Abstract
Alcohol-related liver disease has become the leading indication for liver transplantation in the US, partly due to an increase in the prevalence of high risk drinking behaviour and alcohol use disorder (AUD), particularly among young women. Achievement of sustained alcohol abstinence may not only prevent development and progression of alcohol-related liver disease, but may also lead to significant clinical improvement even in the advanced stages of disease. In this review, we discuss the diagnosis and outpatient management of alcohol-related liver disease with emphasis on treatment options for AUD and assessment of nutritional status.
Keywords: Alcohol-related cirrhosis, alcohol use disorder, abstinence, nutrition, steatohepatitis
Introduction
In the past 10–15 years, the prevalence of high-risk drinking and alcohol use disorder has increased by 30% and 50%, respectively. The greatest increase has been observed among younger women, older adults, racial/ethnic minorities, and socioeconomically disadvantaged individuals.(1) The pattern of drinking has also significantly changed with a rise in binge drinking, particularly among middle-aged and older adults. This has resulted in a higher prevalence of alcohol-related liver disease and a 40% increase in alcohol-related liver disease-associated mortality.(2),(3) In the United States, in 2016, alcohol related cirrhosis became the leading indication for liver transplantation surpassing cirrhosis related to chronic hepatitis C. (4)
Disease spectrum
The spectrum of alcohol-related liver disease ranges from hepatic steatosis and steatohepatitis to cirrhosis and hepatocellular carcinoma (figure 1). Steatosis can be seen within 3 to 7 days of excessive alcohol consumption and is present in 85–90% of patients with high-risk drinking.(5) Among patients with biopsy-proven alcohol-related liver disease, the disease spectrum at presentation includes 27% steatosis, 24% steatohepatitis, 27% mild-moderate hepatic fibrosis and 26% cirrhosis. The annual progression rate to cirrhosis ranges from 3% in patients with steatosis at baseline to 10% in steatohepatitis and 8% in mild-moderate fibrosis stages.(6) The risk of hepatocellular carcinoma in alcohol-related cirrhosis is about 1% per year.(7) Compared to other causes of liver disease, patients with alcohol-related cirrhosis are more likely to present late; at initial diagnosis complications of portal hypertension are often apparent; patients also carry a higher annual risk of hospital admission and readmission. This has resulted in significantly higher costs incurred in the first year after diagnosis of alcohol-related cirrhosis compared to other etiologies of cirrhosis.(8) Alcoholic hepatitis is an acute and severe form of alcohol-related liver disease, which may present at any stage and is associated with high short-term mortality. The management of severe alcoholic hepatitis typically takes place in the hospital setting and is beyond the scope of this review.
Figure 1.
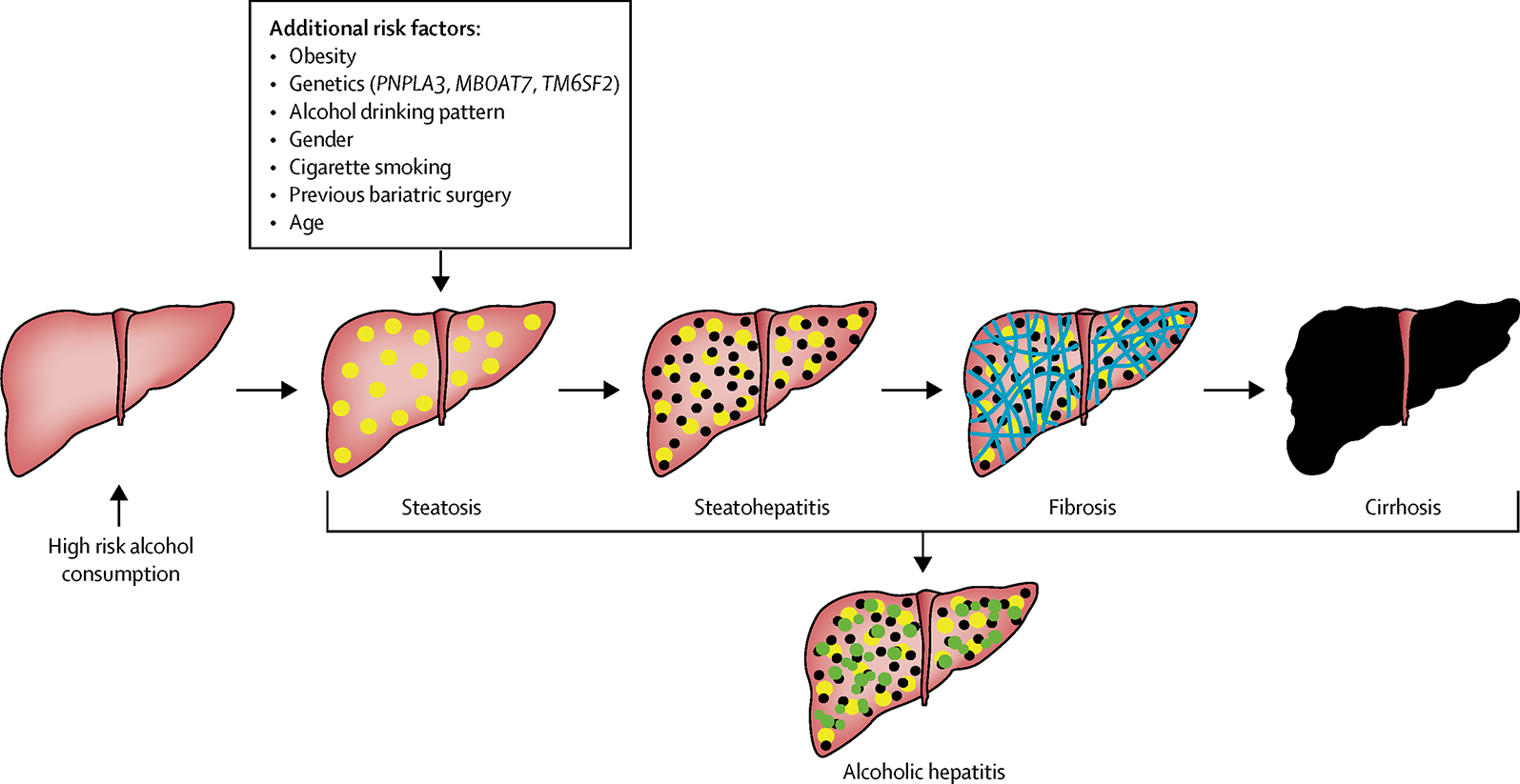
Natural History of Alcohol-related Liver Disease
Screening/diagnosis
Since the development and progression of alcohol-related liver injury is often asymptomatic, the initial step in detection of alcohol-related liver disease is to identify patients at risk. Patients referred to gastroenterology practices are more likely to have advanced disease and cirrhosis, and most do not recognize alcohol use as the cause of their liver disease.(9) Therefore, taking a detailed alcohol consumption history in all patients in primary care practices and at hospital admission to identify those at risk for alcohol related disease is required for early diagnosis and treatment.(10, 11) A remote history of alcohol use in excess should also be investigated as up to a third of patients with alcohol use disorder in sustained remission may be are at risk for alcohol-related liver disease.(12)
Guidelines on safe limits of alcohol consumption vary significantly across different countries, ranging from 10 to 56 grams per day and 84 to 280 grams per week.(13, 14) US, France and Canada have some of the highest limits while the current UK guidelines advise limiting alcohol intake to 112 grams a week. A recent analysis of individual data from 19 high income countries identified the lowest all-cause mortality risk associated with alcohol consumption to be around 100 grams per week; it is even suggested that there is no safe level of alcohol consumption. A linear correlation between alcohol use and severe cardiovascular comorbidities was also observed, including stroke, coronary disease excluding myocardial infarction, heart failure, fatal hypertensive disease and fatal aortic aneurysm.(15) Other studies have demonstrated an increased risk of overall cancer even at the range of light to moderate alcohol use (16, 17). In women, the risk of breast cancer is increased with up to one standard drink per day.(17, 18)
In respect to the threshold for development of alcohol-related liver disease, many factors in addition to amount of alcohol use need to be accounted for as they confer an increased risk of alcohol-related liver injury. Previous studies have suggested that long-term alcohol consumption below 30–40 grams per day does not confer a higher overall risk of alcohol-related liver disease (19–23) or at least of advanced hepatic fibrosis.(24) However, the threshold varies according to the presence or absence of additional risk factors including gender(25), obesity(26), age(27), coffee drinking(28), cigarette smoking (28, 29), prior bariatric surgery(30), drinking pattern(31) and genetics. Women are at higher risk of alcohol-related liver disease with a lower limit of 7–13 drinks per week, compared to 14–27 drinks/week for men.(25) Obesity is also associated with higher risk of alcohol-associated liver injury at a much lower threshold (1 drink or 10–14 grams of alcohol/day).(26) Genetic factors, often unknown to the clinician or to the patient, also play an important role both in increasing or reducing the risk of alcohol-related liver disease. Genetic polymorphism of patatin-like phospholipase domain protein 3 (PNPLA3) gene(26), as well as membrane bound O-acyltransferase domain-containing 7 (MBOAT7) and transmembrane 6 superfamily member 2 (TM6SF2) have been shown to confer a higher risk of alcohol-related cirrhosis(32); whereas a variant in HSD17B13, encoding hydroxysteroid 17-beta dehydrogenase 13, has been associated with a reduced risk of both alcohol-related liver disease and progression from steatosis to steatohepatitis.(33)
Early diagnosis of alcohol-related liver disease, prior to the development of symptomatic liver injury, may lead to successful interventions and reversal of liver disease. However, detection of alcohol-related liver disease at early stages is difficult due to the lack of cost-effective biomarkers. Sole reliance on abnormal liver biochemistry for detection of alcohol-related liver disease leads to the diagnosis being missed in 41% to 75% of the cases.(34) Although a variety of non-invasive tests have been investigated for early detection of advanced liver disease only a few have been validated in alcohol-related liver disease.(35) A recent study assessed screening for alcohol-related liver disease in the workplace utilizing the Southampton Traffic Light (STL) test (36) which comprises hyaluronic acid, procollagen type III N-terminal peptide (PIIINP) and platelet count. Of the patients who reported drinking above the considered safe limits, 30% were found to have liver disease based on the STL test. Another study applying the STL test for individuals reporting high-risk drinking in the community showed that early detection of liver injury and patient feedback can reduce future harmful drinking.(37) Direct comparisons of STL to other non-invasive tools for detection of advanced liver disease are lacking however, and further validation studies are needed. A rigorous prospective study has recently compared 10 different direct and indirect non-invasive tests or markers of advanced liver fibrosis in patients with alcohol-related liver disease with same day liver biopsies as the reference standard. Of patients recruited from primary rehabilitation centers, 6% were found to have advanced fibrosis, compared to 36% in those recruited from health care centers. The commercially available Enhanced Liver Fibrosis (ELF) test, which comprises hyaluronic acid, N-terminal pro-peptide of collagen type III, and tissue inhibitor of metalloproteinase-1, and the FibroTest another commercially available algorithm that combines age, gender, alpha-2-macroglobulin, haptoglobin, apolipoprotein, bilirubin, and gamma-glutamyl transferase, demonstrated the highest diagnostic accuracy for detection of advanced liver fibrosis in high risk drinkers. Both ELF and FibroTest were superior to AST-platelet-ratio index (APRI), age-platelet index, fibrosis-4 index, Forns index, AST:ALT ratio, and GGT-to-platelet ratio, with areas under the receiver operating characteristic curve (AUROC) of 0.92 and 0.90, respectively. (38)
Transient elastography (TE) has been the most validated non-invasive fibrosis assessment tool in alcohol-related liver disease; however the optimal cut-offs for detection of mild to moderate disease remain to be defined.(39) Furthermore, active alcohol-related steatohepatitis results in significantly higher liver stiffness values and cut-off adjustments based on serum AST and bilirubin levels may be needed to improve TE performance.(40) Abstinence leads to significant reduction in liver stiffness, although this is likely due to histologic improvement of steatohepatitis with abstinence.(41) Shear wave elastography appears to be an acceptable alternative to TE with similar accuracy for detecting advanced fibrosis and cirrhosis (area under the curve ≥0.92), although the optimal stiffness cut-offs may differ.(42)
Further studies and initiatives evaluating the cost-effectiveness of routine screening of populations at risk of alcohol-related liver disease are urgently needed. Such initiatives are already on the way, as The Scarred Liver Project led by the Nottingham University Hospital in the UK.
Presentation/Differential diagnosis
With the exception of severe alcoholic hepatitis, the initial presentation of patients with alcohol-related liver disease is often indistinguishable from other causes of chronic liver disease, particularly nonalcoholic steatohepatitis (NASH). Compared to other etiologies, patients with alcohol-related liver disease are seen at later stages of disease, reflecting late referral to specialized hepatology care. (43) Given the lack of liver-related symptoms early in the disease course, the diagnosis should be suspected on the basis of abnormal liver biochemical tests or imaging studies and a history of high risk alcohol use. The National Institute on Alcohol Abuse and Alcoholism (NIAAA) recommends two methods of screening for heavy drinking, either a single question about binge drinking (How often do you have 5 or more drinks on one occasion?) or a written 10-item questionnaire, the Alcohol Use Disorders Identification Test (AUDIT). When obtaining a history of alcohol use it is important to use an empathic and non-judgmental approach and to do so when asking lifestyle questions such as smoking and exercise. Some patients may feel more comfortable filling out a form that can be completed even before the visit. The AUDIT has been extensively validated as a useful tool for detection of high risk drinking and alcohol use disorder.(44) (Table 1) AUDIT scores of ≥8 for men up to age 60 or ≥4 for women, adolescents, and men over 60 are suggestive of risky alcohol use, with scores ≥20 being highly predictive of alcohol use disorder.(45) Alternatively, AUDIT-C, a simplified version composed of 3 consumption-related questions, can be used. AUDIT-C scores ≥ 3 for women and ≥4 for men have a comparable performance to full AUDIT(46) in detecting heavy alcohol use and are superior to traditional CAGE questionnaire.(47)
Table 1.
Alcohol Screening Questionnaire (AUDIT): One drink equals: 12oz of beer, 5oz of wine and 1.5oz of hard liquor.
| 0 points | 1 point | 2 points | 3 points | 4 points | |
|---|---|---|---|---|---|
| 1. How often do you have a drink containing alcohol? | Never | Monthly or less | 2–4 times a month | 2–3 times a week | ≥4 times a week |
| 2. How many drinks containing alcohol do you have on a typical day when you are drinking? | 0–2 | 3–4 | 5–6 | 7–9 | ≥10 |
| 3. How often do you have four or more drinks on one occasion? | Never | Less than monthly | Monthly | Weekly | Daily or almost daily |
| 4. How often during the last year have you found that you were not able to stop drinking once you had started? | Never | Less than monthly | Monthly | Weekly | Daily or almost daily |
| 5. How often during the last year have you failed to do what was normally expected of you because of drinking? | Never | Less than monthly | Monthly | Weekly | Daily or almost daily |
| 6. How often during the last year have you needed a first drink in the morning to get yourself going after a heavy drinking session? | Never | Less than monthly | Monthly | Weekly | Daily or almost daily |
| 7. How often during the last year have you had a feeling of guilt or remorse after drinking? | Never | Less than monthly | Monthly | Weekly | Daily or almost daily |
| 8. How often during the last year have you been unable to remember what happened the night before because of your drinking? | Never | Less than monthly | Monthly | Weekly | Daily or almost daily |
| 9. Have you or someone else been injured because of your drinking? | No | Yes, but not in the last year | Yes, in the last year | ||
| 10. Has a relative, friend, doctor, or other health care worker been concerned about your drinking or suggested you cut down? | No | Yes, but not in the last year | Yes, in the last year | ||
| Add the score of each column: | |||||
| TOTAL SCORE | |||||
Unfortunately, due to persistent social stigma associated with alcohol use disorder(48), many patients may underreport their alcohol consumption leading to a delay in diagnosis and treatment. Without a reliable history, distinction between alcohol-related liver disease and NASH can be particularly difficult given shared radiographic and histologic features.(49) Laboratory tests have historically been used to help differentiate the two with emphasis on the elevated AST to ALT ratio commonly observed in alcohol-related liver disease.(50) The potential reasons for the classic 2:1 AST to ALT ratio in alcohol-related liver disease include a decrease in ALT activity related to hepatic B6 depletion in heavy drinkers and alcohol-induced mitochondrial damage as AST is present in both the cytoplasm and mitochondria of hepatocytes, whereas ALT is only found in the cytoplasm. However, an increased AST to ALT ratio can also observed in cirrhosis, irrespective of etiology, as a result of impaired AST clearance by hepatic sinusoidal cells.(51)
Additionally, the AST/ALT ratio steadily decreases following a short period of abstinence due to more rapid clearance of AST (half-life 18 hours) compared to ALT (half-life 36 hours) and therefore the ratio may only be useful in the setting of active and recent alcohol use. Therefore, combining AST:ALT ratio to other clinical and laboratory variables may improve diagnostic accuracy of alcohol-related liver disease. The ALD/NAFLD Index (ANI) comprises of mean corpuscular volume, body mass index and gender in addition to AST:ALT ratio and has been found superior to conventional biomarkers in distinguishing alcohol-related liver disease from NAFLD.(52)
Besides NAFLD, it is important to recognize additional or alternative etiologies of chronic liver disease. Viral hepatitis C infection is particularly common in patients with AUD with a prevalence of 16%,(53) and therefore, routine screening for viral hepatitis should be considered in this population. Additional diagnostic tests however are not usually necessary, and should be tailored based on clinical suspicion to avoid over-testing. A thorough medication history, including herbals and supplements, is important to rule out drug-induced liver injury (DILI). Certain drugs, such as amiodarone and tamoxifen, may cause steatohepatitis which can be challenging to distinguish from alcohol-related liver disease. Wilson disease may also present with hepatic steatosis and AST to ALT ratio greater than 2 and the diagnosis may need to be ruled out especially in young patients. Liver biopsy is rarely necessary for diagnosis of alcohol-related liver disease, but may be required for diagnosis in the presence of confounding features (i.e. when there is suspicion for autoimmune hepatitis, Wilson’s disease, DILI), lack of consistent alcohol use history or persistent liver test abnormalities despite confirmed and sustained abstinence from alcohol.(54)
Biomarkers of alcohol consumption may be used after discussion with patients to complement the history. Ethyl glucuronide and ethyl sulfate can be detected in the urine for up to 7 days after last alcohol use whereas phosphatidylethanol, a phospholipid formed by the reaction of phosphatidylcholine with ethanol, can test positive for up to 2 weeks after last exposure. Ethyl glucuronide can also be detected in hair samples using liquid chromatography/electrospray tandem mass spectrometry (55, 56), and this method can be used to determine alcohol consumption over the preceding 3 months.(57) These novel biomarkers have replaced the carbohydrate-deficient transferrin test (58) due to greater sensitivity. However, alcohol biomarkers are positive only in the presence of recent consumption and therefore, they should not be used alone for diagnosis of AUD.
Patients with severe alcoholic hepatitis determined by Maddrey score ≥ 32 or MELD score ≥ 21 are managed as in-patients. However, it must be emphasized that patients with non-severe alcoholic hepatitis defined as DF < 32, MELD < 21, ABIC < 6.71 or bilirubin < 85 μmol/L with histological confirmation are also at risk for significant mortality. Mortality in this group of patients was 6% at 28 days, 7% at 90 days and 13% at 1 year. These mortality rates are similar to conditions such as myocardial infarction or community-acquired pneumonia; therefore, non-severe alcoholic hepatitis cannot be considered a benign condition. (59)
Outpatient Management
It is uncertain whether patients with alcohol-related liver disease, including non-severe alcoholic hepatitis, require intervention other than therapies directed at abstinence and nutritional support. An algorithmic approach to evaluation and management by the primary care physician of patients with suspected AUD and alcohol-related liver disease is provided in figure 2. Once alcohol-related liver disease is suspected the patient should be referred to a gastroenterologist or hepatologist. In turn, the specialist should determine whether the patient has advanced liver disease or not, based on clinical, laboratory and imaging findings and non-invasive fibrosis assessment tools if required. In the presence of advanced fibrosis (stages 3–4 or stage 4), then surveillance for hepatocellular carcinoma should be initiated with liver imaging every 6 months. In the absence of advanced liver disease the patient may continue to be managed by the primary care physician.
Figure 2.
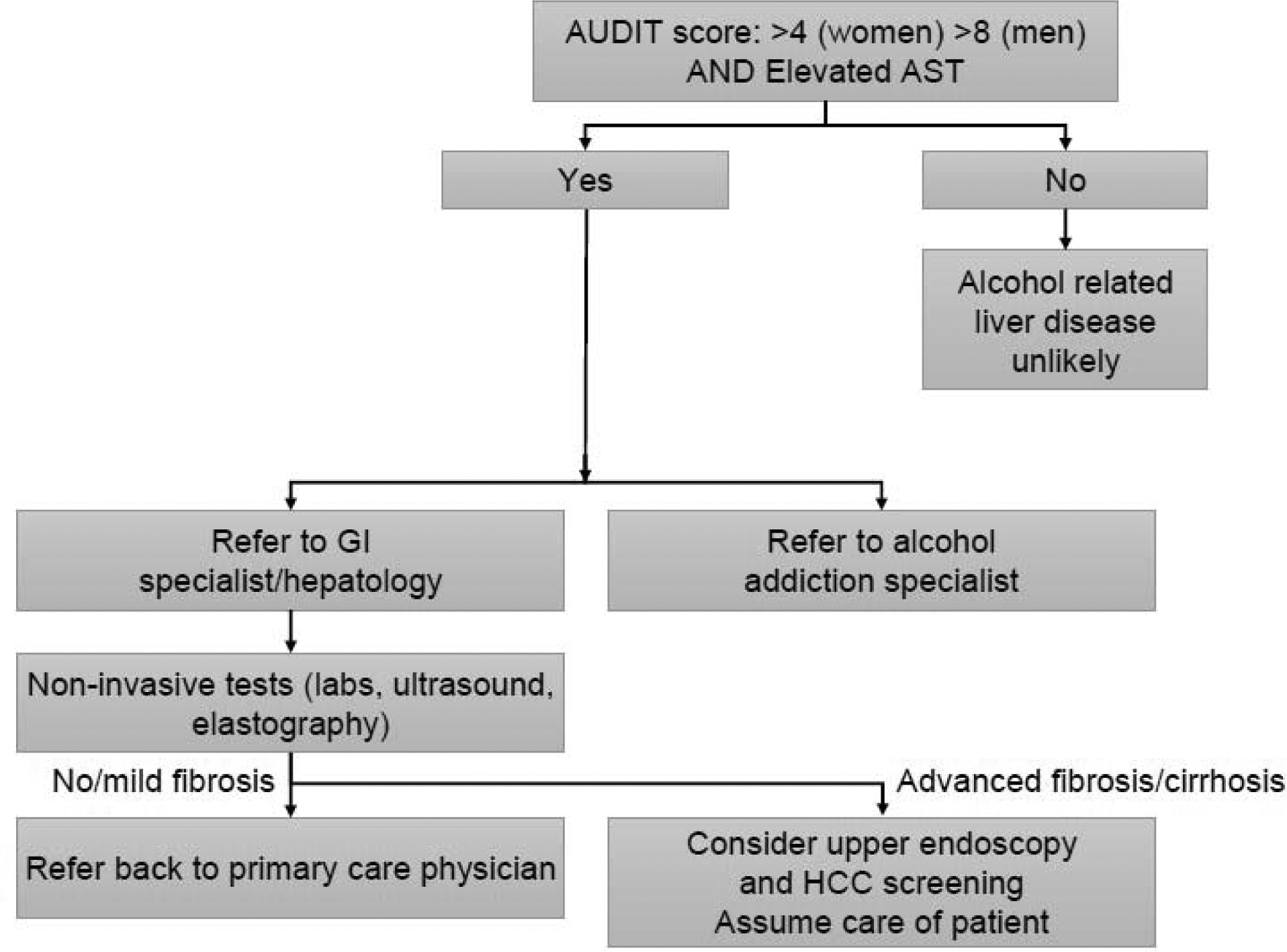
Proposed algorithm for evaluation of alcohol use disorder and alcohol-related liver disease. AUDIT: Alcohol Use Disorder Identification Test; AST: aspartate transferase; HCC: hepatocellular carcinoma
Abstinence
Achievement of abstinence is the key therapeutic goal in the management of all stages of alcohol-related liver disease. Alcohol abstinence improves long term survival in patients with compensated as well as decompensated liver disease (60, 61), although a survival advantage has been best observed with sustained abstinence of greater than 1–2 years.(62) This indicates that additional factors including nutritional status and sepsis play a role in short term survival beyond abstinence. Clinical and laboratory improvement are commonly observed with only sustained alcohol abstinence in patients with decompensated alcohol-related cirrhosis, however studies assessing predictors of response to abstinence and time to improvement are lacking.
The management of AUD requires a multidisciplinary approach led by addiction specialists, counselors, hepatologists, dietitians and social workers. Psychosocial interventions include motivational enhancement therapy (MET), cognitive behavioral therapy (CBT), motivational interviewing, 12-step facilitation (Alcoholic Anonymous) as well as individual, family or group counselling. More recently, a new platform incorporating emerging technology has enabled patients to participate in group counselling from remote locations where local resources are scarce. The so-called avatar-assisted therapy is also an appealing option to individuals concerned about anonymity. (63) However, the effectiveness of psychosocial interventions alone for induction and maintenance of abstinence in patients with alcohol-related liver disease is limited.(64) This is largely due to lack of local resources, limited social support and assistance with transportation, inadequate insurance coverage as well as liver-related complications, such as hepatic encephalopathy. On the other hand, integrating comprehensive medical treatment, psychosocial interventions and pharmacologic therapies for AUD are effective in promoting abstinence and preventing relapse (64, 65).
Pharmacologic treatment of AUD includes a myriad of effective or promising medications. Naltrexone, disulfiram and acamprosate are currently the only drugs approved by Food and Drug Administration (FDA) in the United States for treatment of AUD. Additional drugs that are FDA-approved for other indications have shown promising results in the management of AUD, and include baclofen, gabapentin, topiramate and varenicline. Despite a growing number of potentially effective medications to promote alcohol abstinence and prevent relapse, there is a significant lack of studies assessing their safety in patients with alcohol-related liver disease. None of the FDA-approved drugs have been adequately studied in the alcohol-related liver disease population which limits their use in routine practice. Although disulfiram may be effective in preventing alcohol relapse by producing an aversive reaction when combined with alcohol, results from clinical trials have been conflicting(66, 67), and its use can lead to drug-induced liver injury with significant mortality risk.(68) Naltrexone is also potentially hepatotoxic and is generally avoided; whereas there are no reports of liver injury linked to acamprosate, which does not undergo hepatic metabolism. Baclofen is currently the only drug that has been formally studied in a randomized double-blind placebo-controlled trial in patients with cirrhotic stage alcohol-related liver disease. Baclofen improved the rate of abstinence from 30% to 70%, compared to placebo, and no liver-related side effects were observed.(69) Although most patients in this study had decompensated liver disease (Child-Pugh class B and C), patients with hepatic encephalopathy were excluded. GABA agonists, such as Baclofen and topiramate, may precipitate or worsen hepatic encephalopathy and therefore, they should be used with extreme caution in these patients. Moreover, in a pharmacoepidemiological study comparing baclofen with approved treatments for alcohol use disorders (acamprosate, naltrexone, or nalmefene), patients who received baclofen had a dose-related increase in mortality and risk of hospitalization. (70)
Nutritional Support
Malnutrition is common in alcohol-related liver disease, particularly in patients with alcoholic hepatitis or jaundice, and correlates closely with severity of disease, complications and mortality. Therefore, adequate assessment of nutritional status is imperative. Currently, there is not a single gold standard approach but rather multiple methods to assess for malnutrition including subjective global assessment (SGA), hand-grip strength and dry body mass index (BMI) (table 2). History of weight loss, physical findings of muscle wasting and low dry BMI (adjusted for the presence of ascites) may be the first indicators of malnutrition. Hypoalbuminemia, a common finding in patients with cirrhosis, may not be a reliable nutritional marker due to impaired hepatic synthetic function as well as frequent use of intravenous albumin in this population. Rather, multivariable tools may perform better than a single marker. The SGA, a scoring based system, has been validated for assessment of nutritional status in patients with cirrhosis and includes six categories: nutrient intake, weight change, symptoms affecting oral intake, functional capacity, metabolic demand, and physical examination findings.(71) Sarcopenia is also a robust marker of malnutrition independently associated with increased mortality in cirrhosis.(72) Muscle mass depletion at the third lumbar vertebral level on cross-sectional imaging; thigh thickness on ultrasound(73); fat-free muscle mass on MRI(74); arm-muscle circumference; and hand-grip strength may be used as markers of sarcopenia in patients with alcohol-related cirrhosis.
Table 2.
Recommended tools for assessment of nutritional status in patients with liver disease by the European Society of Clinical Nutrition and Metabolism (ESPEN)
| Tool | Method | Strengths | Limitations |
|---|---|---|---|
| Subjective global assessment (SGA) | Questionnaire collecting information on dietary intake, weight change, and gastrointestinal symptoms | Reliable Validated in patients with liver disease Cost-effective |
Self-reported |
| Hand-grip strength | Hand dynamometer is used to assess grip strength | Objective Superior to SGA to predict of complications of cirrhosis |
Hand dynamometer required |
| Anthropometric parameters | Mid-arm muscle circumference Triceps skin thickness |
Objective Not affected by ascites or volume overload |
Requires experienced providers |
The European Society for Clinical Nutrition and Metabolism (ESPEN) recommends the oral intake of 30–35 kcal/kg/day and 1.5 g of protein/kg/day for patients with cirrhosis based on available evidence.(75) Normal protein consumption has not been shown to cause or worsen hepatic encephalopathy as previously thought, and protein restriction not only does not prevent episodes of HE but it leads to increased protein breakdown.(76) Three to five meals per day as well as a late evening snack may also help prevent catabolism and further loss of muscle mass.(77) When minimal nutritional goals are not met, temporary enteral support should be considered via nasogastric or nasojejunal tubes. Percutaneous endoscopic gastrostomy tube (PEG) placement should be generally avoided in patients with cirrhosis in the presence of portosystemic collaterals and ascites.
Micronutrient and vitamin deficiencies are common in patients with alcohol-related liver disease and screening for deficiencies or empiric supplementation should be considered. Thiamine deficiency, for example, is particularly common in this group and routine replacement is recommended for prevention of Wernicke’s encephalopathy during refeeding state. Deficiency of 25-hydroxyvitamin D is also not only common but it has been associated with severity of liver disease and increased mortality in patients with alcohol-related liver disease.(78) Vitamin A and zinc deficiencies may result in dysgeusia and contribute to poor oral intake, and zinc supplementation may also improve low grade HE when used in combination with lactulose(79) potentially through augmentation of urea production from circulating ammonia.
Additional comorbidities
It is also important to recognize that patients with AUD are at increased risk of additional comorbidities that may require surveillance from the primary care standpoint. Co-occurring mental disorders, such as anxiety, depression and psychosis, are particularly common in patients with AUD as is polysubstance use.(80) Patients with AUD are at risk of replacing alcohol use with other drugs such as benzodiazepines, cannabis or opioids, especially those with untreated mood disorders. Therefore, patients should be informed of the potential impact of alcohol cessation upon other drug use and available strategies to minimise the risk of developing dependence on other drugs should be implemented.
Patients with AUD are also at increased risk of cardiovascular (CV) disease and CV-related death compared to the general population.(81) This is likely driven by a high prevalence of central obesity, hypertriglyceridemia and hypertension in these patients, which range from 38 to 46%.(82) Therefore, early screening and appropriate management of metabolic conditions should be part of the routine care of patients with AUD. This should include the use of statins when indicated. Although there has been a historical concern on the use of statins in chronic liver disease, recent studies have consistently demonstrated the safety of statins in these patients. In fact, a recent systematic review and meta-analysis has suggested that statins may be associated with lower risk of hepatic decompensation, variceal bleeding and mortality.(83)
Summary
With the rise in the prevalence of alcohol-related liver disease worldwide, screening tools such as AUDIT, AUDIT-C or a simple question about binge drinking should become part of routine medical evaluations. Although the ideal screening tool for liver disease does not exist, a variety of noninvasive options is commercially available and should be applied to those at risk. Ultimately, the management and prognosis of alcohol-related liver disease at all stages rely on sustained alcohol abstinence and adequate nutritional support. Therefore, novel and safe therapeutic options for management of alcohol use disorder in patients with liver disease are urgently needed.
Financial Support
This work was supported by grant(s) NIAAA-NIH AA026886-01 and AA 26974.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests
Dr. Shah serves as a consultant to Durect Corporation and Ambys medicine and he is on the advisory board of Akaza Bioscience, Generon Shanghai and Surrozen. The other authors have nothing to disclose.
Search strategy and selection criteria
We searched PubMed from November 8, 2018 through May 1, 2019 using the search terms “alcohol” and “alcohol use disorder” combined with the terms “liver disease”, “cirrhosis” or “steatohepatitis”, restricted to the English language. We selected further relevant publications from the reference lists of articles identified by this search strategy. Relevant publications were selected on the basis of the subheadings used in this Review.
References
- 1.Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74(9):911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grucza RA, Sher KJ, Kerr WC, Krauss MJ, Lui CK, McDowell YE, et al. Trends in Adult Alcohol Use and Binge Drinking in the Early 21st-Century United States: A Meta-Analysis of 6 National Survey Series. Alcohol Clin Exp Res. 2018;42(10):1939–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim D, Li AA, Gadiparthi C, Khan MA, Cholankeril G, Glenn JS, et al. Changing Trends in Etiology-Based Annual Mortality From Chronic Liver Disease, From 2007 Through 2016. Gastroenterology. 2018;155(4):1154–63 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cholankeril G, Ahmed A. Alcoholic Liver Disease Replaces Hepatitis C Virus Infection as the Leading Indication for Liver Transplantation in the United States. Clin Gastroenterol Hepatol. 2018;16(8):1356–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubin E, Lieber CS. Early fine structural changes in the human liver induced by alcohol. Gastroenterology. 1967;52(1):1–13. [PubMed] [Google Scholar]
- 6.Parker R, Aithal GP, Becker U, Gleeson D, Masson S, Wyatt JI, et al. Natural history of histologically proven alcohol-related liver disease: A systematic review. J Hepatol. 2019. [DOI] [PubMed] [Google Scholar]
- 7.Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S87–96. [DOI] [PubMed] [Google Scholar]
- 8.Mellinger JL, Shedden K, Winder GS, Tapper E, Adams M, Fontana RJ, et al. The high burden of alcoholic cirrhosis in privately insured persons in the United States. Hepatology. 2018;68(3):872–82. [DOI] [PubMed] [Google Scholar]
- 9.Sofair AN, Barry V, Manos MM, Thomas A, Zaman A, Terrault NA, et al. The epidemiology and clinical characteristics of patients with newly diagnosed alcohol-related liver disease: results from population-based surveillance. J Clin Gastroenterol. 2010;44(4):301–7. [DOI] [PubMed] [Google Scholar]
- 10.Askgaard G, Leon DA, Kjaer MS, Deleuran T, Gerds TA, Tolstrup JS. Risk for alcoholic liver cirrhosis after an initial hospital contact with alcohol problems: A nationwide prospective cohort study. Hepatology. 2017;65(3):929–37. [DOI] [PubMed] [Google Scholar]
- 11.Westwood G, Meredith P, Atkins S, Greengross P, Schmidt PE, Aspinall RJ. Universal screening for alcohol misuse in acute medical admissions is feasible and identifies patients at high risk of liver disease. J Hepatol. 2017;67(3):559–67. [DOI] [PubMed] [Google Scholar]
- 12.Udo T, Vasquez E, Shaw BA. A lifetime history of alcohol use disorder increases risk for chronic medical conditions after stable remission. Drug Alcohol Depend. 2015;157:68–74. [DOI] [PubMed] [Google Scholar]
- 13.Kalinowski A, Humphreys K. Governmental standard drink definitions and low-risk alcohol consumption guidelines in 37 countries. Addiction. 2016;111(7):1293–8. [DOI] [PubMed] [Google Scholar]
- 14.Rehm J, Patra J. Different guidelines for different countries? On the scientific basis of low-risk drinking guidelines and their implications. Drug Alcohol Rev. 2012;31(2):156–61. [DOI] [PubMed] [Google Scholar]
- 15.Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, et al. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet. 2018;391(10129):1513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rehm J, Baliunas D, Borges GL, Graham K, Irving H, Kehoe T, et al. The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction. 2010;105(5):817–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao Y, Willett WC, Rimm EB, Stampfer MJ, Giovannucci EL. Light to moderate intake of alcohol, drinking patterns, and risk of cancer: results from two prospective US cohort studies. BMJ. 2015;351:h4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306(17):1884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savolainen VT, Liesto K, Mannikko A, Penttila A, Karhunen PJ. Alcohol consumption and alcoholic liver disease: evidence of a threshold level of effects of ethanol. Alcohol Clin Exp Res. 1993;17(5):1112–7. [DOI] [PubMed] [Google Scholar]
- 20.Savolainen V, Perola M, Lalu K, Penttila A, Virtanen I, Karhunen PJ. Early perivenular fibrogenesis--precirrhotic lesions among moderate alcohol consumers and chronic alcoholics. J Hepatol. 1995;23(5):524–31. [DOI] [PubMed] [Google Scholar]
- 21.Bellentani S, Saccoccio G, Costa G, Tiribelli C, Manenti F, Sodde M, et al. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut. 1997;41(6):845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorensen TI, Orholm M, Bentsen KD, Hoybye G, Eghoje K, Christoffersen P. Prospective evaluation of alcohol abuse and alcoholic liver injury in men as predictors of development of cirrhosis. Lancet. 1984;2(8397):241–4. [DOI] [PubMed] [Google Scholar]
- 23.Rehm J, Taylor B, Mohapatra S, Irving H, Baliunas D, Patra J, et al. Alcohol as a risk factor for liver cirrhosis: a systematic review and meta-analysis. Drug Alcohol Rev. 2010;29(4):437–45. [DOI] [PubMed] [Google Scholar]
- 24.Kondili LA, Taliani G, Cerga G, Tosti ME, Babameto A, Resuli B. Correlation of alcohol consumption with liver histological features in non-cirrhotic patients. Eur J Gastroenterol Hepatol. 2005;17(2):155–9. [DOI] [PubMed] [Google Scholar]
- 25.Becker U, Deis A, Sorensen TI, Gronbaek M, Borch-Johnsen K, Muller CF, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23(5):1025–9. [DOI] [PubMed] [Google Scholar]
- 26.Ruhl CE, Everhart JE. Joint effects of body weight and alcohol on elevated serum alanine aminotransferase in the United States population. Clin Gastroenterol Hepatol. 2005;3(12):1260–8. [DOI] [PubMed] [Google Scholar]
- 27.Hagstrom H, Hemmingsson T, Discacciati A, Andreasson A. Alcohol consumption in late adolescence is associated with an increased risk of severe liver disease later in life. J Hepatol. 2018;68(3):505–10. [DOI] [PubMed] [Google Scholar]
- 28.Klatsky AL, Armstrong MA. Alcohol, smoking, coffee, and cirrhosis. Am J Epidemiol. 1992;136(10):1248–57. [DOI] [PubMed] [Google Scholar]
- 29.Dam MK, Flensborg-Madsen T, Eliasen M, Becker U, Tolstrup JS. Smoking and risk of liver cirrhosis: a population-based cohort study. Scand J Gastroenterol. 2013;48(5):585–91. [DOI] [PubMed] [Google Scholar]
- 30.White GE, Courcoulas AP, Richardson GA, Mair C, King WC. Alcohol Use Thresholds for Identifying Alcohol-related Problems Before and Following Roux-en-Y Gastric Bypass. Ann Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 31.Askgaard G, Gronbaek M, Kjaer MS, Tjonneland A, Tolstrup JS. Alcohol drinking pattern and risk of alcoholic liver cirrhosis: a prospective cohort study. J Hepatol. 2015;62(5):1061–7. [DOI] [PubMed] [Google Scholar]
- 32.Buch S, Stickel F, Trepo E, Way M, Herrmann A, Nischalke HD, et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet. 2015;47(12):1443–8. [DOI] [PubMed] [Google Scholar]
- 33.Abul-Husn NS, Cheng X, Li AH, Xin Y, Schurmann C, Stevis P, et al. A Protein-Truncating HSD17B13 Variant and Protection from Chronic Liver Disease. N Engl J Med. 2018;378(12):1096–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris R, Harman DJ, Card TR, Aithal GP, Guha IN. Prevalence of clinically significant liver disease within the general population, as defined by non-invasive markers of liver fibrosis: a systematic review. Lancet Gastroenterol Hepatol. 2017;2(4):288–97. [DOI] [PubMed] [Google Scholar]
- 35.Agbim U, Asrani SK. Non-invasive assessment of liver fibrosis and prognosis: an update on serum and elastography markers. Expert Rev Gastroenterol Hepatol. 2019;13(4):361–74. [DOI] [PubMed] [Google Scholar]
- 36.Cook PA, Morleo M, Billington D, Sanderson-Shortt K, Jones C, Gabbay M, et al. Evaluation of work-based screening for early signs of alcohol-related liver disease in hazardous and harmful drinkers: the PrevAIL study. BMC Public Health. 2015;15:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheron N, Moore M, O’Brien W, Harris S, Roderick P. Feasibility of detection and intervention for alcohol-related liver disease in the community: the Alcohol and Liver Disease Detection study (ALDDeS). Br J Gen Pract. 2013;63(615):e698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thiele M, Madsen BS, Hansen JF, Detlefsen S, Antonsen S, Krag A. Accuracy of the Enhanced Liver Fibrosis Test vs FibroTest, Elastography, and Indirect Markers in Detection of Advanced Fibrosis in Patients With Alcoholic Liver Disease. Gastroenterology. 2018;154(5):1369–79. [DOI] [PubMed] [Google Scholar]
- 39.Pavlov CS, Casazza G, Nikolova D, Tsochatzis E, Gluud C. Systematic review with meta-analysis: diagnostic accuracy of transient elastography for staging of fibrosis in people with alcoholic liver disease. Aliment Pharmacol Ther. 2016;43(5):575–85. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen-Khac E, Thiele M, Voican C, Nahon P, Moreno C, Boursier J, et al. Non-invasive diagnosis of liver fibrosis in patients with alcohol-related liver disease by transient elastography: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2018;3(9):614–25. [DOI] [PubMed] [Google Scholar]
- 41.Gianni E, Forte P, Galli V, Razzolini G, Bardazzi G, Annese V. Prospective Evaluation of Liver Stiffness Using Transient Elastography in Alcoholic Patients Following Abstinence. Alcohol Alcohol. 2017;52(1):42–7. [DOI] [PubMed] [Google Scholar]
- 42.Thiele M, Detlefsen S, Sevelsted Moller L, Madsen BS, Fuglsang Hansen J, Fialla AD, et al. Transient and 2-Dimensional Shear-Wave Elastography Provide Comparable Assessment of Alcoholic Liver Fibrosis and Cirrhosis. Gastroenterology. 2016;150(1):123–33. [DOI] [PubMed] [Google Scholar]
- 43.Shah ND, Ventura-Cots M, Abraldes JG, Alboraie M, Alfadhli A, Argemi J, et al. Alcohol-Related Liver Disease Is Rarely Detected at Early Stages Compared With Liver Diseases of Other Etiologies Worldwide. Clin Gastroenterol Hepatol. 2019;17(11):2320–9 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test (AUDIT): a review of recent research. Alcohol Clin Exp Res. 2002;26(2):272–9. [PubMed] [Google Scholar]
- 45.Fiellin DA, Reid MC, O’Connor PG. Screening for alcohol problems in primary care: a systematic review. Arch Intern Med. 2000;160(13):1977–89. [DOI] [PubMed] [Google Scholar]
- 46.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–95. [DOI] [PubMed] [Google Scholar]
- 47.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31(7):1208–17. [DOI] [PubMed] [Google Scholar]
- 48.Schomerus G, Lucht M, Holzinger A, Matschinger H, Carta MG, Angermeyer MC. The stigma of alcohol dependence compared with other mental disorders: a review of population studies. Alcohol Alcohol. 2011;46(2):105–12. [DOI] [PubMed] [Google Scholar]
- 49.Diehl AM, Goodman Z, Ishak KG. Alcohollike liver disease in nonalcoholics. A clinical and histologic comparison with alcohol-induced liver injury. Gastroenterology. 1988;95(4):1056–62. [PubMed] [Google Scholar]
- 50.Sorbi D, Boynton J, Lindor KD. The ratio of aspartate aminotransferase to alanine aminotransferase: potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am J Gastroenterol. 1999;94(4):1018–22. [DOI] [PubMed] [Google Scholar]
- 51.Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1998;93(1):44–8. [DOI] [PubMed] [Google Scholar]
- 52.Dunn W, Angulo P, Sanderson S, Jamil LH, Stadheim L, Rosen C, et al. Utility of a new model to diagnose an alcohol basis for steatohepatitis. Gastroenterology. 2006;131(4):1057–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muga R, Sanvisens A, Jarrin I, Fuster D, Bolao F, Tor J, et al. Hepatitis C infection substantially reduces survival of alcohol-dependent patients. Clin Epidemiol. 2018;10:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crabb DW, Bataller R, Chalasani NP, Kamath PS, Lucey M, Mathurin P, et al. Standard Definitions and Common Data Elements for Clinical Trials in Patients With Alcoholic Hepatitis: Recommendation From the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology. 2016;150(4):785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morini L, Politi L, Groppi A, Stramesi C, Polettini A. Determination of ethyl glucuronide in hair samples by liquid chromatography/electrospray tandem mass spectrometry. J Mass Spectrom. 2006;41(1):34–42. [DOI] [PubMed] [Google Scholar]
- 56.Appenzeller BM, Agirman R, Neuberg P, Yegles M, Wennig R. Segmental determination of ethyl glucuronide in hair: a pilot study. Forensic Sci Int. 2007;173(2–3):87–92. [DOI] [PubMed] [Google Scholar]
- 57.Verbeek J, Crunelle CL, Leurquin-Sterk G, Michielsen PP, De Doncker M, Monbaliu D, et al. Ethyl Glucuronide in Hair Is an Accurate Biomarker of Chronic Excessive Alcohol Use in Patients With Alcoholic Cirrhosis. Clin Gastroenterol Hepatol. 2018;16(3):454–6. [DOI] [PubMed] [Google Scholar]
- 58.Fagan KJ, Irvine KM, McWhinney BC, Fletcher LM, Horsfall LU, Johnson L, et al. Diagnostic sensitivity of carbohydrate deficient transferrin in heavy drinkers. BMC Gastroenterol. 2014;14:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bennett K, Enki DG, Thursz M, Cramp ME, Dhanda AD. Systematic review with meta-analysis: high mortality in patients with non-severe alcoholic hepatitis. Aliment Pharmacol Ther. 2019;50(3):249–57. [DOI] [PubMed] [Google Scholar]
- 60.Lackner C, Spindelboeck W, Haybaeck J, Douschan P, Rainer F, Terracciano L, et al. Histological parameters and alcohol abstinence determine long-term prognosis in patients with alcoholic liver disease. J Hepatol. 2017;66(3):610–8. [DOI] [PubMed] [Google Scholar]
- 61.Masson S, Emmerson I, Henderson E, Fletcher EH, Burt AD, Day CP, et al. Clinical but not histological factors predict long-term prognosis in patients with histologically advanced nondecompensated alcoholic liver disease. Liver Int. 2014;34(2):235–42. [DOI] [PubMed] [Google Scholar]
- 62.Xie YD, Feng B, Gao Y, Wei L. Effect of abstinence from alcohol on survival of patients with alcoholic cirrhosis: A systematic review and meta-analysis. Hepatol Res. 2014;44(4):436–49. [DOI] [PubMed] [Google Scholar]
- 63.Gordon MS, Carswell SB, Schadegg M, Mangen K, Merkel K, Tangires S, et al. Avatar-assisted therapy: a proof-of-concept pilot study of a novel technology-based intervention to treat substance use disorders. Am J Drug Alcohol Abuse. 2017;43(5):518–24. [DOI] [PubMed] [Google Scholar]
- 64.Khan A, Tansel A, White DL, Kayani WT, Bano S, Lindsay J, et al. Efficacy of Psychosocial Interventions in Inducing and Maintaining Alcohol Abstinence in Patients With Chronic Liver Disease: A Systematic Review. Clin Gastroenterol Hepatol. 2016;14(2):191–202 e1–4; quiz e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–17. [DOI] [PubMed] [Google Scholar]
- 66.Yoshimura A, Kimura M, Nakayama H, Matsui T, Okudaira F, Akazawa S, et al. Efficacy of disulfiram for the treatment of alcohol dependence assessed with a multicenter randomized controlled trial. Alcohol Clin Exp Res. 2014;38(2):572–8. [DOI] [PubMed] [Google Scholar]
- 67.Skinner MD, Lahmek P, Pham H, Aubin HJ. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis. PLoS One. 2014;9(2):e87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bjornsson E, Nordlinder H, Olsson R. Clinical characteristics and prognostic markers in disulfiram-induced liver injury. J Hepatol. 2006;44(4):791–7. [DOI] [PubMed] [Google Scholar]
- 69.Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370(9603):1915–22. [DOI] [PubMed] [Google Scholar]
- 70.Chaignot C, Zureik M, Rey G, Dray-Spira R, Coste J, Weill A. Risk of hospitalisation and death related to baclofen for alcohol use disorders: Comparison with nalmefene, acamprosate, and naltrexone in a cohort study of 165 334 patients between 2009 and 2015 in France. Pharmacoepidemiol Drug Saf. 2018;27(11):1239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morgan MY, Madden AM, Soulsby CT, Morris RW. Derivation and validation of a new global method for assessing nutritional status in patients with cirrhosis. Hepatology. 2006;44(4):823–35. [DOI] [PubMed] [Google Scholar]
- 72.Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS One. 2017;12(10):e0186990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tandon P, Low G, Mourtzakis M, Zenith L, Myers RP, Abraldes JG, et al. A Model to Identify Sarcopenia in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2016;14(10):1473–80 e3. [DOI] [PubMed] [Google Scholar]
- 74.Praktiknjo M, Book M, Luetkens J, Pohlmann A, Meyer C, Thomas D, et al. Fat-free muscle mass in magnetic resonance imaging predicts acute-on-chronic liver failure and survival in decompensated cirrhosis. Hepatology. 2018;67(3):1014–26. [DOI] [PubMed] [Google Scholar]
- 75.Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schutz T, et al. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38(2):485–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cordoba J, Lopez-Hellin J, Planas M, Sabin P, Sanpedro F, Castro F, et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41(1):38–43. [DOI] [PubMed] [Google Scholar]
- 77.Tsien CD, McCullough AJ, Dasarathy S. Late evening snack: exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol. 2012;27(3):430–41. [DOI] [PubMed] [Google Scholar]
- 78.Trepo E, Ouziel R, Pradat P, Momozawa Y, Quertinmont E, Gervy C, et al. Marked 25-hydroxyvitamin D deficiency is associated with poor prognosis in patients with alcoholic liver disease. J Hepatol. 2013;59(2):344–50. [DOI] [PubMed] [Google Scholar]
- 79.Shen YC, Chang YH, Fang CJ, Lin YS. Zinc supplementation in patients with cirrhosis and hepatic encephalopathy: a systematic review and meta-analysis. Nutr J. 2019;18(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Castillo-Carniglia A, Keyes KM, Hasin DS, Cerda M. Psychiatric comorbidities in alcohol use disorder. Lancet Psychiatry. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roerecke M, Rehm J. Cause-specific mortality risk in alcohol use disorder treatment patients: a systematic review and meta-analysis. Int J Epidemiol. 2014;43(3):906–19. [DOI] [PubMed] [Google Scholar]
- 82.Vancampfort D, Hallgren M, Mugisha J, De Hert M, Probst M, Monsieur D, et al. The Prevalence of Metabolic Syndrome in Alcohol Use Disorders: A Systematic Review and Meta-analysis. Alcohol Alcohol. 2016;51(5):515–21. [DOI] [PubMed] [Google Scholar]
- 83.Kim RG, Loomba R, Prokop LJ, Singh S. Statin Use and Risk of Cirrhosis and Related Complications in Patients With Chronic Liver Diseases: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2017;15(10):1521–30 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]


