Abstract
Objective:
To validate subgroups of cognitive impairment on the Montreal Cognitive Assessment (MoCA)—defined as Normal (score of 25–30), Mildly Impaired (score of 20–24), and Moderately Impaired (score less than 19)—by determining whether they differ in rehabilitation gain during inpatient stroke rehabilitation.
Design:
Observational study. Linear regression models were conducted and predictors included MoCA subgroups and relevant baseline demographic and clinical covariates. Separate models included the Cognitive subscale of the Functional Independence Measure (FIM) as a predictor.
Setting:
Inpatient rehabilitation facility of an urban, academic medical center.
Participants:
334 inpatients with mild-moderate strokes who were administered the MoCA on admission.
Interventions:
N/A
Main Outcome Measures:
mean relative functional gain (mRFG) and mean relative functional efficiency (mRFE, which adjusts for length of stay) on the FIM Total.
Results:
MoCA subgroups significantly predicted mRFG and mRFE after accounting for age, gender, education, stroke severity, and recurrent vs. first stroke. The Normal group exhibited greater mRFG and mRFE than the Mildly Impaired group, while the Moderately Impaired had significantly worse mRFG and mRFE than the Mildly Impaired group. The Moderately Impaired group had a significantly smaller proportion of individuals who made a clinically meaningful change on the Total FIM than the Mildly Impaired and Normal groups. MoCA subgroups better accounted for mRFG and mRFE than a standard-of-care cognitive assessment (Cognitive FIM).
Conclusion:
Use of MoCA-defined subgroups can assist providers in predicting functional gain in stroke survivors being treated in inpatient rehabilitation.
ClinicalTrials.gov Identifier: NCT02876783.
Keywords: Cognition, Neuropsychology, Cerebrovascular Disorders, Brain Infarction, Neurological Rehabilitation
Post-stroke cognitive impairment is associated with poorer outcome and greater disability. 41% of stroke survivors demonstrate cognitive deficits at six months and 39% are impaired at two years post-stroke1. The presence of cognitive deficits in the first week following stroke predicts cognitive and functional disability at 6, 12, and 36 months2. Accurate identification and meaningful gradation of cognitive deficits in the early period after stroke can facilitate tailored management and treatment in the acute inpatient rehabilitation setting.
The Montreal Cognitive Assessment (MoCA)3 has emerged as a preferred screening tool in stroke rehabilitation. In the acute/subacute setting, the MoCA is feasible to administer4,5, sensitive to cognitive impairment6, and predicts long-term cognitive dysfunction7. Worse performance on the MoCA is associated with greater dependence in activities of daily living on the Barthel Index assessed cross-sectionally8. Higher MoCA score is also associated longitudinally with better functional outcome at 3–6 months on the modified Rankin Scale9. In the acute inpatient rehabilitation setting specifically, MoCA score is associated with discharge independence in instrumental activities of daily living10 and improvement on the Functional Independence Measures (FIM)11.
Cognitive screening measures often employ a single cutoff point that maximizes sensitivity and specificity and classifies individuals as “impaired” or “not impaired.” However, it may be more clinically useful to quantify the extent of impairment to provide information that extends beyond “is an individual cognitively impaired” to “how impaired is an individual.” The identification of clinically meaningful subgroups based on ranges of scores on the MoCA would allow rehabilitation professionals to better understand and manage cognitive impairments and to more precisely ascertain the functional impact of a particular score on the MoCA.
Previous research has demonstrated clinical utility of MoCA subgroups. Outpatients with vascular disease (including but not limited to stroke) can be stratified into three MoCA subgroups that correspond to low (MoCA = 28–30), intermediate (23–27), and high (≤ 22) risk of cognitive impairment on a criterion neuropsychological test battery12. Patients 6+ months post-stroke or TIA classified on the MoCA as normal (25–30), mildly impaired (20–24), and “significantly” impaired (≤ 19) differ on markers of underlying vascular disease such as hypertensive arteriopathy13. The original developers of the MoCA themselves suggest that scores between 18–25 constitute mild impairment, 10–17 moderate impairment, and 0–10 severe impairment (www.mocatest.org); however, they state specifically that empirical research to validate subgroups is necessary.
There is a need for the investigation of the clinical utility of subgroups specifically in acute inpatient rehabilitation. Previously, we proposed the creation of three subgroups as Normal (MoCA = 25–30), Mildly Impaired (20–24), or Moderately Impaired (≤ 19)6. These subgroups were empirically-defined based on the most sensitive and specific cutoff point (24/25) for identifying mild cognitive impairment6, and the optimal cutoff point (19/20) for identifying deficits in cognitively-based instrumental activities of daily living (bill-paying)10 in stroke inpatients. Initial validation of these three subgroups revealed group differences on neuropsychological measures of processing speed, executive functioning, and memory6.
The goal of the current study was to further validate the clinical utility of these subgroups in individuals with stroke undergoing acute inpatient rehabilitation. We sought to determine whether stroke inpatients classified as Normal, Mildly Impaired, or Moderately Impaired on the MoCA differed in their functional gain during rehabilitation. We hypothesized that individuals in the Moderately Impaired group would make less functional gain than those in the Mildly Impaired group, who in turn would make less functional gain than the Normal group. We additionally explored whether MoCA subgroups would be more predictive of functional gain than a standard-of-care cognitive assessment measure (the cognitive subscale of the Functional Independence Measure [FIM]).
Methods
Participants
The study was conducted in the acute inpatient general rehabilitation unit of a large urban academic medical center. Participants were individuals with stroke admitted to our unit between August, 2012 and July, 2016 who were either identified prospectively and provided informed consent to have their data entered into a clinical research database, or (for those who were not or could not be formally consented) had their clinical data retrospectively collected in a de-identified manner. Both study procedures were approved by the medical center’s Institutional Review Board. Inclusion criteria were 18 years of age or older, medically able to participate in inpatient rehabilitation therapies for three hours daily, and reasonable chance of making functional gains.
Assessments
Demographic variables collected included age, education, gender, ethnicity, employment status, and prior living situation. Stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS) in the emergency department or assessed retrospectively. Medical comorbidity was assessed using the Charlson Comorbidity Index (CCI). We also evaluated days post stroke at admission, side of lesion, first ever stroke vs. recurrent stroke, length of stay, and stroke type (ischemic vs. hemorrhagic).
We used the MoCA and MoCA-defined subgroups as our primary predictor of interest. The MoCA is composed of brief cognitive tasks that assess visuospatial/executive skills, attention, naming, language, abstraction, delayed recall, and orientation3. Administration time is approximately 10 minutes. Scores range from 0–30, with lower scores indicating greater cognitive impairment. Per the standard administration criteria, 1 point was added for individuals whose education level was less than 12 years. The MoCA was administered within 72 hours of rehabilitation admission by the treating occupational therapist to all individuals that could maintain attention and could follow simple commands. Patients did not complete the MoCA if the clinician determined that severe attention/arousal deficits precluded administration. Individuals with a total MoCA score of 25–30 were classified as Normal (N = 55), those with a total MoCA score of 20–24 were classified as Mildly Impaired (N = 109), and those with a total MoCA score less than or equal to 19 were classified as Moderately Impaired (N = 170).
We assessed functional gain in rehabilitation using the FIM14 total score. The FIM is a standardized, clinician-rated instrument for assessing and tracking functioning during rehabilitation. Each item on the FIM is rated on a 1–7 scale, with higher scores representing increased levels of functional independence. It is completed at admission and at discharge. Items assess eating, grooming, bathing, dressing, toileting, transfers, locomotion, communication, and social cognition.
The outcome (dependent) variables included the following: the mean relative FIM gain (mRFG)15, which quantifies the amount of functional gain achieved as a percentage of the total functional gain possible, using the formula (Discharge Total FIM – Admission Total FIM) / (Maximum Total FIM Score [i.e., 126]) – Admission FIM) x 100%. A second outcome was the mean relative FIM efficiency (mRFE)15, which includes the length of stay in the formula and therefore quantifies the relative functional gain achieved per day as a measure of efficiency of rehabilitation. It is calculated as the mRFG / Length of Stay (Days). We included the mRFE as an additional outcome variable because it corrects for length of stay, which could have impacted the amount of gain made. Finally, we calculated the proportion of individuals who met the minimum clinically important difference (MCID) on the Total FIM, which has been established at a threshold of 22 points in stroke rehabilitation16.
Statistical Analyses
All statistical analyses were conducted using IBM SPSS Statistics version 25. One-way analysis of variance (ANOVA) was conducted to evaluate group differences in clinical and demographic variables. Post-hoc comparisons were made using Tukey’s HSD tests. When the homogeneity of variance assumption was violated, we confirmed the presence of statistical significance using a Welch ANOVA and evaluated pairwise differences using the post-hoc Games-Howell test.
To evaluate the effect of MoCA subgroups on rehabilitation gain and to statistically control for possible confounding effects of relevant demographic and clinical factors, we conducted separate linear regression analyses for each outcome variable (mRFG and mRFE). MoCA subgroups were entered as predictors using dummy coding with the Mildly Impaired group as the reference group to which the Moderately Impaired and Normal groups were compared. We also entered as covariates age, education (dummy coded with high school education as the reference group), gender (dummy coded with males as the reference group), recurrent versus first-time stroke (dummy coded with first-time stroke as the reference group), and NIHSS. We chose these variables as covariates because they differed by MoCA subgroup and may have impacted rehabilitation gain. We then ran a secondary analysis with the same linear regression models substituting MoCA subgroups with Cognitive-FIM, in order to explore how the models with MoCA subgroups compared to a model with a standard of care cognitive assessment measure such as the Cognitive-FIM. All p-values are for two-tailed tests with alpha of .05.
Results
Comparison of Included and Excluded Participants
425 individuals with stroke were admitted to the rehabilitation unit between August 2012 and July 2016, 273 who enrolled prospectively and 147 whose data were collected retrospectively. 86 individuals were excluded from analysis because they were not administered the MoCA. The most common reason for not being administered the MoCA was impaired language. Outcome data were missing for five individuals, who were also excluded from analysis. The final sample thus comprised N = 334 individuals with stroke. Compared to our included sample, individuals who were excluded were significantly younger (t=2.15, p=.03), had greater NIHSS scores (t=5.7, p<.001), and had greater functional disability on admission (Total FIM, t=5.62, p<.001; Motor-FIM, t=4.10, p<.001; Cognitive FIM, t=7.67, p<.001). There was also a difference in side of lesion (χ2=9.15, p=.01; higher percentage of left hemisphere strokes in the excluded group).
Demographic Characteristics
As shown in Table 1, individuals in the Moderately Impaired group were older than those in the Mildly Impaired group and Normal group. Education levels differed by group (χ2=23.2, p=.01). The Moderately Impaired group had a smaller proportion of individuals with college or post-college education than the other two groups. Pre-admission employment status differed by group (χ2=17.1, p=.009). The Moderately Impaired group had a greater proportion of individuals who were unemployed or retired.
Table 1.
Demographic Characteristics
| Normal (N=55) | Mildly Impaired (N=109) | Moderately Impaired (N=170) | Significance | Entire Sample (N=334) | |
|---|---|---|---|---|---|
| Age in Years | 67.2 ± 15.9 | 68.1 ± 14.8 | 72.7 ± 13.0 | Moderate > Mild (p=.02) and Normal (p=.03) | 70.3 ± 14.3 |
| Education (Years; N [%]) | |||||
| Less Than High School | 2 (4%) | 9 (8%) | 28 (17%) | 39 (12%) | |
| Completed High School | 9 (16%) | 26 (24%) | 53 (31%) | 88 (26%) | |
| Some College | 6 (10%) | 16 (15%) | 16 (9%) | p=.01 | 38 (11%) |
| Completed College | 19 (35%) | 36 (33%) | 39 (23%)) | 94 (28%) | |
| Graduate Degree | 19 (35%) | 22 (20%) | 32 (19%) | 73 (22%) | |
| Other | 0 (0%) | 0 (0%) | 2 (1%) | 2 (1%) | |
| Ethnicity (N [%]) | |||||
| White | 43 (78%) | 67 (62%) | 90 (53%) | NS (p=.15) | 200 (60%) |
| Black | 4 (7%) | 12 (11%) | 27 (16%) | 43 (13%) | |
| Hispanic | 3 (6%) | 11(10%) | 22 (13%) | 36 (11%) | |
| Asian | 4 (7%) | 11 (10%) | 20 (12%) | 35 (10%) | |
| Other/Undisclosed | 1 (2%) | 8 (7%) | 11 (6%) | 20 (6%) | |
| Gender (N [%]) | |||||
| Male | 32 (58%) | 67 (61%) | 82 (48%) | NS (p=.08) | 181 (54%) |
| Female | 23 (42%) | 42 (39%) | 88 (52%) | 153 (46%) | |
| Pre-Admission Living (N [%]) | |||||
| Home Independent | 44 (83%) | 88 (83%) | 132 (78%) | NS (p=.54) | 264 (81%) |
| Home; Informal Assistance | 6 (11%) | 17 (16%) | 29 (17%) | 52 (16%) | |
| Home; Agency Assistance | 3 (6%) | 1 (1%) | 7 (4%) | 11 (3%) | |
| Pre-Admission Employment | |||||
| Employed | 25 (46%) | 46 (42%) | 42 (24%) | p=.009 | 113 (34%) |
| Unemployed | 3 (5%) | 3 (3%) | 17 (10%) | 23 (7%) | |
| Disabled | 1 (2%) | 4 (4%) | 7 (4%) | 12 (3%) | |
| Retired | 26 (47%) | 55 (51%) | 106 (62%) | 187 (56%) | |
Notes. Values are Mean ± Standard Deviation, unless otherwise noted.
Clinical Characteristics
As shown in Table 2, the Moderately Impaired group had more severe strokes than the Mildly Impaired group and Normal group, though the magnitude of the difference was relatively small. The Moderately Impaired group had a greater percentage of individuals with recurrent stroke (30%) than the Normal group (22%) or the Mildly Impaired group (18%). At admission and discharge, the Moderately Impaired group had greater functional disability on the FIM compared to the Mildly Impaired group, which in turn had greater functional disability than the Normal.
Table 2.
Clinical Characteristics
| Normal (N=55) | Mildly Impaired (N=109) | Moderately Impaired (N=170) | Significance | Entire Sample (N=334) | |
|---|---|---|---|---|---|
| Days Post Stroke | 8.0 ± 6.6 | 9.2 ± 8.7 | 10.7 ± 10.8 | NS (p=.13) | 9.8 ± 9.6 |
| Lesion Side (N [%]) | |||||
| Right | 26 (47%) | 56 (51%) | 87 (51%) | NS (p=.93) | 169 (50%) |
| Left | 25 (46%) | 43 (40%) | 71 (42%) | 139 (42%) | |
| Bilateral | 4 (7%) | 10 (9%) | 12 (7%) | 26 (8%) | |
| Stroke Type (N [%]) | |||||
| Ischemic | 50 (91%) | 101 (93%) | 156 (92%) | NS (p=.92) | 307 (92%) |
| Hemorrhagic | 5 (9%) | 8 (7%) | 14 (8%) | 27 (8%) | |
| Length of Stay in Days | 11.9 ± 6.3 | 13.7 ± 8.0 | 14.6 ± 7.4 | NS (p=.06) | 13.9 ± 7.4 |
| Charlson Comorbidity Index (median [range]) | 1 (0–8) | 1 (0–8) | 1 (0–9) | NS (p=.73) | 1 (0–9) |
| NIHSS | 4.8 ± 4.8 | 5.9 ± 5.3 | 8.4 ± 6.4 | Moderate > Mild (p=.001) and Normal (p<.001) | 7.0 ± 6.0 |
| Recurrent Stroke | 12 (22%) | 20 (18%) | 51 (30%) | p=.06 | 84 (25%) |
| First Stroke | 43 (78%) | 89 (82%) | 119 (70%) | 255 (75%) | |
| Admission Motor-FIM | 41.0 ± 12.6 | 35.8 ± 12.5 | 31.9 ± 13.4 | Normal > Mild (p=.04); Mild > Moderate (p=.04) | 34.7 ± 13.4 |
| Admission Cognitive-FIM | 30.2 ± 6.4 | 27.9 ± 5.8 | 23.7 ± 6.9 | Moderate < Mild (p<.001) and Normal (p<.001) | 26.2 ± 7.0 |
| Admission Total-FIM | 72.8 ± 17.9 | 65.2 ± 16.3 | 57.1 ± 18.4 | Normal > Mild (p=.03); Mild > Moderate (p=.001) | 62.3 ± 18.6 |
| Discharge Motor-FIM | 64.1 ± 10.8 | 55.4 ± 13.7 | 46.5 ± 15.9 | Normal > Mild (p=.001); Mild > Moderate (p<.001) | 52.3 ± 15.9 |
| Discharge Cognitive-FIM | 32.4 ± 2.8 | 30.1 ± 4.2 | 24.8 ± 6.5 | Normal > Mild (p<.001); Mild > Moderate (p<.001) | 27.8 ± 6.2 |
| Discharge Total-FIM | 98.8 ± 13.0 | 87.6 ± 15.9 | 73.0 ± 20.8 | Normal > Mild (p=.001); Mild > Moderate (p<.001) | 82.0 ± 20.7 |
| MoCA Total Score | 26.6 ± 1.6 | 21.9 ± 1.3 | 13.9 ± 4.0 | Normal > Mild (p<.001); Mild > Moderate (p<.001) | 18.6 ± 5.9 |
Notes. Values are Mean ± Standard Deviation, unless otherwise noted.
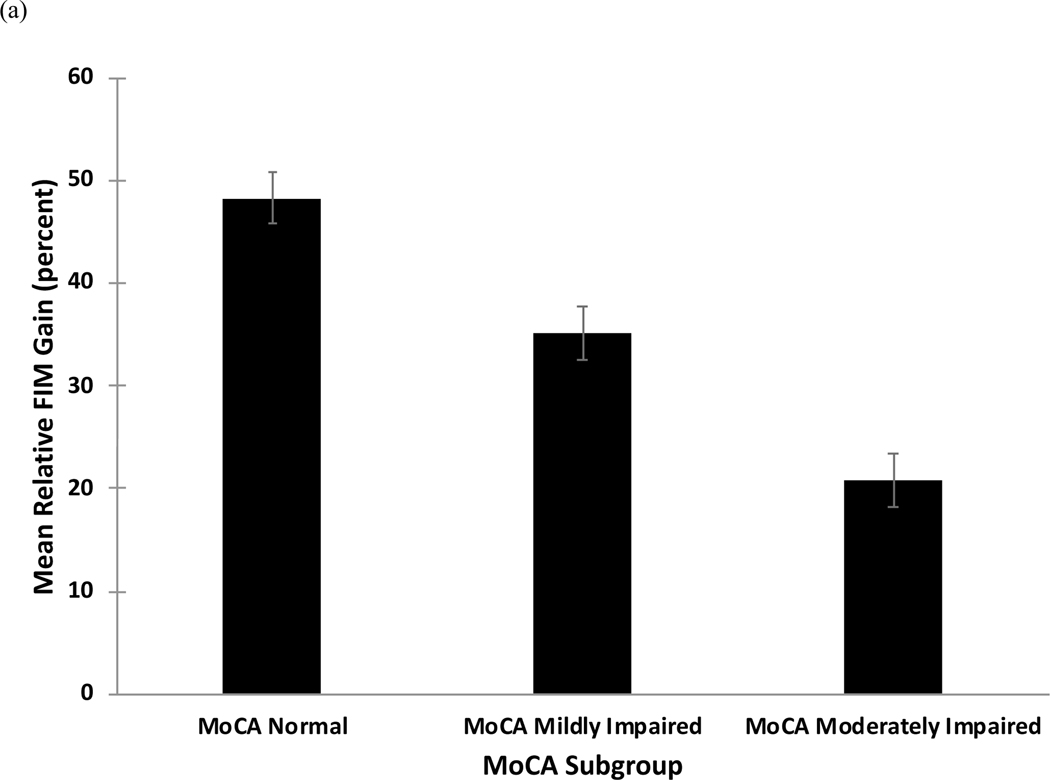
Comparison of mRFG and mRFE by MoCA Subgroup
mRFG values by group are shown in Figure 1a and results of regression analyses are shown in Table 3a. The overall model was significant (F=6.2, p<.001, Adjusted R2=.14). The Moderately Impaired group had a significantly lower mRFG relative to the Mildly Impaired group, while the Normal group had a significantly higher mRFG relative to the Mildly Impaired group. Gender, education, and stroke severity were not significant predictors of mRFG, while age was a significant predictor. Recurrent stroke was a marginally significant predictor of mRFG.
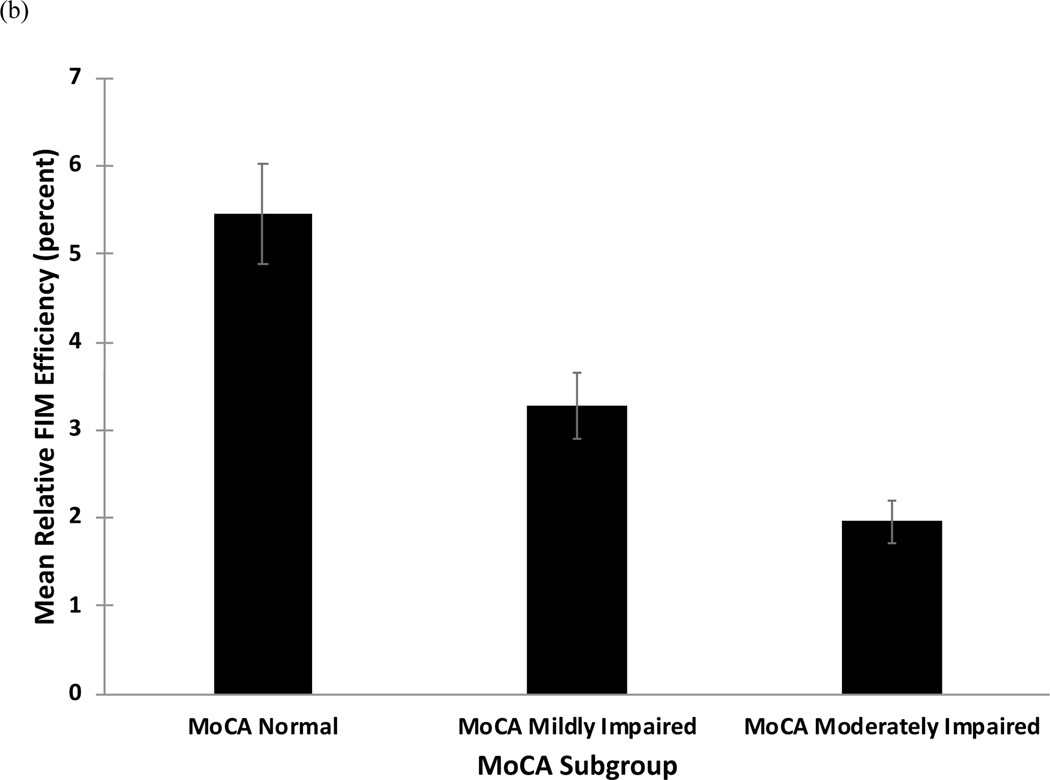
Figure 1.
(a) Mean relative functional gain (mRFG) and (b) mean relative functional efficiency (mRFE) as a function of subgroup on the Montreal Cognitive Assessment (MoCA).
Table 3.
Linear regression models predicting (a) mean relative functional gain (mRFG) and (b) mean relative functional efficiency (mRFE). Note that FIM = Functional Independence Measure; HS = High School; MoCA = Montreal Cognitive Assessment; NIHSS = National Institute of Health Stroke Scale. Gender, education, stroke number, and MoCA subgroup are all dummy coded with the reference group listed as the second variable in parentheses.
| (a) mRFG |
| Predictor | Unstandardized β | Standard Error | 95% Confidence Interval | t | p |
|---|---|---|---|---|---|
| Age | −0.42 | 0.12 | −0.65, −0.18 | −3.53 | <.001 |
| Gender (Female vs. Male) | 0.48 | 3.31 | −6.03, 6.99 | 0.15 | .88 |
| Education (Less than HS vs. HS) | −5.27 | 5.73 | −16.54, 6.00 | −0.92 | .36 |
| Education (Some College vs. HS) | 1.29 | 5.75 | −10.02, 12.60 | 0.22 | .82 |
| Education (College vs. HS) | −1.72 | 4.42 | −10.42, 6.98 | −0.39 | .70 |
| Education (Graduate Degree vs. HS) | −5.77 | 4.80 | −15.21, 3.67 | −1.20 | .23 |
| NIHSS | −0.36 | 0.29 | −0.93, 0.21 | −1.24 | .22 |
| Stroke Number (Recurrent vs. First Stroke) | −7.53 | 3.84 | −15.08, 0.02 | −1.96 | .05 |
| MoCA Subgroup (Normal vs. Mildly Impaired) | 13.24 | 4.92 | 3.56, 22.92 | 2.69 | .007 |
| MoCA Subgroup (Moderately Impaired vs. Mildly Impaired) | −10.48 | 3.84 | −18.03, −2.94 | −2.74 | .007 |
| (b) mRFE |
| Predictor | Unstandardized β | Standard Error | 95% Confidence Interval | t | p |
|---|---|---|---|---|---|
| Age | −0.02 | 0.01 | −0.05, 0.01 | −1.38 | .17 |
| Gender (Female vs. Male) | 0.35 | 0.41 | −0.45, 1.15 | 0.87 | .39 |
| Education (Less than HS vs. HS) | 0.66 | 0.70 | −0.72, 2.04 | 0.94 | .35 |
| Education (Some College vs. HS) | 0.65 | 0.70 | −.73, 2.04 | 0.93 | .35 |
| Education (College vs. HS) | −0.19 | 0.54 | −1.25, 0.88 | −0.35 | .73 |
| Education (Graduate Degree vs. HS) | −0.36 | 0.59 | −1.51, 0.80 | −0.61 | .55 |
| NIHSS | −0.07 | 0.04 | −0.14, −0.01 | −2.00 | .05 |
| Stroke Number (Recurrent vs. First Stroke) | −0.67 | 0.47 | −1.60, 0.25 | −1.43 | .15 |
| MoCA Subgroup (Normal vs. Mildly Impaired) | 2.20 | 0.60 | 1.02, 3.38 | 3.65 | <.001 |
| MoCA Subgroup (Moderately Impaired vs. Mildly Impaired) | −1.07 | 0.47 | −1.99, −0.14 | −2.27 | .02 |
Similar results emerged for the mRFE, for which mean values by group are shown in Figure 1b and regression results are shown in Table 3b. The overall model was significant (F=5.1, p<.001, Adjusted R2=.11). The Moderately Impaired group had a significantly lower mRFE relative to the Mildly Impaired group, while the Normal group had a significantly higher mRFE relative to the Mildly Impaired group. Stroke severity was also a significant predictor of mRFE, while age, gender, and education were not.
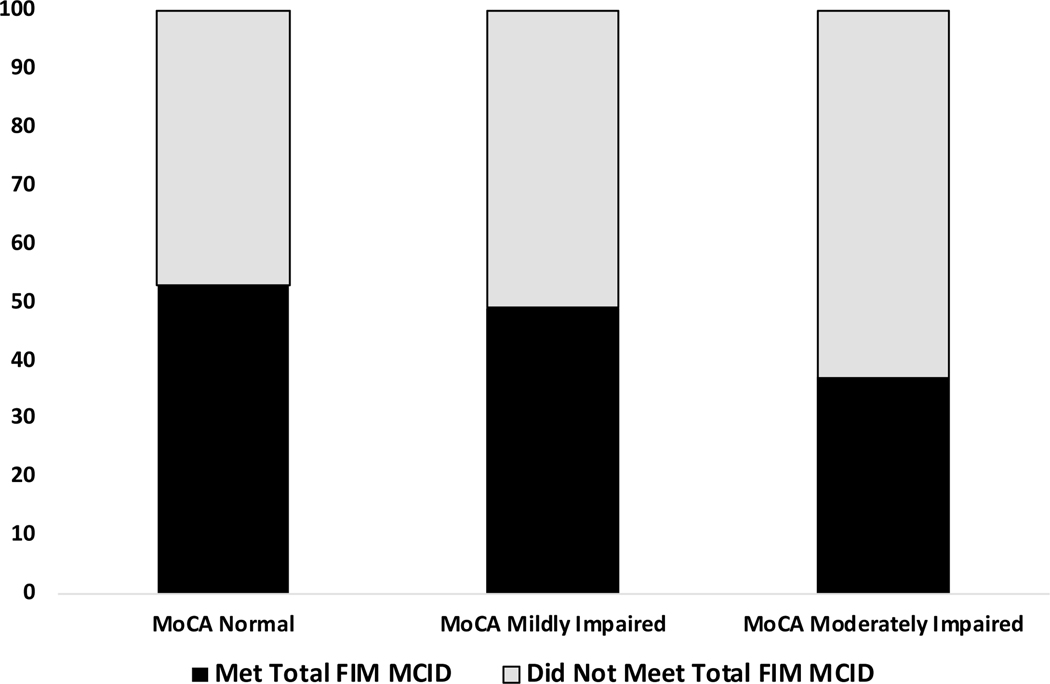
Comparison of FIM MCID by MoCA Subgroup
The percentage of individuals who met the FIM Total MCID by group is shown in Figure 2. A chi-square test revealed a significant group difference (χ2=5.94, p=.05). Comparison of cell proportions indicated that the Moderately Impaired group (37% met MCID) differed significantly from the Mildly Impaired group (49%) and Normal group (53%).
Figure 2.
The percentage of individuals in each subgroup of the Montreal Cognitive Assessment (MoCA) who met the minimal clinically important difference (MCID; 22 points) on the total score of the Functional Independence Measure (FIM).
Exploratory Comparison of MoCA Subgroups and the Cognitive-FIM.
Regressions models that replaced MoCA subgroups with the Cognitive-FIM were also statistically significant, but accounted for a smaller amount of variance in predicting mRFG (F=4.5, p<.001, Adjusted R2=.09) and mRFE (F=2.8, p<.001, Adjusted R2=.05). Within each model, the Cognitive-FIM predicted mRFG (Unstandardized Beta=−0.53, SE=0.25, 95%CI=−1.02 to −0.05, t=−2.16, p=.03) and mRFE (Unstandardized Beta=−0.07, SE=0.03, 95%CI=−0.13 to −0.01, t=−2.42, p=.02).
Discussion
We found that subgroups defined by admission MoCA score differed in their relative functional gain in rehabilitation, the efficiency by which they make gains, and the proportion of individuals who make clinically meaningful gains. As hypothesized, MoCA subgroups were significant predictors of relative functional gain and relative functional efficiency, accounting for age, gender, education, stroke severity, and recurrent stroke. Those with MoCA scores in the Normal range (25–30) made the greatest and most efficient functional gains as assessed by the FIM. This group also had the highest proportion making a clinically meaningful FIM gain. In contrast, those in the Moderately Impaired group (≤ 19) made the least functional gain and least efficient gains, as well as had the smallest proportion making a clinically meaningful FIM gain.
Our findings further validate the clinical utility of these subgroups in inpatient stroke rehabilitation. We previously demonstrated that these MoCA-defined subgroups differ significantly in performance on neuropsychological testing, with those in the Normal group performing on average within normal limits, those in Mildly Impaired group performing on average in the mildly impaired range, and those in the Moderately Impaired group performing in the moderately impaired range6. Our current results suggest that these subgroups also differ in their ability to distinguish among stroke inpatients who make differing levels of functional gain in rehabilitation. Subgroups defined by these ranges of scores have further been shown to differ in their degree of underlying vascular disease13. The ranges that define our subgroups are somewhat different than those proposed by Swartz and colleagues12, although the purpose of their study was to maximize the prediction of risk of future cognitive impairment across a more heterogenous population, while we sought to derive clinically useful subgroups in the inpatient stroke rehabilitation setting. Future studies should directly compare our proposed subgroups with other subgroups classified by different score ranges, in a separate validation sample.
A secondary, exploratory analysis found that the Cognitive-FIM score was also a significant predictor of relative functional gain and relative functional efficiency; however, the amount of variance explained was smaller relative to the models with MoCA subgroups. This finding accords with prior research that has found significant relationships between Cognitive-FIM and rehabilitation outcome, but has argued for the use of performance-based cognitive screening tools such as the MoCA because of their minimal cost and time, relative to the information that can be gained quantitatively and qualitatively17,18. Similarly, we show here that categorizing the degree of cognitive impairment by MoCA subgroup better accounts for rehabilitation gain than the Cognitive-FIM. Because of its greater coverage of cognitive domains, the MoCA can also provide useful qualitative information to inform treatment. For individuals with more severe cognitive and language impairment, the Cognitive-FIM is nonetheless an important tool to assess cognitive status.
Our findings indicate that those individuals with greater levels of cognitive impairment on the MoCA—despite similar lengths of stay—achieve fewer functional gains in rehabilitation, make gains less efficiently, and less often make an amount of functional gain that is clinically meaningful. This finding is consistent with prior studies demonstrating that cognitive impairment predicts poor outcome during acute hospitalization8 and rehabilitation17,19,20. Cognitive impairment after stroke may interfere with attention to and processing of strategies and techniques taught in rehabilitation, with learning and recall of skills, and with comprehending and following multistep instructions, altogether slowing the amount of gain in rehabilitation. The degree of cognitive impairment may also be a marker of decreased neurologic reserve and neuroplastic potential, which corresponds to poorer recovery in rehabilitation. Stroke inpatients with moderate cognitive impairment may especially require additional supports or modification of interventions to promote optimal functional gain. Such interventions should be implemented within a rehabilitation setting that also addresses the unique psychosocial and medical needs of individuals with greater cognitive impairment and disability.
Study Limitations
Our sample of stroke patients had less severe strokes, better functional ability at admission, and a smaller percentage of individuals with left-hemisphere strokes compared to participants excluded from analysis. This limits generalizability of our findings to those who tend to manifest relatively milder symptoms of stroke and are less likely to be aphasic. Assessment of stroke severity with the NIHSS using retrospective medical record review has demonstrated validity21; however, additional error may have been introduced in the measurement of stroke severity by this method. Our Moderately Impaired group contained a large range of MoCA scores and it is possible that finer gradations of impairment may exist within this group (i.e., a “Severely Impaired” subcategory). Additional research into the lower end of the MoCA and its correlates is an important avenue for future research. Our outcome measures of mRFG and mRFE tend to favor individuals with high admission FIM scores, and our results may have differed with use of a different outcome measure such as the raw FIM difference score. Our investigation was also limited to the inpatient rehabilitation setting. Because we do not have information on the mRFG or mRFE post-discharge, the clinical utility of our MoCA subgroups in predicting functioning after inpatient rehabilitation is unknown and warrants further study. We did not have access to neuroimaging to determine how lesion characteristics map onto our MoCA subgroups. This would be an important avenue of future research given that performance on the MoCA is associated with disruption to specific structural networks after stroke22.
Conclusions
We demonstrate that MoCA subgroups differentiate the degree of functional gains and the likelihood of making clinically meaningful gains in inpatient stroke rehabilitation. These and previous findings6,13 indicate that our MoCA subgroups provide empirically-validated and clinically-meaningful gradations of cognitive severity. Clinicians and rehabilitation practitioners would derive benefit from categorizing patients’ severity of impairment based on our MoCA score ranges. This can enable a more individually tailored, personalized medicine approach to care in which individuals scoring between 20–24 and particularly below 20 may require additional cognitive remediation interventions, intensive practice, and/or modification and tailoring of standard of care treatment strategies to mitigate the risk of poorer functional gain. Future research should target cognitive and rehabilitation strategies specifically at these individuals who score in the mildly impaired or moderately impaired range on the MoCA. Future studies should also directly compare the categorization proposed here with others proposed in the literature.
Supplementary Material
Acknowledgements:
This work was conducted at NewYork-Presbyterian Hospital/Weill Cornell Medical Center and supported by the Peter Jay Sharp Foundation and in part by the Clinical and Translational Science Center, National Center for Advancing Translational Sciences (grant no. UL1-TR000457-06).
List of Abbreviations:
- ANOVA
Analysis of Variance
- CCI
Charlson Comorbidity Index
- FIM
Functional Independence Measure
- MCID
minimum clinically important difference
- MoCA
Montreal Cognitive Assessment
- mRFE
mean relative functional efficiency
- mRFG
mean relative functional gain
- NIHSS
National Institutes of Health Stroke Scale
- TIA
transient ischemic attack
Footnotes
Disclosure Statement: The authors have no conflicts of interest to disclose.
References
- 1.Turunen KEA, Laari SPK, Kauranen TV, Uimonen J, Mustanoja S, Tatlisumak T, et al. Domain-specific cognitive recovery after first-ever stroke: A 2-year follow-up. J. Int. Neuropsychol. Soc. 2018;24:117–27. [DOI] [PubMed] [Google Scholar]
- 2.Zietemann V, Georgakis MK, Dondaine T, Müller C, Mendyk AM, Kopczak A, et al. Early MoCA predicts long-term cognitive and functional outcome and mortality after stroke. Neurology. 2018;91:e1838–50. [DOI] [PubMed] [Google Scholar]
- 3.Nasreddine ZS, Phillips NA, Bédirian VA, Charbonneau SI, Whitehead VI, Colllin IS, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–9. [DOI] [PubMed] [Google Scholar]
- 4.Chiti G, Pantoni L. Use of montreal cognitive assessment in patients with stroke. Stroke. 2014;45:3135–40. [DOI] [PubMed] [Google Scholar]
- 5.Blackburn DJ, Bafadhel L, Randall M, Harkness KA. Cognitive screening in the acute stroke setting. Age Ageing. 2013;42:113–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaywant A, Toglia J, Gunning FM, O’Dell MW. The diagnostic accuracy of the Montreal Cognitive Assessment in inpatient stroke rehabilitation. Neuropsychol. Rehabil. 2017; [DOI] [PubMed]
- 7.Salvadori E, Pasi M, Poggesi A, Chiti G, Inzitari D, Pantoni L. Predictive value of MoCA in the acute phase of stroke on the diagnosis of mid-term cognitive impairment. J. Neurol. 2013;260:2220–7. [DOI] [PubMed] [Google Scholar]
- 8.Abzhandadze T, Rafsten L, Lundgren-Nilsson Å, Sunnerhagen KS. Feasibility of cognitive functions screened with the Montreal Cognitive Assessment in determining ADL dependence early after stroke. Front. Neurol. 2018;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong Y, Slavin MJ, Chan BP-L, Venketasubramanian N, Sharma VK, Crawford JD, et al. Cognitive screening improves the predictive value of stroke severity scores for functional outcome 3–6 months after mild stroke and transient ischaemic attack: an observational study. BMJ Open. 2013;3:e003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toglia J, Askin G, Gerber LM, Taub MC, Mastrogiovanni AR, O’Dell MW. Association between 2 measures of cognitive instrumental activities of daily living and their relation to the Montreal Cognitive Assessment in persons with stroke. Arch. Phys. Med. Rehabil. 2017;98:2280–7. [DOI] [PubMed] [Google Scholar]
- 11.Toglia J, Fitzgerald KA, O’Dell MW, Mastrogiovanni AR, Lin CD. The Mini-Mental State Examination and Montreal Cognitive Assessment in persons with mild subacute stroke: Relationship to functional outcome. Arch. Phys. Med. Rehabil. 2011;92:792–8. [DOI] [PubMed] [Google Scholar]
- 12.Swartz RH, Cayley ML, Lanctôt KL, Murray BJ, Smith EE, Sahlas DJ, et al. Validating a Pragmatic Approach to Cognitive Screening in Stroke Prevention Clinics Using the Montreal Cognitive Assessment. Stroke. 2016;47:807–13. [DOI] [PubMed] [Google Scholar]
- 13.Webb AJS, Pendlebury ST, Li L, Simoni M, Lovett N, Mehta Z, et al. Validation of the Montreal Cognitive Assessment versus Mini-Mental State Examination against hypertension and hypertensive arteriopathy after transient ischemic attack or minor stroke. Stroke. 2014;45:3337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keith RA. The functional independence measure: A new tool for rehabilitation. Adv. Clin. Rehabil. 1987;1:6–18. [PubMed] [Google Scholar]
- 15.Koh GC, Chen CH, Petrella R, Thind A. Rehabilitation impact indices and their independent predictors: A systematic review. BMJ Open. 2013;3:e003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beninato M, Gill-Body KM, Salles S, Stark PC, Black-Schaffer RM, Stein J. Determination of the minimal clinically important difference in the FIM instrument in patients with stroke. Arch. Phys. Med. Rehabil. 2006;87:32–9. [DOI] [PubMed] [Google Scholar]
- 17.Heruti RJ, Lusky A, Dankner R, Ring H, Dolgopiat M, Barell V, et al. Rehabilitation outcome of elderly patients after a first stroke: Effect of cognitive status at admission on the functional outcome. Arch. Phys. Med. Rehabil. 2002;83:742–9. [DOI] [PubMed] [Google Scholar]
- 18.Zwecker M, Levenkrohn S, Fleisig Y, Zeilig G, Ohry A, Adunsky A. Mini-Mental State Examination, Cognitive FIM Instrument, and the Loewenstein Occupational Therapy Cognitive Assessment: Relation to Functional Outcome of Stroke Patients. Arch. Phys. Med. Rehabil. 2002;83:342–5. [DOI] [PubMed] [Google Scholar]
- 19.Jaywant A, Toglia J, Gunning FM, O’Dell MW. The clinical utility of a 30-minute neuropsychological assessment battery in inpatient stroke rehabilitation. J. Neurol. Sci. 2018;390:54–62. [DOI] [PubMed] [Google Scholar]
- 20.Toglia J, Askin G, Gerber LM, Taub MC, Mastrogiovanni AR, O’Dell MW. Association between two measures of cognitive instrumental activities of daily living and their relationship to the Montreal Cognitive Assessment In persons with stroke. Arch. Phys. Med. Rehabil. 2017;98:2280–7. [DOI] [PubMed] [Google Scholar]
- 21.Lindsell C, Alwell K, Moomaw C, Kleindorfer D, Woo D, Flaherty M, et al. Validity of a retrospective National Institutes of Health Stroke Scale scoring methodology in patients with severe stroke. J. Stroke Cerebrovasc. Dis. 2005;14:281–3. [DOI] [PubMed] [Google Scholar]
- 22.Kuceyeski A, Navi BB, Kamel H, Relkin N, Villanueva M, Raj A, et al. Exploring the brain’s structural connectome: A quantitative stroke lesion-dysfunction mapping study. Hum. Brain Mapp. 2015;36:2147–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.