Abstract
Objective
Ketamine is an anaesthetic agent with a unique dissociative profile and pharmacological effects ranging from the induction and maintenance of anaesthesia to analgesia and sedation, depending on the dose. This article provides information for the clinical use of ketamine in anaesthesia, in both conventional and special circumstances.
Methods
This is a non-systematic review of the literature, through a PubMed search up to February 2021.
Results
With a favourable pharmacokinetic profile, ketamine is used in hospital and prehospital settings for emergency situations. It is suitable for patients with many heart conditions and, unlike other anaesthetics, its potential for cardiorespiratory depression is low. Furthermore, it may be used when venous access is difficult as it may be administered through various routes. Ketamine is the anaesthetic of choice for patients with bronchospasm thanks to its bronchodilatory and anti-inflammatory properties.
Conclusion
With a favourable pharmacokinetic profile, ketamine is used in hospital and prehospital settings for emergency situations and is suitable for patients with many cardiac and respiratory conditions.
Keywords: anaesthesia, dissociative profile, ketamine
Introduction
Ketamine is an anaesthetic agent with a unique dissociative profile, with pharmacological effects ranging from the induction and maintenance of anaesthesia to analgesia and sedation, depending on the dose. Additional effects include bronchodilation, stimulation of the sympathetic nervous system, catalepsy and psychiatric effects, including rapid and sustained antidepressant activity.1–4 Although these activities may be valuable in anaesthesia representing interesting advantages for special patient subgroups, such as those with respiratory or cardiovascular conditions, a possible psychotropic activity and other central effects have limited the use of ketamine as an anaesthetic in clinical practice.
This non-systematic review of the literature presents useful information for the clinical use of ketamine in anaesthesia, in both conventional and special circumstances.
Methods
For this review of the literature, a non-systematic search was performed in PubMed using the following keywords to retrieve pharmacological data: “ketamine”, “NMDA”, “GABA”, “receptor”, “pharmacodynamics”, “pharmacokinetics”, “pain”, “central nervous system”. A search with the keywords “ketamine”, “anesthesia”, “administration route”, “bronchodilation”, “hemodynamics”, “congenital heart disease”, “burn”, “pain”, “emergence phenomena” and “adverse event” was performed to review clinical uses. Articles in English, published up to February 2021, were included.
Review
Pharmacological aspects
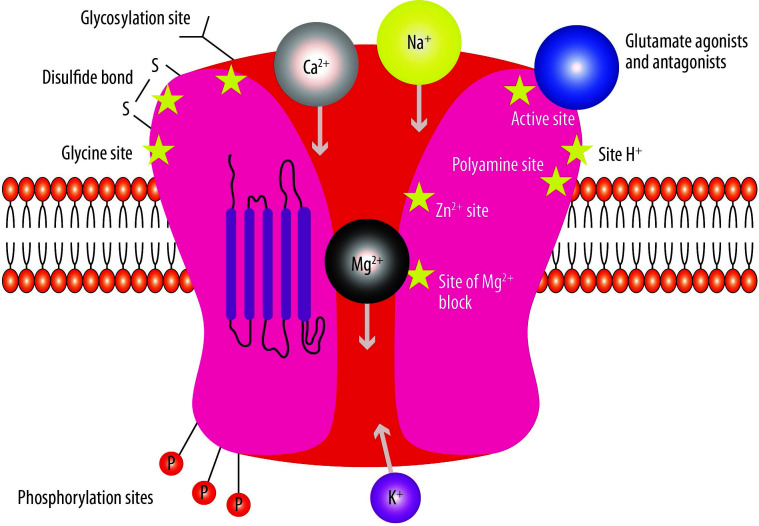
Since its synthesis in 1962, several clinical applications have been described for the phencyclidine derivative ketamine, including anaesthesia, pain management and psychiatry.5 Many therapeutic activities of ketamine have been linked to its antagonism to the N-methyl-D-aspartate (NMDA) receptor (NMDAR) (Figure 1).6 In the early 1980s, it was discovered that ketamine blocks the NMDAR by binding to a specific site (referred to as the phencyclidine (PCP) site) in an non-competitive way.7 As PCP is localized inside the NMDA channel, it can be reached and bound to only when the receptor is activated.8 Furthermore, the ability of ketamine to bind to and dissociate from the PCP binding site in vivo is dictated by the degree of activation of the receptor, which depends on glutamate release in the synapses, cell membrane depolarization and the levels of other modulatory factors (Figure 2).9 The affinity of ketamine to the NMDAR is similar to that of other non-competitive NMDA antagonists. In rodents, higher-affinity NMDA antagonists determine neurotoxicity, neuronal vacuolization and neurodegeneration, although this has not been demonstrated in primates.10,11 High-affinity compounds, which are administered in the therapeutic dose range, determine learning and memory impairment, sedation, ataxia, and psychotomimetic effects, such as hallucinations in humans, whereas low-affinity blockers, such as memantine, seem to have a better therapeutic index and an activity similar to that of magnesium, which is an endogenous NMDA channel blocker.10,12
Figure 1.
The N-methyl-D-aspartate (NMDA) receptor representation.
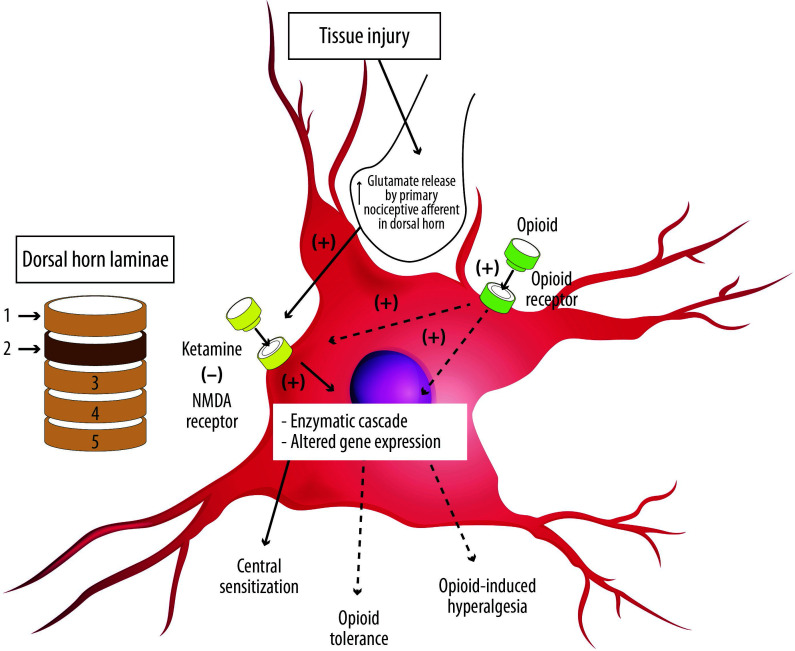
Figure 2.
The activated primary nociceptive afferent from the periphery releases glutamate at the second-order sensory neuron in the dorsal horn of the spinal cord, which binds to N-methyl-D-aspartate (NMDA) receptors. Ketamine blocks the NMDA receptor, which attenuates the development of central sensitization as well as opioid tolerance and hyperalgesia.
− = block; + = increase.
Adapted from Gorlin et al.22.
The effects of ketamine on the central nervous system (CNS) seem to go beyond NMDAR blocking; several molecular targets and neurophysiological properties are known, although many mechanisms of action remain to be understood. Ketamine interacts with opioid receptors13,14 and blocks monoaminergic reuptake15 and muscarinic receptors16,17 as well as voltage-sensitive ion channels (Table 1).18,19
Table 1.
Pharmacological actions of ketamine.
| Molecular target | Mechanism | Potency (μM) | References |
|---|---|---|---|
| NMDA receptor (PCP site) | Antagonism | Ki 0.4–46 IC50 1.6–6.2 |
6,7,9,138 |
| μ-Opioid receptors | Agonism | Ki 27 | 13,14,139 |
| δ-Opioid receptor | Agonism | Ki 101 | 13,139 |
| k-Opioid receptor | Agonism | Ki 85 | 13 |
| Sigma receptor | Agonism | Ki 66 | 13 |
| Noradrenaline transporter | Inhibition of reuptake | Ki 67 | 14,140 |
| Dopamine transporter | Inhibition of reuptake | Ki 162 | 140 |
| Serotonin transporter binding | Inhibition of reuptake | Ki 67 | 140 |
| Muscarinic, nicotinic receptor | Antagonism | IC50: >50 or 100 | 16,17 |
| Voltage-dependent Na2+, Ca2+ channels | Block | Ki 67 | 19,21 |
| Dopamine D2 | Partial agonism | Ki 0.5 | 141,142 |
| Serotonin 5HT2 | Antagonism | Ki 15 | 141 |
IC50, half-maximum inhibitory concentration; Ki, inhibitory constant; NMDA, N-methyl-D-aspartate.
It is well known that the affinity of ketamine to opioid receptors may be relevant at high doses and related to its anaesthetic activity, whilst low doses (≤0.3 mg/kg intravenous (i.v.)) have an analgesic effect.20–22 Topically administered ketamine displays local anaesthetic properties due to the ability to block the conductance of ion channels.23
Due to the lipid solubility of ketamine and its relatively low protein binding (about 20–50%), a considerably large volume of distribution (3–5 L/kg) is attained after either an i.v. or an intramuscular (i.m.) bolus dose.24 In addition, ketamine quickly crosses the blood–brain barrier, and concentrations in the cerebrospinal fluid may be four- to five-fold higher than in plasma.2 Due to these pharmacokinetic features, the analgesic effect of ketamine has a rapid onset.25
Ketamine is mainly metabolized in the liver and several metabolites have been identified. Although there are some dissonant results regarding the contribution of enzymes to clinical ketamine metabolism, CYP2B6, CYP3A4 and CYP2C9 contribute to the production of norketamine via ring hydroxylation and N-demethylation pathways.26 This primary metabolite is pharmacologically active, with 30% of the anaesthetic and analgesic potency compared with the parent compound, and is further metabolized to 4-, 5- and 6-hydroxynorketamine by CYP2B6 and CYP2A6.27 Children are known to require relatively higher doses of ketamine compared with adults although the pharmacokinetics were found to be similar.28 It is possible that pharmacokinetic modelling may not apply to the paediatric population as analyses were scaled to a standardized 70 kg patient. Thus, dosing by titration to effect is advisable in children whilst dosing by body weight may not be reliable. Elderly patients behave as poor metabolizers, hence a lower dosing is recommended.29 In addition, the effect of metabolic enzyme variants or sex on pharmacokinetics is still unknown.30
Ketamine can be administered through various routes. The more conventional route is i.v. but i.m. injection can be used when venous access is not available, retaining a satisfactory bioavailability (93%).24
Ketamine is used in children and adults as an anaesthetic agent for diagnostic and surgical procedures as either i.v. infusion, i.v. injection or i.m. injection. It is often used for short procedures, but it can be used for longer procedures with additional doses or by i.v. infusion. If skeletal muscle relaxation is desired, a muscle relaxant can be used together with ketamine. Ketamine is recommended for the induction of anaesthesia prior to the administration of other general anaesthetic agents and to supplement other anaesthetic agents. To induce anaesthesia, an infusion of 0.5–2 mg/kg is typically administered with a duration of action of ~5–10 min. Anaesthesia may be maintained using a microdrip infusion of 10–45 μg/kg/min.31
The role of NMDAR in the CNS
Some considerations on the role of NMDA in the CNS may be useful to understand the anaesthetic activity of ketamine. NMDARs are widely expressed in the CNS and play critical roles in excitatory synaptic transmission, excitotoxicity and plasticity by participating in neuronal regeneration and circuit formation, coordinating functional circuits and controlling dendritic growth. Hence, NMDARs are essential to memory, to learning and during development. Excitotoxic events involving NMDARs have also been linked to degenerative diseases such as Alzheimer disease and Huntington disease.
The blockade of NMDARs is neuroprotective in animal models of both stroke and seizure but the therapeutic use of NMDA antagonists has failed in humans due to the development of severe side-effects because NMDARs are essential to physiological neuronal function.32,33
An important element is that the NMDAR hypofunction produced by any mechanism can be psychotogenic, possibly resulting in dopaminergic hyperactivity and behavioural changes characteristic of schizophrenia. However, the mechanism linking NMDAR blockade by ketamine and psychosis remains to be established.32
The role of NMDAR in pain
Peripheral nociceptor activation by high-energy stimuli can evoke pain. On peripheral nociceptor activation, glutamate is released in the dorsal horns of the spinal cord and binds to postsynaptic glutamate ionotropic AMPA and kainate subtype receptors that generate excitatory postsynaptic currents. The summation of multiple sub-threshold excitatory postsynaptic currents in the postsynaptic neuron induces firing of the action potential and transmission of the pain message to second-order nociceptive projecting neurons. However, pain responses are enhanced following repetitive stimulation of nociceptive afferents, leading to the well-known ‘wind-up’ phenomenon, a progressive increase of nociceptive response to each successive stimulus.34,35 Wind-up is a form of short-lasting synaptic plasticity that leads to nociceptive pathway potentiation. In humans, pain wind-up results from a temporal summation of either subjective pain intensity or nociceptive flexor reflexes evoked by repetitive noxious stimuli and can be prevented by low-dose ketamine-blocking NMDARs. Wind-up can only be evoked if small-calibre afferents are involved,35,36 eliciting the release of substance P and calcitonin gene-related peptide along with glutamate in the dorsal horn.37–41 The presence of both glutamate and neuropeptides in the synaptic cleft induces a relatively more prolonged postsynaptic depolarization compared to glutamate alone. Under these conditions, the normally inactive NMDA glutamate receptor unblocks and lets calcium in. Indeed, at normal resting membrane potentials, NMDAR is blocked by magnesium ions in a voltage-dependent manner. Sustained membrane depolarization can reduce the blockage because a lower electrical gradient may force magnesium into the channel. Thus, a prolonged and lasting synaptic activity can amplify current flow through open NMDA channels. When nociceptive inputs are intense and prolonged (i.e. induce high-frequency nociceptor firing), NMDAR is involved, inducing wind-up in second-order nociceptive neurons and leading to short-term central sensitization.42 In the context of inflammation or tissue injury, this short-living synaptic plasticity induces secondary hyperalgesia, which may be an excessive response to nociceptive stimuli out of the primary injury site or stimulation site that can endure until healing occurs and inflammation fades. However, repetitive primary afferent stimulation at frequencies much higher than those that evoke wind-up may induce long-term potentiation (LTP) of the nociceptive pathway.43 LTP is a well-described event in several neural networks and is the neural basis of processes such as learning and memory. LTP mechanisms include the NMDA-mediated elevation of cytosolic Ca2+ in the postsynaptic neuron and subsequent downstream activation of signalling pathways and second messenger systems such as kinases (such as MAPK, PKA, PKC, PI3K and Src) as well as the release of nitric oxide by Ca2+-activated neuronal nitric oxide synthase and the release of prostaglandins by cyclooxygenase enzymes, which may further increase the excitability of these neurons in the long term.44,45 Together, these downstream effects of NMDA activation result in the amplification of pain messages.46
Under normal conditions, high-frequency firing able to induce LTP does not usually occur in nociceptive C-fibres. However, bursts of ectopic activity recorded from nociceptive primary afferents in pain patients with nerve injury can be sufficient to trigger sustained NMDA activation and LTP, which is the neural basis of chronic pain within the spinal cord.47
Central sensitization is a major pathophysiological event common to inflammatory and neuropathic pain. It is important to understand that central sensitization is a physiological and reversible adaptive mechanism during inflammatory pain, whereas it is a pathological, hardly reversible and maladaptive event when neuropathic pain occurs. However, the diverse events that converge onto the mechanism of NMDAR-mediated pronociceptive plasticity and central sensitization can potentially lead to chronic pain regardless of the trigger. When peripheral tissue damage occurs, the subsequent inflammatory process induces changes in peripheral nociceptive endings, resulting in peripheral activation and sensitization and a further increased firing rate that leads to rapid-onset homosynaptic and heterosynaptic facilitation in the dorsal horn of the spinal cord in a short-term wind-up-like manner. However, for some reason, the physiological short-term synaptic potentiation may turn into a longer-term LTP-like increase in synaptic strength and maintenance of central sensitization and hence into chronic pain, which most frequently occurs as a result of maladaptive repair of the injured nervous system in neuropathic pain.48
Clinically, manifestations of central sensitization are hyperalgesia (an increased pain response to mild noxious stimuli) and allodynia (abnormal pain caused by normally innocuous stimuli) and reduction in opioid responsiveness or opioid-induced hyperalgesia, in both neuropathic and inflammatory pain. This state can be prevented or reduced by i.v. infusion of low-dose ketamine either in the setting of acute inflammatory pain49–54 or in chronic pain conditions, including osteoarthritic and rheumatoid pain, neuropathic pain, fibromyalgia, irritable bowel syndrome and migraine.55–66
Based on this rationale, ketamine – the most potent of all NMDA antagonists currently available for use in humans – has been used in various pain states, including acute, chronic and neuropathic pain (Figure 2).
Ketamine advantages in anaesthesia
Ketamine has many attributes yet many drawbacks. It affects the CNS by producing a unique dissociative state wherein a patient’s eyes are open but disconnected from the surroundings, in a cataleptic condition with strong analgesia and sedation.67
Ketamine has undoubted advantages for anaesthetists (Table 2). Its unique pharmacokinetics properties that provide a high bioavailability (between 100% when administered by the i.v. route and 93% for i.m. administration) have led to its prevalent use in hospital and prehospital environments for emergencies. Ketamine can also be applied via rapid-sequence induction, producing dissociative anaesthesia ~1–2 min after administration.
Table 2.
Advantages of ketamine in anaesthesia.
| Characteristic | Advantage | References |
|---|---|---|
| Dissociative sedation | Strong sedation and analgesia, useful for emergency in uncooperative subjects | 67 |
| High bioavailability | Rapid action, for emergency in prehospital or hospital setting | 108 |
| No direct interaction with GABA receptors | Cardiorespiratory depression is unlikely | 68,69 |
| Multiple administration routes | Useful when venous access is difficult | 108 |
| Bronchodilatory activity | Anaesthesia in patients with bronchospasm | 29 |
| Preserves haemodynamic stability | Anaesthesia in patients with congenital heart disease, shocked and hypotensive subjects | 76,115,119 |
| Analgesic activity | Useful for postoperative pain control | 129–132 |
Unlike other general anaesthetic agents, ketamine shows no direct interaction with GABA receptors at clinically relevant concentrations. Indeed, subanaesthetic doses of ketamine do not bind to GABA-A receptors in the human brain,68 and anaesthetic concentrations of ketamine do not alter GABA-A receptor function in vitro.69 Thus, at least at subanaesthetic doses, the effect of ketamine on GABA-A receptor activity might only be indirect. As a consequence, relevant cardiorespiratory depression after induction is unlikely, especially if ketamine is given slowly or as monotherapy.70
More recent literature highlights that ketamine may produce a dose-dependent GABA release in specific brain cortical areas, thus altering the overall glutamate–GABA balance. Another work suggests that ketamine may decrease GABA release by blocking NMDAR located on GABAergic interneurons, thus increasing cortical excitability.71 These latter mechanisms may underlie the antidepressant effects of ketamine in treatment-resistant depression.71
The possibility to administer ketamine by different routes has prompted its use when venous access is difficult such as in trauma patients with hypovolemic shock. Indeed, the low cardiorespiratory depressant effects and sympathomimetic effects of ketamine render this drug a valid alternative to other anaesthetic agents in trauma patients as well as in septic shock patients, as several clinical reports have indicated that ketamine produced either no change or a slight increase in arterial pressure and heart rate.1,72 Indeed, it has been observed that ketamine can improve the blood gas and pulmonary function index of patients with acute lung injury caused by mechanical ventilation.73 With a wide variation in individual response, ketamine leads to increased blood pressure, stroke volume and heart rate, and maintains systemic vascular resistance. These effects are commonly observed at a maximum of ~2 min after the injection and resolve over 15–20 min. However, severe hypotension after a ketamine bolus dose has been described as the loss of sympathoadrenal activity that accompanies the loss of consciousness.74
The haemodynamic changes induced by ketamine make it suitable for patients with congenital heart defects and other cardiac conditions.75,76 However, it must be mentioned that ketamine is contraindicated in patients with serious myocardial disease or serious heart failure and when blood hypertension or increased myocardial oxygen consumption may be dangerous.31
Due to its bronchodilatory properties, ketamine is the anaesthetic of choice for patients with bronchospasm29,77,78 and has also been successfully used as a medication in the treatment of status asthmaticus.79 Joint to anticholinergic and spasmolytic actions, ketamine may have some anti-inflammatory effects, which may contribute to its efficacy in asthma patients.80–82 Other mechanisms are the inhibition of catecholamine uptake and L-type Ca2+ channel blocking,83–86 although the precise mechanism by which ketamine induces airway muscle relaxation is still to be elucidated.
Drawbacks of ketamine in anaesthesia
NMDAR occupancy is related to the potential of ketamine to produce adverse symptoms.87 Emergence phenomena, delusions, hallucinations, delirium and confusion, sometimes described as ‘out of body’ and ‘near-death experiences’, are amongst the adverse effects related to the use of ketamine.88 These events are more common in patients older than 16 years, in women, during shorter operative procedures and when large doses of ketamine are administered quickly.89,90 Benzodiazepines effectively prevent these disturbing psychotic phenomena. Midazolam reduced the incidence of unpleasant dreams when compared with diazepam (number needed to treat of 6).91,92 Propofol, lorazepam and diazepam are also effective.93 In clinical practice, and in the opinion of some authors, the regular coadministration of benzodiazepines needs to be increased.94 Finally, a recent trial (n=100) reported that a positive persuasion may reduce unpleasant sensations.95
Intracranial pressure is increased during ketamine use. Cerebral blood flow is increased secondary to a decrease in cerebral vascular resistance. Hence, ketamine should be avoided in patients with intracranial disease or abnormal cerebral blood flow.96
Psychotic symptoms similar to schizophrenia have been described in association with ketamine activity; these need to be managed to reduce any undesired effects.87,97 Moreover, semantic and episodic memory may be impaired by subanaesthetic doses of ketamine.98,99
Other adverse effects have been described after ketamine administration. These include nausea and vomiting in 5–15% of patients100 and hypersalivation, which can be anticipated by atropine.101 Limb purposeless movements and clonus have been occasionally reported.100
Anaesthetic effects
During the ketamine-induced dissociative state, patients may appear awake with preserved airway reflexes and respiratory drive, but they are unable to respond to sensory input.102,103
Clinical use of ketamine
In 1966, the anaesthetic effects of ketamine were reported for the first time in 130 patients aged 6 weeks to 86 years undergoing a total of 133 surgical procedures.104 Ketamine produced profound and rapid analgesia with a unique state of altered consciousness; its duration of effect was limited but could be safely prolonged with repeated administration.105
A well-established use of ketamine is anaesthesia induction in the emergency setting in shocked or hypotensive patients.104 Ketamine was used for anaesthetic induction and maintenance in patients with cardiac tamponade and restrictive pericarditis.106 A study showed that ketamine was as safe and effective as etomidate for endotracheal intubation in critically ill patients with sepsis.107
Ketamine is considered the i.v. anaesthesia induction agent of choice in patients with active bronchospasm because of its bronchodilating properties and allowing the use of high oxygen concentrations.108
Ketamine is the anaesthetic drug of choice for the induction of patients with congenital heart disease with a right to left shunt because it increases systemic vascular resistance, resulting in a reduced right to left shunt.109 In a study, i.v. or i.m. ketamine as induction agents did not significantly affect the proportion of SaO2 in patients with Fallot’s tetralogy.109 Finally, ketamine preserves intraoperative and postoperative haemodynamic stability in patients with congenital heart disease and is an alternative to sevoflurane.76,110
Ketamine has a major role in repeated anaesthesia for burn dressings and for sedation during excision and grafting, both in adults and in children.111–113 The major advantage of ketamine in patients with burns is that it usually preserves airway and spontaneous respiratory function whilst providing good sedation and analgesia.111,114 In addition, the venous access may be difficult in patients with extensive burns and ketamine i.m. administration may be useful.115 Ketamine can be used for burn dressings in adults and children in combination with midazolam/dexmedetomidine or with propofol to obtain effective sedoanalgesia without any significant side effects.111,116
Low-dose ketamine alone (5–25 mg/kg/min infusion) can be used for sedation and analgesia during local or regional anaesthetic procedures.106,117 Low doses (i.v. 0.5 mg/kg) may be combined with i.v. diazepam or midazolam for local and regional anaesthesia techniques, including spinal anaesthesia in adults and children.106 Prophylactic i.v. ketamine 0.5 mg/kg before neuraxial blockade decreases the incidence of shivering, improves the haemodynamic profile, provides good sedation and prevents recall.118 Ketamine 1 mg/kg i.v. given before spinal anaesthesia results in good haemodynamic stability in elderly patients undergoing transurethral resection of the prostate.119
Several authors found that ketamine reduced postoperative pain.120–123 Perioperative low-dose ketamine was found to improve postoperative analgesia following caesarean delivery with general anaesthesia. In a randomized study on 52 women, a ketamine bolus of 0.5 mg/kg i.v. was administered at the time of induction of general anaesthesia. After induction, a ketamine infusion of 0.25 mg/kg/h was started and discontinued at the end of surgery.124 Low-dose regimens (0.25–0.5 mg/kg i.v. as an initial bolus followed by 50–500 μg/kg/h) have been proposed for postoperative analgesia and for the reduction of exogenous opioid-induced hyperalgesia.125 In a recent review on postoperative pain, it was found that ketamine administered in addition to opioids for i.v. patient-controlled analgesia significantly reduced pain scores, cumulative morphine consumption and postoperative desaturation in patients undergoing thoracic surgery.126
Post-tonsillectomy pain was controlled by low-dose ketamine 0.5 mg/kg i.v./subcutaneous at the end of surgery.127 After tonsillectomy, ketamine 0.5 mg/kg added to fentanyl 1 mg/kg improved analgesia without delaying hospital discharge in a study on 60 children.128
In a prospective randomized study on 30 patients undergoing coronary artery bypass grafting surgery, the combination of ketamine compared with propofol, with midazolam and fentanyl for the induction of anaesthesia provided better haemodynamic stability during induction and until the end of sternotomy.129
A systematic review with a meta-analysis of 12 randomized clinical trials, which included patients undergoing major or minor surgery, assessed the effectiveness of ketamine in reducing morphine consumption and pain intensity scores after remifentanil-based general anaesthesia.130 Ketamine reduced the use of morphine in the first 24 postoperative hours whilst postoperative pain intensity was improved in the first 2 hours in the minor and major surgery groups. In addition, ketamine significantly reduced pain intensity in the first 24 hours in the minor surgery group. Patients administered with ketamine and undergoing major surgery had a longer time to the first rescue analgesia.130
Use of ketamine in children
Traditionally, ketamine is considered the agent of choice in children.102 It is suitable for use in paediatrics for analgesia, procedural sedation and anaesthesia, overcoming some barriers to achieving adequate paediatric analgesia, such as a culture of underdosing and difficulty obtaining i.v. access, thanks to the possibility of using a number of routes of administration and the pharmacokinetic profile.102
No major side effects were reported when ketamine was used in 164 awake non-trapped children with blunt trauma for procedural sedation and analgesia.131
In general anaesthesia, ketamine was safely used in addition to fentanyl or other anaesthesia induction, improving analgesia and intubation conditions and preserving haemodynamics in children with congenital heart or oncological disease.110,128,132,133 Ketamine 0.25 mg/kg reduced sevoflurane-induced postoperative agitation (n=60), whilst propofol 1 mg/kg was not effective.134 Less agitation compared with saline was also reported by two other studies.135,136
Ketamine in combination with propofol ensured stable haemodynamics, with reduced incidence of adverse events compared to single agents, during anaesthesia induction in 120 children subjected to short-term elective and urgent interventions.137
Finally, it may be mentioned that the incidence of psychotic phenomena at awakening is lower in children than in adults.31,137
Conclusion
Ketamine can be considered as the most versatile drug for anaesthesia. It can be used solely or in combination with other coadjuvant drugs, increasing their efficacy. Ketamine has been widely used in several clinical settings due to its specific properties, including its neuroprotective and anti-inflammatory effects. In addition, subanaesthetic regimens of ketamine represent a great clinical advantage. With a favourable pharmacokinetic profile, ketamine is used in hospital and prehospital settings for emergency situations. It is suitable for patients with many cardiac conditions.75,76 It may be used when venous access is difficult as it may be administered through different routes.31 Finally, it is the anaesthetic of choice for patients with bronchospasm thanks to its bronchodilatory and anti-inflammatory properties.29
Acknowledgements
Editorial assistance was provided by Laura Brogelli and Aashni Shah (Polistudium SRL, Milan, Italy). Unconditioned support to this assistance was provided by Minerva Medica and Molteni Farmaceutici.
Footnotes
Contributions: SN searched the literature, evaluated it, and prepared the manuscript. The author meets the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, takes responsibility for the integrity of the work as a whole, and has given her approval for this version to be published.
Disclosure and potential conflicts of interest: In the past 2 years, SN received honoraria for an advisory role for Grunenthal Italia, Sandoz and Angelini Spa; she also received honoraria to participate in a speakers’ bureau for Mylan. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2021/03/dic.2020-12-8-COI.pdf
Funding declaration: Minerva Medica and Molteni Farmaceutici (Firenze, Italy) funded editorial assistance to the article.
Correct attribution: Copyright © 2021 Natoli S. https://doi.org/10.7573/dic.2020-12-8. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Article URL: https://www.drugsincontext.com/the-multiple-faces-of-ketamine-in-anaesthesia-and-analgesia
Provenance: Submitted; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Domino EF, Chodoff P, Corssen G. Pharmacologic effects of CI-581, a new dissociative anesthetic, in man. Clin Pharmacol Ther. 1965;6:279–291. doi: 10.1002/cpt196563279. [DOI] [PubMed] [Google Scholar]
- 2.Zanos P, Moaddel R, Morris PJ, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70(3):621–660. doi: 10.1124/pr.117.015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dincer B, Halici Z, Cadirci E. Investigation of the role of stimulation and blockade of 5-HT7 receptors in ketamine anesthesia. J Mol Neurosci. 2020 doi: 10.1007/s12031-020-01732-3. [DOI] [PubMed] [Google Scholar]
- 4.Mihaljević S, Pavlović M, Reiner K, Ćaćić M. Therapeutic mechanisms of ketamine. Psychiatr Danub. 2020;32(3–4):325–333. doi: 10.24869/psyd.2020.325. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Vlisides PE. Ketamine: 50 years of modulating the mind. Front Hum Neurosci. 2016;10:612. doi: 10.3389/fnhum.2016.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lodge D. The history of the pharmacology and cloning of iono-tropic glutamate receptors and the development of idiosyncratic nomenclature. Neuropharmacology. 2009;56:6–21. doi: 10.1016/j.neuropharm.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Zukin SR, Fitz-Syage ML, Nichtenhauser R, Zukin RS. Specific binding of [3H]phencyclidine in rat central nervous tissue: further characterization and technical considerations. Brain Res. 1983;258(2):277–284. doi: 10.1016/0006-8993(83)91151-4. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald JF, Bartlett MC, Mody I, et al. Actions of ketamine, phencyclidine and MK-801 on NMDA receptor currents in cultured mouse hippocampal neurones. J Physiol. 1991;432:483–508. doi: 10.1113/jphysiol.1991.sp018396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parsons CG, Danysz W, Quack G. Glutamate in CNS disorders as a target for drug development: an update. Drug News Perspect. 1998;11(9):523–569. doi: 10.1358/dnp.1998.11.9.863689. [DOI] [PubMed] [Google Scholar]
- 10.Danysz W, Zajaczkowski W, Parsons CG. Modulation of learning processes by ionotropic glutamate receptor ligands. Behav Pharmacol. 1995;6(5):455–474. doi: 10.1097/00008877-199508000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Low SJ, Roland CL. Review of NMDA antagonist-induced neurotoxicity and implications for clinical development. Int J Clin Pharmacol Ther. 2004;42(1):1–14. doi: 10.5414/CPP42001. [DOI] [PubMed] [Google Scholar]
- 12.Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist – a review of preclinical data. Neuropharmacology. 1999;38(6):735–767. doi: 10.1016/S0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 13.Smith DJ, Bouchal RL, deSanctis CA, et al. Properties of the interaction between ketamine and opiate binding sites in vivo and in vitro. Neuropharmacology. 1987;26(9):1253–1260. doi: 10.1016/0028-3908(87)90084-0. [DOI] [PubMed] [Google Scholar]
- 14.Hustveit O, Maurset A, Oye I. Interaction of the chiral forms of ketamine with opioid, phencyclidine, sigma and muscarinic receptors. Pharmacol Toxicol. 1995;77(6):355–359. doi: 10.1111/j.1600-0773.1995.tb01041.x. [DOI] [PubMed] [Google Scholar]
- 15.Crisp T, Perrotti JM, Smith DL, et al. The local monoaminergic dependency of spinal ketamine. Eur J Pharmacol. 1991;194(2–3):167–172. doi: 10.1016/0014-2999(91)90101-U. [DOI] [PubMed] [Google Scholar]
- 16.Mimura M, Namiki A, Kishi R, et al. Central cholinergic action produces antagonism to ketamine anesthesia. Acta Anaesthesiol Scand. 1992;36(5):460–462. doi: 10.1111/j.1399-6576.1992.tb03497.x. [DOI] [PubMed] [Google Scholar]
- 17.Durieux ME. Inhibition by ketamine of muscarinic acetylcholine receptor function. Anesth Analg. 1995;81(1):57–62. doi: 10.1213/00000539-199507000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Baum VC, Tecson ME. Ketamine inhibits transsarcolemmal calcium entry in guinea pig myocardium: direct evidence by single cell voltage clamp. Anesth Analg. 1991;73(6):804–807. doi: 10.1213/00000539-199112000-00022. [DOI] [PubMed] [Google Scholar]
- 19.Yamakage M, Hirshman CA, Croxton TL. Inhibitory effects of thiopental, ketamine, and propofol on voltage-dependent Ca2+ channels in porcine tracheal smooth muscle cells. Anesthesiology. 1995;83(6):1274–1282. doi: 10.1097/00000542-199512000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Smith B, Pinnock C, Fischer B, et al. Unilateral analgesia following injection of fentanyl into the lumbosacral plexus. Lancet. 1987;1(8548):1497–1498. doi: 10.1016/S0140-6736(87)92254-9. [DOI] [PubMed] [Google Scholar]
- 21.Eide PK, Stubhaug A. Relief of glossopharyngeal neuralgia by ketamine-induced N-methyl-aspartate receptor blockade. Neurosurgery. 1997;41(2):505–508. doi: 10.1097/00006123-199708000-00043#. [DOI] [PubMed] [Google Scholar]
- 22.Gorlin AW, Rosenfeld DM, Ramakrishna H. Intravenous sub-anesthetic ketamine for perioperative analgesia. J Anaesthesiol Clin Pharmacol. 2016;32(2):160–167. doi: 10.4103/0970-9185.182085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowdy EG, Kaya K, Gocho Y. Some pharmacologic similarities of ketamine, lidocaine, and procaine. Anesth Analg. 1973;52(5):839–842. doi: 10.1213/00000539-197309000-00039. [DOI] [PubMed] [Google Scholar]
- 24.Schuttler J, Zsigmond EK, White PF. Ketamine and its isomers. In: White PF, editor. Textbook of Intravenous Anesthesia. Media, PA: Williams & Wilkins; 1997. pp. 171–188. [Google Scholar]
- 25.Niesters M, Martini C, Dahan A. Ketamine for chronic pain: risks and benefits. Br J Clin Pharmacol. 2013;72:357–367. doi: 10.1111/bcp.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reves JG, Glass PS, Lubarsky DA, McEvoy MD. Intravenous nonopioid anesthetics. In: Miller RD, Fleisher LA, Johns RA, et al., editors. Miller’s anesthesia. 6th ed. Philadelphia: Elsevier; 2005. pp. 317–378. [Google Scholar]
- 27.Desta Z, Moaddel R, Ogburn ET, et al. Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica. 2012;42(11):1076–1087. doi: 10.3109/00498254.2012.685777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grant IS, Nimmo WS, McNicol LR, Clements JA. Ketamine disposition in children and adults. Br J Anaesth. 1983;55:1107–1111. doi: 10.1093/bja/55.11.1107. [DOI] [PubMed] [Google Scholar]
- 29.Sun LH, Fan YY, Wang X, Zheng HB. Pharmacodynamic elucidation of glutamate & dopamine in ketamine-induced anaesthesia. Chem Biol Interact. 2020;327:109164. doi: 10.1016/j.cbi.2020.109164. [DOI] [PubMed] [Google Scholar]
- 30.Kamp J, Van Velzen M, Olofsen E, et al. Pharmacokinetic and pharmacodynamic considerations for NMDA-receptor antagonist ketamine in the treatment of chronic neuropathic pain: an update of the most recent literature. Expert Opin Drug Metab Toxicol. 2019;15(12):1033–1041. doi: 10.1080/17425255.2019.1689958. [DOI] [PubMed] [Google Scholar]
- 31.EMC: Ketamine SPC. https://www.medicines.org.uk/emc/product/6935/smpc#gref.
- 32.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11(3):327–335. doi: 10.1016/S0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 33.Sepulveda FJ, Bustos FJ, Inostroza E, et al. Differential roles of NMDA receptor subtypes NR2A and NR2B in dendritic branch development and requirement of RasGRF1. J Neurophysiol. 2010;103(4):1758–1770. doi: 10.1152/jn.00823.2009. [DOI] [PubMed] [Google Scholar]
- 34.Mendell LM. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966;16(3):316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- 35.Eide PK. Wind-up and the NMDA receptor complex from a clinical perspective. Eur J Pain. 2000;4(1):5–15. doi: 10.1053/eujp.1999.0154. [DOI] [PubMed] [Google Scholar]
- 36.Herrero JF, Laird JM, López-García JA. Wind-up of spinal cord neurons and pain sensation: much ado about something? Prog Neurobiol. 2000;61(2):169–203. doi: 10.1016/S0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 37.De Biasi S, Rustioni A. Glutamate and substance P coexist in primary afferent terminals in the superficial laminae of spinal cord. Proc Natl Acad Sci USA. 1988;85(20):7820–7824. doi: 10.1073/pnas.85.20.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skilling SR, Smullin DH, Beitz AJ, Larson AA. Extracellular amino acid concentrations in the dorsal spinal cord of freely moving rats following veratridine and nociceptive stimulation. J Neurochem. 1988;51(1):127–132. doi: 10.1111/j.1471-4159.1988.tb04845.x. [DOI] [PubMed] [Google Scholar]
- 39.Merighi A, Polak JM, Theodosis DT. Ultrastructural visualization of glutamate and aspartate immunoreactivities in the rat dorsal horn, with special reference to the co-localization of glutamate, substance P and calcitonin-gene related peptide. Neuroscience. 1991;40(1):67–80. doi: 10.1016/0306-4522(91)90175-N. [DOI] [PubMed] [Google Scholar]
- 40.Jeftinija S, Jeftinija K, Liu F, et al. Excitatory amino acids are released from rat primary afferent neurons in vitro. Neurosci Lett. 1991;125(2):191–194. doi: 10.1016/0304-3940(91)90025-O. [DOI] [PubMed] [Google Scholar]
- 41.Duggan AW, Furmidge LJ. Probing the brain and spinal cord with neuropeptides in pathways related to pain and other functions. Front Neuroendocrinol. 1994;15(3):275–300. doi: 10.1006/frne.1994.1011. [DOI] [PubMed] [Google Scholar]
- 42.Li P, Wilding TJ, Kim SJ, et al. Kainate-receptor-mediated sensory synaptic transmission in mammalian spinal cord. Nature. 1999;397(6715):161–164. doi: 10.1038/16469. [DOI] [PubMed] [Google Scholar]
- 43.Drdla R, Sandkühler J. Long-term potentiation at C-fibre synapses by low-level presynaptic activity in vivo. Mol Pain. 2008;4:18. doi: 10.1186/1744-8069-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8(1):1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- 46.Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-d-aspartate (NMDA) receptors in pain: a review. Anesth Analg. 2003;97:1108–1116. doi: 10.1213/01.ANE.0000081061.12235.55. [DOI] [PubMed] [Google Scholar]
- 47.Ochoa J, Torebjörk HE, Culp WJ, Schady W. Abnormal spontaneous activity in single sensory nerve fibers in humans. Muscle Nerve. 1982;5(9S):S74–S77. [PubMed] [Google Scholar]
- 48.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park KM, Max MB, Robinovitz E, et al. Effects of intravenous ketamine, alfentanil, or placebo on pain, pinprick hyperalgesia, and allodynia produced by intradermal capsaicin in human subjects. Pain. 1995;63(2):163–172. doi: 10.1016/0304-3959(95)00029-R. [DOI] [PubMed] [Google Scholar]
- 50.Andersen OK, Felsby S, Nicolaisen L, et al. The effect of ketamine on stimulation of primary and secondary hyperalgesic areas induced by capsaicin – a double-blind, placebo-controlled, human experimental study. Pain. 1996;66(1):51–62. doi: 10.1016/0304-3959(96)02995-8. [DOI] [PubMed] [Google Scholar]
- 51.Gottrup H, Hansen PO, Arendt-Nielsen L, Jensen TS. Differential effects of systemically administered ketamine and lidocaine on dynamic and static hyperalgesia induced by intradermal capsaicin in humans. Br J Anaesth. 2000;84(2):155–162. doi: 10.1093/oxfordjournals.bja.a013396. [DOI] [PubMed] [Google Scholar]
- 52.Koppert W, Dern SK, Sittl R, et al. A new model of electrically evoked pain and hyperalgesia in human skin: the effects of intravenous alfentanil, S(+)-ketamine, and lidocaine. Anesthesiology. 2001;95(2):395–402. doi: 10.1097/00000542-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 53.Willert RP, Woolf CJ, Hobson AR, et al. The development and maintenance of human visceral pain hypersensitivity is dependent on the N-methyl-D-aspartate receptor. Gastroenterology. 2004;126(3):683–692. doi: 10.1053/j.gastro.2003.11.047. [DOI] [PubMed] [Google Scholar]
- 54.Chizh BA, Headley PM. NMDA antagonists and neuropathic pain – multiple drug targets and multiple uses. Curr Pharm Des. 2005;11(23):2977–2994. doi: 10.2174/1381612054865082. [DOI] [PubMed] [Google Scholar]
- 55.Morris VH, Cruwys SC, Kidd BL. Characterisation of capsaicin-induced mechanical hyperalgesia as a marker for altered nociceptive processing in patients with rheumatoid arthritis. Pain. 1997;71(2):179–186. doi: 10.1016/S0304-3959(97)03361-7. [DOI] [PubMed] [Google Scholar]
- 56.Sörensen J, Graven-Nielsen T, Henriksson KG, et al. Hyperexcitability in fibromyalgia. J Rheumatol. 1998;25(1):152–155. [PubMed] [Google Scholar]
- 57.Attal N, Bouhassira D. Mechanisms of pain in peripheral neuropathy. Acta Neurol Scand Suppl. 1999;173:12–24. doi: 10.1111/j.1600-0404.1999.tb07386.x. discussion 48–52. [DOI] [PubMed] [Google Scholar]
- 58.Petersen KL, Fields HL, Brennum J, et al. Capsaicin evoked pain and allodynia in post-herpetic neuralgia. Pain. 2000;88(2):125–133. doi: 10.1016/S0304-3959(00)00311-0. [DOI] [PubMed] [Google Scholar]
- 59.Farrell M, Gibson S, McMeeken J, Helme R. Pain and hyperalgesia in osteoarthritis of the hands. J Rheumatol. 2000;27(2):441–447. [PubMed] [Google Scholar]
- 60.Burstein R. Deconstructing migraine headache into peripheral and central sensitization. Pain. 2001;89(2–3):107–110. doi: 10.1016/S0304-3959(00)00478-4. [DOI] [PubMed] [Google Scholar]
- 61.Staud R, Smitherman ML. Peripheral and central sensitization in fibromyalgia: pathogenetic role. Curr Pain Headache Rep. 2002;6(4):259–266. doi: 10.1007/s11916-002-0046-1. [DOI] [PubMed] [Google Scholar]
- 62.Price DD, Verne GN. Does the spinothalamic tract to ventroposterior lateral thalamus and somatosensory cortex have roles in both pain sensation and pain-related emotions? J Pain. 2002;3(2):105–108. doi: 10.1054/jpai.2002.122950. discussion 113–114. [DOI] [PubMed] [Google Scholar]
- 63.Graven-Nielsen T, Aspegren Kendall S, Henriksson KG, et al. Ketamine reduces muscle pain, temporal summation, and referred pain in fibromyalgia patients. Pain. 2000;85(3):483–491. doi: 10.1016/S0304-3959(99)00308-5. [DOI] [PubMed] [Google Scholar]
- 64.Schaible HG, Ebersberger A, Von Banchet GS. Mechanisms of pain in arthritis. Ann NY Acad Sci. 2002;966:343–354. doi: 10.1111/j.1749-6632.2002.tb04234.x. [DOI] [PubMed] [Google Scholar]
- 65.Jensen TS, Baron R. Translation of symptoms and signs into mechanisms in neuropathic pain. Pain. 2003;102(1–2):1–8. doi: 10.1016/s0304-3959(03)00006-x. [DOI] [PubMed] [Google Scholar]
- 66.Desmeules JA, Cedraschi C, Rapiti E, et al. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003;48(5):1420–1429. doi: 10.1002/art.10893. [DOI] [PubMed] [Google Scholar]
- 67.Kurdi MS, Theerth KA, Deva RS. Ketamine: current applications in anesthesia, pain, and critical care. Anesth Essays Res. 2014;8(3):283–290. doi: 10.4103/0259-1162.143110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salmi E, Långsjö JW, Aalto S, et al. Subanesthetic ketamine does not affect 11C- flumazenil binding in humans. Anesth Analg. 2005;101:722–725. doi: 10.1213/01.ANE.0000156951.83242.8D. [DOI] [PubMed] [Google Scholar]
- 69.Flood P, Krasowski MD. Intravenous anesthetics differentially modulate ligand-gated ion channels. Anesthesiology. 2000;92:1418–1425. doi: 10.1097/00000542-200005000-00033. [DOI] [PubMed] [Google Scholar]
- 70.Craven R. Ketamine. Anaesthesia. 2007;62(Suppl 1):48–53. doi: 10.1111/j.1365-2044.2007.05298.x. [DOI] [PubMed] [Google Scholar]
- 71.Pham TH, Gardier AM. Fast-acting antidepressant activity of ketamine: highlights on brain serotonin, glutamate, and GABA neurotransmission in preclinical studies. Pharmacol Ther. 2019;199:58–90. doi: 10.1016/j.pharmthera.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 72.White PF, Way WL, Trevor AJ. Ketamine – its pharmacology and therapeutic uses. Anesthesiology. 1982;56(2):119–136. doi: 10.1097/00000542-198202000-00007. [DOI] [PubMed] [Google Scholar]
- 73.Wang WF, Liu S, Xu B. A study of the protective effect and mechanism of ketamine on acute lung injury induced by mechanical ventilation. Eur Rev Med Pharmacol Sci. 2017;21(6):1362–1367. [PubMed] [Google Scholar]
- 74.Hoffman WE, Pelligrino D, Werner C, Kochs E, Albrecht RF, Schulte am Esch J. Ketamine decreases plasma catecholamines and improves outcome from incomplete cerebral ischemia in rats. Anesthesiology. 1992;76(5):755–762. doi: 10.1097/00000542-199205000-00014. [DOI] [PubMed] [Google Scholar]
- 75.Morray JP, Lynn AM, Stamm SJ, Herndon PS, Kawabori I, Stevenson JG. Hemodynamic effects of ketamine in children with congenital heart disease. Anesth Analg. 1984;63:895–899. [PubMed] [Google Scholar]
- 76.Goyal R, Singh S, Bangi A, Singh SK. Case series: Dexmedetomidine and ketamine for anesthesia in patients with uncorrected congenital cyanotic heart disease presenting for non-cardiac surgery. J Anaesthesiol Clin Pharmacol. 2013;29:543–546. doi: 10.4103/0970-9185.119142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lam E, Rochani A, Kaushal G, et al. Pharmacokinetics of ketamine at dissociative doses in an adult patient with refractory status asthmaticus receiving extracorporeal membrane oxygenation therapy. Clin Ther. 2019;41(5):994–999. doi: 10.1016/j.clinthera.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goyal S, Agrawal A. Ketamine in status asthmaticus: a review. Indian J Crit Care Med. 2013;17(3):154–161. doi: 10.4103/0972-5229.117048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sarma VJ. Use of ketamine in acute severe asthma. Acta Anaesthesiologica Scandinavica. 1992;36:106–107. doi: 10.1111/j.1399-6576.1992.tb03432.x. [DOI] [PubMed] [Google Scholar]
- 80.Wilson LE, Hatch DJ, Rehder K. Mechanisms of the relaxant action of ketamine on isolated porcine trachealis muscle. Br J Anaesth. 1993;71(4):544–550. doi: 10.1093/bja/71.4.544. [DOI] [PubMed] [Google Scholar]
- 81.Hirota K, Sato T, Rabito SF, et al. Relaxant effect of ketamine and its isomers on histamine-induced contraction of tracheal smooth muscle. Br J Anaesth. 1996;76:266–270. doi: 10.1093/bja/76.2.266. [DOI] [PubMed] [Google Scholar]
- 82.Schmidt H, Ebeling D, Bauer H, et al. Ketamine attenuates endotoxin-induced leukocyte adherence in rat mesenteric venules. Crit Care Med. 1995;23:2008–2014. doi: 10.1097/00003246-199512000-00009. [DOI] [PubMed] [Google Scholar]
- 83.Zhao Y, Sun L. Antidepressants modulate the in vitro inhibitory effects of propofol and ketamine on norepinephrine and serotonin transporter function. J Clin Neurosci. 2008;15(11):1264–1269. doi: 10.1016/j.jocn.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pabelick CM, Jones KA, Street K, et al. Calcium concentration-dependent mechanisms through which ketamine relaxes canine airway smooth muscle. Anesthesiology. 1997;86:1104–1111. doi: 10.1097/00000542-199705000-00014. [DOI] [PubMed] [Google Scholar]
- 85.Jung I, Jung SH. Vasorelaxant mechanisms of ketamine in rabbit renal artery. Korean J Anesthesiol. 2012;63(6):533–539. doi: 10.4097/kjae.2012.63.6.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gateau O, Bourgain JL, Gaudy JH, Benveniste J. Effects of ketamine on isolated human bronchial preparations. Br J Anaesth. 1989;63(6):692–695. doi: 10.1093/bja/63.6.692. [DOI] [PubMed] [Google Scholar]
- 87.Stone JM, Erlandsson K, Arstad E, et al. Relationship between ketamine-induced psychotic symptoms and NMDA receptor occupancy: a [(123)I]CNS-1261 SPET study. Psychopharmacology. 2008;197(3):401–408. doi: 10.1007/s00213-007-1047-x. [DOI] [PubMed] [Google Scholar]
- 88.Treston G, Bell A, Cardwell R, et al. What is the nature of the emergence phenomenon when using intravenous or intramuscular ketamine for paediatric procedural sedation? Emerg Med Australas. 2009;21(4):315–322. doi: 10.1111/j.1742-6723.2009.01203.x. [DOI] [PubMed] [Google Scholar]
- 89.Mort TC. Preoxygenation in critically ill patients requiring emergency tracheal intubation. Crit Care Med. 2005;33:2672–2675. doi: 10.1097/01.CCM.0000187131.67594.9E. [DOI] [PubMed] [Google Scholar]
- 90.Green SM, Johnson NE. Ketamine sedation for pediatric procedures: part 2. Review and implications. Ann Emerg Med. 1990;19:1033–1046. doi: 10.1016/S0196-0644(05)82569-7. [DOI] [PubMed] [Google Scholar]
- 91.Sener S, Eken C, Schultz CH, et al. Ketamine with and without midazolam for emergency department sedation in adults: a randomized controlled trial. Ann Emerg Med. 2011;57(2):109–114.e2. doi: 10.1016/j.annemergmed.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 92.Cartwright PD, Pingel SM. Midazolam and diazepam in ketamine anaesthesia. Anaesthesia. 1984;39:439–442. doi: 10.1111/j.1365-2044.1984.tb07312.x. [DOI] [PubMed] [Google Scholar]
- 93.Lilburn JK, Dundee JW, Nair SG, et al. Ketamine sequelae. Evaluation of the ability of various premedicants to attenuate its psychic actions. Anaesthesia. 1978;33(4):307–311. doi: 10.1111/j.1365-2044.1978.tb12412.x. [DOI] [PubMed] [Google Scholar]
- 94.Marland S, Ellerton J, Andolfatto G, et al. Ketamine: use in anesthesia. CNS Neurosci Ther. 2013;19(6):381–389. doi: 10.1111/cns.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheong SH, Lee KM, Lim SH, et al. Brief report: the effect of suggestion on unpleasant dreams induced by ketamine administration. Anesth Analg. 2011;112(5):1082–1085. doi: 10.1213/ANE.0b013e31820eeb0e. [DOI] [PubMed] [Google Scholar]
- 96.Gardner AE, Olson BE, Lichtiger M. Cerebrospinal-fluid pressure during dissociation anesthesia with ketamine. Anesthesiology. 1971;35:226–228. doi: 10.1097/00000542-197108000-00029. [DOI] [PubMed] [Google Scholar]
- 97.Yadav M, Parle M, Jindal DK, Dhingra S. Protective effects of stigmasterol against ketamine-induced psychotic symptoms: possible behavioral, biochemical and histopathological changes in mice. Pharmacol Rep. 2018;70(3):591–599. doi: 10.1016/j.pharep.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 98.Fletcher PC, Honey GD. Schizophrenia, ketamine and cannabis: evidence of overlapping memory deficits. Trends Cogn Sci. 2006;10:167–174. doi: 10.1016/j.tics.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 99.Morgan CJ, Mofeez A, Brandner B, Bromley L, Curran HV. Ketamine impairs response inhibition and is positively reinforcing in healthy volunteers: a dose-response study. Psychopharmacology. 2004;172(3):298–308. doi: 10.1007/s00213-003-1656-y. [DOI] [PubMed] [Google Scholar]
- 100.Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med. 2008;26(9):985–1028. doi: 10.1016/j.ajem.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 101.Kye YC, Rhee JE, Kim K, et al. Clinical effects of adjunctive atropine during ketamine sedation in pediatric emergency patients. Am J Emerg Med. 2012;30(9):1981–1985. doi: 10.1016/j.ajem.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 102.Gao M, Rejaei D, Liu H. Ketamine use in current clinical practice. Acta Pharmacol Sin. 2016;37(7):865–872. doi: 10.1038/aps.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Acevedo-Diaz EE, Cavanaugh GW, Greenstein D, et al. Comprehensive assessment of side effects associated with a single dose of ketamine in treatment-resistant depression. J Affect Disord. 2020;263:568–575. doi: 10.1016/j.jad.2019.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morris C, Perris A, Klein J, Mahoney P. Anaesthesia in haemodynamically compromised emergency patients: does ketamine represent the best choice of induction agent? Anaesthesia. 2009;64:532–539. doi: 10.1111/j.1365-2044.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- 105.Corssen G, Domino EF. Dissociative anesthesia: further pharmacologic studies and first clinical experience with the phencyclidine derivative CI-581. Anesth Analg. 1966;45(1):29–40. doi: 10.1213/00000539-196601000-00007. [DOI] [PubMed] [Google Scholar]
- 106.Reves JG, Glass PS, Lubarsky DA, McEvoy MD, Ruiz RM. Intravenous anaesthetics. In: Miller RD, editor. Miller’s Anaesthesia. 7th ed. Philadelphia, USA: Churchill Livingstone; 2010. pp. 719–771. https://drive.google.com/file/d/0B81TNkQfgbpENFlrS2tDN3lZbWc/view. [Google Scholar]
- 107.Jabre P, Combes X, Lapostolle F, et al. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet. 2009;374:293–300. doi: 10.1016/S0140-6736(09)60949-1. [DOI] [PubMed] [Google Scholar]
- 108.Stoelting RK, Hillier SC. Nonbarbiturate intravenous anaesthetic drugs. In: Stoelting RK, Hillier SC, editors. Pharmacology and Physiology in Anaesthetic Practice. 4th ed. Philadelphia: Lippincott Williams and Wilkin; 2006. pp. 155–178. [Google Scholar]
- 109.Tavakollian AR, Allahyary E. The comparison of the effect of three anesthetic induction regimens on the arterial oxygen saturation in children with tetralogy of fallot undergoing cardiac surgery. Iran Red Crescent Med J. 2011;13:702–706. [PMC free article] [PubMed] [Google Scholar]
- 110.Sungur Ulke Z, Kartal U, Orhan Sungur M, et al. Comparison of sevoflurane and ketamine for anesthetic induction in children with congenital heart disease. Paediatr Anaesth. 2008;18:715–721. doi: 10.1111/j.1460-9592.2008.02637.x. [DOI] [PubMed] [Google Scholar]
- 111.Gündüz M, Sakalli S, Güneş Y, et al. Comparison of effects of ketamine, ketamine-dexmedetomidine and ketamine-midazolam on dressing changes of burn patients. J Anaesthesiol Clin Pharmacol. 2011;27:220–224. doi: 10.4103/0970-9185.81823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kolawole IK. Ketamine hydrochloride: a useful but frequently misused drug. Niger J Surg Res. 2001;3:118–125. doi: 10.4314/njsr.v3i3.12232. [DOI] [Google Scholar]
- 113.O’Hara D, Ganeshalingam K, Gerrish H, Richardson P. A 2 year experience of nurse led conscious sedation in paediatric burns. Burns. 2014;40:48–53. doi: 10.1016/j.burns.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 114.Owens VF, Palmieri TL, Comroe CM, et al. Ketamine: a safe and effective agent for painful procedures in the pediatric burn patient. J Burn Care Res. 2006;27:211–216. doi: 10.1097/01.BCR.0000204310.67594.A1. [DOI] [PubMed] [Google Scholar]
- 115.Dundee JW, Wyant GM. Intravenous Anasethesia. New York: Churchill Livingstone; 1988. pp. 135–159. [Google Scholar]
- 116.Samad MA, Islam MS, Ahmed M, Maruf AA. Evaluation of ketofol (ketamine-propofol combination) as total intravenous anaesthetic for burn dressing in adult patient. J Armed Forces Med Coll Bangladesh. 2012;8:20–24. doi: 10.3329/jafmc.v8i1.13534. [DOI] [Google Scholar]
- 117.Tobin HA. Low-dose ketamine and diazepam. Use as an adjunct to local anesthesia in an office operating room. Arch Otolaryngol. 1982;108(7):439–440. doi: 10.1001/archotol.1982.00790550043011. [DOI] [PubMed] [Google Scholar]
- 118.Wason R, Jain N, Gupta P, Gogia AR. Randomized double-blind comparison of prophylactic ketamine, clonidine and tramadol for the control of shivering under neuraxial anaesthesia. Indian J Anaesth. 2012;56:370–375. doi: 10.4103/0019-5049.100821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ozkan F, Kaya Z, Suren M. The effect of intravenous ketamine in prevention of hypotension during spinal anaesthesia in patients with benign prostatic hyperplasia. Nobel Medicus. 2011;7:82–88. [Google Scholar]
- 120.Radvansky BM, Shah K, Parikh A, et al. Role of ketamine in acute postoperative pain management: a narrative review. Biomed Res Int. 2015;2015 doi: 10.1155/2015/749837. 749837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Elia N, Tramer MR. Ketamine and postoperative pain – a quantitative systematic review of randomised trials. Pain. 2005;113(1–2):61–70. doi: 10.1016/j.pain.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 122.Bell RF, Dahl JB, Moore RA, Kalso E. Peri-operative ketamine for acute post-operative pain: a quantitative and qualitative systematic review (Cochrane review) Acta Anaesthesiol Scand. 2005;49(10):1405–1428. doi: 10.1111/j.1399-6576.2005.00814.x. [DOI] [PubMed] [Google Scholar]
- 123.Laskowski K, Stirling A, McKay WP, Lim HJ. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesth. 2011;58(10):911–923. doi: 10.1007/s12630-011-9560-0. [DOI] [PubMed] [Google Scholar]
- 124.Haliloglu M, Ozdemir M, Uzture N, et al. Perioperative low-dose ketamine improves postoperative analgesia following Cesarean delivery with general anesthesia. J Matern Fetal Neonatal Med. 2016;29(6):962–966. doi: 10.3109/14767058.2015.1027190. [DOI] [PubMed] [Google Scholar]
- 125.Berti M, Baciarello M, Troglio R, Fanelli G. Clinical uses of low-dose ketamine in patients undergoing surgery. Curr Drug Targets. 2009;10:707–715. doi: 10.2174/138945009788982496. [DOI] [PubMed] [Google Scholar]
- 126.Costantini R, Affaitati G, Fabrizio A, Giamberardino MA. Controlling pain in the post-operative setting. Int J Clin Pharmacol Ther. 2011;49(2):116–127. doi: 10.5414/CP201401. [DOI] [PubMed] [Google Scholar]
- 127.Javid MJ, Hajijafari M, Hajipour A, et al. Evaluation of a low dose ketamine in post tonsillectomy pain relief: a randomized trial comparing intravenous and subcutaneous ketamine in pediatrics. Anesth Pain Med. 2012;2:85–89. doi: 10.5812/aapm.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Elshammaa N, Chidambaran V, Housny W, et al. Ketamine as an adjunct to fentanyl improves postoperative analgesia and hastens discharge in children following tonsillectomy – a prospective, double-blinded, randomized study. Paediatr Anaesth. 2011;21(10):1009–1014. doi: 10.1111/j.1460-9592.2011.03604.x. [DOI] [PubMed] [Google Scholar]
- 129.Basagan-Mogo E, Goren S, Korfali G, et al. Induction of anesthesia in coronary artery bypass graft surgery: the hemodynamic and analgesic effects of ketamine. Clinics. 2010;65(2):133. doi: 10.1590/S1807-59322010000200003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.García-Henares JF, Moral-Munoz JA, Salazar A, Del Pozo E. Effects of ketamine on postoperative pain after remifentanil-based anesthesia for major and minor surgery in adults: a systematic review and meta-analysis. Front Pharmacol. 2018;9:921. doi: 10.3389/fphar.2018.00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bredmose PP, Grier G, Davies GE, Lockey DJ. Pre-hospital use of ketamine in paediatric trauma. Acta Anaesthesiol Scand. 2009;53(4):543–545. doi: 10.1111/j.1399-6576.2008.01852.x. [DOI] [PubMed] [Google Scholar]
- 132.Kim KS, Kwak HJ, Min SK, et al. The effect of ketamine on tracheal intubating conditions without neuromuscular blockade during sevoflurane induction in children. J Anesth. 2011;25(2):195–199. doi: 10.1007/s00540-011-1092-9. [DOI] [PubMed] [Google Scholar]
- 133.Aouad MT, Moussa AR, Dagher CM, et al. Addition of ketamine to propofol for initiation of procedural anesthesia in children reduces propofol consumption and preserves hemodynamic stability. Acta Anaesthesiol Scand. 2008;52:561–565. doi: 10.1111/j.1399-6576.2008.01584.x. [DOI] [PubMed] [Google Scholar]
- 134.Tsai PS, Hsu YW, Lin CS, et al. Ketamine but not propofol provides additional effects on attenuating sevoflurane-induced emergence agitation in midazolam premedicated pediatric patients. Paediatr Anaesth. 2008;18:1114–1115. doi: 10.1111/j.1460-9592.2008.02593.x. [DOI] [PubMed] [Google Scholar]
- 135.Abu-Shahwan I, Chowdary K. Ketamine is effective in decreasing the incidence of emergence agitation in children undergoing dental repair under sevoflurane general anesthesia. Paediatr Anaesth. 2007;17:846–850. doi: 10.1111/j.1460-9592.2007.02298.x. [DOI] [PubMed] [Google Scholar]
- 136.Dalens BJ, Pinard AM, Létourneau DR, et al. Prevention of emergence agitation after sevoflurane anesthesia for pediatric cerebral magnetic resonance imaging by small doses of ketamine or nalbuphine administered just before discontinuing anesthesia. Anesth Analg. 2006;102(4):1056–1061. doi: 10.1213/01.ane.0000200282.38041.1f. [DOI] [PubMed] [Google Scholar]
- 137.Berlinskiĭ VV, Zhdanov GG, Mushkin VV, et al. Combined anesthesia using Diprivan and ketamine in pediatric surgery. Anesteziol Reanimatol. 2000;3:10–12. [PubMed] [Google Scholar]
- 138.Fisher K, Coderre TJ, Hagen NA. Targeting the N-methyl-D-aspartate receptor for chronic pain management. Preclinical animal studies, recent clinical experience and future research directions. J Pain Symptom Manage. 2000;20(5):358–373. doi: 10.1016/S0885-3924(00)00213-X. [DOI] [PubMed] [Google Scholar]
- 139.Kohrs R, Durieux ME. Ketamine: teaching an old drug new tricks. Anesth Analg. 1998;87(5):1186–1193. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- 140.Nishimura M, Sato K, Okada T, et al. Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells. Anesthesiology. 1998;88(3):768–774. doi: 10.1097/00000542-199803000-00029. [DOI] [PubMed] [Google Scholar]
- 141.Kapur S, Seeman P. NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia. Mol Psychiatry. 2002;7(8):837–844. doi: 10.1038/sj.mp.4001093. [DOI] [PubMed] [Google Scholar]
- 142.Irnaten M, Wang J, Chang KS, et al. Ketamine inhibits sodium currents in identified cardiac parasympathetic neurons in nucleus ambiguus. Anesthesiology. 2002;96(3):659–666. doi: 10.1097/00000542-200203000-00024. [DOI] [PubMed] [Google Scholar]