Abstract
Background
The prevalence and burden of age-related non-AIDS comorbidities (NACMs) are poorly characterized among women living with HIV (WLWH).
Methods
Virologically suppressed WLWH and HIV-seronegative participants followed in the Women’s Interagency HIV Study (WIHS) through at least 2009 (when >80% of WLWH used antiretroviral therapy) were included, with outcomes measured through 31 March 2018. Covariates, NACM number, and prevalence were summarized at most recent WIHS visit. We used linear regression models to determine NACM burden by HIV serostatus and age.
Results
Among 3232 women (2309 WLWH, 923 HIV-seronegative) with median observation of 15.3 years, median age and body mass index (BMI) were 50 years and 30 kg/m2, respectively; 65% were black; 70% ever used cigarettes. WLWH had a higher mean NACM number than HIV-seronegative women (3.6 vs 3.0, P < .0001) and higher prevalence of psychiatric illness, dyslipidemia, non-AIDS cancer, kidney, liver, and bone disease (all P < .01). Prevalent hypertension, diabetes, and cardiovascular and lung disease did not differ by HIV serostatus. Estimated NACM burden was higher among WLWH versus HIV-seronegative women in those aged 40–49 (P < .0001) and ≥60 years (P = .0009) (HIV × age interaction, P = .0978). In adjusted analyses, NACM burden was associated with HIV, age, race, income, BMI, alcohol abstinence, cigarette, and crack/cocaine use; in WLWH, additional HIV-specific indices were not associated, aside from recent abacavir use.
Conclusions
Overall, NACM burden was high in the cohort, but higher in WLWH and in certain age groups. Non-HIV traditional risk factors were significantly associated with NACM burden in WLWH and should be prioritized in clinical guidelines for screening and intervention to mitigate comorbidity burden in this high-risk population.
Keywords: human immunodeficiency virus, women living with HIV, HIV and aging, non-AIDS comorbidities, comorbidity burden
Women living with HIV (WLWH) experienced a greater burden of non-AIDS comorbidities than HIV-seronegative counterparts overall and in certain age groups. Traditional risk factors were commonly associated with multimorbidity and should be prioritized for screening and intervention in aging WLWH.
(See the Editorial Commentary by Huaman and Fichtenbaum on pages 1312–3.)
Combination antiretroviral therapy (cART) has resulted in tremendous improvements in mortality, and as such, nearly half of individuals with diagnosed human immunodeficiency virus (HIV) in the United States are now aged 50 years or older [1, 2]. In those with access to care and treated, age-associated non-AIDS comorbidities (NACMs) increasingly account for morbidity [3–5] and mortality [6, 7]. Compared with HIV-seronegative counterparts, persons living with HIV (PLWH) experience higher risk and severity of NACMs [3, 8], which may accrue at an earlier age [9, 10].
While women account for more than 50% of adults with HIV worldwide [11], they are underrepresented in HIV research [12]. Further, analyses do not always present sex-delineated outcomes [13]. Female-specific biological and sociobehavioral factors influence HIV acquisition, pathogenesis, reservoir, and treatment responses, and likely contribute to comorbidity development; however, additional investigation is warranted [14]. Large, multisite cohort studies describing “multimorbidity” in aging PLWH lack adequate representation of female participants (range, 13–21%) [3, 5, 10, 15]. This is especially concerning given that NACM risk and burden may be amplified in women living with HIV (WLWH) compared with men [5, 16, 17].
A comprehensive understanding of the distribution of age-related comorbidities in WLWH is crucial to optimizing care for this unique aging population. Our objectives were to describe NACM burden and prevalence in WLWH, assess the effects of HIV serostatus and age on NACM burden, and describe risk factors associated with NACM in a large, geographically diverse, exclusively female cohort of WLWH and HIV-seronegative counterparts.
METHODS
The Women’s Interagency HIV Study
We analyzed data from the Women’s Interagency HIV Study (WIHS), the largest prospective US-based cohort of women living with or at risk for HIV infection [18]. Enrollment occurred in 4 waves (1994–1995, 2001–2002, 2011–2012, 2013–2015) from 11 cities (Atlanta, GA; Birmingham, AL; Bronx, NY; Brooklyn, NY; Chapel Hill, NC; Chicago, IL; Jackson, MS; Los Angeles, CA; Miami, FL; San Francisco, CA; Washington, DC). HIV-seropositive (HIV+) or HIV-seronegative (HIV−) women at risk of HIV acquisition (based on sexually transmitted infection history and/or sociobehavioral characteristics) were recruited, as described in more detail previously [18].
Women’s Interagency HIV Study participants complete a biannual interviewer-administered questionnaire, standardized physical examination (including 3 seated blood pressure measurements in the participant’s right arm using an automated Dinamap monitor [Dinamap Procare Series, GE Medical Systems]) and biospecimen collections. Sociodemographic and clinical information, medical and psychiatric comorbidities, medications, and health behaviors are assessed. Blood testing evaluates kidney and liver function, CD4 count, and HIV viral load. The WIHS study protocol has been approved by each site’s institutional review board, and all participants have provided written informed consent.
Study Design
To focus our analysis on age-related NACMs in the era of effective HIV treatment (and to minimize the contribution of AIDS-related pathology), we included all WIHS participants with at least 2 full study visits completed between 2009 (when >80% of WLWH used cART) through the end of observation (31 March 2018) (Supplementary Figure). Longitudinal WIHS data from study enrollment through observation end were cross-sectionalized, such that covariates and NACM prevalence and burden were assessed as of the most recent visit for each participant.
Outcome Measures
We selected 10 NACMs due to their known age association and significant contribution to morbidity and mortality in the general population (Supplementary Table 1). Our primary outcome was NACM burden, defined as the number of total NACMs per participant. Our secondary outcomes were the prevalence of each NACM, defined as the presence of the condition as of the participant’s most recent visit. Non-AIDS comorbidities were defined using up to 3 potential data sources: self-reported diagnosis or medication, clinical measurement, and/or laboratory evidence. Use of pathology and/or imaging modalities to define NACMs (eg, biopsies for cancer, bone densitometry for osteopenia/osteoporosis, transient elastography for liver disease, etc) was not employed given a lack of availability of these data. Non-AIDS comorbidities were assigned if a participant had any history of the comorbidity at the most recent visit, with the exception of certain comorbidities defined by measurements with the potential to fluctuate over time (eg, chronic kidney disease [CKD] and depression), for which criteria were required on 2 or more consecutive visits to satisfy the NACM definition. Once a participant met criteria for a comorbidity, that comorbidity was considered prevalent.
Statistical Analysis
We compared demographic and clinical characteristics of women by HIV serostatus using chi-square tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. Chi-square tests and 2-sample t tests assessed the association of HIV serostatus with the prevalence of each comorbidity and NACM burden, respectively. For the entire cohort and then stratified by HIV serostatus, we assessed for a linear trend by ranked age category (<40, 40–49, 50–59, ≥60 years) using the Wilcoxon test (for individual NACM prevalence) and unadjusted linear regression (for NACM burden).
We performed separate, partially adjusted logistic regression (outcome = individual NACM prevalence) and partially adjusted linear regression (outcome = NACM burden) analyses to assess for associations of HIV serostatus and age; models included HIV serostatus, categorized age, and an HIV × age interaction term and no other covariates.
For the primary outcome (NACM burden), we used a “fully adjusted” linear regression model, controlling for important covariates (derived from the literature or univariate analyses), in addition to HIV serostatus, categorized age, and an HIV × age interaction term to determine model-based estimates of mean NACM burden by HIV × age category and covariates. A separate adjusted linear regression model including only WLWH was performed to assess the effect of age- and HIV-specific indices, controlling for the same covariates as the “fully adjusted” model, on NACM burden. Any variables used to define individual NACMs were not included as covariates in adjusted models. Model fit was assessed through residual plots for linear regression and Hosmer-Lemeshow test for logistic regression.
To evaluate co-occurring NACM dyads, all possible pairs of comorbidities were assessed for prevalence (overall, by HIV serostatus, and by age category, and within each HIV × age category combination) and then ranked in order of co-occurrence regardless of the presence of additional comorbidities.
All analyses were conducted in SAS version 9.4 (SAS Institute) and significance was set at α = 0.05.
RESULTS
Participant Characteristics
Among 3232 women (2309 HIV+, 923 HIV−) included in our analysis (Supplementary Figure) with a median observation of 15.3 years, median age was 50 years and 65% were black (Table 1). Compared with WLWH, HIV-seronegative women had higher systolic blood pressure (126 vs 122 mmHg), a body mass index (BMI) of 30 kg/m2 or greater (57% vs 46%), and current use of cigarettes (44% vs 36%), alcohol (57% vs 41%), and crack/cocaine (9% vs 6%) (all P < .0001). Women living with HIV had a higher prevalence of chronic hepatitis C (13% vs 9%, P = .0026) and hepatitis B viral infection (2% vs 1%, P = .0148) and worse kidney function than HIV-seronegative women (Table 1). Educational level and median depressive symptoms score did not significantly differ by HIV serostatus. Women living with HIV had a median CD4 count of 615 cells/mm3, 81% had virologic suppression, and median time since cART initiation was 12.5 years.
Table 1.
Demographic and Clinical Characteristics of Women Living With or at Risk for Human Immunodeficiency Virus (HIV) Infection at End of Observation in the Women’s Interagency HIV Study
| Characteristics | Entire Cohorta (N = 3232) | HIV Positive (n = 2309) | HIV Negative (n = 923) | P b |
|---|---|---|---|---|
| Age, y | 50 (43–56) | 51 (44–57) | 49 (41–55) | <.0001 |
| Age group, n (%) | <.0001 | |||
| <40 y | 520 (16) | 315 (14) | 205 (22) | |
| 40–49 y | 996 (31) | 711 (31) | 285 (31) | |
| 50–59 y | 1241 (38) | 936 (41) | 305 (33) | |
| ≥60 y | 475 (15) | 347 (15) | 128 (14) | |
| Observation time, y | 15.3 (4.0–18.2) | 15.3 (4.0–18.4) | 15.3 (4.0–17.9) | .6365 |
| Race/ethnicity, n (%) | .0478 | |||
| White, non-Hispanic | 356 (11) | 273 (12) | 83 (9) | |
| Black, non-Hispanic | 2108 (65) | 1486 (64) | 622 (67) | |
| Hispanic | 657 (20) | 477 (21) | 180 (20) | |
| Other | 111 (3) | 73 (3) | 38 (4) | |
| WIHS enrollment wave, n (%) | <.0001 | |||
| 1994–1995 | 1183 (37) | 887 (38) | 296 (32) | |
| 2001–2002 | 867 (27) | 559 (24) | 308 (33) | |
| 2011–2012 | 363 (11) | 271 (12) | 92 (10) | |
| 2013–2015 | 819 (25) | 592 (26) | 227 (25) | |
| BMI, n (%) | <.0001 | |||
| <30 kg/m2 | 1547 (51) | 1172 (54) | 375 (43) | |
| ≥30 | 1492 (49) | 1004 (46) | 488 (57) | |
| SBP, mmHg | 123 (111–138) | 122 (110–136) | 126 (115–141) | <.0001 |
| DBP, mmHg | 75 (68–83) | 75 (68–83) | 76 (69–84) | .0024 |
| Antihypertensive medication use, n (%) | 1298 (40) | 951 (41) | 347 (38) | .0599 |
| Lipid-lowering medication use, n (%) | 578 (18) | 430 (19) | 148 (16) | .0829 |
| eGFR, mL/min per 1.73 m2 (CKD-EPI) | 94.3 (75.4–110.3) | 91.7 (72.6–108.4) | 99.6 (84.0–114.4) | <.0001 |
| CES-D scorec | 9 (3–19) | 9 (3–19) | 8 (3–19) | .6481 |
| Education, n (%) | .1795 | |||
| ≤High school | 2093 (65) | 1513 (66) | 580 (63) | |
| >High school | 1132 (35) | 793 (34) | 339 (37) | |
| Income, n (%) | .0198 | |||
| <$12 000 | 1515 (50) | 1091 (50) | 424 (49) | |
| $12 001–$24 000 | 703 (23) | 521 (24) | 182 (21) | |
| >$24 000 | 828 (27) | 562 (26) | 266 (31) | |
| Insured, n (%) | 3011 (94) | 2245 (98) | 766 (83) | <.0001 |
| Marital status, n (%) | .0038 | |||
| Married/partner | 886 (29) | 629 (28) | 257 (29) | |
| Divorced/widowed/separated | 914 (30) | 690 (31) | 224 (25) | |
| Never married/other | 1295 (42) | 894 (40) | 401 (45) | |
| Own residence, n (%) | 2686 (83) | 1973 (86) | 713 (77) | <.0001 |
| Cigarette use, n (%) | <.0001 | |||
| Never | 1020 (32) | 786 (34) | 234 (25) | |
| Current | 1230 (38) | 820 (36) | 410 (44) | |
| Former | 980 (30) | 701 (30) | 279 (30) | |
| Current alcohol use, n (%) | <.0001 | |||
| None | 1735 (54) | 1339 (58) | 396 (43) | |
| 1–7 drinks/wk | 1200 (37) | 807 (35) | 393 (43) | |
| >7 drinks/wk | 280 (9) | 147 (6) | 133 (14) | |
| Marijuana use, n (%) | <.0001 | |||
| Never | 985 (31) | 782 (34) | 203 (22) | |
| Current | 677 (21) | 450 (20) | 227 (25) | |
| Former | 1551 (48) | 1059 (46) | 492 (53) | |
| Crack/cocaine use, n (%) | <.0001 | |||
| Never | 2239 (70) | 1669 (73) | 570 (62) | |
| Current | 218 (7) | 133 (6) | 85 (9) | |
| Former | 758 (24) | 491 (21) | 267 (29) | |
| Opioid use (heroin/methadone), n (%) | … | … | <.0001 | |
| Never | 2822 (88) | 2056 (90) | 766 (83) | |
| Current | 58 (2) | 38 (2) | 20 (2) | |
| Former | 335 (10) | 199 (9) | 136 (15) | |
| Injection drug use, n (%) | … | … | .4479 | |
| Never | 2622 (82) | 1866 (81) | 756 (82) | |
| Current | 29 (1) | 18 (1) | 11 (1) | |
| Former | 563 (18) | 408 (18) | 155 (17) | |
| Noninjection drug use, n (%) | … | … | <.0001 | |
| Never | 820 (26) | 659 (29) | 161 (17) | |
| Current | 796 (25) | 525 (23) | 271 (29) | |
| Former | 1597 (50) | 1107 (48) | 490 (53) | |
| Chronic HBV, n (%) | 66 (2) | 56 (2) | 10 (1) | .0148 |
| Chronic HCV, n (%) | 393 (12) | 306 (13) | 87 (9) | .0026 |
| CD4 count, cells/mm3 | … | 615 (400–855) | … | |
| CD4 nadir, cells/mm3 | … | 280 (160–414) | … | |
| HIV viral load, n (%) | … | … | … | |
| Suppressedd | … | 1773 (81) | … | |
| 200–999 copies/mL | … | 106 (5) | … | |
| ≥1000 copies/mL | … | 311 (14) | … | |
| Proportion visits HIV suppressed,d % | … | … | … | |
| From baseline visit | … | 69% (44–94%) | … | |
| On/after 2009 | … | 91% (60–100%) | … | |
| Year initiated cART, n (%) | … | … | … | |
| 1995–1999 | … | 865 (37) | … | |
| 2000–2003 | … | 305 (13) | … | |
| 2004–2008 | … | 364 (16) | … | |
| ≥2009 | … | 702 (30) | … | |
| Never initiated cART, n (%) | … | 73 (3) | … | |
| Time since cART initiation, years | … | 12.5 (7.0–17.1) | … | |
| Antiretroviral class,e n (%) | … | … | … | |
| PI | … | 769 (33) | … | |
| NNRTI | … | 591 (26) | … | |
| INSTI | … | 746 (32) | … | |
| Other | … | 24 (1) | … | |
| Not on therapy | … | 179 (8) | … | |
| Specified NRTI,f n (%) | … | … | … | |
| ABC | … | 479 (24) | … | |
| TDF | … | 1156 (57) | … | |
| TAF | … | 480 (24) | … | |
| Antiretroviral adherence, n (%) | … | … | … | |
| ≥95% | … | 1746 (82) | … | |
| <95% | … | 381 (18) | … |
Data are presented as median (Q1–Q3) or n (%). Column percentages may not total 100 due to rounding.
Abbreviations: ABC, abacavir; BMI, body mass index; cART, combined antiretroviral therapy; CES-D, Center for Epidemiologic Studies–Depression; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HBV, hepatitis B virus; HCV, hepatitis C virus; INSTI, integrase strand transfer inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SBP, systolic blood pressure; TAF, tenofovir alafenamide fumarate; TDF, tenofovir disoproxil fumarate; WIHS, Women’s Interagency HIV Study.
aData missing for the following: SBP (n = 169), DBP (n = 169), CES-D (n = 36), CD4 count (n = 81), CD4 nadir (n = 92), time since cART initiation (n = 75).
bχ2 test performed for categorical variables and Wilcoxon rank-sum test for continuous variables.
cRange, 0–60; threshold for depressive symptoms, ≥16.
dHIV viral load <200 copies/mL and/or less than the lower limit of quantification of assay.
eCategorized hierarchically as PI > NNRTI > INSTI > other.
fOf those on any NRTI (n = 2033), not mutually exclusive.
Non-AIDS Comorbidity Burden and Prevalence
Mean (SD) NACM burden increased with each older age category (<40, 40–49, 50–59, ≥60 years)—1.7 (1.4), 2.7 (1.8), 4.0 (2.0), and 5.2 (1.9) (P < .0001)—as did the prevalence of each individual NACM (P < .0001) (Supplementary Table 2). Women living with HIV had a higher mean NACM burden than HIV-seronegative women, 3.6 versus 3.0 (P < .0001), and the following comorbidities were more prevalent in WLWH versus HIV-seronegative women (all P < .01): psychiatric illness (57%/48%), liver disease (45%/26%), dyslipidemia (40%/35%), bone disease (40%/33%), CKD (15%/7%) and non-AIDS cancer (11%/7%) (Supplementary Table 3). The prevalence of hypertension (66%/64%), lung disease (41%/42%), diabetes (22%/24%), and cardiovascular disease (19%/19%) did not significantly differ by HIV serostatus.
Non-AIDS Comorbidity Burden by HIV Serostatus and Age Group
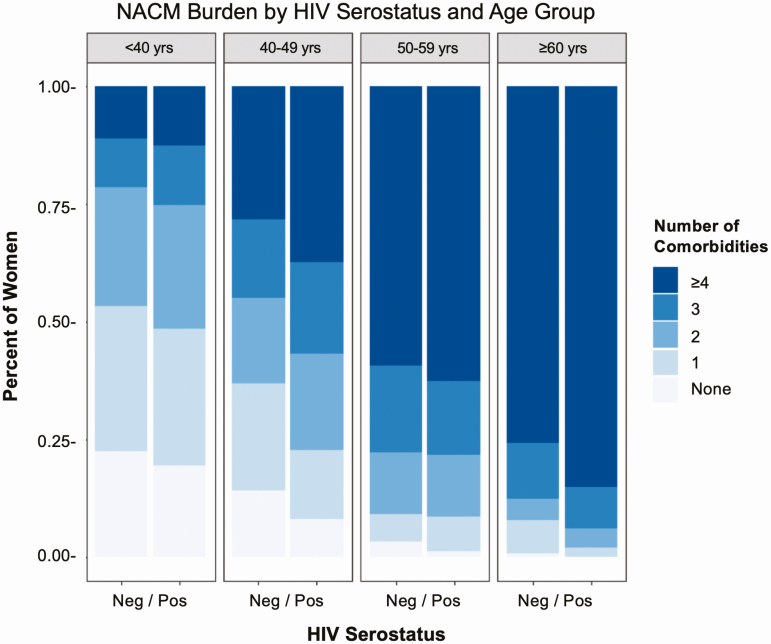
Figure 1 shows the distribution of categorized NACM burden by HIV serostatus and age group. In partially adjusted models, WLWH had greater NACM burden compared with HIV-seronegative women, although this was significant only for those aged 40–49 years (P < .0001) and 60 years or older (P = .0028) (HIV × age interaction, P = .0206) (Table 2). The estimated mean difference in NACM (HIV+/HIV−) for women aged 40–49, 50–59, and 60 years or older, compared with those aged less than 40 years of the same HIV serostatus, was 1.17 (95% confidence interval [CI], .92–1.41)/.73 (95% CI, .40–1.06), 2.35 (95% CI, 2.11–2.58)/2.35 (95% CI, 2.03–2.68), 3.65 (95% CI, 3.37–3.93)/3.18 (95% CI, 2.77–3.58), respectively (all P < .0001).
Figure 1.
Distribution of prevalent non-AIDS comorbidity burden by HIV serostatus and age group demonstrating that women living with HIV have a higher burden of comorbidities overall and specifically in age groups 40–49 and ≥60 years. Abbreviation: HIV, human immunodeficiency virus.
Table 2.
Burden and Prevalence of Non-AIDS Comorbidities in Women Stratified by Human Immunodeficiency Virus Serostatus and Age Group
| NACM | HIV Positive | HIV Negative | HIV × Age Interaction, Pb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| <40 Years (n = 315) | 40–49 Years (n = 711) | 50–59 Years (n = 936) | ≥60 Yearsa (n = 347) | <40 Years (n = 205) | 40–49 Years (n = 285) | 50–59 Years (n = 305) | ≥60 Yearsa (n = 128) | ||
| Hypertension | 99 (31) | 413 (58) | 698 (75) | 322 (93) | 77 (38) | 146 (51) | 246 (81) | 119 (93) | .0169 |
| Psychiatric illness | 122 (39) | 385 (54) | 574 (61) | 231 (67) | 65 (32) | 120 (42) | 187 (61) | 73 (57) | .0816 |
| Lung disease | 91 (29) | 249 (35) | 442 (47) | 172 (50) | 68 (33) | 103 (36) | 157 (51) | 64 (50) | .8503 |
| Liver disease | 76 (24) | 239 (34) | 482 (52) | 233 (67) | 21 (10) | 63 (22) | 94 (31) | 59 (46) | .4073 |
| Dyslipidemia | 54 (17) | 244 (34) | 413 (44) | 215 (62) | 32 (16) | 64 (22) | 150 (49) | 73 (57) | .0023 |
| Bone disease | 41 (13) | 208 (29) | 447 (48) | 233 (67) | 30 (15) | 72 (25) | 126 (41) | 73 (57) | .3940 |
| Diabetes mellitus, type 2 | 23 (7) | 113 (16) | 244 (26) | 122 (35) | 17 (8) | 48 (17) | 105 (34) | 55 (43) | .5365 |
| Cardiovascular disease | 15 (5) | 89 (13) | 210 (22) | 124 (36) | 12 (6) | 37 (13) | 84 (28) | 45 (35) | .6543 |
| Chronic kidney disease | 7 (2) | 37 (5) | 160 (17) | 145 (42) | 0 (0) | 2 (<1) | 26 (9) | 33 (26) | .3004 |
| Cancer, non-AIDS | 8 (3) | 64 (9) | 119 (13) | 60 (17) | 7 (3) | 11 (4) | 32 (10) | 18 (14) | .1914 |
| Mean (SD) NACM burden | 1.7 (1.4) | 2.9 (1.8) | 4.0 (2.0) | 5.4 (1.9) | 1.6 (1.4) | 2.3 (1.8) | 4.0 (2.0) | 4.8 (2.0) | .0206 |
Data are presented as n (%) unless otherwise indicated.
Abbreviations: HIV, human immunodeficiency virus; NACM, non-AIDS comorbidity.
aWilcoxon test performed for linear trend across (ordinal) age groups for categorical variables (ie, prevalence of each comorbidity) and unadjusted linear regression performed for continuous variables (ie, NACM burden) stratified by HIV serostatus: all P values were < .0001 for each prevalent comorbidity and NACM burden within each strata of HIV serostatus.
bPartially adjusted logistic regression performed for the prevalence of each comorbidity and partially adjusted linear regression for NACM burden with HIV serostatus, categorized age, and HIV × age interaction terms in the model.
Non-AIDS Comorbidity Burden Adjusting for Demographic and Clinical Factors
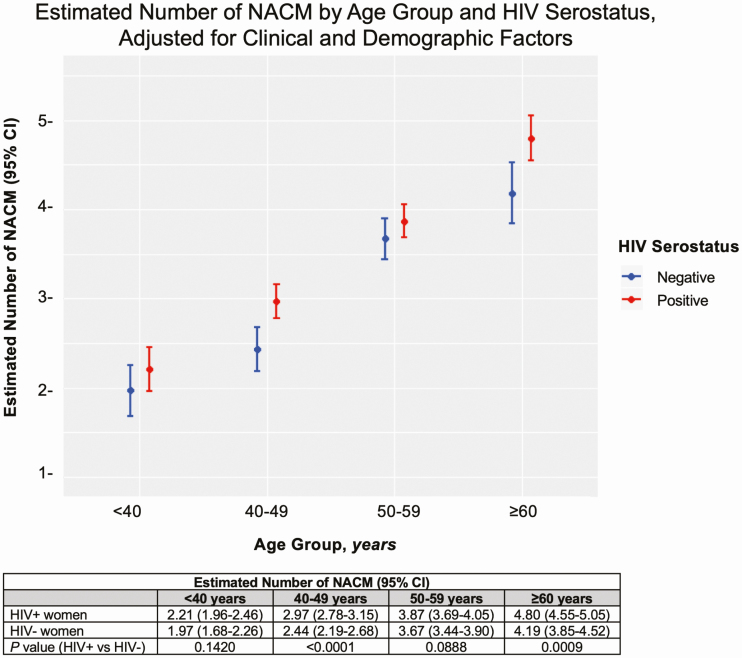
Univariate analysis of factors associated with NACM burden can be found in Supplementary Table 4. In fully adjusted models controlling for race, BMI, education, income, marital status, own residence, and current use of cigarettes, alcohol, and crack/cocaine (in addition to HIV, age, HIV × age), the estimated mean NACM burden differed by HIV serostatus and age group (Figure 2). Non-AIDS comorbidity burden was higher among WLWH compared with HIV-seronegative women in those aged 40–49 years (P < .0001) and 60 years or older (P = .0009), but not in those aged less than 40 years (P = .1420) and 50–59 years (P = .0888). The association between HIV serostatus and age on NACM burden approached significance (HIV × age interaction, P = .0978). In addition, estimated mean NACM burden was significantly higher in women who were older, of white race, and who had HIV, BMI of 30 kg/m2 or higher, income of $24 000 or less, and reported cigarette use, crack/cocaine use, or alcohol abstinence (Table 3).
Figure 2.
Estimated number of non-AIDS comorbidities by HIV serostatus and age group, adjusted for HIV serostatus, categorized age, HIV × age interaction, in addition to the following demographic and clinical factors: race, BMI, education, income, marital status, own residence, and substance use (eg, cigarettes, crack/cocaine and alcohol). Abbreviations: BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus; NACM, non-AIDS comorbidities.
Table 3.
Multivariable Analysis of Risk Factors at End of Observation Associated With the Burden of Non-AIDS Comorbidities in Women Living With or at Risk for Human Immunodeficiency Virus Infection
| Risk Factor | Estimated Mean Number of NACMs (95% CI) | B (±SE) | P a |
|---|---|---|---|
| HIV serostatusb | <.0001 | ||
| Positive | 3.46 (3.30, 3.63) | 0.40 (±0.08) | |
| Negative | 3.07 (2.89, 3.24) | Ref | |
| Age groupb | <.0001 | ||
| ≥60 y | 4.49 (4.25, 4.73) | 2.40 (±0.14) | |
| 50–59 y | 3.77 (3.60, 3.94) | 1.68 (±0.11) | |
| 40–49 y | 2.70 (2.52, 2.88) | 0.61 (±0.10) | |
| <40 y | 2.09 (1.88, 2.30) | Ref | |
| Race | <.0001 | ||
| Non-Hispanic AA | 3.09 (2.95, 3.22) | −0.40 (±0.11) | |
| Hispanic | 3.00 (2.81, 3.18) | −0.50 (±0.12) | |
| Other non- Hispanic | 3.48 (3.13, 3.83) | −0.02 (±0.20) | |
| White | 3.49 (3.27, 3.72) | Ref | |
| Body mass index | <.0001 | ||
| ≥30 kg/m2 | 3.51 (3.34, 3.67) | 0.48 (±0.06) | |
| <30 kg/m2 | 3.02 (2.86, 3.18) | Ref | |
| Education | .3517 | ||
| ≤High school | 3.23 (3.07, 3.39) | −0.07 (±0.07) | |
| >High school | 3.30 (3.12, 3.47) | Ref | |
| Income | <.0001 | ||
| <$12 000 | 3.63 (3.46, 3.79) | 0.84 (±0.08) | |
| $12 001– $24 000 | 3.38 (3.19, 3.57) | 0.59 (±0.09) | |
| >$24 000 | 2.79 (2.60, 2.97) | Ref | |
| Marital status | .1108 | ||
| Had a partner | 3.35 (3.17, 3.53) | 0.09 (±0.09) | |
| Never partner/other | 3.18 (3.01, 3.36) | −0.07 (±0.08) | |
| Married/ partner | 3.26 (3.08, 3.44) | Ref | |
| Own residence | .2937 | ||
| No | 3.22 (3.02, 3.41) | −0.09 (±0.09) | |
| Yes | 3.31 (3.16, 3.46) | Ref | |
| Cigarette use | <.0001 | ||
| Current | 3.61 (3.45, 3.78) | 0.77 (±0.08) | |
| Former | 3.33 (3.15, 3.51) | 0.48 (±0.08) | |
| Never | 2.85 (2.66, 3.04) | Ref | |
| Current alcohol use | .0467 | ||
| >7 drinks/ wk | 3.15 (2.91, 3.39) | −0.24 (±0.12) | |
| 1–7 drinks/ wk | 3.26 (3.09, 3.42) | −0.13 (±0.07) | |
| None | 3.39 (3.22, 3.56) | Ref | |
| Crack/cocaine use | <.0001 | ||
| Current | 3.30 (3.04, 3.56) | 0.24 (±0.13) | |
| Former | 3.43 (3.25, 3.62) | 0.38 (±0.08) | |
| Never | 3.06 (2.91, 3.21) | Ref |
Abbreviations: AA, African American; HIV, human immunodeficiency virus; NACM, non-AIDS comorbidity; Ref, reference; WIHS, Women’s Interagency HIV Study.
aAdjusted linear regression with all covariates listed included in the model plus WIHS enrollment wave; assessing HIV × age interaction: P = .0978.
bAdjusted for HIV serostatus × age interaction.
In an adjusted model including only WLWH (controlling for the aforementioned covariates and HIV-specific indices), recent use of abacavir was the only HIV-related characteristic associated with NACM burden (mean, 3.7 vs 3.3; P < .0001). Other characteristics, including current and nadir CD4 count, measures of HIV virologic suppression, time since cART initiation, and protease inhibitor use, were not associated with NACM burden in WLWH (Supplementary Table 5).
Non-AIDS Comorbidity Dyads
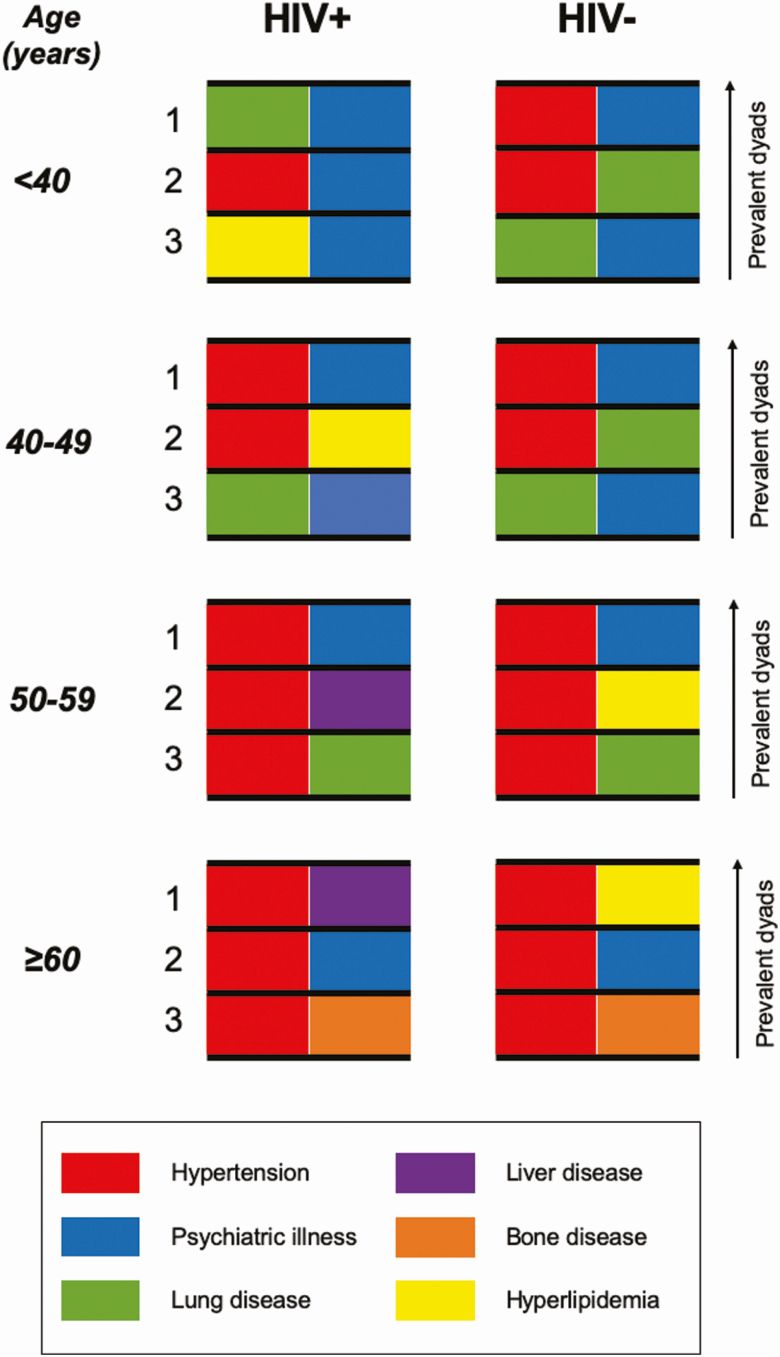
Figure 3 shows the 3 most common co-occurring NACM dyads in women, stratified by HIV serostatus and categorized age. The co-occurrence of hypertension-psychiatric illness ranked in the top 2 for each HIV × age stratification and was prevalent in 60% of all women aged 60 years or older (Supplementary Table 6). In WLWH, the co-occurrence of hypertension and liver disease was the next most prevalent, occurring in 33% overall and 64% of those aged 60 years or older. This pattern differed from HIV-seronegative women in whom hypertension–lung disease and hypertension–dyslipidemia were the next most common comorbidity dyads depending on age category.
Figure 3.
The 3 most common co-occurring non-AIDS comorbidity dyads in women living with or at risk for HIV infection, stratified by HIV serostatus and age group and ranked as most prevalent (1), second most prevalent (2), and third most prevalent (3) by each HIV × age stratum. The dyad of hypertension–psychiatric illness is represented in each stratification of HIV serostatus and age. Abbreviation: HIV, human immunodeficiency virus.
DISCUSSION
In this exclusively female, geographically diverse, US-based cohort of 3232 participants with a median observation of 15.3 years, the burden of NACM was high in WLWH and at-risk HIV-seronegative women, but significantly higher in WLWH overall and in certain age groups. Human immunodeficiency virus infection modified the effect of age on NACM burden, although this interaction was attenuated when adjusting for covariates. Factors significantly associated with NACM burden were HIV seropositivity, older age, white race, obesity, income $24 000 or lower, cigarette use, crack/cocaine use, and alcohol abstinence. For virologically suppressed WLWH, traditional comorbidity risk factors were more commonly associated with NACM burden than were HIV-related clinical indices. To our knowledge, this analysis is the first of its scale to comprehensively examine age-associated NACM prevalence and burden specifically in women. Given that more than 50% of the HIV population is female, and women have unique biological and sociobehavioral risk influencing comorbidity development, this work has broad implications for the clinical care of aging WLWH.
Our findings are consistent with several large cohorts of aging PLWH in developed countries reporting a high burden of NACM [3, 5, 10, 15]. Female representation in these studies ranged from 13% to 21%, the majority of participants were men who have sex with men, and the types and number of comorbidities evaluated varied. Considering these differences, we found a comparatively higher burden of NACM among virologically suppressed WLWH in the United States. For example, Palella et al [5] reported the mean number of 11 NACMs assessed in PLWH (19% female) aged 18–40, 41–50, 51–60, and 61 years or older as 1.4, 2.1, 3.0, and 3.9, respectively, compared with a mean NACM burden of 1.7, 2.9, 4.0, and 5.4 in similarly age-categorized WLWH in our analysis, respectively. Evaluation of 13 age-associated comorbidities among British Columbian women found a greater risk of NACMs in 267 WLWH compared with 276 HIV-seronegative women (incidence rate ratio, 1.58; 95% CI, 1.38–1.81) [19]. Although the median number of NACMs in WLWH was lower than what we observed (2 [interquartile range, 1–4] vs 3 [quartile 1–quartile 3, 2–5], respectively), this group similarly reported a significant interaction between HIV serostatus and age.
In our cross-sectional analysis, NACM burden was higher among WLWH compared with HIV-seronegative women in every age group and significantly so in those aged 40–49 and 60 years or older. We demonstrated that HIV infection significantly modified the effect of age on NACM burden, although this was attenuated when adjusting for covariates. Epidemiologic data suggest that PLWH are at risk of earlier accrual of NACM than their seronegative counterparts, with NACM diagnosed up to a decade earlier in PLWH [9, 10, 19]. Discrepancy in NACM prevalence between PLWH and the general population may even precede the diagnosis of HIV [10], suggesting that non-HIV risk factors may play an important role in the premature onset of age-associated comorbidities. Longitudinal analyses are needed to better understand if age has a greater effect on NACMs in WLWH than in HIV-seronegative women.
Our adjusted analyses of WLWH revealed “traditional” comorbidity risk factors were more commonly associated with multimorbidity than HIV-related clinical indices. The association of low income, cigarette use, and obesity with NACM risk has been previously demonstrated in WLWH [19]. In our analysis, the estimated mean NACM burden was higher in WLWH of white race compared with WLWH of minority races. This finding may represent differences in unmeasured factors such as race-mediated disparities in access to care that may have affected NACM identification [20]. We did not find a relationship between NACM burden and measures of CD4 count, HIV virologic suppression, or cART exposure other than recent abacavir use. This could reflect preferred use of abacavir over tenofovir disoproxil fumarate (prior to the advent of tenofovir alafenamide) in PLWH with certain comorbidities [21, 22]. A recent examination of traditional and HIV-related factors contributing to several comorbidity outcomes found a substantial proportion of NACMs could be prevented by interventions addressing smoking, elevated cholesterol, and hypertension [23]. These findings, corroborated by other large cohorts of virologically suppressed PLWH [5, 10, 24, 25], highlight the need for clinical strategies to screen, prevent, and/or intervene on traditional comorbidity risk factors to mitigate NACM development in aging WLWH.
Of the 10 NACMs evaluated, the most common in the WIHS included hypertension, psychiatric illness, dyslipidemia, and liver and lung disease, with most NACMs significantly more prevalent in WLWH compared with HIV-seronegative women. The individual NACM prevalence ranging between 10% and 66% is modestly higher than that described among other cohorts of PLWH including WLWH [5, 15, 19], although similar to our data, the most commonly occurring NACMs include hypertension, dyslipidemia, psychiatric illness, and liver disease [3–5, 19]. Observed differences by HIV serostatus in the prevalence of certain NACMs are likely driven by the complex interplay of overrepresentation of mental health, including substance-use disorders in PLWH compared with the general population [26, 27]; higher prevalence of viral hepatitis coinfection; virally and cART-mediated effects on lipids, kidney, and bone health [28–30]; and the poorly understood relationship between HIV and oncogenesis [31].
Recent studies suggest that comorbidities in PLWH occur in nonrandom patterns [24, 25, 32]. Understanding NACM co-occurrence is therefore important for addressing shared risk factors in the era of increasing multimorbidity among PLWH [4, 15]. In WIHS women of all ages, we found the most common co-occurring NACM dyad was hypertension–psychiatric illness. Depression is more common in women than men [33] and has been shown to increase the risk of hypertension in women and be strongly associated with fatal cardiovascular disease [34]. In turn, hypertension may lead to incident depression and is frequently comorbid with diabetes, dyslipidemia, and CKD, both in the general population and in PLWH [15, 32, 35, 36]. It is paramount to aggressively screen for and manage hypertension and depression in WLWH as the cumulative burden of depression is linked not only to limited antiretroviral adherence but also to all-cause mortality [37, 38].
We dedicated our analysis to women as they represent a vital yet historically understudied constituency of the aging HIV population [13]. Recently published sex-stratified data demonstrated a higher NACM burden in WLWH than in men living with HIV (median, 3.9 vs 3.4, respectively; P < .05) [5]. This is consistent with prior reports of higher risk of cardiovascular and cerebrovascular events in PLWH, compared with HIV-seronegative individuals, which is amplified in women compared with men [16, 17]. The role of biological sex on the development of age-associated comorbidities warrants further study, especially considering numerous potential mechanistic differences (eg, anatomy, genetics, sex hormones, immunology, microbiome, response to cART, behaviors, and access to care) [14]. Further investigation characterizing sex differences in NACM prevalence and burden could serve to inform sex-tailored clinical guidance on comorbidity screening and management among PLWH.
Our study compared NACMs in WLWH with demographically similar HIV-seronegative women recruited in the WIHS based on sociobehavioral characteristics associated with risk of HIV acquisition [18]. In our study, HIV-seronegative women had significantly higher BMI, blood pressure, and substance use compared with WLWH. It is well established that these characteristics predispose to age-related comorbidities [39]. Therefore, it is possible that the observed differences in NACM burden by HIV serostatus may be even greater if WLWH were compared with HIV-seronegative women from a more generalized population. The US-based Health and Retirement Study assessed multimorbidity (≥2 age-associated health conditions of 8 measured) in 5355 participants (55% women; mean age, 68 years) [33]. In their study, 62% of women reported multimorbidity, compared with 98% of WLWH and 92% of HIV-seronegative women in the WIHS aged 60 years or older. Similarly, in the Australian Longitudinal Study on Women’s Health [40], while 46% of more than 10 000 women aged 45–50 years reported 2 or more chronic comorbidities of 18 assessed, WIHS participants aged 40–49 years reported 2 or more NACMs among 75% of WLWH and 62% of HIV-seronegative women.
We acknowledge several additional limitations. Several diagnoses relied on self-report, which likely underestimated NACM prevalence and burden. Results of pathology and/or imaging modalities to supplement NACM definitions were not available. Given the chronic nature of HIV infection requiring routine medical visits, differences in self-reported NACM diagnoses or treatment could differ by HIV serostatus due to possible ascertainment bias. This may be counterbalanced by the overall poor health status of HIV-seronegative women in WIHS, when compared with a more generalized female population, which could have underestimated the effect of HIV serostatus on NACM burden. Women were included in our analysis from 4 different WIHS recruitment waves, each with varying enrollment criteria in terms of age, cART use, geography, etc; therefore, we adjusted for enrollment wave in multivariable analyses. Given the scope of our study, we were limited in assessing the contribution of specific cART types on individual NACMs, especially considering the possibility of switches over time and nonadherence. Last, since this study focused on prevalent NACMs, we did not evaluate the role of menopause status or timing.
In conclusion, WLWH living in the United States experienced a high overall burden of age-associated NACMs compared with HIV-seronegative counterparts. Human immunodeficiency virus appears to modify the effect of aging on comorbidity burden in women, although longitudinal studies examining the interaction of HIV and age on NACM accrual are needed. Our understanding of how women and men age with HIV is evolving, although dedicated characterization of respective unique biological and sociobehavioral profiles contributing to comorbidity risk is needed. Our study highlights the significant association between non-HIV traditional risk factors and multimorbidity in WLWH. Clinical care guidelines may consider additional emphasis on screening and intervention of social determinants of health and modifiable lifestyle factors to mitigate comorbidity risk in aging WLWH, including those aged less than 50 years, in whom NACM burden is already higher than in their HIV-seronegative counterparts.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments: The authors thank the Women’s Interagency Human Immunodeficiency Virus Study (WIHS) participants who contributed their time and data to this study. In addition, we are grateful to the WIHS for their support and data utilization. Finally, we thank the WIHS site co-investigators for serving as site liaisons for data collaboration.
Disclaimer: The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health.
Financial support: Data in this manuscript were collected by the Women’s Interagency Human Immunodeficiency Virus (HIV) Study (WIHS), now the Multicenter AIDS Cohort Study/WIHS Combined Cohort Study (MWCCS). MWCCS (Principal Investigators): Atlanta Clinical Research Site(s) (CRS) (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01‐HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01‐HL146201; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01‐HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01‐HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01‐HL146193; Chicago‐Cook County CRS (Mardge Cohen and Audrey French), U01‐HL146245; Chicago‐Northwestern CRS (Steven Wolinsky), U01‐HL146240; Connie Wofsy Women’s HIV Study, Northern California CRS (Bradley Aouizerat and Phyllis Tien), U01‐HL146242; Los Angeles CRS (Roger Detels), U01‐HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01‐HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01‐HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01‐HL146208; UAB‐MS CRS (Mirjam‐Colette Kempf and Deborah Konkle‐Parker), U01‐HL146192; University of North Carolina (UNC) CRS (Adaora Adimora), U01‐HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co‐funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Human Genome Research Institute (NHGRI), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). MWCCS data collection is also supported by UL1‐TR000004 (UCSF CTSA), P30‐AI‐050409 (Atlanta CFAR), P30‐AI‐050410 (UNC CFAR), and P30‐AI‐027767 (UAB CFAR). This work was also supported by the Emory Specialized Center of Research Excellence (SCORE) on Sex Differences (grant number U54AG062334; to I. O.). L. F. C. is also supported by the National Center for Advancing Translational Sciences (NCATS) of the NIH (award numbers UL1TR002378 and TL1TR002382). A. N. S. is also supported by National Institute of Allergy and Infectious Diseases (NIAID) of the NIH (award number K23AI114407).
Potential conflicts of interest. A. A. A. reports personal fees from Merck, ViiV, and Gilead, and grants from Gilead, outside the submitted work. A. N. S. reports institutional grants from Gilead Sciences, outside the submitted work. P. C. T. reports grants from Merck and Theratechnologies, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. HIV surveillance report, 2017. Vol 29. Published November 2018. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed 1 June 2019. [Google Scholar]
- 2. Marcus JL, Chao CR, Leyden WA, et al. . Narrowing the gap in life expectancy between HIV-Infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr 2016; 73:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schouten J, Wit FW, Stolte IG, et al. ; AGEhIV Cohort Study Group . Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis 2014; 59:1787–97. [DOI] [PubMed] [Google Scholar]
- 4. Gallant J, Hsue PY, Shreay S, Meyer N. Comorbidities among US patients with prevalent HIV infection—a trend analysis. J Infect Dis 2017; 216:1525–33. [DOI] [PubMed] [Google Scholar]
- 5. Palella FJ, Hart R, Armon C, et al. ; HIV Outpatient Study (HOPS) . Non-AIDS comorbidity burden differs by sex, race, and insurance type in aging adults in HIV care. AIDS 2019; 33:2327–35. [DOI] [PubMed] [Google Scholar]
- 6. Palella FJ Jr, Baker RK, Moorman AC, et al. ; HIV Outpatient Study Investigators . Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006; 43:27–34. [DOI] [PubMed] [Google Scholar]
- 7. Trickey A, May MT, Vehreschild J, et al. ; Antiretroviral Therapy Cohort Collaboration (ART-CC) . Cause-specific mortality in HIV-positive patients who survived ten years after starting antiretroviral therapy. PLoS One 2016; 11:e0160460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Onen NF, Overton ET, Seyfried W, et al. . Aging and HIV infection: a comparison between older HIV-infected persons and the general population. HIV Clin Trials 2010; 11:100–9. [DOI] [PubMed] [Google Scholar]
- 9. Guaraldi G, Orlando G, Zona S, et al. . Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53:1120–6. [DOI] [PubMed] [Google Scholar]
- 10. Ronit A, Gerstoft J, Nielsen L, et al. . Non-AIDS comorbid conditions in persons living with human immunodeficiency virus (HIV) compared with uninfected individuals 10 years before HIV diagnosis. Clin Infect Dis 2018; 67:1291–3. [DOI] [PubMed] [Google Scholar]
- 11.UNAIDS. UNAIDS global AIDS update. 2018. Available at: http://www.unaids.org/en/resources/fact-sheet. Accessed 10 September 2019.
- 12. Grewe ME, Ma Y, Gilbertson A, Rennie S, Tucker JD. Women in HIV cure research: multilevel interventions to improve sex equity in recruitment. J Virus Erad 2016; 2:49–51. [PMC free article] [PubMed] [Google Scholar]
- 13. Gandhi M, Smeaton LM, Vernon C, et al. ; Womenʼs Health Inter-Network Scientific Committee (WHISC) . Low rate of sex-specific analyses in presentations at the Conference on Retroviruses and Opportunistic Infections (CROI) meeting, 2018: room to improve. J Acquir Immune Defic Syndr 2019; 81:e158–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scully EP. Sex differences in HIV infection. Curr HIV/AIDS Rep 2018; 15:136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong C, Gange SJ, Moore RD, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) . Multimorbidity among persons living with human immunodeficiency virus in the United States. Clin Infect Dis 2018; 66:1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chow FC, Regan S, Zanni MV, et al. . Elevated ischemic stroke risk among women living with HIV infection. AIDS 2018; 32:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adimora AA, Ramirez C, Benning L, et al. . Cohort profile: the Women’s Interagency HIV Study (WIHS). Int J Epidemiol 2018; 47:393–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Donaldson MA, Campbell AR, Albert AY, et al. ; CIHR Team on Cellular Aging and HIV Comorbidities in Women and Children (CARMA) . Comorbidity and polypharmacy among women living with HIV in British Columbia. AIDS 2019; 33:2317–26. [DOI] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2010–2016. HIV Surveillance Supplemental Report 2019;24(No. 1). Published February 2019. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed 3 August 2019.
- 21. Wyatt CM, Kitch D, Gupta SK, et al. ; AIDS Clinical Trials Group Study A5224s Team . Changes in proteinuria and albuminuria with initiation of antiretroviral therapy: data from a randomized trial comparing tenofovir disoproxil fumarate/emtricitabine versus abacavir/lamivudine. J Acquir Immune Defic Syndr 2014; 67:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Güerri-Fernández R, Molina-Morant D, Villar-García J, et al. . Bone density, microarchitecture, and tissue quality after long-term treatment with tenofovir/emtricitabine or abacavir/lamivudine. J Acquir Immune Defic Syndr 2017; 75:322–7. [DOI] [PubMed] [Google Scholar]
- 23. Althoff KN, Gebo KA, Moore RD, et al. ; North American AIDS Cohort Collaboration on Research and Design . Contributions of traditional and HIV-related risk factors on non-AIDS-defining cancer, myocardial infarction, and end-stage liver and renal diseases in adults with HIV in the USA and Canada: a collaboration of cohort studies. Lancet HIV 2019; 6:e93–e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Francesco D, Underwood J, Bagkeris E, et al. ; Pharmacokinetic and Clinical Observations in PeoPle Over fiftY (POPPY) Study . Risk factors and impact of patterns of co-occurring comorbidities in people living with HIV. AIDS 2019; 33:1871–80. [DOI] [PubMed] [Google Scholar]
- 25. Maggi P, Santoro CR, Nofri M, et al. . Clusterization of co-morbidities and multi-morbidities among persons living with HIV: a cross-sectional study. BMC Infect Dis 2019; 19:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry 2001; 158:725–30. [DOI] [PubMed] [Google Scholar]
- 27. Mdodo R, Frazier EL, Dube SR, et al. . Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med 2015; 162:335–44. [DOI] [PubMed] [Google Scholar]
- 28. Riddler SA, Smit E, Cole SR, et al. . Impact of HIV infection and HAART on serum lipids in men. JAMA 2003; 289:2978–82. [DOI] [PubMed] [Google Scholar]
- 29. Moran CA, Weitzmann MN, Ofotokun I. Bone loss in HIV infection. Curr Treat Options Infect Dis 2017; 9:52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nadkarni GN, Konstantinidis I, Wyatt CM. HIV and the aging kidney. Curr Opin HIV AIDS 2014; 9:340–5. [DOI] [PubMed] [Google Scholar]
- 31. Deeken JF, Tjen-A-Looi A, Rudek MA, et al. . The rising challenge of non-AIDS-defining cancers in HIV-infected patients. Clin Infect Dis 2012; 55:1228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. De Francesco D, Verboeket SO, Underwood J, et al. ; Pharmacokinetic and Clinical Observations in PeoPle Over fiftY (POPPY) Study and the AGEhIV Cohort Study . Patterns of co-occurring comorbidities in people living with HIV. Open Forum Infect Dis 2018; 5:ofy272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Niedzwiedz CL, Katikireddi SV, Pell JP, Smith DJ. Sex differences in the association between salivary telomere length and multimorbidity within the US Health & Retirement Study. Age Ageing 2019; 48:703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Whang W, Kubzansky LD, Kawachi I, et al. . Depression and risk of sudden cardiac death and coronary heart disease in women: results from the Nurses’ Health Study. J Am Coll Cardiol 2009; 53:950–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wong C, Gange SJ, Buchacz K, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) . First occurrence of diabetes, chronic kidney disease, and hypertension among North American HIV-infected adults, 2000-2013. Clin Infect Dis 2017; 64:459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Y, Chen Y, Ma L. Depression and cardiovascular disease in elderly: current understanding. J Clin Neurosci 2018; 47:1–5. [DOI] [PubMed] [Google Scholar]
- 37. Mills JC, Pence BW, Edmonds A, et al. . The impact of cumulative depression along the HIV care continuum in women living with HIV during the era of universal antiretroviral treatment. J Acquir Immune Defic Syndr 2019; 82:225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mills JC, Pence BW, Todd JV, et al. . Cumulative burden of depression and all-cause mortality in women living with human immunodeficiency virus. Clin Infect Dis 2018; 67:1575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med 2003; 163:427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jackson CA, Dobson AJ, Tooth LR, Mishra GD. Lifestyle and socioeconomic determinants of multimorbidity patterns among mid-aged women: a longitudinal study. PLoS One 2016; 11:e0156804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.