Abstract
Functional gastrointestinal (GI) disorders (FGIDs) result from central and peripheral mechanisms, cause chronic remitting-relapsing symptoms, and are associated with comorbid conditions and impaired quality of life. This article reviews sex- and gender-based differences in the prevalence, pathophysiologic factors, clinical characteristics, and management of functional dyspepsia (FD) and irritable bowel syndrome (IBS) that together affect approximately 1 in 4 people in the United States. These conditions are more common in women. Among IBS patients, women are more likely to have severe symptoms and coexistent anxiety or depression; constipation or bloating, and diarrhea are more common respectively in women and men, perhaps partly because defecatory disorders, which cause constipation, are more common in women. Current concepts suggest that biological disturbances (eg, persistent mucosal inflammation after acute gastroenteritis) interact with other environmental factors (eg, abuse) and psychological stressors, which influence brain and gut to alter GI tract motility and/or sensation, thereby causing symptoms. By comparison to a considerable understanding of sex-based differences in the pathogenesis of visceral hypersensitivity in animal models, we know less about the contribution of these differences to FGID in humans. Slow gastric emptying and colon transit are more common in healthy women than men, but effects of gonadal hormones on colon transit are less important than in rodents. While increased visceral sensation partly explains symptoms, effects of sex on visceral sensation, colonic permeability, and the gut microbiome are less prominent in humans than rodents. Whether sex or gender affects response to medications or behavioral therapy in FD or IBS is unclear because most patients in these studies are women.
Introduction
Roughly one-third of the adult population has 1 or more symptoms (eg, dyspepsia, constipation, diarrhea, chronic abdominal pain, or fecal incontinence).1 Accounting for a majority of gastroenterology consultations, these patients often have chronic remitting-relapsing symptoms, comorbid conditions (eg, fibromyalgia, anxiety, depression), and impaired quality of life (QOL) and use considerable health care resources.2 Initially, these conditions were defined as symptoms, in the absence of an organic explanation (eg, inflammatory bowel disease, peptic ulcer or celiac disease, ischemic bowel disease) for the same by routine laboratory, endoscopy, and imaging studies.3 Currently, these conditions, broadly termed functional gastrointestinal disorders (FGIDs), are conceptualized as disorders of gut functions, especially gastrointestinal (GI) tract motility and sensation. Some tests may show generally subtle abnormalities (eg, microscopic inflammation, altered gut microbiota) in some patients. Psychosocial conditions and stressors contribute to the development, perception, and management of FGIDs.
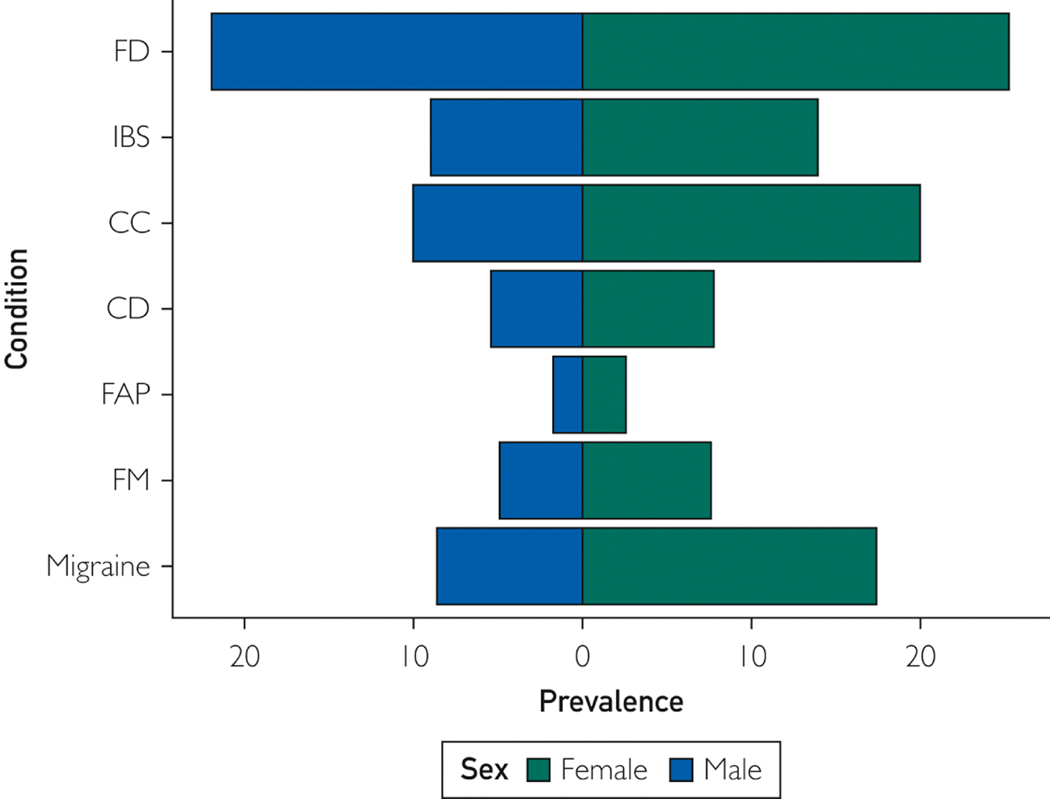
This review covers the 2 most common FGIDs: functional dyspepsia (FD) and irritable bowel syndrome (IBS) (Figure 1). Other FGIDs that are less prevalent but more common in women (eg, chronic abdominal pain and pelvic pain), are not considered herein because the differences between men and women are poorly understood.3, 4 Nor do we consider GI tract motility disorders that are characterized by more pronounced enteric neuronal injury in the stomach (ie, gastroparesis), small intestine (ie, chronic intestinal pseudo-obstruction), or colon (ie, slow transit constipation and megacolon). Geared to practitioners, this review discusses the effects of sex (the biologic differences between men and women) and gender (ie social constructs and behavior) on FD and IBS. Similar to other disorders accompanied by chronic pain, such as fibromyalgia, migraine, and rheumatoid arthritis,5 (Figure 1) FD and IBS are more common in women than men. The pathophysiologic factors, clinical characteristics, and, to a lesser extent, response to therapy also differ between women and men (Table 1). This review highlights the differences between the sexes in humans and animal studies (Table 2).
Figure 1.
Prevalence of Common Functional Gastrointestinal Tract and Somatic Disorders of Men and Women. Values are approximate and are derived from individual population-based studies or systematic reviews. For chronic constipation, the estimate is derived from the average prevalence (16%) and the female to male ratio (1.5:1) in systematic reviews and with the assumption that the female to male ratio is 1:1 in the general population.
Table 1.
Key Concepts About Sex- and Gender-Based Differences in FGID
| Characteristic | Key Concept |
|---|---|
| Prevalence | FD, IBS, and chronic diarrhea are more common in women than men |
| Nature of GI tract symptoms | In FD, the M:F ratio is comparable in patients with epigastric pain syndrome and/or postprandial distress syndrome. Among IBS patients, constipation and diarrhea are more common in women and men, respectively. Bloating is more common in women |
| Variation in GI tract symptoms during menses | Women with IBS have more severe symptoms during menses |
| Extra–GI tract symptoms | |
| Anxiety and depression | Anxiety and to a lesser extent depression are more common in female than male IBS patients. Severity of anxiety correlates with some IBS symptoms in women but not men |
| Gender roles/social factors | Among IBS patients, women described shame because they were not living up to the gender norm expectations in domains of relationships and attractiveness. Expression of pain is socially more acceptable among women than men |
| Pathophysiologic characteristics | |
| Postinfectious IBS | Women are at increased risk for postinfectious IBS after acute gastroenteritis |
| GI tract motility | Gastric emptying and colon transit are slower in healthy women than men |
| GI tract sensation | Sex-based differences in the perception of GI tract distention in healthy people and patients with FGIDs are unclear |
| Central processing of GI tract sensation | Small studies suggest that during rectal distension, greater activation of affective and autonomic regions occurs in women and greater activation of regions belonging to a corticolimbic pain inhibition system occurs in men |
| Early life stress | Associated with increased risk of IBS; effects of sex and gender on this association are unknown |
| Acute stress | In a pharmacologic model (ie, intravenous hydrocortisone), acute stress increased rectal sensation of distention in women but not men |
| Gut microbiome and permeability | No evidence for sex-specific alterations in fecal or colonic mucosal microbiome in FGID |
| Gut inflammation | In IBS, colonic mucosal biopsies have more mast cells but fewer CD3+ and CD8+ T cells for women than men |
| HPA axis and autonomic responses | Men have greater HPA axis and sympathetic responses to stress than women |
| Serotonergic pathways | Higher postprandial plasma serotonin levels in female than male IBS patients and with elevated progesterone and estrogen levels compared with IBS patients with low hormone levels |
Abbreviations: FGID = functional gastrointestinal disorder; GI = gastrointestinal; HPA = hypothalamic-pituitary-adrenal; IBS = irritable bowel syndrome; M:F = male to female; FD = nonulcer dyspepsia.
Table 2.
Comparison of Sex-Related Differences in the Pathogenesis of Functional GI Tract Disorders, From Animal and Human Studies
| Sex-Related Differences |
| Characteristic | Animal Studies | Human Studies |
|---|---|---|
| Visceral sensation in health | Greater in female than male rodents 18 | Unclear18 |
| Effects of early life stress on visceral sensation | Increased perception of colonic distention in female but not male rats 93, in part through epigenetic mechanisms | Unknown |
| Stress-induced visceral hypersensitivity | More pronounced in female than male rodents; increased by estradiol and blocked by testosterone 104 | Hydrocortisone increased rectal sensation in healthy women but not men 101 |
| GI tract motility and transit | In rats, higher hormone levels were accompanied with slower colonic transit 44. Progesterone relaxes circular smooth muscle strips and reduced fecal output in rats with diarrhea 45 |
Slower gastric emptying and colon transit in healthy women than men42. Among women, variations in colonic transit during menstrual cycle were not significant 42. Progesterone accelerated colonic transit in postmenopausal healthy women 47 |
| Intestinal permeability | Estrogen-reduced colonic permeability in ovariectomized rats 113 | Increased psychological stress in healthy people; effects were not different between men and women 112 |
| Intestinal microbiome | Sex hormones selectively modified gut microbiome 117, 118 | No evidence of sex-related differences for fecal or colonic mucosal microbiome in IBS |
Abbreviations: GI = gastrointestinal; IBS = irritable bowel syndrome;
Sex-related differences in the characteristic.
Prevalence
Among 5,931 community adults in the United States, the United Kingdom, and Canada, the overall questionnaire-based prevalence of dyspepsia in 2018 showed a women to men ratio of 11:7.6 For adults older than 65 years, the prevalence was similar in men and women. Absent endoscopy, approximately 15% of these patients may have an organic explanation for their symptoms, such as peptic ulcer disease or erosive esophagitis.7 A meta-analysis of 55 studies showed that FD developed in 25% of women and 22% of men.8
In a systematic review of global, population-based studies that evaluated the prevalence of IBS, the disease was more common in women (14%) than men (9%) (odds ratio [OR], 1.67 [95% CI, 1.53–1.82]).9 In tertiary centers, the female to male ratio is generally higher, (eg, 3:1).10 In a systematic review of population-based studies dealing with the incidence of IBS, female sex was a risk factor in 19 of 31 studies, most of which were from the West.11 In the other 12 studies, of which 6 were from Taiwan, sex was not a risk factor for incident IBS.
GI Tract Symptoms
A meta-analysis of clinic-based studies of IBS found that constipation and diarrhea symptoms are respectively more common in women and men as follows: constipation predominant (women, 40%; men, 21%; OR, 2.38; 95% CI, 1.45–3.92) and diarrhea predominant (women, 31%; men, 50%; OR, 0.45; 95% CI, 0.32–0.65).9 The proportion with mixed IBS (ie, constipation and diarrhea) did not show a statistically significant difference between men and women. Selected symptoms—notably, bloating and/or abdominal distention—are more common and severe for women than men.2, 9, 12, 13 Women also are more likely to report harder stools, greater total somatic symptom burden, less sense of coping, general anxiety and GI tract–specific anxiety, and impaired QOL.12 Other characteristics were not different between men and women: depression, pain, stool frequency, effect on daily life, dissatisfaction with bowel habit, and extracolonic symptoms.12
The National Health and Nutrition Examination Survey defines chronic diarrhea as a predominance of mushy or watery stools but does not consider abdominal pain; hence, it characterizes functional diarrhea but not IBS–diarrhea type (IBS-D), which includes abdominal pain and loose stools.14 Approximately 6.6% of the US population had chronic diarrhea, defined as a predominance of mushy or watery stools. Adjusted for several other important variables (ie, ethnicity, education, depression, body mass index, and higher dietary carbohydrate intake), the prevalence of chronic diarrhea was greater (OR, 1.68; 95% CI, 1.28–2.21) in women (7.8%; 95% CI, 6.9%−8.8%) than men (5.4%; 95% CI, 4.3%−6.6%).14 Microscopic colitis causes diarrhea and is more common in women than men; it may partially explain the greater prevalence of chronic diarrhea in women than men.15, 16
Pelvic floor dysfunction (ie, defecatory disorders [DDs]) and endometriosis cause constipation and are more common in women than men. This distribution may explain at least partly the greater prevalence of IBS–constipation type (IBS-C) in women than men. Social norms for women equate thinness with attractiveness, which may at least partly explain why bloating is more common and bloating is a source of physical discomfort and psychological distress for women.17 A 2016 publication cited, “For many women, the sensation of being overweight, with or without an increase in the size of their abdomen, may evoke worry and shame about their body and therefore about themselves”.18 The physical and psychological distress caused by abdominal discomfort, together with the perception that their pain is minimized or trivialized by health care professionals, may prompt women to become more hypervigilant to any sign of pain or discomfort.
Sex-related differences in the prevalence of upper gastrointestinal symptoms seem smaller than corresponding differences in lower gastrointestinal symptoms. A population-based telephone survey of 21,128 adults from Olmsted County, Minnesota, showed that 26% of women and 20% of men reported early satiety and 12% of women and 7% of men had nausea.{Camilleri, 2005 #5586} The proportion of men and women is comparable with patients who have epigastric pain syndrome or postprandial distress syndrome, or both.{Aziz, 2018 #5835}
Extra–GI Tract Symptoms
Anxiety and Depression
FD is associated with major anxiety (OR, 2.56; 95% CI, 1.06–6.19) 19 and depression (OR, 2.28; 95% CI, 2.02–3.81).20 Compared with healthy control patients, IBS patients were roughly 3-fold more likely to have anxiety,21 which was more common in women (47.8% [95% CI, 36–59.6]) than men (31.5% [95% CI, 15.3–47.7]). To a lesser extent, depressive symptoms also were more common in women (35.1% [95% CI: 23–47.3]) than men (25.9% [95% CI: 11.9–9.9]). Perhaps, the greater anxiety and depression of women alter central pain processing and predispose to more severe GI tract symptoms in women. Indeed, anxiety and depression in women are associated with more severe GI and non-GI somatic symptoms and a lower QOL.22 Among patients with acute gastroenteritis, self-reported anxiety and depression were each associated with a 2-fold increased risk of IBS.23 Among community residents, persons with anxiety and/or depression were at increased risk for incident FD, FGID, or IBS; conversely, persons with FGID at baseline were at increased risk for anxiety and/or depression several years later.24, 25 These outcomes are not different in men compared with women. Together, these studies support a bidirectional relationship between anxiety and/or depression and the FGIDs. For women with IBS-C, the severity of anxiety was associated with abdominal discomfort and abdominal pain but not with abdominal bloating. For men with IBS-C, the severity of anxiety did not show a statistically significant association with abdominal bloating, discomfort, or pain.26
Fibromyalgia
More prevalent in women than men, fibromyalgia affects 26% to 65% of patients with IBS,27, 28 and 32% to 70% of fibromyalgia patients have IBS.27, 29 Among patients with fibromyalgia, IBS was less prevalent in men than women.30 Menstruating and postmenopausal women with IBS had significantly higher somatic symptom scores (eg, backache, headache, joint pain and muscle pain) than men with IBS.31 However, the risk of IBS is not different between men and women with fibromyalgia.32 In contrast, the association between FD and fibromyalgia is poorly characterized.
Other Conditions and QOL
The prevalence of sexual dysfunction (43%) is similar for men and women with FGIDs.33 A higher proportion of women (35%) than men (32%) with overactive bladder have concurrent IBS.34 A small proportion of patients with FGID, and more often women than men, have a history of 35 or an ongoing 36 eating disorder. Among patients with FD or IBS, physical and mental QOL is poorer for women than men 12, 37–39. These differences were limited to selected domains of IBSQoL (ie, emotional, energy, physical functioning, food, and sexual); mental health, sleep, social role, or physical role were not different between men and women with IBS.12 In population-based surveys, differences in sociodemographic and socioeconomic status partly explain lower health-related QoL scores in United States women than men.40 Further studies should compare sex-related differences in health-related QoL in the overall population and people with IBS.
Pathogenesis and Pathophysiologic Characteristics
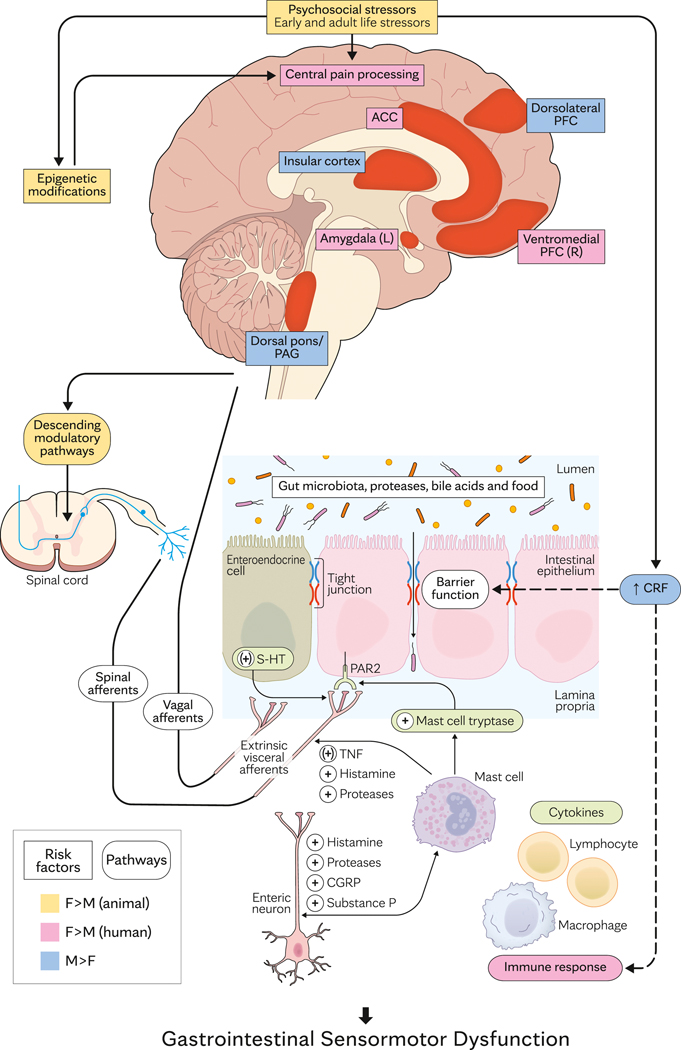
The biopsychosocial model integrates the contribution—and complex interaction—of psychosocial stressors, environmental factors, and diet to the pathogenesis of IBS and FD (Figure 2). These factors affect the GI tract neuromuscular apparatus, microbiota, and/or mucosal epithelial barrier, the central nervous system, and/or the interactions between the big and little brain, resulting in altered GI tract motility and/or sensation.
Figure 2.
Sex- and Gender-Based Differences in the Pathogenesis of Common Functional GI Tract Disorders. Peripheral disturbances (ie, postinfectious irritable bowel syndrome) and central factors (eg, psychological stressors) cause GI tract sensory and motor dysfunctions that contribute to symptoms. The peripheral mechanisms include triggers (eg, postinfectious inflammation, bile acids) that stimulate enterochromaffin cells, may change mucosal permeability, and activate immune mechanisms to stimulate afferent nerves. Central sensitization may result from this peripheral sensitization and/or reduced descending inhibition, which normally gates visceral sensation in the spinal cord. Sex-related differences in activation of brain centers and mechanisms are shown in colors. Difference between male and female sexes in humans are shown in blue and dark pink; corresponding differences only observed in animal models are shown in olive. ACC indicates anterior cingulate cortex; CGRP = calcitonin gene-related peptide; CRF = corticotropin-releasing factor; 5-HT = serotonin; GDNF = glial cell line–derived neurotrophic factor; GI = gastrointestinal; HPA = hypothalamic-pituitary-adrenal; IFNγ = interferon gamma; L = left; PAG = periaqueductal gray; PAR2 = proteinase-activated receptor 2; PFC = prefrontal cortex; R = right; TNF, tumor necrosis factor.
GI Tract Sensorimotor Dysfunctions
While the symptoms of FD and IBS define them, patients also have GI tract sensorimotor dysfunctions, which are associated with the presence and severity of selected symptoms. In the largest series of FD patients (N=560) so far, 37%, 37%, and 23% of patients had gastric hypersensitivity, impaired gastric accommodation, and delayed gastric emptying, respectively.41 Among 407 IBS patients from 3 medical centers, 81% had 1 or more GI tract–related disturbances, including allodynia, hyperalgesia, accelerated or delayed oroanal transit, anxiety, and depression.10 In that study, the number of pathophysiologic abnormalities was associated with the symptom severity but not with the patient’s sex.
GI Tract Transit
Gastric emptying and colonic transit are slower and the intraindividual variation of colonic transit is greater for healthy women than men.42 Female sex is an independent risk factor for delayed gastric emptying for patients with FD.43
In studies of adult female rats, higher hormone levels during proestrus-estrus in nonpregnant rats and in pregnant rats are accompanied by slower colonic transit and vice versa.44 Progesterone (P4) relaxes circular smooth muscle strips and reduces fecal output in rats with diarrhea.45 In contrast to rats, variations in colonic transit across the menstrual cycle in humans are not statistically significant.42 Among women with slow-transit constipation, results of small studies have been suggestive that in vitro colonic contractile response to G protein–independent agonists (eg, acetylcholine, cholecystokinin) is impaired because of the downregulation of G proteins that mediate contraction (eg, Gq/11 protein).46 In addition, G proteins that mediate relaxation are upregulated, which may predispose to colonic relaxation. Both these changes in Gq/11 and Gs proteins are associated with overexpression of P4 receptors in the colon, perhaps rendering its muscle cells more sensitive to physiologic P4 concentrations. They can be induced by pretreating normal colonic muscle cells with P4 for 4 hours. However, among 49 healthy postmenopausal women, P4 (400 mg daily for 7 days) accelerated colonic transit.47
The effects of estrogen on gastrointestinal motility are mediated by the, predominantly nuclear, α and β receptors and the more recently described 7-transmembrane G-protein coupled estrogen receptors (GPER), which are located in the myenteric plexus throughout the gastrointestinal tract including the colon.48–50 While nuclear:cytoplasmic ratio of ER-α ισ greater in male than female mice, the distribution of ER-β and GPER is similar in male and female mice.48 Stimulation of GPER elicits a response within seconds to minutes. By contrast, the responses to stimulation of ER-α and ER-β, which initiate transcription and translation, are delayed in onset and prolonged in duration. G-1, which is a selective GPER agonist, and 17β-estradiol, which also binds to ER-α and ER-β, inhibit colonic response to electric field stimulation motility in mice and humans.50 Both agonists also inhibited in vivo colonic motility in mice. By contrast, treatment with micronized estradiol for 7 days induced lose stools but did not affect colonic transit in healthy people.47 To our knowledge, the physiologic effects of estrogen on colonic motility in humans have not been investigated (eg with a selective estrogen receptor disruptor or modulator). In most but not all animal models, stimulation of GPER increases visceral sensitivity.48
Current etiologic concepts implicate slow colon transit to a loss of colonic nerves and interstitial cells of Cajal.51, 52 More investigation is necessary to determine the contribution of gonadal hormones to slower colon transit in women and men and to slow transit constipation.
Visceral Sensation
Studies have shown that sex hormones affect pain processing in humans.53–55 Visceral pain is often regarded as more unpleasant and is more difficult to localize than somatic pain.56 Visceral hypersensitivity consists of 2 components: allodynia, in which an innocuous stimulus is perceived as painful, and hyperalgesia, defined as a more intense perception of a painful stimulus. 10 Patients with visceral hypersensitivity have more severe IBS symptoms.10, 57 A majority of patients in these studies were women.
Among IBS patients, bowel symptoms and rectal sensation are more intense during menses than during the follicular, luteal, or premenstrual phase.58 Other, small studies suggest that bowel symptoms are worse during menses than other phases of the menstrual cycle for women with IBS.59 The UK General Practice Research Database indicated that postmenopausal women who were being or had been treated with hormone replacement had increased risk of IBS compared with nonusers.60 However, the data are conflicting on the perception of esophageal, duodenal, and rectal distention of male and female healthy persons vs patients with FGIDs.18 This inconsistency is likely due to differences in the patients’ IBS types, rectal distention techniques, ovarian hormone and receptor levels, and levels of stress among study participants. In particular, few studies controlled for the menstrual phase, which, as explained above,58 affects rectal sensation in IBS patients. A wide range of menstrual cycle phases, different definitions of the same phase, and lack of cycle phase confirmation by hormone measures limit the understanding of the relationship between menstrual cycle and pain outside the GI tract.53, 54, 61
Plasma hormone measurements allow for a more refined assessment of the relationship between gonadal hormones and sensation.62 Assessments of somatic sensation (ie, pinprick pain sensitivity, incision-induced pain, and pinprick hyperalgesia) were significantly correlated with plasma P4 and follicle-stimulating hormone and negatively correlated with testosterone in humans. By contrast, few studies have evaluated the relationship between sex, gender, and visceral (gastrointestinal) sensation using such refined techniques. While plasma luteinizing hormone levels were significantly lower in male IBS patients than control patients,63 the differences between male IBS patients and healthy male patients were small. Among IBS patients, rectal sensory thresholds inversely correlated with serum testosterone levels, a result that is counterintuitive since plasma testosterone is protective against somatic pain.62
Assessment of cortical activation or regional changes in cerebral blood flow with functional magnetic resonance imaging or positron emission tomography, respectively, provide insights into the central processing of visceral pain.56 These studies assessed cortical activation at rest and during balloon distention, cortical thickness, and functional connectivity. The spinal and vagal afferents from the GI tract indirectly project to the thalamus, insula, amygdala, prefrontal cortex, primary somatosensory cortex, secondary somatosensory cortex, and cingulate cortices, including the anterior cingulate cortex.56 The somatosensory cortices, the lateral pain system, serve to encode the intensity and location of visceral pain. The cingulate cortex, or the medial pain system, is implicated in the emotional interpretation of the stimulus—specifically, the affective-motivational aspect (pain unpleasantness and related anxiety) and the cognitive-evaluative aspect (anticipation and attention of pain). The insula serves to process of the affective dimension of pain, integrate it with emotional information, and inform the amygdala, hypothalamus, and periaqueductal gray. The amygdala is critical to affective dimension, especially fear, and painful sensation. Together with the periaqueductal gray, the amygdala has a key role in the descending modulation of pain. These studies have uncovered the following salient sex-based differences in the central processing of pain.
At rest, the female brain allocates greater resources to interoceptive awareness (ie, to stimuli originating in the body), whereas the male brain relies more on cognitive function.64
The patterns of central activation during rectal distention and the differences between healthy men and women differ among studies.65–67
The largest study of IBS patients, comprising 42 patients, observed greater activation of the ventromedial prefrontal cortex, right anterior cingulate cortex, and left amygdala in women and greater activation of the right dorsolateral prefrontal cortex, insula, and dorsal pons and periaqueductal gray in men (Figure 1).68 These findings suggest greater activation of the affective and autonomic regions in women and greater activation of regions belonging to a corticolimbic pain inhibition system in men. Similar differences were observed in the anticipation of distention.
Connectivity analysis of the same data suggested that differences in the effective connectivity of emotional arousal circuitry, rather than visceral afferent processing circuitry, underpin the differences between men and women.69
The right pregenual anterior cingulate cortices are thinner but the bilateral anterior insula is thicker in women than healthy men.70 Another study of healthy volunteers found that increased visceral sensitivity to rectal distension correlates with decreased gray matter volume in the brain’s pain processing regions—thalamus, insula, cingulate cortex, ventrolateral and orbitofrontal prefrontal cortices, amygdala, and basal ganglia.71
These sex-related differences are detailed elsewhere.72 One exception 68 is the small studies with 15 or fewer IBS patients.73 Many studies enrolled only men or only women. Another recent study observed that “the published literature on sex differences in brain activation by visceral stimuli is still sparse and somewhat contradictory. Although many reviews emphasized the importance of considering sex-related differences, no conclusive studies have been performed”.74
In rodents, the effects of sex hormones on visceral sensation have been studied through investigation of changes during the estrus cycle and after ovariectomy followed by replacement of estrogen and progesterone.18 The estrus cycle is shorter (4–6 days) and changes in plasma estrogen and P4 levels are smaller in rodents than in humans. The variations in visceral sensation during the estrus cycle are subtle and differ among studies. Estrogen has pro- and antinociceptive effects. When considered together, these observations suggest that sex steroids do not contribute substantially to visceral hypersensitivity in patients with IBS.
Acute Gastroenteritis
Acute gastroenteritis is the most important risk factor for IBS and, to a lesser extent, FD in children 75 and adults.76 After infectious enteritis, IBS symptoms occur in 10.1% (95% CI, 7.2–14.1) at 12 months and 14.5% (95% CI, 7.7–25.5) at more than 12 months.76, 77 Since 1 in 6 persons, or a total of 48 million people, in the United States have acute infectious gastroenteritis every year, postinfectious IBS probably accounts for a substantial proportion of new cases of IBS.
Female sex, younger age, psychological distress during or before acute gastroenteritis, and more severe acute enteritis are risk factors for postinfectious IBS.77 Unknown is whether female sex is an independent risk factor for postinfectious IBS after correction for other risk factors, especially psychosocial stressors. Only 2 studies included psychosocial stressors in the multivariate analysis. In those 2 studies, sex was not a risk factor for development of IBS.78, 79
The mechanism(s) that predispose to a greater rate of developing postinfectious IBS after acute gastroenteritis are unclear. Compared with male IBS patients, female IBS patients had more gastrointestinal mucosal mast cells, which were correlated with the severity of GI tract symptoms, but fewer CD3+ and CD8+ T cells.80 Perhaps these differences are at least partly explained by sex-related differences in the immunologic profile that are mediated by sex hormones, contributions of X chromosome genes, and environmental factors. Indeed, women have a greater prevalence of various autoimmune diseases, including Sjögren syndrome, scleroderma, rheumatoid arthritis, systemic lupus erythematosus, and multiple sclerosis.81 Another possible mechanism is that acute diarrhea is more likely to increase rectal sensitivity, which is a feature of IBS, in women than men.82.
The relationship between sex and the risk of postinfectious FD is unclear. The sex distribution of patients with postinfectious and unspecified-onset FD is comparable 83 or different.84 A meta-analysis of risk factors for postinfectious FD did not study sex.85 Some but not all studies have observed duodenal mucosal inflammation in FD.86, 87
In summary, whether female sex is an independent risk factor for developing postinfectious IBS and FD is unknown. The mechanisms by which female sex may predispose to development of these diseases after acute gastroenteritis are poorly understood.
Psychosocial Stressors
Psychosocial stressors include environmental factors and psychological distress (ie, mood disorders, anxiety, somatization, and cognitive-affective processes) that influence the brain and the gut and are associated with FGIDs. Environmental factors include social constructs and behavior, parenteral anxiety, depression, somatization, and adverse early life experiences (ie, physical, sexual, and emotional abuse), which are all strongly associated with abdominal symptoms.88 Gender-related social or societal expectations such as standards for attractiveness, norms for women’s caretaking role in relationships, and sanctions against anger expression by women can impair health and well-being.18 Among IBS patients, women described shame because they were not living up to gender norm expectations for women in the domains of relationships (taking care of others at the expense of their own needs), attractiveness (because of bloating), and lack of desire to engage in sex.89 Men were more concerned about the effect of IBS symptoms on paid employment and sense of control. Although men also were embarrassed by IBS symptoms, “they had a more relaxed attitude to feces and passing wind” than women.93 During health care visits, women risked being trivialized and men risked being overlooked because IBS may be regarded as a health concern for women.
During childhood, children whose mothers reinforce illness behavior have more severe stomach aches and more school absences than other children.88 More common in persons with IBS than healthy control persons, physical punishment, emotional abuse, and sexual abuse, which often coexist, are related to the severity and clinical outcomes of FGIDs. They may lead to increase health care seeking, which might explain the greater prevalence of abuse in tertiary care than primary care. In a large community-based study from Sweden, the prevalence of childhood and adulthood abuse was more common in women who had FGID (45%) than women in the control group without GI tract symptoms (16%).90 In contrast, the corresponding prevalence was not different in men with FGID (29%) vs without it (24%). Likewise, at a tertiary center, the prevalence of abuse was greater among female IBS patients than control patients compared with male patients vs control patients.91 In meta-analyses, a history of sexual abuse has been associated with FGIDs, nonspecific chronic pain, chronic pelvic pain, and psychogenic seizures but not with fibromyalgia or headache.92 Yet, most of these studies were conducted with adult female participants. The extent to which gender affects the relationship between stressful events, abuse, and IBS is unknown.
In contrast, studies of animals suggest that the effects of early life stress (ELS) on visceral sensation are modified by sex and gender. ELS, and especially unpredictable stress, induced visceral hypersensitivity during colonic distention in female but not male rats.93 This phenomenon may be partly mediated by epigenetic changes associated with chronic prenatal stress 94 and neonate-maternal stress 95 that cause visceral hypersensitivity.94, 96 Chronic prenatal stress induced visceral hypersensitivity that was markedly greater in female than male rats. It was attributed to increased H3 acetylation and expression of spinal cord brain-derived neurotrophic factor (BDNF) in female but not male offspring.94 Of interest, abnormal activation occurred to the amygdala, which regulates visceral sensitivity, after ELS, and in IBS patients; gender differences have not been evaluated.93, 97
ELS increased the expression of corticotrophin-releasing factor (CRF) and glucocorticoid receptors in the amygdala, which modulate nociception and stress, in female but not male rats.93 This sex-based interaction may be at least partly explained by estrogen. This hormone preferentially binds to the CRF promotor region and increases CRF expression, increasing visceral hypersensitivity.93 In adult rats, the ELS-induced visceral hypersensitivity can be reversed through elimination of estrogen cycling and restored with reintroduction of estrogen.98
Effects of Stress
Stress activates the hypothalamic-pituitary-adrenal (HPA) axis. The cortisol response to mental stressors is greater in healthy men than healthy women.99 In response to psychosocial stress, the adrenocorticotropic hormone (ACTH) response was greater in men than in women regardless of their menstrual cycle,100 and the salivary cortisol response to ACTH was comparable in men and women in the luteal phase.99, 100 In both groups, the response was greater than in women during the follicular phase.99, 100
Mediators of stress probably participate in the pathophysiologic characteristics and persistence of chronic pain. Supportive of this concept, hydrocortisone increased the sensitivity for visceral (ie, during rectal distention) but not somatic pain in women vs men.101 However, ratings for pain and unpleasantness were unaffected by hydrocortisone. These findings suggest that cortisol primarily affects visceral sensory-discriminatory aspects, or pain sensitivity, rather than cognitive-evaluative or affective pain components (ie, ratings of intensity and unpleasantness) for healthy persons.
Among IBS patients, the autonomic response (ie, increased sympathetic and decreased parasympathetic activity) to rectosigmoid distension was more pronounced in men than women.102 Unclear is whether the exaggerated autonomic response in men, which was not correlated with the severity of daily symptoms or rectal sensory thresholds, contributed to and/or was a consequence of abdominal discomfort. One large study compared sex differences in responses to CRF and ACTH among healthy control patients and patients with IBS.103 Compared with sex-matched healthy controls, the CRF-induced ACTH release and the ACTH-evoked cortisol response was increased in male IBS patients and decreased in women with IBS.103 The mRNA expression of glucocorticoid receptors in peripheral blood mononuclear cells was less in men but not in female IBS patients vs control patients and was correlated inversely with the severity of IBS symptoms. Whether the reduced mRNA expression of glucocorticoid receptors is a cause or a consequence of activation of the HPA axis is unknown.
Stress-induced visceral hypersensitivity is more pronounced in female rats than male rats, blocked by ovariectomy, and increased by orchiectomy.104 Altered expression of glutaminergic receptors and BDNF may explain the opposing effects of estrogen and testosterone on stress-induced visceral hypersensitivity.104 In summary, intravenous hydrocortisone increased rectal sensitivity in healthy women. The CRF-induced ACTH release and the ACTH-evoked cortisol response were increased for male IBS patients and decreased for women with IBS. In contrast to animal models, no data confirm that the gonadal hormones influence the response to stress or stress-induced visceral sensitivity for healthy people or those with IBS.
Serotoninergic Pathways and the Brain-Gut Axis
Mechanical and chemical stimuli release serotonin from enteric neurons and mucosal enterochromaffin cells. Serotonin initiates motor reflexes and visceral sensation. Sex-related differences in GI tract serotoninergic pathways and the brain-gut axis may contribute to differences among the sexes in IBS. Some 105, 106 but not all 107 studies suggest that the colonic mucosal expression of serotonin transporter (SERT), which terminates serotonergic signaling, is reduced in IBS patients. Reduced SERT expression could predispose to higher serotonin levels and hence to faster colonic transit and greater visceral pain. However, the correlations between mucosal SERT expression, transit, and sensation have not been assessed in humans. Increased colonic extracellular serotonin in female SERT knockout rats is associated with visceral hypersensitivity and hyperexcitability of colon projecting sensory neurons.108 Postprandial plasma serotonin levels were higher in female IBS patients with elevated progesterone and estrogen levels compared with IBS patients with low hormone levels.109 Serotonin turnover is reduced in female IBS patients and elevated serotonin levels may be due to defects in uptake or metabolism.109 In summary, IBS is associated with serotoninergic disturbances, the functional importance of which is unclear.
Intestinal Permeability, Foods, and the Microbiome
Conceptually, increased intestinal permeability in IBS may reflect the sustained immune and inflammatory response that is characteristic of postinfectious IBS and/or long-term intermittent psychological stress, which in male Fischer rats activates release of proinflammatory cytokines to alter tight junction proteins and increase gut permeability.110 Although some patients with postinfectious IBS have increased small intestine permeability, this parameter is not useful for discriminating between healthy control patients and patients with IBS-C or IBS-D.111 Acute psychological stress increased intestinal permeability through corticotropin-releasing hormone and mast cell–dependent mechanism studies in animals and healthy people.112 In that study of 23 healthy volunteers, the effects of acute psychological stress were comparable among men and women. Larger studies are necessary because estrogen reduces colonic permeability through estrogen receptor β and the upregulation of tight junction proteins in ovariectomized rats.113
Certain foods cause GI tract symptoms either directly or after microbiome-induced fermentation of carbohydrates to short-chain fatty acids 114 or metabolism with bile acids.115 Moreover, the diet can modify the gut microbiome. Although the gut microbiome differs between IBS patients and control patients, no specific microbial signature is associated with IBS.116 In addition, sex hormones can alter the gut microbiome in selected mice strains,117,118 yet no evidence shows sex-specific differences in the fecal or colonic mucosal microbiome in IBS.
Genetic Alterations Associated With FGIDs
Family and twin studies suggest there is a heritable component to IBS.119, 120 In a sample of 45,750 Swedish twins, investigators saw no evidence of sex differences in heritability of IBS.121 Subsequently, a multinational study observed a significant association between chromosome 9q31.2 (single-nucleotide polymorphism rs10512344) and women with IBS-C in some countries.122 Intriguingly, this locus is also associated with other conditions and traits (eg, voice breaking in males, body mass index, breast and prostate cancer; GWAS catalog www.ebi.ac.uk/gwas/) are influenced by hormonal stimuli and sex hormones. Further studies are necessary to understand the specific gene(s) at this locus that predispose to IBS, possibly via biological mechanisms that are regulated by sex hormones.
Reporting of Pain, Interpersonal Connections, and Health Care–Seeking Behavior
Men manage their pain through self-distraction and problem-focused challenges, whereas women resort to emotion-focused tactics, catastrophizing, positive self-statements, and social support.123 Expression of pain is socially more acceptable among women,124 whereas men show increased pain tolerance and greater resistance in reporting pain.125 These differences may at least partly explain why women with IBS are more likely to seek health care than men with IBS.126, 127
Since women attach more importance to interpersonal relationships than men,128 perhaps female IBS patients might regard their relationships as more problematic (eg, higher levels of interpersonal problems) than men with IBS. To the contrary, among IBS patients, men reported more interpersonal difficulties (ie, less support from others and more interpersonal problems involving hostile-dominant behaviors) than women with IBS.129 These interpersonal difficulties may influence the estimation of IBS symptoms by physicians, especially among men, and may undermine the quality of the physician-patient relationship.
Conditions That Are Much More Frequent or Occur in Women Only
Defecatory Disorders
Resulting from impaired coordination between rectal propulsive force and relaxation of the anal sphincter and pelvic floor muscles, DDs are a common cause of constipation and are more common in women than men in both clinical practice and the community. Because symptoms of a DD are similar to functional constipation and IBS-C, this condition is overlooked unless anorectal tests are performed. Functional constipation, IBS-C, and DDs are more common among women than men.130 The age-adjusted incidences per 100 000 person-years for women and men are 31.8 (95% CI, 27.4–36.1) and 6.6 (95%CI, 4.4–8.9), respectively.
Endometriosis
Up to 80% of women who present with chronic pelvic pain (defined as noncyclic lower abdominal pain lasting at least 6 months) have endometriosis.3 The typical presenting symptoms include perimenstrual lower abdominal pain and dyspareunia; dysuria, urinary urgency, hematuria are other symptoms. Patients also may have pelvic floor dysfunction with symptoms of a DD. The diagnosis is confirmed with laparoscopy-guided biopsy.
Effect of Sex on Response to Treatment of Functional GI Tract Disorders
Perhaps reflective of the predominance of female patients in clinical practice, most patients enrolled in the pharmacologic and behavioral trials of FD and IBS were, by chance or design, women (Table 3). Hence, our understanding of the efficacy of several drugs and potential sex-based differences in the response to treatment are limited and possibly are biased. For example, on the basis of early drug trials conducted in the United States (alosetron) and Japan (ramosetron), investigators inferred that the serotonin (5-HT3) receptor antagonists ondansetron and ramosetron were more effective for patients with IBS-D, respectively, for women and men.131 Thereafter, ramosetron was shown to be effective for women with IBS-D—but at one-half the dose for men.131 Likewise, alosetron delayed colonic transit to a greater extent for women than men.132 For the other drug treatments and for behavioral therapy, differences in efficacy have not been assessed or observed for men compared with women. Some drugs are approved only for women (eg, alosetron for IBS-D). Although with limited data, other drugs (eg, lubiprostone) are approved for use in men and women for chronic idiopathic constipation but only in women for IBS-C.
Table 3.
Relationship Among Sex, Gender, and Treatment Efficacy for Commonly Used Pharmacologic and Behavioral Therapies for FD and IBS in the United States
| Therapy | Men and/or Women | Differences in Efficacy Between Men and Womena |
|---|---|---|
| Pharmacologic | ||
| IBS-D | ||
| Alosetron (5-HT3 receptor antagonist) | In large trials, 81% women 133 | Retards small bowel and colonic transit to greater extent in women than men 132. Approved for use in women only, under a restricted marketing program |
| Ondansetron | Approximately 75% women 134 | Not assessed |
| Ramosetron | Men 135 and women 131 | In women, effective dose was one-half that in men |
| Bile acid sequestrant colesevelam | Approximately 92% women 136 | Not assessed |
| Loperamide (μ-opioid receptor agonist) | Men and women 137, 138 | Not assessed |
| Eluxadoline (μ- and κ-opioid receptor agonist) | Two-thirds women 145. | Approved for use, no significant differences between men and women |
| IBS-C and chronic constipation | ||
| Fiber | Meta-analysis of 6 RCTs in chronic idiopathic constipation 139; 65%−100% women. An RCT had 78% women 140 | Not assessed |
| PEG | 85% women 141 | None |
| Laxatives: prucalopride, lubiprostone, and linaclotide | Meta-analysis; predominantly women 142 | Not assessed |
| Stimulant and nonstimulant laxatives | Systematic review; sex distribution not provided 143 | Not assessed |
| Bisacodyl | 75% women 144 | Not assessed |
| Prucalopride | Review of all trials. In the 3 pivotal trials, 85% women. Later, another trial enrolled 100% men 145 | Efficacious in men and women |
| Guanylate cyclase-C agonists (linaclotide and plecanatide) | Systematic review 146; most were women | Not assessed |
| Tegaserod (5-HT4 agonist) | Systematic review 133; 83%−100% women in all but 1 study, which had 100% men147 | More extensively evaluated, hence approved for IBS-C in women; limited data suggest that it has more efficacy in men 133 |
| FD | ||
| Helicobacter pylori eradication therapy | Men and women 148; distribution varied among studies | Not assessed |
| PPI therapy | Men and women 149; distribution varied among studies | Not assessed |
| Amitriptyline | 75% women 150 | No significant differences between men and women |
| Prokinetics | Men and women 151, 152; distribution varied among studies | Not assessed |
| Behavioral | ||
| CBT | Women only 153 | |
| CBT | Approximately 80% women 154, 155 | Gender did not affect response to CBT of IBS patients 154; not assessed in second study 151 |
| Hypnotherapy | Men and women, variable response, 62%–87% women 156 | Not assessed |
Abbreviations: CBT = cognitive behavioral therapy; 5-HT = serotonin; IBS = irritable bowel syndrome; IBS-C = irritable bowel syndrome–constipation type; IBS-D, irritable bowel syndrome–diarrhea type; PEG = polyethylene glycol; PPI = proton pump inhibitor; RCT, randomized controlled trial.
Conclusions
Several sex- and gender-related differences exist in the prevalence and clinical features, pathophysiologic characteristics, and management of FD and IBS for women compared with men. The understanding of sex-based differences in the pathogenesis of FGIDs lags behind the understanding of these mechanisms in animals. Additional research into the gaps detailed in the tables not only will provide more insight into the differences between the sexes but more broadly will enhance the understanding of FGIDs, especially the mechanism(s) by which adverse early life experiences predispose to IBS, sex-based differences in the evolution of acute gastroenteritis to postinfectious IBS, the contribution of sex steroids to normal and disordered gastrointestinal sensorimotor functions, and sex-related differences in the response to treatment in these diseases.
Supplementary Material
Acknowledgments:
This study was supported in part by US Public Health Service National Institutes of Health (NIH) Grant R01 DK78924 from the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- ACTH
adrenocorticotropic hormone
- BDNF
brain-derived neurotrophic factor
- CRF
corticotropin-releasing factor
- DD
defecatory disorder
- ELS
early life stress
- FGID
functional gastrointestinal disorder
- FD
functional dyspepsia
- GI
gastrointestinal
- HPA
hypothalamic-pituitary-adrenal
- IBS
irritable bowel syndrome
- IBS-C
irritable bowel syndrome–constipation type
- IBS-D
irritable bowel syndrome–diarrhea type
- OR
odds ratio
- P4
progesterone
- QOL
quality of life
- SERT
serotonin transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aziz I, Palsson OS, Tornblom H, Sperber AD, Whitehead WE, Simren M. The Prevalence and Impact of Overlapping Rome IV-Diagnosed Functional Gastrointestinal Disorders on Somatization, Quality of Life, and Healthcare Utilization: A Cross-Sectional General Population Study in Three Countries. American Journal of Gastroenterology. 2018;113:86–96. [DOI] [PubMed] [Google Scholar]
- 2.Jiang X, Locke GR 3rd, Choung RS, Zinsmeister AR, Schleck CD, Talley NJ. Prevalence and risk factors for abdominal bloating and visible distention: a population-based study. Gut. 2008;57:756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharucha AE, Chakraborty S, Sletten CD. Common Functional Gastroenterological Disorders Associated With Abdominal Pain. Mayo Clinic Proceedings. 2016;91:1118–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharucha AE, Lee TH. Anorectal and Pelvic Pain. Mayo Clin Proc. 2016;91:1471–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkley KJ, Holdcroft A. Sex and Gender differences in pain. In: Wall PD, ed. Textbook of pain. 4 ed. Edinburgh: Churchill Livingstone; 2000:951–965. [Google Scholar]
- 6.Aziz I, Palsson OS, Tornblom H, Sperber AD, Whitehead WE, Simren M. Epidemiology, clinical characteristics, and associations for symptom-based Rome IV functional dyspepsia in adults in the USA, Canada, and the UK: a cross-sectional population-based study. The lancet. Gastroenterology & hepatology. 2018;3:252–262. [DOI] [PubMed] [Google Scholar]
- 7.Ford AC, Marwaha A, Lim A, Moayyedi P. What is the prevalence of clinically significant endoscopic findings in subjects with dyspepsia? Systematic review and meta-analysis. Clinical Gastroenterology & Hepatology. 2010;8:830–837, 837.e831–832. [DOI] [PubMed] [Google Scholar]
- 8.Ford AC, Marwaha A, Sood R, Moayyedi P. Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta-analysis. Gut. 2015;64:1049–1057. [DOI] [PubMed] [Google Scholar]
- 9.Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. American Journal of Gastroenterology. 2012;107:991–1000. [DOI] [PubMed] [Google Scholar]
- 10.Simren M, Tornblom H, Palsson OS, Van Oudenhove L, Whitehead WE, Tack J. Cumulative Effects of Psychologic Distress, Visceral Hypersensitivity, and Abnormal Transit on Patient-reported Outcomes in Irritable Bowel Syndrome. Gastroenterology. 2019;157:391–402.e392. [DOI] [PubMed] [Google Scholar]
- 11.Creed F. Review article: the incidence and risk factors for irritable bowel syndrome in population-based studies. Alimentary Pharmacology & Therapeutics. 2019;50:507–516. [DOI] [PubMed] [Google Scholar]
- 12.Bjorkman I, Jakobsson Ung E, Ringstrom G, Tornblom H, Simren M. More similarities than differences between men and women with irritable bowel syndrome. Neurogastroenterology & Motility. 2015;27:796–804. [DOI] [PubMed] [Google Scholar]
- 13.Schmulson M, Adeyemo M, Gutierrez-Reyes G, et al. Differences in gastrointestinal symptoms according to gender in Rome II positive IBS and dyspepsia in a Latin American population. The American journal of gastroenterology. 2010;105:925–932. [DOI] [PubMed] [Google Scholar]
- 14.Singh P, Mitsuhashi S, Ballou S, et al. Demographic and Dietary Associations of Chronic Diarrhea in a Representative Sample of Adults in the United States. American Journal of Gastroenterology. 2018;113:593–600. [DOI] [PubMed] [Google Scholar]
- 15.Kamp EJ, Kane JS, Ford AC. Irritable Bowel Syndrome and Microscopic Colitis: A Systematic Review and Meta-analysis. Clinical Gastroenterology & Hepatology. 2016;14:659–668.e651; quiz e654–655. [DOI] [PubMed] [Google Scholar]
- 16.Kane JS, Rotimi O, Everett SM, Samji S, Michelotti F, Ford AC. Development and validation of a scoring system to identify patients with microscopic colitis. Clinical Gastroenterology & Hepatology. 2015;13:1125–1131. [DOI] [PubMed] [Google Scholar]
- 17.Cheney AM. “Most girls want to be skinny”: body (dis)satisfaction among ethnically diverse women. Qualitative Health Research. 2011;21:1347–1359. [DOI] [PubMed] [Google Scholar]
- 18.Heitkemper M, Houghton LA, Crowell M, et al. Age, Gender, and Women’s Health and the Patient. In: Drossman D, ed. Rome IV. Functional Gastrointestinal Disorders. Disorders of Gut-Brain Interaction. Vol 1. Raleigh, NC: The Rome Foundation; 2016:307–372. [Google Scholar]
- 19.Aro P, Talley NJ, Ronkainen J, et al. Anxiety is associated with uninvestigated and functional dyspepsia (Rome III criteria) in a Swedish population-based study. Gastroenterology. 2009;137:94–100. [DOI] [PubMed] [Google Scholar]
- 20.Lin S, Gao T, Sun C, Jia M, Liu C, Ma A. The association between functional dyspepsia and depression: a meta-analysis of observational studies. European Journal of Gastroenterology & Hepatology. 2019;31:911–918. [DOI] [PubMed] [Google Scholar]
- 21.Zamani M, Alizadeh-Tabari S, Zamani V. Systematic review with meta-analysis: the prevalence of anxiety and depression in patients with irritable bowel syndrome. Alimentary Pharmacology & Therapeutics. 2019;50:132–143. [DOI] [PubMed] [Google Scholar]
- 22.Midenfjord I, Polster A, Sjovall H, Tornblom H, Simren M. Anxiety and depression in irritable bowel syndrome: Exploring the interaction with other symptoms and pathophysiology using multivariate analyses. Neurogastroenterology & Motility. 2019;31:e13619. [DOI] [PubMed] [Google Scholar]
- 23.Sibelli A, Chalder T, Everitt H, Workman P, Windgassen S, Moss-Morris R. A systematic review with meta-analysis of the role of anxiety and depression in irritable bowel syndrome onset. Psychological Medicine. 2016;46:3065–3080. [DOI] [PubMed] [Google Scholar]
- 24.Koloski NA, Jones M, Kalantar J, Weltman M, Zaguirre J, Talley NJ. The brain--gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut. 2012;61:1284–1290. [DOI] [PubMed] [Google Scholar]
- 25.Aro P, Talley NJ, Johansson SE, Agreus L, Ronkainen J. Anxiety Is Linked to New-Onset Dyspepsia in the Swedish Population: A 10-Year Follow-up Study. Gastroenterology. 2015;148:928–937. [DOI] [PubMed] [Google Scholar]
- 26.Kosako M, Akiho H, Miwa H, Kanazawa M, Fukudo S. Impact of symptoms by gender and age in Japanese subjects with irritable bowel syndrome with constipation (IBS-C): a large population-based internet survey. BioPsychoSocial medicine. 2018;12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sperber AD, Atzmon Y, Neumann L, et al. Fibromyalgia in the irritable bowel syndrome: studies of prevalence and clinical implications. The American journal of gastroenterology. 1999;94:3541–3546. [DOI] [PubMed] [Google Scholar]
- 28.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140–1156. [DOI] [PubMed] [Google Scholar]
- 29.Veale D, Kavanagh G, Fielding JF, Fitzgerald O. Primary fibromyalgia and the irritable bowel syndrome: different expressions of a common pathogenetic process. British journal of rheumatology. 1991;30:220–222. [DOI] [PubMed] [Google Scholar]
- 30.Yunus MB, Inanici F, Aldag JC, Mangold RF. Fibromyalgia in men: comparison of clinical features with women. The Journal of rheumatology. 2000;27:485–490. [PubMed] [Google Scholar]
- 31.Cain KC, Jarrett ME, Burr RL, Rosen S, Hertig VL, Heitkemper MM. Gender differences in gastrointestinal, psychological, and somatic symptoms in irritable bowel syndrome. Digestive Diseases & Sciences. 2009;54:1542–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang TY, Chen CS, Lin CL, Lin WM, Kuo CN, Kao CH. Risk for irritable bowel syndrome in fibromyalgia patients: a national database study. Medicine. 2015;94:e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fass R, Fullerton S, Naliboff B, Hirsh T, Mayer EA. Sexual dysfunction in patients with irritable bowel syndrome and non-ulcer dyspepsia. Digestion. 1998;59:79–85. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto S, Hashizume K, Wada N, et al. Relationship between overactive bladder and irritable bowel syndrome: a large-scale internet survey in Japan using the overactive bladder symptom score and Rome III criteria. BJU international. 2013;111:647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porcelli P, Leandro G, De Carne M. Functional gastrointestinal disorders and eating disorders. Relevance of the association in clinical management. Scandinavian journal of gastroenterology. 1998;33:577–582. [DOI] [PubMed] [Google Scholar]
- 36.Murray HB, Bailey AP, Keshishian AC, et al. Prevalence and Characteristics of Avoidant/Restrictive Food Intake Disorder in Adult Neurogastroenterology Patients. Clinical Gastroenterology & Hepatology. 2019;24:24. [DOI] [PubMed] [Google Scholar]
- 37.Westbrook JI, Talley NJ, Westbrook MT. Gender differences in the symptoms and physical and mental well-being of dyspeptics: a population based study. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2002;11:283–291. [DOI] [PubMed] [Google Scholar]
- 38.Welen K, Faresjo A, Faresjo T. Functional dyspepsia affects women more than men in daily life: a case-control study in primary care. Gender medicine. 2008;5:62–73. [DOI] [PubMed] [Google Scholar]
- 39.Choghakhori R, Abbasnezhad A, Amani R, Alipour M. Sex-Related Differences in Clinical Symptoms, Quality of Life, and Biochemical Factors in Irritable Bowel Syndrome. Digestive diseases and sciences. 2017;62:1550–1560. [DOI] [PubMed] [Google Scholar]
- 40.Cherepanov D, Palta M, Fryback DG, Robert SA. Gender differences in health-related quality-of-life are partly explained by sociodemographic and socioeconomic variation between adult men and women in the US: evidence from four US nationally representative data sets. Quality of Life Research. 2010;19:1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanheel H, Carbone F, Valvekens L, et al. Pathophysiological Abnormalities in Functional Dyspepsia Subgroups According to the Rome III Criteria. American Journal of Gastroenterology. 2017;112:132–140. [DOI] [PubMed] [Google Scholar]
- 42.Degen LP, Phillips SF. Variability of gastrointestinal transit in healthy women and men. Gut. 1996;39:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stanghellini V, Tosetti C, Paternico A, et al. Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology. 1996;110:1036–1042. [DOI] [PubMed] [Google Scholar]
- 44.Ryan JP, Bhojwani A. Colonic transit in rats: effect of ovariectomy, sex steroid hormones, and pregnancy. American Journal of Physiology. 1986;251:G46–50. [DOI] [PubMed] [Google Scholar]
- 45.Li CP, Ling C, Biancani P, Behar J. Effect of progesterone on colonic motility and fecal output in mice with diarrhea. Neurogastroenterology & Motility. 2012;24:392–e174. [DOI] [PubMed] [Google Scholar]
- 46.Cheng L, Biancani P, Behar J. Progesterone receptor A mediates VIP inhibition of contraction. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2010;298:G433–439. [DOI] [PubMed] [Google Scholar]
- 47.Gonenne J, Esfandyari T, Camilleri M, et al. Effect of female sex hormone supplementation and withdrawal on gastrointestinal and colonic transit in postmenopausal women. Neurogastroenterology & Motility. 2006;18:911–918. [DOI] [PubMed] [Google Scholar]
- 48.Lu CL, Herndon C. New roles for neuronal estrogen receptors. Neurogastroenterology & Motility. 2017;29. [DOI] [PubMed] [Google Scholar]
- 49.Liu JYH, Lin G, Fang M, Rudd JA. Localization of estrogen receptor ERalpha, ERbeta and GPR30 on myenteric neurons of the gastrointestinal tract and their role in motility. General & Comparative Endocrinology. 2019;272:63–75. [DOI] [PubMed] [Google Scholar]
- 50.Zielinska M, Fichna J, Bashashati M, et al. G protein-coupled estrogen receptor and estrogen receptor ligands regulate colonic motility and visceral pain. Neurogastroenterology & Motility. 2017;29. [DOI] [PubMed] [Google Scholar]
- 51.Bharucha AE, Wald A. Chronic Constipation. Mayo Clinic Proceedings. 2019;28:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bharucha AE, Lacy BE. Chronic Constipation: Mechanisms, Evaluation and Management. Gastroenterology. 2020;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choiniere M. A systematic literature review of 10 years of research on sex/gender and experimental pain perception - part 1: are there really differences between women and men? Pain. 2012;153:602–618. [DOI] [PubMed] [Google Scholar]
- 54.Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choiniere M. A systematic literature review of 10 years of research on sex/gender and pain perception - part 2: do biopsychosocial factors alter pain sensitivity differently in women and men? Pain. 2012;153:619–635. [DOI] [PubMed] [Google Scholar]
- 55.Iacovides S, Avidon I, Baker FC. Does pain vary across the menstrual cycle? A review. European Journal of Pain. 2015;19:1389–1405. [DOI] [PubMed] [Google Scholar]
- 56.Ruffle JK, Frokjaer JB, Farmer AD. Neuroimaging of Visceral Pain. In: Saba L, ed. Neuroimaging of Pain: Springer; 2017. [Google Scholar]
- 57.Posserud I, Syrous A, Lindstrom L, Tack J, Abrahamsson H, Simren M. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology. 2007;133:1113–1123. [DOI] [PubMed] [Google Scholar]
- 58.Houghton LA, Lea R, Jackson N, Whorwell PJ. The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut. 2002;50:471–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bharadwaj S, Barber MD, Graff LA, Shen B. Symptomatology of irritable bowel syndrome and inflammatory bowel disease during the menstrual cycle. Gastroenterology report. 2015;3:185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruigomez A, Garcia Rodriguez LA, Johansson S, Wallander MA. Is hormone replacement therapy associated with an increased risk of irritable bowel syndrome? Maturitas. 2003;44:133–140. [DOI] [PubMed] [Google Scholar]
- 61.Sherman JJ, LeResche L. Does experimental pain response vary across the menstrual cycle? A methodological review. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2006;291:R245–256. [DOI] [PubMed] [Google Scholar]
- 62.Pogatzki-Zahn EM, Mengersen C, Englbrecht JS, Klein T, Magerl W, Zahn PK. Progesterone relates to enhanced incisional acute pain and pinprick hyperalgesia in the luteal phase of female volunteers. Pain. 2019;21:21. [DOI] [PubMed] [Google Scholar]
- 63.Houghton LA, Jackson NA, Whorwell PJ, Morris J. Do male sex hormones protect from irritable bowel syndrome? The American journal of gastroenterology. 2000;95:2296–2300. [DOI] [PubMed] [Google Scholar]
- 64.Hong JY, Kilpatrick LA, Labus JS, et al. Sex and disease-related alterations of anterior insula functional connectivity in chronic abdominal pain. J Neurosci. 2014;34:14252–14259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kern MK, Jaradeh S, Arndorfer RC, Jesmanowicz A, Hyde J, Shaker R. Gender differences in cortical representation of rectal distension in healthy humans. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2001;281:G1512–1523. [DOI] [PubMed] [Google Scholar]
- 66.Berman SM, Naliboff BD, Suyenobu B, et al. Sex differences in regional brain response to aversive pelvic visceral stimuli. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2006;291:R268–276. [DOI] [PubMed] [Google Scholar]
- 67.Benson S, Kotsis V, Rosenberger C, et al. Behavioural and neural correlates of visceral pain sensitivity in healthy men and women: does sex matter? European Journal of Pain. 2012;16:349–358. [DOI] [PubMed] [Google Scholar]
- 68.Naliboff BD, Berman S, Chang L, et al. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology. 2003;124:1738–1747. [DOI] [PubMed] [Google Scholar]
- 69.Labus JS, Naliboff BN, Fallon J, et al. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41:1032–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang Z, Dinov ID, Labus J, et al. Sex-related differences of cortical thickness in patients with chronic abdominal pain. PLoS ONE [Electronic Resource]. 2013;8:e73932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elsenbruch S, Schmid J, Kullmann JS, et al. Visceral sensitivity correlates with decreased regional gray matter volume in healthy volunteers: a voxel-based morphometry study. Pain. 2014;155:244–249. [DOI] [PubMed] [Google Scholar]
- 72.Mayer EA, Labus J, Aziz Q, et al. Role of brain imaging in disorders of brain-gut interaction: a Rome Working Team Report. Gut. 2019;68:1701–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kano M, Dupont P, Aziz Q, Fukudo S. Understanding Neurogastroenterology From Neuroimaging Perspective: A Comprehensive Review of Functional and Structural Brain Imaging in Functional Gastrointestinal Disorders. Journal of neurogastroenterology and motility. 2018;24:512–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cremon C, Stanghellini V, Pallotti F, et al. Salmonella Gastroenteritis During Childhood Is a Risk Factor for Irritable Bowel Syndrome in Adulthood. Gastroenterology. 2014;147:69–77. [DOI] [PubMed] [Google Scholar]
- 76.Barbara G, Grover M, Bercik P, et al. Rome Foundation Working Team Report on Post-Infection Irritable Bowel Syndrome. Gastroenterology. 2019;156:46–58.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, Risk Factors, and Outcomes of Irritable Bowel Syndrome After Infectious Enteritis: A Systematic Review and Meta-analysis. Gastroenterology. 2017;152:1042–1054.e1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ruigomez A, Garcia Rodriguez LA, Panes J. Risk of irritable bowel syndrome after an episode of bacterial gastroenteritis in general practice: influence of comorbidities. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007;5:465–469. [DOI] [PubMed] [Google Scholar]
- 79.Pitzurra R, Fried M, Rogler G, et al. Irritable bowel syndrome among a cohort of European travelers to resource-limited destinations. Journal of travel medicine. 2011;18:250–256. [DOI] [PubMed] [Google Scholar]
- 80.Cremon C, Gargano L, Morselli-Labate AM, et al. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. The American journal of gastroenterology. 2009;104:392–400. [DOI] [PubMed] [Google Scholar]
- 81.Pennell LM, Galligan CL, Fish EN. Sex affects immunity. Journal of Autoimmunity. 2012;38:J282–291. [DOI] [PubMed] [Google Scholar]
- 82.Houghton LA, Wych J, Whorwell PJ. Acute diarrhoea induces rectal sensitivity in women but not men. Gut. 1995;37:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tack J, Demedts I, Dehondt G, et al. Clinical and pathophysiological characteristics of acute-onset functional dyspepsia. Gastroenterology. 2002;122:1738–1747. [DOI] [PubMed] [Google Scholar]
- 84.Mearin F, Perez-Oliveras M, Perello A, et al. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: one-year follow-up cohort study. Gastroenterology. 2005;129:98–104. [DOI] [PubMed] [Google Scholar]
- 85.Futagami S, Itoh T, Sakamoto C. Systematic review with meta-analysis: post-infectious functional dyspepsia. Aliment Pharmacol Ther. 2015;41:177–188. [DOI] [PubMed] [Google Scholar]
- 86.Du L, Chen B, Kim JJ, Chen X, Dai N. Micro-inflammation in functional dyspepsia: A systematic review and meta-analysis. Neurogastroenterology & Motility. 2018;30:e13304. [DOI] [PubMed] [Google Scholar]
- 87.Puthanmadhom Narayanan S, Linden DR, Peters SA, et al. Duodenal mucosal secretory disturbances in functional dyspepsia. Neurogastroenterology & Motility. 2020:e13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van Oudenhove L, Levy RL, Crowell MD, et al. Biopsychosocial Aspects of Functional Gastrointestinal Disorders: How Central and Environmental Processes Contribute to the Development and Expression of Functional Gastrointestinal Disorders. Gastroenterology. 2016;150:1355–1367.e1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bjorkman I, Dellenborg L, Ringstrom G, Simren M, Jakobsson Ung E. The gendered impact of Irritable Bowel Syndrome: a qualitative study of patients’ experiences. Journal of Advanced Nursing. 2014;70:1334–1343. [DOI] [PubMed] [Google Scholar]
- 90.Alander T, Heimer G, Svardsudd K, Agreus L. Abuse in women and men with and without functional gastrointestinal disorders. Digestive diseases and sciences. 2008;53:1856–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bradford K, Shih W, Videlock EJ, et al. Association between early adverse life events and irritable bowel syndrome. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012;10:385–390.e381–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paras ML, Murad MH, Chen LP, et al. Sexual abuse and lifetime diagnosis of somatic disorders: a systematic review and meta-analysis. JAMA. 2009;302:550–561. [DOI] [PubMed] [Google Scholar]
- 93.Prusator DK, Greenwood-Van Meerveld B. Amygdala-mediated mechanisms regulate visceral hypersensitivity in adult females following early life stress: importance of the glucocorticoid receptor and corticotropin-releasing factor. Pain. 2017;158:296–305. [DOI] [PubMed] [Google Scholar]
- 94.Winston JH, Li Q, Sarna SK. Chronic prenatal stress epigenetically modifies spinal cord BDNF expression to induce sex-specific visceral hypersensitivity in offspring. Neurogastroenterology & Motility. 2014;26:715–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van den Wijngaard RM, Stanisor OI, van Diest SA, et al. Susceptibility to stress induced visceral hypersensitivity in maternally separated rats is transferred across generations. Neurogastroent Motil. 2013;25:E780–E790. [DOI] [PubMed] [Google Scholar]
- 96.Aguirre JE, Winston JH, Sarna SK. Neonatal immune challenge followed by adult immune challenge induces epigenetic-susceptibility to aggravated visceral hypersensitivity. Neurogastroenterol Motil. 2017;29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilder-Smith CH. The balancing act: endogenous modulation of pain in functional gastrointestinal disorders. Gut. 2011;60:1589–1599. [DOI] [PubMed] [Google Scholar]
- 98.Chaloner A, Greenwood-Van Meerveld B. Sexually Dimorphic Effects of Unpredictable Early Life Adversity on Visceral Pain Behavior in a Rodent Model. J Pain. 2013;14:270–280. [DOI] [PubMed] [Google Scholar]
- 99.Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosomatic Medicine. 1992;54:648–657. [DOI] [PubMed] [Google Scholar]
- 100.Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. [DOI] [PubMed] [Google Scholar]
- 101.Benson S, Siebert C, Koenen LR, et al. Cortisol affects pain sensitivity and pain-related emotional learning in experimental visceral but not somatic pain: a randomized controlled study in healthy men and women. Pain. 2019;06:06. [DOI] [PubMed] [Google Scholar]
- 102.Tillisch K, Mayer EA, Labus JS, Stains J, Chang L, Naliboff BD. Sex specific alterations in autonomic function among patients with irritable bowel syndrome. Gut. 2005;54:1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Videlock EJ, Shih W, Adeyemo M, et al. The effect of sex and irritable bowel syndrome on HPA axis response and peripheral glucocorticoid receptor expression. Psychoneuroendocrinology. 2016;69:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ji Y, Hu B, Li J, Traub RJ. Opposing Roles of Estradiol and Testosterone on Stress-Induced Visceral Hypersensitivity in Rats. J Pain. 2018;19:764–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. [DOI] [PubMed] [Google Scholar]
- 106.Kerckhoffs AP, ter Linde JJ, Akkermans LM, Samsom M. SERT and TPH-1 mRNA expression are reduced in irritable bowel syndrome patients regardless of visceral sensitivity state in large intestine. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2012;302:G1053–1060. [DOI] [PubMed] [Google Scholar]
- 107.Camilleri M, Andrews CN, Bharucha AE, et al. Alterations in expression of p11 and SERT in mucosal biopsy specimens of patients with irritable bowel syndrome. Gastroenterology. 2007;132:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Galligan JJ, Patel BA, Schneider SP, et al. Visceral hypersensitivity in female but not in male serotonin transporter knockout rats. Neurogastroenterology & Motility. 2013;25:e373–381. [DOI] [PubMed] [Google Scholar]
- 109.Houghton LA, Brown H, Atkinson W, et al. 5-hydroxytryptamine signalling in irritable bowel syndrome with diarrhoea: effects of gender and menstrual status. Alimentary Pharmacology & Therapeutics. 2009;30:919–929. [DOI] [PubMed] [Google Scholar]
- 110.Hattay P, Prusator DK, Tran L, Greenwood-Van Meerveld B. Psychological stress-induced colonic barrier dysfunction: Role of immune-mediated mechanisms. Neurogastroenterology & Motility. 2017;29. [DOI] [PubMed] [Google Scholar]
- 111.Camilleri M, Shin A, Busciglio I, et al. Validating biomarkers of treatable mechanisms in irritable bowel syndrome. Neurogastroenterology & Motility. 2014;26:1677–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vanuytsel T, van Wanrooy S, Vanheel H, et al. Psychological stress and corticotrophin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63:1293–1299. [DOI] [PubMed] [Google Scholar]
- 113.Braniste V, Leveque M, Buisson-Brenac C, Bueno L, Fioramonti J, Houdeau E. Oestradiol decreases colonic permeability through oestrogen receptor beta-mediated upregulation of occludin and junctional adhesion molecule-A in epithelial cells. The Journal of physiology. 2009;587:3317–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Simren M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Appleby RN, Walters JRF. The role of bile acids in functional GI disorders. Neurogastroent Motil. 2014;26:1057–1069. [DOI] [PubMed] [Google Scholar]
- 116.Pittayanon R, Lau JT, Yuan Y, et al. Gut Microbiota in Patients With Irritable Bowel Syndrome-A Systematic Review. Gastroenterology. 2019;157:97–108. [DOI] [PubMed] [Google Scholar]
- 117.Menon R, Watson SE, Thomas LN, et al. Diet complexity and estrogen receptor beta status affect the composition of the murine intestinal microbiota. Applied and environmental microbiology. 2013;79:5763–5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Markle JG, Frank DN, Mortin-Toth S, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science (New York, N.Y.). 2013;339:1084–1088. [DOI] [PubMed] [Google Scholar]
- 119.Saito YA. The role of genetics in IBS. Gastroenterology Clinics of North America. 2011;40:45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Waehrens R, Ohlsson H, Sundquist J, Sundquist K, Zöller B. Risk of irritable bowel syndrome in first-degree, second-degree and thirddegree relatives of affected individuals: a nationwide family study in Sweden. Gut. 2015;64:215–221. [DOI] [PubMed] [Google Scholar]
- 121.Svedberg P, Johansson S, Wallander MA, Pedersen NL. No evidence of sex differences in heritability of irritable bowel syndrome in Swedish twins. Twin research and human genetics : the official journal of the International Society for Twin Studies. 2008;11:197–203. [DOI] [PubMed] [Google Scholar]
- 122.Bonfiglio F, Zheng T, Garcia-Etxebarria K, et al. Female-Specific Association Between Variants on Chromosome 9 and Self-Reported Diagnosis of Irritable Bowel Syndrome. Gastroenterology. 2018;155:168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. British journal of anaesthesia. 2013;111:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sluka KA, Berkley KJ, O’Connor MI, et al. Neural and psychosocial contributions to sex differences in knee osteoarthritic pain. Biology of sex differences. 2012;3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Robinson ME, Wise EA, Gagnon C, Fillingim RB, Price DD. Influences of gender role and anxiety on sex differences in temporal summation of pain. J Pain. 2004;5:77–82. [DOI] [PubMed] [Google Scholar]
- 126.Koloski NA, Talley NJ, Boyce PM. Epidemiology and health care seeking in the functional GI disorders: a population-based study. American Journal of Gastroenterology. 2002;97:2290–2299. [DOI] [PubMed] [Google Scholar]
- 127.Heitkemper M, Jarrett M, Bond EF, Chang L. Impact of sex and gender on irritable bowel syndrome. Biological research for nursing. 2003;5:56–65. [DOI] [PubMed] [Google Scholar]
- 128.Feingold A. Gender differences in personality: a meta-analysis. Psychological Bulletin. 1994;116:429–456. [DOI] [PubMed] [Google Scholar]
- 129.Thakur ER, Gurtman MB, Keefer L, Brenner DM, Lackner JM, Representing the IBSOSRG. Gender differences in irritable bowel syndrome: the interpersonal connection. Neurogastroenterology & Motility. 2015;27:1478–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mearin F, Lacy BE, Chang L, et al. Bowel Disorders. Gastroenterology. 2016;18:18. [DOI] [PubMed] [Google Scholar]
- 131.Fukudo S, Kinoshita Y, Okumura T, et al. Ramosetron Reduces Symptoms of Irritable Bowel Syndrome With Diarrhea and Improves Quality of Life in Women. Gastroenterology. 2016;150:358–366.e358. [DOI] [PubMed] [Google Scholar]
- 132.Viramontes BE, Camilleri M, McKinzie S, Pardi DS, Burton D, Thomforde GM. Gender-related differences in slowing colonic transit by a 5-HT3 antagonist in subjects with diarrhea-predominant irritable bowel syndrome. American Journal of Gastroenterology. 2001;96:2671–2676. [DOI] [PubMed] [Google Scholar]
- 133.Ford AC, Brandt LJ, Young C, Chey WD, Foxx-Orenstein AE, Moayyedi P. Efficacy of 5-HT3 antagonists and 5-HT4 agonists in irritable bowel syndrome: systematic review and meta-analysis. The American journal of gastroenterology. 2009;104:1831–1843; quiz 1844. [DOI] [PubMed] [Google Scholar]
- 134.Garsed K, Chernova J, Hastings M, et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut. 2014;63:1617–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fukudo S, Ida M, Akiho H, Nakashima Y, Matsueda K. Effect of ramosetron on stool consistency in male patients with irritable bowel syndrome with diarrhea. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12:953–959.e954. [DOI] [PubMed] [Google Scholar]
- 136.Camilleri M, Acosta A, Busciglio I, et al. Effect of colesevelam on faecal bile acids and bowel functions in diarrhoea-predominant irritable bowel syndrome. Alimentary Pharmacology and Therapeutics. 2015;41:438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cann PA, Read NW, Holdsworth CD, Barends D. Role of loperamide and placebo in management of irritable bowel syndrome (IBS). Digestive diseases and sciences. 1984;29:239–247. [DOI] [PubMed] [Google Scholar]
- 138.Efskind PS, Bernklev T, Vatn MH. A double-blind placebo-controlled trial with loperamide in irritable bowel syndrome. Scandinavian journal of gastroenterology. 1996;31:463–468. [DOI] [PubMed] [Google Scholar]
- 139.Suares NC, Ford AC. Systematic review: the effects of fibre in the management of chronic idiopathic constipation. Alimentary Pharmacology & Therapeutics. 2011;33:895–901. [DOI] [PubMed] [Google Scholar]
- 140.Bijkerk CJ, de Wit NJ, Muris JW, Whorwell PJ, Knottnerus JA, Hoes AW. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. BMJ (Clinical research ed.). 2009;339:b3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dipalma JA, Cleveland MV, McGowan J, Herrera JL. A randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. The American journal of gastroenterology. 2007;102:1436–1441. [DOI] [PubMed] [Google Scholar]
- 142.Ford AC, Suares NC. Effect of laxatives and pharmacological therapies in chronic idiopathic constipation: systematic review and meta-analysis. Gut. 2011;60:209–218. [DOI] [PubMed] [Google Scholar]
- 143.Pare P, Fedorak RN. Systematic review of stimulant and nonstimulant laxatives for the treatment of functional constipation. Canadian journal of gastroenterology & hepatology. 2014;28:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kamm MA, Mueller-Lissner S, Wald A, Richter E, Swallow R, Gessner U. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9:577–583. [DOI] [PubMed] [Google Scholar]
- 145.Omer A, Quigley EMM. An update on prucalopride in the treatment of chronic constipation. Therapeutic advances in gastroenterology. 2017;10:877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shah ED, Kim HM, Schoenfeld P. Efficacy and Tolerability of Guanylate Cyclase-C Agonists for Irritable Bowel Syndrome with Constipation and Chronic Idiopathic Constipation: A Systematic Review and Meta-Analysis. American Journal of Gastroenterology. 2018;113:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Harish K, Hazeena K, Thomas V, Kumar S, Jose T, Narayanan P. Effect of tegaserod on colonic transit time in male patients with constipation-predominant irritable bowel syndrome. Journal of gastroenterology and hepatology. 2007;22:1183–1189. [DOI] [PubMed] [Google Scholar]
- 148.Kang SJ, Park B, Shin CM. Helicobacter pylori Eradication Therapy for Functional Dyspepsia: A Meta-Analysis by Region and H. pylori Prevalence. Journal of clinical medicine. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pinto-Sanchez MI, Yuan Y, Hassan A, Bercik P, Moayyedi P. Proton pump inhibitors for functional dyspepsia. The Cochrane database of systematic reviews. 2017;11:Cd011194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Talley NJ, Locke GR, Saito YA, et al. Effect of Amitriptyline and Escitalopram on Functional Dyspepsia: A Multicenter, Randomized Controlled Study. Gastroenterology. 2015;149:340–349.e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang YJ, Bang CS, Baik GH, et al. Prokinetics for the treatment of functional dyspepsia: Bayesian network meta-analysis. BMC gastroenterology. 2017;17:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pittayanon R, Yuan Y, Bollegala NP, Khanna R, Leontiadis GI, Moayyedi P. Prokinetics for functional dyspepsia. The Cochrane database of systematic reviews. 2018;10:Cd009431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19–31. [DOI] [PubMed] [Google Scholar]
- 154.Lackner JM, Jaccard J, Krasner SS, Katz LA, Gudleski GD, Blanchard EB. How does cognitive behavior therapy for irritable bowel syndrome work? A mediational analysis of a randomized clinical trial. Gastroenterology. 2007;133:433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lackner JM, Jaccard J, Group IBSOSR. Factors Associated With Efficacy of Cognitive Behavior Therapy vs Education for Patients With Irritable Bowel Syndrome. Clinical Gastroenterology & Hepatology. 2019;17:1500–1508.e1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee HH, Choi YY, Choi MG. The Efficacy of Hypnotherapy in the Treatment of Irritable Bowel Syndrome: A Systematic Review and Meta-analysis. J Neurogastroenterol Motil. 2014;20:152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.