Abstract
Objective
To evaluate the clinical outcomes, histopathological features, and obstetric and oncological outcomes of uterine smooth muscle tumor of uncertain malignant potential (STUMP).
Methods
We conducted a single-center, database review of patients with STUMP between January 2001 and December 2015. We investigated the clinical, operative, histopathologic, recurrence, and fertility outcomes of the included cases.
Results
Nineteen patients with STUMP were studied. Three were reclassified as sarcoma after slide review, and 16 patients were finally included in the study. The mean age was 45 years. Ki-67 expression was ≥10% in 25.0% of cases and 30% in the only recurrent case. Recurrence occurred 52 months after a diagnosis of STUMP in a 56-year-old female patient who underwent hysterectomy. Two of six patients who underwent myomectomy had fertility requirements, and both successfully delivered babies without recurrence. Recurrence was not related to mitosis, degree of atypia, or necrosis. There was also no relationship between type of surgery or surgical approach and recurrence.
Conclusions
Patients with STUMP warrant a pathological review process in centers with experience. Fertility-preservation is worth attempting, but young patients must be followed-up closely. Ki-67 might be a valuable marker predicting recurrence.
Keywords: Uterine smooth muscle tumor of uncertain malignant potential, recurrence, leiomyosarcoma, myomectomy, fertility preservation, Ki-67
Introduction
The 2014 World Health Organization (WHO) criteria classify uterine smooth muscle tumors that cannot be identified as definitely malignant or benign as uterine smooth muscle tumors of uncertain malignant potential (STUMPs). The diagnosis and treatment of STUMP can be challenging because its behavior is unpredictable and because it may be difficult to differentiate from other uterine smooth muscle tumors with atypical histology, such as leiomyosarcoma (LMS) and atypical leiomyoma (LM).1–4
STUMPs are distinct and relatively rare lesions.2,5 The diagnosis, treatment, and follow-up of STUMP remains controversial, especially in young women with a desire to retain their fertility.6 This study aimed to review a single institution’s experience of STUMP, and to update the information regarding the clinical management, histological features, treatment, and follow-up of this rare uterine neoplasm.
Materials and methods
Case selection
We retrospectively searched the surgical pathology files of our Pathology Department for patients with a diagnosis of STUMP between January 2001 and December 2015. Only patients with available slides and a minimum follow-up period of 2 years were included (Figure 1). The choice of uterus-preserving surgery depended largely on the patient’s wish to preserve their uterus for future childbearing. Recurrence was defined as the occurrence of STUMP or LMS after previous surgery. Uterine LM or other benign diagnoses were not considered as recurrence.
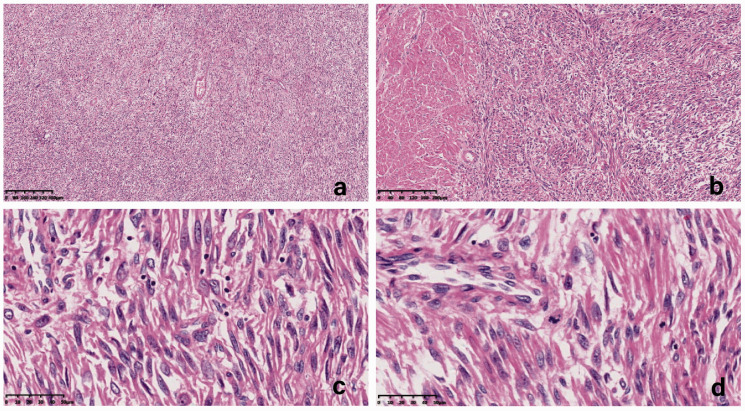
Figure 1.
Microscopic findings of cases that met the criteria for uterine smooth muscle tumor of uncertain malignant potential (STUMP). (a) Coagulative tumor cell necrosis was not found (hematoxylin and eosin (H&E; ×40). (b) Clear tumor margins, abundant cells, and obvious cellular atypia compared with the surrounding myometrium (H&E ×100). (c) Significant (moderate-severe) atypia (H&E ×400). (D) Mitotic index <10 mitoses per 10 high-power fields (H&E ×400).
Slide review process
The pathological materials for all patients were re-reviewed by two coauthors experienced in gynecologic pathology. Cases were defined as STUMP in our institution if they met one of the following 2014 WHO criteria: (1) tumor cell necrosis (TCN), no atypia, and a mitotic index <10 mitoses per 10 high-power fields (HPFs); (2) moderate-to-severe atypia, no TCN, and a mitotic index <10 mitoses per 10 HPFs; and (3) no TCN, no atypia, and a mitotic index ≥15 mitoses per 10 HPFs. Cases that did not fit the above criteria were reclassified or excluded. Tumor mitotic activity was categorized as <10 mitoses, 10 to 15 mitoses, and >15 mitoses per 10 HPFs, using the highest count in 10 HPFs at ×400 magnification. TCN was noted and typed if present. Cases were considered indeterminate if the necrosis type was difficult to classify.
Clinical and follow-up data
Information on immunohistochemical staining for Ki-67, p53, desmin, smooth muscle actin (SMA), caldesmon, CD10, progesterone receptor (PR), and estrogen receptor (ER) was available for all patients. Medical records included information on the patients’ clinical features, treatment results, recurrence-free survival, and obstetric outcomes. After primary treatment, patients were followed-up with clinical visits including gynecological examinations every 6 months for the first 3 years and annually for the following 2 years. Chest computed tomography was performed every 12 months. For recurrent cases, we recorded the site of recurrence, the size and pathologic features of the recurrent lesions, and subsequent treatment.
Ethics statement
This study was approved by the Ethics Committee of the Obstetrics and Gynecology Hospital, Fudan University, Shanghai, China (approval number: 2019-09). All patient records/information were anonymized and de-identified prior to analysis. All patients provided written informed consent for publication of these case details.
Statistical analysis
A P-value <0.05 was considered statistically significant. Categorical variables were recorded as numbers and percentages, and numerical variables as mean and standard deviation, or median and range. Means were compared using Fisher’s exact test and the Wilcoxon rank-sum test. Statistical analysis was carried out using Stata 15.1 software (StataCorp, College Station, TX, USA).
Results
Slides were reviewed for 19 patients with a diagnosis of STUMP. Three (15.8%) were interpreted as LMS, undifferentiated endometrial sarcoma and low-grade endometrial stromal sarcoma, respectively, and were subsequently excluded. Sixteen patients (11 in-house and 5 consultations) with a final diagnosis of STUMP were therefore enrolled. The clinical characteristics of the included patients are summarized in Table 1. Three cases (18.8%) had a history of previous laparoscopic myomectomy, including the relapsed case. The other patients had no related surgical history. None of the patients had received hormone replacement therapy or pelvic irradiation prior to being diagnosed with STUMP, and none received adjuvant chemotherapy or radiotherapy after the initial diagnosis of STUMP.
Table 1.
Clinical characteristics of the patients.
| Overall |
|||
|---|---|---|---|
| Characteristic | STUMP | Revised diagnosis | |
| (n = 16) | (n = 3) | P-value | |
| Median age (years) | 45 (23–56) | 45 (35–67) | 0.822 |
| Prior treatment | |||
| Prior myomectomy history (%) | 3 (18.8) | 1 (33.3) | >0.95 |
| Median follow-up time (months) | 59.5 (41–87) | 62 (24–72) | 0.502 |
| Surgical approach | 0.546 | ||
| Laparoscopy (%) | 10 (62.5) | 1 (33.3) | |
| Laparotomy (%) | 6 (37.5) | 2 (66.7) | |
| Surgery type | 0.458 | ||
| Myomectomy (%) | 6 (37.5) | 1 (33.3) | |
| Subtotal hysterectomy+/−BSO (%) | 1 (6.3) | 1 (33.3) | |
| Hysterectomy+/−BSO (%) | 9 (56.3) | 1 (33.3) | |
| Ovary-conserving (%) | 10 (62.5) | 3 (100) | 0.517 |
| Alive with no evidence of disease at last follow-up (%) | 16 (100) | 1 (33.3) | 0.018 |
| Recurrence rate (%) | 1 (6.3) | 2 (66.7) | 0.051 |
STUMP, uterine smooth muscle tumor of uncertain malignant potential; BSO, bilateral salpingo-oophorectomy.
Among the 16 patients, four (25%) underwent total hysterectomy (TH) alone, five (31.3%) had TH with bilateral salpingo-oophorectomy (BSO), one (6.3%) was treated with subtotal hysterectomy with BSO, and six patients (37.5%) underwent myomectomy. Ten patients (62.5%) were treated by laparoscopic surgery and six (37.5%) by laparotomy. Of the five patients who underwent total laparoscopic hysterectomy with BSO, one had a relapse of STUMP 52 months after surgery. There were no recurrences in patients treated with laparotomy or myomectomy. Neither type of surgery (uterus-preserving versus hysterectomy) nor surgical approach (laparotomy versus laparoscopy) was predictive of recurrence.
Microscopic evaluation of the 16 STUMP cases revealed moderate to severe atypia in two cases (12.5%) (Table 2). Fifteen (93.7%) individuals had <10 mitoses/10 HPFs. Necrosis was present in one of the 16 (6.3%) cases, but no TCN was found. According to the 2014 WHO classification of STUMP, 15 cases met the criteria of diffuse atypia, no TCN, and a mitotic index <10 mitoses/10 HPFs (Table 2, Figure 1). The other case who met the criteria of no TCN, no atypia, and a mitotic index >15 mitoses/10 HPFs had a relapse of STUMP (Table 2, Figure 2). The results of immunohistochemical analyses are shown in Table 3. Six (37.5%) cases were positive for p53 and 13 (81.3%) were positive for SMA. Twelve tumors were positive for PR expression (75.0%) and 10 were positive for ER expression. The patient who developed recurrence (Case 16) was positive for desmin, caldesmon, SMA, PR, and ER, but negative for p53. Ki-67 expression was ≥10% in four (25.0%) cases, and was 30% in the recurrent case.
Table 2.
Clinical features of patients with uterine smooth muscle tumors of uncertain malignant potential.
| Histological features |
|||||||
|---|---|---|---|---|---|---|---|
| Case | Age (years) | Surgery | Cellularity | Atypia | MI | Necrosis | Recurrence |
| 1 | 34 | Abdominal myomectomy | high | mild to moderate | <10 | absent | No |
| 2 | 44 | Laparoscopic myomectomy | moderate | mild | <10 | absent | No |
| 3 | 51 | TAH, BSO | high | mild | <10 | absent | No |
| 4 | 38 | TAH, BSO | moderate | mild | <10 | absent | No |
| 5 | 38 | TLH | high | mild to moderate | <10 | absent | No |
| 6 | 51 | TAH | moderate | mild | <10 | absent | No |
| 7 | 53 | TLH, BSO | high | mild to moderate | <10 | absent | No |
| 8 | 50 | TAH | moderate | moderate | <10 | absent | No |
| 9 | 46 | TLH | high | moderate | <10 | absent | No |
| 10 | 40 | Laparoscopic myomectomy | moderate | moderate to severe | <10 | absent | No |
| 11 | 53 | Laparoscopic myomectomy | moderate | moderate | <10 | absent | No |
| 12 | 23 | Laparoscopic myomectomy | moderate | mild | <10 | absent | No |
| 13 | 38 | Subtotal LH, BSO | moderate | mild | <10 | absent | No |
| 14 | 52 | TAH, BSO | moderate | moderate to severe | <10 | absent | No |
| 15 | 38 | Laparoscopic myomectomy | high | mild to moderate | <10 | multifocal | No |
| 16 | 56 | TLH, BSO | high | mild to moderate | ≥10 | absent | Yes (as STUMP) |
STUMP, uterine smooth muscle tumor of uncertain malignant potential; TAH, total abdominal hysterectomy; TLH, total laparoscopic hysterectomy; BSO, bilateral salpingo-oophorectomy; LH, laparoscopic hysterectomy; MI, mitotic index; MF, mitotic figure; HPF, high-power field.
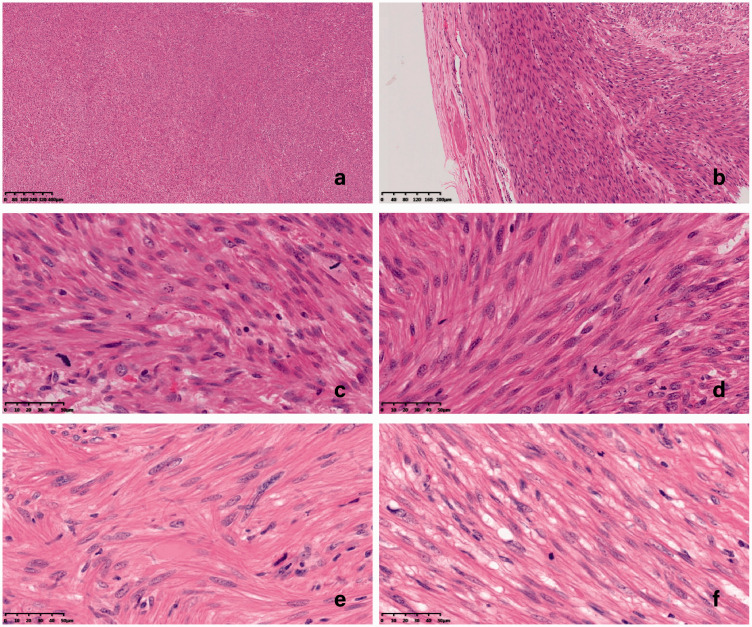
Figure 2.
Microscopic findings of primary and recurrent lesions in a patient with recurrent uterine smooth muscle tumor of uncertain malignant potential (STUMP). (a) Coagulative tumor cell necrosis was not found (hematoxylin and eosin (H&E ×40). (b) Clear tumor margins, abundant cells, but no obvious cellular atypia compared with the surrounding myometrium (H&E ×100). (c) Minimal atypia: regular nuclear membrane, fine granules on staining, and small nucleoli (H&E ×400). (D) Mitotic index >15 mitoses per 10 high-power fields (HPFs) (H&E ×400). (e, f) Recurrent tumors: minimal atypia and mitotic index >15 mitoses per 10 HPFs, no histological progression (H&E ×400).
Table 3.
Immunochemical results in patients with uterine smooth muscle tumors of uncertain malignant potential.
| Case | p53 | Ki-67 (%) | SMA | Desmin | Caldesmon | PR | ER | CD10 | Recurrence |
|---|---|---|---|---|---|---|---|---|---|
| 1 | – | <5 | + | + | + | + | No | ||
| 2 | – | 10 | + | + | + | + | – | No | |
| 3 | – | <5 | + | + | + | + | + | – | No |
| 4 | – | 10 | + | + | + | + | + | – | No |
| 5 | + | 1 | + | + | + | + | + | – | No |
| 6 | 60% | 5 | + | + | + | + | + | – | No |
| 7 | – | 5 | + | + | + | – | No | ||
| 8 | – | 5 | + | + | + | + | + | – | No |
| 9 | – | 5 | + | + | No | ||||
| 10 | + | 2 | + | + | + | + | – | No | |
| 11 | + | 1 | + | + | + | + | + | + | No |
| 12 | 2 | + | + | + | + | – | – | No | |
| 13 | – | 15 | + | + | – | + | No | ||
| 14 | + | 1 | + | + | + | No | |||
| 15 | + | 5 | + | + | – | – | + | No | |
| 16 | – | 30 | + | + | + | + | + | Yes (as STUMP) |
PR, progesterone receptor; ER, estrogen receptor; SMA, smooth muscle actin.
The only patient with recurrent STUMP was a 56-year-old woman who underwent total laparoscopic hysterectomy and BSO. Recurrent lesions were detected in the retroperitoneum 52 months after her first diagnosis of STUMP. She had undergone laparoscopic myomectomy 3 years previously and the pathologic diagnosis was benign myoma. Abdominal pelvic mass excision and exploration were performed to remove the recurrent lesions and the final pathologic diagnosis based on that surgery was STUMP, with the same mitotic activity as the initial STUMP tissue slices. She remained alive at last follow-up with no evidence of disease 60 months after the initial surgery for STUMP.
Although not defined as recurrence, one patient developed a uterine mass 3 years after the initial diagnosis of STUMP and continued to be followed-up. The overall recurrence rate was therefore 6.3%. There were no significant differences in clinical characteristics between STUMP cases with and without relapse. In contrast, two patients with a revised diagnosis of sarcoma had rapid recurrence as endometrial stromal sarcoma and LMS, respectively (Table 1). The difference in recurrence rate between patients with STUMP (6.3%) and the reclassified group (66.7%) was borderline significant (P = 0.051).
Two of six patients who underwent myomectomy wished to retain their fertility (Table 4). Both patients subsequently successfully delivered full-term live babies by Cesarean section, with no complications. The patients were alive with no evidence of disease at 78 and 71 months after the surgery, respectively.
Table 4.
Obstetric outcomes in patients who attempted pregnancy after uterus-preserving treatment for uterine smooth muscle tumors of uncertain malignant potential (n=2).
| Patient | Age at diagnosis (years) | Gestational age (weeks) | Initial surgery | Pregnancy outcome | Follow-up (months), status |
|---|---|---|---|---|---|
| 1 | 34 | 38 | Abdominal myomectomy | Live birth by C/S | 78, ANED |
| 2 | 38 | 38 | Laparoscopic myomectomy | Live birth by C/S | 71, ANED |
C/S, cesarean section; ANED, alive with no evidence of disease.
Discussion
We evaluated the clinicopathological features, and obstetric and oncological outcomes of patients with STUMP. Only one of 16 patients experienced recurrence of STUMP (6.3%), giving a lower recurrence rate compared with previous reports for STUMP (7.3%–26.7%) and stage I uterine LMS (72.3%).5,7–11 The detailed slide review performed during case selection ruled out three patients with sarcomas that were originally reported as STUMP, which might have reduced the recurrence rate.5,12 Derman et al.13 reported that six of 21 patients with a final diagnosis of STUMP were initially evaluated with equivocal smooth muscle tumors or LMS before pathologic review. The interchangeability and vagueness of the diagnosis presents a major problem in patients who present with uterus smooth muscle tumors. We therefore strongly recommend that a slide review should be carried out in centers with experience of STUMP diagnosis.
We found no significant difference in age, prior myomectomy history, uterus-conserving surgery, or surgical approach (laparotomy versus laparoscopy) between patients with and without recurrence. This was consistent with the findings of previous studies.5,8,14 Guntupalli et al.5 investigated risk factors for STUMP recurrence by comparing demographics between patients with and without recurrence but found no relationships. However, the low incidence and limited number of recurrent events make it difficult to draw conclusions regarding the risk factors or management of STUMP.
We failed to identify any pathological factors associated with recurrence or death among the patients with STUMP. However, the only patient to experience a relapse in this study had a mitotic count >15/10 HPFs, suggesting that increased proliferation, not sufficient to cause malignant behavior, may still be an important parameter. Notably, the recurrent case had no moderate-severe nuclear atypia or TCF, whereas patients with tumors with moderate-to-severe atypia (2 cases) had no adverse events at last follow-up. Although there was no significant associations between mitosis, degree of atypia, or necrosis and relapse, some other parameters have recently been reported in patients with malignant tumors. Gupta et al.15 found that several morphological parameters, including vascular space involvement, infiltrative margins, and atypical mitosis, appeared to distinguish between malignant tumors and benign lesions in STUMP cases. Sabrina et al.16 found that genomic analysis using array-comparative genomic hybridization provided an improved classification method for predicting malignant uterine smooth muscle tumors, especially those with equivocal morphological features. These approaches need to be validated in future prospective studies.
Given that the diagnosis of STUMP is difficult, markers such as Ki-67, p16, and p53 have been investigated.8,17 Several studies reported that Ki-67 expression differed among STUMP, LMS, and LM,18,19 with significantly higher expression in STUMP and LMS compared with LM. Ki-67 expression was also higher in LMS compared with STUMP, but the difference was not significant. Ip et al.8,17 reported that recurrent cases were strongly positive for p16, p53, and Ki-67 expression, and Qing et al.20 reported that ER and PR expression were significantly lower in LMS than in STUMP. Furthermore, high PR expression was significantly associated with longer overall survival.20 In the current study, 75% of STUMP tumors (12/16) showed PR expression and 63% (10/16) showed ER expression. The recurrent case showed 30% Ki-67 and was negative for p53 and positive for PR and ER. Ki-67 expression might not be the most useful marker for differentiating between LMS and STUMP, but might be valuable for predicting recurrence.18,19
The patient with recurrence in our study underwent TH with BSO and developed a late relapse of STUMP 52 months after surgery. According to previous reports, STUMP can recur as STUMP or LMS.2,7,8,14 Ip et al.14 reviewed previous cases using the Stanford diagnostic criteria and identified 10 cases of recurrence among 91 cases of STUMP. The average recurrence interval was 51 months after the primary treatment (range 15 months to 9 years).21 Yoon et al.22 reported a rapid relapse as LMS 6 months after a STUMP diagnosis and myomectomy. These studies confirmed that STUMP could have rapid or late recurrence, suggesting that patients should undergo regular follow-up examinations for the first 5 years, with further long-term follow-up.6,21,23
There were no remarkable differences in recurrence rates between patients treated with myomectomy and hysterectomy.12,17,24–28 In the present study, six patients with STUMP underwent myomectomy and none experienced recurrence. Both patients with fertility requirements subsequently delivered full-term live babies. They retained their uterus after childbirth and remained alive with no evidence of disease. George et al.6 conducted a literature review of the histopathologic analysis and clinical outcomes of patients with STUMP diagnosed from myomectomy specimens and those who subsequently underwent hysterectomy. Residual lesions were found in 2 of 14 patients (14.3%) who underwent hysterectomy following initial myomectomy, suggesting that recurrence after myomectomy might result from incomplete excision. Although the current results suggest that fertility-preserving management should be considered, we still recommend hysterectomy as the gold standard for women who have completed their childbearing, considering the proven possibilities of recurrence and incomplete excision. Full exploration should be carried out in patients undergoing fertility-preserving surgery to ensure that no residual lesions remain, and close follow-up should be provided.
There were some limitations to this study, including the relatively small sample size and its retrospective design.
In conclusion, pathological review of lesions diagnosed as STUMP can reduce inappropriate labeling and may help to select suitable data for predicting long-term outcomes. Patients with STUMP may require closer surveillance throughout their life to detect rapid or slow recurrence. Ki-67 and sex steroid hormone receptors might be valuable markers for differentiating between LMS and STUMP. Future large studies including a comprehensive review of the literature and immunohistochemical and molecular analyses might provide information to aid the prospective diagnosis of patients with STUMP at higher risk of recurrence.
Footnotes
Presentation: Presented in part as an oral presentation at the 5th Biennial Meeting of the Asian Society of Gynecologic Oncology, December 2017, Tokyo, Japan.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This study was supported by the Natural Science Foundation of Shanghai [grant number 20ZR1408800].
ORCID iD: Chao Gu https://orcid.org/0000-0001-6045-9532
References
- 1.Layfield LJ, Liu K, Dodge R, et al. Uterine smooth muscle tumors: utility of classification by proliferation, ploidy, and prognostic markers versus traditional histopathology. Arch Pathol Lab Med 2000; 124: 221–227. [DOI] [PubMed] [Google Scholar]
- 2.Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol 1994; 18: 535–558. [PubMed] [Google Scholar]
- 3.Veras E, Zivanovic O, Jacks L, et al. “Low-grade leiomyosarcoma” and late-recurring smooth muscle tumors of the uterus: a heterogenous collection of frequently misdiagnosed tumors associated with an overall favorable prognosis relative to conventional uterine leiomyosarcomas. Am J Surg Pathol 2011; 35: 1626–1637. [DOI] [PubMed] [Google Scholar]
- 4.Deodhar KK, Goyal P, Rekhi B, et al. Uterine smooth muscle tumors of uncertain malignant potential and atypical leiomyoma: a morphological study of these grey zones with clinical correlation. Indian J Pathol Microbiol 2011; 54: 706–711. [DOI] [PubMed] [Google Scholar]
- 5.Guntupalli SR, Ramirez PT, Anderson ML, et al. Uterine smooth muscle tumor of uncertain malignant potential: a retrospective analysis. Gynecol Oncol 2009; 113: 324–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vilos GA, Marks J, Ettler HC, et al. Uterine smooth muscle tumors of uncertain malignant potential: diagnostic challenges and therapeutic dilemmas. Report of 2 cases and review of the literature. J Minim Invasive Gynecol 2012; 19: 288–295. [DOI] [PubMed] [Google Scholar]
- 7.Peters WA, 3rd, Howard DR, Andersen WA, et al. Uterine smooth-muscle tumors of uncertain malignant potential. Obstet Gynecol 1994; 83: 1015–1020. [DOI] [PubMed] [Google Scholar]
- 8.Ip PP, Cheung AN, Clement PB. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): a clinicopathologic analysis of 16 cases. Am J Surg Pathol 2009; 33: 992–1005. [DOI] [PubMed] [Google Scholar]
- 9.Ng JS, Han A, Chew SH, et al. A clinicopathologic study of uterine smooth muscle tumours of uncertain malignant potential (STUMP). Ann Acad Med Singap 2010; 39: 625–628. [PubMed] [Google Scholar]
- 10.Dall'Asta A, Gizzo S, Musarò A, et al. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): pathology, follow-up and recurrence. Int J Clin Exp Pathol 2014; 7: 8136–8142. [PMC free article] [PubMed] [Google Scholar]
- 11.Pedra Nobre S, Hensley ML, So M, et al. The impact of tumor fragmentation in patients with stage I uterine leiomyosarcoma on patterns of recurrence and oncologic outcome. Gynecol Oncol 2020; 160: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ha HI, Choi MC, Heo JH, et al. A clinicopathologic review and obstetric outcome of uterine smooth muscle tumor of uncertain malignant potential (STUMP) in a single institution. Eur J Obstet Gynecol Reprod Biol 2018; 228: 1–5. [DOI] [PubMed] [Google Scholar]
- 13.Basaran D, Usubutun A, Salman MC, et al. The clinicopathological study of 21 cases with uterine smooth muscle tumors of uncertain malignant potential: centralized review can purify the diagnosis. Int J Gynecol Cancer 2018; 28: 233–240. [DOI] [PubMed] [Google Scholar]
- 14.Ip PP, Tse KY, Tam KF. Uterine smooth muscle tumors other than the ordinary leiomyomas and leiomyosarcomas: a review of selected variants with emphasis on recent advances and unusual morphology that may cause concern for malignancy. Adv Anat Pathol 2010; 17: 91–112. [DOI] [PubMed] [Google Scholar]
- 15.Gupta M, Laury AL, Nucci MR, et al. Predictors of adverse outcome in uterine smooth muscle tumours of uncertain malignant potential (STUMP): a clinicopathological analysis of 22 cases with a proposal for the inclusion of additional histological parameters. Histopathology 2018; 73: 284–298. [DOI] [PubMed] [Google Scholar]
- 16.Croce S, Ribeiro A, Brulard C, et al. Uterine smooth muscle tumor analysis by comparative genomic hybridization: a useful diagnostic tool in challenging lesions. Mod Pathol 2015; 28: 1001–1010. [DOI] [PubMed] [Google Scholar]
- 17.Karatasli V, Çakır İ, Ayaz D, et al. Clinicopathologic evaluation of uterine smooth muscle tumors of uncertain malignant potential (STUMP): A single center experience. J Gynecol Obstet Hum Reprod 2019; 48: 637–642. [DOI] [PubMed] [Google Scholar]
- 18.Petrovic D, Babić D, Forko JI, et al. Expression of Ki-67, P53 and progesterone receptors in uterine smooth muscle tumors. Diagnostic value. Coll Antropol 2010; 34: 93–97. [PubMed] [Google Scholar]
- 19.Rubisz P, Ciebiera M, Hirnle L, et al. The usefulness of immunohistochemistry in the differential diagnosis of lesions originating from the myometrium. Int J Mol Sci 2019; 20: 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q, Kanis MJ, Ubago J, et al. The selected biomarker analysis in 5 types of uterine smooth muscle tumors. Hum Pathol 2018; 76: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gadducci A, Zannoni GF. Uterine smooth muscle tumors of unknown malignant potential: A challenging question. Gynecol Oncol 2019; 154: 631–637. [DOI] [PubMed] [Google Scholar]
- 22.Yoon BS, Seong SJ, Park H. Rapid recurrence of uterine smooth muscle tumor of uncertain malignant potential as leiomyosarcoma. Int J Gynaecol Obstet 2011; 113: 244–245. [DOI] [PubMed] [Google Scholar]
- 23.Bacanakgil BH, Deveci M, Karabuk E, et al. Uterine smooth muscle tumor of uncertain malignant potential: clinicopathologic-sonographic characteristics, follow-up andr. World J Oncol 2017; 8: 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clauss S, Höller S, Hegi L, et al. [“STUMP” (smooth muscle tumour of uncertain malignant potential), a tumour of the uterus in pregnancy–a diagnostic and therapeutic challenge]. Z Geburtshilfe Neonatol 2010; 214: 74–77. [DOI] [PubMed] [Google Scholar]
- 25.Campbell JE, Knudtson JF, Valente PT, et al. Successful pregnancy following myomectomy for uterine smooth muscle tumor of uncertain malignant potential: A case report and review of the literature. Gynecol Oncol Rep 2016; 15: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller Vranjes A, Sijanović S, Vidosavljević D, et al. Surgical treatment of large smooth muscle tumor of uncertain malignant potential during pregnancy. Med Glas (Zenica) 2011; 8: 290–292. [PubMed] [Google Scholar]
- 27.Sahin H, Karatas F, Coban G, et al. Uterine smooth muscle tumor of uncertain malignant potential: fertility and clinical outcomes. J Gynecol Oncol 2019; 30: e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huo L, Wang D, Wang W, et al. Oncologic and reproductive outcomes of uterine smooth muscle tumor of uncertain malignant potential: a single center retrospective study of 67 cases. Front Oncol 2020; 10: 647. [DOI] [PMC free article] [PubMed] [Google Scholar]