cancer Institute, National Institutes of Health, R01 CA142840 (PI: Kong), Merit Review I01 CX000911 from the Department of Veterans Affairs (PI: Curtis), and Shenzhen Science and Technology grant KQTD20180411185028798 (PI: Kong).
Footnotes
【Conflict of Interest】
The authors declare no potential conflict of interest related to this work.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. : Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Tyldesley S, Boyd C, Schulze K, et al. : Estimating the need for radiotherapy for lung cancer: an evidence-based, epidemiologic approach. Int J Radiat Oncol Biol Phys 49:973–85, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bradley JD, Paulus R, Komaki R, et al. : Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 16:187–99, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senan S, Brade A, Wang LH, et al. : PROCLAIM: Randomized phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 34:953–62, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Timmerman R, Paulus R, Galvin J, et al. : Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 303:1070–6, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley JD, Hu C, Komaki RU, et al. : Long-Term Results of RTOG 0617: A Randomized Phase 3 Comparison of Standard Dose Versus High Dose Conformal Chemoradiation Therapy +/− Cetuximab for Stage III NSCLC. International Journal of Radiation Oncology • Biology • Physics 99:S105, 2017 [Google Scholar]
- 7.Kelsey CR, Kahn D, Hollis DR, et al. : Radiation-induced narrowing of the tracheobronchial tree: an in-depth analysis. Lung Cancer 52:111–6, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Miller KL, Shafman TD, Anscher MS, et al. : Bronchial stenosis: an underreported complication of high-dose external beam radiotherapy for lung cancer? Int J Radiat Oncol Biol Phys 61:64–9, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Nomura M, Kodaira T, Furutani K, et al. : Predictive factors for radiation pneumonitis in oesophageal cancer patients treated with chemoradiotherapy without prophylactic nodal irradiation. Br J Radiol 85:813–8, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deasy JO, Bentzen SM, Jackson A, et al. : Improving normal tissue complication probability models: the need to adopt a “data-pooling” culture. Int J Radiat Oncol Biol Phys 76:S151–4, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson A, Marks LB, Bentzen SM, et al. : The lessons of QUANTEC: recommendations for reporting and gathering data on dose-volume dependencies of treatment outcome. Int J Radiat Oncol Biol Phys 76:S155–60, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong FM, Ten Haken RK, Schipper M, et al. : Effect of Midtreatment PET/CT-Adapted Radiation Therapy With Concurrent Chemotherapy in Patients With Locally Advanced Non-Small-Cell Lung Cancer A Phase 2 Clinical Trial. Jama Oncology 3:1358–1365, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannon DM, Mehta MP, Adkison JB, et al. : Dose-limiting toxicity after hypofractionated dose-escalated radiotherapy in non-small-cell lung cancer. J Clin Oncol 31:4343–8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CB, Stinchcombe TE, Moore DT, et al. : Late complications of high-dose (>/=66 Gy) thoracic conformal radiation therapy in combined modality trials in unresectable stage III non-small cell lung cancer. J Thorac Oncol 4:74–9, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Dechambre S, Dorzee J, Fastrez J, et al. : Bronchial stenosis and sclerosing mediastinitis: an uncommon complication of external thoracic radiotherapy. Eur Respir J 11:1188–90, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Videtic GM, Hu C, Singh AK, et al. : A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer: NRG Oncology RTOG 0915 (NCCTG N0927). Int J Radiat Oncol Biol Phys 93:757–64, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timmerman RD, Paulus R, Pass HI, et al. : Stereotactic Body Radiation Therapy for Operable Early-Stage Lung Cancer: Findings From the NRG Oncology RTOG 0618 Trial. JAMA Oncol 4:1263–1266, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlsson K, Nyman J, Baumann P, et al. : Retrospective cohort study of bronchial doses and radiation-induced atelectasis after stereotactic body radiation therapy of lung tumors located close to the bronchial tree. Int J Radiat Oncol Biol Phys 87:590–5, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


