Abstract
Plesiadapiform mammals, as stem primates, are key to understanding the evolutionary and ecological origins of Pan-Primates and Euarchonta. The Purgatoriidae, as the geologically oldest and most primitive known plesiadapiforms and one of the oldest known placental groups, are also central to the evolutionary radiation of placentals and the Cretaceous-Palaeogene biotic recovery on land. Here, we report new dental fossils of Purgatorius from early Palaeocene (early Puercan) age deposits in northeastern Montana that represent the earliest dated occurrences of plesiadapiforms. We constrain the age of these earliest purgatoriids to magnetochron C29R and most likely to within 105–139 thousand years post-K/Pg boundary. Given the occurrence of at least two species, Purgatorius janisae and a new species, at the locality, we provide the strongest support to date that purgatoriids and, by extension, Pan-Primates, Euarchonta and Placentalia probably originated by the Late Cretaceous. Within 1 million years of their arrival in northeastern Montana, plesiadapiforms outstripped archaic ungulates in numerical abundance and dominated the arboreal omnivore–frugivore niche in mammalian local faunas.
Keywords: primates, Purgatoriidae, plesiadapiforms, Cretaceous–Palaeogene boundary, frugivory
1. Introduction
Plesiadapiforms are crucial to understanding the evolutionary and ecological origins of primates and other euarchontans (treeshrews and colugos) as well as the traits that separate those groups from other mammals [1]. Plesiadapiforms are a paraphyletic assemblage of 11 extinct families and more than 150 species from the Palaeocene and Eocene of North America, Europe and Asia (see [2] for most up-to-date species list). Researchers have debated the systematic affinities of this group, with some suggesting that some or all plesiadapiform taxa are more closely related to other euarchontans (e.g. dermopterans) than they are to primates, and others interpreting plesiadapiforms as stem primates (see [2] for a review). Recent phylogenetic analyses, which have benefited from newly discovered, exceptionally complete fossils, support the latter hypothesis and consistently place plesiadapiforms as successive sister-taxa to Primates (e.g. [3,4]; but see [5]). Here we use Pan-Primates [6] to designate the total clade that includes the crown clade (Primates [7]) and all stem primates (including plesiadapiforms). The Purgatoriidae, as the oldest and most primitive known plesiadapiform family [8–10], are particularly important to understanding primate ancestry. Researchers can use their morphology to inform character-state polarity in phylogenetic analyses, resolve broader relationships among stem and crown primates, and characterize the ecology of the earliest members of Pan-Primates. Purgatoriids were also among the first known placental mammals to diversify, both taxonomically and ecologically, following the Cretaceous–Palaeogene (K/Pg) mass extinction that resulted in the loss of all non-avian dinosaurs [11,12]. Understanding the detailed pattern of this seminal diversification event thus has implications for understanding the evolutionary radiation of placentals and the K/Pg biotic recovery on land.
The Purgatoriidae comprise two genera, Purgatorius and Ursolestes, and seven named species [2]. Van Valen and Sloan [13] named two species, both from northeastern Montana: the type species, P. unio, on the basis of six isolated teeth from the early Palaeocene (Pu3 subinterval of the Puercan North American land mammal ‘age’) Purgatory Hill locality, and P. ceratops from the Harbicht Hill locality, then considered latest Cretaceous in age. Revised stratigraphic interpretations at Harbicht Hill suggest that the fossil assemblage is a mixture of both earliest Palaeocene and reworked latest Cretaceous taxa [14,15]. In addition to its age uncertainties, P. ceratops is known by only a single, poorly preserved lower molar that most authors consider non-diagnostic (e.g. [16,17]). The first large sample of isolated teeth and dentigerous dentary fragments of Purgatorius were reported from the Pu3 Garbani Channel localities in northeastern Montana [18]. Initially referred to P. unio, Van Valen [19] later placed them in a new taxon, P. janisae. Buckley [20] also described a large sample of teeth from the Pu3 Simpson Quarry in southcentral Montana, for which he named P. titusi, although we provisionally follow Silcox [21] in considering P. titusi a junior synonym of P. unio. The only other known purgatoriid genus, Ursolestes, is also from Simpson Quarry and includes but a single species, U. perpetior, that has dental dimensions more than twice as large as those of all species of Purgatorius [22]. Two other plesiadapiform species, both members of Pandemonium (Family incertae sedis), also occur in the Puercan: Pandemonium dis from the Pu3 Purgatory Hill locality of northeastern Montana [19] and Pandemonium hibernalis from the ?Pu2 Schowalter locality of southern Alberta [23]. In the light of the uncertainties about Purgatorius ceratops, the earliest purgatoriid (and plesiadapiform) known to date is P. coracis, erected on the basis of 29 isolated teeth and two fragmentary jaws from the Medicine Hat Brick and Tile Quarry, Rav W-1 horizon, from southwestern Saskatchewan [24]. That locality occurs in magnetochron C29R but preserves other mammals thought to be of Pu2-aspect. Another taxon, P. pinecreeensis, was reported from the Pine Cree Park locality near Rav W-1, but that locality is more questionably considered Pu2 in age [25].
Here, we report on the discovery of even earlier occurrences of Purgatorius. Five isolated teeth were recovered from the early Palaeocene Harley's Point locality in the lowermost part of the Tullock Member of the Fort Union Formation in northeastern Montana (figure 1). Two lower molars from this sample are referred to P. janisae, which is otherwise only known from stratigraphically higher deposits in the Tullock Member (i.e. Garbani Channel localities); another lower molar is morphologically distinct from all known purgatoriid species, leading us to erect a new taxon; and two upper molars are tentatively referred to that new taxon. During our comparative study, we encountered three dentigerous dentary fragments from the Pu3 Garbani Channel assemblage that also represent the new taxon. With new stratigraphic, biochronological, sedimentological and geochronological data from the Harley's Point locality and surrounding outcrop (see the electronic supplementary material), we constrain the age of the earliest purgatoriids to the early Puercan (Pu1), to magnetochron C29R, and to within 208 thousand years (kyr) after the K/Pg boundary (KPB), with a likelihood that their age could be as little as 105–139 kyr post-KPB. Note that Purgatorius coracis from southwestern Saskatchewan also occurs in C29R, but the associated Pu2-aspect mammalian fauna suggests that it is younger than the Pu1 specimens described herein. Thus, the occurrence of at least two species of Purgatorius at the Pu1 Harley's Point locality provides the strongest support to date that purgatoriids and, by extension, Pan-Primates, Euarchonta and Placentalia must have originated in the Late Cretaceous [24].
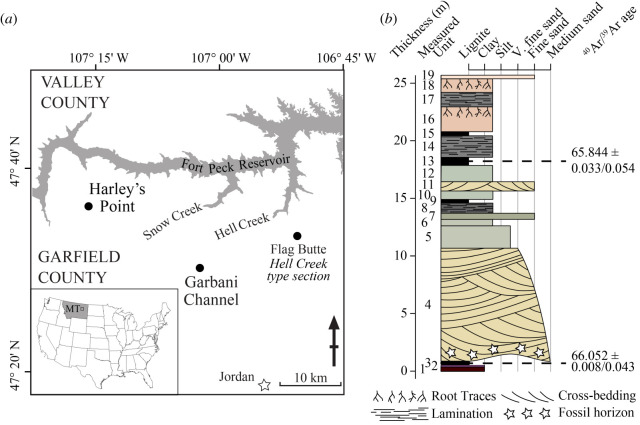
Figure 1.
(a) Location map showing the study area in northeastern Montana, USA. Fort Peck Reservoir is shown in grey. Circles show locations of localities discussed here including Harley's Point and Garbani Channel. The location of the Hell Creek type section, Flag Butte, is also shown. (b) Stratigraphy of a composite section compiled from the most proximal outcrops to Harley's Point locality (shown in electronic supplementary material, figure S1b). Ages shown were determined in [26], for details see the electronic supplementary material.
1.1. Institutional abbreviations and conventions
LACM, Los Angeles County Museum, Los Angeles, California, USA; UCMP, University of California Museum of Paleontology, Berkeley, California, USA; UWBM, University of Washington Burke Museum, Seattle, Washington, USA. We use standard convention in referring to lower dentition with lower-case letters (p, premolar and m, molar) and upper dentition with upper-case letters (P and M, respectively). The number following the letter designates tooth position in the series.
2. Results
2.1. Systematic palaeontology
The fossil specimens described herein are permanently stored in the collections of the UCMP. All digital models are available on MorphoSource (https://www.morphosource.org/).
Primates Linnaeus, 1758; Purgatoriidae Gunnell, 1989; Purgatorius Van Valen and Sloan, 1965.
Purgatorius janisae Van Valen, 1994
Referred specimens. UCMP 150018, right m1, and UCMP 192398, left m3 (figure 2a,b,e–l).
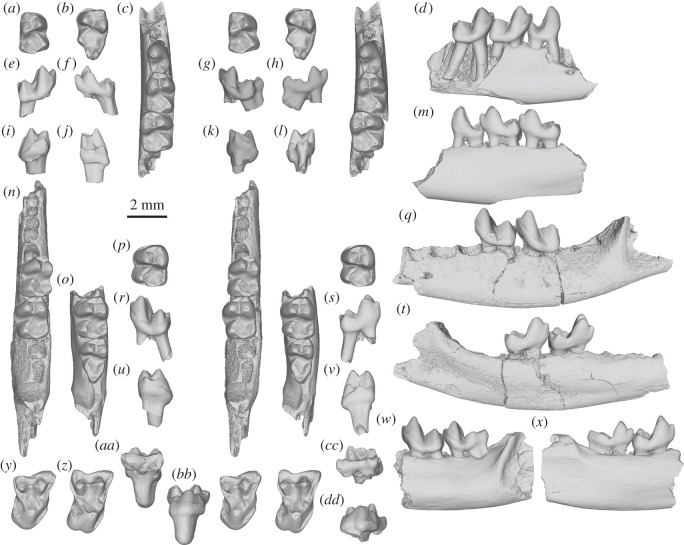
Figure 2.
Purgatorius from Harley's Point (Pu1) and Garbani Channel (Pu3) localities of northeastern Montana, USA. Images are three-dimensional surface renderings derived from µCT scans: P. janisae in stereo occlusal, buccal, lingual, mesial and distal views (a,e,f,i,j) UCMP 150018, right m1 and (b,g,h,k,l) UCMP 192398, left m3; P. mckeeveri sp. nov. in stereo occlusal, buccal and lingual views (c,d,m) UCMP 157977 (holotype), incomplete right dentary with p4–m2, (n,q,t) UCMP 111586, incomplete left dentary with m1–2, (o,w,x) UCMP 189505, incomplete left dentary with m2–3 and in stereo occlusal, buccal, lingual, mesial and distal views (p,r,s,u,v) UCMP 150021, left m2; and Purgatorius cf. P. mckeeveri in stereo occlusal, buccal and lingual views (y,aa,bb) UCMP 150019, right M2, and (z,cc,dd) UCMP 150020, right M2.
Horizon locality. The early Puercan (Pu1) ‘Harley's Point’ UCMP locality V77087 from the lowermost Palaeocene Tullock Member, Fort Union Formation, Garfield County, Montana, USA.
Description. See the electronic supplementary material.
Purgatorius mckeeveri sp. nov.
Etymology. ‘McKeever’ honours Frank McKeever, who was among the first residents of Garfield County to facilitate the fieldwork of Harley J. Garbani in 1965, and the family of John and Cathy McKeever, who have since supported our fieldwork at the Harley's Point locality, where the oldest specimen of this new taxon was recovered.
Holotype. UCMP 157977, right dentary fragment with p4–m2 (figure 2c,d,m).
Hypodigm. UCMP 111586, left dentary fragment with m1–2, UCMP 189505, left dentary fragment with m2–m3, and UCMP 150021, left m2 (figure 2n–x).
Horizon localities. The ‘Garbani Channel-NW Harley's High’ UCMP locality V73080 (holotype and UCMP 111586) and the ‘Garbani Channel-NW Main Quarry’ UCMP locality V73082 (UCMP 189505), both from late Puercan (Pu3), and the ‘Harley's Point’ UCMP locality V77087 (UCMP 150021) from early Puercan (Pu1), all from the lowermost Palaeocene Tullock Member, Fort Union Formation, Garfield County, Montana, USA.
Diagnosis. Differs from all other purgatoriids in having lower molars with more inflated cusps, rounded crests, and a paraconid that is higher on the trigonid and more appressed to the metaconid (especially on m2). Further differs from P. pinecreeensis and P. ceratops in having slightly larger lower molar dimensions. Further differs from P. janisae, P. unio and P. coracis in having a paraconid that is distinct from the paracristid and a shallower protocristid notch (on m1 and m2) with closer proximity of the metaconid and protoconid apices. Further differs from P. janisae, P. unio, P. ceratops and P. pinecreeensis in having a J-shaped (not L-shaped) paracristid on m2. Further differs from P. janisae and P. pinecreeensis in having a m3 that is narrower (particularly the talonid) relative to m2 with smaller talonid cusps and a shallower talonid basin. Further differs from P. unio and P. coracis in having lower molars with a larger paraconid and a more transversely oblique postvallid. Further differs from P. janisae in having a p4 with a lower protoconid and poorly developed talonid and without a paraconid, in having lower molars with a smaller, not mesially projecting paraconid, and a less transversely oblique postvallid, and in having a lower p4:m1 length ratio and a lower trigonid:talonid length ratio on m1 and m2. Further differs from P. coracis in having lower molars with a paraconid that is more lingual in position and a shallower talonid basin. Further differs from P. unio in lacking a p4 paraconid (variably present in P. unio), having a p4 talonid with a less distinct lingual cusp (entoconid) and having molars with smaller, less distinct talonid cusps and a higher trigonid:talonid length ratio on m1 and m2.
Description. See the electronic supplementary material.
Purgatorius cf. P. mckeeveri
Referred specimens. UCMP 150019, right M2, and UCMP 150020, right M2 (figure 2y–dd).
Horizon locality. The early Puercan (Pu1) ‘Harley's Point’ UCMP locality V77087 from the lowermost Palaeocene Tullock Member, Fort Union Formation, Garfield County, Montana, USA.
Description. See the electronic supplementary material.
2.2. Comparisons
On the basis of dental morphological comparisons with a broad range of latest Cretaceous (Lancian) and early Palaeocene (Puercan and Torrejonian) mammals, we conclude that these specimens are most similar to those of purgatoriid plesiadapiforms, specifically to species of Purgatorius.
The two lower molars (UCMP 150018 and UCMP 192398) from the Harley's Point locality that we refer to P. janisae are almost identical to those of the holotype (UCMP 107406 [18,19]). The incipient double hypoconulid on the m3 (UCMP 192398; figure 2b,l) is not developed on the holotype but occurs on some m3s in the Garbani Channel sample of P. janisae [18].
The new species, Purgatorius mckeeveri, from the Harley's Point and Garbani localities is distinguishable from Ursolestes perpetior notably in its smaller size but also in dental morphology (e.g. more developed p4 talonid basin [22]). It is most similar in size to P. janisae and P. unio (slightly larger than P. coracis, P. pinecreeensis and P. ceratops). It is morphologically distinct from all of those taxa in having lower molars with (i) a relatively taller molar trigonid (trigonid:talonid height ratio on m1 and m2) with (ii) more rounded crests, (iii) more inflated cusps, and (iv) a paraconid (especially on m2) that is higher on the trigonid and more appressed to the metaconid (no distinct valley separating them). Additional comparisons with each Purgatorius species can be found in the electronic supplementary material.
The Harley's Point upper molar specimens (UCMP 150019 and 150020) are morphologically distinct from those of all other purgatoriid species. They are notably smaller than the M2 of Ursolestes perpetior [22], larger than the M1 of Purgatorius pinecreeensis, and similar in size to the upper molars of all other purgatoriids. They are more transverse than M2s referred to P. janisae but not as transverse as those of P. unio, P. coracis, U. perpetior and the M1 of P. pinecreeensis. The protocone in both UCMP 150019 and 150020 is not as mesiodistally compressed as in P. unio and P. coracis and the M1 of P. pinecreeensis; and it is in a more lingual position on the crown compared with that of P. unio. The Harley's Point specimens differ from M2s of P. janisae in that the buccal margin of the crown is asymmetrical (i.e. divided into unequal lobes by the ectoflexus), the metacone is slightly more buccal on the crown, the protocone apex is slightly more buccal on the crown (slightly less so in UCMP 150020), and the protocone base is more lingually expanded (but not as much as in P. unio); in turn, the lingual part of the crown is more twisted [19,21] than in P. janisae (but less than so than in P. unio). UCMP 150020 has a faint furrow (or cleft) that extends ventrally from the lingual base of the protocone and fades away midway to the apex (figure 2z,dd). This furrow creates a slightly bilobed outline of the lingual margin in occlusal view that is unique among known purgatoriid species but reminiscent of the more pronounced bilobed condition of some palaechthonid and paromomyid plesiadapiforms, such as Paromomys farrandi [27]. Thus, despite a number of features shared with the upper molars of known purgatoriid species, these specimens are not attributable to any taxon that is known from upper molars. Because lower molars of P. mckeeveri occur at this locality, we tentatively assigned these specimens to the new taxon. The coronal dimensions and occlusal morphology of the upper molars are consistent with this hypothesis.
2.3. Taxonomic diversity of early Palaeocene plesiadapiforms versus archaic ungulates
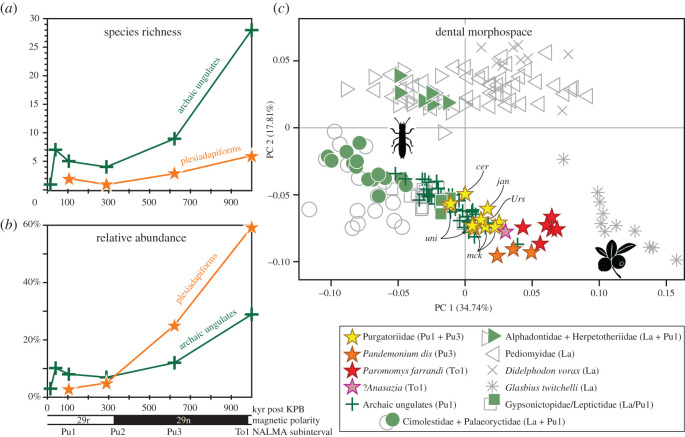
Plesiadapiforms are absent from the oldest local faunas of the Palaeocene of northeastern Montana (the Z-Line local fauna [28] and the Worm Coulee 1 local fauna [12,29], from ca 25 and 80 kyr post-KPB, respectively; figure 3a,b). Their absence is probably not an artefact of sampling, given that the Worm Coulee 1 local fauna is represented by greater than 900 specimens of many other small-bodied mammals [12]. Plesiadapiforms first appear in the Harley's Point local fauna and the slightly younger Coke's Clemmys local fauna (ca 250 to 328 kyr post-KPB [28]), represented by up to three Purgatorius spp. and less than 5% of all mammalian individuals (figure 3b). In the younger Garbani Channel local fauna (ca 311 to 934 kyr post-KPB, with a likely age between 584 and 691 kyr post-KPB, see electronic supplementary material), plesiadapiforms are represented by slightly more species [12,13,19] and a much greater relative abundance (25%). Although the relative abundance of purgatoriids is much lower in the Horsethief Canyon (ca 855 to 1.148 Myr post-KPB [32]) and Farrand local faunas (ca 934 kyr to 1.01 Myr post-KPB), the paromomyid plesiadapiform Paromomys farrandi makes up approximately 56% of all individuals [27,30]. In comparison, archaic ungulates occur earlier and are represented by many more species (seven species in the Worm Coulee 1 local fauna and 28 species in the Horsethief Canyon and Farrand local faunas) but have fairly low relative abundance (less than or equal to 12%) until ca 1 Myr post-KPB (29%).
Figure 3.
Taxonomic diversity and dental morphospace of early Palaeocene plesiadapiforms versus archaic ungulates. (a) Species richness and (b) relative abundances of plesiadapiforms (stars) and archaic ungulates (plus signs) in mammalian local faunas from northeastern Montana, USA. Data are from [12,28,30] and are provided in the electronic supplementary material. (c) PC1 versus PC2 plot of two-dimensional geometric morphometric analysis of lower molars illustrating dental morphospace occupancy of Lancian and early Puercan (Pu1) therians from northeastern Montana with additional plesiadapiforms from later in the Puercan (Pu3) and the early Torrejonian (To1) NALMA subintervals. Purgatorius ceratops (cer) = ?Pu1; P. janisae (jan) and P. mckeeveri (mck) = Pu1 and Pu3; and P. unio (uni) and Ursolestes perpetior (Urs) = Pu3. Data are from [31] and this study. The specimen list is provided in the electronic supplementary material.
2.4. Dental morphospace occupation of early Palaeocene plesiadapiforms
In the two-dimensional geometric morphometric (2DGM) analysis of Lancian and early Puercan therians from northeastern Montana, archaic ungulates (plus signs) and purgatoriids (yellow stars) occupy a similar region of the morphospace, as represented by the plot of PC1 versus PC2 (figure 3c). That region reflects lower molars that are relatively inflated and have broad talonid basins—morphological adaptations toward omnivory and frugivory [33]. Plesiadapiforms from later in the Puercan and earliest Torrejonian (yellow, orange, pink and red stars) plot farther toward the lower-right corner of the dental morphospace, away from the early Puercan archaic ungulates and toward the region previously occupied by the latest Cretaceous frugivorous stem marsupial Glasbius [31,34,35].
3. Discussion
3.1. Evolutionary implications
New fossils from the Harley's Point locality document two purgatoriid taxa from the early Puercan (Pu1) of northeastern Montana, USA: Purgatorius janisae, previously only known from the late Puercan (Pu3) Garbani Channel localities [16], and the new taxon, P. mckeeveri, which we also document from the Pu3 Garbani Channel localities. These new occurrences of Purgatorius at the Harley's Point locality represent the oldest confirmed record of Pan-Primates and Euarchonta known. Their co-occurrence with Protungulatum donnae, Mimatuta minuial and Thylacodon montanensis at the Harley's Point locality [36] supports a Pu1 age. The associated, high-resolution stratigraphic and geochronological data further constrain the age of the locality to within 208 kyr post-KPB (66.052–65.844 Ma), with a likelihood that the age could be as early as 105–139 kyr post-KPB (65.946–65.912 Ma). Harley's Point locality is possibly on the younger end of this range, given that Purgatorius does not occur at the Pu1 Z-Line localities [28] or the well-sampled Pu1 Worm Coulee 1 locality [12,29] from ca 25 and 80 kyr post-KPB, respectively. The slightly younger age for Harley's Point is also consistent with the substantial thickness of its associated channel sequence (approx. 17 m) and the occurrence of a younger-aspect archaic ungulate (?periptychid, LACM 112903 [29]) from the same channel sequence (nearby McKeever Ranch 1 locality) that is unknown from those other well-sampled Pu1 localities. With few exceptions (see the electronic supplementary material), all other known occurrences of plesiadapiforms are from Pu3 (ca 65.6 Ma [26]) or younger [2].
A Cretaceous origin of Pan-Primates has long been hypothesized by palaeontologists, in part based on the initial description of Purgatorius, including P. ceratops, which until more recently [14,15] was considered latest Cretaceous in age [13]. A consensus later emerged that despite the lack of unambiguous records of Cretaceous plesiadapiforms, a pre-KPB origin of Pan-Primates was still within the realm of possibility (e.g. [2,16]). Fox & Scott [24] recently speculated that the early (Pu2) occurrence and derived characteristics of Purgatorius coracis imply that the ancestral purgatoriid was from the Late Cretaceous. Fossils reported here (i) derive from an older (Pu1) locality, less than 208 kyr and most likely to within 105–139 kyr post-KPB, and (ii) represent two sympatric species of Purgatorius, each with a uniquely accumulated suite of dental specializations that evolved following divergence from a common ancestor. These data provide even stronger evidence that the origin of plesiadapiforms, and in turn Pan-Primates, Euarchonta and Placentalia extends back into the Late Cretaceous.
As is the case for many other mammalian clades, there is considerable discrepancy between the fossil record and molecular-clock estimates for the timing of the origin of Primates, Euarchonta and Placentalia [37,38]. Fossil evidence is generally in accord with Placentalia and stem members of some placental orders having originated either just before or after the KPB (Soft and Hard Explosive Models, respectively) and the placental ordinal crown groups having originated and diversified in the early Cenozoic; molecular-clock studies, in contrast, support the origin of Placentalia and placental ordinal stem groups earlier in the Cretaceous and the origination and diversification of placental ordinal crown groups either shortly thereafter (Short-Fuse Model) or after the KPB (Long-Fuse Model) [39,40]. The co-occurrence of two species of Purgatorius documented here at ca 66 Ma establishes a slightly older divergence date for Pan-Primates (with Pan-Primates splitting either from Dermoptera [assuming Primatomorpha is valid] or Sundatheria [Dermoptera + Scandentia]) and for Euarchonta (with Euarchonta splitting from Glires) than previously documented in the fossil record. Minimum fossil calibrations used in recent analyses have been younger (e.g. 65 Ma for Purgatorius representing Euarchonta [41]; 64.85 Ma for Purgatorius coracis representing Euarchonta and 64.85 Ma for Protungulatum donnae representing Placentalia [37]) and have resulted in Pan-Primates diversifying after or just before the KPB. Although a difference of 1 Myr for a minimum fossil calibration is not necessarily regarded as significant in the context of deep time, two species of Purgatorius at 66 Ma might be an especially important observation given their close proximity to the KPB. The oldest date for Pan-Primates and/or Euarchonta established here provides additional support for a Cretaceous origin or a more explosive evolution of Pan-Primates in the earliest Palaeocene.
3.2. Palaeoecological implications
The new fossils reported here also shed light on the palaeoecology of early plesiadapiforms and, by extension, Pan-Primates and Euarchonta in relation to the post-KPB biotic recovery and the evolutionary radiation of other placental mammals. In northeastern Montana, which we use as a model for the post-KPB biotic recovery (but see also [42]), plesiadapiforms were not part of the earliest ‘disaster’ or ‘survival’ phase [12,28]. They first appear, as immigrants into the area [43], in the Harley's Point local fauna, which we estimate is slightly younger than the ‘disaster faunas’ (i.e. ‘recovery’ phase) but still within ca 208 kyr post-KPB (figure 3a, stars). We speculate that the delayed arrival and initially low relative abundance of plesiadapiforms (figure 3b, stars) might have been tied to their arboreal ecology [4,44,45] and the temporary loss of arboreal habitats across the KPB in the region [46]. By the ‘fully recovered’ phase (ca 328 to 847 kyr post-KPB), plesiadapiforms were represented by at least three Purgatorius spp. and Pandemonium dis [12,13,19] and were numerically abundant (figure 3b [12]). Although purgatoriids declined in relative abundance thereafter (ca 934 kyr to 1.01 Myr post-KPB), the paromomyid Paromomys farrandi made up more than half of all individuals in the local faunas of northeastern Montana [27,30].
An illuminating contrast is the corresponding pattern for archaic ungulates (figure 3a,b, plus signs), a group considered central to the post-KPB recovery and placental radiation [12,47,48]. They too arrived in northeastern Montana as immigrants but prior to plesiadapiforms, in the earliest ‘disaster’ phase of the post-KPB recovery (see [49,50] for possible older occurrences elsewhere). Despite their greater taxonomic richness, archaic ungulates were surpassed in numerical abundance by plesiadapiforms during the ‘fully recovered’ phase (25% versus 12%) and that gap widened markedly by 1 Myr post-KPB (59% versus 29%). Although these two taxonomic groups had distinct patterns of species richness and relative abundance, together they dominated the initial phase of the early Palaeocene placental radiation [12].
The diversification of these two groups, which continued through most of the Palaeocene, was characterized by a trend toward omnivory and herbivory [51]. Results of our 2DGM analysis of lower molars show the initiation of this dietary trend (figure 3c). Overlap in dental morphospace between archaic ungulates and plesiadapiforms (especially purgatoriids) should not necessarily be interpreted as direct competition for food resources between these early placental groups. Although the early Palaeocene fossil record of mammalian postcrania is sparse, the tarsals attributed to earliest Palaeocene archaic ungulates (e.g. cf. Protungulatum) differ from those of plesiadapiforms and other euarchontan mammals in lacking features related to arboreality [44,52]. Therefore, the immigration of Purgatorius probably represented the introduction of a unique arboreal mammal that had direct access to angiosperm products and associated insect pollinators that were probably not as readily available to contemporary terrestrial mammals including archaic ungulates [44].
The origin of Pan-Primates has long been thought to relate in part to a shift from a more insectivorous diet toward a more herbivorous diet [1,53,54]. This hypothesis is based in part on the observation that the earliest plesiadapiforms, such as Purgatorius, had lower crowned molars, rounder cusps, and broader talonid basins than those of many Late Cretaceous and contemporaneous early Palaeocene small-bodied mammals. Although many plesiadapiforms appear to have been at least partly specialized for increased consumption of non-leafy plant resources (e.g. fruit), it seems likely that all Purgatorius species also relied on insects to varying extents given their small body size and aspects of their molar morphology. Most previous studies on the dietary features of purgatoriid teeth have been qualitative, but a recent study [55] used dental topographic analyses to assess the diet of paromomyid plesiadapiforms and included casts of two teeth of Purgatorius janisae (p4, m2) and one tooth of P. coracis (m2) for comparative purposes. Their results suggested that P. janisae was an insectivore and P. coracis was a less strict insectivore (i.e. insectivore–omnivore), whereas the paromomyid Paromomys farrandi was an omnivore–frugivore [55].
Our 2DGM results are consistent with previous studies (e.g. [53,55]); purgatoriid species from northeastern Montana occupy a more insectivorous–omnivorous morphospace than larger (e.g. Pandemonium) and more specialized (e.g. lower crowned, more bunodont molars of Paromomys) plesiadapiforms that occupy more herbivorous morphospace. The two oldest known species of Purgatorius (this paper) evolved distinct dental specializations to capitalize on a mixed diet of insects and plant products in different ways. Purgatorius janisae has molars with shorter trigonids, pointed cusps and sharper crests, whereas P. mckeeveri has molars with relatively taller trigonids with more inflated cusps and rounded crests. We posit that such dental features for omnivory coupled with postcranial specializations for arboreality led to the rapid evolutionary success of the plesiadapiforms following the K/Pg mass extinction.
Supplementary Material
Acknowledgements
We acknowledge that the fossils in this paper were collected on lands that are the traditional territory of the Fort Belknap Assiniboine & Gros Ventre Tribes and Fort Peck Assiniboine & Sioux Tribes. Future field trips will be respectful to the original peoples and sovereignty. We thank the McKeever and Engdahl families for land access and support, H.J. Garbani for the site discovery, volunteers from the UCMP, UW Biology, Burke Museum, and UNM Honours College for field and laboratory work, Drs P. Holroyd, D. Boyer and M. Silcox for access to specimens, casts and µCT scans of comparative material, H. Fulghum and J. Crowell for µCT processing, post-processing and help with specimen plates, M. Holland and S. Olroyd for 3D printing of specimens, and Drs L. DeBey, M. dos Reis, D. Grossnickle, S. Smith, T. Tobin, G. Weissmann, J. Banaszak, A. Brannick, J. Claytor, J. Crowell, H. Fulghum, D. McDonald, A. Tholt, L. Weaver, P. Wilson and two anonymous reviewers for useful input. We dedicate this work to the memory of our co-author William A. Clemens, who passed away while this paper was in revision. We gratefully acknowledge Bill's immense contributions to study of the Hell Creek area and early Palaeocene mammals, his thoughtful mentorship, and his loyal friendship over many decades.
Contributor Information
Gregory P. Wilson Mantilla, Email: gpwilson@uw.edu.
Stephen G. B. Chester, Email: stephenchester@brooklyn.cuny.edu.
Ethics
Fieldwork to collect fossil and geological data were done with permissions of the US Bureau of Land Management and oral permission of private landowners (Bob and Jane Engdahl and John and Cathy McKeever). The BLM Paleontological Resources Use Permit is MTM 110439. The contact information of landowners can be provided upon request. We have also obtained permission from the University of California Museum of Paleontology to study, image (photo, CT), and publish on fossils and casts in their collections. We have received a letter from the Director of the UCMP Charles Marshall attesting to this agreement.
Data accessibility
Associated data are available in the electronic supplementary material. All µCT tiff stacks are available via Morphosource (www.morphosource.org).
Authors' contributions
G.P.W.M., S.G.B.C. and W.A.C. designed the research; G.P.W.M., W.A.C., J.R.M., C.J.S., B.T.H., W.W.M. and P.R.R. performed field research; all authors analysed data and interpreted results; G.P.W.M. and S.G.B.C. wrote the main text; G.P.W.M., S.G.B.C., J.R.M., C.J.S. and W.W.M. wrote the electronic supplementary material; and all authors critically revised the manuscript.
Competing interests
We declare we have no competing interests.
Funding
The discovery and initial collecting at the various localities were largely funded by the UCMP and the NSF. Further research was supported by the Hell Creek Project from the Myhrvold and Havranek Charitable Family Fund, UW Biology, and Burke Museum (G.P.W.M.), a Leakey Foundation General Research Grant, a PSC CUNY Award, jointly funded by The Professional Staff Congress and The City University of New York (S.G.B.C.), a NSF Graduate Research Fellowship (C.J.S.) and UW Earth and Space Sciences (B.T.H.).
References
- 1.Szalay FS. 1968. The beginnings of Primates. Evolution 22, 19-36. ( 10.1111/j.1558-5646.1968.tb03445.x) [DOI] [PubMed] [Google Scholar]
- 2.Silcox MT, Bloch JI, Boyer DM, Chester SGB, López-Torres S. 2017. The evolutionary radiation of plesiadapiforms. Evol. Anthropol. 26, 39-98. ( 10.1002/evan.21526) [DOI] [PubMed] [Google Scholar]
- 3.Bloch JI, Silcox MT, Boyer DM, Sargis EJ. 2007. New Paleocene skeletons and the relationship of plesiadapiforms to crown-clade primates. Proc. Natl Acad. Sci. USA 104, 1159-1164. ( 10.1073/pnas.0610579104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chester SGB, Williamson TE, Bloch JI, Silcox MT, Sargis EJ. 2017. Oldest skeleton of a plesiadapiform provides additional evidence for an exclusively arboreal radiation of stem primates in the Palaeocene. R. Soc. Open Sci. 4, 170329. ( 10.1098/rsos.170329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ni X, Li Q, Li L, Beard KC. 2016. Oligocene primates from China reveal divergence between African and Asian primate evolution. Science 352, 673-677. ( 10.1126/science.aaf2107) [DOI] [PubMed] [Google Scholar]
- 6.Chester SGB, Sargis EJ. 2020. Pan-Primates. In Phylonyms: A companion to the PhyloCode (eds de Queiroz K, Cantino PD, Gauthier JA), pp. 903-906. Boca Raton, FL: CRC Press. [Google Scholar]
- 7.Gunnell GF, Yoder AD. 2020. Primates C. Linnaeus 1758. In Phylonyms: A companion to the PhyloCode (eds de Queiroz K, Cantino PD, Gauthier J), pp. 907-914. Boca Raton, FL: CRC Press. [Google Scholar]
- 8.Savage DE, Russell DE, Waters BT. 1977. Critique of certain Eocene primate taxa. Géobios 10, 159-164. ( 10.1016/S0016-6995(77)80015-6) [DOI] [Google Scholar]
- 9.Gunnell GF. 1989. Evolutionary history of Microsyopoidea (Mammalia, ?Primates) and the relationship between Plesiadapiformes and Primates. Univ. Michigan Pap. Paleontol. 27, 1-157. [Google Scholar]
- 10.Rose KD. 1995. The earliest primates. Evol. Anthropol.: Issues News Rev. 3, 159-173. ( 10.1002/evan.1360030505) [DOI] [Google Scholar]
- 11.Clemens WA. 2002. Evolution of the mammalian fauna across the Cretaceous-Tertiary boundary in northeastern Montana and other areas of the Western Interior. In The Hell Creek Formation and the Cretaceous-Tertiary boundary in the northern Great Plains: an integrated continental record of the end of the Cretaceous (eds Hartman JH, Johnson KR, Nichols DJ), pp. 217-245. Geological Society of America Special Paper 361. [Google Scholar]
- 12.Wilson GP. 2014. Mammalian extinction, survival, and recovery dynamics across the Cretaceous-Paleogene boundary in northeastern Montana, USA. In Through the end of the Cretaceous in the type locality of the Hell Creek Formation in Montana and adjacent areas (eds Wilson GP, Clemens WA, Horner JR, Hartman JH), pp. 365-392. Geological Society of America Special Paper 503. [Google Scholar]
- 13.Van Valen L, Sloan RE. 1965. The earliest primates. Science 150, 743-745. ( 10.1126/science.150.3697.743) [DOI] [PubMed] [Google Scholar]
- 14.Lofgren DL. 1995. The Bug Creek problem and the Cretaceous-Tertiary transition at McGuire Creek, Montana. Univ. Calif. Publ. Geol. Sci. 140, 1-185. [Google Scholar]
- 15.Smit J, Van Der Kaars S. 1984. Terminal Cretaceous extinctions in the Hell Creek area, Montana: compatible with catastrophic extinction. Science 223, 1177-1179. ( 10.1126/science.223.4641.1177) [DOI] [PubMed] [Google Scholar]
- 16.Clemens WA. 2004. Purgatorius (Plesiadapiformes, Primates?, Mammalia), a Paleocene immigrant into northeastern Montana: Stratigraphic occurrences and incisor proportions. Bull. Carnegie Mus. Nat. Hist. 36, 3-13. ( 10.2992/0145-9058(2004)36[3:PPPMAP]2.0.CO;2) [DOI] [Google Scholar]
- 17.Silcox MT. 2017. Purgatorius. In The international encyclopedia of primatology (ed. Fuentes A), pp. 1-2. New York, NY: John Wiley & Sons. [Google Scholar]
- 18.Clemens WA. 1974. Purgatorius, an early paromomyid primate. Science 184, 903-905. ( 10.1126/science.184.4139.903) [DOI] [PubMed] [Google Scholar]
- 19.Van Valen LM. 1994. The origin of the plesiadapid primates and the nature of Purgatorius. Evol. Monogr. 15, 1-79. [Google Scholar]
- 20.Buckley GA. 1997. A new species of Purgatorius (Mammalia; Primatomorpha) from the lower Paleocene Bear Formation, Crazy Mountains Basin, south-central Montana. J. Paleontol. 71, 149-155. ( 10.1017/S0022336000039032) [DOI] [Google Scholar]
- 21.Silcox MT. 2001. A phylogenetic analysis of the plesiadapiformes and their relationship to euprimates and other archontans. PhD dissertation, Johns Hopkins University, Baltimore, MD. [Google Scholar]
- 22.Fox RC, Scott CS, Buckley GA. 2015. A ‘giant' purgatoriid (Plesiadapiformes) from the Paleocene of Montana, USA: mosaic evolution in the earliest primates. Palaeontology 58, 277-291. ( 10.1111/pala.12141) [DOI] [Google Scholar]
- 23.Fox RC, Rankin BD, Scott CS, Sweet AR. 2014. Second known occurrence of the early Paleocene plesiadapiform Pandemonium (Mammalia: Primates), with description of a new species. Can. J. Earth Sci. 51, 1059-1066. ( 10.1139/cjes-2014-0113) [DOI] [Google Scholar]
- 24.Fox RC, Scott CS. 2011. A new, early Puercan (earliest Paleocene) species of Purgatorius (Plesiadapiformes, Primates) from Saskatchewan, Canada. J. Paleontol. 85, 537-548. ( 10.1666/10-059.1) [DOI] [Google Scholar]
- 25.Scott CS, Fox RC, Redman CM. 2016. A new species of the basal plesiadapiform Purgatorius (Mammalia, Primates) from the early Paleocene Ravenscrag Formation, Cypress Hills, southwest Saskatchewan, Canada: further taxonomic and dietary diversity in the earliest primates. Can. J. Earth Sci. 53, 343-354. ( 10.1139/cjes-2015-0238) [DOI] [Google Scholar]
- 26.Sprain CJ, Renne PR, Clemens WA, Wilson GP. 2018. Calibration of chron C29r: New high-precision geochronologic and paleomagnetic constraints from the Hell Creek region, Montana. Geol. Soc. Am. Bull. 130, 1615-1644. ( 10.1130/B31890.1) [DOI] [Google Scholar]
- 27.Clemens WA, Wilson GP. 2009. Early Torrejonian mammalian local faunas from northeastern Montana, U.S.A. Mus. Northern Arizona Bull. 65, 111-158. [Google Scholar]
- 28.Smith SM, Sprain CJ, Clemens WA, Lofgren DL, Renne PR, Wilson GP. 2018. Early mammalian recovery after the end-Cretaceous mass extinction: a high-resolution view from McGuire Creek area, Montana, USA. Geol. Soc. Am. Bull. 130, 2000-2014. ( 10.1130/B31692.1) [DOI] [Google Scholar]
- 29.Archibald JD. 1982. A study of Mammalia and geology across the Cretaceous-Tertiary boundary in Garfield County, Montana. Univ. Calif. Publ. Geol. Sci. 122, 1-286. [Google Scholar]
- 30.Hovatter BT, Wilson GP. 2015. Faunal analysis of earliest Torrejonian (To1) mammals from northeastern Montana, U.S.A. In Journal of Vertebrate Paleontology, Programs and Abstracts, 75th Annual Meeting of the Society of Vertebrate Paleontology, Dallas, TX, 14–17 October. [Google Scholar]
- 31.Wilson GP. 2013. Mammals across the K/Pg boundary in northeastern Montana, U.S.A.: dental morphology and body-size patterns reveal extinction selectivity and immigrant-fueled ecospace filling. Paleobiology 39, 429-469. ( 10.5061/dryad.gv06d) [DOI] [Google Scholar]
- 32.Sprain CJ, Renne PR, Wilson GP, Clemens WA. 2015. High-resolution chronostratigraphy of the terrestrial Cretaceous-Paleogene transition and recovery interval in the Hell Creek region, Montana. Geol. Soc. Am. Bull. 127, 393-409. ( 10.1130/B31076.1) [DOI] [Google Scholar]
- 33.Kay RF. 1975. The functional adaptations of primate molar teeth. Am. J. Phys. Anthropol. 43, 195-216. ( 10.1002/ajpa.1330430207) [DOI] [PubMed] [Google Scholar]
- 34.Clemens WA. 1966. Fossil mammals of the type Lance Formation, Wyoming: Part II. Marsupialia. Univ. Calif. Publ. Geol. Sci. 62, 1-122. [Google Scholar]
- 35.Gordon CL. 2003. Functional morphology and diet of Late Cretaceous mammals of North America. PhD dissertation, University of Oklahoma, Norman, OK. [Google Scholar]
- 36.Lofgren DL, Lillegraven JA, Clemens WA, Gingerich PD, Williamson TE. 2004. Paleocene biochronology: the Puercan through Clarkforkian land mammal ages. In Late Cretaceous and Cenozoic mammals of North America: biostratigraphy and geochronology (ed. Woodburne MO), pp. 43-105. New York, NY: Columbia University Press. [Google Scholar]
- 37.O'Leary MA, et al. 2013. The placental mammal ancestor and the post–K-Pg radiation of placentals. Science 339, 662-667. ( 10.1126/science.1229237) [DOI] [PubMed] [Google Scholar]
- 38.dos Reis M, Donoghue PC, Yang Z. 2014. Neither phylogenomic nor palaeontological data support a Palaeogene origin of placental mammals. Biol. Lett. 10, 20131003. ( 10.1098/rsbl.2013.1003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Archibald JD, Deutschman DH. 2001. Quantitative analysis of the timing of the origin and diversification of extant placental orders. J. Mamm. Evol. 8, 107-124. ( 10.1023/A:1011317930838) [DOI] [Google Scholar]
- 40.Grossnickle DM, Smith SM, Wilson GP. 2019. Untangling the multiple ecological radiations of early mammals. Trends Ecol. Evol. 34, 936-949. ( 10.1016/j.tree.2019.05.008) [DOI] [PubMed] [Google Scholar]
- 41.dos Reis M, Gunnell GF, Barba-Montoya J, Wilkins A, Yang Z, Yoder AD. 2018. Using phylogenomic data to explore the effects of relaxed clocks and calibration strategies on divergence time estimation: primates as a test case. Syst. Biol. 67, 594-615. ( 10.1093/sysbio/syy001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lyson TR, et al. 2019. Exceptional continental record of biotic recovery after the Cretaceous–Paleogene mass extinction. Science 366, 977-983. ( 10.1126/science.aay2268) [DOI] [PubMed] [Google Scholar]
- 43.Clemens WA. 2010. Were immigrants a significant part of the earliest Paleocene mammalian fauna of the North American Western Interior? Vertebrata PalAsiatica 48, 285-307. [Google Scholar]
- 44.Chester SGB, Bloch JI, Boyer DM, Clemens WA. 2015. Oldest known euarchontan tarsals and affinities of Paleocene Purgatorius to Primates. Proc. Natl Acad. Sci. USA 112, 1487-1492. ( 10.1073/pnas.1421707112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeBey LB, Wilson GP. 2017. Mammalian distal humerus fossils from eastern Montana, USA with implications for the Cretaceous-Paleogene mass extinction and the adaptive radiation of placentals. Palaeontol. Electron. 20, 1-92. ( 10.26879/694) [DOI] [Google Scholar]
- 46.Field DJ, Bercovici A, Berv JS, Dunn R, Fastovsky DE, Lyson TR, Vajda V, Gauthier JA. 2018. Early evolution of modern birds structured by global forest collapse at the end-Cretaceous mass extinction. Current Biology 28, 1825-1831. e1822. ( 10.1016/j.cub.2018.04.062) [DOI] [PubMed] [Google Scholar]
- 47.Archibald JD. 1983. Structure of the KT mammal radiation in North America: speculations on turnover rates and trophic structure. Acta Palaeontol. Pol. 28, 7-17. [Google Scholar]
- 48.Sloan RE, Rigby JK Jr, Van Valen L, Gabriel D. 1986. Gradual dinosaur extinction and simultaneous ungulate radiation in the Hell Creek Formation. Science 232, 629-633. ( 10.1126/science.232.4750.629) [DOI] [PubMed] [Google Scholar]
- 49.Kelly TS. 2014. Preliminary report on the mammals from Lane's Little Jaw Site Quarry: a latest Cretaceous (earliest Puercan?) local fauna, Hell Creek Formation, southeastern Montana. Paludicola 10, 50-91. [Google Scholar]
- 50.Archibald JD, Zhang Y, Harper T, Cifelli RL. 2011. Protungulatum, confirmed Cretaceous occurrence of an otherwise eutherian (placental?) mammal. J. Mamm. Evol. 18, 153-161. ( 10.1007/s10914-011-9162-1) [DOI] [Google Scholar]
- 51.Maas MC, Krause DW. 1994. Mammalian turnover and community structure in the Paleocene of North America. Hist. Biol. 8, 91-128. ( 10.1080/10292389409380473) [DOI] [Google Scholar]
- 52.Szalay FS, Decker RL. 1974. Origins, evolution, and function of the tarsus in Late Cretaceous Eutheria and Paleocene primates. In Primate locomotion (ed. Jenkins FA), pp. 223-259. Cambridge, UK: Academic Press. [Google Scholar]
- 53.Kay RF, Cartmill M. 1977. Cranial morphology and adaptations of Palaechthon nacimienti and other Paromomyidae (Plesiadapoidea, ?Primates), with a description of a new genus and species. J. Hum. Evol. 6, 19-53. ( 10.1016/S0047-2484(77)80040-7) [DOI] [Google Scholar]
- 54.Sussman RW, Raven PH. 1978. Pollination by lemurs and marsupials: an archaic coevolutionary system. Science 200, 731-736. ( 10.1126/science.200.4343.731) [DOI] [PubMed] [Google Scholar]
- 55.López-Torres S, Selig KR, Prufrock KA, Lin D, Silcox MT. 2018. Dental topographic analysis of paromomyid (Plesiadapiformes, Primates) cheek teeth: more than 15 million years of changing surfaces and shifting ecologies. Hist. Biol. 30, 76-88. ( 10.1080/08912963.2017.1289378) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Associated data are available in the electronic supplementary material. All µCT tiff stacks are available via Morphosource (www.morphosource.org).