Abstract
Background and purpose:
Epilepsy is recognized as a chronic neurologic disease. Increasing evidence has addressed the antioxidant and anti-inflammatory roles of olive leaf extract (OLE) in neurodegenerative diseases. So, the current study aimed to investigate the neuroprotective roles of OLE in epilepsy.
Experimental approach:
Forty rats were divided into 4 groups including a control group, sham group, kainic acid (KA) group, and KA + OLE group. KA (4 μg/rat) was injected intrahippocampal, and OLE (300 mg/kg) was orally administrated for 4 weeks. Animals were sacrificed, and their hippocampi were isolated. KA- induced seizure activity was recorded. Oxidative stress index was assessed by measuring its indicators including malondialdehyde (MDA), nitrite, nitrate, and glutathione (GSH) as well as the catalase (CAT) activity. The supernatant concentration of tumor necrosis factor-α (TNF-α) and the apoptosis rate in neurons were measured.
Findings/Results:
Treatment with OLE significantly reduced the seizure score. OLE decreased oxidative stress index by reducing the concentration of MDA, nitrite, and nitrate as well as increasing the level of GSH. OLE had a significant anti-apoptotic effect on neurons. However, CAT activity and the level of TNF-α were not affected.
Conclusion and implications:
Our findings indicated neuroprotective properties of OLE, which is mainly mediated by its antioxidant and anti-apoptotic effects, therefore, could be considered as a valuable therapeutic supplement for epilepsy.
Keywords: Epilepsy, Inflammation, Kainic acid, Olive leaf extract, Oxidative stress
INTRODUCTION
Epilepsy is recognized as one of the most widespread and serious neurologic disease, which is characterized by recurrent epileptic seizures and emotional dysfunction (1). This disorder affects 0.5-1% of the world population (≈50 million people worldwide) (2). Epilepsy causes several momentous physical, psychological, and economic consequences (1,3). In spite of the availability of various antiepileptic drugs (such as acetazolamide, carbamazepine, clobazam, rivotril, and so on), epilepsy is not completely curable and about one-third of patients with this disorder suffer from endurable seizures that do not respond to antiepileptic drugs (4).
Temporal lobe epilepsy, as one of the most persistent types of epilepsy, is associated with a structural alteration in the hippocampus which is known as hippocampal sclerosis, the formation of neurodegeneration, and extensive reorganization of hippocampal circuits. This situation is distinguished by a specific pattern of neuronal death. The most sensitive neurons are in the CA1 and CA3 regions and the hilus of the dentate gyrus (5,6).
Increasing research evidence has addressed the crucial role of inflammation in the pathophysiology of several neurodegenerative and autoimmune brain diseases such as Parkinson’s disease, multiple sclerosis, and, Alzheimer’s disease (7,8,9). Moreover, data from several clinical studies indicated the clinical efficacy of anti-inflammatory drugs for the treatment of epilepsy by suppressing inflammation, which could be considered as one of the potential mechanisms in the pathophysiology of epilepsy (8,10). Additional evidence emerged from experimental studies and demonstrated that brain inflammation is the key aspect of epilepsy (8). During chronic inflammation in the brain, several pro- inflammatory mediators are produced by peripheral immune cells and brain resident cells including endothelial cells of the blood-brain barrier, astrocytes, and microglia (11,12). Tumor necrosis factor-α (TNF-α) is one of the most important pro-inflammatory mediators and is a major cause of hyperexcitability and seizures (7,12). On the other hand, oxidative stress, which is closely related to inflammation, is another key mechanism involved in neuronal death and seizures (13,14). It has been demonstrated that increased production of free radicals causes cellular damage, neuronal irritability, and excitability, which might contribute to the pathophysiology of several neurodegenerative disorders (15). With regard to this mounting evidence, inflammation and oxidative stress can be considered as two main targets for the treatment of epilepsy.
The last two decades have seen a growing interest in the use of medicinal plant-derived extract or compounds for the treatment or control of several diseases (16,17). In this regard, olive leaf extract (OLE) has been studied by many researchers (18,19). The therapeutic potential of OLE is mainly associated with the olive phenolic compounds (such as oleuropein), which have powerful antiinflammatory and antioxidant properties (19,20,21). Moreover, the anti-apoptotic effects of OLE have been shown in several experimental studies (22,23,24). Recent work by Sarbishegi et al. has indicated that hydro-alcoholic extract of olive leaf effectively reduced both oxidative stress index and neuronal apoptosis in an experimental model of Parkinson’s disease (25). Up to now, however, no previous study has addressed the protective roles of OLE in epilepsy. So, this study set out to investigate the potential anti-inflammatory, antioxidant, and anti-apoptotic roles of OLE in hippocampus neurons under conditions of kainic acid (KA)- induced temporal lobe epilepsy in rats.
MATERIALS AND METHODS
Reagents
KA, cresyl violet, and ketamine hydrochloride/xylazine hydrochloride solution purchased from Sigma-Aldrich (Germany). Diethyl ether was purchased from Merck (Germany). Lipid peroxidation (malondialdehyde, MDA) assay kit, nitrite/nitrate assay kit, and glutathione (GSH) assay kit were procured from Sigma-Aldrich (Germany). Catalase (CAT) activity assay kit was supplied by Abcam (UK).
Preparation of OLE
The extract was provided in the Herbarium of the School of Pharmacy (Shahid Beheshti University of Medical Sciences, Tehran, Iran). The olive leaf powder extract was purchased from the local herbal medicines market (Tehran, Iran) in 2018.
The preparation of the olive extract was performed with ethanol as an extracting solvent by the maceration method. All extracted samples were then condensed using a rotary evaporator. Finally, 15 g of dried extracts were obtained. In order to prepare OLE gel, 6% polymer Carbopol® (base gel) was added to the obtained extract. Total phenolic content of OLE assayed using Folin’s phenol reagent (Folin- Ciocalteu colorimetric method; Sigma-Aldrich, Germany), and the absorbance read at 760 nm and compared with gallic acid as the calibrator, according to the previous study (26). Total flavonoid content of OLE measured and calculated per mg equivalents of quercetin/g of the extract, as previously reported (27).
Animals
Forty male Wistar rats (weighing 200-250 g) were purchased from Pasteur Institute (Tehran, Iran). A 12/12-h light/dark cycle was maintained and the rats kept three per cage with free access to water and pellet chow. The Ethics Committee of Tehran University of Medical Science (Tehran, IRAN) approved all procedures of the current animal study (Ethic code: IR. TUMS. VCR. REC. 1395. 1380). All rats received human care in conformity with the National Institutes of Health (NIH) guidelines.
Experimental procedure
Four groups of rats (n = 10) determined as follows: (1) control group, didn’t receive any interference; (2) sham group, received the intrahippocampal injection of normal saline and gavaged with water; (3) KA group, injected intrahippocampal with 4 μg of KA dissolved in 5 μL of normal saline at a rate of 1 μL/min using a Hamilton microsyringe; and (4) KA + OLE group, injected with 4 μg of KA dissolved in 5 μL of normal saline and orally treated with OLE at a dose of 300 mg/kg/daily for 4 weeks before surgery (25).
For intrahippocampal injections, rats anesthetized with xylazine (5 mg/kg, ip) and ketamine (60 mg/kg, ip). The dorsal surface of the skull exposed and a burr hole was drilled, according to the atlas of Paxinos Thebregma point used as the reference: anteroposterior: 4.1 mm, Lateral: 4.1 mm, and ventral to the dura: 4 mm (28).
Behavioral assessment of seizures
Rats rated for KA-induced seizure activity during a 4-h period after the surgery, according to Racine’s classification, as previously described (14,29).(0), No reaction; (1) stereotypic mounting, eye blinking, and/or mild facial clonus; (2) head nodding and/or multiple facial clonus; (3) myoclonic jerks in the forelimbs; (4) clonic convulsions in the forelimbs with rearing; and (5) generalized clonic convulsions and loss of balance.
Hippocampi isolation and preparation
After 24 h, rats decapitated under diethyl ether anesthesia. The brains removed and the hippocampi were isolated and prepared, as previously described (30). Briefly, brains were removed and hippocampi isolated and prepared as a 10% tissue homogenate in ice-cold 0.9% saline solution containing protease inhibitor cocktail. After centrifugation (1,000 g, 4 °C, 10 min), the supernatant was aliquoted and stored at -70 °C until assayed.
Assessment of oxidative stress markers
Measurement of hippocampal MDA
The concentration of MDA, as an in vitro marker of lipid peroxidation, was determined by measuring thiobarbituric acid- reactive substances in the supernatant, as previously described (31). Briefly, the reaction was carried out at pH 2-3 and 90 °C for 90 min. After cooling samples on ice, centrifuging was carried out at 1000 g for 10 min, and then, the absorbance of the supernatant read at 532 nm. Thiobarbituric acid-reactive substances were determined using tetramethoxypropan as standard and MDA concentration presented as nanomoles/mg of protein.
Measurement of hippocampal nitrite and nitrate concentration
Supernatant nitrite and nitrate concentration measured using Griess assay (14). Briefly, 1 mL of the homogeneous tissue and 1 mL of Griess solution kept at room temperature for 10 min. Then, the absorbance determined at 540 nm, using a spectrophotometer (752N, Jiangsu, China).
Hippocampal GSH measurement
GSH was assayed, using Ellman’s method (32). Briefly, to 0.1 mL of the homogenate supernatant, 2 mL of phosphate buffer (pH 8.4), 0.5 mL of 5’5 dithiobis (2-nitrobenzoic acid) (DTNB or Ellman’s reagent), and 0.4 mL of double distilled water were added. The absorbance measured at 412 nm within 15 min, using a spectrophotometer (752N, Jiangsu, China). The final concentration of these optical absorbencies calculated using the standard curve.
Antioxidant activity of CAT
The antioxidant activity of CAT was measured, according to the procedure of Claiborne (30,31). Briefly, the rates of H2O2 decomposition in the supernatant, containing 50 mM potassium phosphate buffer (pH 7.0), measured by monitoring the optical absorbance changes at 240 nm for 2 min, using a spectrophotometer (752N, Jiangsu, China). The antioxidant activity of CAT is expressed as unit/mg protein. One unit of catalase activity is defined as 1 μmol of H2O2 that is decomposed in 1 min.
Protein assay
The protein concentration of the hippocampus tissue supernatant assayed, according to the procedure used by Bradford (14). Briefly, 100 mg of Coomassie Brilliant Blue (G-250) was dissolved in 50 mL of 95% ethanol and added to 100 mL of phosphoric acid 85%. When the color was completely dissolved, the volume adjusted to 1 L and passed the solution to the paper before use. Then, 5 mL of reagent and 100 μL of the sample were added and after 5 min, the absorbance read at 595 nm, using a spectrophotometer (752N, Jiangsu, China).
Cytokine assay
The supernatant concentration of TNF-α measured using a TNF-α ELISA kit (Blue Gene Biotech Co., Shanghai, China), according to the manufacturer’s protocol. Samples measured in duplicate.
Neuronal death detection
Cell death detection ELISA plus kit (Sigma- Aldrich, USA; #11287100) was used to assess DNA fragmentation as an index of apoptosis using a plate reader (BioTek, USA).
Statistical analysis
All analyses were carried out, using the GraphPad Prism software program (Version 6.07, San Diego, California). Descriptive data were generated for all variables and presented as mean ± SEM. The significance level was set at P < 0.05.
RESULTS
The phenol and flavonoid content of OLE
The total content of phenol and flavonoid in the extract has been shown in Table 1.
Table 1.
Total phenols and flavonoids in the olive leaf extract
| Total phenols; mg equivalent of gallic acid /g of the extract | 6.72 mg/g |
| Total flavonoids; mg equivalent of Alcl3 /g of the extract | 0.36 mg/g |
The effect of OLE on seizure activity and behavior
As can be seen from Fig. 1, no seizure symptoms were observed in the control and sham groups. Besides, treatment with OLE markedly reduced the mean score of seizure strength compared with the KA group (P < 0.01).
Fig. 1.
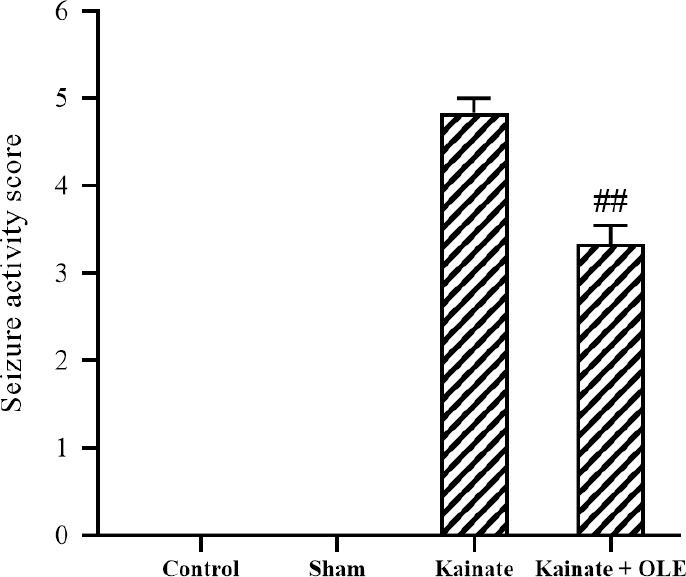
Seizure strength score in different groups. Data are presented as mean ± SEM. ##P < 0.01 Indicates significant difference compared with kainate group. OLE, olive leaf extract.
Oxidative stress index
The effect of OLE on membrane lipid peroxidation
In Fig. 2, the concentration of MDA, as a marker of membrane lipid peroxidation, was shown in the hippocampal supernatant. Treatment with OLE markedly reduced MDA concentration in the treatment group in comparison with the KA group (P <0.01).
Fig. 2.
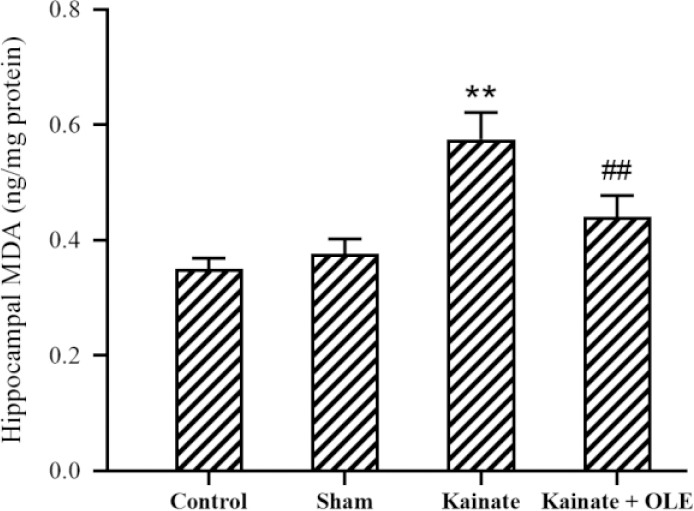
Alterations of hippocampus MDA in different groups. Data are presented as mean ± SEM. **P < 0.01 Indicates significant difference compared with sham group and ##P < 0.01 vs kainate group. MDA, Malondialdehyde; OLE, olive leaf extract.
Oxidative stress index
The effect of OLE on membrane lipid peroxidation
In Fig. 2, the concentration of MDA, as a marker of membrane lipid peroxidation, was shown in the hippocampal supernatant. Treatment with OLE markedly reduced MDA concentration in the treatment group in comparison with the KA group (P <0.01).
The effects of OLE on nitrite and nitrate concentration
As shown in Fig. 3, an increase in the hippocampal concentration of nitrite and nitrate was observed in the KA group which significantly lowered under oral treatment with OLE (P < 0.05).
Fig. 3.
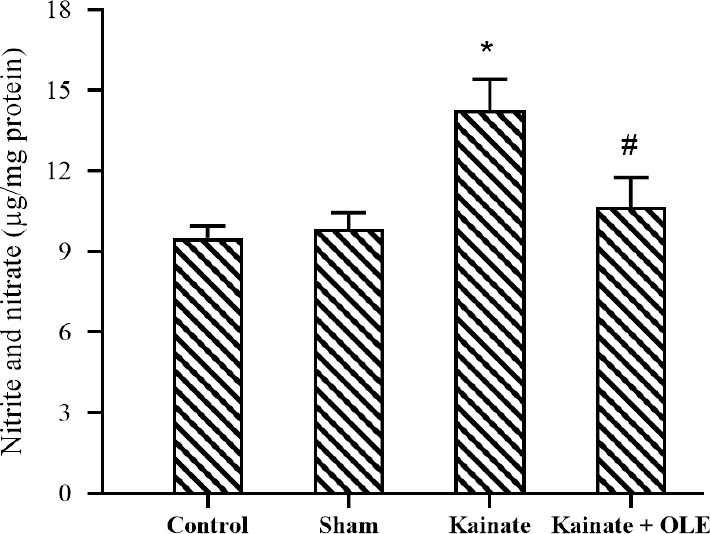
Alterations of hippocampus nitrite and nitrate in different groups. Data are presented as mean ± SEM. *P < 0.05 Indicates significant difference compared with sham group and #P < 0.05 vs kainate group. OLE, Olive leaf extract.
The effect of OLE on GSH level
In Fig. 4, the level of GSH was significantly decreased in the KA group (P <0.05) compared to the sham group; however, there is a clear increase in the level of GSH in hippocampal supernatant of OLE-treated rats (P < 0.05).
Fig. 4.
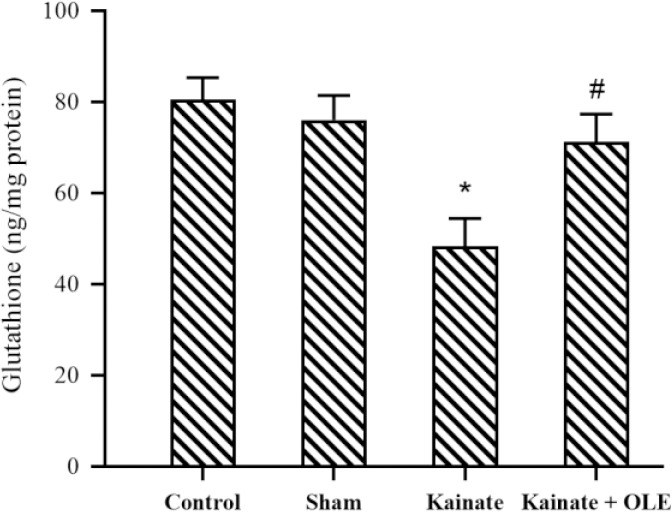
Alterations of hippocampus glutathione in different groups. Data are presented as mean ± SEM. *P < 0.05 Indicates significant difference compared with sham group and #P < 0.05 vs kainate group. OLE, Olive leaf extract.
Antioxidant enzyme activity
As shown in Fig. 5, despite the elevated enzyme activity of CAT following the treatment with OLE, however, this increased activity of CAT was not statistically significant in comparison with the KA group (P >0.05).
Fig. 5.
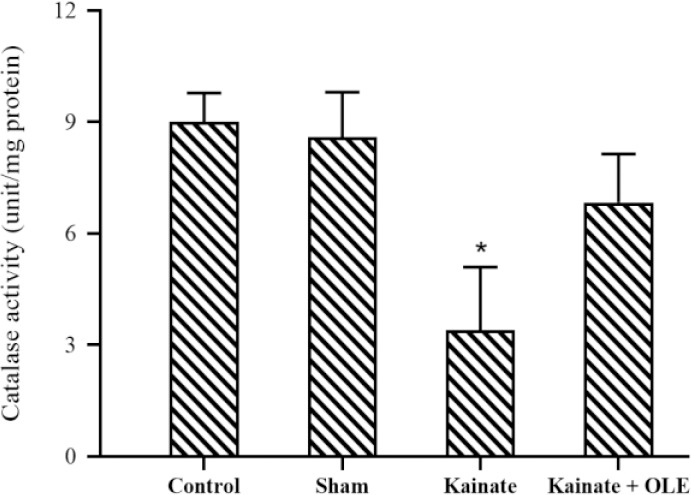
Alterations of hippocampus catalase activity in different groups. Data are presented as mean ± SEM. *P < 0.05 Indicates significant difference compared with the sham group and. OLE, Olive leaf extract.
Proinflammatory cytokine concentration
According to Fig. 6, the high concentration of TNF-α was observed in the hippocampal supernatants of rats treated with KA (P < 0.05), however, a clear anti-inflammatory effect of OLE in the prevention of TNF-α could not be identified in the treatment group (P > 0.05).
Fig. 6.
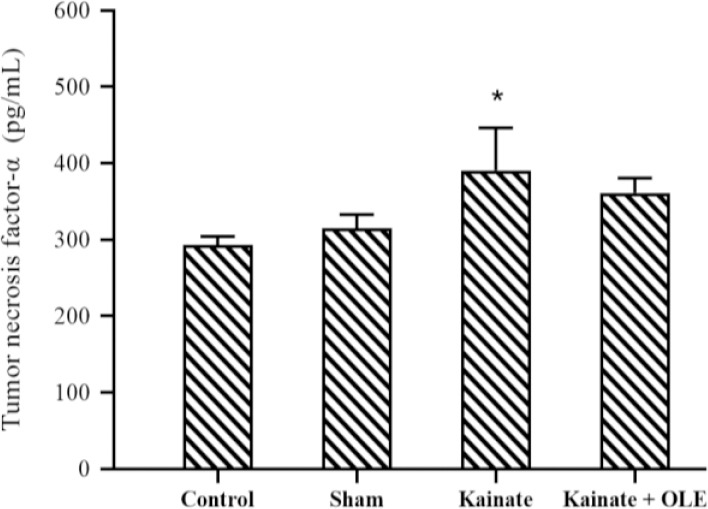
Alterations of hippocampus tumor necrosis factor-α in different groups. Data are presented as mean ± SEM. P < 0.05 Indicates significant difference compared with the sham group and. OLE, Olive leaf extract.
Neuronal death
The apoptosis rate of the CA3 neurons was measured using the in situ assay and results are presented in Fig. 7. Generally, the fragmentation of DNA, as a marker of apoptosis, in the neurons was significantly increased under intervention with the KA (P < 0.01). Interestingly, after treatment with OLE, a lower apoptosis rate in CA3 neurons was found compared with the KA group (P < 0.01).
Fig. 7.
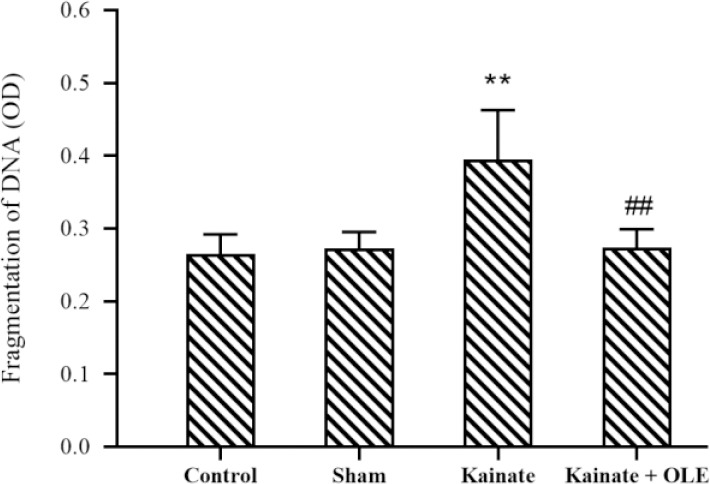
Alteration of hippocampus fragmentation of DNA in different groups. Data are presented as mean ± SEM. **P < 0.01 Indicates significant difference compared with sham group and ##P < 0.01 vs kainate group. OLE, Olive leaf extract.
DISCUSSION
Understanding the mechanisms of epilepsy has long been the subject of human research. Despite extensive research in this field, the mechanism of this disorder is still unknown, so it is important to achieve animal models that are most similar to humans. Currently, one of the most similar animal models of epilepsy is temporal lobe epilepsy induced by KA (33).
Hence, KA can be the best substance to create an animal model of temporal lobe epilepsy. The CA3 region in the hippocampus has a high density of glutamate receptors. The activation of the glutamate receptor increases the intake of sodium ions and calcium into the cell, where calcium ions can be the initiator of apoptosis and neuronal death (34).
Prior research studies have addressed the anti-inflammatory, antioxidant, and anti- apoptotic roles of OLE in several neurodegenerative disorders (19,20,21,22,23). So, the current study was designed to assess the potential neuroprotective roles of OLE in a rat model of epilepsy. In this study, OLE was found to have neuroprotective effects against the temporal lobe epilepsy model in rats.
Increasing evidence has shown that oxi dative stress is a common feature of several neurodegenerative disorders such as Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, stroke, and dementia, which causes neural dysfunction or death, and neuronal excitability (15). Interestingly, several research-reports indicate that neurodegenerative disorders may get some aspects of epilepsy with time (35,36). Therefore, oxidative stress is considered as a possible mechanism in epileptogenesis (37). High levels of polyunsaturated fatty acids in the brain are potent substrates for oxidation (38). Moreover, defect in the elimination of free radicals by antioxidant mechanisms is a major cause that promotes the increased generation of the reactive oxygen species (ROS) in the brain. As a consequence of these events, free radicals damage susceptible targets including phospholipids, proteins, and mitochondrial
DNA of neuronal cells (38,39). On the other hand, several reports have shown that mitochondrial ROS is increased after durable epilepsy, which causes lipid peroxidation, DNA damage, gliosis, impairment of intracellular calcium homeostasis, and neuronal overexcitability in hippocampus neurons (39,40,41,42). Increased ROS production has been related to the pathology of diseases as stroke, amyotrophic lateral sclerosis, diabetes, cancer, atherosclerosis, and neurodegenerative illnesses such as Alzheimer’s and Parkinson’s diseases and epilepsy. Extracellular levels of ROS cause injury to lipids, nucleic acids, proteins, and membranes which can begin cell death signaling pathways, for example, apoptosis (43). In the current study, epilepsy was induced by KA injection. This model emulates many pathophysiological features of epilepsy such as increased-ROS production, impaired-mitochondrial function, impairment of intracellular calcium homeostasis, and neuronal apoptosis in different areas of the brain, especially in the hippocampus (44,45).
However, treatment with OLE for 4 weeks significantly decreased KA-induced oxidative stress by reducing the concentration of MAD, nitrate, nitrite, and increasing the level of GSH in the hippocampus. These findings accord with prior research reports, in which OLE effectively reduced the production of free radicals and lipid peroxidation (23,24,25). As we mentioned above, the potential antioxidant properties of OLE arise from its two important bioactive components including polyphenols and flavonoids (19,20). Oleuropein as a main phenolic constitute of OLE effectively decreases the level of MDA in the hippocampus of an animal model of Alzheimer’s disease (46). Concerning this precise evidence, OLE has antioxidant effects in epilepsy.
Apoptosis of neurons or other susceptible cells in the brain is one of the major causes in the pathogenesis of neurodegenerative diseases (47). Elevated levels of oxidative stress by damaging to mitochondrial and nucleus DNA are the most important stimulator of apoptosis in neurons (38,39). It has been shown that KA injections (systemic or intracerebral) by increasing generation of free radicals contribute to the neuronal death via apoptosis in the CA3 region of the hippocampus, which causes epilepsy in the experimental temporal lobe model (48). Moreover, inhibition of the NO formation in the brain prevents KA-induced neuronal apoptosis in the CA3 area (49). In the current study, oral treatment with OLE effectively protected neurons against apoptosis induced by KA. In line with our findings, Sarbishegi et al. indicated that administration of OLE inhibits rotenone-induced apoptosis of dopaminergic cells in Parkinson’s disease rat model (25). Our finding also accords with earlier in vitro and in vivo observations, which showed that OLE has an anti-apoptotic role against neuronal death induced by neurotoxin and aging (50,51,52). Molecular mechanisms by which OLE plays its anti-apoptotic role has been described. The first mechanism of OLE’s anti-apoptotic action is tightly associated with its antioxidant effects. OLE by suppressing the production of the free radicals (such as hydroxyl •OH), superoxide (O2-), and NO), and increasing the level of GSH indirectly inhibits the neurons’ death via apoptosis. The second mechanism is related to the function of hydroxytyrosol, which is one of the important phenolic constituents of OLE. Hydroxytyrosol by increasing the mRNA levels of NAD(P)H quinone oxidoreductase 1 (NQO1) effectively prevents quinone cytotoxicity to protects neurons against cell death (53,54). Moreover, it has been reported that oleuropein by up- regulating the expression of BCL-2, a main anti-apoptotic member of BCL-2 family, and downregulating the expression of BAX, a main pro-apoptotic member of BCL-2 family, play crucial roles to protect neurons against apoptosis induced by ischemia (22).
Existing researches recognize the critical roles played by inflammatory mediators in the epileptogenic process (7). Pro-inflammatory mediators including cytokines and chemokines are produced by brain resident cells such as microglial cells, astrocytes, and endothelial cells of the blood-brain barrier, leading to vascular inflammation and increased infiltration of peripheral leukocytes into sites of inflammation in the brain (11,12). Precise evidence has been supported that neuro- inflammation might be both a consequence and cause of seizures and epilepsy (11). TNF-α is one of the most important inflammatory mediators involved in the pathogenesis of seizures by increasing the excitability of the neurons (7). In the current study, intrahippocampal injection of KA significantly increased the level of TNF-α in the rat model of epilepsy. However, the finding of the current study does not support the previous research reports, in which administration of OLE, significantly reduced the mRNA or protein levels of TNF-α (24,55). There are several possible explanations for this result including the period time and the dose of treatment, and also different animal models of diseases. Finally, parallel to the reduced-oxidative stress index and apoptosis, treatment with OLE also significantly attenuated the seizure activity score in the rat model of epilepsy, therefore represents the clinical benefit of OLE for the treatment of temporal lobe epilepsy.
CONCLUSION
Collectively, the findings of the current study provide important insights into the neuroprotective properties of OLE, which is mainly mediated by its antioxidant and anti- apoptotic effects, therefore, could be considered as a valuable supplement for the treatment of epilepsy. However, more research on this topic needs to be undertaken to confirm the potential neuroprotective effects of OLE in human temporal lobe epilepsy.
Conflict of interest statement
All authors declared no conflict of interest in this study.
Author’s contribution
S. Khamse was responsible for the selection and implementation of the design subject, responsible for statistical analysis, and helped with the performance of the oxidative stress parameters, assistance in reviewing the manuscript and final edition of the manuscript. S. Mohammadian Haftcheshmeh contributed to writing this article, responsible for reviewing the manuscript and the final edition of the manuscript. SS. Sadr contributed to the monitoring and design of the study. M. Roghani was responsible for the performance of the oxidative stress and ELISA test. M. Kamalinejad was responsible for preparation of the aqueous extract. R. Golchoobian helped with the performance of the ELISA test. P. Mohseni Moghaddam was responsible for the animal’s works. F. Ebrahimi helped to animal’s works and record the behavior of the animal. All authors monitored the article.
Acknowledgments
This study was financially supported by the Electrophysiology Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran under Grant No. 36110. The authors thank the Neurophysiology Research Center, Shahed University, Tehran, Iran.
REFERENCES
- 1.Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367(9516):1087–1100. doi: 10.1016/S0140-6736(06)68477-8. DOI: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- 2.Harden CL. The co-morbidity of depression and epilepsy: epidemiology, etiology, and treatment. Neurology. 2002;59(6 Suppl 4):S48–S55. doi: 10.1212/wnl.59.6_suppl_4.s48. DOI: 10.1212/wnl.59.6_suppl_4.s48. [DOI] [PubMed] [Google Scholar]
- 3.De Boer HM. Out of the shadows: a global campaign against epilepsy. Epilepsia. 2002;43(Supple 6):7–8. doi: 10.1046/j.1528-1157.43.s.6.4.x. DOI: 10.1046/j.1528-1157.43.s.6.4.x. [DOI] [PubMed] [Google Scholar]
- 4.Perucca E, French J, Bialer M. Development of new antiepileptic drugs: challenges, incentives, and recent advances. Lancet Neurol. 2007;6(9):793–804. doi: 10.1016/S1474-4422(07)70215-6. DOI: 10.1016/S1474-4422(07)70215-6. [DOI] [PubMed] [Google Scholar]
- 5.Noor NA, Mohammed HS, Khadrawy YA, Ezz HSA, Radwan NM. Evaluation of the neuroprotective effect of taurine and green tea extract against oxidative stress induced by pilocarpine during status epilepticus. J Basic Appl Zool. 2015;72:8–15. DOI: 10.1016/j.jobaz.2015.02.001. [Google Scholar]
- 6.Pitkanen A, Lukasiuk K. Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav. 2009;14(Suppl 1):16–25. doi: 10.1016/j.yebeh.2008.09.023. DOI: 10.1016/j.yebeh.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Lorigados Pedre L, Morales Chacon LM, Orozco Suarez S, Pavon Fuentes N, Estupinan Diaz B, Serrano Sanchez T, et al. Inflammatory mediators in epilepsy. Curr Pharm Des. 2013;19(38):6766–6772. doi: 10.2174/1381612811319380009. DOI: 10.2174/1381612811319380009. [DOI] [PubMed] [Google Scholar]
- 8.Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46(11):1724–1743. doi: 10.1111/j.1528-1167.2005.00298.x. DOI: 10.1111/j. 1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 9.McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat Disord. 2004;10(Suppl 1):S3–S7. doi: 10.1016/j.parkreldis.2004.01.005. DOI: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Riikonen R. Infantile spasms: therapy and outcome. J Child Neurol. 2004;19(6):401–404. doi: 10.1177/088307380401900601. DOI: 10.1177/088307380401900601. [DOI] [PubMed] [Google Scholar]
- 11.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7(1):31–40. doi: 10.1038/nrneurol.2010.178. DOI: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vezzani A, Lang B, Aronica E. Immunity and inflammation in epilepsy. Cold Spring Harb Perspect Med. 2015;6(2):a022699,1–21. doi: 10.1101/cshperspect.a022699. DOI: 10.1101/cshperspect.a022699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked. Free Radic Biol Med. 2010;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. DOI: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khamse S, Sadr SS, Roghani M, Hasanzadeh G, Mohammadian M. Rosmarinic acid exerts a neuroprotective effect in the kainate rat model of temporal lobe epilepsy: underlying mechanisms. Pharm Biol. 2015;53(12):1818–1825. doi: 10.3109/13880209.2015.1010738. DOI: 10.3109/13880209.2015.1010738. [DOI] [PubMed] [Google Scholar]
- 15.Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3(3):205–214. doi: 10.1038/nrd1330. DOI: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 16.Momtazi-Borojeni AA, Haftcheshmeh SM, Esmaeili SA, Johnston TP, Abdollahi E, Sahebkar A. Curcumin: a natural modulator of immune cells in systemic lupus erythematosus. Autoimmun Rev. 2018;17(2):125–135. doi: 10.1016/j.autrev.2017.11.016. DOI: 10.1016/j.autrev.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Kahkhaie KR, Mirhosseini A, Aliabadi A, Mohammadi A, Mousavi MJ, Haftcheshmeh SM, et al. Curcumin: a modulator of inflammatory signaling pathways in the immune system. Inflammopharmacology. 2019;27(5):885–900. doi: 10.1007/s10787-019-00607-3. DOI: 10.1007/s10787-019-00607-3. [DOI] [PubMed] [Google Scholar]
- 18.El SN, Karakaya S. Olive tree (Olea europaea) leaves: potential beneficial effects on human health. Nutr Rev. 2009;67(11):632–638. doi: 10.1111/j.1753-4887.2009.00248.x. DOI: 10.1111/j. 1753-4887.2009.00248.x. [DOI] [PubMed] [Google Scholar]
- 19.Gorzynik-Debicka M, Przychodzen P, Cappello F, Kuban-Jankowska A, Marino Gammazza AM, Knap N, et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. 2018;19(3):686–698. doi: 10.3390/ijms19030686. DOI: 10.3390/ijms19030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fito M, de la Torre R, Covas MI. Olive oil and oxidative stress. Mol Nutr Food Res. 2007;51(10):1215–1224. doi: 10.1002/mnfr.200600308. DOI: 10.1002/mnfr.200600308. [DOI] [PubMed] [Google Scholar]
- 21.Martin-Pelaez S, Covas MI, Fito M, Kusar A, Pravst I. Health effects of olive oil polyphenols: recent advances and possibilities for the use of health claims. Mol Nutr Food Res. 2013;57(5):760–771. doi: 10.1002/mnfr.201200421. DOI: 10.1002/mnfr.201200421. [DOI] [PubMed] [Google Scholar]
- 22.Yu H, Liu P, Tang H, Jing J, Lv X, Chen L, et al. Oleuropein, a natural extract from plants, offers neuroprotection in focal cerebral ischemia/reperfusion injury in mice. Eur J Pharmacol. 2016;775:113–119. doi: 10.1016/j.ejphar.2016.02.027. DOI: 10.1016/j.ejphar.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 23.Dekanski D, Selakovic V, Piperski V, Radulovic Z, Korenic A, Radenovic L. Protective effect of olive leaf extract on hippocampal injury induced by transient global cerebral ischemia and reperfusion in Mongolian gerbils. Phytomedicme. 2011;18(13):1137–1143. doi: 10.1016/j.phymed.2011.05.010. DOI: 10.1016/j.phymed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Khalatbary AR, Ahmadvand H. Neuroprotective effect of oleuropein following spinal cord injury in rats. Neurol Res. 2012;34(1):44–51. doi: 10.1179/1743132811Y.0000000058. DOI: 10.1179/1743132811Y.0000000058. [DOI] [PubMed] [Google Scholar]
- 25.Sarbishegi M, Charkhat Gorgich EA, Khajavi O, Komeili G, Salimi S. The neuroprotective effects of hydro-alcoholic extract of olive (Olea europaea L.) leaf on rotenone-induced Parkinson's disease in rat. Metab Brain Dis. 2018;33(1):79–88. doi: 10.1007/s11011-017-0131-0. DOI: 10.1007/s11011-017-0131-0. [DOI] [PubMed] [Google Scholar]
- 26.Singleton VL, Orthofer R, Lamuela-Raventos R. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin- ciocalteu reagent. Meth Enz. 1999;299:152–178. DOI.org/10.1016/S0076-6879(99)99017-1. [Google Scholar]
- 27.Lin JY, Tang CY. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007;101(1):140–147. DOI: 10.1016/j.foodchem.2006.01.014. [Google Scholar]
- 28.Khamse S, Sadr SS, Roghani M, Rashvand M, Mohammadian M, Marefati N, et al. The effect of rosmarinic acid on apoptosis and nNOS immunoreactivity following intrahippocampal kainic acid injections in rats. Basic Clin Neurosci. 2020;11(1):41–48. doi: 10.32598/bcn.9.10.340. DOI: 10.32598/bcn.9.10.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohseni Moghaddam P, Sadr SS, Roghani M, Arabzadeh S, Khamse S, Zamani E, et al. Huperzine A ameliorates cognitive dysfunction and neuroinflammation in kainic acid induced epileptic rats by antioxidant activity and NLRP 3/caspase1 pathway inhibition. Clin Exp Pharmacol Physiol. 2019;46(4):360–372. doi: 10.1111/1440-1681.13064. DOI: 10.1111/1440-1681.13064. [DOI] [PubMed] [Google Scholar]
- 30.Ebrahimi F, Sadr SS, Roghani M, Khamse S, Mohammadian Haftcheshmeh S, Navid Hamidi M, et al. Assessment of the protective effect of KN-93 drug in systemic epilepsy disorders induced by pilocarpine in male rat. J Cell Biochem. 2019;120(9):15906–15914. doi: 10.1002/jcb.28864. DOI: 10.1002/jcb.28864. [DOI] [PubMed] [Google Scholar]
- 31.Baluchnejadmojarad T, Roghani M. Chronic epigallocatechin-3-gallate ameliorates learning and memory deficits in diabetic rats via modulation of nitric oxide and oxidative stress. Behav Brain Res. 2011;224(2):305–310. doi: 10.1016/j.bbr.2011.06.007. DOI: 10.1016/j.bbr.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Sedaghat R, Taab Y, Kiasalari Z, Afshin-Majd S, Baluchnejadmojarad T, Roghani M. Berberine ameliorates intrahippocampal kainate-induced status epilepticus and consequent epileptogenic process in the rat: underlying mechanisms. Biomed Pharmacother. 2017;87:200–208. doi: 10.1016/j.biopha.2016.12.109. DOI: 10.1016/j.biopha.2016.12.109. [DOI] [PubMed] [Google Scholar]
- 33.Tchekalarova J, Petkova Z, Pechlivanova D, Moyanova S, Kortenska L, Mitreva R, et al. Prophylactic treatment with melatonin after status epilepticus: effects on epileptogenesis, neuronal damage, and behavioral changes in a kainate model of temporal lobe epilepsy. Epilepsy Behav. 2013;27(1):174–187. doi: 10.1016/j.yebeh.2013.01.009. DOI: 10.1016/j.yebeh.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Kanada A, Nishimura Y, Yamaguchi JY, Kobayashi M, Mishima K, Horimoto K, et al. Extract of Ginkgo biloba leaves attenuates kainate-induced increase in intracellular Ca2+ concentration of rat cerebellar granule neurons. Biol Pharm Bull. 2005;28(5):934–936. doi: 10.1248/bpb.28.934. DOI: 10.1248/bpb.28.934. [DOI] [PubMed] [Google Scholar]
- 35.Amatniek JC, Hauser WA, DelCastillo-Castaneda C, Jacobs DM, Marder K, Bell K, et al. Incidence and predictors of seizures in patients with Alzheimer’s disease. Epilepsia. 2006;47(5):867–872. doi: 10.1111/j.1528-1167.2006.00554.x. DOI: 10.1111/j.1528-1167.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- 36.Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34(4-5):325–337. doi: 10.1016/s0143-4160(03)00141-6. DOI: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 37.Aguiar CC, Almeida AB, Araujo PV, de Abreu RN, Chaves EM, do Vale OC, et al. Oxidative stress and epilepsy: literature review. Oxid Med Cell Longev. 2012;2012:795259. doi: 10.1155/2012/795259. DOI: 10.1155/2012/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid â-peptide. Trends Mol Med. 2001;7(12):548–554. doi: 10.1016/s1471-4914(01)02173-6. DOI: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- 39.Liang LP, Patel M. Seizure-induced changes in mitochondrial redox status. Free Radic Biol Med. 2006;40(2):316–322. doi: 10.1016/j.freeradbiomed.2005.08.026. DOI: 10.1016/j.freeradbiomed.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 40.Cock HR. The role of mitochondria and oxidative stress in neuronal damage after brief and prolonged seizures. Prog Brain Res. 2002;135:187–196. doi: 10.1016/S0079-6123(02)35018-0. DOI: 10.1016/S0079-6123(02)35018-0. [DOI] [PubMed] [Google Scholar]
- 41.Patel M. Mitochondrial dysfunction and oxidative stress: cause and consequence of epileptic seizures. Free Radic Biol Med. 2004;37(12):1951–1962. doi: 10.1016/j.freeradbiomed.2004.08.021. DOI: 10.1016/j.freeradbiomed.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 42.Gluck MR, Jayatilleke E, Shaw S, Rowan AJ, Haroutunian V. CNS oxidative stress associated with the kainic acid rodent model of experimental epilepsy. Epilepsy Res. 2000;39(1):63–71. doi: 10.1016/s0920-1211(99)00111-4. DOI: 10.1016/s0920-1211(99)00111-4. [DOI] [PubMed] [Google Scholar]
- 43.Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863(12):2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. DOI: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Hsieh PF, Hou CW, Yao PW, Wu SP, Peng YF, Shen ML, et al. Sesamin ameliorates oxidative stress and mortality in kainic acid-induced status epilepticus by inhibition of MAPK and COX-2 activation. J Neuroinflammation. 2011;;8:57–66. doi: 10.1186/1742-2094-8-57. DOI: 10.1186/1742-2094-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang LP, Ho YS, Patel M. Mitochondrial superoxide production in kainate-induced hippocampal damage. Neuroscience. 2000;101(3):563–570. doi: 10.1016/s0306-4522(00)00397-3. DOI: 10.1016/s0306-4522(00)00397-3. [DOI] [PubMed] [Google Scholar]
- 46.Pourkhodadad S, Alirezaei M, Moghaddasi M, Ahmadvand H, Karami M, Delfan B, et al. Neuroprotective effects of oleuropein against cognitive dysfunction induced by colchicine in hippocampal CA1 area in rats. J Physiol Sci. 2016;66(5):397–405. doi: 10.1007/s12576-016-0437-4. DOI: 10.1007/s12576-016-0437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1(2):120–129. doi: 10.1038/35040009. DOI: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 48.Chuang YC, Chen SD, Lin TK, Liou CW, Chang WN, Chan SHH, et al. Upregulation of nitric oxide synthase II contributes to apoptotic cell death in the hippocampal CA3 subfield via a cytochrome c/caspase-3 signaling cascade following induction of experimental temporal lobe status epilepticus in the rat. Neuropharmacology. 2007;52(5):1263–1273. doi: 10.1016/j.neuropharm.2007.01.010. DOI: 10.1016/j.neuropharm.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 49.Montecot C, Rondi-Reig L, Springhetti V, Seylaz J, Pinard E. Inhibition of neuronal (type 1) nitric oxide synthase prevents hyperaemia and hippocampal lesions resulting from kainate-induced seizures. Neuroscience. 1998;84(3):791–800. doi: 10.1016/s0306-4522(97)00566-6. DOI: 10.1016/s0306-4522(97)00566-6. [DOI] [PubMed] [Google Scholar]
- 50.Pasban-Aliabadi H, Esmaeili-Mahani S, Sheibani V, Abbasnejad M, Mehdizadeh A, Yaghoobi MM. Inhibition of 6-hydroxydopamine-induced PC12 cell apoptosis by olive (Olea europaea L.) leaf extract is performed by its main component oleuropein. Rejuvenation Res. 2013;16(2):134–142. doi: 10.1089/rej.2012.1384. DOI: 10.1089/rej.2012.1384. [DOI] [PubMed] [Google Scholar]
- 51.Achour I, Arel-Dubeau AM, Renaud J, Legrand M, Attard E, Germain M, et al. Oleuropein prevents neuronal death, mitigates mitochondrial superoxide production and modulates autophagy in a dopaminergic cellular model. Int J Mol Sci. 2016;17(8):1293–1309. doi: 10.3390/ijms17081293. DOI: 10.3390/ijms17081293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarbishegi M, Mehraein F, Soleimani M. Antioxidant role of oleuropein on midbrain and dopaminergic neurons of substantia nigra in aged rats. Iran Biomed J. 2014;18(1):16–22. doi: 10.6091/ibj.1274.2013. DOI: 10.6091/ibj.1274.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu G, Deng A, Tang W, Ma J, Yuan C, Ma J. Hydroxytyrosol induces phase II detoxifying enzyme expression and effectively protects dopaminergic cells against dopamine- and 6-hydroxydopamine induced cytotoxicity. Neurochem Int. 2016;96:113–120. doi: 10.1016/j.neuint.2016.03.005. DOI: 10.1016/j.neuint.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 54.Zafar KS, Inayat-Hussain SH, Siegel D, Bao A, Shieh B, Ross D. Overexpression of NQO1 protects human SK-N-MC neuroblastoma cells against dopamine- induced cell death. Toxicol Lett. 2006;166(3):261–267. doi: 10.1016/j.toxlet.2006.07.340. DOI: 10.1016/j.toxlet.2006.07.340. [DOI] [PubMed] [Google Scholar]
- 55.Wang L, Geng C, Jiang L, Gong D, Liu D, Yoshimura H, et al. The anti-atherosclerotic effect of olive leaf extract is related to suppressed inflammatory response in rabbits with experimental atherosclerosis. Eur J Nutr. 2008;47(5):235–243. doi: 10.1007/s00394-008-0717-8. DOI: 10.1007/s00394-008-0717-8. [DOI] [PubMed] [Google Scholar]


