ABSTRACT
Introduction: A novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was reported via nucleic acid identification in December, 2019. Accuracy of SARS-CoV-2 diagnostic assays has emerged as a major barrier to COVID-19 diagnosis, particularly in cases requiring urgent or emergent treatment.
Areas covered: In this review, we explore the major reasons for false-positive and false-negative SARS-CoV-2 test results. How clinical characteristics, specific respiratory comorbidities and SARS-CoV-2 vaccination impact on existing diagnostic assays are highlighted. Different COVID-19 management algorithms based on each test and limitations are thoroughly presented.
Expert opinion: The diagnostic accuracy and the capacity of every available assay, which need to be interpreted in the light of the background incidence of SARS-CoV-2 infection in the communities in which they are used, are essential in order to minimize the number of falsely tested cases. Automated testing platforms may enhance diagnostic accuracy by minimizing the potential for human error in assays’ performance. Prior immunization against SARS-CoV-2 impairs the utility of serologic testing of suspected COVID-19 cases. Future avenues of research to evaluate lung tissue innate immune responses hold promise as a target for research to optimize SARS-CoV-2 and future infections’ testing accuracy.
KEYWORDS: COVID-19, false-negative, false-positive, management, respiratory, vaccination
1. Introduction
Three highly pathogenic coronaviruses have impacted substantially on human populations since the beginning of the 21st century. In December 2019, a novel coronavirus (SARS-CoV-2) was reported from a cluster of pneumonia cases in Wuhan, China [1]. As defined by World Health Organization (WHO), a confirmed case is detected from nucleic acid amplification tests (NAAT) for SARS-CoV-2, such as real-time reverse transcription polymerase chain reaction (rRT-PCR), that is worldwide preferred [2]. Nowadays, immunometric assays (IMAs) are performed for detecting the immune response to COVID-19, while several test manufacturers have launched various rapid diagnostic tests (RDTs), to facilitate SARS-CoV-2 detection at point-of-care.
It is widely accepted that tests are not completely foolproof, and, thus no single ‘gold standard’ assay exists. One or more negative tests do not rule out the possibility of SARS-CoV-2 infection [2–4]. Retrospectively, test positivity does not always show an infection existing in reality. The false-negative rRT-PCR test probability for SARS-CoV-2 depends on various sampling and technical factors, whereas the chances of obtaining a true positive result decreases over time, and with decreasing viral titers in clinical specimens [2–5]. Principally, false-positive tests refer to a wrong indication for a particular infection to be present, while false-negative tests pertain to patients labeled as being ‘uninfected’, despite being infected.
The current massive use of RDTs by inexperienced individuals, and poor rRT-PCR laboratory procedures, increase the risk of a false-positive test result. Consequently, challenges arise in hospitalizations and treatments when needed, epidemiological studies may overestimate the extent of disease, financial and business losses emerge from forced isolation in response to false-positive tests, and adverse psychological and societal effects arise through lockdown policies which are designed to limit transmission of SARS-CoV-2 in communities [6].
Reversely, people infected with SARS-CoV-2 but tested negative, remain unaware of their infection status, and may develop a false sense of security based on their test results, and pose a risk for onward transmission of the virus. This would give rise to a situation that perpetuates local epidemics, placing people at high risk for a severe COVID-19.
False-positive tests have generally attracted more attention than false-negative tests, but both are important in successful management of local disease epidemics. The accuracy of available tests must be optimized, particularly within the context of increasing access to SARS-CoV-2 vaccination which further impacts on interpretation of serologic tests for COVID-19.
2. False COVID-19 cases
2.1. False-positive test results
Initially, rapid diagnosis was recommended by WHO mainly in research; however, low-cost technologies with a high degree of accuracy, rapid turnaround times, and which can be implemented by inexperienced laboratory staff, have become widely available in clinical practice. Most RDTs are designed on the basis of lateral-flow immunoassay (LFIA), and they are currently used for a qualitative and to some extent quantitative COVID-19 monitoring in public or private non-laboratory environments. Sajid et al. [7] present Ag-RDTs (antigen-RDTs) as devices consisting of prefabricated strips of a carrier material with dry reagents, activated when applying the recommended specimen. Similar assays, but which rapidly detect antibodies targeting SARS-CoV-2, include Ig-RDTs (immunoglobulin-RDTs).
Five type of false-positive Ag-RDT interpretations are recognized: 1) errors in test operation, 2) poorly specific Ag-RDTs, 3) detection of inactive or residual SARS-CoV-2, 4) cross-contamination and 5) cross-reactions with other substances in clinical samples.
False Ag-RDT results arise when test procedures are incorrectly followed (improper sample handling, contamination of test kits or clinical specimens, deviation from flow through the sequence of test performance, lack of test validation) or by untutored users. Ag-RDT positivity does not exclude other infection, or co-infection with coronaviruses other than SARS-CoV-2, as many test kits are designed to detect highly conserved proteins [8,9]. Highly sensitive tests may detect inactive virus, or virus at low density in clinical specimens. Endogenous (e.g. blood) or exogenous (e.g. nasal spray ions, or chemicals that affect the pH of the test cassette) may impact on test performance, giving rise to false-positive results [10]. LFIAs may be susceptible to temperature fluctuations, humidity, and positioning of the cassette during the testing procedure [11,12].
Tzouvelekis et al. [13] reported the first false-positive Ig-RDT case in July 2020; yet, other authors have announced cross-reactivities with other viruses [14,15]. Heretofore, false-positive Ig-RDTs are present due to:1) an erroneous Ig-RDT operation, 2) use of poorly specific Ig-RDT assays, 3) inattention to the time constraints imposed during a single test, and 4) cross-reactions with other sample substances.
The use of Ig-RDTs during the resolution of SARS-CoV-2 infection may be misleading, as there is uncertainty as to the duration of persistence of IgG following primary and recurrent SARS-CoV-2 infection [16,17]. Endogenous factors, such as hematocrit levels or other blood substances, can affect the whole LFIA procedure in different ways [18,19]. Certain Ig-RDTs detect SARS-CoV-2 specific antibodies, antibodies to other viruses, antinuclear antibodies and other autoantibodies [15,20]. Wang et al reported false-positive SARS-CoV-2 antibody tests in the face of rheumatoid factor, while Tan et al described possible cross-reactivity with HIV [21,22]. LFIAs may also be affected by the presence of heterophilic antibodies, such as human anti-mouse antibodies (HAMA), which have also been described as giving rise to false-positive results [23,24].
Laboratory IMAs include enzyme immunoassay (EIA) and enzyme-linked immunosorbent assay (ELISA), radioimmunoassay (RIA), fluoroimmunoassay (FIA), chemiluminescent immunoassay (CLIA) and counting immunoassay (CIA), and all of them are affected by: 1) technical reasons in each type, 2) testing in window period, and 3) antibody-related parameters. Generally, IMAs are affected by specific endogenous antibodies (heterophile, autoantibodies, antinuclear or anti-animal) or exogenous administered antibodies (Ig-drugs) that interfere and give a falsely elevated result in one assay or a lower result in another assay, even in the same individual [25].
False test results are present in each IMA type, interpretation of serological tests’ sensitivity varies [26], and false-positive serology test results have been reported in COVID-19 [27,28]. Serological assays show a various sensitivity range [29,30].
Real-time PCR is the technique of collecting data throughout the PCR process as it occurs, and rPCR can amplify DNA, or, when preceded by a reverse transcription, RNA. The threshold cycle (Ct) is the point of time at which the target amplification is first detected, and fluorescence intensity is greater than background fluorescence [31,32]. Viral load is inversely related to the Ct value, with lower Ct values correlating to higher viral density in clinical specimens. Yet, it is not determined as varies among diagnostic technologies and fluorescence systems, and several manufacturers have launched different RT-PCRs [33,34]. Nowadays, several rapid PCR assays are utilized, even combining lateral flow technologies, or named as closed PCRs (classical hand-performed are opened PCRs) that are known to be ‘RNA-RDTs’. Yet, several rapid assays lack in control existence and, thus, test validity is risky; additionally, they are affected by bloody and viscous samples.
In routine laboratory PCR testing, some false-positive results can be managed through standard curve or interim controls [35]. However, misleading results can occur due to: 1) inadequate laboratory rRT-PCR experience, 2) SARS-CoV-2 cross-contaminations, 3) detection of unspecified coronaviruses, 4) SARS-CoV-2 inactive/residual detections, 5) cross-reaction with nucleic acids from other pathogens or tissue cells, and 6) technical reasons relating to kit primers, probes and fluorescence type.
Generally, cross-contaminations in laboratories, especially in two-step rRT-PCR (processing RNA extraction and polymerization in different tubes), while sampling or handling, are possible [2,36]. Temperature is crucial for whole PCR procedure. Van Kasteren et al report that some assays detect both SARS-CoV-2 and SARS-CoV, because targeted genetic regions share homology [37], other pathogens, respiratory tract or colon organisms. Lan et al report positive RT-PCR tests in cases who have recovered from COVID-19 [38], but the assay cannot distinguish between viable virus and noninfectious or residual RNA, whereas viral shedding is related to infectivity [39]. Regarding fluorescence, prime-dimers (detected in classical RT-qPCR via melting curve), short oligonucleotide primers and probes, or fluorescent dyes that bind nonspecifically to dsDNA even to ssDNA, can give rise to false-positive results, while various methods use different genes and different probes that may not be equivalent, and, thus, there is a 100-fold difference in limit of detection (LoD) between some assays [40,41].
2.2. False-negative test results
As previously stated, a negative result does not rule out the presence of SARS-CoV-2 infection. Common causes of false-negative Ag-RDT tests include: 1) faulty technique in operating the assay, 2) insufficient clinical specimens, 3) inhibitors, and 4) antigen degradation.
LFIA's performance depends on numerous factors, while luminescent and fluorescent LFIAs have higher sensitivity [42]. Inexperienced operators who may be deployed to run large volumes of Ag-RDTs for COVID-19 monitoring, may handle assay materials poorly or interpret tests incorrectly, compounding the rates of false-negative tests. Each tests’ result is affected by the dynamics of SARS-CoV-2 viral load in early pre-symptomatic and later stages of viral shedding [43]. An insufficient sample or viral mutation can cause a false result, and an individual may have the virus, but the swab might have not collected it from nose or throat. Apart from possible exogenous substances, endogenous molecules could clog the membrane at the cassettes’ conjugate pad in high concentrations. Certain ‘sandwich’ LFIAs may give rise to false-negative results when samples are saturated with antigen: the so-called Hook effect [44].
Ig-RDTs can test negative in the presence of SARS-CoV-2 infection due to: 1) erroneous operation of the assay, 2) factors which may impair antibody production, 3) insufficient samples, 4) inhibitors, and 5) antibody degradation.
Palma et al. [45], Childs et al. [46], Taneja [47] and other authors report several factors that impact on antibody production, such as sex, diet, genetics, adjuvants, vaccines and other parameters affecting immunity, and, thus, rapid or laboratory IMA's results are comparably affected. Moreover, handling and sampling that lead to antibody degradation can give rise to false-negative results. Autoimmune conditions and treatment thereof may also give rise to false-negative tests for SARS-CoV-2 antibody [48,49]. Deeks et al. [3] report that most cases of symptomatic SARS-CoV-2 infection will test positive for antibodies directed against the virus. When a patient is early ill, IgM/IgA antibodies may not be peripherally detectable, and IgA, IgM and IgG antibodies present a sensitivity heterogeneity [3]. Also, endogenous and exogenous factors can affect the final result. Hematocrit, triglycerides, cholesterol (as the cellulose-based material into the cassette LFIA is hydrophilic and affected by viscosity), hemoglobin, and sample temperature, could affect the final result in some cases [18,19]. In traditional lateral flow serodiagnostic formats, the degree of detectable binding is reduced in the presence of high concentrations of nonspecific immunoglobulin. Laboratory IMAs’ negativity is being affected by antibody interference at the same way as the positivity, but in the first case, the extra antibodies interfere by separating and binding to the control and the targeting antibodies, thus blocking the reaction.
Approaching the ‘gold-standard’ rRT-PCR and the extraction-free technologies, some common false-negative types occur in: 1) inadequate laboratory rRT-PCR performance, 2) sample deficiency or degradation, 3) technical reasons relating to kit primers, probes and fluorescence type, 4) SARS-CoV-2 mutations and 5) RT-PCR inhibitors. Faulty sample collection, processing, transportation, or degradation of the SARS-CoV-2 RNA during shipping/storage, can lead to suboptimal rRT-PCR test performance and false-negative SARS-CoV-2 results.
Viral load and Ct affect result accuracy, while applying a cutoff could reduce false-positive and increase false-negative test results [30]. However, in some tests, false-negative results occurring through lack of cell material in the sample are controlled for by simultaneous detection of a universally expressed human gene. Most tests present a LoD for the number of viral copies that can be detected, and false-negative tests may arise if the viral load is lower than that detection limit [2]. Poor sample quality or collection in very early or late infection could give rise to a false-negative test, depending on the assay’s sensitivity [2]. Also, sample degradation is a possible etiology for a false-negative PCR test result. Furthermore, SARS-CoV-2 may not be detected, or may give rise to ambiguous test results, if the genome expressing target genes is mutated [2]. The fluorescent system, which plays a crucial role in the final result, may be affected by PCR inhibitors in the sample. Also, pooling strategies, as laboratory methods in PCR assays can be risky for giving rise to false-negative test results. A brief synopsis of the etiologies for false test results is illustrated in Table 1.
Table 1.
Synopsis of false COVID-19 test results potential reasons in all test types. Each test varies in specificity and sensitivity, and a positive test does not exclude the presence of another pathogen or co-infection. SARS-CoV-2 vaccination does not exclude other pathogen or co-infection
| Potential reasons for COVID-19 false test results | |||
|---|---|---|---|
|
erroneous test administration – untutored use – deviation from testing protocol | |||
| False-positive test result | False-negative test result | ||
| Antigen | Antibody | Antigen | Antibody |
| non-clear place – sampling/handling contaminations time of implementation – humidity – position – sample viscosity – temperature | poor sampling – humidity – position – sample viscosity – temperature – time to evaluation (early or late reading of the test result) – destroyed cassette – sample degradation – time of evaluation – mutations | ||
| cross-reactions with other antigens | cross-reactions with other antibodies | Hook effect | antibody production (e.g. age, sex, diet, smoking, adjuvants, vaccines, genetics,etc) |
| SARS-COV detection | SARS-COV-2 vaccination | SARS-CoV-2 inadequacy | exogenous/endogenous other antibodies |
| inactivate virus detection | IgG positive long after initial infection | late test implementation (long after infection) | early test implementation (pre-symptomatic or asymptomatic cases) |
| exogenous factors (e.g. high concentrations of nasal spray, chemical substances or ions) | exogenous factors (e.g. high concentrations of nasal spray, chemical substances or ions) | exogenous factors (e.g. high concentrations of nasal spray, chemical substances or ions) | exogenous factors (e.g. Ig-drugs, etc) |
| endogenous factors (e.g. blood-impurity derived substances) | endogenous factors (e.g. hematocrit, etc) | endogenous factors (e.g. blood-impurity derived substances) | endogenous factors (e.g. hematocrit, etc) |
| nucleic acid amplification test (RT-PCR) | nucleic acid amplification test (RT-PCR) | ||
| nonclear place – sampling/handling contaminations – temperature | deficient sampling – suboptimal processing/RNA extraction – temperature | ||
| technical reasons (e.g. prime-dimers, short/nonspecific primers, probes, fluorescence) | technical reasons (e.g. destroyed reagents – nonspecific primers, probes, fluorescence) | ||
| Ct cutoff value/control in different test interim guidances | Ct cutoff value/control in different test interim guidances | ||
| cross-contaminations in sampling, handling, laboratory (especially in 2-step RT-PCR) | PCR inhibitors | ||
| cross-reactions with other pathogens/tissue nucleic acids or SARS-COV detection | SARS-COV-2 nucleic acid degradation | ||
| inactive/residual SARS-COV-2 detection | SARS-COV-2 genome mutations | ||
3. Respiratory prevention
NAAT are currently the ‘gold standard’ assays for the detection of SARS-CoV-2 in clinical specimens. Because of suboptimal test sensitivity and specificity, false-negative and false-positive results may occur. False-negative results, which lead to a failure of detecting persons who are infected with SARS-CoV-2, are potentially more damaging than false-positive tests. It is essential that such false-negative test results be minimized, so that respiratory physicians and other clinical staff caring for such patients are alerted to the correct diagnosis as soon as possible, especially when hospitalization and further treatment strategies are necessary. The PCR assay on respiratory specimens may be inhibited in several ways, apart from a SARS-CoV-2 mutant that cannot be detected by the assay, and respiratory physicians should be trained to preempt false-negative test results, in COVID-19 or other pathogens requiring a PCR assay identification.
PCR inhibitors act on one or more essential stages of the PCR testing procedure, from nucleic acid binding, capture or degradation, DNA polymerase inhibition, or ionic buffer alteration which may increase the Ct value and give rise to false-negative test results. When referring to RT-PCR, reverse transcriptase can be inhibited, too. Schrader et al. [50], Wilson et al. [51] and Sidstedt et al. [52,53] present several substances (including hemoglobin, lactoferrin, melanin, IgG, myoglobin, NaCl, tannic acids, urea, bile salts, bilirubin, cellulose, heparin, free radicals and ethanol) which, when present in high concentrations, have been found to affect test performance. Combs et al. [54] and Kuffel et al. [55] report metal ions that affect PCR performance, and Marino-Merlo et al. [56] show that reverse transcriptase can be inhibited from antiretroviral drugs. The capacity of each PCR test kit to perform in the presence of different inhibitors have been presented in interim guidance documents. Some PCR kits have controls to detect inhibitors, while others cannot detect these substances.
Respiratory tract samples include tissue residues and respiratory secretions, with endogenous and exogenous factors, initially deposited in the respiratory mucosa or lung parenchyma which, in high concentrations, can inhibit inexpertly conducted or low-sensitivity PCR tests. Lower respiratory tract specimens are particularly prone to being affected by different pulmonary pathologies which lead to variability of specimen adequacy (bloody, viscous, etc), while upper respiratory tract specimens tend to be affected by exogenous factors such as drugs and inhaled toxins.
Generally, occupational and allergic lung diseases, such as occupational asthma or chronic obstructive lung disease (COPD) with an ongoing exposure, could affect respiratory sample testing by containing specific known inhibitors. Occupational and interstitial lung diseases (ILD), such as asbestosis, silicosis, alveolar microlithiasis, chemical pneumonia, melanoma or hemorrhage-related diseases, could affect a PCR test by presenting sample inhibitors (metal ions or blood substances). Respiratory conditions (endogenous factors) that may impact on the performance of RT-PCR are presented in Figure 1.
Figure 1.
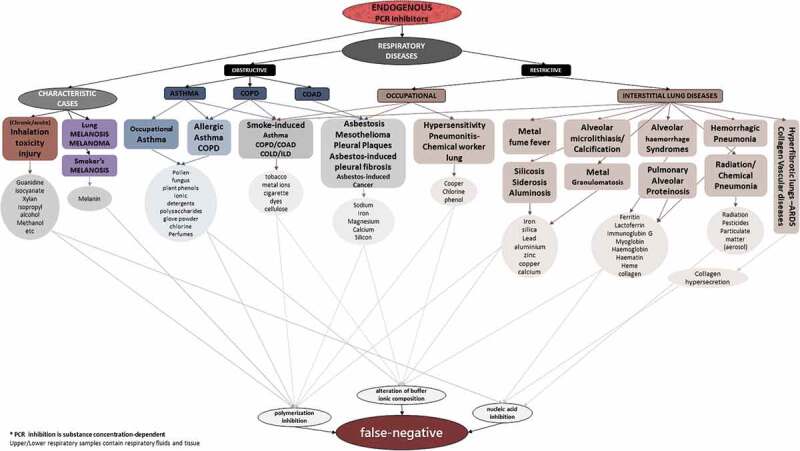
Respiratory prediction of an endogenous inhibited PCR result
High concentrations of endogenous or exogenous substances, if present in upper or lower respiratory tract samples, can lead to false-negative PCR results. These inhibitory factors apply to PCR testing for all respiratory pathogens tested in PCR assay, and are not limited to SARS-CoV-2 testing.
PCR assay is affected by ethanol, contained in a sample, thus consuming alcohol just before sampling could be risky, and there exists a report of a COVID-19 case with a false-negative PCR assay, presenting a past medical history of alcohol use disorder, but it remains unknown if alcohol was consumed before sampling [50,57]. Important exogenous factors that affect PCR assays are specific drugs that may exist in respiratory tract specimens, such as nasal sprays including humic and fulvic acids’ derivatives, phenolic ions, polysaccharides, polyamines, etc., that mainly inhibit DNA polymerization. For instance, inhaled drugs with specific chemical substances, such as inhaled heparin for pulmonary function, or inhaled D-cycloserine, may affect the final result, while a high concentration of intranasal cellulose powder may lead to nucleic acid or polymerization inhibition. Furthermore, chemotherapy drugs, such as bleomycin, may affect PCR, as the inhibition mechanism is almost the same, and especially bleomycin can give rise to bleomycin-induced pneumonitis (BIP) which provides a saturated bronchoalveolar lavage (BAL) specimen for a low sensitive PCR assay. Last but not least, antiretroviral therapy could inhibit RT-PCR assay, since some antiretroviral drugs are even being tested for their effectiveness in reverse transcriptase inhibition assays.
As it appears, cases with preexisting conditions that could yield false-negative test results, should be reported from physicians to laboratory experts. Alternative and more sensitive assays can be performed in laboratories -than the classical methods for non-bloody or non-viscous samples-, or combination of PCR assay and IMAs, so as to prevent PCR inhibition. Thus, a potential false-negative COVID-19 case can be prevented.
4. Preexisting conditions and testing assays
As it appears, not only the clinical diagnosis but also the laboratory test result interpretation is affected by a case’s preexisting diseases and total health status. In general, false-positive COVID-19 cases are more likely to exist in a low COVID-19 prevalence in the community where other viruses abound, and false-negative COVID-19 are more likely to exist in a community rating other comorbidities. Since COVID-19 Ag-RDTs were initially based on SARS-CoV highly conserved genetic loci, it is certain that they can present a false-positive result for SARS-CoV-2 [8], and less accurate Ag-RDTs show a positivity to influenzas, other viruses, or bacteria. If sample contains blood, there is a possibility for blood-derived substances and antibodies to interfere in the assay, as occurring in the IMAs.
Rapid (Ig-RDTs) and laboratory IMAs (such as ELISA) are more likely to be affected by the preexisted individual’s immunity, such as several autoimmune diseases [13,22]. Also, Ig-RDTs have indicated a false-positivity even in pregnant women [58]. IMAs’ interference has been reported in specific diseases producing heterophile antibodies, such as infectious mononucleosis (IM) [59]. More than a half of the patients’ samples contain HAAAs [25,60] that interfere in IMAs by presenting both positivity and negativity, while existing mainly in serum of animal workers, people living with indoor pets or patients being administered genetically engineered mouse monoclonal antibodies (Ig-drugs) for therapy or imaging. In reality, all human beings present autoantibodies interfering in IMAs and, thus, every serological test should be interpreted according to an individual’s total health condition [61,62]. Rheumatoid factor (RF) and antinuclear antibodies have long been reported for serological interferences [63–65]. HIV, hepatitis, syphilis, malaria, lupus, vasculitis, hyper-gamma-globulinaemia and presence of HLA-DR antibodies have long been correlated with false IMAs test results and antibody interference [66–69].
Importantly, while trying to detect the real infection with another disease’s IMA, instead of targeting COVID-19, a false result can occur again, and arise suspicions for a SARS-CoV-2 infection, especially when the total health status is ambiguous. As mentioned before, anti-SARS-CoV-2 antibodies can interfere in other IMAs.
PCR assays can be affected by preexisting medical conditions, such as jaundice and hyperbilirubinemia-related disorders, since high concentrations of bilirubin and bile salts found in human samples can inhibit PCR [50]. Concomitantly, PCR assay is affected by the material of the sample collector (swab, etc). Background medical conditions leading to an excess of specific proteins (collagen, ferritin, lactoferrin, myoglobin, IgG, hemoglobin and heme) in human samples, can be crucial in estimating a PCR test result [50–53]. Phenolic, citrates, polyamines or polysaccharides found in human samples, due to preexisting conditions or because of specific drugs’ consumption and metabolism, need to be further taken into account, in accordance with the interim guidances of each implemented assay.
Alternatively, these cases with preexisting conditions that could affect a testing assay performance should be reported from physicians to laboratory experts. Better and more sensitive assays can be utilized instead of, and may some false results be prevented and eliminated.
5. Management strategies
The WHO recommends that challenging cases, so-called challenging COVID-like diseases (CLD), be tested for other respiratory pathogens, as co-infection with other respiratory pathogens is frequent. Parallel testing platforms are becoming more widely available [70,71]. SARS-CoV-2 is now ubiquitous, and any suspected case should be tested for SARS-CoV-2 regardless of whether another respiratory pathogen is detected [2].
Management decisions rely on NAAT, combined with medical examination, epidemiological information, and patient history, and the results of the diagnostic work-up inclusive of all radiological, biochemical and microbiological tests. Espy et al. [35] consider IMAs as an essential tool for the diagnosis of viral infections. During the course of the pandemic, SARS-CoV-2 should be considered in all acutely ill patients presenting with respiratory failure, and the virus, or its mutants, may or may not be present in the patient’s respiratory secretions. A combined assessment is critical for the rational management of such patients. An example of such a COVID-19 management algorithm is presented in Figure 2.
Figure 2..

Algorithm for the management of suspected COVID-19 cases during the course of the pandemic
Ag-RDT: Antigen rapid diagnostic test, Ig-RDT: Immunoglubin rapid diagnostic test. Color represents symptomatic (sympt), suspected (susp), contacted (cont), and non-suspected (non-susp) cases. Symptomatic and suspected cases, as well as cases with possible/confirmed contacts, or cases with no history, and evidence for infection with SARS-CoV-2, is shown here. As no test is 100% accurate and false results can occur, COVID-19 positivity is stated as ‘current evidence’ with precautions. Both false-positive and false-negative test result possibility is depicted in each test type. Regarding Ig-RDTs, positivity for IgM/IgA and/or IgG should be combined with all the aforementioned criteria. CLDs should be considered in every testing type and further management. RDTs are not to be existed necessarily, the algorithm is simply summarizing the whole testing cases.
False-positive COVID-19 place patients at risk through cohorting with other COVID-19 cases, while false-negative COVID-19 place healthcare workers, other patients and the general public at risk for infection from an undiagnosed source case. Both scenarios have the potential to impact substantially on patient-level care and public safety. A positive test does not exclude co-infection with other respiratory pathogens, while a negative test does not exclude SARS-CoV-2 infection, particularly in the context of infection with viral mutants. Where the clinical index of suspicion is high, repeated testing should be undertaken.
Despite the false COVID-19 tested cases, it is clear that a result represents a unique tested sample in a particular point of time; therefore, the whole case condition can be different, and, exclusively, each cases’ various samples can manifest different results. Comparing different clinical cases with false results, in different test types, methods and kits, when even an individuals’ samples vary in the same test kit, seems groundless. Instead of randomly reporting and analyzing clinical cases with false results in numerous test types, precluding each tests’ limitations, it would be more successful to detect the specific tests’ molecular reason of a false result and prevent further misleading results.
6. SARS-CoV-2 vaccination
In the era of growing access to SARS-CoV-2 vaccination, serologic testing to establish a diagnosis of SARS-CoV-2 infection will become more complicated. Numerous vaccine platforms, ranging from mRNA vaccines, antigen-based vaccines and viral vector vaccines, are currently under investigation or have been implemented in mass immunization campaigns. Ag-RDTs do not detect SARS-CoV-2 antigen derived from antigen-based vaccines; nevertheless, it remains unknown as to whether tissue or blood impurities in respiratory specimens could give rise to false-positive Ag-RDT test results.
Ig-RDTs cannot be relied upon to establish a diagnosis of SARS-CoV-2 infection in individuals that have received SARS-CoV-2 vaccines. It remains to be seen how vaccine-induced SARS-CoV-2 antibody responses may cross-react with serologic tests for other pathogens, giving rise to false-positive test results for those pathogens. SARS-CoV-2 vaccination history, and timing thereof, should be established when consideration is being made to use Ig-RDTs to screen for current or past SARS-CoV-2 infection. It would be appropriate to use Ig-RDTs that target different antigen-antibody loci from the vaccine antigen when using Ig-RDTs in persons who have received SARS-CoV-2 vaccination.
Considering that various platforms are being under consideration SARS-CoV-2 vaccination, including multi-epitope or reverse vaccinology and immunoinformatics [72–75], may these technologies be more precise in immunogenic responses. Also, may multi-allelic vaccines be more advantageous in non-interfering in IMAs targeting the real antibodies, as the natural antibody is different. However, in this case, if a vaccine induces multi-epitope immune responses, it gives rise to additional produced antibodies in the body and, as a result, the possibility for a general IMAs’ possible interference is equivalently increasing.
NAATs done on respiratory samples cannot detect vaccine-derived SARS-CoV-2 nucleic acids which were administered via the intramuscular route. It remains unknown as to whether blood or tissue contamination of respiratory fluids could give rise to false-positive SARS-CoV-2 NAATs in persons that have been administered nucleic acid-containing SARS-CoV-2 vaccines, however.
7. Conclusion
This review article has summarized what is currently known about false-negative and false-positive tests for SARS-CoV-2, and clinical scenarios that may give rise to such erroneous results. All tests should be interpreted with caution, within the context of the individual patient’s clinical status, exposure history, and the results of ancillary tests, as well as in the context of the prevalence of SARS-CoV-2 infection in the wider community at the time of testing. In situations where SARS-CoV-2 is circulating widely, the positive predictive value of the available tests increases. Where clinical suspicion is high, despite an initial negative test of SARS-CoV-2, tests should be repeated in order to establish a firm diagnosis of the condition.
It seems obvious that not only is a microcosmic high-affinity reaction crucial for confirming a realistic COVID-19 case, but also clinicians and respiratory physicians should be in a great macrocosmic interaction, for an accurate test result, further respiratory medical management and treatment perspectives. COVID-19 can be such an illusory disease; yet, chemistry cannot be deceived, and some false result cases can be predicted.
Relentless pursuit of SARS-CoV-2 in a patient who has multiple negative tests, despite using different assay types and test kits, should not be encouraged, however. Management strategies can be precise and straightaway, when assessing each test result at the angle of each testing method guidelines – concerning vaccination-, and, respiratory physicians can prevent some potential false tested cases for a better and on-the-spot response to emergent COVID-19 cases.
8. Expert opinion
After almost a year of SARS-CoV-2 pandemic, the various reports of falsely tested cases, precluding each tests’ limitations, have revealed that physicians are far away from the real tests’ capacity, so their sole point-of-care performing, could be more effective in estimating a test result. In the decades of multiplexed and rapid NAATs, rapid LFIAs, and golden nanoparticles which present no research endpoint, the testing automation, provides better accuracy and sample handling, shorter turnarounds and cost-effective administrations for the improvement of a pathogens’ detection. However, ‘self-tests’ performed by non-guidanced arise awareness about the sampling quality and adequacy, the if-swab safe usage, and the final assessment regarding background status.Easier automated methods may be designed, such as ‘licking-devices’, since a virus exists in droplets and aerosols.Public should be informed about the importance of a test’s interim guidances. Also, more sensitive rapid LFIAs, different for winter/summer use – regarding humidity/temperature conditions, could be designed.
New technologies have loss of standardization as the countless PCR kits vary in methods and cutoff values, thus, test results are paralleled in unassociated weights, and a realistic comparison between cases is trammeled. Thus, by preserving the existence of misleading COVID-19 cases in such way, scientific community is being prevented from clear-sighted advances. Since PCR assay cannot distinguish between active and residual RNA, a better assay – maybe with an active viral amplicon size cutoff value- needs to be designed. Also, a further development of the widely successful CRISPR-Cas9 method for a better detection and differentiation of amplification products could be seen in near future. The false-positive COVID-19 test results in other existing pathogens need further analysis. Besides, it remains unknown, to what extent, in cases with a negative NAAT and positive IMA, the final result could be a negative COVID-19 case, as antibodies are such difficult to be assessed. Also, the real time that SARS-CoV-2 is detected active and inactive in deceased cases is unclear.
SARS-CoV-2 vaccination era boosted the mRNA technology and lipid nanoparticles with mainly the intramuscular way; therefore, gene markers, such as GFP (green fluorescent protein) could be more useful, in detecting exactly in which tissue cells apart from muscle cells can these vaccine nanoparticles exist, for an accurate prognosis and exclusion of a possible false-positive result. Also, it would be essential for determining further vaccine side-effects when analyzing exactly the nanoparticles’ tissue route. Furthermore, the in vivo vaccine-produced anti-SARS-CoV-2 antibodies should be analyzed for possible cross-reactions in other pathogens’ serology assays – a possible false-positive test result in other pathogen.
The kinetics of pulmonary route need to be further studied for a future vaccine or therapy progression, against COVID-19 or other lung infections, even for preventing the so-called coming ‘Disease X’ severity. Preclinical models and researches for inhaled antibodies or vaccines need to speed up, for lung-targeted viral drugs or pulmonary-based vaccinations. Inhalation-based or intravenous strategies targeting solely the lungs or lung-designed vaccines/drugs with lung-cell signal peptides, are more successful. They may need lower doses reducing chances for exposing toxic side-effects in other tissues. Targeting the primordial system of the lung tissue-resident innate immunity, could be a more promising strategy for SARS-CoV-2 or other current or future lung infections’ drug or vaccine formulation. Lung dendritic cells (DCs) named the lung sentinels, have proved to be important in the initiation of antiviral responses that lead to general viral clearance, so they could be a future potential vaccines’ antigen expression target. However, lung immunity and DCs need to be thoroughly analyzed, as there are several functional DCs’ questions in common respiratory diseases, such as COPD, to prevent possible side effects.
It is speculated that, in the near future, communities will have acculturated SARS-CoV-2 and its mutants, but false-tested cases cannot be excluded for all pathogens, as it is extremely difficult for the whole medical community to follow a same and unique route of pathogens’ management, beginning with the countless testing assays. Regarding SARS-CoV-2 vaccination, the inactivated virus-based vaccines will trouble IMAs, as vaccine-induced antibodies will be produced for all genetic loci, and, Ig-RDTs could be used for manifesting the individuals’ immunity. Vaccination global rhythms vary, and long-lasting vaccines are required for a concomitant universal herd immunity. Vulnerable cases may be prone to re-infection, for instance in ADE phenomenon (antibody-dependent enhancement), and vaccinations even drug platforms will be needed systematically. Vaccines for stable SARS-CoV-2 genetic loci are required to compete viral mutations, different vaccine doses may be needed for generations, or different vaccine types for cases with background diseases, for standard recurrent administration, so as to present a cutoff antibody threshold against SARS-CoV-2.
Funding Statement
This paper was not funded.
Article highlights
False-positive COVID-19 cases occur in erroneous testing and cross-reactions, and place patients at risk through cohorting with other COVID-19 cases.
False-negative COVID-19 cases occur through sample deficiency, concurrent respiratory infection, or test inhibitors, and place healthcare workers, other patients, and the general public at risk for infection from an undiagnosed source case.
SARS-CoV-2 vaccination produces an antibody response, which renders serologic testing for COVID-19 less reliable.
prevailing community incidence of covid-19, together with diagnostic test accuracy, must be considered in the management of all suspected covid-19 cases
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Zhu N, Zhang D, Wang W, et al. China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO [Internet] . Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases; [cited 2021. February 16] Available from: https://apps.who.int/iris/bitstream/handle/10665/331329/WHO-COVID-19-laboratory-2020.4-eng.pdf?sequence=1&isAllowed=y
- 3.Deeks JJ, Dinnes J, Takwoingi Y, et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020. June. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watson J, Whiting PF, Brush JE.. Interpreting a covid-19 test result. BMJ. 2020;369:m1808. [DOI] [PubMed] [Google Scholar]
- 5.Wikramaratna PS, Paton RS, Ghafari M, et al. Estimating the false-negative test probability of SARS-CoV-2 by RT-PCR. Euro Surveill. 2020;25(50):2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surkova E, Nikolayevskyy V, Drobniewski F. False-positive COVID-19 results: hidden problems and costs. The Lancet Resp Med. 2020;8(12):1167–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sajid M, Kawde AN, Daud M. Designs, formats and applications of lateral flow assay: a literature review. J Saudi Chem Soc. 2015;19(6):689–705. [Google Scholar]
- 8.WHO [Internet] . Antigen-detection in the diagnosis of SARS-CoV-2 infection using rapid immunoassays COVID-19: laboratory and diagnosis; [cited 2021. February 16] Available from: https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays
- 9.Dinnes J, Deeks JJ, Adriano A, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2020;8:CD013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinkel R, Jacobi J, Ziefuβ AR, et al. Role of citrate and NaBr at the surface of colloidal gold nanoparticles during functionalization. J Phys Chem C. 2018;122(48):27383–27391. [Google Scholar]
- 11.Wang J, Obermeyer J, Filipe CDM, et al. Effects of temperature and relative humidity on the stability of paper-immobilized antibodies. Biomacromolecules. 2012;13(2):559–564. [DOI] [PubMed] [Google Scholar]
- 12.O’Farrell B. Evolution in lateral flow–Based immunoassay systems. In: Wong R, Tse H, editors. Lateral flow immunoassay. [place unknown]: Humana Press; 2009. p. 1–33. [Google Scholar]; •• this book chapter is important for analyzing the principles for the current used LFIAs
- 13.Tzouvelekis A, Karampitsakos T, Krompa A, et al. False positive COVID-19 antibody test in a case of granulomatosis with Polyangiitis. Front Med (Lausanne). 2020;7:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibata S, Ishiguro T, Kobayashi Y, et al. High incidence of false-positive results of IgG antibody against SARS-CoV-2 with rapid immunochromatographic antibody test due to human common cold coronavirus infection. Resp. Med. Case Reports. 2020;31:101180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lustig Y, Keler S, Kolodny R, et al. Potential antigenic cross-reactivity between SARS-CoV-2 and dengue viruses. Clin Infect Dis. 2020;14:ciaa1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Figueiredo-Campos P, Blankenhaus B, Mota C, et al. Seroprevalence of anti-SARS-CoV-2 antibodies in COVID-19 patients and healthy volunteers up to 6 months’ post disease onset. Eur J Immunol. 2020;50(12):2025–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou W, Xu X, Chang Z, et al. The dynamic changes of serum IgM and IgG against SARS-CoV-2 in patients with COVID-19. J Med Virol. 2021;93(2):924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Hegener MA, Pauletti GM, et al. Flow reproducibility of whole blood and other bodily fluids in simplified no reaction lateral flow assay devices. Biomicrofluidics. 2017;11(2):024116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laureano AFS, Riboldi M. The different tests for the diagnosis of COVID-19 - A review in Brazil so far. JBRA Assist Reprod. 2020;24(3):340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer B, Drosten C, Muller M. Serological assays for emerging coronaviruses: challenges and pitfalls. Virus Res. 2014;194:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Du B, Guo B, et al. A method to prevent SARS-CoV-2 IgM false positives in gold immunochromatography and Enzyme-Linked immunosorbent assays. J Clin Microbiol. 2020;58(6):e00375–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan SS, Chew KL, Saw S, et al. Cross-reactivity of SARS-CoV-2 with HIV chemiluminescent assay leading to false-positive results. J Clin Pathol. 2020. September 09. 10.1136/jclinpath-2020-206942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolstad N, Warren DJ, Nustad K. Heterophilic antibody interference in immunometric assays. Best Pract Res Clin Endocrinol Metab. 2013;27(5):647–661. [DOI] [PubMed] [Google Scholar]; •• this article is crucial for IMAs, analyzing basic interference types
- 24.Mohammadi MM, Bozorgi S. Investigating the presence of human anti-mouse antibodies (HAMA) in the blood of laboratory animal care workers. J Lab Med. 2019;43(2):287–291. [Google Scholar]
- 25.Tate J, Ward G. Interferences in immunoassay. Clin Biochem Rev. 2004;25(2):105–120. [PMC free article] [PubMed] [Google Scholar]
- 26.Lisboa Bastos M, Tavaziva G, Abidi SK, et al. Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ. 2020;370:m2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.To KK, Chua GT, Kwok KL, et al. False-positive SARS-CoV-2 serology in 3 children with Kawasaki disease. Diagn Microbiol Infect Dis. 2020;98(3):115141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boukli N, Le Mene M, Schnuriger A, et al. High incidence of false-positive results in patients with acute infections other than COVID-19 by the Liaison SARS-CoV-2 commercial chemiluminescent microparticle immunoassay for detection of IgG Anti-SARS-CoV-2 antibodies. J Clin Microbiol. 2020;58(11):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cota G, Freire ML, De Souza CS, et al. Diagnostic performance of commercially available COVID-19 serology tests in Brazil. Int J Infect Dis. 2020;101:382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swadźba J, Bednarczyk M, Anyszek T, et al. The real life performance of 7 automated anti-SARS-CoV-2 IgG and IgM/IgA immunoassays. Pract Lab Med. 2021;25:e00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heid CA, Stevens J, Livak KJ, et al. Real time quantitative PCR. Genome Res. 1996;6(10):986–994. [DOI] [PubMed] [Google Scholar]
- 32.Wittwer CT, Herrmann MG, Moss AA, et al. Continuous fluorescence monitoring of rapid cycle DNA amplification. Biotechniques. 1997;22(130–1):134–138. [DOI] [PubMed] [Google Scholar]
- 33.Mackay IM, Arden KE, Real-time NA. PCR in virology. Nucl Acids Res. 2002;30(6):1292–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukumoto T, Iwasaki S, Fujisawa S, et al. Efficacy of a novel SARS-CoV-2 detection kit without RNA extraction and purification. Int J Infect Dis. 2020;98:16–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Espy MJ, Uhl JR, Sloan LM, et al. Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clin Microbiol Rev. 2006;19:165–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shanti NA, Saini A, Stewart CE. Two-step versus one-step RNA-to-CT™ 2-Step and one-step RNA-to-CT™ 1-Step: validity, sensitivity, and efficiency. J Biomol Tech. 2009;20(3):172–179. [PMC free article] [PubMed] [Google Scholar]
- 37.Van Kasteren PB, Van Der Veer B, Van Den Brink S, et al. Comparison of seven commercial RT-PCR diagnostic kits for COVID-19. J Clin Virol. 2020;128:104412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lan L, Xu D, Ye G, et al. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020. February;323(15):1502–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atkinson B, Petersen E. SARS-CoV-2 shedding and infectivity, lancet. Corresp. 2020;395:1339–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernández F, Gutiérrez J, Sorlózano A, et al. Comparison of the SYBR green and the hybridization probe format for real-time PCR detection of HHV-6. Microbiol Res. 2006;161(2):158–163. [DOI] [PubMed] [Google Scholar]
- 41.Rosati B, Grau F, Kuehler A, et al. Comparison of different probe-level analysis techniques for oligonucleotide microarrays. Biotechniques. 2004;36(2):316–322. [DOI] [PubMed] [Google Scholar]
- 42.Posthuma-Trumpie GA, Korf J, Van Amerongen A. Lateral flow (immuno)assay: its strengths, weaknesses, opportunities and threats. A literature survey. Anal Bioanal Chem. 2009;393(2):569–582. [DOI] [PubMed] [Google Scholar]
- 43.Weiss A, Jellingsø M, Sommer MOA. Spatial and temporal dynamics of SARS-CoV-2 in COVID-19 patients: a systematic review and meta-analysis. EBioMed. 2020;58:102916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross GMS, Filippini D, Nielen MWF, et al. Unraveling the Hook effect: a comprehensive study of high antigen concentration effects in sandwich lateral flow immunoassays. Anal Chem. 2020;92(23):15587–15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palma J, Tokarz-Deptula B, Deptula J, et al. Natural antibodies – facts known and unknown. Cent Eur J Immunol. 2018;43(4):466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Childs CE, Calder PC, Miles EA. Diet and immune function. Nutrients. 2019;11(8):1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taneja V. Sex hormones determine immune response. Front Immunol. 2018;9:1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choy KW. SARS-CoV-2 serological cross-reactivity with autoantibodies. The Lancet Rheumatol Correspondence. 2021;3:1,E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schrader C, Schielke A, Ellerbroek L, et al. PCR inhibitors – occurrence, properties and removal. J Appl Microbiol. 113(5): 1014–1026. 2012. . [DOI] [PubMed] [Google Scholar]; •• this article is significant revealing the PCR inhibitors.
- 51.Wilson IG. Inhibition and facilitation of nucleic acid amplification. Appl Env Microbiol. 1997;63(10):3741–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sidstedt M, Hedman J, Romsos EL, et al. Inhibition mechanisms of hemoglobin, immunoglobulin G, and whole blood in digital and real-time PCR. Anal Bioanal Chem. 2018;410(10):2569–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sidstedt M, Rådström P, Hedman J. PCR inhibition in qPCR, dPCR and MPS—mechanisms and solutions. Anal Bioanal Chem. 2020;412(9):2009–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Combs LG, Warren JE, Huynh V, et al. The effects of metal ion PCR inhibitors on results obtained with the quantifiler (®) human DNA quantification kit. Forensic Sci Int Genet. 2015;19:180–189. [DOI] [PubMed] [Google Scholar]
- 55.Kuffel A, Gray A, Daeid NN. Impact of metal ions on PCR inhibition and RT-PCR efficiency. Int J Legal Med. 2021;135(1):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marino-Merlo F, Frezza C, Papaianni E, et al. Development and evaluation of a simple and effective RT-qPCR inhibitory assay for detection of the efficacy of compounds towards HIV reverse transcriptase. Appl Microbiol Biotechnol. 2017;101(22):8249–8258. [DOI] [PubMed] [Google Scholar]
- 57.Wiseman J, D’Amico TA, Zawadzka S, et al. False negative SARS-CoV-2 PCR - A case report and literature review. Respir Med Case Rep. 2020;31:101140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fabre M, Ruiz-Martinez S, Monserrat Cantera ME, et al. SARS-CoV-2 immunochromatographic IgM/IgG rapid test in pregnancy: a false friend? Ann Clin Biochem. 2021;58(2):149–152. [DOI] [PubMed] [Google Scholar]
- 59.Fisher BAC, Bhalara S. False-positive result provided by rapid heterophile antibody test in a case of acute infection with hepatitis E Virus. J Clin Microbiol. 2004;42(9):4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kricka LJ. Human anti-animal antibody interferences in immunological assays. Clin Chem. 1999;45(7):942–956. [PubMed] [Google Scholar]
- 61.Andersen DC, Koch C, Jensen CH, et al. High prevalence of human anti‐bovine IgG antibodies as the major cause of false positive reactions in two‐site immunoassays based on monoclonal antibodies. J Immunoassay Immunochem. 2004;25(1):17–30. [DOI] [PubMed] [Google Scholar]
- 62.Hennig C, Rink L, Kirchner H. Evidence for presence of lgG4 anti-immunoglobulin autoantibodies in all human beings. Lancet. 2000;355(9215):1617–1618. [DOI] [PubMed] [Google Scholar]; •• this article is important since it reveals that all humans present autoantibodies
- 63.Sato H, Ito S, Nakazono K, et al. False-positive semiquantitative immunochromatography assays for procalcitonin in three patients with rheumatoid arthritis-A case series. Clin Case Rep. 2020;8(9):1704–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee J-H, Jang JW, Cho CH, et al. False-positive results for rapid diagnostic tests for malaria in patients with rheumatoid factor. J Clin Microbiol. 2014;52(10):3784–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grygiel-Górniak B, Rogacka N, Puszczewicz M. Antinuclear antibodies in healthy people and non-rheumatic diseases - diagnostic and clinical implications. Reumatologia. 2018;56(4):243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Noh J, Ko HH, Yun Y, et al. [Evaluation of performance and false positivity of Mediace RPR test that uses a chemistry autoanalyzer]. Korean J Lab Med. 2008;28(4):312–318. [DOI] [PubMed] [Google Scholar]
- 67.Monos DS, Frank TS, Senior MB, et al. Delineation of false-positive HIV antibody response in patients with renal failure and history of multiple transfusions. Transfusion. 1989;29(2):119–123. [DOI] [PubMed] [Google Scholar]
- 68.Vitali C, Sciuto M, Neri R, et al. Anti-hepatitis C virus antibodies in primary Sjögren’s syndrome: false positive results are related to hyper-gamma-globulinaemia. Clin Exp Rheumatol. 1992;10(1):103–104. [PubMed] [Google Scholar]
- 69.Hunter JB, Menitove JE. HLA antibodies detected by ELISA HTLV-III antibody kits. Lancet. 1985;2(8451):397. [DOI] [PubMed] [Google Scholar]
- 70.Ling L, Se K, Jc L, et al. Parallel validation of three molecular devices for simultaneous detection and identification of influenza A and B and respiratory syncytial viruses. J Clin Microbiol. 2018;56(3):e01691–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.[internet] Number of deaths involving coronavirus disease 2019. (COVID-19), pneumonia, and influenza in the U.S. as of January 2, 2021. [2021 February16] Available from: https://www.statista.com/statistics/1113051/number-reported-deaths-from-covid-pneumonia-and-flu-us/
- 72.Sarkar B, Ullah M, Johora FT, et al. Immunoinformatics-guided designing of epitope-based subunit vaccines against the SARS Coronavirus-2 (SARS-CoV-2). Immunobiology. 2020;225(3):151955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhattacharya M, Sharma AR, Patra P. A SARS-CoV-2 vaccine candidate: in-silico cloning and validation. Inf Med Unlocked. 2020;20:100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rahman N, Ali F, Basharat Z, et al. Vaccine design from the ensemble of surface glycoprotein epitopes of SARS-CoV-2: an immunoinformatics approach. Vaccines (Basel). 2020;8(3):423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhattacharya M, Sharma AR, Patra P, et al. Development of epitope-based peptide vaccine against novel coronavirus 2019 (SARS-COV-2): immunoinformatics approach. J Med Virol. 2020;92(6):618–631. [DOI] [PMC free article] [PubMed] [Google Scholar]


