Abstract
Climate change (CC) is predicted to increase the risk of aflatoxin (AF) contamination in maize, as highlighted by a project supported by EFSA in 2009. We performed a comprehensive literature search using the Scopus search engine to extract peer-reviewed studies citing this study. A total of 224 papers were identified after step I filtering (187 + 37), while step II filtering identified 25 of these papers for quantitative analysis. The unselected papers (199) were categorized as “actions” because they provided a sounding board for the expected impact of CC on AFB1 contamination, without adding new data on the topic. The remaining papers were considered as “reactions” of the scientific community because they went a step further in their data and ideas. Interesting statements taken from the “reactions” could be summarized with the following keywords: Chain and multi-actor approach, intersectoral and multidisciplinary, resilience, human and animal health, and global vision. In addition, fields meriting increased research efforts were summarized as the improvement of predictive modeling; extension to different crops and geographic areas; and the impact of CC on fungi and mycotoxin co-occurrence, both in crops and their value chains, up to consumers.
Keywords: Aspergillus flavus, mycotoxin, crop modeling, predictive model, co-occurrence, food, feed, risk assessment, safety
1. Conceptual Framework
The mycotoxins of greatest concern to food and feed safety are produced by members of a few genera of filamentous fungi, with Aspergillus, Fusarium and Penicillium playing a key role. These fungi colonize many crops and are adapted to a wide range of environmental conditions, having different but partially overlapping ecological niches [1]. A key point of interest in relation to maize are the aflatoxin (AF) producers Aspergillus flavus and A. parasiticus, Fusarium verticillioides and F. proliferatum, known for fumonisin (FB) production, and F. graminearum, able to biosynthesize both trichothecenes, such as deoxynivalenol (DON), and zearalenones (ZEN) [2,3]. Among staple crops, maize is of concern for mycotoxin contamination; mycotoxins are regulated in Europe and in several other countries worldwide, and several co-occurring fungal organisms are often detected [4].
Knowledge of environmental factors affecting fungal survival, growth, metabolic activity and interaction with other organisms, including host plants, is essential for understanding their dynamics and the resulting toxin contamination [5]. The environment provides all the leading factors for mycotoxin prevalence. In particular, high temperatures and drought stress directly affect maize and the occurrence of A. flavus, favoring fungal growth, conidiation and spore dispersal, and impairing the growth and development of maize [6]. FB-producing fungi can be found wherever maize is grown, but their occurrence varies geographically. FB occurrence is typically higher in maize-growing areas at low latitudes and elevations, where conditions are relatively warmer compared with those of high-latitude or high-altitude maize-growing regions where [7,8,9], on the contrary, DON is commonly dominant [2].
Climate change (CC) is predicted to have a significant impact on the security of staple commodities. Based on available data, atmospheric concentrations of CO2 are expected to double or triple (from 350–400 to 800–1200 ppb) in the next 25–50 years. Therefore, different regions of Europe is expected to face increases in temperature of 2–5 °C coupled with elevated CO2 (800–1200 ppm) and drought episodes, with concomitant effects on pests and diseases and ultimately crop yield [10,11,12], as well as mycotoxins. Until a few years ago, AFs had not been identified as a matter of concern for primary production in Europe. However, the year 2003 saw the first alarming contamination of maize in Italy [13]. AFs are potent carcinogens existing as four primary structural analogues: AFB1, AFB2, AFG1 and AFG2. The International Agency for Research on Cancer (IARC) has classified AFB1 as a Group 1A carcinogen, i.e., carcinogenic to humans [14]. In addition to hepatocellular carcinoma, AFs are associated with occasional outbreaks of acute aflatoxicoses, leading to death shortly after exposure [15].
The European Food Safety Authority (EFSA), with a mandate to identify emerging risks in the food and feed sectors, has identified changing patterns in mycotoxin production in cereals due to CC as a potential matter of concern. Therefore, in 2009, the EFSA’s Emerging Risks Unit delivered a call for scientific information (CFP/EFSA/EMRISK/2009/01), based on models and scenarios, to predict the possible increase of AFs in cereals in the EU due to CC. Two CC scenarios, +2 °C and +5 °C above pre-industrial levels, which consider whether or not mitigation strategies for CC are applied, in addition to the present (baseline) scenario were considered in the funded project, MODMAP-AFLA. These scenarios provided the data input for AFLA-maize [16], a mechanistic model, able to predict AF contamination risk using weather data as input. The project’s output predicted an increased risk of AF contamination in maize in the future [17,18]. Findings also suggested that CC effects will be (a) regional; and (b) detrimental or advantageous depending on geographical region and the CC scenario considered [18]. In northern Europe, the effects may be positive, with the enlargement of maize growing area without or with minimal AF risk. Conversely, the Mediterranean basin is expected to be a hot spot of many adverse effects, with extreme changes in rainfall/drought, elevated temperatures and CO2 impacting food production and AF contamination in maize.
In this study, we identified the actions and reactions of the scientific community based on the results of the MODMAP-AFLA project [17,18].
1.1. Dataset Creation: Scientific Paper Search, Filtering, and Selection
A comprehensive literature search was performed using the Scopus search engine to extract peer-reviewed studies that were published until the end of 2020 (Scopus last access 28 March 2021). The citations included, either (a) the EFSA report: Modelling, predicting and mapping the emergence of aflatoxins in cereals in the EU due to climate change [17]; or (b) the accompanying manuscript: AFB1 contamination in maize in Europe increases due to CC [18].
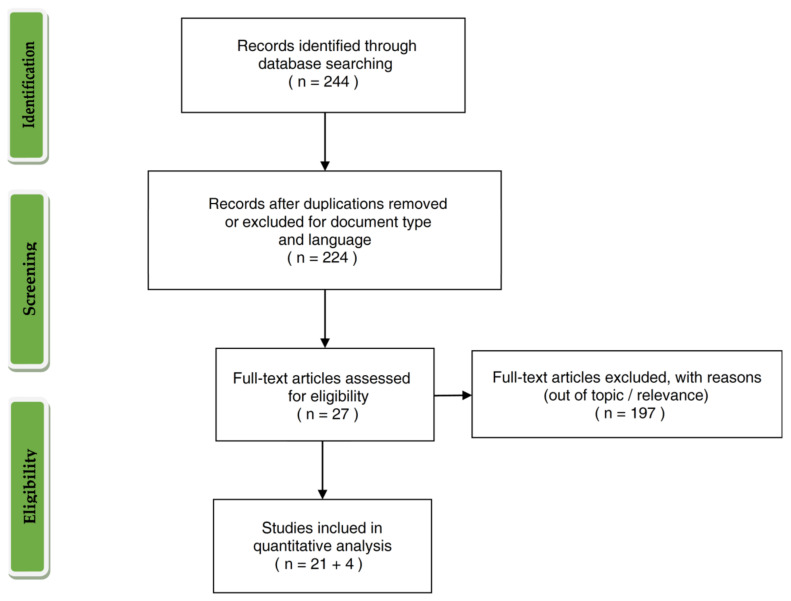
Two-step filtering was conducted during database creation. The step I exploited the exclusion criteria available directly in the Scopus search engine: Document type, and language (Figure 1). Only papers, conference papers, and book chapters published in English were selected.
Figure 1.
Workflow showing the phases of paper selection.
Bibliometric metadata for the selected research papers were then exported from the Scopus search engine. Metadata text files were elaborated using the scientific mapping software VOSviewer [19].
1.2. Topic Categorization and Other Classification Criteria
A second level of filtering was performed to determine eligibility of the selected research papers, based on the following exclusion criteria: (a) Adequacy of the paper topic to match the objectives of aflatoxin and CC; (b) mixed criterion accounting for at least one topic within (a) crop model, (b) fungal model, (c) weather data, (d) climate data, (e) current impact, (f) future impact and (g) single occurrence or co-occurrence (Table 1). For all papers compliant with at least one of the aforementioned criteria, the authors extracted information about the area of study and matrix. The authors then proceeded with a careful reading of the full text of each eligible article.
2. Motivations Underpinning Action-Reaction Analysis
This review considers all papers citing the output of EFSA project MODMAP-AFLA [17] on the effect of CC on A. flavus and AF contamination in maize across Europe [18].
Step I filtering identified 224 papers [5,6,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242]: 187 citing Battilani, et al. [18] and 37 citing Battilani, et al. [17]. Step II filtering identified 25 papers (Table 1; 21 citing [18] and 4 citing [17]) relevant to the study, which were included in a deeper analysis. These papers were categorized as “reactions” to the cited results because they went a step further. All the other papers (199) were considered “actions” following those publications; they played the role of sounding board for the expected impact of CC on AFB1 contamination, without adding new data on the topic.
Table 1.
Overall research paper dataset tabulated according to topic categorization. Reference number refers to bibliography reference; Study area as ISO 3166-1 alpha-2 country code, otherwise Continents or Global for larger study area; aw = water activity; AFB1 = aflatoxin B1; WOFOST = WOrld FOod STudies; DON = deoxynivalenol; JRC MARS = Joint Research Centre Monitoring Agricultural ResourceS; DAYMET = daily weather observation data; CRONOS = Climate Retrieval and Observations Network Of the Southeast; ECHAM5 = Global climate model 5th generation; HadCM3Q0 = Hadley Centre Coupled Model version 3, A1B Special Report on Emissions Scenarios; HadGEM2-ES = Hadley Centre Global Environment Model version 2 Earth System; RACMO2 = Regional Atmospheric Climate Model version 2; HADRM3Q0 = Hadley Center Regional Model version 3, A1B Special Report on Emissions Scenarios; AFM1 = aflatoxin M1; OTA = ochratoxin A; AFs = aflatoxins; FBs = fumonisins; NIV = nivalenol; ZEN = zearalenone.
| Reference | Study Area | Matrix | Model Approach | Weather Data | Climate Scenario | Current Impact | Future Impact | Mycotoxin Occurrence | Co-Occurrence |
|---|---|---|---|---|---|---|---|---|---|
| Djekic, et al. [64] | RS | Milk and dairy products | NO | Speculative | Speculative | 2015–2018 | NO | AFM1 (AFB1 in feed) | NO |
| Hiatt and Beyeler [94] | Global | Speculative | Speculative | Speculative | Speculative | Speculative | Speculative | General | NO |
| Adhikari, et al. [21] | Global | Coffee | Speculative | Speculative | Speculative | Speculative | Speculative | OTA-AFs-FBs | NO |
| Fouché, et al. [78] | Global | Soil/Food/Feed | Speculative | Speculative | Speculative | Speculative | Speculative | AFs | NO |
| Cervini, et al. [47] | IT * | Grape | Water/light/temperature in lab conditions | LAB conditions | Speculative | Speculative | Speculative | OTA | NO |
| Camardo Leggieri, et al. [45] | IT | Maize | aridity index-correlation index | Air temperature, rainfall, relative humidity | Speculative | 2014 | Speculative | NIV-DON-T2-HT2-ZEN-FBs-AFB1 | YES |
| Pleadin, et al. [151] | Europe | Food/Feed | Speculative | Speculative | Speculative | Speculative | Speculative | AFB1-OTA-FBs-PATULINE-DON | NO |
| Gasperini, et al. [85] | BR/MX ** | Maize | Pre/post harvest + interactions of Air temperature × CO2 × aw | LAB conditions | Speculative | Speculative | Speculative | AFB1 | NO |
| Van der Fels-Klerx, et al. [191] | NL/UA | Maize feed in UA/Milk in NL | 3 climate models + AFB1 model + WOFOST+ 5 carryover models |
JRC MARS | ECHAM5, HadCM3Q0 | 2005–2017 | 2030 | AFB1-AFM1 | NO |
| Moretti, et al. [131] | Europe | Food | Speculative | Speculative | Speculative | Speculative | Speculative | AFs-DON | NO |
| Labanca, et al. [118] | IT | Maize for feed | Speculative | Speculative | Speculative | Speculative | Speculative | AFs | NO |
| Ricciardi, et al. [159] | Global | Food | Speculative | Speculative | Speculative | Speculative | Speculative | General | NO |
| Cervini, et al. [48] | IT | Grape | NO | LAB conditions | NO | Speculative | Speculative | OTA | NO |
| Iizumi [99] | Global | Speculative | Speculative | Speculative | Speculative | Speculative | Speculative | General | NO |
| Bailly, et al. [31] | FR | Maize | Speculative | Speculative | Speculative | Speculative | Speculative | AFB1 | NO |
| Damianidis, et al. [57] | US | Maize | Logistic regression | Weather stations, DAYMET, CRONOS | NO | Speculative | Speculative | AFs | NO |
| Fanzo, et al. [72] | US | Food/ Feed | Speculative | Speculative | Speculative | Speculative | Speculative | General | NO |
| Assunção, et al. [30] | PT | Dietary exposure | NO | Speculative | Speculative | Speculative | Speculative | AFs | NO |
| Medina, et al. [128] | GB | Food | Speculative | Speculative | Speculative | Speculative | Speculative | General | YES |
| Raiten and Aimone [157] | CA/US | Speculative | Speculative | Speculative | Speculative | Speculative | Speculative | General | NO |
| Magan and Medina [121] | GB | Maize and Coffee | Linear regression | Lab conditions | Speculative | Speculative | Speculative | All mycotoxins | NO |
| Van de Perre, et al. [241] | ES/PL | Tomato | Climate + Alternaria model | Weather stations | HadGEM2-ES | 1981–2000 | 2031–2050 2081–2100 |
Alternaria | NO |
| Giorni, et al. [211] | GB/IT | Maize | NO | NO | NO | NO | NO | AFs | NO |
| Van der Fels-Klerx, et al. [242] | Europe *** | Wheat | Wheat phenology + Climate + DON model | JRC MARS | RACMO2, HADRM3Q0 | 1975–1994 | 2031–2050 | DON | NO |
| Medina, et al. [226] | Global | Feed/Food | Data from review + in vitro data | Speculative | Speculative | Speculative | Speculative | All mycotoxins | NO |
* Lab/in vitro study reproducing climatic conditions of Apulia region (Italy); ** combination of in situ and in vitro studies; *** refers to north-western Europe.
The overall workflow of database creation with single steps and corresponding number of selected or excluded papers is shown in Figure 1.
3. Overview of Selected Papers
The results of the scientific mapping, including papers categorized as “actions” and “reactions,” are summarized in four figures highlighting the journal where papers were published, keywords and their link to each other, and the countries to which the authors were affiliated (Figure 2, Figure 3, Figure 4 and Figure 5).
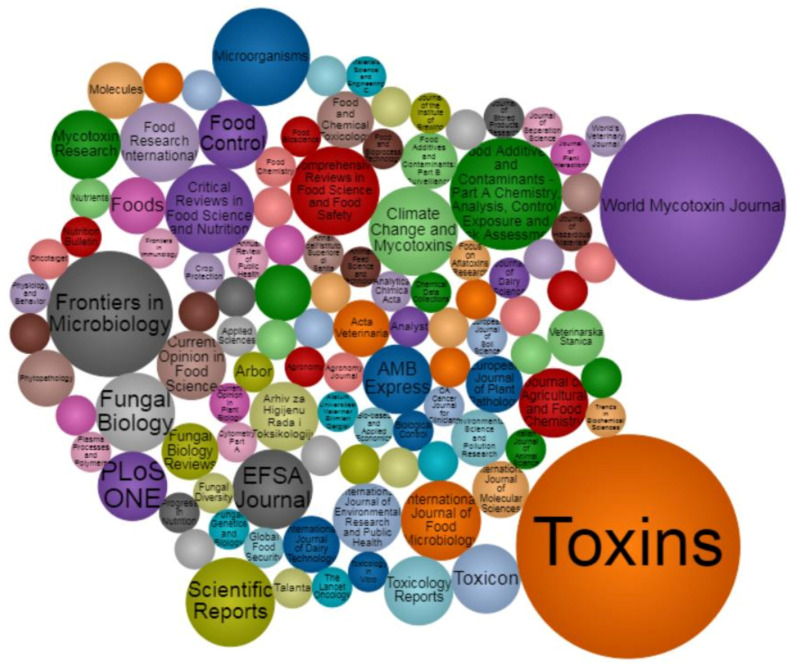
Figure 2.
Treemap of all source titles for the records (paper and report citations) identified during step I filtering. Treemap elaborated and created using the DrasticData online tool [243].
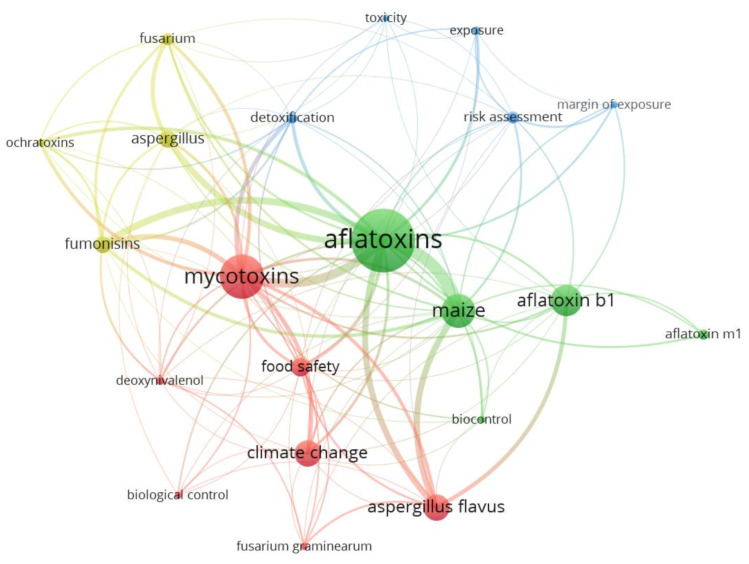
Figure 3.
Scientific mapping of all keyword networks based on records (paper and report citations) from step I filtering.
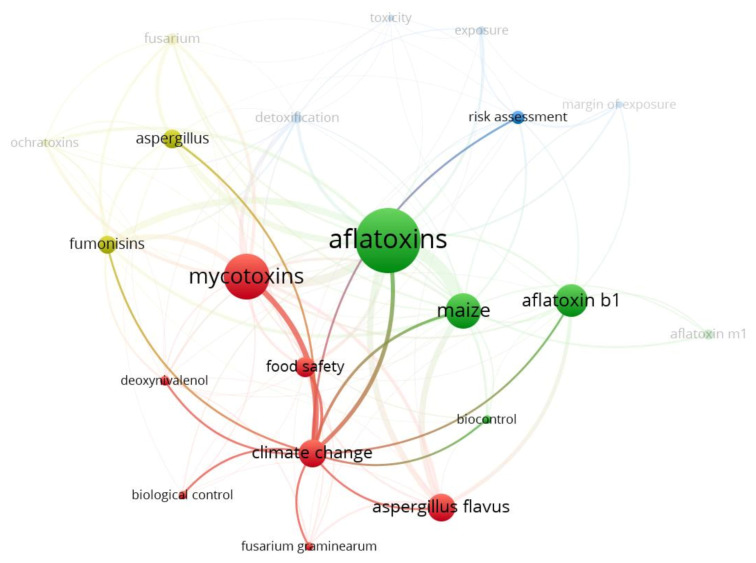
Figure 4.
Scientific mapping of strictly linked networks for climate change as keyword, based on records (paper and report citations) from step I filtering.
Figure 5.
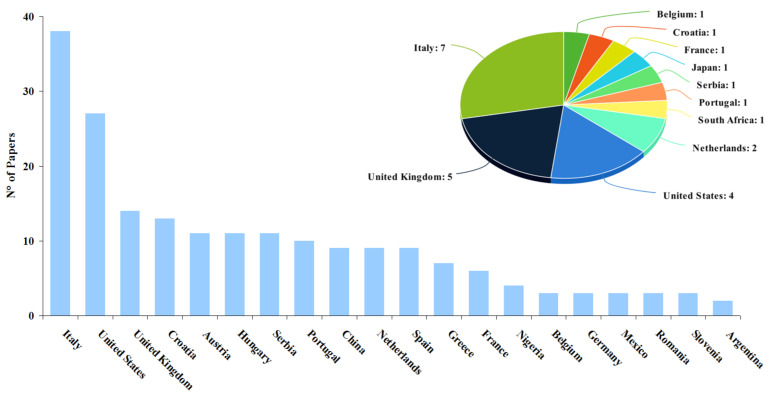
Bar graph showing the top 20 countries affiliated with authors of records from step I filtering. [Others: 3 papers each from Belgium, Germany, Mexico, Romania, Slovenia; 2 papers each from Argentina, Canada, India, Iran, Malawi, Malaysia, Philippines, Poland, South Africa, Switzerland, Thailand, Turkey; 1 paper each from Algeria, Brazil, Cyprus, Egypt, El Salvador, Ghana, Haiti, Indonesia, Ireland, Japan, Lithuania, North Macedonia, Pakistan, Saudi Arabia]. Pie chart (upper corner right) refers to the authors’ countries for the 25 studies selected for quantitative analysis.
The source titles for all research papers filtered through the exclusion criteria during the screening process (step I—224 papers) are shown in Figure 2. Toxins (MDPI) turned out to be, by far, the most popular journal for publication, accounting for 14.3% (32 papers) of the filtered publications, followed by World Mycotoxin Journal (9.8%, 22 papers—Wageningen Academic Publishers), Frontiers in Microbiology (4.5%. 10 papers—Frontiers Media), Food Additives and Contaminants—Part A Chemistry, Analysis, Control, Exposure and Risk Assessment (3.6%, 8 papers—Taylor & Francis Online) and Microorganism (2.7%, 6 papers—MDPI).
Despite most of the selected articles (89%, 199 papers) citing Battilani, et al. [17] and Battilani, et al. [18] only in the introduction, or not providing substantial advances to the topic covered by these two publications, our keywords occurrence analysis (Figure 3 and Figure 4) resulted in a well-defined pattern clustering the keywords into four groups, with colored lines indicating strong co-occurrence links between them. In the network mapping shown in Figure 3, (a) the first cluster (red color) comprises the keywords “Aspergillus flavus,” “biological control,” “climate change,” “deoxynivalenol,” “food safety,” “Fusarium graminearum” and “mycotoxins”; (b) the second cluster (green color), includes “aflatoxin B1,” “aflatoxin M1,” “aflatoxins,” “biocontrol” and “maize”; (c) the third cluster (light blue color) encompasses “detoxification,” “exposure,” “margin of exposure,” “risk assessment” and “toxicity”; while (d) the fourth cluster covers “Aspergillus,” “fumonisins,” “Fusarium” and “ochratoxins.” An in-depth analysis of the co-occurrence of keywords from different clusters (Figure 4) revealed “climate change” as the key element for most papers, with this keyword strongly linked (thick lines) to most of the main keywords of other clusters such as “fumonisins,” “Aspergillus,” “aflatoxins,” “maize,” “aflatoxin B1” and “risk assessment.”
The bar graph in Figure 5 displays the top 20 countries affiliated with authors of the selected papers. Italy and the United States were the leading countries where researchers citing Battilani, et al. [17] and Battilani, et al. [18] came from, with 38 and 27 papers, respectively. There were also scientists from the United Kingdom (14), Croatia (13) and Austria (11) together with Hungary and Serbia. This top 20 highlight a deficit of papers from some continents where mycotoxin contamination is considered a major problem, with implications that affect human and animal health (i.e., Africa and Asia). Indeed, only Nigeria (4 papers) and China (9 papers) ranked in this top 20 list. The pie chart (Figure 5—upper corner right) illustrates the authors’ countries for the 25 studies selected for quantitative analysis, considered as “reactions”: Here also, Italy (7), the United Kingdom (5) and the United States (4) were the countries with the largest number of articles.
4. Reactions
We selected 25 papers from the final dataset, accounting for the scientific community’s reactions to the topic (Table 1). The eligible research studies were tabulated, according to study area, matrix, model approach, weather data, climate scenario, current and future impact, and mycotoxin occurrence and co-occurrence, in order to highlight the availability of data and to outline some statements based on the above-mentioned tabulating criteria. Most of the matrices analyzed were related to both food and feed (general), while maize was the most represented crop. Milk and dairy products were also present, as well as coffee, tomato, grapes and wheat. The majority (64%) of studies did not implement any models, such as climate models, plant phenology or algorithms, or just referred to the results published in other studies. As expected, most of the work was focused on AFs (AFB1, AFM1 and total AFs), while their co-occurrence with other mycotoxins (FBs and DON) in the same matrix was only considered in two cases. The analysis of the impact of current climate conditions on mycotoxin contamination was limited to six studies, which was further reduced to three studies if the assessment of the impact of future climate scenarios was also studied.
4.1. CC Impact on Aspergillus flavus and Aflatoxin Contamination
First confirmations of the predicted increase in risk of AFB1 occurrence in maize under CC scenarios arrived soon after publication of the MODMAP-AFLA report in 2012 [17], with an event occurring in Serbia in the same year [244,245]. This was also the case for France, where, in 2015, exceptionally hot and dry climatic conditions caused 6% of maize fields to be contaminated by aflatoxins. Strains of Aspergillus section Flavi were isolated from maize samples, and A. flavus was the prevalent species (69% of strains), confirming the presence of these potent toxin-producers in fields in France [31], in addition to those reported in Italy before [13,246] and after publication of the report [247].
The same approach reported in the reference papers [17,18] was used effectively to study the outcome of CC on A. flavus in maize in Malawi [248]. Malawi is projected to get warmer (by 1–2.5 °C) and drier (reduction of 0–4% in annual rainfall levels) in all regions, with some uncertainty regarding precipitation. These conditions are expected to shorten the maize growing season, with a major impact on long-development varieties, causing the pre-harvest conditions for Malawian maize to become more favorable for AFB1 contamination. This was the only study that considered all components of CC, with particular regards to the effect of climate on maize crop phenology, A. flavus ecology and expected AFB1 contamination of grain.
The effect of CC was also reviewed in the context of mycotoxigenic fungi in coffee cultivation regions, Mesoamerica and central Africa in particular [21]. CC is expected to modulate the prevalence of fungal species, with a decline in Penicillium species and an increase in aflatoxin-producing Aspergilli species. In addition, the impact on OTA production seems species dependent. In fact, only for A. westerdijkiae, high CO2 (1000 ppm), high temperature (30–35 °C) and sub-optimal aw (0.90, 0.95 and 0.97), significantly stimulated OTA production in coffee beans. Suitable coffee growing areas will be affected by CC as well. Predictions suggest that suitable coffee cultivation areas could decrease by ~50% by 2050, both for Arabica and Robusta varieties. All indications showed that CC will have an extremely negative effect on future coffee production worldwide, in terms of both loss of cultivation areas and increase in mycotoxin contamination. In particular, suitable areas will migrate to higher altitudes where temperatures are cooler. Generally, Arabica is expected to fare worse than Robusta. However, more research is needed to understand how shifts in suitable areas for Arabica and Robusta will impact fungi and their mycotoxins under various CC scenarios.
An interesting approach evaluated grain contamination and considered the impact of CC on the maize-milk chain. This case study was based on maize grown in eastern Europe and imported to the Netherlands to be fed—as part of compound feed—to dairy cows. Three different climate models, one AFB1 prediction model and five different carryover models (carryover intended as the passage from AFB1 in the feed to AFM1, its hydroxylated metabolite, in the milk) were used and combined to obtain a predictive tool based on Monte Carlo simulations [191]. The results showed that, given the case study and the scenarios and models used, AFM1 contamination in milk is expected to be comparable or to increase in future climates. The outputs were sometimes in disagreement, depending on the model used; nevertheless, this study merits attention for the chain approach suggested.
The exposure of Serbia’s adult population to AFM1 from milk and dairy product consumption in 2015–2018 was examined by Djekic, et al. [64] and confirmed the previous data. In fact, these authors showed a moderate exposure risk compared with similarly managed studies worldwide, but the research underlined the importance of promoting continuous monitoring of feed and dairy supply chains and providing exposure assessment updates, with the exposure variable depending on the monitoring year.
However, all the studies mentioned above were missing essential aspects of fungal and plant interaction. Medina, et al. [128] stressed this critical aspect, underlining the importance of ecological studies to assess how fungal resilience is affected by interacting CC factors. Camardo Leggieri, et al. [45] recently confirmed this concern by using maize grown in 2014 in northern Italy as a case study. Wide unevenness in mycotoxin occurrence was noticed, even within a small area, with changes in the prevalent compound and in the level of contamination. This variability was attributed to CC effects on fungal complex interaction, with the dominant fungal species alternating during the growing season.
The challenging topic of defining the impact of fungal co-occurrence under different meteorological/ecological conditions on mycotoxin contamination was addressed by Giorni, et al. [249], and Camardo Leggieri, et al. [44], respectively, in field and in vitro. A. flavus, F. verticillioides and F. graminearum were artificially inoculated on maize grown in northern Italy in the two-year period 2016–2017. In parallel, A. flavus and F. verticillioides were inoculated on cornmeal medium and incubated under a wide range of temperature and water activity (aw) conditions. Therefore, fungal interactions could be observed under natural conditions, but the impact of temperature and aw could also be studied in detail and modeled. Under natural conditions, AFB1 accumulation was stimulated by the presence of F. graminearum, while no effects on FBs or DON, caused by F. verticillioides—F. graminearum co-occurrence were noticed. Interestingly, the co-occurrence of A. flavus with F. verticillioides or F. graminearum significantly reduced both FBs and DON production. Only A. flavus and F. verticillioides were included in the in vitro study, and each fungus was affected by the co-occurrence of the other; in particular, showing a decrease in colony diameter of 10%, and 44%, respectively, when they were grown together compared with growth alone. On the contrary, the dynamics of toxin production under different temperature regimes followed a similar trend for fungi grown alone, or together, but with a decrease in production rate and a shift in optimal temperature for AFB1 production. Although these preliminary results seem in partial disagreement, they need attention and careful elaboration. They provide basic knowledge for inclusion in predictive models to account for fungi co-occurrence in the CC scenario and to predict resulting mycotoxin co-occurrence.
Several researchers underlined the importance of acquiring detailed data in vitro on fungal responses to ecological conditions in the context of CC. In particular, Giorni, et al. [211] studied the effect of temperature and relative humidity on A. flavus sclerotia sporulation; data obtained were used to develop equations included in the AFLA-maize predictive model [16,204].
A step forward in ecological study was explored by Magan and Medina [121]. They examined the relationship between three-way interacting environmental factors, representative of CC scenarios (water stress × temperature + 2/4 °C × elevated CO2 650/1000 ppm) on growth and mycotoxin gene cluster expression for A. flavus. This impacted significantly on AFB1 production both on maize based medium (around 80 x the control) and on maize grain (x 3–4 the control). Studies on species of the Aspergillus section Circumdati and A. section Nigri on maize grain and coffee suggested that, while fungal growth may not be significantly affected, mycotoxin production seems to be stimulated by CC factors, Comparable conclusions were reported by Raiten and Aimone [157], based on ecological studies with a CC perspective on maize grain and coffee. Apart from revealing the up- or down-regulation of genes, a genomic approach represents a powerful tool for exploiting relative toxin production under extreme stress conditions, such as CC scenarios.
Most of the research efforts during recent years have focused on harvest or post-harvest contamination of AFs in feed/food commodities, but the soil ecosystem has been poorly considered. Fouché, et al. [78] recently reviewed studies that addressed the environmental and toxicological consequences of AF contamination, with the aim of clarifying the eventual risk that AF contamination poses to soil ecosystems. Many aspects of AF occurrence, degradation and the effects of its transformation products in the soil environment are still unknown and remain an essential area of research for both soil health and soil productivity. In terms of soil moisture and air temperature changes, a climatic approach is important for future risk assessments of AF contamination.
4.2. CC Impact on Other Pathosystems
Following the prediction of CC impact on A. flavus and AFB1 in maize under CC scenarios, another pathosystem, Alternaria spp. in tomatoes and related mycotoxins, was analyzed, this being an emerging matter of concern. Van de Perre, et al. [241] evaluated the effect of CC in two regions, Badajoz in Spain and Krobia in Poland. There was a significant difference in the potential growth of Alternaria among time frame scenarios in Poland, with far future > near future > current time frame. The results suggested that Poland’s situation in the far future (2081–2100) will become similar to Spain’s situation in the present time frame (1981–2000), showing a geographic shift in the problem. There were no significant differences among the scenarios studied for Spain because the higher temperatures predicted will become limiting for Alternaria spp.
Similarly, DON production in wheat was assessed for north-western Europe, indicating that both flowering and complete maturation of wheat will be earlier in the season because of CC effects. At the same time, DON contamination was expected to increase in most of the regions studied, raising initial concentrations by up to three times [242]. Fusarium species involved in Fusarium head blight (FHB) of cereals in the CC context were also addressed by Moretti, et al. [131] in 2019. In-depth modifications to the profile of toxigenic Fusarium species occurring on kernels at maturity in different global geographical areas are expected. A substantial modification in mycotoxin occurrence profile will most likely cause the advent of new mycotoxin risks in specific regions due to the shift of Fusarium species into new environments.
The CC scenarios examined by Cervini, et al. [48], considering an increase of more than 2.5 times CO2 concentration in the northern Apulia region (southern Italy), predicted an increase in colonization rate by A. carbonarius and ochratoxin A (OTA) production in grapes, a matter of concern in that Italian region. Furthermore, preliminary evidence indicated that temperature increase, likely to happen in the same area, may reduce both berry spoilage caused by A. carbonarius and OTA production in grapes [47]. In particular, with a temperature range 18/31 °C and under water stress conditions (0.93 aw), the fungal growth rate was slower than at 0.99 aw, but an over-expression of OTA genes was observed. On the contrary, at 20/37 °C a higher growth rate was observed at 0.93 aw. Therefore, high T and water stress seem not favorable for OTA production. Predictions of CO2 and temperature increase, resulting from CC seem to lead to contrasting results that need to be verified in the future.
Overall, in the context of ecological studies, only one work [85] addressed the resilience of non-toxigenic strains of A. flavus to CC factors to ensure they have the necessary ecological competence to compete effectively and reduce toxin contamination pre- or post-harvest. The efficacy of non-toxigenic strains in controlling AFB1 production was supported by expression of target structural and regulatory genes; they maintained biocontrol of AFB1 contamination under elevated CC interacting factors (37 °C × 1000 ppm CO2 and drought stress).
4.3. CC Impact on Human and Animal Health
During recent years, research has focused on studying or reviewing CC impact on fungal behavior and toxin production, as well as on related human health risks. Fanzo, et al. [72] examined the relationships between CC, diets and nutrition through a food system lens. They included food safety issues that were not only focused on mycotoxins, and identified adaptation and mitigation interventions for each step of the food supply chain to move towards a more climate-smart, nutrition-sensitive food system. The authors proposed that climate-smart agriculture is a promising approach for mitigating direct CC constraints. However, more action is needed to link climate-smart approaches to diets and nutrition, especially for the most vulnerable individuals in the population. Hiatt and Beyeler [94] provided a review synopsis of what is known about CC-induced exposure and its relevance for cancer events. Considering the predicted increase in AFs with CC, of etiological importance for liver cancer, no evidence of increases in hepatocellular cancer associated with CC has been directly attributed to AFs.
The food system appears to show good resilience to CC, but this is apparently not the case for livestock, where two specific and possible impacts on the production system were underlined: (i) contamination of livestock feed by mycotoxins; and (ii) animal health under heat stress (HS) conditions [118]. This suggests the importance of linking feed safety with the integrated approach proposed to adequately tackle food safety risks associated with CC, including perspectives from different natural and social sciences [30]. The potential consequences of an incompletely explored perspective of CC must be considered.
Taking account of the impact of CC as a whole on social and environmental health elements, and of the increased risk of adverse health effects, especially on the most vulnerable groups in the population, such as children and the elderly, the Symposium “Health and Climate Change” was organized in Rome in 2018 as a joint initiative of the Italian Institute of Health and EFSA. The meeting aimed to promote an inter-sectoral and multidisciplinary approach to CC-related events to counteract expected adverse health effects; the launch of the International Charter on Health and Climate was the concrete output [159].
5. Steps Forward and Perspectives
On a global level, CC is expected to have significant impacts on plant biogeography and fungal populations, with effects on mycotoxin patterns, as confirmed by predictive approaches and field surveys. AFB1 is expected to increase in Europe as a result of CC; this prediction is based on the AFLA-maize model and confirmed by field surveys. This result has captured the scientific community’s attention, as confirmed by the numerous citations gained by the papers reporting this data [17,18].
Predictive models have become crucial for addressing future uncertainties and highlighting risk conditions on a geographic basis. They are likely to be essential tools for mycotoxin prediction, in production chain management and as support for all stakeholders, farmers, extension services and policymakers [250,251]. Scientific mapping of keyword networks of papers citing the EFSA project results [17,18] revealed the total absence of “crop modeling” as a keyword, although the studies analyzed contemplate most of the topics for a holistic approach. In fact, advances in modeling the impact of CC were very limited, as detailed in “reactions”. This is undoubtedly one of the areas where research needs to be encouraged, together with extension to crops other than maize, as pointed out by Van Der Fels-Klerx, et al. [190], as well as other interacting factors, such as insects pests [252]. Furthermore, when evaluating the pressure risk of mycotoxins based on CC, we strongly advise not neglecting a pre-analysis of the suitability of countries/study areas for cultivation and the specific crop for which the current and future impact of mycotoxins must be assessed.
An increased risk of AFs is paired with fungal and related mycotoxin co-occurrence. The modeling approach should therefore include this event. Scarce data is available on this topic, and it is apparently not easy to interpret and convert into quantitative models. Therefore, new efforts should be addressed towards this research field, possibly integrated with the support of omics methodologies.
The top 20 authors’ countries identified Italy, the USA and the UK as leading actors in this area, but surely does not reflect the main countries where AFs are a matter of concern for people’s health, as highlighted very recently [155]. Therefore, major involvement of developing countries in studies aimed at predicting the impact of CC on AF occurrence is strongly desirable.
Several aspects related to AFB1 and CC need more attention, based on our literature review; nevertheless, interesting statements can be captured, which can be summarized using the following keywords: chain and multi-actor approach, intersectoral and multidisciplinary, resilience, human and animal health, global vision. To further summarize, the food system should be considered as a whole [253], taking advantage of smart agriculture [23]. We can learn from each other, both from different steps in the chain and from different geographic areas. Scenario analyses build on multi-actor, intersectoral and multidisciplinary approaches, which can provide all stakeholders, policymakers and risk managers the best support in facing health threats, related to CC, and build the needed resilience.
Author Contributions
Conceptualization, P.B.; methodology, P.T.; formal analysis M.C.L. and P.T.; data curation, P.T. and M.C.L.; writing—original draft preparation, P.B., P.T. and M.C.L.; writing—review and editing, P.B., P.T. and M.C.L.; supervision, P.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
Advances in modeling the impact of climate change (CC) on aflatoxin occurrence in maize over the last decade have been limited, mainly being considered by Italy, the United Kingdom and the United States, with few contributions from the continents where mycotoxin contamination is a major problem (Africa and Asia). Interestingly, related topics have been purposed, such as the co-occurrence of fungi and their impact on mycotoxin contamination, the chain approach (from cropping season to final products of the value chain), and the link between the expected increase in aflatoxin occurrence resulting from CC and its impact on human and animal health.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Perrone G., Ferrara M., Medina A., Pascale M., Magan N. Toxigenic fungi and mycotoxins in a climate change scenario: Ecology, genomics, distribution, prediction and prevention of the risk. Microorganisms. 2020;8:1496. doi: 10.3390/microorganisms8101496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Logrieco A., Bottalico A., Mule G., Moretti A., Perrone G. Epidemiology of toxigenic fungi and their associated mycotoxins for some mediterranean crops. Eur. J. Plant Pathol. 2003;109:645–667. doi: 10.1023/A:1026033021542. [DOI] [Google Scholar]
- 3.Bottalico A. Fusarium disease of cereals: Species complex and related mycotoxin profile in europe. J. Plant Pathol. 1998;80:84–103. [Google Scholar]
- 4.Palumbo R., Crisci A., Venâncio A., Cortiñas Abrahantes J., Dorne J.L., Battilani P., Toscano P. Occurrence and co-occurrence of mycotoxins in cereal-based feed and food. Microorganisms. 2020;8:74. doi: 10.3390/microorganisms8010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medina Á., González-Jartín J.M., Sainz M.J. Impact of global warming on mycotoxins. Curr. Opin. Food Sci. 2017;18:76–81. doi: 10.1016/j.cofs.2017.11.009. [DOI] [Google Scholar]
- 6.Ojiambo P.S., Battilani P., Cary J.W., Blum B.H., Carbone I. Cultural and genetic approaches to manage aflatoxin contamination: Recent insights provide opportunities for improved control. Phytopathology. 2018;108:1024–1037. doi: 10.1094/PHYTO-04-18-0134-RVW. [DOI] [PubMed] [Google Scholar]
- 7.Bush B.J., Carson M.L., Cubeta M.A., Hagler W.M., Payne G.A. Infection and fumonisin production by Fusarium verticillioides in developing maize kernels. Phytopathology. 2004;94:88–93. doi: 10.1094/PHYTO.2004.94.1.88. [DOI] [PubMed] [Google Scholar]
- 8.Miller J.D. Factors that affect the occurrence of fumonisin. Environ. Health Perspect. 2001;109(Suppl. 2):321–324. doi: 10.1289/ehp.01109s2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu F., Bhatnagar D., Bui-Klimke T., Carbone I., Hellmich R., Munkvold G., Paul P., Payne G., Takle E. Climate change impacts on mycotoxin risks in us maize. World Mycotoxin J. 2011;4:79–93. doi: 10.3920/WMJ2010.1246. [DOI] [Google Scholar]
- 10.Gregory P.J., Johnson S.N., Newton A.C., Ingram J.S. Integrating pests and pathogens into the climate change/food security debate. J. Exp. Bot. 2009;60:2827–2838. doi: 10.1093/jxb/erp080. [DOI] [PubMed] [Google Scholar]
- 11.Bebber D.P., Ramotowski M.A.T., Gurr S.J. Crop pests and pathogens move polewards in a warming world. Nat. Clim. Chang. 2013;3:985–988. doi: 10.1038/nclimate1990. [DOI] [Google Scholar]
- 12.Bebber D.P., Gurr S.J. Crop-destroying fungal and oomycete pathogens challenge food security. Fungal Genet. Biol. 2015;74:4. doi: 10.1016/j.fgb.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Piva G., Battilani P., Pietri A. Emerging issues in southern europe: Aflatoxins in italy. In: Barug D., Bhatnagar D., Egmond H.P.V., Kamp J.W.V.D., Osenbruggen W.A.V., Visconti A., editors. The Mycotoxin Factbook. Food & Feed Topics. Wageningen Academic Publishers; Wageningen, The Netherlands: 2006. pp. 139–153. [Google Scholar]
- 14.IARC . Iarc monographs on the evaluation of carcinogenic risks to humans. In: World Health Organization, editor. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. Volume 56. IARC Press; Lyon, France: 1993. pp. 445–466. [Google Scholar]
- 15.Azziz-Baumgartner E., Lindblade K., Gieseker K., Rogers H.S., Kieszak S., Njapau H., Schleicher R., McCoy L.F., Misore A., DeCock K., et al. Case-control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environ. Health Perspect. 2005;113:1779–1783. doi: 10.1289/ehp.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Battilani P., Camardo Leggieri M., Rossi V., Giorni P. Afla-maize, a mechanistic model for Aspergillus flavus infection and aflatoxin b1 contamination in maize. Comput. Electron. Agric. 2013;94:38–46. doi: 10.1016/j.compag.2013.03.005. [DOI] [Google Scholar]
- 17.Battilani P., Rossi V., Giorni P., Pietri A., Gualla A., Van der Fels-Klerx H.J., Booij C.J.H., Moretti A., Logrieco A., Toscano P. Modelling, predicting and mapping the emergence of aflatoxins in cereals in the eu due to climate change. EFSA Sci. Tech. Rep. 2012;9:223E. doi: 10.2903/sp.efsa.2012.EN-223. [DOI] [Google Scholar]
- 18.Battilani P., Toscano P., Van der Fels-Klerx H.J., Moretti A., Camardo Leggieri M., Brera C., Rortais A., Goumperis T., Robinson T. Aflatoxin b1 contamination in maize in europe increases due to climate change. Sci. Rep. 2016;6:24328. doi: 10.1038/srep24328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vosviewer—Visualizing Scientific Landscapes. [(accessed on 26 February 2021)]; Available online: https://www.vosviewer.com/
- 20.Adegbeye M.J., Reddy P.R.K., Chilaka C.A., Balogun O.B., Elghandour M.M.M.Y., Rivas-Caceres R.R., Salem A.Z.M. Mycotoxin toxicity and residue in animal products: Prevalence, consumer exposure and reduction strategies—a review. Toxicon. 2020;177:96–108. doi: 10.1016/j.toxicon.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Adhikari M., Isaac E.L., Paterson R.R.M., Maslin M.A. A review of potential impacts of climate change on coffee cultivation and mycotoxigenic fungi. Microorganisms. 2020;8:1625. doi: 10.3390/microorganisms8101625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agbetiameh D., Ortega-Beltran A., Awuah R.T., Atehnkeng J., Elzein A., Cotty P.J., Bandyopadhyay R. Field efficacy of two atoxigenic biocontrol products for mitigation of aflatoxin contamination in maize and groundnut in ghana. Biol. Control. 2020;150 doi: 10.1016/j.biocontrol.2020.104351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agrimonti C., Lauro M., Visioli G. Smart agriculture for food quality: Facing climate change in the 21st century. Crit. Rev. Food Sci. Nutr. 2020;61:971–981. doi: 10.1080/10408398.2020.1749555. [DOI] [PubMed] [Google Scholar]
- 24.Agriopoulou S., Stamatelopoulou E., Varzakas T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods. 2020;9:137. doi: 10.3390/foods9020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali S., Ejaz S., Anjum M.A., Nawaz A., Ahmad S. Plant Ecophysiology and Adaptation Under Climate Change: Mechanisms and Perspectives i: General Consequences and Plant Responses. Springer; Singapore: 2020. Impact of climate change on postharvest physiology of edible plant products; pp. 87–115. [Google Scholar]
- 26.Alshannaq A.F., Gibbons J.G., Lee M.-K., Han K.-H., Hong S.-B., Yu J.-H. Controlling aflatoxin contamination and propagation of Aspergillus flavus by a soy-fermenting Aspergillus oryzae strain. Sci. Rep. 2018;8:1–14. doi: 10.1038/s41598-018-35246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antiga L., La Starza S.R., Miccoli C., D’Angeli S., Scala V., Zaccaria M., Shu X., Obrian G., Beccaccioli M., Payne G.A., et al. Aspergillus flavus Exploits Maize Kernels Using an “Orphan” Secondary Metabolite Cluster. Int. J. Mol. Sci. 2020;21:8213. doi: 10.3390/ijms21218213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arce-López B., Lizarraga E., Vettorazzi A., González-Peñas E. Human Biomonitoring of Mycotoxins in Blood, Plasma and Serum in Recent Years: A Review. Toxins. 2020;12:147. doi: 10.3390/toxins12030147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aristil J., Venturini G., Maddalena G., Toffolatti S.L., Spada A. Fungal contamination and aflatoxin content of maize, moringa and peanut foods from rural subsistence farms in South Haiti. J. Stored Prod. Res. 2020;85:101550. doi: 10.1016/j.jspr.2019.101550. [DOI] [Google Scholar]
- 30.Assunção R., Martins C., Viegas S., Viegas C., Jakobsen L.S., Pires S., Alvito P. Climate change and the health impact of aflatoxins exposure in portugal—An overview. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018;35:1610–1621. doi: 10.1080/19440049.2018.1447691. [DOI] [PubMed] [Google Scholar]
- 31.Bailly S., El Mahgubi A., Carvajal-Campos A., Lorber S., Puel O., Oswald I.P., Bailly J.D., Orlando B. Occurrence and identification of Aspergillus section flavi in the context of the emergence of aflatoxins in french maize. Toxins. 2018;10:525. doi: 10.3390/toxins10120525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bandyopadhyay R., Ortega-Beltran A., Akande A., Mutegi C., Atehnkeng J., Kaptoge L., Senghor A., Adhikari B., Cotty P. Biological control of aflatoxins in Africa: Current status and potential challenges in the face of climate change. World Mycotoxin J. 2016;9:771–789. doi: 10.3920/WMJ2016.2130. [DOI] [Google Scholar]
- 33.Barukčić I., Bilandžić N., Markov K., Jakopović K.L., Božanić R. Reduction in aflatoxin m1 concentration during production and storage of selected fermented milks. Int. J. Dairy Technol. 2018;71:734–740. doi: 10.1111/1471-0307.12490. [DOI] [Google Scholar]
- 34.Battilani P. Recent advances in modeling the risk of mycotoxin contamination in crops. Curr. Opin. Food Sci. 2016;11:10–15. doi: 10.1016/j.cofs.2016.08.009. [DOI] [Google Scholar]
- 35.Battilani P., Stroka J., Magan N. Foreword: Mycotoxins in a changing world. World Mycotoxin J. 2016;9:647–651. doi: 10.3920/WMJ2016.x004. [DOI] [Google Scholar]
- 36.Bellingeri A., Cabrera V., Gallo A., Liang D., Masoero F. A survey of dairy cattle management, crop planning, and forages cost of production in Northern Italy. Ital. J. Anim. Sci. 2019;18:786–798. doi: 10.1080/1828051X.2019.1580153. [DOI] [Google Scholar]
- 37.Bellingeri A., Gallo A., Liang D., Masoero F., Cabrera V. Development of a linear programming model for the optimal allocation of nutritional resources in a dairy herd. J. Dairy Sci. 2020;103:10898–10916. doi: 10.3168/jds.2020-18157. [DOI] [PubMed] [Google Scholar]
- 38.Benkerroum N. Retrospective and Prospective Look at Aflatoxin Research and Development from a Practical Standpoint. Int. J. Environ. Res. Public Health. 2019;16:3633. doi: 10.3390/ijerph16193633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bessaire T., Mujahid C., Mottier P., Desmarchelier A. Multiple mycotoxins determination in food by lc-ms/ms: An international collaborative study. Toxins. 2019;11:658. doi: 10.3390/toxins11110658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braun H., Woitsch L., Hetzer B., Geisen R., Zange B., Schmidt-Heydt M. Trichoderma harzianum: Inhibition of mycotoxin producing fungi and toxin biosynthesis. Int. J. Food Microbiol. 2018;280:10–16. doi: 10.1016/j.ijfoodmicro.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 41.Caceres I., El Khoury R., Bailly S., Oswald I.P., Puel O., Bailly J.-D. Piperine inhibits aflatoxin B1 production in Aspergillus flavus by modulating fungal oxidative stress response. Fungal Genet. Biol. 2017;107:77–85. doi: 10.1016/j.fgb.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Caceres I., Khoury A.A., El Khoury R., Lorber S., Oswald I.P., El Khoury A., Atoui A., Puel O., Bailly J.D. Aflatoxin biosynthesis and genetic regulation: A review. Toxins. 2020;12:150. doi: 10.3390/toxins12030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caceres I., Snini S.P., Puel O., Mathieu F. Streptomyces roseolus, A Promising Biocontrol Agent Against Aspergillus flavus, the Main Aflatoxin B1 Producer. Toxins. 2018;10:442. doi: 10.3390/toxins10110442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leggieri M.C., Giorni P., Pietri A., Battilani P. Aspergillus flavus and Fusarium verticillioides Interaction: Modeling the Impact on Mycotoxin Production. Front. Microbiol. 2019;10:2653. doi: 10.3389/fmicb.2019.02653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Camardo Leggieri M., Lanubile A., Dall’Asta C., Pietri A., Battilani P. The impact of seasonal weather variation on mycotoxins: Maize crop in 2014 in northern italy as a case study. World Mycotoxin J. 2020;13:25–36. doi: 10.3920/WMJ2019.2475. [DOI] [Google Scholar]
- 46.Çatak J., Yaman M., Uǧur H. Investigation of aflatoxin levels in chips by hplc using postcolumn uv derivatization system. Prog. Nutr. 2020;22:214–223. [Google Scholar]
- 47.Cervini C., Gallo A., Piemontese L., Magistà D., Logrieco A.F., Ferrara M., Solfrizzo M., Perrone G. Effects of temperature and water activity change on ecophysiology of ochratoxigenic Aspergillus carbonarius in field-simulating conditions. Int. J. Food Microbiol. 2020;315:108420. doi: 10.1016/j.ijfoodmicro.2019.108420. [DOI] [PubMed] [Google Scholar]
- 48.Cervini C., Verheecke-Vaessen C., Ferrara M., García-Cela E., Magistà D., Medina A., Gallo A., Magan N., Perrone G. Interacting climate change factors (CO2 and temperature cycles) effects on growth, secondary metabolite gene expression and phenotypic ochratoxin A production by Aspergillus carbonarius strains on a grape-based matrix. Fungal Biol. 2021;125:115–122. doi: 10.1016/j.funbio.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Chaudhari A.K., Singh V.K., Das S., Deepika, Singh B.K., Dubey N.K. Antimicrobial, aflatoxin b1 inhibitory and lipid oxidation suppressing potential of anethole-based chitosan nanoemulsion as novel preservative for protection of stored maize. Food Bioprocess Technol. 2020;13:1462–1477. doi: 10.1007/s11947-020-02479-w. [DOI] [Google Scholar]
- 50.Chulze S.N., Palazzini J.M., Lullien-Pellerin V., Ramirez M.L., Cuniberti M., Magan N. Wheat Quality for Improving Processing and Human Health. Springer International Publishing; Berlin, Germany: 2020. Fusarium species infection in wheat: Impact on quality and mycotoxin accumulation; pp. 421–452. [Google Scholar]
- 51.Cohen S.P., Leach J.E. High temperature-induced plant disease susceptibility: More than the sum of its parts. Curr. Opin. Plant Biol. 2020;56:235–241. doi: 10.1016/j.pbi.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 52.Cowger C., Brown J.K.M. Durability of quantitative resistance in crops: Greater than we know? Annu. Rev. Phytopathol. 2019;57:253–277. doi: 10.1146/annurev-phyto-082718-100016. [DOI] [PubMed] [Google Scholar]
- 53.Czéh Á., Mézes M., Mandy F., Szőke Z., Nagyéri G., Laufer N., Kőszegi B., Koczka T., Kunsági-Máté S., Lustyik G. Flow cytometry based rapid duplexed immunoassay for Fusarium mycotoxins. Cytom. Part A. 2017;91:190–196. doi: 10.1002/cyto.a.23018. [DOI] [PubMed] [Google Scholar]
- 54.Dallabona C., Pioli M., Spadola G., Orsoni N., Bisceglie F., Lodi T., Pelosi G., Restivo F.M., Degola F. Sabotage at the Powerhouse? Unraveling the Molecular Target of 2-Isopropylbenzaldehyde Thiosemicarbazone, a Specific Inhibitor of Aflatoxin Biosynthesis and Sclerotia Development in Aspergillus flavus, Using Yeast as a Model System. Molecules. 2019;24:2971. doi: 10.3390/molecules24162971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dall’Asta C., Battilani P. Fumonisins and their modified forms, a matter of concern in future scenario? World Mycotoxin J. 2016;9:727–739. doi: 10.3920/WMJ2016.2058. [DOI] [Google Scholar]
- 56.Damianidis D., Ortiz B.V., Bowen K.L., Windham G.L., Hoogenboom G., Hagan A., Knappenberger T., Abbas H.K., Scully B.T., Mourtzinis S. Minimum temperature, rainfall, and agronomic management impacts on corn grain aflatoxin contamination. Agron. J. 2018;110:1697–1708. doi: 10.2134/agronj2017.11.0628. [DOI] [Google Scholar]
- 57.Damianidis D., Ortiz B., Windham G., Bowen K., Hoogenboom G., Scully B., Hagan A., Knappenberger T., Woli P., Williams W. Evaluating a generic drought index as a predictive tool for aflatoxin contamination of corn: From plot to regional level. Crop. Prot. 2018;113:64–74. doi: 10.1016/j.cropro.2018.07.013. [DOI] [Google Scholar]
- 58.De Santis B., Debegnach F., Gregori E., Russo S., Marchegiani F., Moracci G., Brera C. Development of a LC-MS/MS Method for the Multi-Mycotoxin Determination in Composite Cereal-Based Samples. Toxins. 2017;9:169. doi: 10.3390/toxins9050169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Debegnach F., Brera C., Mazzilli G., Sonego E., Buiarelli F., Ferri F., Rossi P.G., Collini G., De Santis B. Optimization and validation of a LC-HRMS method for aflatoxins determination in urine samples. Mycotoxin Res. 2020;36:257–266. doi: 10.1007/s12550-020-00389-6. [DOI] [PubMed] [Google Scholar]
- 60.Dellafiora L., Dall’Asta C. Masked mycotoxins: An emerging issue that makes renegotiable what is ordinary. Food Chem. 2016;213:534–535. doi: 10.1016/j.foodchem.2016.06.112. [DOI] [PubMed] [Google Scholar]
- 61.Nieto C.D., Granero A., Garcia D., Nesci A., Barros G., Zon M., Fernández H. Development of a third-generation biosensor to determine sterigmatocystin mycotoxin: An early warning system to detect aflatoxin B1. Talanta. 2019;194:253–258. doi: 10.1016/j.talanta.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 62.Dimitrieska-Stojkovikj E. Increased Health Impact of Aflatoxins Due to Climate Change: Prospective Risk Management Strategies. J. Food Qual. Hazards Control. 2018;5:38–39. doi: 10.29252/jfqhc.5.2.1. [DOI] [Google Scholar]
- 63.Djaaboub S., Moussaoui A., Meddah B., Gouri S., Benyahia K. Prevalence of Mycoflora and Fusarium graminearum Chemotype DON in Wheat in Bechar Province of South-Western Algeria. Acta Phytopathol. Èntomol. Hung. 2020;55:11–26. doi: 10.1556/038.55.2020.002. [DOI] [Google Scholar]
- 64.Djekic I., Petrovic J., Jovetic M., Redzepovic-Djordjevic A., Stulic M., Lorenzo J.M., Iammarino M., Tomasevic I. Aflatoxins in Milk and Dairy Products: Occurrence and Exposure Assessment for the Serbian Population. Appl. Sci. 2020;10:7420. doi: 10.3390/app10217420. [DOI] [Google Scholar]
- 65.Dong Y., Fan L., Liang J., Wang L., Yuan X., Wang Y., Zhao S. Risk assessment of mycotoxins in stored maize: Case study of Shandong, China. World Mycotoxin J. 2020;13:313–320. doi: 10.3920/WMJ2019.2449. [DOI] [Google Scholar]
- 66.Dowd P.F., Johnson E.T. Insect damage influences heat and water stress resistance gene expression in field-grown popcorn: Implications in developing crop varieties adapted to climate change. Mitig. Adapt. Strat. Glob. Chang. 2017;23:1063–1081. doi: 10.1007/s11027-017-9772-x. [DOI] [Google Scholar]
- 67.Echarri E., Vettorazzi A., Lizarraga E., Arce-López B., González-Peñas E. Aflatoxins: Biochemistry, Toxicology, Public Health, Policies and Modern Methods of Analysis. Nova Science Publisher; Hauppauge, NY, USA: 2019. Review of the analytical methodologies and occurrence data of aflatoxins in cereals and cereal-based foods in spain; pp. 207–243. [Google Scholar]
- 68.Elgioushy M.M., Elgaml S.A., El-Adl M.M., Hegazy A.M., Hashish E.A. Aflatoxicosis in cattle: Clinical findings and biochemical alterations. Environ. Sci. Pollut. Res. 2020;27:35526–35534. doi: 10.1007/s11356-020-09489-3. [DOI] [PubMed] [Google Scholar]
- 69.Elzupir A.O., Abdulkhair B.Y. Health risk from aflatoxins in processed meat products in Riyadh, KSA. Toxicon. 2020;181:1–5. doi: 10.1016/j.toxicon.2020.04.092. [DOI] [PubMed] [Google Scholar]
- 70.Eskola M., Elliott C.T., HajšLová J., Steiner D., Krska R. Towards a dietary-exposome assessment of chemicals in food: An update on the chronic health risks for the European consumer. Crit. Rev. Food Sci. Nutr. 2019;60:1890–1911. doi: 10.1080/10408398.2019.1612320. [DOI] [PubMed] [Google Scholar]
- 71.Eskola M., Kos G., Elliott C.T., HajšLová J., Mayar S., Krska R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25% Crit. Rev. Food Sci. Nutr. 2020;60:2773–2789. doi: 10.1080/10408398.2019.1658570. [DOI] [PubMed] [Google Scholar]
- 72.Fanzo J., Davis C., McLaren R., Choufani J. The effect of climate change across food systems: Implications for nutrition outcomes. Glob. Food Secur. 2018;18:12–19. doi: 10.1016/j.gfs.2018.06.001. [DOI] [Google Scholar]
- 73.Fanzo J., Hood A., Davis C. Eating our way through the Anthropocene. Physiol. Behav. 2020;222:112929. doi: 10.1016/j.physbeh.2020.112929. [DOI] [PubMed] [Google Scholar]
- 74.Fapohunda S.O., Esan A.O., Anjorin T.S. Biological control of mycotoxins: An update. World’s Vet. J. 2017;7:117–127. [Google Scholar]
- 75.Ferri F., Brera C., De Santis B., Collini G., Crespi E., Debegnach F., Gargano A., Gattei D., Magnani I., Mancuso P., et al. Association between Urinary Levels of Aflatoxin and Consumption of Food Linked to Maize or Cow Milk or Dairy Products. Int. J. Environ. Res. Public Heal. 2020;17:2510. doi: 10.3390/ijerph17072510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ferri F., Brera C., De Santis B., Fedrizzi G., Bacci T., Bedogni L., Capanni S., Collini G., Crespi E., Debegnach F., et al. Survey on Urinary Levels of Aflatoxins in Professionally Exposed Workers. Toxins. 2017;9:117. doi: 10.3390/toxins9040117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferrigo D., Mondin M., Scopel C., Maso E.D., Stefenatti M., Raiola A., Causin R. Effects of a prothioconazole- and tebuconazole-based fungicide on Aspergillus flavus development under laboratory and field conditions. Eur. J. Plant Pathol. 2019;155:151–161. doi: 10.1007/s10658-019-01757-4. [DOI] [Google Scholar]
- 78.Fouché T., Claassens S., Maboeta M. Aflatoxins in the soil ecosystem: An overview of its occurrence, fate, effects and future perspectives. Mycotoxin Res. 2020;36:303–309. doi: 10.1007/s12550-020-00393-w. [DOI] [PubMed] [Google Scholar]
- 79.Frumkin H., Haines A. Global environmental change and noncommunicable disease risks. Annu. Public Health. 2019;40:261–282. doi: 10.1146/annurev-publhealth-040218-043706. [DOI] [PubMed] [Google Scholar]
- 80.Fusco V., Chieffi D., Fanelli F., Logrieco A.F., Cho G., Kabisch J., Böhnlein C., Franz C.M.A.P. Microbial quality and safety of milk and milk products in the 21st century. Compr. Rev. Food Sci. Food Saf. 2020;19:2013–2049. doi: 10.1111/1541-4337.12568. [DOI] [PubMed] [Google Scholar]
- 81.Gagiu V. Triticale crop and contamination with mycotoxins under the influence of climate change—Global study. J. Hyg. Eng. Des. 2018;23:30–45. [Google Scholar]
- 82.Gagiu V., Mateescu E., Armeanu I., Dobre A.A., Smeu I., Cucu M.E., Oprea O.A., Iorga E., Belc N. Post-harvest contamination with mycotoxins in the context of the geographic and agroclimatic conditions in romania. Toxins. 2018;10:533. doi: 10.3390/toxins10120533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.García-Díaz M., Gil-Serna J., Vázquez C., Botia M.N., Patiño B. A comprehensive study on the occurrence of mycotoxins and their producing fungi during the maize production cycle in Spain. Microorganisms. 2020;8:141. doi: 10.3390/microorganisms8010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.García-Díaz M., Patiño B., Vázquez C., Gil-Serna J. A novel niosome-encapsulated essential oil formulation to prevent Aspergillus flavus growth and aflatoxin contamination of maize grains during storage. Toxins. 2019;11:646. doi: 10.3390/toxins11110646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gasperini A.M., Rodriguez-Sixtos A., Verheecke-Vaessen C., Garcia-Cela E., Medina A., Magan N. Resilience of Biocontrol for Aflatoxin Minimization Strategies: Climate Change Abiotic Factors May Affect Control in Non-GM and GM-Maize Cultivars. Front. Microbiol. 2019;10:2525. doi: 10.3389/fmicb.2019.02525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gauthier T., Duarte-Hospital C., Vignard J., Boutet-Robinet E., Sulyok M., Snini S.P., Alassane-Kpembi I., Lippi Y., Puel S., Oswald I.P., et al. Versicolorin A, a precursor in aflatoxins biosynthesis, is a food contaminant toxic for human intestinal cells. Environ. Int. 2020;137:105568. doi: 10.1016/j.envint.2020.105568. [DOI] [PubMed] [Google Scholar]
- 87.Gering E., Incorvaia D., Henriksen R., Wright D., Getty T. Maladaptation in feral and domesticated animals. Evol. Appl. 2019;12:1274–1286. doi: 10.1111/eva.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ghadiri S., Spalenza V., Dellafiora L., Badino P., Barbarossa A., Dall’Asta C., Nebbia C., Girolami F. Modulation of aflatoxin b1 cytotoxicity and aflatoxin m1 synthesis by natural antioxidants in a bovine mammary epithelial cell line. Toxicol. In Vitro. 2019;57:174–183. doi: 10.1016/j.tiv.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 89.Gilbert Sandoval I., Wesseling S., Rietjens I.M.C.M. Aflatoxin b1 in nixtamalized maize in Mexico; occurrence and accompanying risk assessment. Toxicol. Rep. 2019;6:1135–1142. doi: 10.1016/j.toxrep.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Girona A.J.R., Sillué S.M., Gahete F.M., Donat P.V., Almenar V.S. Mycotoxins: The silent enemy. Arbor. 2020;196:1–13. [Google Scholar]
- 91.Gömöri C., Nacsa-Farkas E., Kerekes E., Vidács A., Bencsik O., Kocsubé S., Khaled J., Alharbi N., Vágvölgyi C., Krisch J. Effect of essential oil vapours on aflatoxin production of Aspergillus parasiticus. World Mycotoxin J. 2018;11:579–588. doi: 10.3920/WMJ2017.2260. [DOI] [Google Scholar]
- 92.Gonçalves A., Gkrillas A., Dorne J.L., Dall’Asta C., Palumbo R., Lima N., Battilani P., Venâncio A., Giorni P. Pre- and Postharvest Strategies to Minimize Mycotoxin Contamination in the Rice Food Chain. Compr. Rev. Food Sci. Food Saf. 2019;18:441–454. doi: 10.1111/1541-4337.12420. [DOI] [PubMed] [Google Scholar]
- 93.Gruber-Dorninger C., Novak B., Nagl V., Berthiller F. Emerging mycotoxins: Beyond traditionally determined food contaminants. J. Agric. Food Chem. 2017;65:7052–7070. doi: 10.1021/acs.jafc.6b03413. [DOI] [PubMed] [Google Scholar]
- 94.Hiatt R.A., Beyeler N. Cancer and climate change. Lancet Oncol. 2020;21:e519–e527. doi: 10.1016/S1470-2045(20)30448-4. [DOI] [PubMed] [Google Scholar]
- 95.Hojnik N., Modic M., Walsh J.L., Žigon D., Javornik U., Plavec J., Žegura B., Filipič M., Cvelbar U. Unravelling the pathways of air plasma induced aflatoxin B1 degradation and detoxification. J. Hazard. Mater. 2021;403:123593. doi: 10.1016/j.jhazmat.2020.123593. [DOI] [PubMed] [Google Scholar]
- 96.Hojnik N., Modic M., Žigon D., Kovač J., Jurov A., Dickenson A., Walsh J.L., Cvelbar U. Cold atmospheric pressure plasma-assisted removal of aflatoxin B 1 from contaminated corn kernels. Plasma Process. Polym. 2021;18 doi: 10.1002/ppap.202000163. [DOI] [Google Scholar]
- 97.Hruska Z., Yao H., Kincaid R., Brown R.L., Bhatnagar D., Cleveland T.E. Temporal effects on internal fluorescence emissions associated with aflatoxin contamination from corn kernel cross-sections inoculated with toxigenic and atoxigenic Aspergillus flavus. Front. Microbiol. 2017;8:1718. doi: 10.3389/fmicb.2017.01718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hyde K.D., Al-Hatmi A.M.S., Andersen B., Boekhout T., Buzina W., Dawson T.L., Eastwood D.C., Jones E.B.G., de Hoog S., Kang Y., et al. The world’s ten most feared fungi. Fungal Divers. 2018;93:161–194. doi: 10.1007/s13225-018-0413-9. [DOI] [Google Scholar]
- 99.Iizumi T. Adaptation to Climate Change in Agriculture: Research and Practices. Springer International Publishing; Berlin, Germany: 2019. Emerging adaptation to climate change in agriculture; pp. 3–16. [Google Scholar]
- 100.Janić Hajnal E., Kos J., Krulj J., Krstović S., Jajić I., Pezo L., Šarić B., Nedeljković N. Aflatoxins contamination of maize in serbia: The impact of weather conditions in 2015. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017;34:1999–2010. doi: 10.1080/19440049.2017.1331047. [DOI] [PubMed] [Google Scholar]
- 101.Jesmin R., Chanda A. Restricting mycotoxins without killing the producers: A new paradigm in nano-fungal interactions. Appl. Microbiol. Biotechnol. 2020;104:2803–2813. doi: 10.1007/s00253-020-10373-w. [DOI] [PubMed] [Google Scholar]
- 102.Kaminiaris M.D., Tsitsigiannis D.I. Aflatoxins: Biochemistry, Toxicology, Public Health, Policies and Modern Methods of Analysis. Nova Science Publisher; Hauppauge, NY, USA: 2019. Pre-harvest management strategies to control aflatoxin contamination in crops; pp. 247–285. [Google Scholar]
- 103.Kaynarca H.D., Hecer C., Ulusoy B. Mycotoxin hazard in meat and meat products. Ataturk Univ. Vet. Bilimleri Derg. 2019;14:90–97. doi: 10.17094/ataunivbd.449705. [DOI] [Google Scholar]
- 104.Keriene I., Mankeviciene A., Cesnuleviciene R. Risk factors for mycotoxin contamination of buckwheat grain and its products. World Mycotoxin J. 2018;11:519–529. doi: 10.3920/WMJ2018.2299. [DOI] [Google Scholar]
- 105.Klvana M., Bren U. Aflatoxin B1–Formamidopyrimidine DNA Adducts: Relationships between Structures, Free Energies, and Melting Temperatures. Molecules. 2019;24:150. doi: 10.3390/molecules24010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Knutsen H.K., Alexander J., Barregard L., Bignami M., Brüschweiler B., Ceccatelli S., Cottrill B., DiNovi M., Edler L., Grasl-Kraupp B. Effect on public health of a possible increase of the maximum level for ‘aflatoxin total’ from 4 to 10 μg/kg in peanuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs. EFSA J. 2018;16 doi: 10.2903/j.efsa.2018.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kovač T., Borišev I., Crevar B., Čačić Kenjerić F., Kovač M., Strelec I., Ezekiel C.N., Sulyok M., Krska R., Šarkanj B. Fullerol c60(oh)24 nanoparticles modulate aflatoxin b1 biosynthesis in Aspergillus flavus. Sci. Rep. 2018;8:1–8. doi: 10.1038/s41598-018-31305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kovač T., Borišev I., Kovač M., Lončarić A., Čačić Kenjerić F., Djordjevic A., Strelec I., Ezekiel C.N., Sulyok M., Krska R., et al. Impact of fullerol c60(oh)24 nanoparticles on the production of emerging toxins by Aspergillus flavus. Sci. Rep. 2020;10:1–10. doi: 10.1038/s41598-020-57706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kovač T., Kovač M., Strelec I., Nevistić A., Molnar M. Antifungal and antiaflatoxigenic activities of coumarinyl thiosemicarbazides against Aspergillus flavus nrrl 3251. Arh. za Hig. Rada i Toksikol. 2017;68:9–15. doi: 10.1515/aiht-2017-68-2883. [DOI] [PubMed] [Google Scholar]
- 110.Kovač T., Šarkanj B., Borišev I., Djordjevic A., Jović D., Lončarić A., Babić J., Jozinović A., Krska T., Gangl J., et al. Fullerol c60(oh)24 nanoparticles affect secondary metabolite profile of important foodborne mycotoxigenic fungi in vitro. Toxins. 2020;12:213. doi: 10.3390/toxins12040213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kovač T., Šarkanj B., Crevar B., Kovač M., Lončarić A., Strelec I., Ezekiel C.N., Sulyok M., Krska R. Aspergillus flavus nrrl 3251 growth, oxidative status, and aflatoxins production ability in vitro under different illumination regimes. Toxins. 2018;10:528. doi: 10.3390/toxins10120528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kovač T., Šarkanj B., Klapec T., Borišev I., Kovač M., Nevistić A., Strelec I. Antiaflatoxigenic effect of fullerene c60 nanoparticles at environmentally plausible concentrations. AMB Express. 2018;8:1–8. doi: 10.1186/s13568-018-0544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kövesi B., Cserháti M., Erdélyi M., Zándoki E., Mézes M., Balogh K. Lack of dose- and time-dependent effects of aflatoxin b1 on gene expression and enzymes associated with lipid peroxidation and the glutathione redox system in chicken. Toxins. 2020;12:84. doi: 10.3390/toxins12020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Krska R., De Nijs M., McNerney O., Pichler M., Gilbert J., Edwards S., Suman M., Magan N., Rossi V., Van Der Fels-Klerx H., et al. Safe food and feed through an integrated toolbox for mycotoxin management: The MyToolBox approach. World Mycotoxin J. 2016;9:487–495. doi: 10.3920/WMJ2016.2136. [DOI] [Google Scholar]
- 115.Krulj J., Đisalov J., Bocarov-Stancic A., Pezo L., Kojic J., Vidaković A., Solarov M.B. Occurrence of aflatoxin B1 in Triticum species inoculated with Aspergillus flavus. World Mycotoxin J. 2018;11:247–257. doi: 10.3920/WMJ2017.2229. [DOI] [Google Scholar]
- 116.Ksenija N. Mycotoxins—Climate impact and steps to prevention based on prediction. Acta Vet. 2018;68:1–15. doi: 10.2478/acve-2018-0001. [DOI] [Google Scholar]
- 117.Kumphanda J., Matumba L., Whitaker T., Kasapila W., Sandahl J. Maize meal slurry mixing: An economical recipe for precise aflatoxin quantitation. World Mycotoxin J. 2019;12:203–212. doi: 10.3920/WMJ2018.2415. [DOI] [Google Scholar]
- 118.Labanca F., Raimondi A., Fontanelli M., Pisuttu C., Rallo G., Galli F., Conte G., Pellegrini E. The effects of climate change on livestock production systems: The cases of mycotoxins in animal feed and animal heat stress. Agrochimica. 2019;2019:99–106. [Google Scholar]
- 119.Lanubile A., Maschietto V., Battilani P., Marocco A. Infection with toxigenic and atoxigenic strains of Aspergillus flavus induces different transcriptional signatures in maize kernels. J. Plant Interact. 2017;12:21–30. doi: 10.1080/17429145.2016.1274062. [DOI] [Google Scholar]
- 120.Leong Y.H., Ahmad N.I., Awang R. Focus on Aflatoxins Research. Nova Science Publisher; Hauppauge, NY, USA: 2017. Occurrence, human exposure and the current trends of exposure measurements for aflatoxins; pp. 1–44. [Google Scholar]
- 121.Magan N., Medina Á. Integrating gene expression, ecology and mycotoxin production by Fusarium and Aspergillus species in relation to interacting environmental factors. World Mycotoxin J. 2016;9:673–684. doi: 10.3920/WMJ2016.2076. [DOI] [Google Scholar]
- 122.Mahato D.K., Lee K.E., Kamle M., Devi S., Dewangan K.N., Kumar P., Kang S.G. Aflatoxins in Food and Feed: An Overview on Prevalence, Detection and Control Strategies. Front. Microbiol. 2019;10:2266. doi: 10.3389/fmicb.2019.02266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mangasuli S.N. Synthesis of novel Isatin-Dithiocarbamate hybrids: An approach to microwave and potent antimicrobial agents. Chem. Data Collect. 2020;29:100515. doi: 10.1016/j.cdc.2020.100515. [DOI] [Google Scholar]
- 124.Martins C., Vidal A., De Boevre M., De Saeger S., Nunes C., Torres D., Goios A., Lopes C., Alvito P., Assunção R. Burden of disease associated with dietary exposure to carcinogenic aflatoxins in portugal using human biomonitoring approach. Food Res. Int. 2020;134:109210. doi: 10.1016/j.foodres.2020.109210. [DOI] [PubMed] [Google Scholar]
- 125.Masiello M., Somma S., Ghionna V., Francesco Logrieco A., Moretti A. In vitro and in field response of different fungicides against Aspergillus flavus and Fusarium species causing ear rot disease of maize. Toxins. 2019;11:11. doi: 10.3390/toxins11010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Masiello M., Somma S., Haidukowski M., Logrieco A.F., Moretti A. Genetic polymorphisms associated to sdhi fungicides resistance in selected Aspergillus flavus strains and relation with aflatoxin production. Int. J. Microbiol. 2020;334:108799. doi: 10.1016/j.ijfoodmicro.2020.108799. [DOI] [PubMed] [Google Scholar]
- 127.Mauro A., Garcia-Cela E., Pietri A., Cotty P.J., Battilani P. Biological control products for aflatoxin prevention in italy: Commercial field evaluation of atoxigenic Aspergillus flavus active ingredients. Toxins. 2018;10:30. doi: 10.3390/toxins10010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Medina A., Akbar A., Baazeem A., Rodriguez A., Magan N. Climate change, food security and mycotoxins: Do we know enough? Fungal Biol. Rev. 2017;31:143–154. doi: 10.1016/j.fbr.2017.04.002. [DOI] [Google Scholar]
- 129.Mesterhazy A., Toth E.T.T., Szel S., Varga M., Toth B. Resistance of Maize Hybrids to Fusarium graminearum, F. culmorum, and F. verticillioides Ear Rots with Toothpick and Silk Channel Inoculation, as Well as Their Toxin Production. Agronomy. 2020;10:1283. doi: 10.3390/agronomy10091283. [DOI] [Google Scholar]
- 130.Michelmore R., Coaker G., Bart R., Beattie G., Bent A., Bruce T., Cameron D., Dangl J., Dinesh-Kumar S., Edwards R., et al. Foundational and Translational Research Opportunities to Improve Plant Health. Mol. Plant-Microbe Interact. 2017;30:515–516. doi: 10.1094/MPMI-01-17-0010-CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moretti A., Pascale M., Logrieco A.F. Mycotoxin risks under a climate change scenario in Europe. Trends Food Sci. Technol. 2019;84:38–40. doi: 10.1016/j.tifs.2018.03.008. [DOI] [Google Scholar]
- 132.Mshelia L.P., Selamat J., Samsudin N.I.P., Rafii M.Y., Mutalib N.-A.A., Nordin N., Berthiller F. Effect of Temperature, Water Activity and Carbon Dioxide on Fungal Growth and Mycotoxin Production of Acclimatised Isolates of Fusarium verticillioides and F. graminearum. Toxins. 2020;12:478. doi: 10.3390/toxins12080478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Munkvold G.P., Arias S., Taschl I., Gruber-Dorninger C. Corn: Chemistry and Technology. 3rd ed. Elsevier; Duxford, UK: 2018. Mycotoxins in corn: Occurrence, impacts, and management; pp. 235–287. [Google Scholar]
- 134.Myndrul V., Coy E., Bechelany M., Iatsunskyi I. Photoluminescence label-free immunosensor for the detection of Aflatoxin B1 using polyacrylonitrile/zinc oxide nanofibers. Mater. Sci. Eng. C. 2021;118:111401. doi: 10.1016/j.msec.2020.111401. [DOI] [PubMed] [Google Scholar]
- 135.Nabwire W.R., Ombaka J., Dick C.P., Strickland C., Tang L., Xue K.S., Wang J.-S. Aflatoxin in household maize for human consumption in Kenya, East Africa. Food Addit. Contam. Part B. 2019;13:45–51. doi: 10.1080/19393210.2019.1690053. [DOI] [PubMed] [Google Scholar]
- 136.Nazhand A., Durazzo A., Lucarini M., Souto E.B., Santini A. Characteristics, Occurrence, Detection and Detoxification of Aflatoxins in Foods and Feeds. Foods. 2020;9:644. doi: 10.3390/foods9050644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nogueira L.M., Yabroff K.R., Bernstein A. Climate change and cancer. CA Cancer J. Clin. 2020;70:239–244. doi: 10.3322/caac.21610. [DOI] [PubMed] [Google Scholar]
- 138.Nugent A.P., Thielecke F. Wholegrains and health: Many benefits but do contaminants pose any risk? Nutr. Bull. 2019;44:107–115. doi: 10.1111/nbu.12379. [DOI] [Google Scholar]
- 139.Nugraha A., Khotimah K., Rietjens I.M. Risk assessment of aflatoxin B1 exposure from maize and peanut consumption in Indonesia using the margin of exposure and liver cancer risk estimation approaches. Food Chem. Toxicol. 2018;113:134–144. doi: 10.1016/j.fct.2018.01.036. [DOI] [PubMed] [Google Scholar]
- 140.Nurerk P., Bunkoed W., Kanatharana P., Bunkoed O. A miniaturized solid-phase extraction adsorbent of calix[4]arene-functionalized graphene oxide/polydopamine-coated cellulose acetate for the analysis of aflatoxins in corn. J. Sep. Sci. 2018;41:3892–3901. doi: 10.1002/jssc.201800440. [DOI] [PubMed] [Google Scholar]
- 141.Oliveira M., Vasconcelos V. Occurrence of Mycotoxins in Fish Feed and Its Effects: A Review. Toxins. 2020;12:160. doi: 10.3390/toxins12030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ortega-Beltran A., Cotty P.J. Frequent shifts in Aspergillus flavus populations associated with maize production in sonora, mexico. Phytopathology. 2018;108:412–420. doi: 10.1094/PHYTO-08-17-0281-R. [DOI] [PubMed] [Google Scholar]
- 143.Palacios-Rojas N., McCulley L., Kaeppler M., Titcomb T.J., Gunaratna N.S., Lopez-Ridaura S., Tanumihardjo S.A. Mining maize diversity and improving its nutritional aspects within agro-food systems. Compr. Rev. Food Sci. Food Saf. 2020;19:1809–1834. doi: 10.1111/1541-4337.12552. [DOI] [PubMed] [Google Scholar]
- 144.Pascale M., Logrieco A.F., Graeber M., Hirschberger M., Reichel M., Lippolis V., De Girolamo A., Lattanzio V.M.T., Slettengren K. Aflatoxin reduction in maize by industrial-scale cleaning solutions. Toxins. 2020;12:331. doi: 10.3390/toxins12050331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Paterson R.R.M., Venâncio A., Lima N., Guilloux-Bénatier M., Rousseaux S. Predominant mycotoxins, mycotoxigenic fungi and climate change related to wine. Food Res. Int. 2018;103:478–491. doi: 10.1016/j.foodres.2017.09.080. [DOI] [PubMed] [Google Scholar]
- 146.Peles F., Sipos P., Győri Z., Pfliegler W.P., Giacometti F., Serraino A., Pagliuca G., Gazzotti T., Pócsi I. Adverse effects, transformation and channeling of aflatoxins into food raw materials in livestock. Front. Microbiol. 2019;10:2861. doi: 10.3389/fmicb.2019.02861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Peña-Rodas O., Martinez-Lopez R., Hernandez-Rauda R. Occurrence of Aflatoxin M1 in cow milk in El Salvador: Results from a two-year survey. Toxicol. Rep. 2018;5:671–678. doi: 10.1016/j.toxrep.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Peña-Rodas O., Martinez-Lopez R., Pineda-Rivas M., Hernandez-Rauda R. Aflatoxin M1 in Nicaraguan and locally made hard white cheeses marketed in El Salvador. Toxicol. Rep. 2020;7:1157–1163. doi: 10.1016/j.toxrep.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Perczak A., Goliński P., Bryła M., Waśkiewicz A. The efficiency of lactic acid bacteria against pathogenic fungi and mycotoxins. Arh. za Hig. Rada i Toksikol. 2018;69:32–45. doi: 10.2478/aiht-2018-69-3051. [DOI] [PubMed] [Google Scholar]
- 150.Pimpitak U., Rengpipat S., Phutong S., Buakeaw A., Komolpis K. Development and validation of a lateral flow immunoassay for the detection of aflatoxin m1 in raw and commercialised milks. Int. J. Dairy Technol. 2020;73:695–705. doi: 10.1111/1471-0307.12728. [DOI] [Google Scholar]
- 151.Pleadin J., Zadravec M., Lešić T., Frece J., Markov K., Vasilj V. Climate change—A potential threat for increasing occurrences of mycotoxins. Vet. Stanica. 2020;51 [Google Scholar]
- 152.Ponce-García N., Serna-Saldivar S.O., Garcia-Lara S. Fumonisins and their analogues in contaminated corn and its processed foods—A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018;35:2183–2203. doi: 10.1080/19440049.2018.1502476. [DOI] [PubMed] [Google Scholar]
- 153.Poór M., Bálint M., Hetényi C., Gődér B., Kunsági-Máté S., Kőszegi T., Lemli B. Investigation of non-covalent interactions of aflatoxins (b1, b2, g1, g2, and m1) with serum albumin. Toxins. 2017;9:339. doi: 10.3390/toxins9110339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Porto Y.D., Trombete F.M., Freitas-Silva O., de Castro I.M., Direito G.M., Ascheri J.L.R. Gaseous ozonation to reduce aflatoxins levels and microbial contamination in corn grits. Microorganisms. 2019;7:220. doi: 10.3390/microorganisms7080220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Qin M., Liang J., Yang D., Yang X., Cao P., Wang X., Ma N., Zhang L. Spatial analysis of dietary exposure of aflatoxins in peanuts and peanut oil in different areas of China. Food Res. Int. 2021;140:109899. doi: 10.1016/j.foodres.2020.109899. [DOI] [PubMed] [Google Scholar]
- 156.Ráduly Z., Szabó L., Madar A., Pócsi I., Csernoch L. Toxicological and medical aspects of Aspergillus-derived mycotoxins entering the feed and food chain. Front. Microbiol. 2020;10:2908. doi: 10.3389/fmicb.2019.02908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Raiten D.J., Aimone A.M. The intersection of climate/environment, food, nutrition and health: Crisis and opportunity. Curr. Opin. Biotechnol. 2017;44:52–62. doi: 10.1016/j.copbio.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 158.Renaud J.B., Miller J.D., Sumarah M.W. Mycotoxin testing paradigm: Challenges and opportunities for the future. J. AOAC Int. 2019;102:1681–1688. doi: 10.1093/jaoac/102.6.1681. [DOI] [PubMed] [Google Scholar]
- 159.Ricciardi W., Marcheggiani S., Puccinelli C., Carere M., Sofia T., Giuliano F., Dogliotti E., Mancini L., Agrimi U., Alleva E., et al. Health and climate change: Science calls for global action. Ann. dell’Istituto Super. di Sanita. 2019;55:323–329. doi: 10.4415/ANN_19_04_04. [DOI] [PubMed] [Google Scholar]
- 160.Righetti L., Paglia G., Galaverna G., Dall’Asta C. Recent advances and future challenges in modified mycotoxin analysis: Why hrms has become a key instrument in food contaminant research. Toxins. 2016;8:361. doi: 10.3390/toxins8120361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rushing B.R., Selim M.I. Adduction to arginine detoxifies aflatoxin b1 by eliminating genotoxicity and altering in vitro toxicokinetic profiles. Oncotarget. 2018;9:4559–4570. doi: 10.18632/oncotarget.23382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rushing B.R., Selim M.I. Aflatoxin b1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019;124:81–100. doi: 10.1016/j.fct.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 163.Šarkanj B., Ezekiel C.N., Turner P.C., Abia W.A., Rychlik M., Krska R., Sulyok M., Warth B. Ultra-sensitive, stable isotope assisted quantification of multiple urinary mycotoxin exposure biomarkers. Anal. Chim. Acta. 2018;1019:84–92. doi: 10.1016/j.aca.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 164.Sarrocco S., Mauro A., Battilani P. Use of competitive filamentous fungi as an alternative approach for mycotoxin risk reduction in staple cereals: State of art and future perspectives. Toxins. 2019;11:701. doi: 10.3390/toxins11120701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Satterlee T., Cary J.W., Calvo A.M. Rmta, a putative arginine methyltransferase, regulates secondary metabolism and development in Aspergillus flavus. PLoS ONE. 2016;11:e0155575. doi: 10.1371/journal.pone.0155575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Savić Z., Dudaš T., Loc M., Grahovac M., Budakov D., Jajić I., Krstović S., Barošević T., Krska R., Sulyok M., et al. Biological control of aflatoxin in maize grown in serbia. Toxins. 2020;12:162. doi: 10.3390/toxins12030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schaarschmidt S., Fauhl-Hassek C. The fate of mycotoxins during secondary food processing of maize for human consumption. Compr. Rev. Food Sci. Food Saf. 2021;20:91–148. doi: 10.1111/1541-4337.12657. [DOI] [PubMed] [Google Scholar]
- 168.Schaarschmidt S., Fauhl-Hassek C. The fate of mycotoxins during the primary food processing of maize. Food Control. 2021;121:107651. doi: 10.1016/j.foodcont.2020.107651. [DOI] [PubMed] [Google Scholar]
- 169.Schrenk D., Bignami M., Bodin L., Chipman J.K., del Mazo J., Grasl-Kraupp B., Hogstrand C., Hoogenboom L., Leblanc J.C., Nebbia C.S., et al. Risk assessment of aflatoxins in food. EFSA J. 2020;18:e06040. doi: 10.2903/j.efsa.2020.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Singh P., Callicott K.A., Orbach M.J., Cotty P.J. Molecular Analysis of S-morphology Aflatoxin Producers from the United States Reveals Previously Unknown Diversity and Two New Taxa. Front. Microbiol. 2020;11:1236. doi: 10.3389/fmicb.2020.01236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Smith J.W., Groopman J.D. Encyclopedia of Cancer. Elsevier; Amsterdam, The Netherlands: 2018. Aflatoxins; pp. 30–43. [Google Scholar]
- 172.Soares R.R.G., Ricelli A., Fanelli C., Caputo D., De Cesare G., Chu V., Aires-Barros M.R., Conde J.P. Advances, challenges and opportunities for point-of-need screening of mycotoxins in foods and feeds. Analyst. 2018;143:1015–1035. doi: 10.1039/C7AN01762F. [DOI] [PubMed] [Google Scholar]
- 173.Sojinrin T., Liu K., Wang K., Cui D., Byrne H.J., Curtin J.F., Tian F. Developing Gold Nanoparticles-Conjugated Aflatoxin B1 Antifungal Strips. Int. J. Mol. Sci. 2019;20:6260. doi: 10.3390/ijms20246260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Söylemez T., Yamaç M., Yıldız Z. Statistical optimization of cultural variables for enzymatic degradation of aflatoxin b1 by Panus neostrigosus. Toxicon. 2020;186:141–150. doi: 10.1016/j.toxicon.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 175.Steiner D., Sulyok M., Malachová A., Mueller A., Krska R. Realizing the simultaneous liquid chromatography-tandem mass spectrometry based quantification of >1200 biotoxins, pesticides and veterinary drugs in complex feed. J. Chromatogr. A. 2020;1629:461502. doi: 10.1016/j.chroma.2020.461502. [DOI] [PubMed] [Google Scholar]
- 176.Stepman F. Scaling-up the impact of aflatoxin research in africa. The role of social sciences. Toxins. 2018;10:136. doi: 10.3390/toxins10040136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sun Y., Liu Z., Liu D., Chen J., Gan F., Huang K. Low-Level Aflatoxin B1 Promotes Influenza Infection and Modulates a Switch in Macrophage Polarization from M1 to M2. Cell. Physiol. Biochem. 2018;49:1151–1167. doi: 10.1159/000493294. [DOI] [PubMed] [Google Scholar]
- 178.Sun Y., Su J., Liu Z., Liu D., Gan F., Chen X., Huang K. Aflatoxin b1 promotes influenza replication and increases virus related lung damage via activation of tlr4 signaling. Front. Immunol. 2018;9:2297. doi: 10.3389/fimmu.2018.02297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sun Y., Su J., Yang S., Liu Z., Liu D., Gan F., Chen X., Huang K. Mannan oligosaccharide protects against the aflatoxin-b1-promoted influenza replication and tissue damages in a toll-like-receptor-4-dependent manner. J. Agric. Food Chem. 2019;67:735–745. doi: 10.1021/acs.jafc.8b05829. [DOI] [PubMed] [Google Scholar]
- 180.Szabo B., Toth B., Toldine E.T., Varga M., Kovacs N., Varga J., Kocsube S., Palagyi A., Bagi F., Budakov D., et al. A new concept to secure food safety standards against Fusarium species and Aspergillus flavus and their toxins in maize. Toxins. 2018;10:372. doi: 10.3390/toxins10090372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tacconi C., Cucina M., Pezzolla D., Zadra C., Gigliotti G. Effect of the mycotoxin aflatoxin b1 on a semi-continuous anaerobic digestion process. Waste Manag. 2018;78:467–473. doi: 10.1016/j.wasman.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 182.Thielecke F., Nugent A.P. Contaminants in grain—A major risk for whole grain safety? Nutrients. 2018;10:1213. doi: 10.3390/nu10091213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Toreti A., Bassu S., Ceglar A., Zampieri M. Encyclopedia of Food Security and Sustainability. Elsevier; Amsterdam, The Netherlands: 2018. Climate change and crop yields; pp. 223–227. [Google Scholar]
- 184.Udovicki B., Audenaert K., De Saeger S., Rajkovic A. Overview on the mycotoxins incidence in serbia in the period 2004–2016. Toxins. 2018;10:279. doi: 10.3390/toxins10070279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Udovicki B., Djekic I., Gajdos Kljusuric J., Papageorgiou M., Skendi A., Djugum J., Rajkovic A. Exposure assessment and risk characterization of aflatoxins intake through consumption of maize products in the adult populations of Serbia, Croatia and Greece. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019;36:940–951. doi: 10.1080/19440049.2019.1600748. [DOI] [PubMed] [Google Scholar]
- 186.Udovicki B., Djekic I., Stankovic S., Obradovic A., Rajkovic A. Impact of climatic conditions on fumonisins in maize grown in Serbia. World Mycotoxin J. 2019;12:183–190. doi: 10.3920/WMJ2018.2364. [DOI] [Google Scholar]
- 187.Uka V., Cary J.W., Lebar M.D., Puel O., De Saeger S., Diana Di Mavungu J. Chemical repertoire and biosynthetic machinery of the Aspergillus flavus secondary metabolome: A review. Compr. Rev. Food Sci. Food Saf. 2020;19:2797–2842. doi: 10.1111/1541-4337.12638. [DOI] [PubMed] [Google Scholar]
- 188.Valencia-Quintana R., Milić M., Jakšić D., Klarić M.Š., Tenorio-Arvide M.G., Pérez-Flores G.A., Bonassi S., Sánchez-Alarcón J. Environment changes, aflatoxins, and health issues, a review. Int. J. Environ. Res. Public Health. 2020;17:7850. doi: 10.3390/ijerph17217850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.van der Fels-Klerx H.J., Camenzuli L. Effects of milk yield, feed composition, and feed contamination with aflatoxin b1 on the aflatoxin m1 concentration in dairy cows’ milk investigated using monte carlo simulation modelling. Toxins. 2016;8:290. doi: 10.3390/toxins8100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Van Der Fels-Klerx H.J., Liu C., Battilani P. Modelling climate change impacts on mycotoxin contamination. World Mycotoxin J. 2016;9:717–726. doi: 10.3920/WMJ2016.2066. [DOI] [Google Scholar]
- 191.Van der Fels-Klerx H.J., Vermeulen L.C., Gavai A.K., Liu C. Climate change impacts on aflatoxin b1 in maize and aflatoxin m1 in milk: A case study of maize grown in eastern europe and imported to the netherlands. PLoS ONE. 2019;14:e0218956. doi: 10.1371/journal.pone.0218956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vandicke J., De Visschere K., Croubels S., De Saeger S., Audenaert K., Haesaert G. Mycotoxins in flanders’ fields: Occurrence and correlations with Fusarium species in whole-plant harvested maize. Microorganisms. 2019;7:571. doi: 10.3390/microorganisms7110571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Verheecke C., Liboz T., Mathieu F. Microbial degradation of aflatoxin b1: Current status and future advances. Int. J. Food Microbiol. 2016;237:1–9. doi: 10.1016/j.ijfoodmicro.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 194.Viegas S., Assunção R., Martins C., Nunes C., Osteresch B., Twarużek M., Kosicki R., Grajewski J., Ribeiro E., Viegas C. Occupational exposure to mycotoxins in swine production: Environmental and biological monitoring approaches. Toxins. 2019;11:78. doi: 10.3390/toxins11020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Viegas S., Assunção R., Nunes C., Osteresch B., Twarużek M., Kosicki R., Grajewski J., Martins C., Alvito P., Almeida A., et al. Exposure assessment to mycotoxins in a portuguese fresh bread dough company by using a multi-biomarker approach. Toxins. 2018;10:342. doi: 10.3390/toxins10090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Viegas S., Assunção R., Twarużek M., Kosicki R., Grajewski J., Viegas C. Mycotoxins feed contamination in a dairy farm—Potential implications for milk contamination and workers’ exposure in a one health approach. J. Sci. Food Agric. 2020;100:1118–1123. doi: 10.1002/jsfa.10120. [DOI] [PubMed] [Google Scholar]
- 197.Wacoo A.P., Wendiro D., Nanyonga S., Hawumba J.F., Sybesma W., Kort R. Feasibility of a novel on-site detection method for aflatoxin in maize flour from markets and selected households in kampala, uganda. Toxins. 2018;10:327. doi: 10.3390/toxins10080327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Windham G.L., Williams W.P., Mylroie J.E., Reid C.X., Womack E.D. A histological study of Aspergillus flavus colonization of wound inoculated maize kernels of resistant and susceptible maize hybrids in the field. Front. Microbiol. 2018;9:799. doi: 10.3389/fmicb.2018.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Winter G., Pereg L. A review on the relation between soil and mycotoxins: Effect of aflatoxin on field, food and finance. Eur. J. Soil Sci. 2019;70:882–897. doi: 10.1111/ejss.12813. [DOI] [Google Scholar]
- 200.Yu J., Hennessy D.A., Wu F. The impact of bt corn on aflatoxin-related insurance claims in the united states. Sci. Rep. 2020;10:1–10. doi: 10.1038/s41598-020-75643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhao X., Wei J., Zhou Y., Kong W., Yang M. Quality evaluation of alpinia oxyphylla after Aspergillus flavus infection for storage conditions optimization. AMB Express. 2017;7:151. doi: 10.1186/s13568-017-0450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhou X., Gan F., Hou L., Liu Z., Su J., Lin Z., Le G., Huang K. Aflatoxin b1 induces immunotoxicity through the DNA methyltransferase-mediated jak2/stat3 pathway in 3d4/21 cells. J. Agric. Food Chem. 2019;67:3772–3780. doi: 10.1021/acs.jafc.8b07309. [DOI] [PubMed] [Google Scholar]
- 203.Review of the Draft Interagency Report on the Impacts of Climate Change on Human Health in the United States. The National Academies Press; Washington, DC, USA: 2015. pp. 1–78. [Google Scholar]
- 204.Battilani P., Camardo Leggieri M. Predictive modelling of aflatoxin contamination to support maize chain management. World Mycotoxin J. 2015;8:161–170. doi: 10.3920/WMJ2014.1740. [DOI] [Google Scholar]
- 205.Clark G.C., Casewell N.R., Elliott C.T., Harvey A.L., Jamieson A.G., Strong P.N., Turner A.D. Friends or foes? Emerging impacts of biological toxins. Trends Biochem. Sci. 2019;44:365–379. doi: 10.1016/j.tibs.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 206.Csáki K.F., Szabó M.S., Túri M.S. Possibilities for the decrease of aflatoxin contamination in food chain. Elelmvizsg. Kozl. 2014;60:68–79. [Google Scholar]
- 207.De Nijs M., Mengelers M.J.B., Boon P.E., Heyndrickx E., Hoogenboom L.A.P., Lopez P., Mol H.G.J. Strategies for estimating human exposure to mycotoxins via food. World Mycotoxin J. 2016;9:831–845. doi: 10.3920/WMJ2016.2045. [DOI] [Google Scholar]
- 208.Donohoe T., Garnett K., Lansink A.O., Afonso A., Noteborn H. Emerging risks identification on food and feed—Efsa. EFSA J. 2018;16:e05359. doi: 10.2903/j.efsa.2018.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Eskola M., Altieri A., Galobart J. Overview of the activities of the european food safety authority on mycotoxins in food and feed. World Mycotoxin J. 2018;11:277–289. doi: 10.3920/WMJ2017.2270. [DOI] [Google Scholar]
- 210.Gilbert M.K., Mack B.M., Payne G.A., Bhatnagar D. Use of functional genomics to assess the climate change impact on Aspergillus flavus and aflatoxin production. World Mycotoxin J. 2016;9:665–672. doi: 10.3920/WMJ2016.2049. [DOI] [Google Scholar]
- 211.Giorni P., Camardo Leggieri M., Magan N., Battilani P. Comparison of temperature and moisture requirements for sporulation of Aspergillus flavus sclerotia on natural and artificial substrates. Fungal Biol. 2012;116:637–642. doi: 10.1016/j.funbio.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 212.Gómez J.V., Tarazona A., Mateo F., Jiménez M., Mateo E.M. Potential impact of engineered silver nanoparticles in the control of aflatoxins, ochratoxin a and the main aflatoxigenic and ochratoxigenic species affecting foods. Food Control. 2019;101:58–68. doi: 10.1016/j.foodcont.2019.02.019. [DOI] [Google Scholar]
- 213.Gruber-Dorninger C., Jenkins T., Schatzmayr G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins. 2019;11:375. doi: 10.3390/toxins11070375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hernández-Martínez R., Navarro-Blasco I. Surveillance of aflatoxin content in dairy cow feedstuff from navarra (Spain) Anim. Feed Sci. Technol. 2015;200:35–46. doi: 10.1016/j.anifeedsci.2014.12.002. [DOI] [Google Scholar]
- 215.Kanapitsas A., Batrinou A., Aravantinos A., Sflomos C., Markaki P. Gamma radiation inhibits the production of ochratoxin a by Aspergillus carbonarius. Development of a method for ota determination in raisins. Food Biosci. 2016;15:42–48. doi: 10.1016/j.fbio.2016.05.001. [DOI] [Google Scholar]
- 216.Kovač T., Šarkanj B., Klapec T., Borišev I., Kovač M., Nevistić A., Strelec I. Fullerol c60(oh)24 nanoparticles and mycotoxigenic fungi: A preliminary investigation into modulation of mycotoxin production. Environ. Sci. Pollut. Res. 2017;24:16673–16681. doi: 10.1007/s11356-017-9214-z. [DOI] [PubMed] [Google Scholar]
- 217.Lagogianni C.S., Tsitsigiannis D.I. Effective chemical management for prevention of aflatoxins in maize. Phytopathol. Mediterr. 2018;57:186–197. [Google Scholar]
- 218.Lagogianni C.S., Tsitsigiannis D.I. Effective biopesticides and biostimulants to reduce aflatoxins in maize fields. Front. Microbiol. 2019;10:2645. doi: 10.3389/fmicb.2019.02645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lulamba T.E., Stafford R.A., Njobeh P.B. A sub-saharan african perspective on mycotoxins in beer—A review. J. Inst. Brew. 2019;125:184–199. doi: 10.1002/jib.558. [DOI] [Google Scholar]
- 220.Magan N., Medina A. Mycotoxins, food security and climate change: Do we know enough? Microbiol. Today. 2016;43:10–13. [Google Scholar]
- 221.Malissiova E., Manouras A. Monitoring aflatoxin m1 levels in donkey milk produced in greece, intended for human consumption. World Mycotoxin J. 2017;10:203–206. doi: 10.3920/WMJ2017.2169. [DOI] [Google Scholar]
- 222.Malissiova E., Tsakalof A., Arvanitoyannis I.S., Katsafliaka A., Katsioulis A., Tserkezou P., Koureas M., Govaris A., Hadjichristodoulou C. Monitoring aflatoxin m1 levels in ewe’s and goat’s milk in thessaly, greece; potential risk factors under organic and conventional production schemes. Food Control. 2013;34:241–248. doi: 10.1016/j.foodcont.2013.04.035. [DOI] [Google Scholar]
- 223.Manouras A., Malissiova E. Occurrence of aflatoxins in compound feeds and feed materials for dairy livestock in central greece. Hell. Veter- Med Soc. 2015;66:169–176. doi: 10.12681/jhvms.15860. [DOI] [Google Scholar]
- 224.Mateo E.M., Gómez J.V., Gimeno-Adelantado J.V., Romera D., Mateo-Castro R., Jiménez M. Assessment of azole fungicides as a tool to control growth of Aspergillus flavus and aflatoxin b1 and b2 production in maize. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017;34:1039–1051. doi: 10.1080/19440049.2017.1310400. [DOI] [PubMed] [Google Scholar]
- 225.Matumba L., Sulyok M., Monjerezi M., Biswick T., Krska R. Fungal metabolites diversity in maize and associated human dietary exposures relate to micro-climatic patterns in malawi. World Mycotoxin J. 2015;8:269–282. doi: 10.3920/WMJ2014.1773. [DOI] [Google Scholar]
- 226.Medina A., Rodriguez A., Magan N. Changes in environmental factors driven by climate change: Effects on the ecophysiology of mycotoxigenic fungi. In: Botana M.J., Sainz L.M., editors. Climate Change and Mycotoxins. Walter de Gruyter GmbH; Berlin, Germany: 2015. pp. 71–90. [Google Scholar]
- 227.Medina Á., Rodríguez A., Magan N. Climate change and mycotoxigenic fungi: Impacts on mycotoxin production. Curr. Opin. Food Sci. 2015;5:99–104. doi: 10.1016/j.cofs.2015.11.002. [DOI] [Google Scholar]
- 228.Moretti A., Logrieco A.F. Climate change effects on the biodiversity of mycotoxigenic fungi and their mycotoxins in preharvest conditions in europe. In: Botana M.J., Sainz L.M., editors. Climate Change and Mycotoxins. Walter de Gruyter GmbH; Berlin, Germany: 2015. pp. 91–108. [Google Scholar]
- 229.Nazari L., Manstretta V., Rossi V. A non-linear model for temperature-dependent sporulation and t-2 and ht-2 production of Fusarium langsethiae and Fusarium sporotrichioides. Fungal Biol. 2016;120:562–571. doi: 10.1016/j.funbio.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 230.Pangga I.B., Hanan J., Chakraborty S. Climate change impacts on plant canopy architecture: Implications for pest and pathogen management. European J. Plant Pathol. 2013;135:595–610. doi: 10.1007/s10658-012-0118-y. [DOI] [Google Scholar]
- 231.Pangga I.B., Salvacion A.R., Cumagun C.J.R. Climate change and plant diseases caused by mycotoxigenic fungi: Implications for food security. In: Botana M.J., Sainz L.M., editors. Climate Change and Mycotoxins. Walter de Gruyter GmbH; Berlin, Germany: 2015. pp. 1–28. [Google Scholar]
- 232.Paris M.P.K., Liu Y.J., Nahrer K., Binder E.M. Climate change impacts on mycotoxin production. In: Botana M.J., Sainz L.M., editors. Climate Change and Mycotoxins. Walter de Gruyter GmbH; Berlin, Germany: 2015. pp. 133–151. [Google Scholar]
- 233.Pautasso M., Petter F., Rortais A., Roy A.S. Emerging risks to plant health: A european perspective. CAB Reviews: Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2015;10:1–16. doi: 10.1079/PAVSNNR201510021. [DOI] [Google Scholar]
- 234.Pecorelli I., Guarducci N., Von Holst C., Bibi R., Pascale M., Ciasca B., Logrieco A.F., Lattanzio V.M.T. Critical comparison of analytical performances of two immunoassay methods for rapid detection of aflatoxin m1 in milk. Toxins. 2020;12:270. doi: 10.3390/toxins12040270. [DOI] [Google Scholar]
- 235.Robinson T., Altieri A., Chiusolo A., Dorne J.L., Goumperis T., Rortais A., Deluyker H., Silano V., Liem D. Efsa’s approach to identifying emerging risks in food and feed: Taking stock and looking forward. EFSA J. 2012;10:s1015. doi: 10.2903/j.efsa.2012.s1015. [DOI] [Google Scholar]
- 236.Rodríguez-Blanco M., Ramos A.J., Prim M., Sanchis V., Marín S. Usefulness of the analytical control of aflatoxins in feedstuffs for dairy cows for the prevention of aflatoxin m1 in milk. Mycotoxin Res. 2020;36:11–22. doi: 10.1007/s12550-019-00362-y. [DOI] [PubMed] [Google Scholar]
- 237.Sainz M.J., Alfonso A., Botana L.M. Climate Change and Mycotoxins. De Gruyter; Berlin, Germany: 2015. Considerations about international mycotoxin legislation, food security, and climate change; pp. 153–179. [Google Scholar]
- 238.Sckokai P., Veneziani M., Moro D., Castellari E. Consumer willingness to pay for food safety: The case of mycotoxins in milk. Bio-Based Appl. Econ. 2014;3:63–81. [Google Scholar]
- 239.Torović L. Aflatoxin m1 in processed milk and infant formulae and corresponding exposure of adult population in serbia in 2013–2014. Food Addit. Contam. Part B Surveill. 2015;8:235–244. doi: 10.1080/19393210.2015.1063094. [DOI] [PubMed] [Google Scholar]
- 240.Trevisani M., Farkas Z., Serraino A., Zambrini A.V., Pizzamiglio V., Giacometti F., Ambrus Á. Analysis of industry-generated data. Part 1: A baseline for the development of a tool to assist the milk industry in designing sampling plans for controlling aflatoxin M1 in milk. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014;31:1246–1256. doi: 10.1080/19440049.2014.925587. [DOI] [PubMed] [Google Scholar]
- 241.Van de Perre E., Jacxsens L., Liu C., Devlieghere F., De Meulenaer B. Climate impact on alternaria moulds and their mycotoxins in fresh produce: The case of the tomato chain. Food Res. Int. 2015;68:41–46. doi: 10.1016/j.foodres.2014.10.014. [DOI] [Google Scholar]
- 242.Van der Fels-Klerx H.J., Olesen J.E., Madsen M.S., Goedhart P.W. Climate change increases deoxynivalenol contamination of wheat in north-western europe. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012;29:1593–1604. doi: 10.1080/19440049.2012.691555. [DOI] [PubMed] [Google Scholar]
- 243.Drasticdata Tool. [(accessed on 26 February 2021)]; Available online: https://www.drasticdata.nl/index.htm.
- 244.Dobolyi C., Sebok F., Varga J., Kocsube S., Szigeti G., Baranyi N., Szecsi A., Toth B., Varga M., Kriszt B., et al. Occurrence of aflatoxin producing Aspergillus flavus isolates in maize kernel in hungary. Acta Aliment. (Budapest) 2013;42:451–459. doi: 10.1556/AAlim.42.2013.3.18. [DOI] [Google Scholar]
- 245.Levic J., Gosic-Dondo S., Ivanovic D., Stankovic S., Krnjaja V., Bocarov-Stancic A., Stepanic A. An outbreak of Aspergillus species in response to environmental conditions in Serbia. Pestic. i Fitomedicina. 2013;28:167–179. doi: 10.2298/PIF1303167L. [DOI] [Google Scholar]
- 246.Giorni P., Magan N., Pietri A., Bertuzzi T., Battilani P. Studies on Aspergillus section flavi isolated in northern Italy from maize. Int. J. Food Microbiol. 2007;113:330–338. doi: 10.1016/j.ijfoodmicro.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 247.Mauro A., Battilani P., Cotty P.J. Atoxigenic Aspergillus flavus endemic to italy for biocontrol of aflatoxins in maize. BioControl. 2015;60:125–134. doi: 10.1007/s10526-014-9624-5. [DOI] [Google Scholar]
- 248.Warnatzsch E.A., Reay D.S., Camardo Leggieri M., Battilani P. Climate change impact on aflatoxin contamination risk in malawi’s maize crops. Front. Sustain. Food Syst. 2020;4:238. doi: 10.3389/fsufs.2020.591792. [DOI] [Google Scholar]
- 249.Giorni P., Bertuzzi T., Battilani P. Impact of fungi co-occurrence on mycotoxin contamination in maize during the growing season. Front. Microbiol. 2019;10:1265. doi: 10.3389/fmicb.2019.01265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Palumbo R., Goncalves A., Gkrillas A., Logrieco A., Dorne J.L., Dall’Asta C., Venancio A., Battilani P. Mycotoxins in maize: Mitigation actions, with a chain management approach. Phytopathol. Mediterr. 2020;59:5–28. [Google Scholar]
- 251.Marín S., Freire L., Femenias A., Sant’Ana A.S. Use of predictive modelling as tool for prevention of fungal spoilage at different points of the food chain. Curr. Opin. Food Sci. 2021;41:1–7. doi: 10.1016/j.cofs.2021.02.006. [DOI] [Google Scholar]
- 252.Miedaner T., Juroszek P. Global warming and increasing maize cultivation demand comprehensive efforts in disease and insect resistance breeding in north-western europe. Plant Pathol. 2021 doi: 10.1111/ppa.13365. [DOI] [Google Scholar]
- 253.Fanzo J., Bellows A.L., Spiker M.L., Thorne-Lyman A.L., Bloem M.W. The importance of food systems and the environment for nutrition. Am. J. Clin. Nutr. 2021;113:7–16. doi: 10.1093/ajcn/nqaa313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.