Abstract
Background:
The opioid crisis has reached epidemic proportions, yet risk of persistent opioid use following curative intent surgery for cancer and factors influencing this risk are not well understood.
Methods:
We used electronic health record data from 3,901 adult patients who received a prescription for an opioid analgesic related to hysterectomy or large bowel surgery from January 1st, 2013 through June 30th, 2018. Patients with and without a cancer diagnosis were matched based on demographic, clinical, and procedural variables and compared for persistent opioid use.
Results:
Cancer diagnosis was associated with greater risk for persistent opioid use after hysterectomy [18.9% vs 9.6%, adjusted odds radio (aOR) 2.26; 95% confidence interval (CI): 1.38 – 3.69, p = 0.001] but not after large bowel surgery [28.3% vs 24.1%, aOR 1.25; 95% CI: 0.97 – 1.59, p = 0.09]. In the cancer hysterectomy cohort, persistent opioid use was associated with cancer stage (increased rates among those with stage III cancer compared to stage I) and use of neoadjuvant or adjuvant chemotherapy; however, these factors were not associated with persistent opioid use in the large bowel cohort.
Conclusions:
Patients with cancer may have an increased risk of persistent opioid use following hysterectomy.
Impact:
Risks and benefits of opioid analgesia for surgical pain among patients with cancer undergoing hysterectomy should be carefully considered.
Introduction
Prescription opioid abuse has become a major public health crisis 1,2. More than 70% of patients undergoing surgery in the US obtain opioid prescriptions 3, but the majority (67–92%) report receiving more opioids than needed to manage post-operative pain 4. Patients with cancer exposed to opioids for curative intent surgery may be especially vulnerable to persistent opioid use due to high levels of anxiety and depression 5, comorbid medical conditions 6, and concomitant medications 7,8.
Guidelines for opioid prescribing have largely exempted the cancer population from consideration under the precept that cancer pain should be treated differently than non-cancer pain due to the unique nature of the disease and its treatment 9–11. Opioid misuse among cancer patients may be underappreciated, even though 1 in 5 cancer patients are at risk of abusing opioids 12. Recent studies suggest that 10–18% of previously opioid-naïve cancer patients who received an opioid prescription following curative intent surgery continue to use opioids after the post-operative healing period is complete 13–17, which is a risk factor for developing chronic post-surgical pain 18; among patients with prior opioid exposure, this proportion is 30–50% 15,17.
To our knowledge, no study has directly compared rates of persistent opioid use after similar major surgeries in patients with cancer compared to those without. Furthermore, no studies have examined whether the risk associated with cancer may differ for patients undergoing different surgeries. An improved understanding of the factors associated with progression to persistent opioid use in oncology would help identify patients at greatest risk who might benefit from alternative approaches to pain management.
To address this knowledge gap, we conducted a retrospective, observational study utilizing data from the University of Pennsylvania Health System (UPHS) electronic health record (EHR) to examine differences in the risk of persistent opioid use between patients with and without cancer following exposure to prescription opioids after hysterectomy or after large bowel (colorectal) surgery. We chose these surgeries because they are prevalent and because they are exemplars of similar surgical procedures performed for both cancer and non-cancer indications. This enabled an analytic approach designed to assess the association of cancer versus non-cancer diagnoses with persistent opioid use after surgery. We further examined patient-level, provider-level, procedural, geographic, and clinical/disease-related factors which might be associated with the likelihood of transition to new persistent opioid use following surgery among patients with cancer and those without.
Methods
Data sources
The UPHS Epic Clarity EHR comprises longitudinal inpatient, outpatient, physician, and pharmacy data for patients treated at 5 hospitals in Pennsylvania. The data includes information on patient characteristics (such as demographics and clinical history) and medical care use (such as visit records, diagnosis and procedure codes) linked to physician National Provider Identifier. These data were augmented with UPHS Tumor Registry data, which contains tumor records (such as primary site, histopathologic type, and tumor stage as well as a custom ICD Oncology 3-to-ICD 9/10 diagnosis code mapping table). We also linked the EHR with the 2017 American Community Survey by patient zip code to obtain census tract median household income. The study was conducted in accordance with the Declaration of Helsinki, approved by the University of Pennsylvania Institutional Review Board and granted a waiver of informed consent.
Study Population
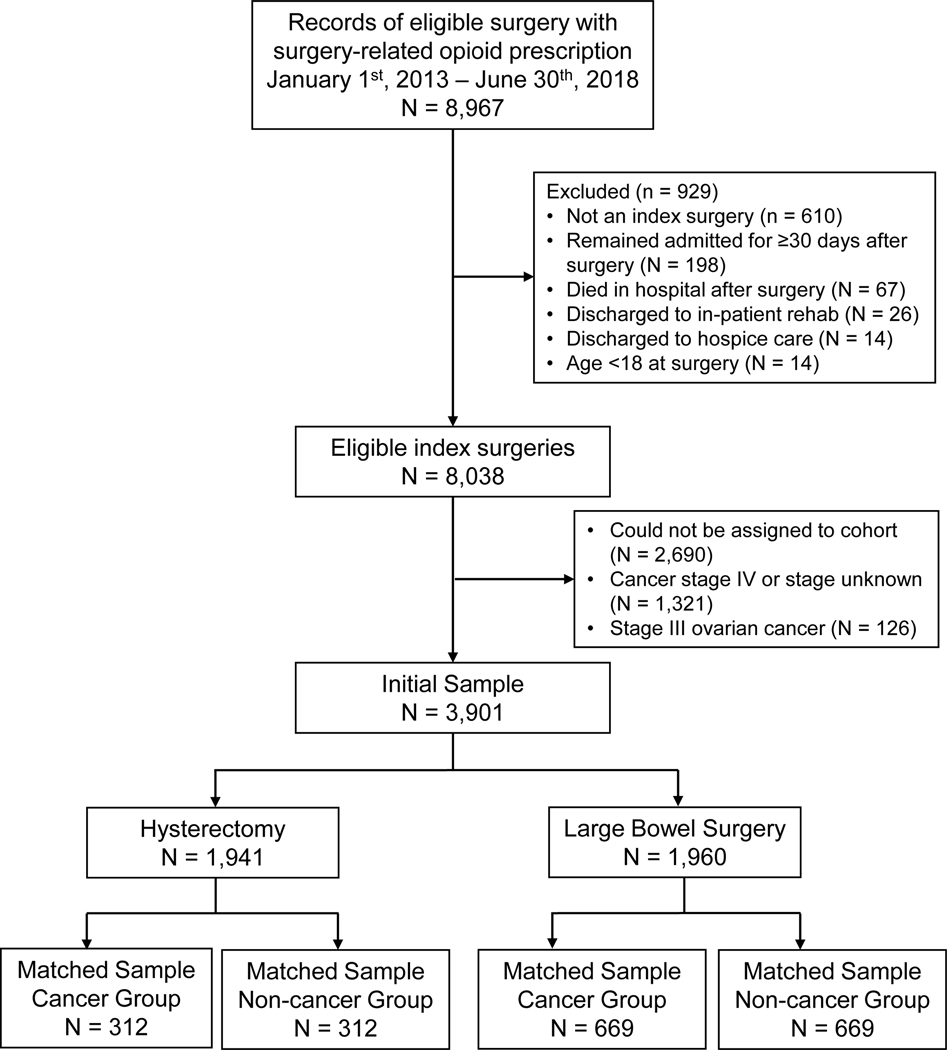
We included adults aged 18 years or older who underwent elective hysterectomy or large bowel surgery between January 1st, 2013, and June 30th, 2018 (180 days prior to records retrieval), received a surgery-related opioid prescription, and could be reliably assigned to a cohort (see Cohort definitions below). These types of surgery were chosen because they involve similar procedures for patients with cancer and non-cancer diagnoses, affording an opportunity to isolate the effects of cancer. Current procedural terminology (CPT) codes were used to identify eligible surgeries and categorize patients into surgical cohorts (Supplemental Table 1). Surgery-related prescriptions were prescriptions for opioids issued from 30 days before to 14 days after the date of the index surgery based on definitions used in prior studies of persistent opioid use following surgery 13,19. Patients were excluded if they were discharged to hospice care or in-patient rehabilitation; remained admitted to the hospital for >30 days; or died in the hospital following the surgery. Figure 1 details the study definition.
Figure 1. Study flow diagram.
Defining cancer and non-cancer groups
We categorized patients into cancer or non-cancer groups based on whether the surgery was related to a cancer diagnosis or not. The cancer group included patients who received curative intent hysterectomy for cervical, uterine, or ovarian cancer, or received curative intent large bowel surgery (including colectomy, rectal excision, and/or proctectomy) for colorectal cancer. The non-cancer group included patients who were not diagnosed with cancer and who received hysterectomy or large bowel surgery for benign conditions. Group classifications were based on ICD9/10 diagnosis codes associated with the surgery (Supplemental Table 2), verified with the Tumor Registry. We excluded patients with stage IV cancer, stage III ovarian cancer (such patients may receive debulking surgery rather than curative intent), missing/unknown stage, or whose surgery could not be matched to a diagnosis code of interest or the Tumor Registry (Figure 1).
Covariates
We used the EHR linked to census data to define patient-level, procedural, provider-level, and geographic covariates. Patient-level covariates included demographics (age, sex, and self-reported race) and clinical history (prior opioid exposure, comorbidities, concomitant medications, and body mass index (BMI) at the time of surgery). For patients with cancer, stage and treatment [including neoadjuvant and adjuvant radiotherapy (RT) or systemic chemotherapy (SYS)] were extracted from the Tumor Registry.
Patients were classified as chronic, intermittent, or naïve opioid users. Chronic opioid users had at least one opioid prescription with a 120-day supply between 31 and 365 days prior to surgery or at least 3 opioid prescriptions in the 3 consecutive months prior to surgery; intermittent users had at least one opioid prescription between 31 and 365 days prior to surgery, but did not meet criteria for chronic use; and opioid naïve patients had no opioid prescriptions from 31–365 days prior to surgery 13.
Comorbidity burden was determined based on the Elixhauser Comorbidity Index 20,21, modified to omit cancer-related comorbidities to minimize confounding (Supplemental Table 3). Concomitant medications previously associated with risk of persistent opioid use were identified (Supplemental Table 4) 14,22–25. Procedural variables included measures of surgical complexity (minimally invasive vs. open; partial -ectomy vs. complete or multiple organ removal [complete/extensive]; operating room time; and estimated blood loss). We included a facility-level variable that captured the hospital in which the surgery took place (operating room location). Census tract median household income was included as an ecologic variable to reflect socioeconomic status.
Primary outcome assessment
The primary outcome was persistent opioid use following surgery, defined as at least one opioid prescription issued 60–180 days post-surgery. This time frame was chosen based on the International Association for the Study of Pain definition of persistent post-operative pain and the expectation that standard procedures for the surgeries included in this study would not require opioid treatment for more than 60 days 26.
Statistical analyses
Our primary analysis used a propensity score matching approach for cancer and non-cancer cases implemented separately by surgical cohort with a nearest-neighbor matching algorithm 27. Propensity scores represent the probability that patients are undergoing surgery for cancer versus non-cancer indications based on observed covariates, and can be used to reduce bias due to systematic differences in distributions of potential confounders between groups at baseline 28,29. To create propensity scores, we used logistic regression with cancer diagnosis as the primary outcome and age, race, BMI, opioid history, Elixhauser comorbidity index, concomitant medications, surgical complexity, surgical extent, operating room time, estimated blood loss, operating room location, and household income as predictors. Matching was performed using a nearest-neighbor matching algorithm with a 1:1 match ratio and proper caliper values 27,30. We calculated the adjusted odds ratio (aOR) of persistent opioid use between cancer and non-cancer groups and conducted matched Chi-squared test (i.e. Cochran–Mantel–Haenszel Test) in the matched samples 31,32. To validate, we also calculated aOR by multivariate logistic regression in the matched samples. Analyses were done using the R package MatchIt 33. All statistical tests were performed at the 0.05 level of significance.
In the secondary analyses, we investigated heterogeneity in associations between cancer and persistent opioid use by surgical cohort based on pre-specified hypotheses on stratifying variables. Specifically, we hypothesized that the likelihood of persistent opioid use would be more pronounced among patients with cancer compared to those without cancer for those with age 65 or older 16,17,24,34, male gender 16,23, prior opioid use 13,22,35, and history of depression 23. Gender differences were examined only in the large bowel surgery cohort. We further examined the effects of cancer stage, surgery extent, and use of neoadjuvant or adjuvant treatments in each cancer surgery cohort by a logistic regression.
Although we retrieved all prescriptions issued within UPHS, it is possible that patients may have obtained opioid prescriptions from an outside provider within the follow-up window; these patients would not have been counted as persistent opioid users in our analysis. To assess the potential impact of unobserved opioid use, we conducted sensitivity analyses to evaluate the robustness of our primary result to outcome misclassification 36. Prior research has shown persistent opioid use following surgery in 5–30% of patients with and without cancer 13,15,16,19,37. Therefore, we randomly selected 10%, 20%, and 30% of patients in the matched samples with no identified persistent opioid use and reversed their outcomes 36. The odds ratio for persistent opioid use in the cancer versus non-cancer group was then calculated based on the re-assigned outcomes. This procedure was repeated 100 times and the average odds ratios and confidence intervals were calculated 36.
Results
Descriptive characteristics in matched samples
After propensity score matching, two matched samples were obtained by surgical cohort: 624 hysterectomy patients (312 each with and without cancer) and 1,338 large bowel surgery patients (669 each with and without cancer). Descriptive characteristics for the matched samples are summarized in Table 1. Patients in the hysterectomy cohort were younger (mean age = 56.7 years, SD 12.3), had a higher BMI (mean = 30.4, SD = 8.2), and were less likely to be white (68.9%) than the large bowel surgery cohort (mean age = 60.4 years, SD = 14.0; mean BMI = 28.5, SD = 6.3; percent white = 80.3%; all ps < 0.001). There were no significant differences in prior opioid usage between the two surgical cohorts (percent opioid naïve = 72.6% and 73.9%, respectively; p = 0.57).
Table 1:
Baseline characteristics by group in the matched samples
| Surgery Type | Hysterectomy | Large Bowel Surgery | ||||
|---|---|---|---|---|---|---|
| Group | Cancer (n = 312) | Non-cancer (n = 312) | p-value | Cancer (n = 669) | Non-cancer (n = 669) | p-value |
| Age, m (SD) | 56.7 (12.5) | 56.6 (12.1) | 0.904 | 61.1 (14.3) | 59.7 (13.6) | 0.009 |
| Sex, n (%) female | 312 (100) | 312 (100) | NA | 335 (50.1) | 278 (41.6) | 0.002 |
| Race, n (%) | ||||||
| White | 219 (70.2) | 211 (67.6) | 540 (80.7) | 534 (79.8) | ||
| Non-white | 93 (29.8) | 101 (32.4) | 0.484 | 129 (19.3) | 135 (20.2) | 0.728 |
| BMI, m (SD) | 30.3 (8.4) | 30.5 (7.9) | 0.674 | 28.4 (6.3) | 28.6 (6.3) | 0.713 |
| Opioid history, n (%) | ||||||
| Naïve | 230 (73.7) | 223 (71.5) | 499 (74.6) | 490 (73.2) | ||
| Intermittent | 64 (20.5) | 75 (24.0) | 0.483 | 128 (19.1) | 133 (19.9) | 0.828 |
| Chronic | 18 (5.8) | 14 (4.5) | 42 (6.3) | 46 (6.9) | ||
| Elixhauser comorbidity score*, m (SD) | 0.5 (4.1) | 0.2 (4.0) | 0.341 | 2.0 (5.7) | 2.0 (5.4) | 0.876 |
| Concomitant meds, n (%) | ||||||
| Benzodiazepines | 24 (7.7) | 11 (3.5) | 0.043 | 48 (7.2) | 62 (9.3) | 0.189 |
| Non-opioid analgesics | 20 (6.4) | 35 (11.2) | 0.050 | 70 (10.5) | 57 (8.5) | 0.263 |
| NSAIDs | 20 (6.4) | 12 (3.6) | 0.216 | 59 (8.8) | 35 (5.2) | 0.016 |
| SSRIs | 11 (3.5) | 4 (1.7) | 0.121 | 27 (4.0) | 24 (3.6) | 0.779 |
| SNRIs | 5 (1.6) | 7 (0.6) | 0.773 | 9 (1.3) | 2 (0.3) | 0.070 |
| NBA/SHs | 2 (0.6) | 2 (0.8) | 1.000 | 6 (0.9) | 9 (1.3) | 0.606 |
| OR time (hours), m (SD) | 4.5 (1.8) | 4.5 (1.4) | 0.886 | 4.6 (2.1) | 4.6 (1.7) | 0.731 |
| Estimated blood loss (100 mL), m (SD) | 3.3 (4.8) | 3.1 (4.8) | 0.624 | 2.0 (2.7) | 1.9 (2.6) | 0.294 |
| Surgical extent, n (%) | ||||||
| Partial | 19 (6.1) | 14 (4.5) | 0.472 | 605 (90.4) | 592 (88.5) | 0.255 |
| Complete/extensive | 293 (93.9) | 298 (95.5) | 64 (9.6) | 77 (11.5) | ||
| OR location, n (%) | ||||||
| OR 1 | 154 (49.4) | 142 (45.5) | 417 (62.3) | 425 (63.5) | ||
| OR 2 | 137 (43.9) | 148 (47.4) | 196 (29.3) | 181 (27.1) | ||
| OR 3 | 0 (0.0) | 9 (2.9) | 0.017 | 18 (2.7) | 40 (6.0) | 0.003 |
| OR 4 | 17 (5.4) | 9 (2.9) | 38 (6.7) | 23 (3.7) | ||
| Other | 4 (1.3) | 4 (1.3) | 0 (0.0) | 0 (0.0) | ||
| Other treatment, n (%) | ||||||
| Neoadjuvant RT | 3 (1.0) | NA | 117 (17.5) | NA | ||
| Adjuvant RT | 87 (27.9) | NA | NA | 28 (4.2) | NA | NA |
| Neoadjuvant SYS | 14 (4.5) | NA | 56 (8.4) | NA | ||
| Adjuvant SYS | 161 (51.6) | NA | 192 (28.7) | NA | ||
| Zip code median annual household income ($1,000), m (SD) | 76.0 (28.8) | 74.7 (30.9) | 0.597 | 75.7 (28.3) | 76.1 (29.9) | 0.819 |
Elixhauser comorbidity score excludes cancer-related items to avoid confounding by cohort.
NBA/SHs: non-benzodiazepine anxiolytics/sedative-hypnotics; NSAIDs: non-steroidal anti-inflammatory drugs; RT: radiotherapy; SSRIs: selective serotonin reuptake inhibitors; SNRIs: selective norepinephrine reuptake inhibitors; SYS: systemic chemotherapy.
The majority of characteristics and covariates were balanced in cancer and non-cancer groups after the matching; age and sex were not balanced in the large bowel surgery cohort, and OR location and concomitant medications were not balanced in either cohort due to the small sample sizes within some of these subgroups (Table 1).
Primary outcome
In the matched samples, 89 patients who received hysterectomy and 350 patients who received large bowel surgery showed persistent opioid use. Cancer was associated with greater odds of persistent opioid use after hysterectomy [18.9% vs 9.6%, adjusted odds ratio (aOR) 2.26; 95% confidence interval (CI): 1.38 – 3.69, p = 0.001]. However, the association with cancer was not significant for patients who received large bowel surgery [28.3% vs 24.1%, aOR 1.25; 95% CI: 0.97 – 1.59, p = 0.09]. The adjusted differences in risk of persistent opioid use for patients with and without cancer were significantly different between the hysterectomy and large bowel cohorts (difference of odds ratios 1.01, 95% CI 0.07 – 2.46, p = 0.03). Multivariate logistic regression (Supplemental Table 5) in the matched samples provided results similar to our main findings.
Subgroup analysis
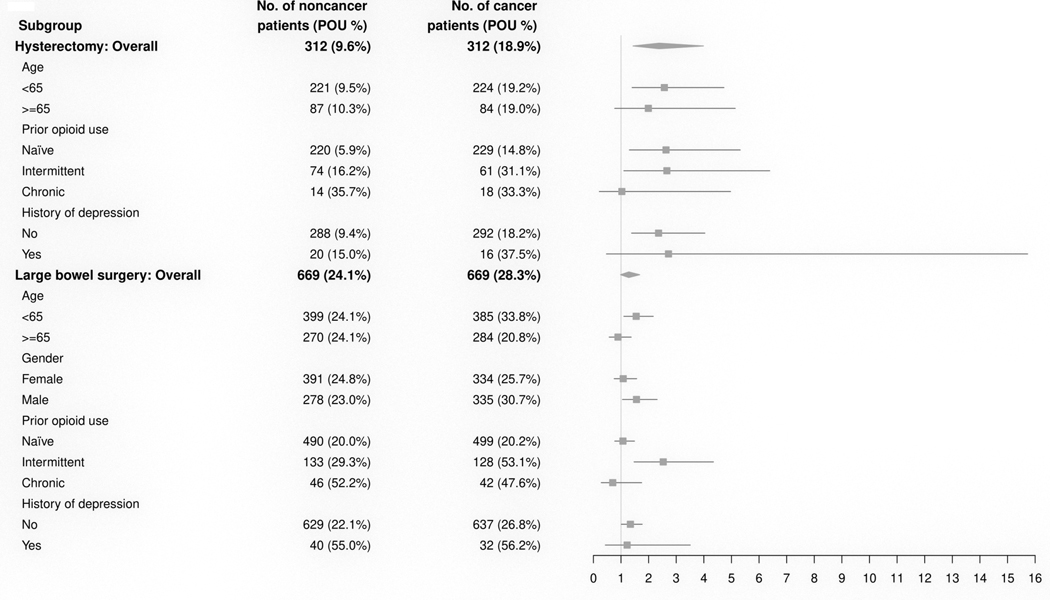
In the secondary analyses of heterogeneity, we found consistent associations with cancer in subgroups in the hysterectomy cohort, and consistent lack of associations with cancer in subgroups in the large bowel cohort (Figure 2). There were no significant interaction effects between cancer and age or history of depression in either cohort, and no interaction between cancer and gender in the large bowel surgery group (p-value > 0.05). In the analysis within cancer groups, patients in the hysterectomy cohort were more likely to show persistent opioid use if they had stage III cancer (compared to stage I) (aOR 2.83, 95% CI 1.32 – 6.08, p = 0.008), or if they received systemic chemotherapy (neoadjuvant SYS aOR 4.82, 95% CI 1.13 – 20.6, p = 0.034; adjuvant SYS aOR 3.14, 95% CI 1.44 – 6.85, p = 0.004) (Supplemental Table 6). However, these factors were not associated with persistent opioid use in the large bowel surgery cohort (ps > 0.05). Patients with cancer in the large bowel surgery cohort were more likely to show persistent opioid use if they received more extensive surgery (aOR = 1.85, 95% CI 1.02 – 3.37, p = 0.044), consistent with a main effect of surgical extent in the large bowel surgery cohort in the matched sample (p < 0.001; Supplemental Table 5).
Figure 2. Forest plot of subgroup analyses in matched samples.
Number of patients and percentage of persistent opioid use (POU) in each subgroup with forest plot of estimated aOR for the heterogeneity analysis. There was a significant difference in the effect of cancer between the hysterectomy and large bowel surgery groups (difference of odds ratios 1.01, 95% CI [0.07, 2.46], p = 0.03), but no significant interactions between cancer and any of the subgroups.
Sensitivity analyses
In sensitivity analyses for the hysterectomy cohort, random reassignment of 10%, 20%, or 30% of patients to the persistent opioid use outcome did not significantly or meaningfully change the results. The average estimated aOR for persistent opioid use in the cancer group compared to the non-cancer group was greater than 1.0 for each of the estimated misclassification rates (Table 2). This analysis suggests that the estimated aORs in the propensity matched samples are largely robust to the potential misclassification of the outcome.
Table 2.
Results of sensitivity analysis in the hysterectomy cohort
| Outcome misclassification rate | aOR | 95% CI |
|---|---|---|
| 10% | 1.69 | (1.15, 2.50) |
| 20% | 1.44 | (1.02, 2.03) |
| 30% | 1.36 | (0.98, 1.88) |
Average adjusted odds ratios for persistent opioid use in the cancer group in the hysterectomy cohort calculated for the estimated misclassification rates, obtained by randomly reassigning 10%, 20%, and 30% of individuals without persistent opioid use to the opposite outcome. This process was repeated 100 times for each misclassification rate and the resulting odds ratios were averaged. These results show a significant increase in risk of persistent opioid use in the cancer group for up to almost 30% misclassification rate in the hysterectomy cohort.
Discussion
In the first study to directly compare opioid use between matched samples of patients undergoing surgery for cancer and non-cancer indications, patients who received hysterectomy for cancer were ~2.3-fold more likely to develop persistent opioid use compared to those who received the same surgery for non-cancer indications. There was no significant association with cancer in the large bowel surgery cohort. We found no significant difference in the association with cancer based on age, gender, or history of depression in either cohort. In subgroup analyses within the cancer groups, patients receiving hysterectomy were more likely to show persistent opioid use if they had advanced (stage III) cancer or if they received adjuvant or neoadjuvant systemic chemotherapy; large bowel surgery patients were more likely to show persistent opioid use if they received more extensive surgery, consistent with the main effect of surgical extent in the large bowel surgery cohort. Collectively, these findings highlight the need for careful consideration of the risks of opioid prescribing for patients with cancer who are undergoing curative intent hysterectomy, and for additional research examining particular risk factors for these vulnerable groups.
Our findings are consistent with and extend prior studies demonstrating the risks of persistent prescription opioid use following exposure to opioids for surgical pain. In the hysterectomy cohort, we found that ~10% of patients without cancer and ~19% of patients with cancer showed persistent opioid use after surgery. Studies of patients without cancer undergoing surgery found that 3–10% of patients were still using opioids 3–6 months later 19,24,25,38; in cancer patients, this rate has been shown to be 10–30% 13–15. Because we used propensity score matching to balance sociodemographic, clinical, and procedural factors between patients with and without cancer in each surgery cohort, it is unlikely that the interaction between cancer and surgery cohort reflect differences in rates of these factors between the cohorts. Additional research is necessary to examine the factors that contribute to differences in risk of persistent opioid use between patients with different cancers undergoing different types of surgery.
Several possible reasons have been proposed to explain why cancer patients might experience greater risk from prescription opioid exposure following surgery compared to patients without cancer. It has been suggested that anxiety and depression may contribute to risk of persistent opioid use among cancer patients 5. Although a clinical diagnosis of depression was not more common in cancer patients in our sample and did not modify the association between cancer group and persistent opioid use, it is possible that a cancer diagnosis may increase subjective symptoms of anxiety and depression in the absence of clinical diagnosis. Patients diagnosed with cancer may also experience chronic pain resulting from adjuvant treatment, such as neuropathy, visceral pain, and musculoskeletal pain, and clinical trials often report pain as an independent side effect of adjuvant treatments 39,40; in our sample, adjuvant and neoadjuvant chemotherapy were associated with greater risk of persistent opioid use in patients with cancer in the hysterectomy cohort, but not in the large bowel surgery cohort. It is possible that different types of cancer and resulting treatment are associated with different degrees of psychological impact or pain that are not routinely captured in the EHR. Cancer patients require analgesics during and after cancer treatment 41; however, there is limited evidence for the efficacy of opioids for treating cancer pain 42. Physicians who are treating patients with cancer who develop chronic pain following surgery and/or adjuvant treatment should consider alternative analgesics after the acute recovery phase is completed.
We also examined potential interactions between cancer diagnosis and other risk factors for persistent opioid use, and found that the associations were largely consistent across the subgroups examined. Prior studies have shown increased risk of persistent opioid use following surgery among cancer patients with a history of opioid use, consistent with the main effect of opioid history in our sample 13,17. Younger age, male gender, and history of depression have previously been associated with elevated risk of persistent opioid use in patients undergoing surgery 13,16,17,23,24, but our findings suggest that these factors generally do not modify the associations between cancer and opioid use.
Our study has strengths and limitations. Strengths include the large sample size and use of propensity score matching to directly compare patients with and without cancer who received two different types of surgery. A limitation is that we were only able to track opioid prescriptions issued within our health system; although the Pennsylvania state prescription drug monitoring program was implemented in 2016, it is not currently structured to allow for research use 43. It is possible that some patients obtained opioid prescriptions from another provider outside of UPHS either prior to surgery or within the follow-up window, and these patients would not have been counted as prior opioid users or persistent opioid users in our analysis. However, our sensitivity analysis shows consistent results estimated with up to almost 30% misclassification rate, suggesting that our findings are robust to misclassification. Other prescriptions, including benzodiazepine prescriptions, may be underrepresented if patients obtained these prescriptions from other providers. Second, our analysis of comorbid risk factors relied on medical history entered into the EHR, which did not consistently capture important risk factors (such as tobacco use). Future studies examining prescription opioid outcomes in cancer patients using a prospective design to fully capture known risk factors would be beneficial. In addition, because type of surgery received and diagnosis are confounded, we cannot say whether the association of a cancer diagnosis with persistent opioid use in cancer in patients receiving hysterectomy versus large bowel surgery is due to different impacts of cancer type or the surgery itself. Although we expect that patients undergoing curative intent surgery would not experience cancer progression in the 6 months following surgery, it is possible that some patients may have had cancer progression, which may in turn have influenced persistent opioid use. Additional research is needed to examine the relationship between cancer progression and long-term opioid use. Future studies might further probe the mechanisms that contribute to persistent opioid use among patients with cancer.
Our findings of greater risk of persistent prescription opioid use following hysterectomy in patients with cancer contribute to the growing body of literature demonstrating the need for evidence-based guidelines for prescription opioid treatment in cancer patients undergoing curative intent surgery 13,14. Historically, cancer pain has been treated differently than non-cancer pain, and current opioid prescribing guidelines explicitly exclude cancer patients 10,44,45. Improvements in cancer care means more patients are surviving longer than ever before 46; therefore, the risks associated with prescription opioid pain management for cancer patients must be carefully considered in terms of the impact on survivors. These risks must be balanced against the need for adequate pain control in light of studies showing that pain management often falls short of cancer patients’ needs 9. Additional research is necessary to examine mechanisms contributing to different risk factors among cancer patients, and to evaluate optimal opioid prescribing strategies for reducing risks and managing surgical pain in cancer patients.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Institutes of Health (R35 CA197461 to C. Lerman) and P30CA014089 (to USC Norris Comprehensive Cancer Center).
Footnotes
Conflicts of Interest
The authors declare no potential conflicts of interest.
References
- 1.Hedegaard H, Arialdi A, Warner M: Drug Overdose Deaths in the United States, 1999–2017. NCHS Data Brief No. 329. National Center for Health Statistics, November 2018., 2018 [Google Scholar]
- 2.Seth P, Rudd RA, Noonan RK, et al. : Quantifying the Epidemic of Prescription Opioid Overdose Deaths. Am J Public Health 108:500–502, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ladha KS, Neuman MD, Broms G, et al. : Opioid Prescribing After Surgery in the United States, Canada, and Sweden. JAMA Netw Open 2:e1910734, 2019 [Google Scholar]
- 4.Bicket MC, Long JJ, Pronovost PJ, et al. : Prescription Opioid Analgesics Commonly Unused After Surgery: A Systematic Review. JAMA Surg 152:1066–1071, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell AJ, Chan M, Bhatti H, et al. : Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol 12:160–74, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Sarfati D, Koczwara B, Jackson C: The impact of comorbidity on cancer and its treatment. CA Cancer J Clin 66:337–50, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Aroke HA, Vyas AM, Buchanan AL, et al. : Prevalence of Psychotropic Polypharmacy and Associated Healthcare Resource Utilization during Initial Phase of Care among Adults with Cancer in USA. Drugs Real World Outcomes 6:73–82, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nightingale G, Hajjar E, Swartz K, et al. : Evaluation of a pharmacist-led medication assessment used to identify prevalence of and associations with polypharmacy and potentially inappropriate medication use among ambulatory senior adults with cancer. J Clin Oncol 33:1453–9, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Page R, Blanchard E: Opioids and Cancer Pain: Patients’ Needs and Access Challenges. J Oncol Pract 15:229–231, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Meghani SH, Vapiwala N: Bridging the Critical Divide in Pain Management Guidelines From the CDC, NCCN, and ASCO for Cancer Survivors. JAMA Oncol 4:1323–1324, 2018 [DOI] [PubMed] [Google Scholar]
- 11.American Society of Clinical Oncology: ASCO Policy Statement on Opioid Therapy: Protecting Access to Treatment for Cancer-Related Pain. https://www.asco.org/sites/new-www.asco.org/files/content-files/advocacy-and-policy/documents/2016-ASCO-Policy-Statement-Opioid-Therapy.pdf Accessed 1/8/2020., 2016
- 12.Carmichael AN, Morgan L, Del Fabbro E: Identifying and assessing the risk of opioid abuse in patients with cancer: an integrative review. Subst Abuse Rehabil 7:71–9, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JS, Hu HM, Edelman AL, et al. : New Persistent Opioid Use Among Patients With Cancer After Curative-Intent Surgery. J Clin Oncol 35:4042–4049, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cass AS, Alese JT, Kim C, et al. : Analysis of Opioid Use Following Curative Cancer Treatment at a Large Urban Safety-net Hospital. Clin J Pain 34:885–889, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Saraswathula A, Chen MM, Mudumbai SC, et al. : Persistent Postoperative Opioid Use in Older Head and Neck Cancer Patients. Otolaryngol Head Neck Surg 160:380–387, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Brescia AA, Harrington CA, Mazurek AA, et al. : Factors Associated With New Persistent Opioid Usage After Lung Resection. Ann Thorac Surg 107:363–368, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermott JD, Eguchi M, Stokes WA, et al. : Short- and Long-term Opioid Use in Patients with Oral and Oropharynx Cancer. Otolaryngol Head Neck Surg 160:409–419, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glare P, Aubrey KR, Myles PS: Transition from acute to chronic pain after surgery. Lancet 393:1537–1546, 2019 [DOI] [PubMed] [Google Scholar]
- 19.Brummett CM, Waljee JF, Goesling J, et al. : New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg 152:e170504, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quan H, Sundararajan V, Halfon P, et al. : Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43:1130–9, 2005 [DOI] [PubMed] [Google Scholar]
- 21.van Walraven C, Austin PC, Jennings A, et al. : A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care 47:626–33, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Anciano Granadillo V, Cancienne JM, Gwathmey FW, et al. : Perioperative Opioid Analgesics and Hip Arthroscopy: Trends, Risk Factors for Prolonged Use, and Complications. Arthroscopy 34:2359–2367, 2018 [DOI] [PubMed] [Google Scholar]
- 23.Brat GA, Agniel D, Beam A, et al. : Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ 360:j5790, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke H, Soneji N, Ko DT, et al. : Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ 348:g1251, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun EC, Darnall BD, Baker LC, et al. : Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period. JAMA Intern Med 176:1286–93, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macrae WA: Chronic post-surgical pain: 10 years on. Br J Anaesth 101:77–86, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Austin PC: An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 46:399–424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenbaum P, Rubin D: The central role of the propensity score in observational studies for causal effects. Biometrika 70:41–55, 1983 [Google Scholar]
- 29.Rosenbaum P: Design of observational studies. Springer; New York, NY., 2010. [Google Scholar]
- 30.Austin PC: Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 10:150–61, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantel N, Haenszel W: Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22:719–48, 1959 [PubMed] [Google Scholar]
- 32.Agresti A: Categorical data analysis. John Wiley & Sons; Hoboken, NJ., 2003 [Google Scholar]
- 33.Ho D, Kosuke I, King G, et al. : MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. Journal of Statistical Software 42:1–28, 2011 [Google Scholar]
- 34.Weston E, Raker C, Huang D, et al. : Opioid use after minimally invasive hysterectomy in gynecologic oncology patients. Gynecol Oncol 155:119–125, 2019 [DOI] [PubMed] [Google Scholar]
- 35.Rao AG, Chan PH, Prentice HA, et al. : Risk factors for postoperative opioid use after elective shoulder arthroplasty. J Shoulder Elbow Surg 27:1960–1968, 2018 [DOI] [PubMed] [Google Scholar]
- 36.Little R, Rubin D: Statistical analysis with missing data. John Wiley & Sons; Hoboken, NJ., 2019 [Google Scholar]
- 37.Santosa KB, Hu HM, Brummett CM, et al. : New persistent opioid use among older patients following surgery: A Medicare claims analysis. Surgery, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stark N, Kerr S, Stevens J: Prevalence and predictors of persistent post-surgical opioid use: a prospective observational cohort study. Anaesth Intensive Care 45:700–706, 2017 [DOI] [PubMed] [Google Scholar]
- 39.Kahn KL, Adams JL, Weeks JC, et al. : Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer. JAMA 303:1037–45, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaiser LD, Melemed AS, Preston AJ, et al. : Optimizing collection of adverse event data in cancer clinical trials supporting supplemental indications. J Clin Oncol 28:5046–53, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, et al. : Update on Prevalence of Pain in Patients With Cancer: Systematic Review and Meta-Analysis. J Pain Symptom Manage 51:1070–1090 e9, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Wiffen PJ, Wee B, Derry S, et al. : Opioids for cancer pain - an overview of Cochrane reviews. Cochrane Database Syst Rev 7:CD012592, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.2014 Act 191: ACHIEVING BETTER CARE BY MONITORING ALL PRESCRIPTIONS PROGRAM (ABC-MAP) ACT - ENACTMENT. Pennsylvania General Assembly. https://www.legis.state.pa.us/cfdocs/legis/li/uconsCheck.cfm?yr=2014&sessInd=0&act=191.,
- 44.Vu JV, Howard RA, Gunaseelan V, et al. : Statewide Implementation of Postoperative Opioid Prescribing Guidelines. N Engl J Med 381:680–682, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Overton HN, Hanna MN, Bruhn WE, et al. : Opioid-Prescribing Guidelines for Common Surgical Procedures: An Expert Panel Consensus. J Am Coll Surg 227:411–418, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shapiro CL: Cancer Survivorship. N Engl J Med 379:2438–2450, 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.