Abstract
Neocortical Layer 1 consists of a dense mesh of excitatory and inhibitory axons, dendrites of pyramidal neurons, as well as neuromodulatory inputs from diverse brain regions. Layer 1 also consists of a sparse population of inhibitory interneurons, which are appropriately positioned to receive and integrate the information from these regions of the brain and modulate cortical processing. Despite being among the sparsest neuronal population in the cortex, Layer 1 interneurons perform powerful computations and have elaborate morphologies. Here we review recent studies characterizing their origin, morphology, physiology, and molecular profiles, as well as their connectivity and in vivo response properties.
General introduction
Cortical interneurons can be divided into four cardinal classes: PV, SST, VIP, and Id2 (5HT3aR non-VIP) [1]. Of these, three of the four have been long studied with regards to their diversity and function. Until recently, the fourth class (Id2 interneurons), the nearly sole cellular component of cortical layer 1 (L1), has been mostly ignored. The reasons for this are myriad, not the least of which is the sense that because L1 is largely acellular it is somehow less interesting. Despite this bias, L1 has long been recognized as a nexus that interfaces bottom-up signaling with top-down contextual input. It represents a critical convergence point between thalamic, intercortical, as well as basal forebrain and brain stem neuromodulatory systems (e.g. serotonergic and cholinergic) afferents. Indeed, David Hubel in his now classic 1982 piece on cortical neurobiology dubbed this layer the cortex’s ‘crowning mystery’ [2]. The fact that L1 has languored as a little-explored corner of cortical function reflects on the paucity of tools to target these cells and the lack of understanding of their origin or composition. Nevertheless, the last three years have seen an explosion in our understanding of the origins, molecular composition, and function of this population. In this review, we will outline the emerging lines of evidence revealing the diversity and developmental origins of these cells. These efforts are rapidly leading to the development of genetic tools, which, when coupled with anatomical and physiological approaches, promise to reveal the logic by which L1 interneurons shape function within the cortex.
Developmental origins (fate and genes)
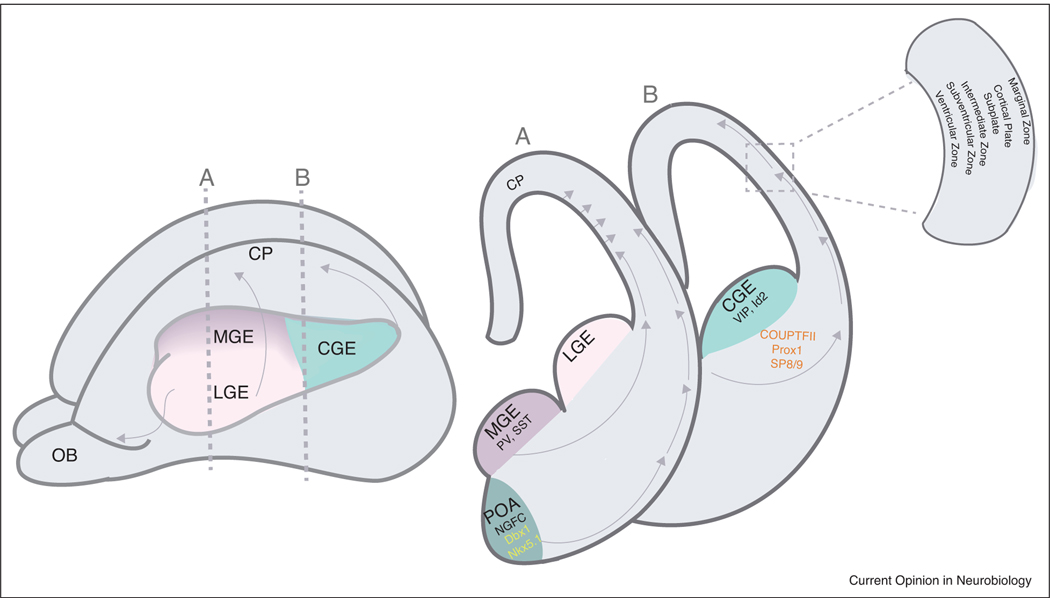
Fate mapping studies have shown that L1 interneurons predominantly originate from a progenitor zone in the ventral telencephalon known as the caudal ganglionic eminence (CGE, [3,4,5]), with a small minority arising from the Dbx-1 positive region of the preoptic area (POA; [7]), see Figure 1. Unlike the parvalbumin and somatostatin interneurons that sequentially arise from the medial ganglionic eminence, VIP and L1 interneuron populations arise concurrently, leading to speculation that they represent two distinct progenitor populations [6]. While the precise origin of each of these cardinal classes remains uncertain, work from the Dayer laboratory indicates that L1 neurogliaform cells (NGFCs) may arise from Nkx5.1 precursors in the vicinity of the POA [8]. However, as outlined below, NGFCs only represent roughly 30 percent of neurons in L1.
Figure 1.
Developmental origin of Layer 1 interneurons.
Left, schematic diagram of an embryonic mouse brain (~E14) highlighting the ganglionic eminences from which inhibitory interneurons are derived. Medial ganglionic eminence (MGE) gives rise to PV and SST interneurons, Lateral ganglionic eminence (LGE) gives rise to the interneurons of the olfactory bulb (OB); and Caudal ganglionic eminence (CGE) gives rise to the VIP and Id2 interneuron populations. Vertical dashed lines indicate the levels of the coronal sections in the right panel.
Right panel, coronal sections through the three eminences as well as the preoptic area (POA). Layer 1 interneurons are derived from the CGE and POA. Arrows indicate the routes of migration that the interneurons prefer to reach the cortical plate. Boxed inset highlights the structures surrounding the cortical plate present at this embryonic age.
The different works from both the Nakajima and Studer laboratories, as well as our own, have documented streams of interneurons emanating from the CGE and migrating caudally and dorsally to invade the cortex [5,9,10]. The use of genetic strategies to target these populations early in development has begun to reveal some of the molecular components involved in their generation. L1 interneurons express a variety of transcription factors, including Prox1, CoupTF2, and SP8/9 during embryogenesis [11–13]. In Prox1 loss-of-function animals, there was a significant reduction in the number of interneurons in L1, with a corresponding increase in deep layers [14,13]. Similarly, the combined loss of Sp8 and Sp9 (compound mutant) appears to impact both the development of VIP and L1 interneurons [15]. Moreover, given that Prox1 gene expression is lost in this compound mutant implies that Prox1 functions downstream of SP8/9. Interestingly, loss of either Prox1 or Sp8/9 strongly affects migration and integration of both VIP and L1 interneurons into the cortex [13] and in the latter case this partly reflects the loss of guidance cues such as Robo1 and Cxcl14 [15]. Together, a molecular appreciation of the key regulatory genes that direct their development is beginning to emerge.
Migration and developmental cues
Interneurons reach the cortex during embryogenesis via two stereotyped routes: the marginal zone (MZ), which is the predecessor for L1 in the adult cortex, and the subventricular zone (SVZ) below the cortex (Figure 1). As might be expected, L1 interneurons preferentially utilize the MZ [9]. Moreover, increasing evidence suggests that L1 contains an abundance of local guidance cues (e.g. Cxcl12 (i.e. SDF1), Cxcl14, Sema3C, and reelin), some of which likely derive from the pia. In addition, within developing L1 reside the Cajal Retzius (CR) cells, a transient glutamatergic population derived from the ventral pallium, cortical hem, and septum [16–18] that undergo apoptosis and disappear from the cortex by the second postnatal week. During their brief lifespan, CR cells are a major source of the glycoprotein reelin, which they secrete into the extracellular matrix of the MZ. Reelin diffuses through the developing cortex, binding to receptors expressed by radial glia cells and migrating neurons [19]. Absence of reelin in the cortex causes massively abnormal neuronal migration, positioning, and lamination, and similar findings are seen with ablation of CR cells [20–26]. Whether CR cell-produced reelin affects L1 interneuron migration is uncertain, but their proximity to migrating L1 precursors certainly positions them to provide developmental cues. Interestingly, L1 interneurons also express reelin. It is unclear whether this source of reelin is important for migration but given its relatively late appearance (approximately P4 in mice), it seems unlikely. One of the best markers for L1 interneurons is neuron-derived neurotrophic factor (NDNF) [27] and, in addition to reelin, it will be interesting to explore whether this peptide provides signaling during development or in adults. As will be discussed below, L1 interneurons are ideally positioned to control the integration of bottom-up and top-down cortical information. Interestingly, many of the Simons Foundation Autism Research Initiative (SFARI) autism genes appear to be enriched within L1 interneurons [28]. As such, an appealing hypothesis is that developmental insults that affect L1 interneurons might provide an etiology for neuropsychiatric disorders, including schizophrenia and autism spectrum disorders.
Electrophysiological properties of L1 interneurons
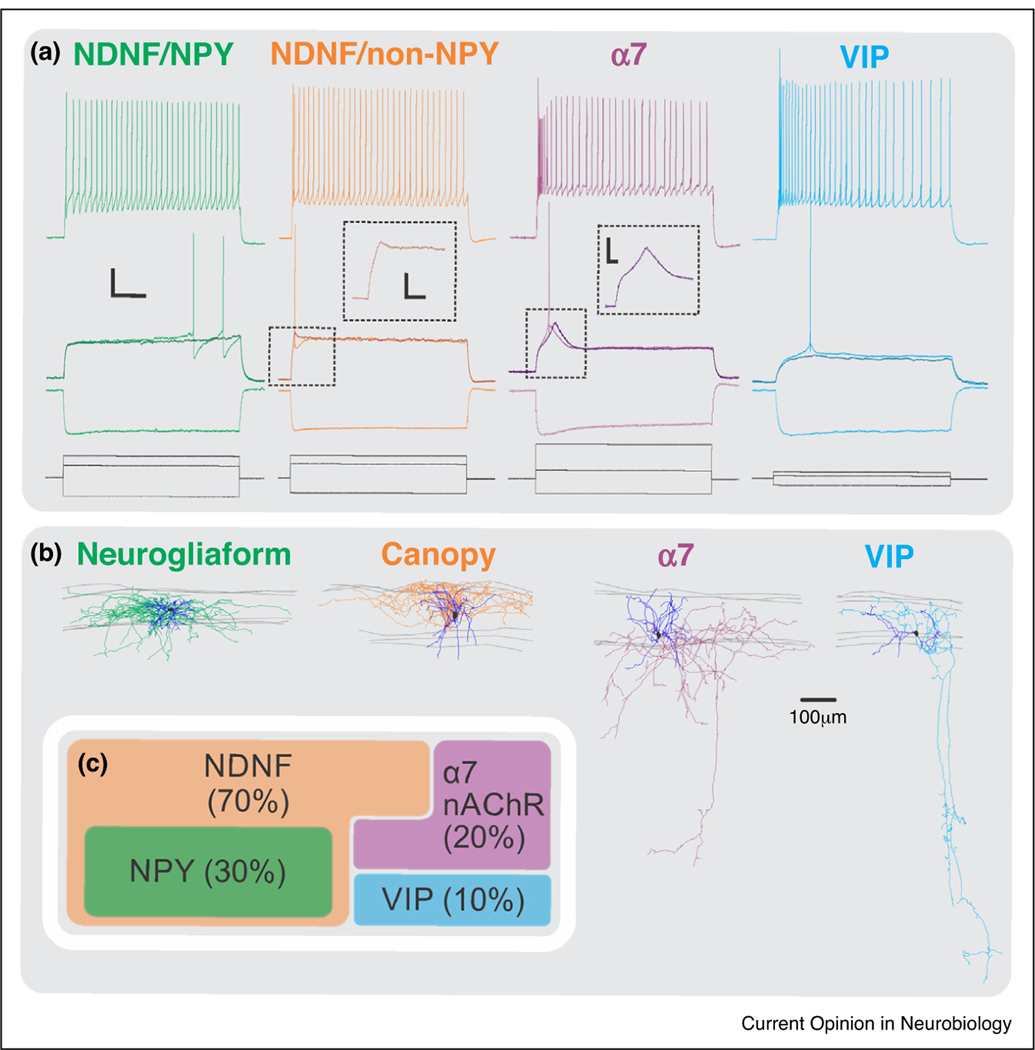
Recent work from the Rudy lab [29] has done the most thorough characterization to date of the diversity of interneurons in L1, both in terms of morphology and physiology. This work has also provided specific molecular markers for each of the constituent subtypes, which are comprised by four novel molecularly defined subtypes: 1) NDNF/Neuropeptide Y (NPY) double positive, 2) NDNF/non-NPY, 3) alpha-7 nicotinic acetylcholine receptor (Chrna7 or α7) positive, and 4) vasoactive intestinal peptide positive (VIP) cells (Figure 2). The NDNF/NPY cells were morphologically found to be NGFCs and electrophysiologically late-spiking neurons, a firing pattern previously associated with NGFCs [30,31,51–54]. Additionally, paired recordings showed that they displayed a high degree of connectivity to nearby pyramidal neurons in Layer 2/3, suggesting that L1 NGFCs can inhibit the distal dendrites of pyramidal cells. NGFCs were also capable of producing unitary GABAB-mediated responses and were found to be highly connected to NDNF/non-NPY cells in L1 [32–35]. In contrast, the NDNF/non-NPY population, dubbed ‘canopy cells’, although morphologically similar to NGFCs, did not display late-spiking properties but were regular spiking, with an onset spike at the beginning of the depolarization threshold. Moreover, in contrast to NGFCs, canopy cells were poorly connected to nearby pyramidal neurons. They were connected to L1 NGFCs; however, the synaptic strength of this connection was significantly smaller than the reciprocal connection. Their main postsynaptic target(s) still remains to be discovered. Abs et al. [36••] showed that light activation of NDNF interneurons expressing channelrhodopsin-2 (ChR2) in acute brain slices of adult auditory cortex elicited inhibitory postsynaptic currents in L2/3 pyramidal cells. Taking into account the results from paired recordings described above [29], these results likely reflect light-mediated recruitment of the NGFCs within the NDNF population. The VIP and α7 subgroups were characterized by a prominent translaminar descending axon (Figure 2).
Figure 2.
Subtypes of Layer 1 interneurons.
(a) Electrophysiological properties of the four different L1 subtypes: NDNF/NPY positive; NDNF/NPY negative, VIP, and alpha-7. Middle panel illustrates the response of the neurons to a threshold current injection. Notice that the NDNF/NPY positive population has a late spiking property, and the alpha-7 population has a depolarizing hump near threshold.
(b) The morphologies associated with the four subtypes. Notice the elaborate axonal and dendritic arborization of the neurogliaform and the canopy cells (NDNF population) mostly restricted to Layer 1; whereas the alpha-7 and VIP possess a descending axon projecting down to deeper layers.
(c) Relative proportions of the four interneuron subtypes in Layer 1.
These are probably the subgroups that were previously characterized as single bouquet cells (SBCs) [30], with an axon projecting down as deep as L5. A distinguishing feature of the α7 subgroup was a depolarizing hump at near-threshold potentials, mediated by T-type calcium channels [29]. The connectivity of these populations has not been characterized.
Previous studies have suggested that the two major morphological subtypes in L1, the SBC and NGFC types, are thought to be involved in disinhibiting pyramidal cells in the same column (center disinhibition) and inhibiting pyramidal cells across multiple columns (surround inhibition) respectively [30,37]. Together, they may play a role in selecting attentional and salient signals [30,37]. However, with the new data emerging from the Rudy lab and the discovery of the canopy cell, this idea may need to be revisited. We speculate that the canopy cell may also be capable of mediating disinhibition via their presumed connectivity to other interneurons in L1-3 and due to the lack of a very strong inhibition onto L2/3 pyramidal cells.
Input connectivity of L1 interneurons
It has long been speculated that inputs within L1 do not merely modulate the integration of bottom-up signaling and top-down signaling but are part of it. This is evident simply from the topography of the top ~100 μm of the cortex, which consists of the apical dendrites of excitatory neurons in layers 2/3 and 5, as well as dense axonal innervation from a wide variety of cortical and subcortical inputs. Among this mesh of axons and dendrites, reside the L1 interneurons.
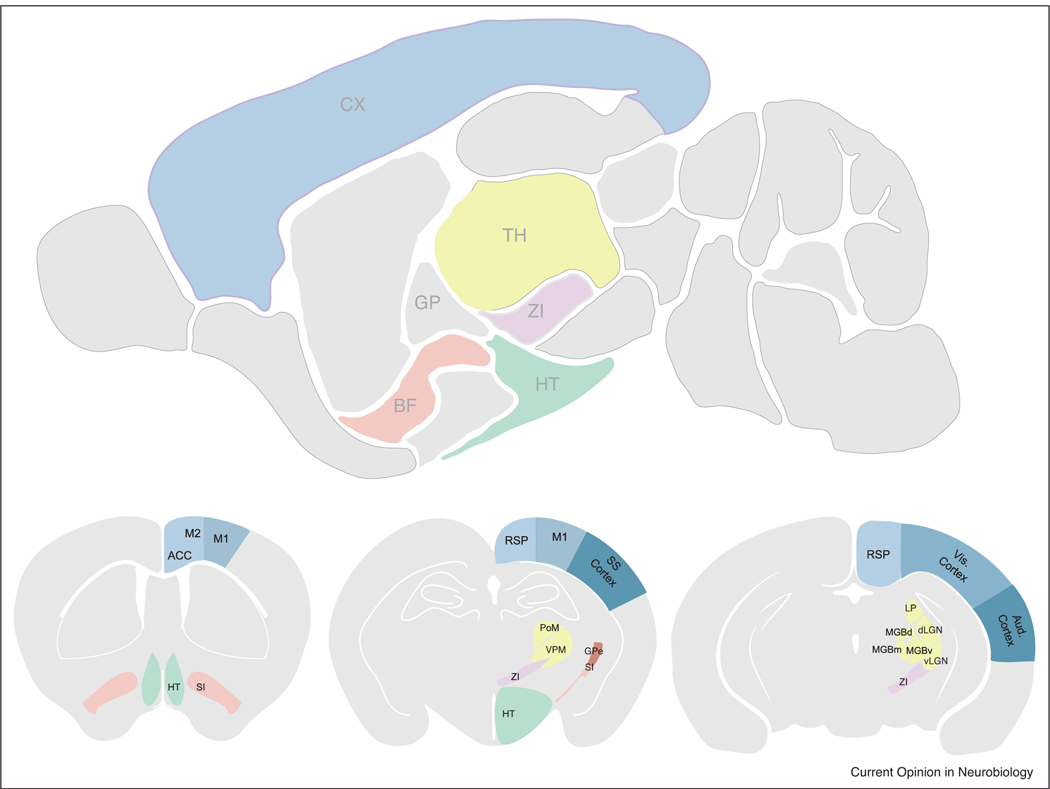
Data from the Allen Institute’s brain connectivity atlas, as well as other studies, reveal that a wide variety of brain structures project to the superficial layers of the cortex. Consistent with these observations, multiple input sources to NDNF interneurons in auditory cortex were revealed using rabies tracing. Sources of input included contralateral and ipsilateral somatosensory and visual cortices, motor and association areas (e.g. retrosplenial, cingulate, infralimbic), several thalamic nuclei, and cholinergic areas in the basal forebrain [36••], see Figure 3). Work from our lab is attempting to systematically characterize the inputs that distinct L1 neurons receive in the major sensory regions of the brain (primary somatosensory cortex, S1, primary visual cortex, V1, and primary auditory cortex, A1) in both the developing and adult sensory cortices in mice. Our unpublished work indicates that there is strong bottom-up (primary thalamic) sensory input onto L1 interneurons during development that is reduced but persists at the termination of the critical period. This early primary thalamic input is largely replaced by long-range corticocortical feedback in the adult. How these two sources of inputs get integrated within L1 and how they modulate cortical output is an area of active investigation. Whether these top-down feedback inputs mediate prediction [38,39] via the recruitment of L1 interneurons and whether sensory experience is required for the maturation of these feedback projections is still an open question.
Figure 3.
Connectivity of Layer 1 interneurons.
Upper panel, saggital section of an adult mouse brain highlighting the relevant brain regions that project to Layer 1. Thalamus (TH), Cortex (CX), Zona Incerta (ZI), Basal Forebrain (BF), Hypothalamus (HT).
Bottom panels, coronal sections detailing the structures in the saggital section above. Cg (cingulate cortex), M2 (premotor cortex), M1 (motor cortex), SI (substantia innominata; part of BF), RSP (retrosplenial cortex), Som Cx (Somatosensory cortex), LP (Lateral posterior nucleus), dLGN (dorsal lateral geniculate nucleus), vLGN (ventral lateral geniculate nucleus), MGB (medial geniculate nucleus). MGB consists of d (dorsal) m (medial) and v (ventral) subdivisions.
Note: These highlighted structures illustrate a general pattern of connectivity of Layer 1 interneurons in the sensory cortices. For example, Layer 1 interneurons in Som cortex receive projections from M1, M2, Cg, RSP, and BF, as well as their respective thalamic nuclei PO and VPM. In the visual cortex, L1 interneurons also receive inputs from M1, M2, Cg, RSP and BF, as well as their respective thalamic nuclei, LGN, and LP.
Also note, this is not an exhaustive list of structures projecting to L1 interneurons in specific brain regions but a general pattern observed across multiple areas of the sensory cortex.
In vivo responses of L1 interneurons
Whether all projections found in L1 actually synapse onto L1 interneurons is not fully known. However, L1 interneurons have been shown to respond to direct sensory stimulation. For example, in V1, visual stimulation has been shown to elicit responses in L1 interneurons [40]. Similarly, in A1, it has been found that electrical stimulation of the MGBv causes activation in L1 interneurons at a similar latency and strength compared to L4 [41]. These observations are supported by optogenetic stimulation of thalamic nuclei in brain slices. Activation of both the dLGN and MGB elicited monosynaptic responses in L1 interneurons [42]. Additionally, imaging studies have revealed the presence of both first order and higher order thalamic fibers in the superficial layers of the visual cortex [43,44]. These thalamic fibers could potentially innervate L1 interneurons themselves. In contrast, in the barrel cortex, there is more skepticism regarding a direct sensory influence on interneurons in L1. One study [31] demonstrated that L1 neurons responded to whisker stimulation and the short latency of these responses suggests that they are due to direct bottom-up sensory inputs. In addition to direct sensory activation, there have been reports suggesting that cross-modal sensory inputs also activate L1 interneurons [40]. L1 interneurons in V1 received input from the auditory cortex and were shown to respond to sound stimulation. This activation led to an auditory mediated strengthening of orientation selectivity in the underlying L2/3 pyramidal cells [40,45]. Similarly, other cross-modal inputs could potentially activate L1 interneurons directly [46]. Hyperpolarization in L2/3 pyramidal neurons in V1 was observed as a result of S1 activation; as well as in S1, as a result of A1 activation. These studies suggest that L1 interneurons can integrate bottom-up sensory information with sensory inputs from other modalities.
As discussed in the previous section, L1 also receives dense projections from higher-order associational cortices and L1 interneurons have been shown to directly respond to some of these inputs. For example, almost 85% of L1 interneurons in V1 responded to optogenetic stimulation of the premotor cortex (M2)/anterior cingulate (ACC) fibers [39]. Whether L1 interneurons in this circuit can mediate some of the visual flow predictions observed in V1 remains to be investigated. Furthermore, callosal axons responsible for interhemispheric communication have been shown to target L1 interneurons in S1 and this connectivity has been suggested to be important for interhemispheric inhibition [47]. Similarly, neuromodulatory centers also project densely to L1. Basal forebrain, the largest source of acetylcholine in the cortex, caused activation in the majority of L1 interneurons tested [48]. This suggests that L1 interneurons can potentially be activated by multiple diverse inputs such as sensory, cross-modal, neuromodulatory, and other higher-order afferents. However, how these diverse inputs shape L1 responses still remains an open question. Letzkus et al. [49] reported that L1 interneurons in both A1 and V1 can be activated by foot shocks. This activation was mediated by nicotinic currents and led to disinhibition of pyramidal neurons, facilitating auditory fear learning. More recently, Mesik et al. [50] characterized L1 responses in V1 to a wide range of sensory and motor stimulations and examined the conditions under which L1 interneurons become activated. They show that about half of L1 neurons responded to visual stimuli and that at least half responded during locomotion. Locomotion increased the responses to visual stimuli, as well elicited responses in L1 interneurons on its own. Furthermore, approximately half of the neurons responded to sound and a fraction responded to whisker stimulation; again, suggesting wide cross-modal integration in the cortex and the recruitment of L1 interneurons. However, all these studies did not take into the account the diversity within the L1 interneuron population. Whether the same interneuron subtype could respond to one or multiple of these stimuli still needs to be investigated. Lastly, L1 has been suggested to be involved in memory processes [36••]. Abs et al., studied the effect of fear conditioning on L1 NDNF neurons in auditory cortex and found that 30% of NDNF interneurons responded to a conditioned stimulus. After fear conditioning, both the response amplitude and the proportion of NDNF interneurons that responded increased (from 30% to 45%) resulting from stronger excitation and reduced inhibition during fear memory expression. In contrast, Doron et al. (2019), have suggested a role for L1 in memory formation. Chemogenetic inhibition of perirhinal inputs to L1 of S1 impaired the learning of a hippocampal-dependent task. However, induction of the chemogenetic inhibition in animals that had already learned the task had no effect. These studies shed more light on the diverse nature of processes in which L1 interneurons have a role, including memory.
Taken together these findings demonstrate that L1 function encompasses a breadth of circuit motifs whose function belies a unitary explanation of their contributions. In the broadest sense, it seems likely that depending on their timing of engagement, specific afferents, as well as co-recruitment of neighboring L1 subtypes, L1 interneurons dynamically integrate bottom-up and top-down signals based on circumstance.
Conclusion
While many aspects concerning the origins, diversity, and function of L1 neurons remain, it seems the cortex’s crowning mystery is at last being unveiled. The rapid emergence of new genetic tools will allow us to precisely manipulate each individual subtype and study effects on the underlying sensory processing. Nonetheless, it seems likely L1 will yield more surprises regarding how cortical function is initialized and how this leads to cognitive function with regard to both representation and contextual modulation. Through the growing availability of new genetic tools, high-density recording approaches and increasingly sophisticated behavioral monitoring, a real understanding of how bottom-up and top-down information flow is controlled by the newly appreciated host of L1 interneurons is at last forthcoming. These insights will no doubt provide clarity in our understanding of cortical function and hopefully also reveal whether developmental insults to L1 interneurons provide an etiology for neuropsychiatric disorders.
Acknowledgements
Work in the GF lab is supported by the following N.I.H. grants: R01 NS081297, R01 MH071679, UG3 MH120096, P01 NS074972 and by the Simons Foundation SFARI. Work in the BR lab is supported by P01 NS074972. LAI is supported by the Hearst Foundation Postdoctoral fellowship.
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
•• of outstanding interest
- 1.Rudy B, Fishell G, Lee S, Hjerling-Leffler J: Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol 2011, 71:45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hubel DH: Cortical neurobiology: a slanted historical perspective. Annu Rev Neurosci 1982, 5:363–370. [DOI] [PubMed] [Google Scholar]
- 3.Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA: Origins of cortical interneuron subtypes. J Neurosci 2004, 24:2612–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G: The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron 2005, 48:591–604. [DOI] [PubMed] [Google Scholar]
- 5.Yozu M, Tabata H, Nakajima K: The caudal migratory stream: a novel migratory stream of interneurons derived from the caudal ganglionic eminence in the developing mouse forebrain. J Neurosci 2005, 25:7268–7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, Battiste J, Johnson JE, Machold RP, Fishell G: Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci 2010, 30:1582–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelman D, Griveau A, Dehorter N, Teissier A, Varela C, Pla R, Pierani A, Marín O: A wide diversity of cortical GABAergic interneurons derives from the embryonic preoptic area. J Neurosci 2011, 31:16570–16580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niquille M, Limoni G, Markopoulos F, Cadilhac C, Prados J, Holtmaat A, Dayer A: Neurogliaform cortical interneurons derive from cells in the preoptic area. eLife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyoshi G, Fishell G: GABAergic interneuron lineages selectively sort into specific cortical layers during early postnatal development. Cereb Cortex 2011, 21:845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Touzot A, Ruiz-Reig N, Vitalis T, Studer M: Molecular control of two novel migratory paths for CGE-derived interneurons in the developing mouse brain. Development 2016, 143:1753–1765. [DOI] [PubMed] [Google Scholar]
- 11.Kanatani S, Yozu M, Tabata H, Nakajima K: COUP-TFII is preferentially expressed in the caudal ganglionic eminence and is involved in the caudal migratory stream. J Neurosci 2008, 28:13582–13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma T, Zhang Q, Cai Y, You Y, Rubenstein JL, Yang Z: A subpopulation of dorsal lateral/caudal ganglionic eminence-derived neocortical interneurons expresses the transcription factor Sp8. Cereb Cortex 2012, 22:2120–2130. [DOI] [PubMed] [Google Scholar]
- 13.Miyoshi G, Young A, Petros T, Karayannis T, McKenzie Chang M, Lavado A, Iwano T, Nakajima M, Taniguchi H, Huang ZJ et al. : Prox1 regulates the subtype-specific development of caudal ganglionic eminence-derived GABAergic cortical interneurons. J Neurosci 2015, 35:12869–12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubin AN, Kessaris N: PROX1: a lineage tracer for cortical interneurons originating in the lateral/caudal ganglionic eminence and preoptic area. PLoS One 2013, 8:e77339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei S, Du H, Li Z, Tao G, Xu Z, Song X, Shang Z, Su Z, Chen H, Wen Y et al. : Transcription factors Sp8 and Sp9 regulate the development of caudal ganglionic eminence-derived cortical interneurons. J Comp Neurol 2019, 527:2860–2874. [DOI] [PubMed] [Google Scholar]
- 16.Takiguchi-Hayashi K, Sekiguchi M, Ashigaki S, Takamatsu M, Hasegawa H, Suzuki-Migishima R, Yokoyama M, Nakanishi S, Tanabe Y: Generation of reelin-positive marginal zone cells from the caudomedial wall of telencephalic vesicles. J Neurosci 2004, 24:2286–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bielle F, Griveau A, Narboux-Nême N, Vigneau S, Sigrist M, Arber S, Wassef M, Pierani A: Multiple origins of Cajal-Retzius cells at the borders of thedeveloping pallium. NatNeurosci 2005, 8:1002–1012. [DOI] [PubMed] [Google Scholar]
- 18.García-Moreno F, López-Mascaraque L, De Carlos JA: Origins and migratory routes of murine Cajal-Retzius cells. J Comp Neurol 2007, 500:419–432. [DOI] [PubMed] [Google Scholar]
- 19.Tissir F, Goffinet AM: Reelin and brain development. Nat Rev Neurosci 2003, 4:496–505. [DOI] [PubMed] [Google Scholar]
- 20.Yang A, Walker N, Bronson R, Kaghad M, Oosterwegel M, Bonnin J, Vagner C, Bonnet H, Dikkes P, Sharpe A et al. : p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature 2000, 404:99–103. [DOI] [PubMed] [Google Scholar]
- 21.Hevner RF, Shi L, Justice N, Hsueh Y, Sheng M, Smiga S, Bulfone A, Goffinet AM, Campagnoni AT, Rubenstein JL: Tbr1 regulates differentiation of the preplate and layer 6. Neuron 2001, 29:353–366. [DOI] [PubMed] [Google Scholar]
- 22.Meyer G, Perez-Garcia CG, Abraham H, Caput D: Expression of p73 and Reelin in the developing human cortex. J Neurosci 2002, 22:4973–4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shinozaki K, Miyagi T, Yoshida M, Miyata T, Ogawa M, Aizawa S, Suda Y: Absence of a Cajal-Retzius cells and subplate neurons associated with defects of tangential cell migration from ganglionic eminence in Emx1/2 double mutant cerebral cortex. Development 2002, 129:3479–3492. [DOI] [PubMed] [Google Scholar]
- 24.Lyu YL, Wang JC: Aberrant lamination in the cerebral cortex of mouse embryos lacking DNA topoisomerase IIbeta. Proc Natl Acad Sci U S A 2003, 100:7123–7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer G, Cabrera Socorro A, Perez Garcia CG, Martinez Millan L, Walker N, Caput D: Developmental roles of p73 in Cajal-Retzius cells and cortical patterning. J Neurosci 2004, 24:9878–9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wines-Samuelson M, Handler M, Shen J: Role of presenilin-1 in cortical lamination and survival of Cajal-Retzius neurons. Dev Biol 2005, 277:332–346. [DOI] [PubMed] [Google Scholar]
- 27.Tasic B, Menon V, Nguyen TN, Kim TK, Jarsky T, Yao Z, Levi B, Gray LT, Sorensen SA, Dolbeare T et al. : Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci 2016, 19:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer C, Hafemeister C, Bandler RC, Machold R, Batista Brito R, Jaglin X, Allaway K, Butler A, Fishell G, Satija R: Developmental diversification of cortical inhibitory interneurons. Nature 2018, 555:457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuman B, Machold RP, Hashikawa Y, Fuzik J, Fishell GJ, Rudy B: Four unique interneuron populations reside in neocortical layer 1. J Neurosci 2019, 39:125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang X, Wang G, Lee AJ, Stornetta RL, Zhu JJ: The organization of two new cortical interneuronal circuits. Nat Neurosci 2013, 16:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu Y, Zhu JJ: Rapid arrival and integration of ascending sensory information in layer 1 nonpyramidal neurons and tuft dendrites of layer 5 pyramidal neurons of the neocortex. J Neurosci 2004, 24:1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hestrin S, Armstrong WE: Morphology and physiology of cortical neurons in layer I. J Neurosci 1996, 16:5290–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oláh S, Füle M, Komlósi G, Varga C, Báldi R, Barzó P, Tamás G: Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature 2009, 461:1278–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wozny C, Williams SR: Specificity of synaptic connectivity between layer 1 inhibitory interneurons and layer 2/3 pyramidal neurons in the rat neocortex. Cereb Cortex 2011, 21:1818–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muralidhar S, Wang Y, Markram H: Synaptic and cellular organization of layer 1 of the developing rat somatosensory cortex. Front Neuroanat 2013, 7:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.••.Abs E, Poorthuis RB, Apelblat D, Muhammad K, Pardi MB, Enke L, Kushinsky D, Pu DL, Eizinger MF, Conzelmann KK et al. : Learning-related plasticity in dendrite-targeting layer 1 interneurons. Neuron 2018, 100:684–699.e686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee AJ, Wang G, Jiang X, Johnson SM, Hoang ET, Lanté F, Stornetta RL, Beenhakker MP, Shen Y, Julius Zhu J: Canonical organization of layer 1 neuron-led cortical inhibitory and disinhibitory interneuronal circuits. Cereb Cortex 2015, 25:2114–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao RP, Ballard DH: Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat Neurosci 1999, 2:79–87. [DOI] [PubMed] [Google Scholar]
- 39.Leinweber M, Ward DR, Sobczak JM, Attinger A, Keller GB: A sensorimotor circuit in mouse cortex for visual flow predictions. Neuron 2017, 96:1204. [DOI] [PubMed] [Google Scholar]
- 40.Ibrahim LA, Mesik L, Ji XY, Fang Q, Li HF, Li YT, Zingg B, Zhang LI, Tao HW: Cross-modality sharpening of visual cortical processing through layer-1-mediated inhibition and disinhibition. Neuron 2016, 89:1031–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takesian AE, Bogart LJ, Lichtman JW, Hensch TK: Inhibitory circuit gating of auditory critical-period plasticity. Nat Neurosci 2018, 21:218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji XY, Zingg B, Mesik L, Xiao Z, Zhang LI, Tao HW: Thalamocortical innervation pattern in mouse auditory and visual cortex: laminar and cell-type specificity. Cereb Cortex 2016, 26:2612–2625 10.1093/cercor/bhv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cruz-Martín A, El-Danaf RN, Osakada F, Sriram B, Dhande OS, Nguyen PL, Callaway EM, Ghosh A, Huberman AD: A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex. Nature 2014, 507:358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roth MM, Dahmen JC, Muir DR, Imhof F, Martini FJ, Hofer SB: Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex. Nat Neurosci 2016, 19:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McClure JP, Polack PO: Pure tones modulate the representation of orientation and direction in the primary visual cortex. J Neurophysiol 2019, 121:2202–2214. [DOI] [PubMed] [Google Scholar]
- 46.Iurilli G, Ghezzi D, Olcese U, Lassi G, Nazzaro C, Tonini R, Tucci V, Benfenati F, Medini P: Sound-driven synaptic inhibition in primary visual cortex. Neuron 2012, 73:814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palmer LM, Schulz JM, Murphy SC, Ledergerber D, Murayama M, Larkum ME: The cellular basis of GABA(B)-mediated interhemispheric inhibition. Science 2012, 335:989–993. [DOI] [PubMed] [Google Scholar]
- 48.Alitto HJ, Dan Y: Cell-type-specific modulation of neocortical activity by basal forebrain input. Front Syst Neurosci 2012, 6:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, Lüthi A: A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 2011, 480:331–335. [DOI] [PubMed] [Google Scholar]
- 50.Mesik L, Huang JJ, Zhang LI, Tao HW: Sensory and motor-related responses of layer 1 neurons in the mouse visual cortex. J Neurosci 2019, 4:1722–19 10.1523/JNEUROSCI.1722-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang X, Shen S, Cadwell CR, Berens P, Sinz F, Ecker AS, Patel S, Tolias AS: Principles of connectivity among morphologically defined cell types in adult neocortex. Scienc 2015:ac9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B: The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci 2010, 30:16796–16808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cruikshank SJ, Ahmed OJ, Stevens TR, Patrick SL, Gonzalez AN, Elmaleh M, Connors BW: Thalamic control of layer 1 circuits in prefrontal cortex. J Neurosci 2012, 32:17813–17823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawaguchi Y, Kubota Y: GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex 1997, 7:476–486. [DOI] [PubMed] [Google Scholar]