Abstract
While lymphopenia has been a common finding in COVID-19 infection, particularly in severe cases, febrile neutropenia has been very rarely reported in immunocompetent patients with COVID-19. Herein, we report the case of a 76-year-old hypertensive and diabetic man who was hospitalised with severe COVID-19 infection and developed delayed-onset severe neutropenia with neutropenic fever, which responded to treatment with antibiotics and granulocyte colony-stimulating factor. This case highlights the importance of identifying a rare complication (febrile neutropenia on the fifth week) of COVID-19 infection in hospitalised patients by intensive monitoring and aggressive management for favourable outcomes.
Keywords: COVID-19, haematology (drugs and medicines), infectious diseases, adult intensive care
Background
In December 2019, pneumonia of unknown aetiology was reported in Wuhan China, which was later named COVID-19 due to the emergence of a new strain of corona virus (CoV), namely, SARS CoV-2, as a causative agent of respiratory tract infection, and the WHO declared a pandemic in March 2020.1 COVID-19 can present with various atypical presentations apart from initial influenza-like symptoms. In a case series of over 1000 patients in China, observed 83% of patients had lymphopenia, but neutropenia has been very rarely reported.2
Severe COVID-19 can present with febrile neutropenia, and our case exemplifies it. We report the case with delayed presentation of neutropenic fever while suffering from COVID-19.
Case presentation
A 76-year-old man presented to the emergency department with a history of dry cough of 10 days’ duration and progressive shortness of breath of 3 days. There was no history of chest pain, fever or haemoptysis. He had hypertension for the past 10 years (well controlled with amlodipine 10 mg/day) and type 2 diabetes mellitus for 10 years with good glycaemic control on oral antidiabetic agents. He was started on tablet azithromycin 500 mg once daily for 1 day for respiratory symptoms before hospitalisation. On presentation, he was conscious and oriented, afebrile and normotensive (blood pressure: 130/80 mm Hg) but had tachycardia (pulse rate: 110/min) and tachypnoea (respiratory rate: 32/min), and he was unable to maintain O2 saturation on room air (SPO2: 89%). He required O2 inhalation via O2 mask at 10 L/min to maintain SPO2 above 95%.
Investigation
Chest X-ray showed bilateral fluffy opacities in all the zones, more in the periphery. Baseline lab investigations showed a total leucocyte count (TLC) of 5.3×109/L and absolute neutrophil count (ANC) of 4.61×109/L. COVID-19 infection was diagnosed by RT-PCR of the nasopharyngeal swab. His high-resolution computed tomography (CT) chest showed a CT severity score of 33/40 with CORADS-6.
Treatment
He was treated as per institutional protocol for severe COVID-19 illness that included amoxicillin 500 mg and azithromycin 500 mg (empirical community-acquired pneumonia coverage as there was high suspicion of bacterial infection as evident from serum procalcitonin of 1.21 (normal range: <0.5 ng/mL), dexamethasone (6 mg intravenously once daily for 10 days), enoxaparin (0.4 mL subcutaneously once daily until hospitalisation) and remdesivir. Injection of remdesivir was administered, as 200 mg intravenous start followed by 100 mg intravenously once daily for 5 days. He also received insulin and amlodipine. Azithromycin 500 mg was stopped after 5-day course and amoxicillin after 7 days. Gradually, his O2 requirements decreased, and the dexamethasone dose was tapered and stopped. The patient required O2 for a prolonged period of more than 3 weeks of illness with nasal prongs at 2 L/min. ECG was normal, and transthoracic echocardiogram (2D) showed concentric left ventricular hypertrophy with grade 1 diastolic dysfunction.
On day 32 of COVID-19 illness, his TLC suddenly decreased to 1.1×109/L, with ANC of 0.39×109/L, haemoglobin of 11.85 g/dL and platelet count of 135×109/L. Peripheral blood smear did not show any abnormal cells or parasites (Plasmodium), and serum vitamin B12 and folate levels were normal. He developed fever (101.4℉) on day 33 of COVID-19 illness, with further worsening of leucopenia and neutropenia (TLC of 1.03×109/L and ANC of 0.33×109/L). Rapid diagnostic tests for malaria, dengue, viral hepatitis, HIV, scrub typhus and enteric fever were negative. Chest X-ray didn’t reveal any new infections, and ultrasonography of whole abdomen ruled out any pathology including hepatosplenomegaly, and he was immediately started on empirical broad-spectrum antibiotic therapy (injection of piperacillin–tazobactam 4.5 gm intravenous infusion q6h) for febrile neutropenia. Bone marrow aspiration and biopsy were done, and injection of filgrastim (recombinant human granulocyte colony-stimulating factor, G-CSF) 300 µg was administered subcutaneously once daily for 2 days (on days 33 and 34). His neutropenia recovered with G-CSF and fever subsided after 2 days. Blood and urine cultures revealed no growth, and the bone marrow aspiration cytology and biopsy were mildly hypercellular for age, with adequate trilineage haematopoiesis; mild maturation arrest in neutrophilic series; and no dysplasia, abnormal cells or marrow fibrosis. The trend of TLC and ANC during hospitalisation and their relation with therapeutic interventions are showed in figure 1.
Figure 1.
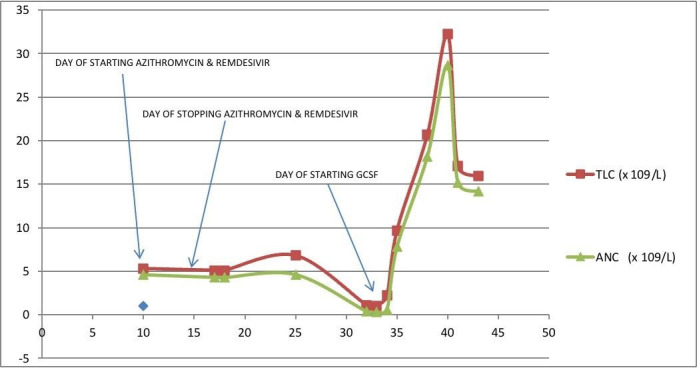
Graph showing trend of total leucocyte count (TLC) and absolute neutrophil count (ANC) with x-axis representing days of illness and y-axis representing values of TLC and ANC in terms of 109/litre.
Outcome and follow-up
The subsequent course in the hospital was uneventful, and he was discharged after 5 days of intravenous antibiotic therapy. He is doing well on follow-up, and his last blood counts were normal.
Discussion
COVID-19 infection is associated with significant alterations in the haematopoietic system and haemostasis. Lymphopenia is a cardinal haematological finding and has prognostic significance.3 Guan et al reported that the majority of patients with COVID-19 had lymphocytopenia (83.2%) at presentation, followed by thrombocytopenia (36.2%) and leucopenia (33.7%).2 Neutrophilic leucocytosis is significantly associated with increased risk of acute respiratory distress syndrome and mortality in patients with COVID-19.3 However, neutropenia (ANC: <1.5×109/L) due to COVID-19 is very rarely described, despite viral infections being one of the the most common aetiologies of transient neutropenia in immunocompetent individuals.4
López-Pereira et al have reported one case of acquired severe neutropenia, in a 33-year-old woman with no relevant medical history.5 The patient presented with severe neutropenia (0.33×109/L), asthenia and mild generalised lymphadenopathy 11 days after the COVID-19 diagnosis. She had no fever associated with neutropenia, and her neutrophil counts recovered after a single dose of G-CSF. The authors proposed transient agranulocytosis occurring early in COVID-19 infection or peripheral neutrophil consumption as potential mechanisms of transient neutropenia.5 Spencer et al reported a 51-year-old man with NK-cell large granular lymphocytic leukaemia who had stable pancytopenia on cyclosporine therapy and presented with febrile illness and worsening of neutropenia from baseline when he was diagnosed with COVID-19 infection; he was successfully managed with antibiotics and G-CSF.6
Our patient had normal baseline leucocyte and neutrophil counts when he was hospitalised for COVID-19 infection, and he subsequently developed delayed-onset, transient, severe neutropenia together with neutropenic fever, around the fifth week of illness. Probability of azithromycin was not considered because it is associated mainly with mild neutropenia and as an early complication.7 8 Laboratory workup including bone marrow study excluded any other underlying aetiology of leucopenia and severe neutropenia; there was no suggestion of drug-induced neutropenia on review of his treatment records. His transient severe neutropenia and febrile neutropenic episodes were attributed to COVID-19 infection as the most likely aetiology.
Febrile neutropenia, although an extremely rare complication of COVID-19 infection in immunocompetent patients, can be associated with significant morbidity and mortality. Early identification of COVID-19-associated neutropenia in hospitalised patients by intensive monitoring, workup to exclude unrelated causes and early treatment with empirical antibiotics and G-CSF can be rewarded with a favourable outcome.
Patient’s perspective.
I had a cough and suddenly started having shortness of breath, which was mild as I thought because it stopped me from usual activities but it was relieved with rest; for that, I consulted a local doctor. I had been told to be COVID-19 positive, and my shortness of breath increased for which I went to All India Institute of Medical Sciences—Rishikesh. I was put on an oxygen mask and shifted to COVID-19-positive ward directly after the X-ray and CT scan were done. Initially few days after admission, my shortness of breath did not improve much, and they started injection of steroids with remdesivir after taking my consent. I started feeling better, and oxygen requirements gradually decreased. Blood sugars were more on the higher side for which my insulin requirement was increased.
The steroid was slowly tapered, blood sugar was controlled and I was put on oxygen on an interval basis. Almost all the medications as far as I knew were stopped, and I was about to get discharged soon; that’s what I felt; then, I have been told my whole blood count had reduced a lot, and I could get any infection at any time if proper precautions were not taken. Then next day itself, I developed fever, increased pulse rate and a slight fall in blood pressure for which I was transferred to intensive care. I was given granulocyte colony-stimulating factor injection to increase my whole blood count, and all precautions were taken. Fever was present for 2 days, and all injectable antibiotics were started again for fever. Blood cultures and urine cultures were all taken. Then after stabilisation, I had been shifted back to COVID-19-positive ward, and my whole blood count had improved. I started walking, which I was not able to; sugars were controlled with insulin; all medications for my recent hospitalisation were stopped; and I was discharged with advice to follow up. What I feel is I have been timely diagnosed; whenever there is any change in my blood investigations, all my examinations and investigations were done on time for which I was able to recover soon. Right now, I am fine except for some weakness in doing strenuous activities. I am following up on regular basis and taking their advice.
Learning points.
Febrile neutropenia can be an unusual haematological complication of COVID-19 infection in an immunocompetent patient.
Neutropenia can present on the fifth week of COVID-19 infection, should be monitored accordingly.
If present, it should be treated aggressively to prevent the adverse outcome.
Acknowledgments
Thanks to the Covid-19 management team who is working hard day and night after wearing personal protective equipment. It is sometimes unbearable to wear a full-covered suit. Still, the team is dedicated to isolating, diagnosing and treating patients since the onset of the pandemic. A wholehearted gratitude to them.
Footnotes
Contributors: YMK and AS collected the data and drafted and approved the manuscript; PKP and UKN gave design, critically reviewed the draft and finally approved the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer-reviewed.
References
- 1.Yi-Chai W, Ching-Sunga C, Yu-Jiuna C. The outbreak of COVID-19. J Chin Med Assoc 2020;83:217–20. 10.1097/JCMA.0000000000000270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terpos E, Ntanasis-Stathopoulos I, Elalamy I. Hematological findings and complications of COVID-19. Am J Hematol 2020:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newburger PE, Dale DC. Evaluation and management of patients with isolated neutropenia. Semin Hematol 2013;50:198–206. 10.1053/j.seminhematol.2013.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.López-Pereira P, Iturrate I, de La Cámara R, et al. Can COVID-19 cause severe neutropenia? Clin Case Rep 2020;8:3349–51. 10.1002/ccr3.3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spencer HC, Wurzburger R. COVID-19 presenting as neutropenic fever. Ann Hematol 2020;99:1939–40. 10.1007/s00277-020-04128-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higa F, Saito A. [Clinical safety of azithromycin]. Jpn J Antibiot 2000;53 Suppl B:125–35. [PubMed] [Google Scholar]
- 8.Kajiguchi T, Ohno T. Azithromycin-related agranulocytosis in an elderly man with acute otitis media. Intern Med 2009;48:1089–91. 10.2169/internalmedicine.48.2093 [DOI] [PubMed] [Google Scholar]


