Abstract
Objectives:
To project the clinical impact of routine glaucoma screening on visual outcomes in middle-aged African American individuals and help guide glaucoma screening policy.
Methods:
Using data from the Eye Diseases Prevalence Research Group and Baltimore Eye Study, we developed a microsimulation model to project visual outcomes in African American individuals screened for glaucoma under a national screening policy using frequency-doubling technology. We projected the impact of universal screening on glaucoma-related visual impairment (acuity worse than 20/40 but better than 20/200 in the better-seeing eye) and blindness (acuity 20/200 or worse in the better-seeing eye). The diagnostic characteristics of frequency-doubling technology and the hazard ratio for glaucoma progression in treated patients were informed by meta-analyses of randomized controlled trials.
Results:
Implementation of a national glaucoma screening policy for a cohort of African American individuals between the ages of 50 and 59 years without known glaucoma would reduce the lifetime prevalence of undiagnosed glaucoma from 50% to 27%, the prevalence of glaucoma-related visual impairment from 4.6% to 4.4% (4.1% relative decrease), and the prevalence of glaucoma-related blindness from 6.1% to 5.6% (7.1% relative decrease). We project the cost of the program to be $80 per screened individual, considering only the cost of frequency-doubling technology and confirmatory eye examinations. The number needed to screen to diagnose 1 person with glaucoma is 58. The number needed to screen to prevent 1 person from developing visual impairment is 875.
Conclusions:
Routine glaucoma screening for middle-aged African American individuals is potentially clinically effective but its impact on visual impairment and blindness may be modest. However, we did not assess the impact on visual field loss.
PRIMARY OPEN-ANGLE GLAU-coma is a chronic, degenerative disease1 that affects more than 2.2 million Americans and 1.9% of Americans older than 40 years.2 Ocular hypotensive therapy for open-angle glaucoma slows the progression of optic nerve degeneration,3 but half of patients with glaucoma are unaware they have the disease.4,5 The high prevalence of undiagnosed glaucoma contributes to visual loss, an outcome that is disproportionately common in African American individuals, where as many as 11% of elderly patients develop blindness.4,6 African American individuals also develop visual impairment earlier than white individuals and are frequently diagnosed at more advanced disease states.7 Despite this significant disease burden, no consensus exists among health care payers and policy bodies about the effectiveness of glaucoma screening in African American individuals or other high-risk populations.8,9
Medicare currently reimburses screening for glaucoma as a preventive intervention in high-risk beneficiaries10 but the US Preventive Services Task Force11 cited insufficient evidence to support this practice. Their decision was based primarily on the paucity of evidence supporting the effectiveness of screening for preventing vision loss,12 though some researchers and health care professionals and researchers disagreed with their assessment.13–15 In the absence of randomized trial data evaluating glaucoma screening, we aimed to help bridge this evidence gap by developing a model to project clinical outcomes associated with glaucoma screening in African American individuals. We focused on this population because they are considered a high-risk group covered by the Medicare screening benefit.
METHODS
DECISION ANALYSIS MODEL
We developed a Monte Carlo microsimulation model to compare universal glaucoma screening among middle-aged African American individuals and usual care. A Monte Carlo microsimulation model is a computer-based mathematical model in which patients are tracked individually through the model and transitions are determined by sampling from probability distributions.16 We calibrated the model to reproduce current age-specific rates of glaucoma (both diagnosed and undiagnosed) and glaucoma-related visual impairment and blindness using data from the Eye Diseases Prevalence Research Group (EDPRG) and Baltimore Eye Study (BES).4,6,17 Visual impairment was defined as a visual acuity worse than 20/40 but better than 20/200 in the better-seeing eye attributable to glaucoma.17 Blindness was defined as a visual acuity of 20/200 or worse in the better-seeing eye attributable to glaucoma.17 Patients advanced through the model annually after being screened and were at risk of developing glaucoma, exhibiting progressive optic nerve damage ultimately producing visual impairment or legal blindness, or dying (Figure). Event probabilities were applied annually and the patient, not the eye, was the unit of analysis (Table 1). The model was programmed with TreeAge Pro 2009 (TreeAge Software Inc) and analyzed with Microsoft Excel (Microsoft Inc) and Intercooled Stata version 9.2 (StataCorp).
Figure.
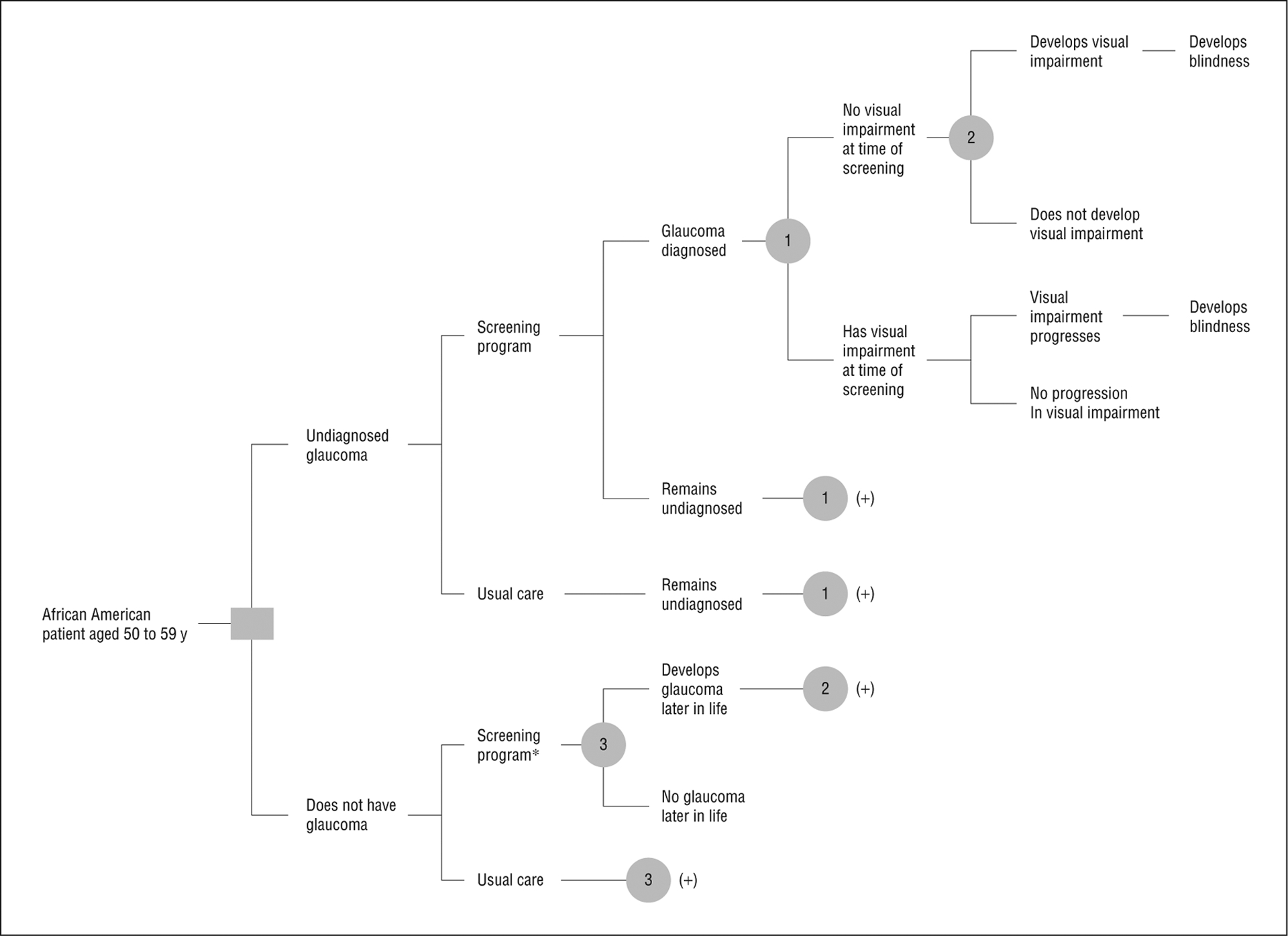
Model structure and stages of glaucoma progression in patients undergoing glaucoma screening or usual care management. Patients were African American, between the ages of 50 and 59 years, with no history of glaucoma undergoing universal screening or usual care (sporadic screening that results in 50% of patients with glaucoma being undiagnosed). Patients with diagnosed glaucoma were treated and all patients with glaucoma were at risk of developing new or progressively worsening glaucoma-related visual loss. *Confirmatory eye examination identified patients with false-positive frequency-doubling technology results. 1, 2, and 3 are probability nodes; (+) represents replication of branches at node (n), where n=1, 2, or 3.
Table 1.
Probabilities of Health Outcomes and Costs of Interventions
| Variable | Base Case Estimate (95% CI) | Source |
|---|---|---|
| Probability of diagnosed glaucoma | 0.5 | Tielsch et al,4 Rudnicka et al5 |
| Initial probability of having glaucoma | ||
| 50–59 y | 0.027–0.049 | Quigley and Vitale18 |
| Annual probability of developing glaucoma | ||
| 50–59 y | 0.002–0.003 | Quigley and Vitale18 |
| 60–69 y | 0.003–0.005 | Quigley and Vitale18 |
| 70–79 y | 0.005–0.006 | Quigley and Vitale18 |
| ≥80 y | 0.006–0.01 | Quigley and Vitale18 |
| Annual probability of progressing to visual impairment | ||
| 50–59 y | 0.007 | Congdon et al17 |
| 60–69 y | 0.01 | Congdon et al17 |
| 70–79 y | 0.01 | Congdon et al17 |
| ≥80 y | 0.01 | Congdon et al17 |
| Annual probability of progressing to blindness | ||
| 50–59 y | 0.35 | Sommer et al6 |
| 60–69 y | 0.20 | Sommer et al6 |
| 70–79 y | 0.02 | Sommer et al6 |
| ≥80 y | 0.02 | Sommer et al6 |
| Hazard ratio for progression with treatment | ||
| Development of visual impairmenta | 0.65 (0.49–0.87) | Maier et al3 |
| Development of blindnessb | 0.65 (0.49–0.87) | Maier et al3 |
| FDT screening test characteristics | ||
| Sensitivity | 0.92 (0.65–0.99) | Burr et al19 |
| Specificity | 0.94 (0.73–0.97) | Burr et al19 |
| Screening cost,$ | ||
| FDT visual field testing | 73 (110)c | Medicare fee schedule20 |
| Eye examination | 127 (190)c | Medicare fee schedule20 |
Abbreviation: FDT, frequency-doubling technology.
Hazard ratio for patients with glaucoma and no visual impairment.
Hazard ratio for patients with glaucoma and visual impairment.
For both the cost of FDT visual field testing and the cost of an eye examination, the base case was fixed (eg, $73) and then we used a higher estimate (eg, $110) for the sensitivity analysis.
SCREENING POPULATION
The screening population comprised African American individuals between the ages of 50 and 59 years. We focused on this age group because the prevalence of glaucoma rises sharply then, from 1.6% in patients between the ages of 40 and 49 years to 4.7% in our target population.4 The initial age distribution was derived using US census data.21 Based on these data, the portion of patients in each annual age stratum ranged from 11.1% for patients between the ages of 50 and 51 years to 8.7% for patients between the ages of 59 and 60 years. The mean initial age was 54.3 years.
DIAGNOSTIC TESTING
We selected the frequency-doubling technology (FDT) (program C-20–1) examination as our gateway screening tool because of its favorable diagnostic characteristics19,22 and the relative feasibility of incorporating it into a community-based screening program.23,24 Other comparative advantages of FDT include its portability, speed of use, and the relatively limited training required to operate it properly.23,25 We assumed that all patients with threshold FDT findings triggering referral would undergo a confirmatory eye examination performed by an ophthalmologist, and this served as the reference standard for glaucoma diagnosis.
ESTIMATING THE PREVALENCE AND INCIDENCE OF GLAUCOMA
Quigley and Vitale18 estimated that the prevalence of glaucoma among African American individuals was modeled by the quadratic equation:
This equation predicts prevalence rates of 2.7%, 5.2%, 8.8%, or 13.5% in African American individuals who are aged 50, 60, 70, or 80 years, respectively (Table 2).4 The incidence of glaucoma in patients without glaucoma was estimated using the equation:
In our model, at the time of disease onset or in the first stage of the simulation, half of patients with glaucoma were assumed to be unaware that they had the disease, a finding that has been duplicated in studies both within and outside of the United States.4,5 As an example, 55-year-old men would have an initial probability of glaucoma of 3.8% (equal to prevalence [age 55 years]). Half of these patients would be undiagnosed (1.9%) and this subpopulation would undergo screening. The remaining 96.2% of patients would face a 0.3% risk of developing glaucoma in the first year (equal to incidence [age 55 years]).
Table 2.
Prevalence of Visual Impairment and Blindness in African American Individuals With Glaucoma Under Usual Care
| Age, y | % | |||
|---|---|---|---|---|
| Visual Impairment | Blindness | |||
| BES Estimatea | Model Prediction | BES Estimatea,b | Model Prediction | |
| 50–59 | 0.9 | 1.7 | 6.5 | 5.8 |
| 60–69 | 3.1 | 3.7 | 6.5 | 6.3 |
| 70–79 | 6.3 | 6.6 | 6.5 | 6.4 |
| ≥80 | 12.0 | 10.5 | 6.5 | 5.7 |
Abbreviation: BES, Baltimore Eye Study.
Prevalence rates derived using data from the BES and Eye Diseases Prevalence Research Group.
Prevalence of blindness among patients with glaucoma was based on a pooled rate of 6.5% from the BES.
ESTIMATING THE PREVALENCE AND INCIDENCE OF GLAUCOMA-RELATED VISUAL IMPAIRMENT
We contacted researchers from the EDPRG for specific data on the prevalence of glaucoma-related visual impairment in African American individuals (Nathan Congdon, MD, MPH, former chairperson of the Writing Group for the EDPRG, and Benita O’Colmain, MPH, PhD, member of the Writing Group, unpublished data, 2010).17 Pooling data from the BES and the Salisbury Eye Study, they estimated that 15.9% of African American individuals with visual impairment were visually impaired because of glaucoma. However, the data were not adequately robust to calculate age-specific values. We therefore estimated age-specific rates by multiplying the prevalence of visual impairment provided in the EDPRG study17 by 15.9% and dividing by the prevalence of glaucoma at each age strata. This yielded estimates of 0.9%, 3.1%, 6.3%, and 12.0% for the prevalence of glaucoma-related visual impairment in African American individuals with glaucoma between ages 50 and 59 years, 60 and 69 years, 70 and 79 years, and 80 years or older, respectively (Table 2).
ESTIMATING THE PREVALENCE AND INCIDENCE OF GLAUCOMA-RELATED BLINDNESS
The prevalence of blindness was derived using BES data on the number of African American individuals with glaucoma-related bilateral blindness and dividing by the number of patients with glaucoma.4,6 These data were also used to empirically estimate the incidence of progressing from visual impairment to blindness (Table 1). This yielded estimates of 6.2%, 2.4%, 10.9%, and 7.2% for African American individuals with glaucoma between ages 50 and 59 years, 60 and 69 years, 70 and 79 years, and 80 years or older, respectively. We pooled these estimates to simplify the analysis and calculated the overall rate of blindness among patients with glaucoma as 6.5% (estimated by dividing 8, the number of patients with glaucoma-related blindness, by 124, the number of patients with glaucoma). This is similar to the estimate of 7.9% derived by Quigley and Vitale,18 who also used BES data but employed a different method. Patients with diagnosed glaucoma were assumed to receive treatment, and the hazard ratio for glaucoma progression to visual impairment and subsequently to blindness in treated patients was 0.65 (95% CI, 0.49–0.87; P=.003), based on a recent meta-analysis (Table 1).3 This meta-analysis combined the results of the Early Manifest Glaucoma Trial26 and the Collaborative Normal-Tension Glaucoma Study27 to estimate the effect of treatment on glaucoma progression using visual field or optic disc deterioration as metrics. We acknowledge that people of African ancestry likely compose only a small portion of the collective population of these 2 studies, but there is evidence that their response to glaucoma treatment is similar to individuals of European ancestry based on data from the Ocular Hypertension Treatment Study.28 Patients with visual impairment or blindness of unknown cause were allowed to undergo screening. We categorized patients as having either diagnosed or undiagnosed glaucoma at the time of glaucoma development in the model, and they retained this status for a lifetime, unless detected by screening (Figure). While in reality patients do transition from an undiagnosed state to a diagnosed state, there were limited data to inform this rate.
MODEL CALIBRATION
We calibrated the model to reproduce prevalence rates of visual impairment and blindness using estimated transition probabilities and simple exponential functions. Our exponential functions take the form P=1−e−r×t where P is the probability of an event occurring by time t, r is the hazard rate, and e is the mathematical constant approximately equal to 2.718.29 We assumed that patients with glaucoma transitioned sequentially from having no visual impairment to having visual impairment to blindness (Figure). Transition probabilities were estimated in a step-wise fashion, with estimates for younger populations derived prior to those for older populations. The calibrated transition probabilities are shown in Table 1, and the predicted prevalence rates of glaucoma and visual decline, compared with rates reported in epidemiological data, are shown in Table 2. To model survival, we used average mortality rates for African American individuals from US life tables21 because a meta-analysis found that glaucoma does not reduce life expectancy,30 though we acknowledge that no consensus exists on the relationship between glaucoma, treatment, and longevity.31
SCREENING COSTS
Costs were estimated using Current Procedural Terminology (CPT) codes and national Medicare reimbursement rates for automatic threshold perimetry (FDT examination; CPT code 92083) and an ophthalmologic examination (CPT code 92018).20 This accounted for the cost of a glaucoma screening test and a confirmatory appointment with an ophthalmologist. Using this source, the cost of FDT was $73 and the cost of an ophthalmologic examination was $127 in 2009 US dollars. We did not account for present or future costs of glaucoma treatment or for disability in patients who developed vision loss.
SENSITIVITY ANALYSES
We varied key parameters over plausible ranges to explore their impact on our results. Sensitivity analyses included the following: (1) the frequency of screening was increased and occurred at baseline and at ages 60 and 70 years; (2) the hazard ratio for glaucoma progression (developing visual impairment or blindness) in treated patients was varied from 0.49 to 0.87, based on the 95% confidence interval from the meta-analysis that estimated this value3; (3) the rate of follow-up eye examinations was reduced to 50%; (4) the sensitivity and specificity of FDT were varied from 0.65 to 0.99 and 0.73 to 0.97, respectively, based on the 95% confidence interval from the meta-analysis that estimated this value19; and (5) the costs of FDT and an ophthalmologic eye examination were varied.
RESULTS
Model validation results and projected health outcomes are displayed in Table 3 and Table 4. Implementation of a national, universal glaucoma screening policy for African American individuals between the ages of 50 and 59 years with no prior diagnosis of glaucoma would reduce the lifetime prevalence of undiagnosed glaucoma from 50% to 27%, the prevalence of glaucoma-related visual impairment from 4.6% to 4.4% (4.1% decrease), and the prevalence of glaucoma-related blindness from 6.1% to 5.6% (7.1% decrease). The lifetime prevalence of blindness in African American individuals is higher than the prevalence of visual impairment because the prevalence of glaucoma-related visual impairment, as reported by the BES, is relatively low in younger African American individuals (who constitute a larger portion of the screened population), while the pooled prevalence of glaucoma-related blindness is relatively high in this population. The prevalence of undiagnosed glaucoma would be lowest at 19% among patients between the ages of 50 and 59 years and rise to 40% in patients older than 80 years. The largest benefits for blindness prevention would accrue in patients older than 80 years, where the prevalence of blindness would fall from 5.7% to 5.1% (10.9% decrease). The benefit of screening was modest because of a combination of factors, including that the number of African American individuals diagnosed during screening is less than half of the total number of African American individuals who will develop glaucoma and that we separately report visual impairment and blindness (instead of a combined outcome).
Table 3.
Model Predictions of Visual Impairment Prevalence in African American Individuals With Glaucoma
| Age, y | Usual Care Visual Impairment, %a | Screening Policy | ||
|---|---|---|---|---|
| Visual Impairment, %a | Absolute Reduction | Relative Reduction, % | ||
| 50–59 | 1.7 | 1.6 | −0.1 | −3.5 |
| 60–69 | 3.7 | 3.5 | −0.2 | −6.2 |
| 70–79 | 6.6 | 6.3 | −0.3 | −3.9 |
| ≥80 | 10.5 | 10.1 | −0.3 | −3.0 |
| Overalla | 4.6 | 4.4 | −0.2 | −4.1 |
Age-adjusted rate.
Table 4.
Model Predictions of Blindness Prevalence in African American Individuals With Glaucoma
| Age, y | Usual Care Blindness, %a | Screening Policy | ||
|---|---|---|---|---|
| Blindness, %a | Absolute Reduction | Relative Reduction, % | ||
| 50–59 | 5.8 | 5.7 | −0.1 | −1.6 |
| 60–69 | 6.3 | 5.7 | −0.5 | −8.4 |
| 70–79 | 6.4 | 5.7 | −0.7 | −10.7 |
| ≥80 | 5.7 | 5.1 | −0.6 | −10.9 |
| Overalla | 6.1 | 5.6 | −0.4 | −7.1 |
Age-adjusted rate.
The program would cost $80 per screened individual, $4750 per new diagnosis of glaucoma, $71 130 per incident of visual impairment avoided, and $98 970 per incident of blindness avoided, considering only the cost of FDT and confirmatory eye examinations. Our model suggests that 58 people would need to be screened to diagnose 1 person with glaucoma. Further, 875 people would need to be screened to prevent 1 case of visual impairment, and 1220 people would need to be screened to prevent 1 case of blindness.
The results were sensitive to the frequency of screening and other factors (Table 5). If screening were performed at baseline and again at ages 60 and 70 years, the lifetime prevalence of undiagnosed glaucoma would fall from 50% to 17%, and the prevalence of glaucoma-related visual impairment and blindness would fall by 6.8% and 9.9%, respectively. The cost of screening would rise from $80 to $176 per screened individual, and the number needed to screen to prevent 1 case of visual impairment or blindness would rise to 1200 or 1930, respectively. The number needed to screen to diagnose 1 patient with glaucoma would rise to 68.
Table 5.
Sensitivity Analysis: Screening Cost per Incident of Visual Impairment or Blindness Prevented Under Various Scenarios
| Scenario | Screening Cost per Incident Prevented, $ per Incident | |
|---|---|---|
| Visual Impairment | Blindness | |
| Screen between ages 50 and 59 years, base case | 71 000 | 99 000 |
| Additional screening at ages 60 and 70 years | 82 000 | 132 000 |
| Incomplete patient follow-up after positive FDT results, 50% follow-up | 108 000 | 167 000 |
| Low treatment efficacy (hazard ratio for glaucoma progression = 0.87) | 281 000 | 393 000 |
| High treatment efficacy (hazard ratio for glaucoma progression = 0.49) | 38 000 | 52 000 |
| Low FDT sensitivity (0.65) and specificity (0.73) | 110 000 | 130 000 |
| High FDT sensitivity (0.99) and specificity (0.97) | 57 000 | 94 000 |
| Less expensive FDT examination ($45) | 42 000 | 58 000 |
| More expensive FDT examination ($110) | 114 000 | 177 000 |
Abbreviation: FDT, frequency-doubling technology.
We also varied the hazard ratio for glaucoma progression in treated patients from 0.49 to 0.87, based on the 95% confidence interval from the meta-analysis that estimated this value. When the hazard ratio was 0.49, the prevalence of visual impairment and blindness fell by 7.1% and 12.2% in the screened population, and the number needed to screen to prevent 1 case of visual impairment or blindness fell to 465 or 645, respectively. The corresponding values were 1.3% and 1.6% with a number needed to screen to prevent 1 case of visual impairment or blindness of 3455 or 4835 when the hazard ratio rose to 0.87, respectively. If only half of patients underwent a follow-up eye examination after positive screening, the prevalence of glaucoma-related visual impairment and blindness would fall by 2.3% and 3.3%, respectively.
We varied the sensitivity and specificity of FDT from 0.65 to 0.99 and 0.73 to 0.97, respectively. The most significant changes occurred when the sensitivity fell to 0.65 and the specificity fell to 0.73. In this setting, the prevalence of visual impairment and blindness fell by 2.0% and 5.0% compared with 4.1% and 7.1% in the base case, respectively, and the prevalence of undiagnosed glaucoma rose to 34% compared with 27%. The screening cost per patient rose to $104 and the number needed to screen to prevent 1 case of visual impairment or blindness was 1330 or 1665, respectively.
The results were also sensitive to the cost of FDT and ophthalmologic evaluation. When the cost of FDT or an ophthalmologic eye examination rose by 50% to $110 or $191, the average screening cost per patient rose to $115 or $85, respectively. When the cost of FDT fell to $45, the average screening cost per patient was $53.
COMMENT
MAIN FINDINGS AND IMPLICATIONS
We found that universal, community-based glaucoma screening for middle-aged African American individuals is potentially clinically effective, though its overall impact on visual impairment and blindness is relatively modest. Sensitivity analyses indicated that optimizing treatment efficacy or increasing screening frequency would have the most favorable impact on the effectiveness of screening.
Our study extends prior work by projecting the potential clinical impact of screening in a high-risk population and by focusing on visual impairment and blindness, vision states more clearly associated with a reduction in health-related quality of life.32 Previous research has often focused on visual field assessment as a marker of glaucoma progression.26,27,33–36 While this addresses an important outcome, studies often use different methods for assessing visual fields, and quantifying the degree to which reductions in visual field are associated with decrements in health-related quality of life can be challenging.37 For example, in the Early Manifest Glaucoma Trial, patients completed the 25-item National Eye Institute Visual Function Questionnaire after 6 years of follow-up.26,33 While this survey was unavailable at the time of study initiation, it found no difference in the scores of treated and untreated patients at 6 years’ follow-up, whereas differences in progression were present. However, other studies have found substantial decrements in vision-related quality of life, even among patients with previously undiagnosed glaucoma.38
COMPARISON WITH OTHER SCREENING TESTS
We found that the number needed to screen to prevent 1 case of visual impairment was 875. This figure is relatively comparable with several screening technologies, including colorectal cancer screening (number needed to screen to prevent 1 colorectal cancer diagnosis or death of 191 and 489, respectively)39; blood pressure screening (number needed to screen to prevent 1 death over 5 years of 274 to 1307)40; and mammography (number needed to screen to prevent 1 breast cancer death of 1224).41 Glaucoma screening is not as effective as annual screening to prevent diabetic retinopathy, where the number needed to screen to prevent blindness ranges from 8 in high-risk patients to 200 in low-risk patients.42 In sensitivity analyses, the number needed to screen to prevent 1 case of visual impairment ranged from 425 to 5330, depending on the effectiveness of treatment and frequency of screening studies.
LIMITATIONS
We did not account for visual field loss; rather, we used bilateral visual acuity alone to define visual impairment and blindness. Incorporating visual field loss, along with accounting for unilateral vision loss, would have improved our estimates of the effectiveness of screening. Related to this, our estimate for the impact of glaucoma treatment on preventing visual impairment or blindness was drawn from a meta-analysis of 2 clinical studies that focused on visual fields or optic nerve injury.26,27 We adapted the results of this meta-analysis for our study of visual acuity. The hazard ratio associated with a decline in visual acuity may be different from that for visual fields. We partially addressed this in a sensitivity analysis in which we varied the hazard ratio associated with glaucoma treatment.
The current analysis drew from national data on the prevalence of glaucoma, visual impairment, and blindness among African American individuals in the United States. It assumed homogeneity in the rate of glaucoma progression among individuals with glaucoma, rather than allowing some patients to face a different likelihood of progression than others. In reality, clinical research suggests that patients are heterogeneous in their likelihood of developing visual impairment or blindness, but we designed the model with homogeneous transition rates because data informing patterns of heterogeneity were limited.43 Further, the prevalence rates reported by the BES may differ from current epidemiological patterns, and our use of these data for model calibration may underestimate the benefit of treatment.
We used cross-sectional data from the BES to project visual impairment and blindness outcomes but longitudinal data would have been more appropriate. However, these data were unavailable for our patient population. The estimated rates of transitioning from no visual impairment to visual impairment and subsequently to blindness in patients with glaucoma were informed from model calibration using these cross-sectional data. In particular, our estimate of the rate of transitioning from visual impairment to blindness was limited by the use of a pooled blindness prevalence that did not vary by age. We pooled blindness prevalence, however, because BES data on the relationship between blindness and age were not monotonic. Our model likely overestimates the incidence of blindness in patients with visual impairment in the 50-to 59-year age group for this reason. Further analyses of the implications of using cross-sectional data vs longitudinal data for calibration and varying transition rates would improve future studies of glaucoma screening policy.
We incorporated the cost of screening and a follow-up eye examination, but we did not explicitly model the cost of treatment or changes in health-related quality of life associated with visual decline. This is an important outcome, as the cost of medical and surgical interventions to reduce intraocular pressure can be high,44 and vision loss can be rapid and significantly reduce health-related quality of life.32,45 We also did not consider the cost of visual rehabilitation, disability, or long-term care in patients with blindness, which are substantial.46 The EDPRG estimated that 37 000 African American individuals will have glaucoma-related visual impairment and 50 000 African American individuals will have glaucoma-related blindness by 2020, up from 22 000 and 29 000 in 2000, respectively.2 In an analysis using Medical Expenditure Panel Survey data, Frick and colleagues47 estimated that the incremental medical expenditures and informal care costs in patients with vision impairment or blindness range from $1050 to $2400 annually, depending on the severity of vision loss. Approximating the impact of glaucoma screening by applying a 6% to 7% reduction to the prevalence of vision loss in that year, universal glaucoma screening could translate into annual savings of more than $10 million dollars in medical expenditures alone. This does not include the cost of lost productivity, long-term care, nursing homes, and other public services for patients with vision loss. Rein and colleagues46 estimated these expenditures to exceed $11 billion in the visually impaired US population, of which African American individuals constitute 9%.17
We conclude that routine screening for glaucoma in African American individuals is a potentially clinically effective and economical method to reduce the burden of glaucoma-related visual impairment and blindness, though its absolute benefit is likely to be modest. Health policy informing glaucoma screening should continue to evaluate effective and efficient methods to reduce the incidence of undiagnosed glaucoma and glaucoma-related visual impairment. Future studies should also consider long-term costs associated with treatment and the impact of delaying visual impairment on health-related quality of life.
Funding/Support:
Funding for this research was provided through a Physician Scientist Award from Research to Prevent Blindness (Dr Pasquale) and National Institutes of Health grant R01 EY015473 (Dr Pasquale).
Additional Contributions:
We thank Nathan Congdon, MD, MPH, and Benita O’Colmain, MPH, PhD, for providing unpublished data on visual impairment epidemiology.
Footnotes
Financial Disclosure: None reported.
Previous Presentation: This work was presented at the 2011 American Glaucoma Society Meeting; March 4, 2011; Dana Point, California.
REFERENCES
- 1.Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009;360(11):1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman DS, Wolfs RC, O’Colmain BJ, et al. ; Eye Diseases Prevalence Research Group. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122(4):532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maier PC, Funk J, Schwarzer G, Antes G, Falck-Ytter YT. Treatment of ocular hypertension and open angle glaucoma: meta-analysis of randomised controlled trials. BMJ. 2005;331(7509):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma: the Baltimore Eye Survey. JAMA. 1991;266(3):369–374. [PubMed] [Google Scholar]
- 5.Rudnicka AR, Mt-Isa S, Owen CG, Cook DG, Ashby D. Variations in primary open-angle glaucoma prevalence by age, gender, and race: a Bayesian meta-analysis. Invest Ophthalmol Vis Sci. 2006;47(10):4254–4261. [DOI] [PubMed] [Google Scholar]
- 6.Sommer A, Tielsch JM, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N Engl J Med. 1991;325(20):1412–1417. [DOI] [PubMed] [Google Scholar]
- 7.Martin MJ, Sommer A, Gold EB, Diamond EL. Race and primary open-angle glaucoma. Am J Ophthalmol. 1985;99(4):383–387. [DOI] [PubMed] [Google Scholar]
- 8.Friedman DS. Issues in screening for glaucoma. Ophthalmic Epidemiol. 2007;14 (3):101–102. [DOI] [PubMed] [Google Scholar]
- 9.Quigley HA. Current and future approaches to glaucoma screening. J Glaucoma. 1998;7(3):210–220. [PubMed] [Google Scholar]
- 10.Medicare’s glaucoma tests coverage. Medicare.gov Web site. http://www.medicare.gov/navigation/manage-your-health/preventive-services/glaucoma-tests.aspx. Accessed August 3, 2010.
- 11.Fleming C, Whitlock EP, Beil T, Smit B, Harris RP. Screening for primary open-angle glaucoma in the primary care setting: an update for the US Preventive Services Task Force. Ann Fam Med. 2005;3(2):167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatt S, Wormald R, Burr J. Screening for prevention of optic nerve damage due to chronic open angle glaucoma. Cochrane Database Syst Rev. 2006;(4):CD006129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Javitt J, Lee P, Lum F. The value of regular examinations to detect glaucoma and other chronic conditions among older Americans. Ophthalmology. 2007;114 (5):833–834. [DOI] [PubMed] [Google Scholar]
- 14.Mills RP. Glaucoma screening: the value is in the details. Am J Ophthalmol. 2008; 145(1):3–4. [DOI] [PubMed] [Google Scholar]
- 15.Maul EA, Jampel HD. Glaucoma screening in the real world. Ophthalmology. 2010; 117(9):1665–1666. [DOI] [PubMed] [Google Scholar]
- 16.Hunink MGM. Decision Making in Health and Medicine: Integrating Evidence and Values. Cambridge, NY: Cambridge University Press; 2001. [Google Scholar]
- 17.Congdon N, O’Colmain B, Klaver CC, et al. ; Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. [DOI] [PubMed] [Google Scholar]
- 18.Quigley HA, Vitale S. Models of open-angle glaucoma prevalence and incidence in the United States. Invest Ophthalmol Vis Sci. 1997;38(1):83–91. [PubMed] [Google Scholar]
- 19.Burr JM, Mowatt G, Hernández R, et al. The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation. Health Technol Assess. 2007;11(41):iii-iv, ix-x, 1–190. [DOI] [PubMed] [Google Scholar]
- 20.Physician fee schedule relative value files. Centers for Medicare & Medicaid Services. http://www.cms.hhs.gov/PhysicianFeeSched/PFSRVF/list.asp#TopOfPage. Accessed April 27, 2007.
- 21.Arias E, Rostron BL, Tejada-Vera B. United States life tables, 2005. Natl Vital Stat Rep. 2010;58(10):1–132. [PubMed] [Google Scholar]
- 22.Mowatt G, Burr JM, Cook JA, et al. ; OAG Screening Project. Screening tests for detecting open-angle glaucoma: systematic review and meta-analysis. Invest Ophthalmol Vis Sci. 2008;49(12):5373–5385. [DOI] [PubMed] [Google Scholar]
- 23.Anderson AJ, Johnson CA. Frequency-doubling technology perimetry. Ophthalmol Clin North Am. 2003;16(2):213–225. [DOI] [PubMed] [Google Scholar]
- 24.Nehmad L, Madonna RJ. Optimizing the use of frequency doubling technology perimetry in community vision screenings. Optom Vis Sci. 2008;85(7):559–565. [DOI] [PubMed] [Google Scholar]
- 25.Frequency Doubling Technology (FDT) perimetry. Imaging and Perimetry Society Web site. http://webeye.ophth.uiowa.edu/ips/PerimetryHistory/FDP/index.htm. Accessed December 28, 2010.
- 26.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M; Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002; 120(10):1268–1279. [DOI] [PubMed] [Google Scholar]
- 27.Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126(4):498–505. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Application of the International Classification of Diseases to Neurology: ICD-NA. 2nd ed. Geneva, Switzerland: World Health Organization; 1997. [Google Scholar]
- 29.Kuntz KM, Weinstein MC. Modeling in economic evaluation. In: Drummond M, McGuire A eds. Economic Evaluation in Health Care: Merging Theory With Practice. New York, NY: Oxford University Press; 2001:150–152. [Google Scholar]
- 30.Stein JD, Newman-Casey PA, Niziol LM, Gillespie BW, Lichter PR, Musch DC. Association between the use of glaucoma medications and mortality. Arch Ophthalmol. 2010;128(2):235–240. [DOI] [PubMed] [Google Scholar]
- 31.Akbari M, Akbari S, Pasquale LR. The association of primary open-angle glaucoma with mortality: a meta-analysis of observational studies. Arch Ophthalmol. 2009;127(2):204–210. [DOI] [PubMed] [Google Scholar]
- 32.Kymes SM, Lee BS. Preference-based quality of life measures in people with visual impairment. Optom Vis Sci. 2007;84(8):809–816. [DOI] [PubMed] [Google Scholar]
- 33.Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E; Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121(1): 48–56. [DOI] [PubMed] [Google Scholar]
- 34.Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126(4):487–497. [DOI] [PubMed] [Google Scholar]
- 35.Kymes SM, Kass MA, Anderson DR, Miller JP, Gordon MO; Ocular Hypertension Treatment Study Group (OHTS). Management of ocular hypertension: a cost-effectiveness approach from the Ocular Hypertension Treatment Study. Am J Ophthalmol. 2006;141(6):997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rein DB, Wittenborn JS, Lee PP, et al. The cost-effectiveness of routine office-based identification and subsequent medical treatment of primary open-angle glaucoma in the United States. Ophthalmology. 2009;116(5):823–832. [DOI] [PubMed] [Google Scholar]
- 37.Parrish RK II, Gedde SJ, Scott IU, et al. Visual function and quality of life among patients with glaucoma. Arch Ophthalmol. 1997;115(11):1447–1455. [DOI] [PubMed] [Google Scholar]
- 38.McKean-Cowdin R, Wang Y, Wu J, Azen SP, Varma R; Los Angeles Latino Eye Study Group. Impact of visual field loss on health-related quality of life in glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2008;115(6):941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atkin WS, Edwards R, Kralj-Hans I, et al. ; UK Flexible Sigmoidoscopy Trial Investigators. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375(9726): 1624–1633. [DOI] [PubMed] [Google Scholar]
- 40.Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ. 1998;317(7154):307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Humphrey L, Chan BKS, Detlefsen S, Helfand M. Screening for Breast Cancer. Rockville, MD: Agency for Healthcare Research and Quality; 2010. [PubMed] [Google Scholar]
- 42.Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA. 2000; 283(7):889–896. [DOI] [PubMed] [Google Scholar]
- 43.Broman AT, Quigley HA, West SK, et al. Estimating the rate of progressive visual field damage in those with open-angle glaucoma, from cross-sectional data. Invest Ophthalmol Vis Sci. 2008;49(1):66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee PP, Walt JG, Doyle JJ, et al. A multicenter, retrospective pilot study of resource use and costs associated with severity of disease in glaucoma. Arch Ophthalmol. 2006;124(1):12–19. [DOI] [PubMed] [Google Scholar]
- 45.Wilson MR, Kosoko O, Cowan CL Jr, et al. Progression of visual field loss in untreated glaucoma patients and glaucoma suspects in St. Lucia, West Indies. Am J Ophthalmol. 2002;134(3):399–405. [DOI] [PubMed] [Google Scholar]
- 46.Rein DB, Zhang P, Wirth KE, et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124(12):1754–1760. [DOI] [PubMed] [Google Scholar]
- 47.Frick KD, Gower EW, Kempen JH, Wolff JL. Economic impact of visual impairment and blindness in the United States. Arch Ophthalmol. 2007;125(4):544–550. [DOI] [PubMed] [Google Scholar]


