Abstract
Background
Cryptosporidiosis has been identified as one of the major causes of diarrhea and diarrhea-associated deaths in young children in sub-Saharan Africa. This study traces back Cryptosporidium-positive children to their human and animal contacts to identify transmission networks.
Methods
Stool samples were collected from children < 5 years of age with diarrhea in Gabon, Ghana, Madagascar, and Tanzania. Cryptosporidium-positive and -negative initial cases (ICs) were followed to the community, where stool samples from households, neighbors, and animal contacts were obtained. Samples were screened for Cryptosporidium species by immunochromatographic tests and by sequencing the 18S ribosomal RNA gene and further subtyped at the 60 kDa glycoprotein gene (gp60). Transmission clusters were identified and risk ratios (RRs) calculated.
Results
Among 1363 pediatric ICs, 184 (13%) were diagnosed with Cryptosporidium species. One hundred eight contact networks were sampled from Cryptosporidium-positive and 68 from negative ICs. Identical gp60 subtypes were detected among 2 or more contacts in 39 (36%) of the networks from positive ICs and in 1 contact (1%) from negative ICs. In comparison to Cryptosporidium-negative ICs, positive ICs had an increased risk of having Cryptosporidium-positive household members (RR, 3.6 [95% confidence interval {CI}, 1.7–7.5]) or positive neighboring children (RR, 2.9 [95% CI, 1.6–5.1]), but no increased risk of having positive animals (RR, 1.2 [95% CI, .8–1.9]) in their contact network.
Conclusions
Cryptosporidiosis in rural sub-Saharan Africa is characterized by infection clusters among human contacts, to which zoonotic transmission appears to contribute only marginally.
Keywords: cryptosporidium, transmission, molecular epidemiology, Africa
A contact network analysis in 4 countries of sub-Saharan Africa revealed Cryptosporidium neighborhood clusters in rural communities with human-to-human transmission being the predominant transmission route, while zoonotic transmission appears to contribute only marginally.
(See the Editorial Commentary by Korpe on pages 1367–8.)
The apicomplexan parasite Cryptosporidium is the causative agent of cryptosporidiosis in a wide range of vertebrate hosts [1]. Infections can remain asymptomatic but may lead to malnutrition, persistent growth retardation, and cognitive deficits [2, 3]. Cryptosporidium species are responsible for large waterborne outbreaks, especially in industrialized countries [4, 5], and have been identified as a major cause of diarrhea and diarrhea-associated deaths in young children in sub-Saharan Africa [6, 7]. In this region, an estimated 2.9 million cases occur annually in children < 2 years of age. Despite this tremendous public health burden, there is only suboptimal treatment, and no vaccine is currently available [8].
So far, 38 Cryptosporidium species have been recognized, with Cryptosporidium hominis and Cryptosporidium parvum being the main human pathogens. Data from genotyping studies have supported human-to-human (anthroponotic) transmission as the major route for pediatric infection in endemic regions, despite close human–animal contact in these settings [9–11]. However, studies have been restricted to single-site locations and specific study populations, such as people living with human immunodeficiency virus or immunocompromised hosts, patients in tertiary care hospitals, or symptomatic patients with diarrhea [11]. Furthermore, although the One Health approach has been widely promoted in recent years, the majority of studies on cryptosporidiosis thus far have focused on either animals or humans, limiting our understanding of Cryptosporidium transmission and epidemiology.
The present study aims to identify Cryptosporidium transmission networks and reservoirs in Gabon, Ghana, Madagascar, and Tanzania by tracing back infected children to their closest human and animal contacts.
METHODS
Study Sites
The study took place at 4 study sites in sub-Saharan Africa, namely the Albert Schweitzer Hospital and Georges Rawiri Hospital in Lambaréné, Gabon, the Agogo Presbyterian Hospital in the Ashanti Region of Ghana, the Korogwe District Hospital in the Tanga Region of Tanzania, and the Imerintsiatosika Health Post in the rural outskirts of Antananarivo, Madagascar. Ethical approval for the study was obtained from the respective ethics committees (see Supplementary Methods for country details).
Sampling Procedure
Between November 2016 and April 2018, stool samples were collected from all children < 5 years of age presenting to outpatient departments with diarrhea or a history of diarrhea, defined as at least 3 loose stools in 24 hours within the past 3 days. Stool samples from all children were screened for Cryptosporidium species with an immunochromatographic rapid test (Certest Biotech, Zaragoza, Spain). In case of a positive test result, 1 stool sample per contact of the initial case (IC) was collected as follows: (1) household contact (HC), defined as somebody who lives on the same compound sharing food on a regular basis with the IC; (2) neighboring children (NC) < 5 years of age living in the proximity of the IC with regular contact to the IC’s family; and (3) animal contacts (ACs), defined as ruminants (ie, goats, sheep, cows) and dogs belonging to the family or kept within a 100-m radius around the respective household. Chickens, although common, were not considered due to difficulties in obtaining fresh fecal specimens. Samples from contacts were only considered for further analysis when collected within 1 week after an IC was identified.
A subset of children with a negative rapid diagnostic test for Cryptosporidium species (later confirmed by polymerase chain reaction [PCR] as described below) were followed to their homes and contact samples were collected as described above. Data from these children and their contacts were included in the analysis as negative control data. Contact networks from positive ICs are hereinafter referred to as positive IC networks and those from negative ICs as negative IC networks.
Molecular Characterization
The molecular analyses are described in detail in the Supplementary Methods. In brief, all study stool samples arrived within 24 hours in the laboratory and DNA was extracted using the Qiagen DNeasy PowerSoil Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Cryptosporidium species were identified using a nested PCR protocol for the amplification of the 18S ribosomal RNA gene as published previously [12]. All samples positive for C. hominis or C. parvum were further subtyped by sequencing a 850-bp fragment of the gp60 gene using a nested PCR as previously described [13, 14]. For a sample subset, 4 loci containing short sequence repeats (TP14, MS9, MM18, and MM19) were amplified by PCR as previously described [15] to confirm identified gp60 subtype clusters.
Data Analysis
Transmission clusters were defined as at least 2 Cryptosporidium cases with the same gp60 subtype diagnosed among contacts (HCs, NCs, or ACs) of an individual IC—that is, occurring within the same contact network. This cluster analysis was also applied to the negative IC networks, and the number of clusters within negative IC networks was compared to the number of clusters within positive IC networks. The statistical analysis is described in detail in the Supplementary Methods.
RESULTS
Between May 2016 and April 2018, 1363 ICs with diarrhea were recruited. Of these, 184 (13%) tested positive for Cryptosporidium species. The highest proportion of positive ICs was identified in Gabon (n = 44 [21%]), while the prevalence at the other study sites ranged between 11% and 15% (Table 1). Contacts of positive ICs were sampled, resulting in a total of 350 HCs, 258 NCs, and 338 ACs, of which 47 (13%), 60 (23%), and 45 (13%), respectively, were positive for Cryptosporidium species. The fraction of positive samples was therefore highest among NCs, even higher than in ICs. In Gabon, no samples from NCs or ACs were available.
Table 1.
Characteristics of the Study Participants and Identified Cryptosporidium Species, by Study Site and Sampling Group
| Characteristic | Initial Cases | Household Contacts | Neighboring Children | Animal Contacts |
|---|---|---|---|---|
| Gabon | ||||
| Observations, No. | 214 | 79 | 0 | 0 |
| Age, y, median (IQR) | 0 (0–1) | 15 (6–25) | … | … |
| Female sex | 93 (43) | 48 (62) | … | … |
| Cryptosporidium spp | 44 (21) | 16 (20) | … | … |
| C. hominis | 35 (80) | 13 (81) | … | … |
| C. parvum | 8 (18) | 2 (13) | … | … |
| C. meleagridis | 1 (2) | … | … | |
| C. felis | 1 (6) | … | … | |
| Ghana | ||||
| Observations, No. | 410 | 105 | 97 | 133 |
| Age, y, median (IQR) | 1 (0–1) | 15 (6–31) | 3 (0–5) | ND |
| Female sex | 183 (45) | 75 (71) | 43 (44) | ND |
| Cryptosporidium spp | 47 (11) | 11 (10) | 18 (19) | 20 (15) |
| C. hominis | 26 (55) | 4 (36) | 8 (44) | |
| C. parvum | 15 (32) | 6 (55) | 7 (39) | 2 (10) |
| C. meleagridis | 3 (6) | … | … | … |
| C. felis | 2 (4) | 1 (9) | 3 (17) | |
| C. xiaovi/bovis | 1 (2) | … | … | 17 (85) |
| C. ubiquitum | … | … | … | 1 (5) |
| Madagascar | ||||
| Observations, No. | 209 | 52 | 50 | 80 |
| Age, y, median (IQR) | 1 (0–1) | 21 (18–36) | 3 (1–3) | ND |
| Female sex | 106 (51) | 35 (67) | 23 (46) | ND |
| Cryptosporidium spp | 25 (12) | 4 (8) | 18 (36) | 6 (8) |
| C. hominis | 20 (80) | 4 (100) | 16 (89) | 3 (50) |
| C. meleagridis | 5 (20) | … | 2 (11) | … |
| C. xiaoi/bovis | … | … | … | 1 (17) |
| C. canis | … | … | … | 1 (17) |
| C. avian genotype III | … | … | … | 1 (17) |
| Tanzania | ||||
| Observations, No. | 462 | 114 | 111 | 125 |
| Age, y, median (IQR) | 1 (0–1) | 18 (7–30) | 3 (1–3) | ND |
| Female sex | 216 (47) | 67 (59) | 61 (55) | ND |
| Cryptosporidium spp | 68 (15) | 16 (14) | 24 (22) | 19 (15) |
| C. hominis | 63 (94) | 15 (94) | 19 (79) | 10 (53) |
| C. parvum | 3 (4) | 1 (6) | 2 (8) | … |
| C. meleagridis | 1 (1) | … | 1 (4) | … |
| C. felis | … | … | 2 (8) | … |
| C. xiaoi/bovis | … | … | … | 6 (32) |
| C. ubiquitum | … | … | … | 2 (11) |
| C. ryanae | … | … | … | 1 (5) |
| Negative initial cases and contacts (all countries) | ||||
| Observations, No. | 68 | 215 | 162 | 244 |
| Age, y, median (IQR) | 1 (0–1) | 20 (8–32) | 2 (1–3) | ND |
| Female sex | 22 (32) | 122 (57) | 92 (57) | ND |
| Cryptosporidium spp | 0 (0) | 8 (4) | 13 (8) | 27 (11) |
| C. hominis | … | 4 (50) | 10 (77) | 8 (30) |
| C. parvum | … | 2 (25) | 1 (8) | … |
| C. meleagridis | … | 1 (13) | … | … |
| C. felis | … | 1 (13) | 1 (8) | … |
| C. suis | … | … | 1 (8) | 1 (4) |
| C. xiaoi/bovis | … | … | … | 11 (41) |
| C. canis | … | … | … | 2 (7) |
| C. ubiquitum | … | … | … | 2 (7) |
| C. ryanae | … | … | … | 2 (7) |
| C. baileyi | … | … | … | 1 (4) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: IQR, interquartile range; ND, not determined.
In addition to the contacts of positive ICs, contacts from 68 negative patients were investigated to construct negative control contact networks. Characteristics of the positive ICs contacts from the 4 study countries and the pooled negative IC contacts are summarized in Table 1.
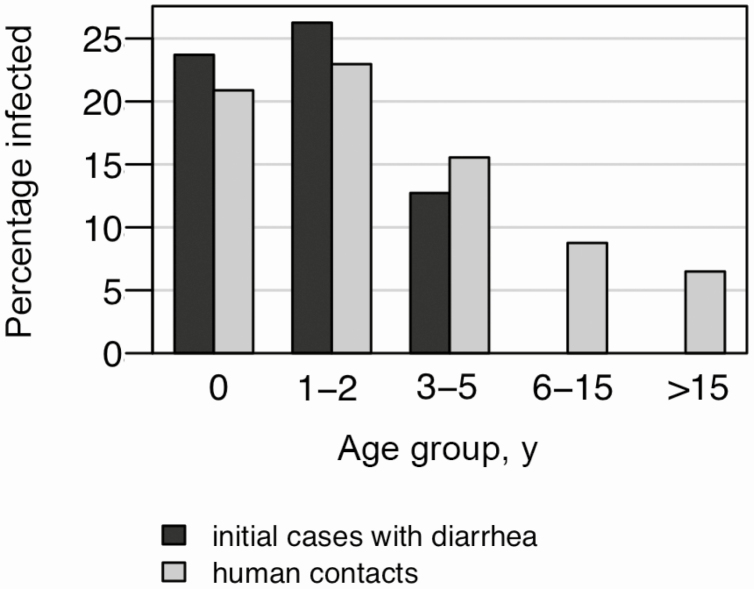
The proportion of Cryptosporidium infections in all study individuals was highest in participants aged 1–2 years and decreased with increasing age (Figure 1). Comparable age-stratified infection rates were observed in ICs with diarrhea and their human contacts.
Figure 1.
Proportion of Cryptosporidium species cases for initial patients and human contacts (household contacts and neighboring children), stratified by age.
Distribution of Cryptosporidium Species and gp60 Subtypes
The distribution of Cryptosporidium species differed among humans and animals. Apart from Madagascar, where no C. parvum was detected, C. hominis (n = 237 [76%]) and C. parvum (n = 47 [15%]) were the most frequently detected species among humans, followed by Cryptosporidium maleagridis (n = 14 [5%]) and Cryptosporidium felis (n = 11 [4%]). Among animals, C. hominis was the most frequently detected species in Madagascar (n = 4 [57%]) and Tanzania (n = 17 [57%]), whereas Cryptosporidium xiaoi/bovis (n = 24 [89%]) was the most frequently detected species in Ghana (Table 1). Ghana was the only country where C. parvum was detected in animals (n = 2 [7%]). The distribution of Cryptosporidium species in the different animal hosts is described in Supplementary Table 1.
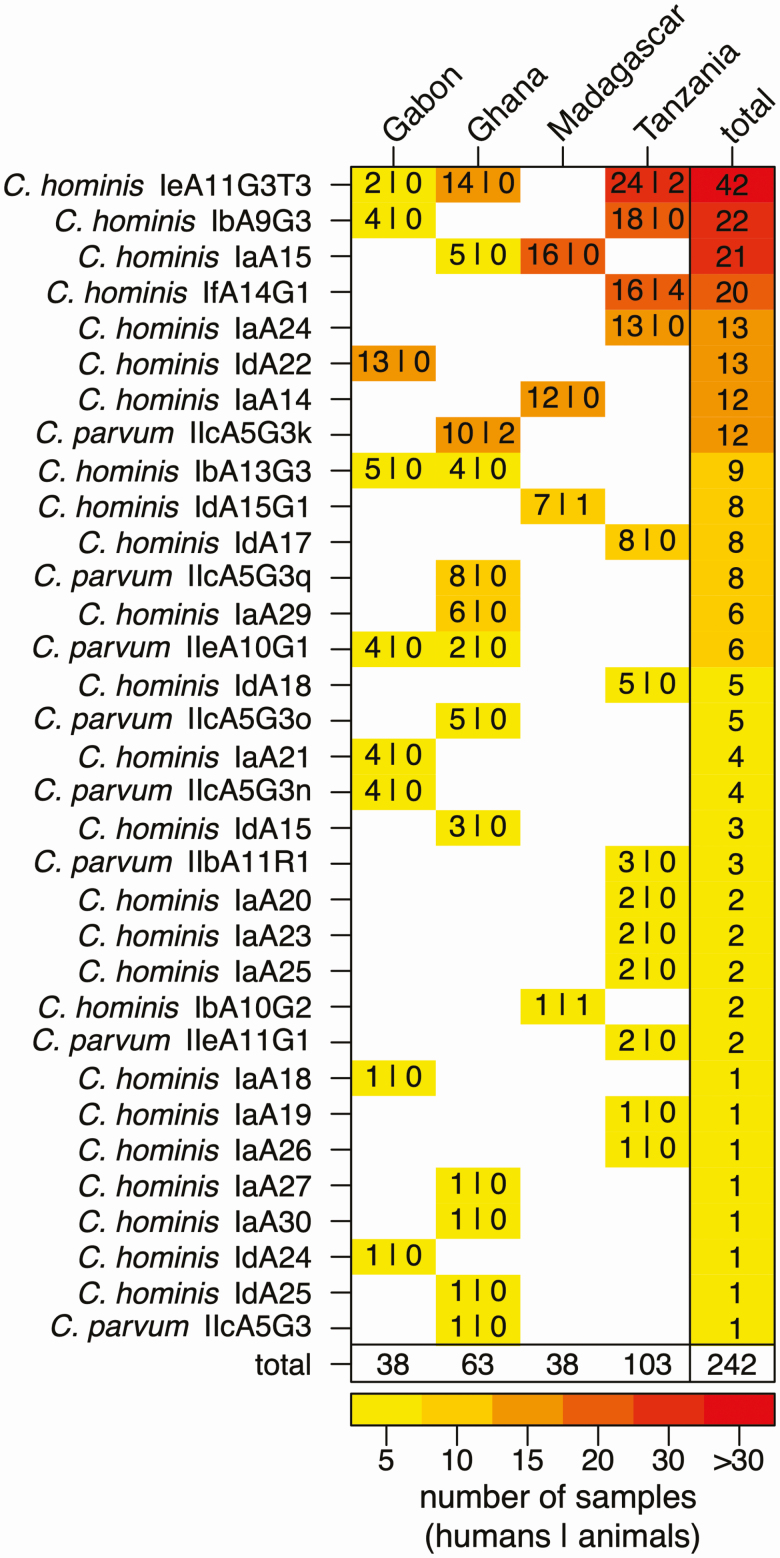
Subtyping for gp60 was applied to C. hominis and C. parvum species to identify closely related strains, and data were available for 242 of 307 (79%) specimens; gp60 subtyping revealed 25 C. hominis and 8 C. parvum subtypes, which were heterogeneously distributed over the study sites (Figure 2). Among the 8 C. parvum subtypes, 5 belonged to the IIc subtype family, likely to be of anthroponotic origin. Only 4 C. hominis and 1 C. parvum subtype were detected at > 1 study site, and no single gp60 subtype was detected at all study locations.
Figure 2.
Frequency of Cryptosporidium gp60 subtypes at the 4 study sites. Frequencies of gp60 subtypes at the 4 study sites are presented for humans (first number) and animals (second number) along with strain and study site totals. The heatmap displays the lowest frequencies in yellow and the highest frequencies in red.
Six gp60 subtypes were responsible for > 50% of infections in humans and animals, namely C. hominis IeA11G3T3 (n = 42 [17%]), C. hominis IbA9G3 (n = 22 [9%]), C. hominis IaA15 (n = 21 [9%]), C. hominis IfA14G1 (n = 20 [8%]), C. hominis IdA22 (n = 13 [5%]), and C. hominis IaA24 (n = 13 [5%]). gp60 diversity was highest in Gabon and Ghana, where the ratio of observed gp60 subtypes over infected subjects was 0.24 (95% confidence interval [CI], .11–.40) and 0.21 (95% CI, .11–.33), respectively, compared to 0.13 (95% CI, .07–.21) in Tanzania and 0.11 (95% CI, .03–.25) in Madagascar.
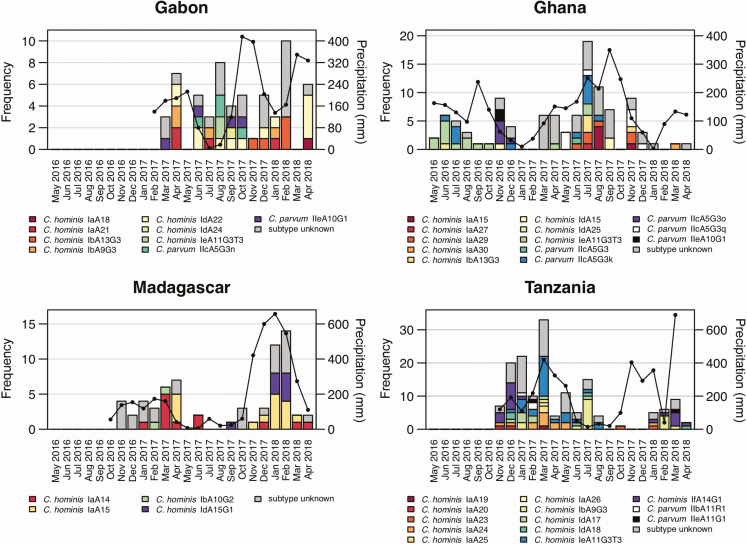
Seasonal Distribution and Temporal Clustering
The seasonal occurrence of gp60 subtypes in human and animal samples along with the monthly precipitation within the study regions are displayed in Figure 3. Seasonal patterns were visible in Ghana and Madagascar, where rising case numbers followed increasing precipitation. In all countries, particular gp60 subtypes were detected repeatedly during the study period while other subtypes were only detected sporadically at distinct time points. In Ghana, for example, C. hominis IeA11G3T3 (n = 14) occurred within 8 months over a period of 15 months, while C. parvum IIeA10G1 (n = 2) was detected during 1 month only.
Figure 3.
Occurrence of gp60 subtypes over time at the 4 study sites. Gray bars represent Cryptosporidium species infections of unknown subtype. Black lines represent monthly precipitation (in millimeters, z-axis).
Overall, 112 of 177 (63%) infections belonged to a temporal cluster, defined as the occurrence of at least 2 isolates with the same gp60 subtype occurring within a 2-week period at a study site. The proportion of subjects belonging to temporal clusters was considerably lower in Gabon (n = 4/21 [19%]). However, the overall contact network size was smaller in Gabon, containing ICs and HCs only, and therefore decreasing the likelihood of detecting identical Cryptosporidium subtypes. In total, among all countries, 55 temporal clusters were detected, which were composed of a median number of 3 (interquartile [IQR], 2–4) subjects and which persisted over a median time of 5 days (IQR, 2–12 days). The largest temporal cluster contained 11 humans and 2 animals infected with C. hominis IeA11G3T3 and occurred in Tanzania over a period of 32 days. However, nearly half of the temporal clusters (n = 27/55 [49%]) contained only 2 infected subjects.
Contact Networks and Transmission Clusters
In total, 108 positive IC contact networks were sampled: 17 in Gabon, 33 in Ghana, 19 in Madagascar, and 39 in Tanzania. As a comparison, contact networks from 68 Cryptosporidium-negative ICs were sampled (5 from Gabon, 11 from Ghana, 26 from Madagascar, and 26 from Tanzania). The composition of all contact networks, including proportions of HCs, NCs, and ACs within each network, is presented in Supplementary Figure 1.
The proportion of positive HCs (risk ratio [RR], 3.6 [95% CI, 1.7–7.5]) and positive NCs (RR, 2.9 [95% CI, 1.6–5.1]) was higher in positive IC networks compared to negative IC networks. However, the proportion of positive ACs was comparable between both groups (RR, 1.2 [95% CI, .8–1.9]). Thus, positive ICs were more likely to have Cryptosporidium-positive individuals than Cryptosporidium-positive animals in their households and neighborhood networks.
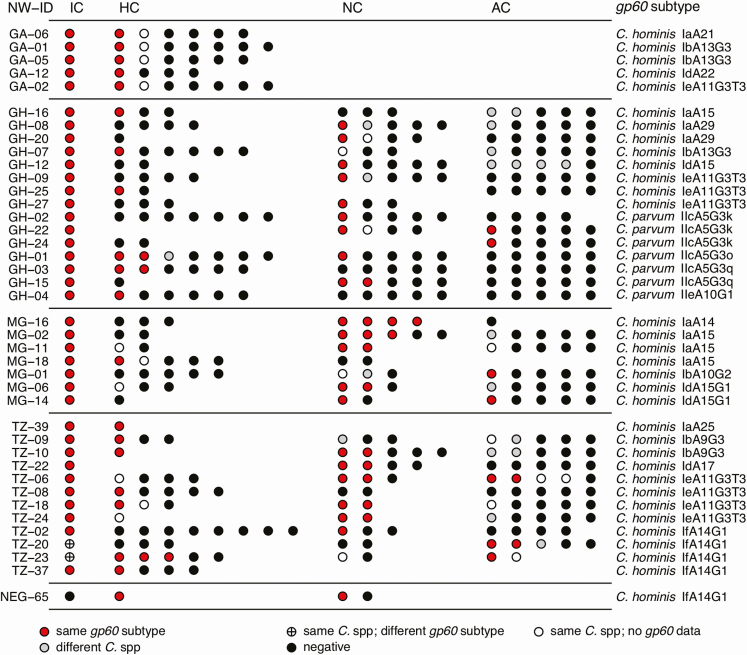
Transmission clusters were defined as at least 2 Cryptosporidium cases with the same gp60 subtype diagnosed within the contact network of an individual IC. Among the 108 positive IC networks, 39 contained transmission clusters: 5 of 17 (29%) in Gabon, 15 of 33 (45%) in Ghana, 7 of 19 (37%) in Madagascar, and 12 of 39 (31%) in Tanzania, while only a single transmission cluster was identified among the negative IC networks (1/68 [1%]) (Figure 4). There was an 8.9 (95% CI, 1.2–65.7) times higher risk of transmission clusters occurring within positive compared to negative IC networks.
Figure 4.
Transmission clusters of individual study subjects (circles) with identical gp60 subtypes among contact networks at the different study sites. Cluster structures are displayed, including study subjects infected with the same Cryptosporidium species but lacking gp60 data, which could potentially be related to the detected cases. The occurrence of differing, non-cluster-defining Cryptosporidium species within networks is also shown. Abbreviations: AC, animal contact; C., Cryptosporidium; GA, Gabon; GH, Ghana; HC, household contact; IC, initial case; MG, Madagascar; NC, neighboring child; NW-ID, network identifier as defined in Supplementary Figure 1; TZ, Tanzania.
Of the 433 study subjects (ICs, HCs, NCs, and ACs) who belonged to contact networks with transmission clusters, 147 (34%) tested positive for Cryptosporidium species, and of those, 104 (71%) were infected with the gp60 subtype defining the cluster. Within these networks, 17% (24/144) of HCs and 31% (34/108) of NCs were positive for the cluster-defining gp60 subtype (Figure 4). Animals were less likely to be positive for the respective gp60 cluster subtype (9/141 [6%]).
The 40 contact networks with transmission clusters could be allocated to 36 temporal clusters (occurrence of at least 2 identical gp60 strains within a time window of 2 weeks). Temporal clusters containing multiple transmission clusters of the same gp60 subtype were only identified in Tanzania. The transmission clusters within networks TZ-06 and TZ-18, with 9 infected subjects, were nested within a C. hominis IeA11G3T3 temporal cluster, which lasted over 32 days with 13 infected subjects in total. The transmission clusters within networks TZ-20, TZ-23, TZ-37, and NEG-65, with 10 infected subjects, occurred within a C. hominis IfA14G1 temporal cluster, which lasted over 18 days with 11 infected subjects in total. GPS-coordinates indicate that TZ-06 and TZ-18 as well as TZ-20 and TZ-37 are neighboring families living within a distance of < 50 m.
Multilocus Variable Number of Tandem Repeats Analysis
To corroborate gp60 clusters, 6 clusters with 23 Cryptosporidium species samples, for which sufficient genetic material remained available, were selected for further variable number of tandem repeats (VNTR) analysis (Supplementary Table 2). Amplification of fragments encompassing tandem repeats was successful for 20 isolates (87%) at the TP14 locus, 19 isolates (83%) at the MM19 locus, 18 isolates (78%) at the MS9 locus, and 14 isolates (61%) at the MM18 locus. Notably, most animal samples positive for C. hominis could not be amplified at any of these loci. The combined analysis identified 4 different multilocus types, 1 of which was shared by 3 clusters of C. parvum IIc subtype in Ghana, while the remaining 3 were each found in 1 of the 3 C. hominis clusters from Tanzania (IeA11G3T3 and IdA17) and Madagascar (IaA14) (Supplementary Table 2). The VNTR analysis therefore supported the previously defined gp60 clusters.
DISCUSSION
Across all study sites, 13% of hospital-attending children with diarrhea (in Gabon, up to 21%) were positive for Cryptosporidium, in agreement with the high prevalence in sub-Saharan Africa described by the Global Enteric Multicenter Study in 2013 [6]. Prevalence from other African studies varies widely, and comparisons are difficult to draw due to differences in age groups and detection methods [11]. Interestingly, our study detected a similarly high prevalence of Cryptosporidium species (18%) in the community, which suggests a high number of asymptomatic or mild diarrheal cases, which are missed by hospital-based studies. The clinical relevance of such cases has been demonstrated in Peru, where even asymptomatic infections were associated with growth retardation in children [16].
The high infection rate among household contacts and neighbors points toward the existence of Cryptosporidium transmission clusters. Indeed, gp60 subtyping more frequently detected transmission clusters in neighborhoods with a positive initial child when compared to negative controls. While household members and neighboring children were significantly more likely to be part of transmission clusters, animals do seem to play a minor role for Cryptosporidium transmission.
Subtyping data from animals and humans in this study suggest a predominant anthroponotic transmission of Cryptosporidium species in sub-Saharan Africa. Previous studies from sub-Saharan Africa have similarly hypothesized a predominantly anthroponotic transmission in the region, but are based on either animal or human data [9, 11, 17]. Surveys including both human and animal samples combined with geospatial data collection for the identification of transmission clusters are lacking in sub-Saharan Africa [11]. A few existing One Health studies from Ghana and Nigeria do not allow any final conclusions on the extent of zoonotic transmission [18, 19]. A recent meta-regression analysis of C. parvum IIc prevalence data suggests that anthroponotic transmission prevails in lower-income countries due to limited access to sanitation facilities [10]. The high subtype diversity, which is commonly seen in sub-Saharan Africa [20] and also in the current study, is thought to reflect this intensive and stable anthroponotic transmission.
A distinct geographic distribution was observed in the present study, with only 5 of 33 C. parvum and C. hominis gp60 subtypes detected in > 1 country. For instance, subtype IdA22 was the most frequently detected subtype in Gabon, but was not detected at any other study site. However, this C. hominis subtype has previously been described in Kenya [17]. Similarly, the most commonly detected human C. parvum subtypes in Ghana (IIcA5G3k, IIcA5G3q, and IIcA5G3o) were exclusively found at this site. Such geographic segregation of C. parvum subpopulations has been observed before in European countries, Israel, and New Zealand [21, 22]. Interestingly, no C. parvum was identified in either humans or animals in Madagascar. Cryptosporidium parvum may not be a clinically relevant species in Madagascar; 2 previous studies from this region found only 1 child infected with C. parvum, while C. hominis and Cryptosporidium suis were the predominant human species [23, 24].
Although anthroponotic transmission appears to predominate in the study areas, there is evidence for the occurrence of zoonotic transmission. Cryptosporidium hominis was detected in 11 cows, 5 goats, and 1 sheep in Tanzania, and from 1 cow and 3 dogs in Madagascar. There is indeed increasing evidence that C. hominis not only infects humans, but can also be observed in symptomatic and asymptomatic animals [25]. The detection of C. hominis in animals, however, does not infer direct transmission from humans, as it may simply reflect circulation of C. hominis in the animal reservoir of the study region. Nevertheless, the present data suggest potential zoonotic transmission of the C. hominis subtypes IeA11G3T3 and IfA14G1 within Tanzanian clusters (detected in humans, cows, and goats) and IbA15G1 and IbA10G2 within Malagasy clusters (detected in humans and dogs). Furthermore, C. parvum IIcA5G3k was found in humans and goats in a Ghanaian transmission cluster. IIcA5G3k has so far only been described in humans from Nigeria [26, 27]. Formerly considered an anthroponotic subtype, other IIcA5G3 variants have since been detected in a goat (IIcA5G3q) from Ghana and from hedgehogs (IIcA5G3j) in the United Kingdom and Germany [19, 28, 29]. Zoonotic IIa subtypes, prevalent in some European countries, Australia, Canada, and the United States [20], were not observed in this study and indeed are rarely seen in sub-Saharan Africa [11].
The high proportion of HCs (17%) and NCs (31%) positive for cluster-defining gp60 subtypes supports the hypothesis of predominantly anthroponotic transmission within households and neighborhoods. The higher proportion among neighboring children can be explained by the fact that cryptosporidiosis primarily affects young children < 2 years of age, whereas adults were included among household contacts. This transmission pattern points to a common neighborhood source, such as shared sanitation facilities or water sources.
In agreement with our results, a study from Bangladesh identified person-to-person transmission of Cryptosporidium species among urban households characterized by crowded living conditions and shared sanitation facilities [30]. The present study design did not allow further risk factor analysis due to very similar household characteristics, such as sanitation facilities or housing conditions. Furthermore, the geographical extent of the identified neighborhood clusters could not be estimated, as samples from no more than 5 neighboring families were collected.
The VNTR analysis data supported the gp60 subtype-defined transmission clusters identified in this study. Within spatiotemporally confined clusters, gp60 subtyping may therefore represent a useful marker for epidemiological analysis, providing a good proxy for genome-wide variability. However, in high-transmission settings, discriminatory power may be limited due to extensive genetic recombination.
CONCLUSIONS
Cryptosporidiosis in rural sub-Saharan Africa is characterized by high subtype diversity, distinct geographic subtype distributions, and high prevalence, not only among children with diarrhea, but also among their household and neighborhood contacts. Animal infections contributed only marginally to the epidemiologically linked transmission clusters observed within neighborhoods. The data from all study sites provide evidence for the central role of anthroponotic transmission within neighborhoods and suggest common infection sources within the community. Water sources or shared sanitation facilities likely represent suitable targets for cryptosporidiosis prevention measures and integrated public health interventions.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
CRYPTO Study Group. Denise Dekker, Anna Jaeger, Benedikt Hogan, Maike Lamshöft, Thorsten Thye, Kathrin Schuldt, Doris Winter, Egbert Tannich, Christina Rohmann, and Sophia Melhem (Infectious Disease Epidemiology, Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany); Kennedy Gyau Boahen, Charity Wiafe Akenten, Nimako Sarpong, and Kwabena Oppong (Kumasi Centre for Collaborative Research in Tropical Medicine, Kumasi, Ghana); Gereon Schares and Franz Conraths (Institute of Epidemiology, Friedrich-Loeffler-Institut, Greifswald-Insel Riems, Germany); Peter G. Kremsner, Prince Manouana, Mirabeau Mbong, and Natalie Byrne (Centre de Recherches Médicales de Lambaréné, Lambaréné, Gabon); Samwel Gesase and Daniel T. R. Minja (National Tanga Research Centre, Institute for Medical Research, Tanga, United Republic of Tanzania); and Anna Rosa Sannella (Department of Infectious Diseases, Istituto Superiore di Sanità, Rome, Italy).
Acknowledgments. The authors express their gratitude to all of the study participants and their caregivers for participating in this study, and to the dedicated field workers and study nurses, namely, Richard Afreh, Grace Owusu, Felix Osei Boateng, and Emmanuel Baah.
Financial support. This work was supported by the German Research Foundation (grant number EI 1044/1-1).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
CRYPTO Study Group:
Tony Stark, Denise Dekker, Anna Jaeger, Benedikt Hogan, Maike Lamshöft, Thorsten Thye, Kathrin Schuldt, Doris Winter, Egbert Tannich, Christina Rohmann, Sophia Melhem, Kennedy Gyau Boahen, Charity Wiafe Akenten, Nimako Sarpong, Kwabena Oppong, Gereon Schares, Franz Conraths, Peter G Kremsner, Prince Manouana, Mirabeau Mbong, Natalie Byrne, Samwel Gesase, Daniel T R Minja, and Anna Rosa Sannella
References
- 1. Feng Y, Ryan UM, Xiao L. Genetic diversity and population structure of Cryptosporidium. Trends Parasitol 2018; 34:997–1011. [DOI] [PubMed] [Google Scholar]
- 2. Mølbak K, Andersen M, Aaby P, et al. . Cryptosporidium infection in infancy as a cause of malnutrition: a community study from Guinea-Bissau, West Africa. Am J Clin Nutr 1997; 65:149–52. [DOI] [PubMed] [Google Scholar]
- 3. Steiner KL, Ahmed S, Gilchrist CA, et al. . Species of cryptosporidia causing subclinical infection associated with growth faltering in rural and urban Bangladesh: a birth cohort study. Clin Infect Dis 2018; 67:1347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gertler M, Dürr M, Renner P, et al. . Outbreak of Cryptosporidium hominis following river flooding in the city of Halle (Saale), Germany, August 2013. BMC Infect Dis 2015; 15:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mac Kenzie WR, Hoxie NJ, Proctor ME, et al. . A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N Engl J Med 1994; 331:161–7. [DOI] [PubMed] [Google Scholar]
- 6. Kotloff KL, Nataro JP, Blackwelder WC, et al. . Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. [DOI] [PubMed] [Google Scholar]
- 7. Krumkamp R, Sarpong N, Schwarz NG, et al. . Gastrointestinal infections and diarrheal disease in Ghanaian infants and children: an outpatient case-control study. PLoS Negl Trop Dis 2015; 9:e0003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sow SO, Muhsen K, Nasrin D, et al. . The burden of Cryptosporidium diarrheal disease among children <24 months of age in moderate/high mortality regions of sub-Saharan Africa and South Asia, utilizing data from the global enteric Multicenter Study (GEMS). PLoS Negl Trop Dis 2016; 10:e0004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eibach D, Krumkamp R, Al-Emran HM, et al. . Molecular characterization of Cryptosporidium spp. among children in rural Ghana. PLoS Negl Trop Dis 2015; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. King P, Tyler KM, Hunter PR. Anthroponotic transmission of Cryptosporidium parvum predominates in countries with poorer sanitation: a systematic review and meta-analysis. Parasit Vectors 2019; 12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Squire SA, Ryan U. Cryptosporidium and Giardia in Africa: current and future challenges. Parasit Vectors 2017; 10:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xiao L, Morgan UM, Limor J, et al. . Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Appl Environ Microbiol 1999; 65:3386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alves M, Xiao L, Sulaiman I, Lal AA, Matos O, Antunes F. Subgenotype analysis of Cryptosporidium isolates from humans, cattle, and zoo ruminants in Portugal. J Clin Microbiol 2003; 41:2744–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Strong WB, Gut J, Nelson RG. Cloning and sequence analysis of a highly polymorphic Cryptosporidium parvum gene encoding a 60-kilodalton glycoprotein and characterization of its 15- and 45-kilodalton zoite surface antigen products. Infect Immun 2000; 68:4117–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Drumo R, Widmer G, Morrison LJ, et al. . Evidence of host-associated populations of Cryptosporidium parvum in Italy. Appl Environ Microbiol 2012; 78: 3523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Checkley W, Gilman RH, Epstein LD, et al. . Asymptomatic and symptomatic cryptosporidiosis: their acute effect on weight gain in Peruvian children. Am J Epidemiol 1997; 145:156–63. [DOI] [PubMed] [Google Scholar]
- 17. Mbae C, Mulinge E, Waruru A, Ngugi B, Wainaina J, Kariuki S. Genetic diversity of Cryptosporidium in children in an urban informal settlement of Nairobi, Kenya. PLoS One 2015; 10:e0142055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ayinmode AB, Oliveira BCM, Obebe OO, Dada-Adgebola HO, Ayede AI, Widmer G. Genotypic characterization of Cryptosporidium species in humans and peri-domestic animals in Ekiti and Oyo States, Nigeria. J Parasitol 2018; 104:639–44. [DOI] [PubMed] [Google Scholar]
- 19. Squire SA, Yang R, Robertson I, Ayi I, Ryan U. Molecular characterization of Cryptosporidium and Giardia in farmers and their ruminant livestock from the coastal savannah zone of Ghana. Infect Genet Evol 2017; 55:236–43. [DOI] [PubMed] [Google Scholar]
- 20. Xiao L. Molecular epidemiology of cryptosporidiosis: an update. Exp Parasitol 2010; 124:80–9. [DOI] [PubMed] [Google Scholar]
- 21. Tanriverdi S, Grinberg A, Chalmers RM, et al. . Inferences about the global population structures of Cryptosporidium parvum and Cryptosporidium hominis. Appl Environ Microbiol 2008; 74:7227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cacciò SM, de Waele V, Widmer G. Geographical segregation of Cryptosporidium parvum multilocus genotypes in Europe. Infect Genet Evol 2015; 31: 245–9. [DOI] [PubMed] [Google Scholar]
- 23. Areeshi M, Dove W, Papaventsis D, et al. . Cryptosporidium species causing acute diarrhoea in children in Antananarivo, Madagascar. Ann Trop Med Parasitol 2008; 102:309–15. [DOI] [PubMed] [Google Scholar]
- 24. Bodager JR, Parsons MB, Wright PC, et al. . Complex epidemiology and zoonotic potential for Cryptosporidium suis in rural Madagascar. Vet Parasitol 2015; 207:140–3. [DOI] [PubMed] [Google Scholar]
- 25. Razakandrainibe R, Diawara EHI, Costa D, et al. . Common occurrence of Cryptosporidium hominis in asymptomatic and symptomatic calves in France. PLoS Negl Trop Dis 2018; 12:e0006355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ojuromi OT, Duan L, Izquierdo F, et al. . Genotypes of Cryptosporidium spp. and Enterocytozoon bieneusi in human immunodeficiency virus-infected patients in Lagos, Nigeria. J Eukaryot Microbiol 2016; 63:414–8. [DOI] [PubMed] [Google Scholar]
- 27. Ayinmode AB, Fagbemi BO, Xiao L. Molecular characterization of Cryptosporidium in children in Oyo State, Nigeria: implications for infection sources. Parasitol Res 2012; 110:479–81. [DOI] [PubMed] [Google Scholar]
- 28. Dyachenko V, Kuhnert Y, Schmaeschke R, Etzold M, Pantchev N, Daugschies A. Occurrence and molecular characterization of Cryptosporidium spp. genotypes in European hedgehogs (Erinaceus europaeus L.) in Germany. Parasitology 2010; 137:205–16. [DOI] [PubMed] [Google Scholar]
- 29. Sangster L, Blake DP, Robinson G, et al. . Detection and molecular characterisation of Cryptosporidium parvum in British European hedgehogs (Erinaceus europaeus). Vet Parasitol 2016; 217:39–44. [DOI] [PubMed] [Google Scholar]
- 30. Korpe PS, Gilchrist C, Burkey C, et al. . Case-control study of Cryptosporidium transmission in Bangladeshi households. Clin Infect Dis 2019; 68:1073–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.