Abstract
Background
Severe hepatotoxicity in people with human immunodeficiency virus (HIV) receiving efavirenz (EFV) has been reported. We assessed the incidence and risk factors of hepatotoxicity in women of childbearing age initiating EFV-containing regimens.
Methods
In the Promoting Maternal and Infant Survival Everywhere (PROMISE) trial, ART-naive pregnant women with HIV and CD4 count ≥ 350 cells/μL and alanine aminotransferase ≤ 2.5 the upper limit of normal were randomized during the antepartum and postpartum periods to antiretroviral therapy (ART) strategies to assess HIV vertical transmission, safety, and maternal disease progression. Hepatotoxicity was defined per the Division of AIDS Toxicity Tables. Cox proportional hazards models were constructed with covariates including participant characteristics, ART regimens, and timing of EFV initiation.
Results
Among 3576 women, 2435 (68%) initiated EFV at a median 121.1 weeks post delivery. After EFV initiation, 2.5% (61/2435) had severe (grade 3 or higher) hepatotoxicity with an incidence of 2.3 (95% confidence interval [CI], 2.0–2.6) per 100 person-years. Events occurred between 1 and 132 weeks postpartum. Of those with severe hepatotoxicity, 8.2% (5/61) were symptomatic, and 3.3% (2/61) of those with severe hepatotoxicity died from EFV-related hepatotoxicity, 1 of whom was symptomatic. The incidence of liver-related mortality was 0.07 (95% CI, .06–.08) per 100 person-years. In multivariable analysis, older age was associated with severe hepatotoxicity (adjusted hazard ratio per 5 years, 1.35 [95% CI, 1.06–1.70]).
Conclusions
Severe hepatotoxicity after EFV initiation occurred in 2.5% of women and liver-related mortality occurred in 3% of those with severe hepatotoxicity. The occurrence of fatal events underscores the need for safer treatments for women of childbearing age.
Keywords: HIV, real-world, hepatotoxicity, liver enzyme elevation
Severe hepatotoxicity occurred in 2.5% of women with HIV who initiated efavirenz-containing regimens. Liver-related mortality occurred in 3% of those with severe hepatotoxicity. Older age was predictive of severe hepatotoxicity. Safer antiretroviral therapy regimens are needed in women of childbearing age.
Efavirenz (EFV)–containing antiretroviral therapy (EFV-ART) is currently a World Health Organization–recommended first-line regimen. EFV is a nonnucleoside reverse transcriptase inhibitor. Another agent in the same class, nevirapine (NVP), has been associated with fulminant hepatotoxicity, especially at high CD4 cell counts [1–3]. However, the extent of severe hepatotoxicity associated with EFV-ART in women of childbearing age with high CD4 cell counts in resource-limited settings has not been well characterized.
A large meta-analysis of 8 randomized trials and 26 prospective cohorts identified severe hepatotoxicity in 2.3% (95% confidence interval [CI], 1.42%–3.21%) of individuals receiving EFV-ART [4]. In African observational adult cohorts on EFV-ART, severe hepatotoxicity was found in 1%–5% of patients, with concomitant hepatitis B virus (HBV) infection and tuberculosis (TB) individually conferring higher overall risks. These observational cohorts did not include a high proportion of women or individuals with high CD4 cell counts [5–7].
Risk factors for NVP-associated hepatotoxicity include coinfection with viral hepatitis, female sex, and CD4 count > 250 cells/μL [3, 8, 9]. Hepatotoxicity occurs within the first 6–8 weeks of therapy initiation [10–12]. NVP has been associated with rare cases of fulminant liver-related mortality in 0.1% of patients [2]. EFV-ART has generally been regarded as safe, although EFV-ART has also occasionally been associated with fulminant hepatic failure [13–15]. More recently, investigators in South Africa have described 3 novel patterns of drug-induced liver injury among patients with human immunodeficiency virus (HIV) initiating EFV-ART [16], including submassive hepatic necrosis. Additionally, the period after delivery may be a risk factor for drug-related hepatotoxicity, as has been seen with isoniazid during TB treatment [17, 18].
Given these reports of hepatotoxicity with EFV-ART and the widespread implementation of EFV-ART including in prevention of mother-to-child transmission (PMTCT) programs in many African settings, we sought to characterize the incidence and risk factors for severe hepatotoxicity in women of childbearing age with high CD4 cell counts initiating EFV-ART therapy in a large PMTCT trial, PROMISE (Promoting Maternal and Infant Survival Everywhere) 1077BF/1077FF.
METHODS
The PROMISE multicountry randomized trial [19] compared antepartum and postpartum HIV PMTCT strategies in pregnant women with high CD4 cell counts through sequential antepartum and postpartum randomizations. The full methodology has been presented previously [19, 20]. In brief, antepartum women were randomized to 1 of 3 regimens: (1) zidovudine (ZDV) plus intrapartum single-dose nevirapine (sd-NVP) followed by 6–14 days of tenofovir disproxil fumarate (TDF) and emtricitabine (FTC) “tail” postpartum (ZDV alone); (2) ZDV, lamivudine, and ritonavir-boosted lopinavir (LPV/r) (ZDV-based ART); or (3) TDF, FTC, and LPV/r (TDF-based ART). After delivery, women were randomized again to postpartum ART or no ART. In the last randomization (maternal health component), mothers on ART were randomized to either continue or stop ART. Mothers who did not want to be randomized or meet criteria for randomization were followed in observational follow-up. The first randomization occurred in 2011 with follow-up ending in September 2016. Supplementary Figure 1 outlines the PROMISE randomizations.
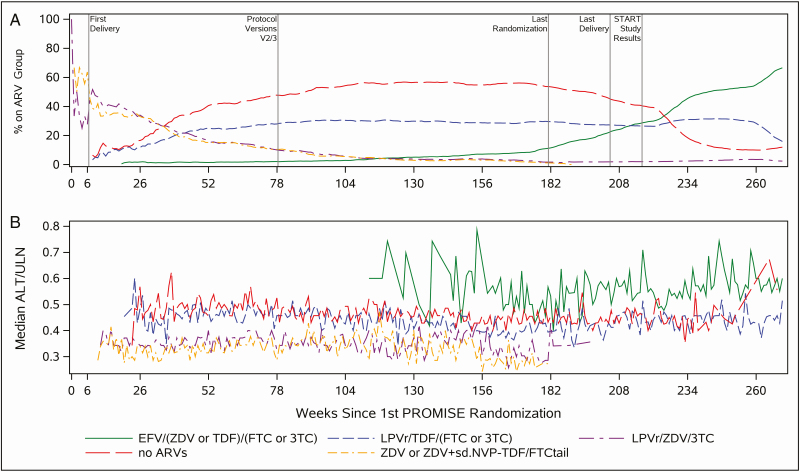
On 7 July 2015, PROMISE sites were notified of the results of the Strategic Timing of Antiretroviral Therapy (START) study, which demonstrated a lower risk of developing AIDS or other serious illnesses with ART initiation in early asymptomatic HIV infection [21], and sites were instructed to recommend that all women enrolled in PROMISE initiate ART regardless of prior randomization or CD4 cell count. Sites offered either local standard of care ART (generally EFV-ART) or, if access was unavailable, study-supplied ART. Consequently, EFV-ART became more common in the last 15 months of the study. This analysis focuses on women enrolled in PROMISE who received EFV-ART, the majority of who first received EFV-ART after delivery. Figure 1A demonstrates the uptake of EFV-ART and other ART regimens during the study period from the first randomization.
Figure 1.
Antiretroviral therapy (ART) distribution and median alanine aminotransferase (ALT) over Promoting Maternal and Infant Survival Everywhere (PROMISE) study period. A, Proportion of women in major antiretroviral regimen groups as percentage in each ART group across time (week) since the first PROMISE randomization. B, Median ALT/upper limit of normal in each ART group across time (week) since the first PROMISE randomization (1-week time window for sample sizes > 10). Abbreviations: 3TC, lamivudine; ALT, alanine aminotransferase; ARV, antiretroviral; EFV, efavirenz; FTC, emtricitabine; LPV/r, ritonavir-boosted lopinavir; sd-NVP, single-dose nevirapine; START, Strategic Timing of Antiretroviral Therapy; TDF, tenofovir disoproxil fumarate; TDF-FTC tail, 1 week of tenofovir disoproxil fumarate–emtricitabine administered postpartum in the women who were randomized to ZDV alone in the antepartum period; ULN, upper limit of normal; ZDV, zidovudine.
Toxicity management included drug discontinuation for any symptomatic alanine aminotransferase (ALT) elevation deemed possibly, probably, or definitely related to EFV-ART and study treatment hold for asymptomatic grade 3 or higher ALT elevation. Permanent discontinuation of individual ART regimens was performed with guidance from a clinical monitoring committee.
The PROMISE1077BF/1077FF study inclusion criteria included pretreatment CD4 count ≥ 350 cells/μL, or greater than or equal to the country-specific threshold of treatment initiation if the threshold was > 350 cells/μL; gestation ≥ 14 weeks; no previous use of triple ART except for PMTCT in previous pregnancies; a hemoglobin level of at least 7.5 g/dL; an absolute neutrophil count of ≥ 750 cells/μL; an ALT ≤ 2.5 times the upper limit of normal (ULN); a calculated creatinine clearance of at least 60 mL/minute; and no serious pregnancy complications.
After delivery, ALT was assessed at weeks 1, 6, 14, 26, and 50 and every 24 weeks until the end of follow-up. In the maternal health component, ALT was assessed at screening, entry, and at weeks 4, 12, 24, and every 24 weeks until the end of follow-up. Additional ALT measurements occurred at early ART discontinuation and when women met criteria for ART initiation; ALT was then repeated 4 weeks afterward. ALT was also assessed at an event-driven visit, defined as confirmation of immunologic or virologic failure, discontinuation of ART for toxicity reasons, or a clinically significant event suggestive of acute exacerbation of hepatitis B. Women who were hepatitis B surface antigen (HBsAg) positive at enrollment had an additional ALT assessment at postpartum week 38.
Severe hepatotoxicity was defined as grade 3 (5.1–10.0) or grade 4 (> 10.0) × ULN ALT elevation using the Division of AIDS toxicity tables (2004/2009) [22]; grade 2 ALT elevation was defined as 1.25–2.5 times the ULN. Symptomatic events were defined as scleral icterus or jaundice, abdominal, or lower extremity swelling, or otherwise unexplained petechiae or abdominal pain. We defined a medication to be hepatotoxic if it was 1 of 72 medications identified as a cause of drug-induced liver injury (DILI) in 5 or more adjudicated DILI cases in 3 DILI registries [23]. We also included albendazole and antimalarial medications. Cox proportional hazards models were run for each covariate and entered in a multivariable model. Covariates included age, body mass index, ALT, prior ALT elevation, HBsAg status, ART regimen prior to EFV-ART and duration, CD4 cell count, country, EFV-ART initiation date, time from delivery to EFV-ART initiation, receipt of EFV-ART prior to delivery, nucleos(t)ide reverse transcriptase inhibitor (NRTI) in EFV-ART, and antepartum and postpartum randomizations. Two-sided 95% confidence intervals (CIs) are presented and associations were assessed at a .05 significance level. Analyses were carried out in SAS software version 9.4.
RESULTS
Characteristics of Women Initiating EFV-ART
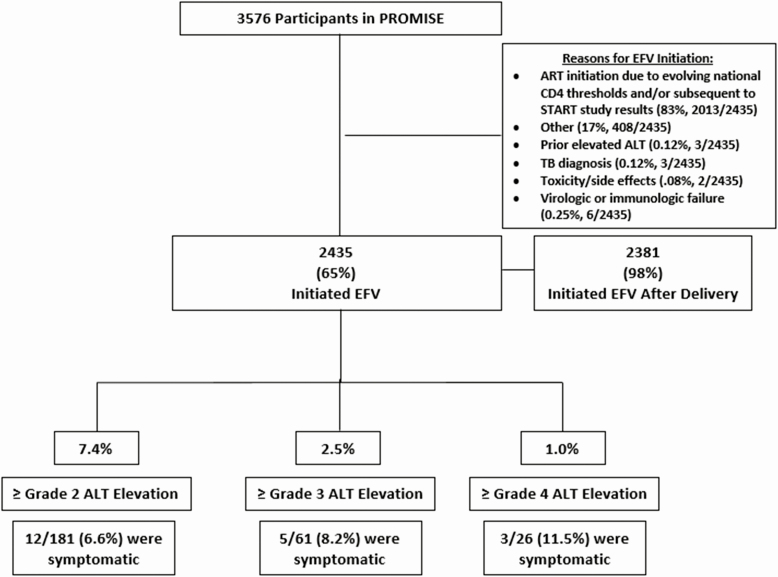
Among 3576 participants, 2435 (65%) initiated EFV and 2381 (98%) did so after delivery (Figure 2). The majority started EFV-ART as countries’ CD4 ART initiation threshold increased over time and after the START study results. Three (0.12%) initiated EFV-ART for prior elevated ALT, 3 (0.12%) for TB (0.12%), 2 for toxicity (0.08%), and 6 for virologic or immunologic failure (0.25%).
Figure 2.
Cohort diagram of women who initiated efavirenz-containing antiretroviral therapy and incidence of hepatotoxicity. Abbreviations: ALT, alanine aminotransferase; ART, antiretroviral therapy; EFV, efavirenz; PROMISE, Promoting Maternal and Infant Survival Everywhere; START, Strategic Timing of Antiretroviral Therapy; TB, tuberculosis.
Table 1 describes the participant characteristics at EFV-ART initiation. The median age was 29.3 years and the median CD4 count was 623 cells/μL. Most (90%) were from Malawi, South Africa, and Zimbabwe. The median time of EFV-ART initiation after delivery was 121.1 weeks. Ninety-one women (4%) were HBsAg positive. Twenty-five (1%) and 43 women (2%) had TB or were on isoniazid (INH), respectively, prior to EFV-ART.
Table 1.
Characteristics of Study Population at Initiation of Efavirenz-Containing Antiretroviral Therapy
| Characteristic | Total(N = 2435) |
|---|---|
| Prior ART regimen | |
| PI + 2 NRTIs | 750 (31) |
| No ARTa | 1546 (63) |
| ZDV or ZDV + sd-NVP–TDF/FTC tail | 50 (2) |
| Other | 89 (4) |
| CD4 count, cells/μL | |
| Min–Max | 75–1938 |
| Median (Q1–Q3) | 623 (463–839) |
| Country | |
| India | 34 (1) |
| Malawi | 841 (35) |
| South Africa | 785 (32) |
| Tanzania | 29 (1) |
| Uganda | 136 (6) |
| Zambia | 60 (2) |
| Zimbabwe | 550 (23) |
| EFV-ART initiation year | |
| 2011–2014 | 661 (27) |
| 6 July 2015 (START date)b | 423 (17) |
| Later than 6 July 2015 | 1351 (55) |
| EFV-ART initiation, wk after delivery | |
| Min–Max | −21.1 to 255.7 |
| Median (Q1–Q3) | 121.1 (73.7–160.9) |
| Before delivery | 52 (2) |
| 0–29 d | 90 (4) |
| > 30 d to 6 wk | 15 (1) |
| > 6 wk to 26 wk | 80 (3) |
| > 26 wk | 2198 (90) |
| Receipt of EFV-ART before delivery | |
| No | 2381 (98) |
| Yes | 54 (2) |
| Age, y | |
| Min–Max | 18.5–50.5 |
| Median (Q1–Q3) | 29.3 (25.5–33.2) |
| HBsAg positive | |
| No | 2344 (96) |
| Yes | 91 (4) |
| Grade 3/4 ALT elevation at EFV-ART initiation | |
| Any grade 3/4 elevation | 72 (3) |
| No grade 3/4 elevation | 363 (97) |
| Grade 3/4 ALT elevation before or during delivery | |
| Any grade 3/4 elevation | 25 (1) |
| No grade 3/4 elevation | 2329 (96) |
| Randomization in BP late presenterc | 81 (3) |
| NRTI in EFV-ART regimen | |
| 3TC/TDF | 1428 (59) |
| FTC/TDF | 937 (38) |
| Other | 70 (3) |
| Antepartum randomization | |
| ZDV + sd-NVP–TDF/FTC tail | 1029 (42) |
| LPV/r/ZDV/3TC | 1019 (42) |
| LPV/r/TDF/3TC | 304 (12) |
| Late presenterc | 83 (3) |
| Postpartum first randomization | |
| Triple ART | 906 (37) |
| No ART | 990 (41) |
| Observationd | 539 (22) |
| New TB prior to EFV-ART initiation | |
| Yes | 25 (1) |
| No | 2410 (99) |
| INH in 3 mo before EFV-ART initation | |
| Yes | 43 (2) |
| No | 2392 (98) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: 3TC, lamivudine; ALT, alanine aminotransferase; ART, antiretroviral therapy; BMI, body mass index; BP, breastfeeding postpartum component; EFV, efavirenz; FTC, emtricitabine; HBsAg, hepatitis B surface antigen; INH, isoniazid; LPV/r, ritonavir-boosted lopinavir; NRTI, nucleos(t)ide reverse transcriptase inhibitor; PI, protease inhibitor; Q1–Q3, first–third quartile; sd-NVP, single-dose nevirapine; START, Strategic Timing of Antiretroviral Therapy; TB, tuberculosis; TDF, tenofovir disoproxil fumarate; TDF-FTC tail, 1 week of tenofovir disoproxil fumarate–emtricitabine administered postpartum in the women who were randomized to no antiretrovirals in the antepartum period; ZDV, zidovudine.
aSixty-three percent were not on ART prior to starting EFV-ART as subsets of participants were randomized to discontinue ART (unless they met country treatment initiation criteria) in the postpartum and maternal health randomizations or may not have been eligible to be randomized to the next step.
bSTART date refers to date of the dissemination of START study findings.
cLate-presenter women did not access antiretroviral strategies for prevention of perinatal transmission during pregnancy.
dWomen in observation were those who did not randomize to ART/no ART postpartum or were ineligible to be randomized.
Incidence, Clinical Characteristics, and Course of Hepatotoxicity
Figure 1 demonstrates the proportion of women on ART regimens over time and the median ALT with each regimen over time. Median ALT levels were higher with EFV-ART over time. After EFV-ART, 7.4% (181/2435), 2.5% (61/2435), and 1.0% (26/2435) had grade 2 or higher, grade 3 or higher, and grade 4 elevated ALT, respectively, with an incidence of severe (grade 3 or higher) hepatotoxicity of 2.3 (95% CI, 2.0–2.6) per 100 person-years (PY) (Figure 2). Among women who had grade 1 or higher ALT elevation after EFV initiation, 5% (25/533) had hepatitis-specific symptoms. Of the 181 women who had grade 2 or higher ALT, 12 of 181 (6.6%) were symptomatic; 2 had jaundice, 4 had a rash, and 6 had abdominal pain within 1 week of the grade 2 or higher ALT. In 141 participants (78%) with grade 2 or lower ALT elevation, ALT resolved to grade 1 or lower, and 119 (66%) resolved to a normal ALT value.
Focusing on those with severe hepatotoxicity, the majority (42 of 61 [69%]) were not on ART prior to initiating EFV-ART. The median CD4 count was 635 cells/μL and the median age was 29.8 years. Only 1 participant was HBsAg positive and 7% (4/61) had a grade 3/4 ALT elevation prior to initiating EFV-ART. Of the 61 with severe (ie, grade 3/4) hepatotoxicity, 5 of 61 (8.2%) were symptomatic, 2 had jaundice, 2 had a rash, and 1 had abdominal pain. Severe hepatotoxicity occurred a median of 20.6 weeks after EFV initiation. In 47 participants (77%), severe hepatotoxicity resolved to grade 2 or lower ALT elevation. The median time to grade 2 ALT decline was 6.9 weeks and the time to grade 2 ALT did not differ by whether EFV was discontinued before a follow-up measurement to the initial ALT elevation (P = .74). Among these 61 women with severe hepatotoxicity, 7 (11%) discontinued EFV a median of 0.14 weeks before severe hepatotoxicity and 11 (18%) discontinued EFV a median of 2.43 weeks after their ALT elevation.
We also evaluated other factors associated with hepatotoxicity in those with severe hepatotoxicity. Among the 61 women on EFV-ART with severe hepatotoxicity, only 2 (3.2%) were on monotherapy or combination INH or had a TB diagnosis prior to their initial elevation. The incidence per 100 PY of severe hepatotoxicity was 0.8 (95% CI, .4–1.7 [2 events]) among 140 who initiated INH for TB disease treatment or had TB within 3 months prior to or after EFV initiation, and 2.4 (95% CI, 2.1–2.7 [59 events]) among 2995 who did not report taking INH or have a TB diagnosis. None had diagnoses of viral hepatitis. Reported alcohol use was uncommon; 11% (7/61) reported drinking > 1 drink per month of alcohol at least once during follow-up. None reported the use of traditional medications. Two women were diagnosed after EFV initiation with pelvic inflammatory disease or a reproductive disorder, which may sometimes cause liver dysfunction. Among all 2435 women, 43% used potentially hepatotoxic medications at the time of EFV-ART initiation. Focusing on those with severe hepatotoxicity, 43% used potentially hepatotoxic drugs within 4 weeks of ALT elevation. The most common medications were acetaminophen-, co-trimoxazole-, and diclofenac/ibuprofen– containing regimens in 16%, 15%, and 13% of women, respectively. Other medications included other antibiotics (10%), enalapril (2%), phenytoin (2%), and ranitidine (2%).
Mortality While on EFV-ART
Thirteen women died after EFV initiation through 30 September 2016 (end of PROMISE). Two had severe hepatotoxicity prior to death. Death was deemed as possibly or probably related to EFV in these 2 women and occurred 1 week after hospitalization for both. They died 105 and 77 weeks after delivery, 16 and 25 weeks after EFV initiation, and 3 and 3.14 weeks after ALT elevation, respectively. The incidence of maternal mortality assessed as related to EFV was 0.07 (95% CI, .06–.08) per 100 PY over 2878 total PY. As the clinical monitoring committee was notified of ongoing toxicities off-study, we learned that 4 additional women on EFV died with hepatic complications following the end of PROMISE, all of which were considered potentially related to efavirenz. We did not include these as outcomes in the analyses as they occurred after the end of PROMISE, but we include them in the descriptive table (Table 2). Among the 6 deaths in combined follow-up, the last previous normal ALT during PROMISE follow-up was measured a median of 4 weeks after EFV initiation and a median of 37.9 weeks prior to death. One woman was receiving INH and 1 had malaria, but these were still thought to be related to EFV. None had heavy alcohol use, use of traditional medications, diagnoses of viral hepatitis, sexually transmitted diseases, or opportunistic infections.
Table 2.
Maternal Hepatitis Deaths Among Women Enrolled in the Promoting Maternal and Infant Survival Everywhere (PROMISE) 1077BF Trial Who Initiated Efavirenz
| Participant Number | ||||||
|---|---|---|---|---|---|---|
| Characteristic | 1 | 2 | 3 | 4 | 5 | 6 |
| Death observed during PROMISE follow-up | Yes | Yes | No | No | No | No |
| Weeks between EFV initiation and death | 25 wk | 16 wk | 59 wk | 22 wk | 52 wk | 102 wk |
| Last CD4 count, cells/μL | 497 | 575 | 604 | 596 | 387 | 483 |
| Site assessment of relation of death to EFVa | Possibly related | Probably related | Potentially related | Potentially related | Potentially related | Potentially related |
| Last CD4 count, cells/μL | 497 | 575 | 604 | 596 | 387 | 483 |
| Last measured normal ALT on PROMISE | ||||||
| Weeks after EFV initiation | 3.86 | 4.14 | 18.57 | −12.57b | 9.0 | −12.29b |
| Weeks before death | 21.14 | 12.14 | 40.71 | 35.14 | 43.0 | 114.57 |
| Laboratory results | ||||||
| Hepatitis E IgM | NR | … | … | … | … | … |
| Hepatitis A IgM | NR | … | … | … | … | … |
| HBV DNA | ND | … | … | … | … | … |
| HCV RNA | SI | … | … | … | … | … |
| Additional relevant diagnoses | ||||||
| TB or INH receipt | No | No | No | No | No | Yes |
| HBsAg positive at screening | No | No | No | No | No | No |
| Malaria | No | No | No | No | Yes | No |
| DVT or PE | No | No | No | No | No | No |
| Hepatobiliary diagnosesc | Nod | Noe | No | No | No | No |
| Genitourinary/sexually transmitted diseasesf | No | No | No | No | No | No |
This table describes the maternal deaths attributable to EFV that occurred during PROMISE follow-up and afterward. INH and malaria may have been contributing causes in each of 2 individuals.
Abbreviations: ALT, alanine aminotransferase; DVT, deep vein thrombosis; EFV, efavirenz; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; IgM, immunoglobulin M; INH, isoniazid; ND, not detectable; NR, nonreactive: PE, pulmonary embolism; SI, sample insufficient; TB, tuberculosis.
aDeaths of participants 1 and 2 were designated as possibly and probably related to EFV by site investigators. As deaths in participants 3–6 occurred off-study, investigators were not required to designate relatedness for reporting purposes. However, site investigators, the clinical monitoring committee, and/or study chairs felt that these deaths were potentially related to EFV in email communications.
bNegative integer represents time before EFV-containing antiretroviral therapy initiation.
cHepatobiliary diagnoses were obtained from case report forms that included hepatitis A and B.
dFor participant 1, “hepatitis, etiology unknown (death)” and “hepatitis, drug induced” were listed, respectively, consistent with no alternate etiology for hepatitis.
eFor participant 2, “hepatitis, etiology unknown (death)” and “hepatitis, drug induced” were listed, respectively, consistent with no alternate etiology for hepatitis.
fGenitourinary/sexually transmitted diseases were obtained from case report forms with relevant diagnoses, including syphilis, chancroid, pelvic inflammatory disease, trichomoniasis, urethritis, and/or venereal disease.
Risk Factors of EFV-ART Hepatotoxicity
Cox proportional hazards model analysis of EFV hepatotoxicity risk factors are presented in Table 3. Older age was significantly associated with an increased risk of severe hepatotoxicity in both unadjusted and adjusted analysis (adjusted hazard ratio per 5 years older, 1.35 [95% CI, 1.06–1.70]). Neither CD4 cell count, HBV coinfection, NRTI selection in EFV regimen, nor prior ARV regimen were significantly associated with severe hepatotoxicity (P ≥ .08).
Table 3.
Predictors of Grade 3/4 Alanine Aminotransferase (ALT) Elevation After Efavirenz Antiretroviral Therapy (EFV-ART) Initiation
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| Covariate at EFV-ART Initiation and Category | Hazard Ratio (95% CI) | P Value (Overall) | Hazard Ratio (95% CI) | P Value (Overall) |
| Prior ART (ref: PI + 2 NRTIs) | ||||
| No ART | 0.75 (.42–1.41) | .35 (.80) | 0.89 (.44–1.87) | .76 (.82) |
| ZDV or ZDV + sd-NVP–TDF/FTC tail | 0.94 (.15–3.35) | .93 | 1.88 (.24–9.36) | .48 |
| Other | 0.66 (.10–2.35) | .58 | 0.69 (.10–2.84) | .65 |
| CD4 count (per 100 cells/μL) | 1.08 (.99–1.17) | .08 | 1.07 (.96–1.17) | .19 |
| EFV-ART initiation study y (per 1 y) | 1.14 (.86–1.56) | .39 | 1.17 (.81–1.74) | .41 |
| EFV-ART initiation study y (ref: 2011–2014)a | ||||
| 6 July 2015 (START dateb) | 0.52 (.21–1.18) | .14 (.09) | … | |
| Later than 6 July 2015 | 1.29 (.73–2.34) | .38 | … | |
| Age (per 5 y older) | 1.25 (1.00–1.55) | .05 | 1.35 (1.06–1.70) | .01 |
| BMI (per 1 kg/m2 higher) | 1.00 (.95–1.04) | .88 | 0.99 (.94–1.04) | .78 |
| HBsAg positive (yes) | 0.44 (.02–1.99) | .40 | 0.43 (.01–1.98) | .41 |
| ALT/ALT × ULN (per 1 higher) | 1.07 (.81–1.21) | .43 | 1.09 (.82–1.24) | .36 |
| ALT grade 3/4a elevation prior to EFV-ART initiation (ref: no grade 3/4 elevation) | 1.86 (.56–4.53) | .23 | … | |
Covariates not presented in the table that were not statistically significant (P ≥ .15) in univariate or multivariable analysis: country enrolled, EFV initiation weeks from delivery, receipt of EFV prior to delivery, categorical BMI, ALT elevation prior to delivery, CD4 cell count by quartile, NRTI in regimen, antepartum and postpartum (ART vs no ART) assignments. The values in the parenthesis of the hazard ratio are the 95% confidence intervals. For grade 3/4 ALT elevation before delivery, there were no postpartum events among those with a prior event, so this covariate was not included in the adjusted model. Continuous covariates were adjusted as applicable.
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; HBsAg, hepatitis B surface antigen; NRTI, nucleos(t)ide reverse transcriptase inhibitor; PI, protease inhibitor; sd-NVP, single-dose nevirapine; START, Strategic Timing of Antiretroviral Therapy; TDF-FTC tail, 1 week of tenofovir disoproxil fumarate–emtricitabine administered postpartum in the women who were randomized to no antiretrovirals in the antepartum period; ULN, upper limit of normal; ZDV, zidovudine.
aResults were similar in an adjusted model with categorical versions for covariates.
bSTART date refers to date of the dissemination of START study findings.
DISCUSSION
Severe hepatotoxicity occurred in 2.5% of women initiating EFV-ART, of whom only 4 of 61 were symptomatic. Older age was associated with increased risk of severe hepatotoxicity. Notably, there were 2 deaths related to EFV-ART hepatotoxicity and 4 additional hepatic-related deaths among participants on EFV-ART that occurred in the year after PROMISE ended. The 2 hepatotoxicity events leading to death during PROMISE follow-up occurred late, up to 6 months after EFV-ART initiation, in contrast to reports of fulminant hepatotoxicity with nevirapine, which occur within the first 6–8 weeks [10–12].
Risk factors for liver-related mortality with EFV-ART are not well understood. Shubber et al found that < 1% of deaths were related to toxicity among patients on EFV-ART, but they did not specify whether the toxicity was hepatotoxicity [4]. Our study supports the findings of other smaller case series of EFV-ART hepatotoxicity and fulminant liver failure. In South Africa, Sonderup et al reported novel patterns of EFV-ART DILI including submassive hepatic necrosis associated with an “immune-allergic pattern” of inflammatory cell infiltrates of eosinophils, plasma cells, and lymphocytes on liver biopsy, a mixed cholestatic hepatitis associated with grade 2–3 hepatotoxicity, and a nonspecific mild hepatitis [16]. Risk factors for each pattern differed with female sex, higher CD4 cell count, and younger age predictive for submassive hepatic necrosis, whereas older age and lower CD4 count were associated with the mixed cholestatic pattern. Participants in the PROMISE study may have had various patterns of hepatic injury, accounting for our differing results with regards to age.
Although our general hepatotoxicity rates were similar to other African cohorts [5–7], our findings of liver-related mortality from hepatotoxicity with relatively late onset after EFV-ART initiation has not been consistently reported in other studies [5–7]. The findings of liver-related mortality among women living with HIV raise concern, particularly since there are no recommendations to routinely monitor liver function tests in HIV treatment programs. It is possible that the deaths reported would not have occurred if hepatotoxicity had been recognized and EFV-ART discontinued earlier.
Also notable was the mild but persistent greater median ALT over time with EFV-ART (Figure 1). Our study population was predominantly black African. There are supporting data that persons of black African ethnicity are at increased risk of severe hepatotoxicity, hospitalizations, and liver transplant or liver-related death after DILI [24]. Mechanisms for an increased risk of hepatotoxicity to EFV in African cohorts are unknown but may be related to the genetics of EFV metabolism. EFV is metabolized by the cytochrome P450 (CYP) 2B6 and CYP3A4/5 liver enzymes [25]. A CYP2B6 variant allele that was found in 43%–49% of South African and Zimbabwean populations was associated with higher plasma EFV concentrations [26, 27] and, in 1 study in Tanzania, was associated with an increased risk of DILI [7]. One hypothesis is that elevated serum EFV may predispose to higher rates of hepatotoxicity or hypersensitivity reactions.
We could not compare rates of hepatotoxicity to women with lower CD4 counts. We cannot exclude the possibility that such an association would have been present if the cohort had included more women with lower CD4 counts.
Hepatitis B infection was not a risk factor for severe hepatotoxicity. The prevalence of hepatitis B e antigenemia and elevated HBV viral loads, both risk factors for hepatotoxicity, are low in African populations [5]. Additionally, participants with grade 2 or higher ALT were excluded, potentially enriching this cohort for less hepatic inflammation.
Limitations of our study include the lack of corroborative liver biopsy and pharmacogenomics or pharmacokinetic information. However, this information is rarely available in resource-limited settings and only endorses the need for more regular ALT monitoring as a proxy for clinically important hepatic events. More than 40% of the overall population received potentially hepatotoxic medications. We did not have dosing information for these medications but given that equivalent proportions of the overall cohort and those with severe hepatotoxicity received potentially hepatotoxic medications and that the most common medications included acetaminophen, nonsteroidal anti-inflammatory medications, and co-trimoxazole–containing regimens, we did not believe that this was a significant contributing factor, although this cannot be excluded. Another limitation was that participants were excluded at study entry if their ALT was > 2.5 times the ULN (grade 2 or higher). Given that prior ALT elevation is a previously reported risk factor for subsequent ALT elevation with EFV-ART [28, 29], rates may have been higher in a population that included this group. To address this, we assessed ALT elevations after entry and screening failures as proxies for potential exclusionary ALT. Only 1.7% had grade 2 or higher ALT after entry and within 3 months before EFV-ART. Among 2723 recorded screening failures, only 5 indicated ALT as reason for failure. These data suggest that only a small proportion of otherwise eligible participants would have been excluded due to an elevated ALT. The PROMISE study was also not specifically designed or powered for the objectives of this secondary analysis and the number of severe hepatotoxicity events was relatively small. Finally, follow-up ended 30 September 2016 with the possibility of later events occurring after the end of data collection.
In summary, we found that in women living with HIV of childbearing age who were initiating EFV at a median of 121 weeks postdelivery, severe (grade 3 or 4) hepatotoxicity after EFV-containing ART initiation occurred at rates seen in prior cohorts. However, liver-related mortality from EFV drug-induced liver injury was unexpected and concerning, as severe hepatotoxicity and mortality occurred late after EFV initiation, generally with no early symptoms. As ART continues to roll out to millions of individuals in sub-Saharan Africa living with HIV, clinicians should be aware of the rare but notable risk of liver-related mortality with EFV-ART and the risk-benefit ratio should be closely weighed. It remains unclear how best to identify women who are at an increased risk of hepatotoxicity. We advise that ALT monitoring should be considered with EFV-ART in older women of childbearing age. Further investigation into the risk factors for DILI in persons living with HIV and receiving EFV-ART should be conducted. With the rollout of lifelong ART for women living with HIV of childbearing age, these results underscore the need for safer treatment options.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health, all components of the National Institutes of Health (NIH), under award numbers UM1AI068632 (IMPAACT Leadership Operations Center), UM1AI068616 (IMPAACT Statistical and Data Management Center), and UM1AI106716 (IMPAACT Leadership Center), and by NICHD contract number HHSN275201800001I. Additional funding was provided by the NIAID (grant numbers UM1AI069465 to A. G. and R01 01AI100748-01) and the NICHD (grant number R01 HD085862 to D. B.). Study products were provided free of charge by AbbVie, Gilead Sciences, Boehringer Ingelheim, and ViiV/GlaxoSmithKline.
Potential conflicts of interest. D. B. reports grants from the National Institutes of Health (NIH), during the conduct of the study; and research support for AIDS Clinical Trials Group (ACTG) clinical trials from AbbVie and Regeneron, outside the submitted work. M. G. P. reports personal fees from Abbott Diagnostics, Atea, Aligos, and Antios outside the submitted work. A. G. and C. T. report grants from the NIH during the conduct of the study. J. S. C. reports grants from Theratechnologies and research support from Merck and Company outside the submitted work. D. G. is an employee of Cepheid, Inc. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sulkowski MS, Thomas DL, Mehta SH, Chaisson RE, Moore RD. Hepatotoxicity associated with nevirapine or efavirenz-containing antiretroviral therapy: role of hepatitis C and B infections. Hepatology 2002; 35:182–9. [DOI] [PubMed] [Google Scholar]
- 2. Stern JO, Robinson PA, Love J, Lanes S, Imperiale MS, Mayers DL. A comprehensive hepatic safety analysis of nevirapine in different populations of HIV infected patients. J Acquir Immune Defic Syndr 2003; 34(Suppl 1):S21–33. [DOI] [PubMed] [Google Scholar]
- 3. Sanne I, Mommeja-Marin H, Hinkle J, et al. Severe hepatotoxicity associated with nevirapine use in HIV-infected subjects. J Infect Dis 2005; 191:825–9. [DOI] [PubMed] [Google Scholar]
- 4. Shubber Z, Calmy A, Andrieux-Meyer I, et al. Adverse events associated with nevirapine and efavirenz-based first-line antiretroviral therapy: a systematic review and meta-analysis. AIDS 2013; 27:1403–12. [DOI] [PubMed] [Google Scholar]
- 5. Hoffmann CJ, Charalambous S, Thio CL, et al. Hepatotoxicity in an African antiretroviral therapy cohort: the effect of tuberculosis and hepatitis B. AIDS 2007; 21:1301–8. [DOI] [PubMed] [Google Scholar]
- 6. Kalyesubula R, Kagimu M, Opio KC, et al. Hepatotoxicity from first line antiretroviral therapy: an experience from a resource limited setting. Afr Health Sci 2011; 11:16–23. [PMC free article] [PubMed] [Google Scholar]
- 7. Mugusi S, Ngaimisi E, Janabi M, et al. Liver enzyme abnormalities and associated risk factors in HIV patients on efavirenz-based HAART with or without tuberculosis co-infection in Tanzania. PLoS One 2012; 7:e40180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Becker S. Liver toxicity in epidemiological cohorts. Clin Infect Dis 2004; 38:S49–55. [DOI] [PubMed] [Google Scholar]
- 9. Luz MC, Marina N, Juan GL, Vincent S. Incidence of liver injury after beginning antiretroviral therapy with efavirenz or nevirapine. HIV Clinical Trials 2003; 4:115–20. [DOI] [PubMed] [Google Scholar]
- 10. Buyse S, Vibert E, Sebagh M, et al. Liver transplantation for fulminant hepatitis related to nevirapine therapy. Liver Transplant 2006; 12:1880–2. [DOI] [PubMed] [Google Scholar]
- 11. Knudtson E, Para M, Boswell H, Fan-Havard P. Drug rash with eosinophilia and systemic symptoms syndrome and renal toxicity with a nevirapine-containing regimen in a pregnant patient with human immunodeficiency virus. Obstet Gynecol 2003; 101:1094–7. [DOI] [PubMed] [Google Scholar]
- 12. Dart Trial Team. Twenty-four-week safety and tolerability of nevirapine vs. abacavir in combination with zidovudine/lamivudine as first-line antiretroviral therapy: a randomized double-blind trial (NORA). Trop Med Int Health 2008; 13:6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abrescia N, D’Abbraccio M, Figoni M, et al. Fulminant hepatic failure after the start of an efavirenz-based HAART regimen in a treatment-naive female AIDS patient without hepatitis virus co-infection. J Antimicrob Chemother 2002; 50:763–5. [DOI] [PubMed] [Google Scholar]
- 14. Turkova A, Ball C, Gilmour-White S, Rela M, Mieli-Vergani G. A paediatric case of acute liver failure associated with efavirenz-based highly active antiretroviral therapy and effective use of raltegravir in combination antiretroviral treatment after liver transplantation. J Antimicrob Chemother 2009; 63:623–5. [DOI] [PubMed] [Google Scholar]
- 15. Clark SJ, Creighton S, Portmann B, Taylor C, Wendon JA, Cramp ME. Acute liver failure associated with antiretroviral treatment for HIV: a report of six cases. J Hepatol 2002; 36:295–301. [DOI] [PubMed] [Google Scholar]
- 16. Sonderup MW, Maughan D, Gogela N, et al. Identification of a novel and severe pattern of efavirenz drug-induced liver injury in South Africa. AIDS 2016; 30:1483–5. [DOI] [PubMed] [Google Scholar]
- 17. Franks AL, Binkin NJ, Snider DE Jr., Rokaw WM, Becker S. Isoniazid hepatitis among pregnant and postpartum Hispanic patients. Public Health Rep 1989; 104:151–5. [PMC free article] [PubMed] [Google Scholar]
- 18. Gupta A, Montepiedra G, Aaron L, et al. Randomized trial of safety of isoniazid preventive therapy during or after pregnancy [abstract 142]. In: 2018 Conference on Retroviruses and Opportunistic Infections, Boston, MA, 2018; 26:60. [Google Scholar]
- 19. Fowler MG, Qin M, Fiscus SA, et al. Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med 2016; 375:1726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Currier JS, Britto P, Hoffman RM, et al. Randomized trial of stopping or continuing ART among postpartum women with pre-ART CD4 ≥ 400 cells/mm3. PLoS One 2017; 12:e0176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Division of AIDS, National Institute of Allergy and Infectious Diseases, US Department of Health and Human Services. Division of AIDS table for grading the severity of adult and pediatric adverse events, version 1.0. XXX, XXX: Department of Health and Human Services, 2004/2009. [Google Scholar]
- 23. Suzuki A, Andrade RJ, Bjornsson E, et al. Drugs associated with hepatotoxicity and their reporting frequency of liver adverse events in VigiBase: unified list based on international collaborative work. Drug Safety 2010; 33: 503–22. [DOI] [PubMed] [Google Scholar]
- 24. Chalasani N, Reddy KRK, Fontana RJ, et al. Idiosyncratic drug induced liver injury in African-Americans is associated with greater morbidity and mortality compared to Caucasians. Am J Gastroenterol 2017; 112:1382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Desta Z, Saussele T, Ward B, et al. Impact of CYP2B6 polymorphism on hepatic efavirenz metabolism in vitro. Pharmacogenomics 2007; 8:547–58. [DOI] [PubMed] [Google Scholar]
- 26. Gounden V, van Niekerk C, Snyman T, George JA. Presence of the CYP2B6 516G> T polymorphism, increased plasma efavirenz concentrations and early neuropsychiatric side effects in South African HIV-infected patients. AIDS Res Ther 2010; 7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nyakutira C, Röshammar D, Chigutsa E, et al. High prevalence of the CYP2B6 516G→T(*6) variant and effect on the population pharmacokinetics of efavirenz in HIV/AIDS outpatients in Zimbabwe. Eur J Clin Pharmacol 2008; 64:357–65. [DOI] [PubMed] [Google Scholar]
- 28. Wit FWNM, Weverling GJ, Weel J, Jurriaans S, Lange JMA. Incidence of and risk factors for severe hepatotoxicity associated with antiretroviral combination therapy. J Infect Dis 2002; 186:23–31. [DOI] [PubMed] [Google Scholar]
- 29. Gao S, Gui XE, Deng L, et al. Antiretroviral therapy hepatotoxicity: prevalence, risk factors, and clinical characteristics in a cohort of Han Chinese. Hepatol Res 2010; 40:287–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.