Abstract
Objective:
To compare the difference in pre- to postoperative speech performance of patients qualifying for a cochlear implant (CI) in quiet, +10 dB signal-to-noise ratio (SNR) and +5 dB SNR.
Study Design:
Retrospective.
Setting:
Tertiary referral center.
Patients:
Fifty-eight post-lingually deafened, unilateral CI recipients from three Groups were included: 1) those who met CI candidacy criteria with AzBio sentences in quiet, 2) in noise at +10 dB SNR but not in quiet, 3) and in noise at +5 dB SNR but not in quiet or +10 dB SNR.
Intervention:
Unilateral CI.
Main Outcome Measures:
Pre- and one year postoperative speech recognition scores.
Results:
Best-aided AzBio speech recognition of individuals in Group 1 improved significantly for all test conditions and improved significantly for Groups 2 and 3 in the +10 and +5 dB SNR test conditions postoperatively. When tested with their CI alone however, while AzBio speech recognition of individuals in Group 1 and Group 2 improved significantly in the quiet and +10 dB SNR conditions, speech recognition was not significantly changed postoperatively under any testing condition for individuals in Group 3.
Conclusions:
While individuals qualifying for a CI only in the +5 dB SNR condition may derive significant benefit from implantation in best aided conditions, speech understanding outcomes can be more variable thus warranting additional counseling prior to implantation and case-by-case consideration of listening needs and goals.
Introduction
In 1985, the United States Food and Drug Administration (FDA) approved the first multichannel cochlear implant (CI) for the treatment of individuals with profound sensorineural hearing loss. Since that time, advancements in technology, improved surgical techniques, and increased understanding of audiological management have led to a gradual expansion of CI candidacy criteria (1, 2). As of 2012, approximately 58,000 adults in the United States had received a CI (3). Although many individuals have received CIs, the estimated utilization rate among the population of individuals who may benefit from CI technology remains low, approximately 5% (3). Studies investigating reasons for a low utilization rate show that this is primarily due to a lack of awareness regarding candidacy criteria and outcomes (3, 4). In recent years, low utilization rates in combination with findings that greater residual hearing and shorter duration of deafness are associated with higher levels of CI benefit have led to a push to evaluate CI candidates sooner (5, 6). One method for doing this involves the use of testing speech recognition in the presence of noise.
Current candidacy criteria and follow-up testing (as used in the United States) recommended by the Minimum Speech Test Battery (MSTB) indicates the use of speech recognition testing with AzBio sentences presented in quiet and in noise (with the signal to noise ratio (SNR) set at +10 or +5 dB), consonant-nucleus-consonant (CNC) words, and Bamford-Kowal-Bench speech-in-noise (BKB-SIN) sentences if time allows (7). Despite these recommendations, variance in candidacy protocols are prevalent across CI centers. In a survey assessing differences in protocols, investigators found significant variance in test batteries used across implant centers. Ninety six percent of survey respondents reported regular testing in noise during candidacy assessments with 26% of respondents only testing in noise presented at +10 dB SNR, 16% testing only in noise presented at +5 dB SNR, and 55% of respondents using both +10 and +5 dB SNRs. Surveys also indicated that 100% of respondents use AzBio sentences in candidacy assessments while only 52% use CNC words in addition to sentences during candidacy assessments (2).
Current CI candidacy protocols at our cochlear implant center follow MSTB recommendations utilizing CNC word recognition and AzBio sentence recognition in quiet and in noise. Testing in noise is conducted in both +10 and +5 dB SNR conditions for all potential candidates. The decision to modify the clinical protocol to test with both the +10 and +5 dB SNRs was made in 2015 based on (a) MSTB guidelines (7), (b) discussions with other centers locally and nationally regarding candidacy protocol (2, 8), (c) identification of many individuals with hearing loss whose greatest complaint was listening in noise (d) retrospective examination of patient outcomes for individuals who qualified in the +10 dB SNR condition, and (e) demonstrations that +5 dB is representative of real-world SNR (9, 10).
While it is clear that in recent years candidacy protocols have changed and more individuals are qualifying for a CI when tested in noise, what remains unknown is whether these individuals are achieving the same amount of success observed in candidates qualifying under more conservative protocols. Although research by Mudery et al. (2016) examining postoperative hearing outcomes of older patients who qualified for a CI in noise but not in quiet found that speech recognition typically improved regardless of qualification condition, conclusions drawn from this work are limited as no distinction was made between SNR levels within the group that qualified in noise (11).
The primary goal of the current analysis was to assess whether differences in CI candidacy protocols (testing in quiet or variable levels of noise) result in variable levels of success based on post-operative assessments. Specifically, the study addressed the following questions regarding the speech recognition outcomes one-year post-implantation: Do patients who qualify for a CI only in conditions of +10 and/or +5 dB SNR improve in speech recognition at one year postoperatively? Are there factors on preoperative evaluation for CI that predict limited benefit from implantation postoperatively? We hypothesize that patients who qualify for a CI only in testing conditions with background noise will have smaller changes in performance postoperatively.
Methods
An IRB-approved retrospective electronic medical record review was completed for fifty-eight consecutive individuals (mean age: 72.26 years, SD: 14.39 years; age range: 20.46–90.63 years) who received their first cochlear implant at a tertiary care center CI program between June 2015 (the surgery date of the first patient qualifying in the +5 dB SNR condition) and December 2017 (to allow for a cohort of patients with one-year post-operative results). Individuals represented all three primary CI manufacturers and eight device types. Inclusion was restricted to post-lingually deafened, unilateral CI users with bilateral sensorineural hearing loss. Only patients with pre-operative CNC word and AzBio sentence recognition scores as well as at least one postoperative speech recognition score obtained between 10–14 months post-activation (considered the patient’s “one-year post-operative score”) were included for analysis. Individuals who received a second CI in the contralateral ear within 12 months post-activation of the initial implant, received reimplantation or revision surgery for any reason, had a known retro-cochlear abnormality, or had no audiometric assessment test results between 10 and 14 months post-activation were excluded from the analysis (see Table 1).
Table 1.
Demographic table summarizing age, electro-acoustic stimulation use, contralateral hearing aid use, pre-operative implant-ear pure tone average, and pre-operative non-implant ear pure tone average by qualifying group.
| Covariate | Overall | Quality at +5 | Qualify at +10 | Qualify in Quiet |
|---|---|---|---|---|
| (N=58) | (N=30) | (N=16) | (N=12) | |
| Age | 72.3 (14.4) | 72.5 (13.3) | 75.4 (10.0) | 67.4 (20.8) |
| EAS | 11 (19.0%) | 8 (26.7%) | 0 (0.0%) | 3 (25.0%) |
| Contralateral Hearing Aid | 55 (94.8%) | 30 (100%) | 15 (93.8%) | 10 (83.3%) |
| PTA (Implant Ear) | 81.7 (18.5) | 77.8 (20.2) | 80.9 (12.5) | 92.4 (17.7) |
| PTA (Non-Implant Ear) | 70.3 (16.9) | 65.1 (12.5) | 67.9 (13.1) | 86.7 (21.4) |
Standard comprehensive audiometric measures are included in the CI candidacy protocol including tympanometry and acoustic reflexes. CI candidacy-specific measures were completed aided using the individual’s personal or clinic-provided hearing aids programmed to NAL-NL1 targets (12). These specific measures included word recognition using CNC word lists for each ear individually with the contralateral ear plugged, sentence recognition in quiet using AzBio sentence lists for each ear aided individually and bilaterally aided, and sentence recognition in noise using AzBio sentence lists tested at both +10 dB SNR and +5 dB SNR for each ear aided individually and aided bilaterally. All testing was performed at zero degree azimuth, with speech and noise co-located.
Candidacy for cochlear implantation was determined based upon insurance-specific requirements. For patients with Medicare, Medicaid or Tricare insurance, this requirement was recorded sentence recognition scores ≤40% correct in the best-aided condition (13). For patients with private insurance sentence recognition scores must be ≤50% correct in the ear to be implanted and ≤60% in the opposite ear or binaurally (14). Patients meeting these criteria for the current analysis were divided into three groups based on their qualifying test condition: sentence in quiet qualifiers (Group 1, n = 12), sentence in noise at +10 dB SNR qualifiers (Group 2, n = 16), and sentence in noise at +5 dB SNR qualifiers (Group 3, n = 30). Patients in Group 1 also met criteria in both noise conditions. Patients in Group 2 had qualified also in the +5 dB SNR condition but not in quiet. Patients in Group 3 qualified only in the +5 dB SNR conditions but not in the +10 dB SNR nor in quiet conditions. Patients using an electric-acoustic stimulation strategy were not excluded from analysis, and this factor was examined for relevance to outcomes (total n=11; n Group 1=3, n Group 3=8).
Patient speech recognition progress was monitored during appointments at 1, 3, 6, and 12 months post-activation prior to re-mapping. Once the first year of implant use had passed, patients returned for testing and mapping annually unless otherwise indicated. Postoperative speech recognition measures for the implanted ear included word recognition using CNC word lists and sentence recognition using AzBio sentence lists. During this testing, the non-implanted ear was plugged if thresholds less than 60 dB HL were present. Postoperatively, if the patient scored 75% or greater during sentence testing in quiet, testing in noise was administered at +10 dB SNR. If the patient scored 75% or greater in the +10 dB SNR condition, testing in noise was then administered at +5 dB SNR. For patients with aidable hearing (determined by thresholds, speech recognition performance using the hearing aid, and patient subjective determination) in the nonimplanted ear, AzBio testing was performed in the bimodal (CI + contralateral HA) in addition to the CI alone condition.
Statistical Analysis
We performed statistical analysis of the changes seen in CNC word recognition comparing pre-operative scores of the aided operative ear to CI alone post-operative scores. We then describe changes in AzBio sentence recognition comparing best-aided pre-operative scores (right hearing aid, left hearing aid, or bilaterally aided – whichever condition the patient performed best in) to post-operative scores with the CI alone and best-aided (CI alone or bimodal depending on the patient). The change in CNC and AzBio scores from pre-operative visit to 1-year post-implantation were tested within aided status, qualifying group, and testing condition using one-sample t-tests against a null change of 0 percentage points. Linear regression models were fit to compare post-op scores between groups adjusting for age and EAS for quiet testing conditions under different aided statuses to explore the possible effects of age or EAS. All analyses and figures were created using R v3.6.0 with p-values less than 0.05 considered significant.
Results
Patients qualifying in quiet (Group 1)
Unilateral pre- vs. CI-alone postoperative CNC words and AzBio
On average, post-operative CNC word recognition for individuals qualifying in quiet (Group 1) improved by 45.6 percentage points [95% CI: (31.0, 60.2), p <0.001] (Table 2). Post-operative AzBio sentence recognition with the CI alone improved in quiet for all individuals by an average of 74.9 percentage points [95% CI: (64.9, 84.9), p<0.001] compared to pre-operative scores of the implant ear alone (Table 2).
Table 2.
Mean change in AzBio and CNC testing for each qualifying group (quiet qualifiers (Group 1), +10 dB SNR qualifiers (Group 2), and +5 dB SNR qualifiers (Group 3) for each testing condition.
| Pre-Op Aided | Post-Op Aided | Qualifying Group | Testing Condition | N | Mean Change | 95% CI | p-value |
|---|---|---|---|---|---|---|---|
| Implant-Ear Alone | CI Alone | Quiet Only | CNC | 10 | 45.6 | (31.0, 60.2) | <0.001 |
| +10 Only | 15 | 33.5 | (19.4, 47.5) | <0.001 | |||
| +5 Only | 30 | 25.6 | (16.3, 35.0) | <0.001 | |||
| CI Alone | Quiet Only | AzBio Quiet | 8 | 74.9 | (64.9, 84.9) | <0.001 | |
| +10 Only | 13 | 52.2 | (36.0, 68.3) | <0.001 | |||
| +5 Only | 16 | 28.7 | (10.3, 47.1) | 0.005 | |||
| Best-Aided | CI Alone | Quiet Only | AzBio Quiet | 12 | 53.4 | (38.4, 68.4) | <0.001 |
| AzBio +10 | 3 | 44.3 | (8.8, 79.8) | 0.033 | |||
| AzBio +5 | 0 | - | - | - | |||
| +10 Only | AzBio Quiet | 14 | 19.5 | (9.7, 29.3) | <0.001 | ||
| AzBio +10 | 10 | 39.2 | (26.9, 51.5) | <0.001 | |||
| AzBio +5 | 4 | 27.5 | (−5.7, 60.7) | 0.078 | |||
| +5 Only | AzBio Quiet | 22 | −1.8 | (−9.5, 5.8) | 0.626 | ||
| AzBio +10 | 11 | −10.7 | (−27.3, 5.8) | 0.179 | |||
| AzBio +5 | 5 | −3.6 | (−27.8, 20.6) | 0.701 | |||
| Best-Aided | Quiet Only | AzBio Quiet | 5 | 46.8 | (22.0, 71.6) | 0.006 | |
| AzBio +10 | 3 | 53.0 | (19.6, 86.4) | 0.021 | |||
| AzBio +5 | 3 | 42.3 | (5.0, 79.6) | 0.039 | |||
| +10 Only | AzBio Quiet | 6 | 12.5 | (−11.5, 36.5) | 0.238 | ||
| AzBio +10 | 9 | 30.9 | (12.4, 49.3) | 0.005 | |||
| AzBio +5 | 6 | 22.5 | (1.0, 44.0) | 0.043 | |||
| +5 Only | AzBio Quiet | 8 | 2.8 | (−8.0, 13.5) | 0.563 | ||
| AzBio +10 | 13 | 16.1 | (4.2, 28.0) | 0.012 | |||
| AzBio +5 | 13 | 14.1 | (5.0, 23.1) | 0.005 | |||
Best-aided pre- vs. best-aided and CI-alone postoperative AzBio
Postoperative AzBio sentence recognition with the CI alone in quiet and in noise at +10 dB SNR improved by 53.4 percentage points [95% CI: (38.4, 68.4), p<0.001] and 44.3 percentage points [95% CI: (8.8, 79.8), p=0.033] respectively when compared to pre-operative best-aided scores (Table 2). Sentence recognition with the CI alone in noise at +5 dB SNR could not be reported as there was no data in this condition for Group 1. Post-operative AzBio sentence recognition in the best-aided condition in quiet improved by 46.8 percentage points [95% CI: (22.0, 71.6), p=0.006], improved in noise at +10 dB SNR by 53 percentage points [95% CI: (19.6, 86.4), p=0.021], and improved in noise at +5 dB SNR by 42.3 percentage points [95% CI: (5.0, 79.6), p=0.039] (Table 2).
Patients qualifying in noise at +10 dB SNR (Group 2)
Unilateral pre- vs. CI-alone postoperative CNC words and AzBio
On average, post-operative CNC word recognition for individuals qualifying in noise at +10 dB SNR (Group 2) improved by 33.5 percentage points [95% CI: (19.4, 47.5), p<0.001] (Table 2). Post-operative AzBio sentence recognition with the CI alone compared to pre-operative scores of the implant ear alone improved in quiet by 52.2 percentage points [95% CI: (36.0, 68.3), p<0.001] (Table 2).
Best-aided pre- vs. best-aided and CI-alone postoperative AzBio
Postoperative AzBio sentence recognition with the CI alone compared to pre-operative best-aided scores improved in quiet by 19.5 percentage points [95% CI: (9.7, 29.3), p<0.001] and in noise at + 10 dB SNR by 39.2 percentage points [95% CI: (26.9, 51.5), p<0.001]. While AzBio sentence recognition with the CI alone in noise at +5 dB SNR trended positively by 27.5 percentage points, this improvement was not significant [95% CI: (−5.7, 60.7), p = 0.078]. On average, post-operative AzBio sentence recognition in the best-aided condition in quiet improved by 12.5 percentage points, though this improvement was not significant [95% CI: (−11.5, 36.5), p=0.238]. AzBio sentence recognition in the best-aided condition in noise at +10 dB SNR improved by 30.9 percentage points [95% CI: (12.4, 49.3), p=0.005] and improved at +5 dB SNR by 22.5 percentage points [95% CI: (1.0, 44.0), p=0.043] (Table 2).
Patients qualifying in noise at +5 dB SNR (Group 3)
Unilateral pre- vs. CI-alone postoperative CNC words and AzBio
On average, post-operative CNC word recognition for individuals qualifying in noise at +5 dB SNR (Group 3) improved by 25.6 percentage points [95% CI: (16.3, 35.0), p<0.001] (Table 2). AzBio sentence recognition with the CI alone compared to pre-operative scores of the implant ear alone improved in quiet by 28.7 percentage points [95% CI: (10.3, 47.1), p<0.001] (Table 2).
Best-aided pre- vs. best-aided and CI-alone postoperative AzBio
Post-operative AzBio sentence recognition with the CI alone exhibited an average decrement in the quiet, +10 dB SNR, and +5 dB SNR conditions of −1.8 percentage points [95% CI: (09.5, 5.8), p=0.626], −10.7 percentage points [95% CI: (−27.3, 5.8), p=0.179], and −3.6 percentage points [95% CI: (−27.8, 20.6), p=0.701] respectively, though this decrement was not statistically significant. While improvement in the best-aided condition in quiet trended positively by 2.8 percentage points [95% CI: (−8.0, 13.5), p=0.563], this improvement was not significant. Improvement in AzBio sentence recognition in the best-aided condition in noise at +10 dB SNR and + 5 dB SNR however, was significant, increasing by 16.1 percentage points [95% CI: (4.2, 28.0), p=0.012] and 14.1 percentage points [95% CI: (5.0, 23.1), p=0.005] respectively.
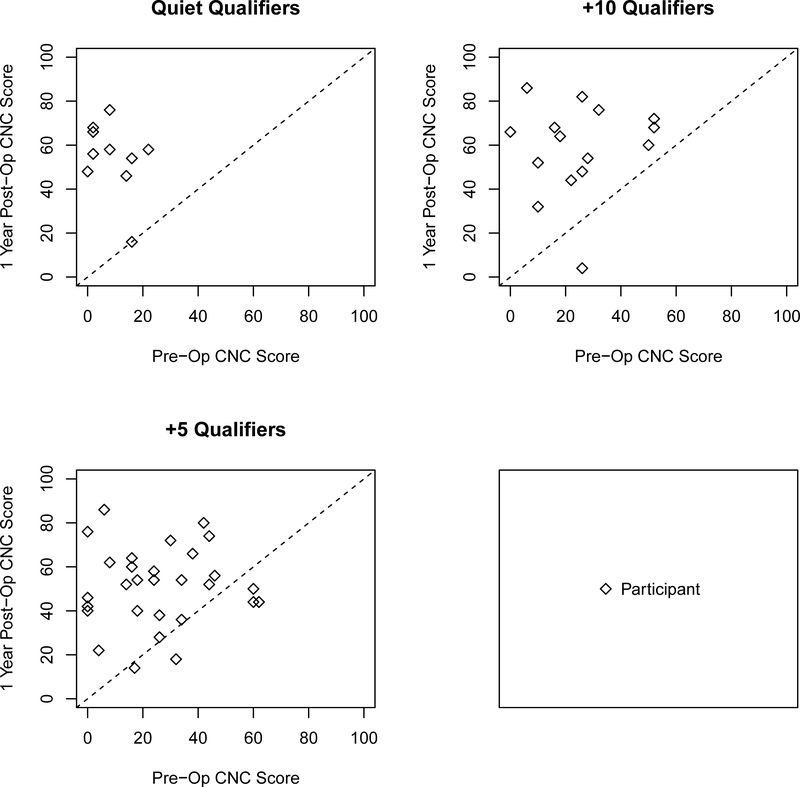
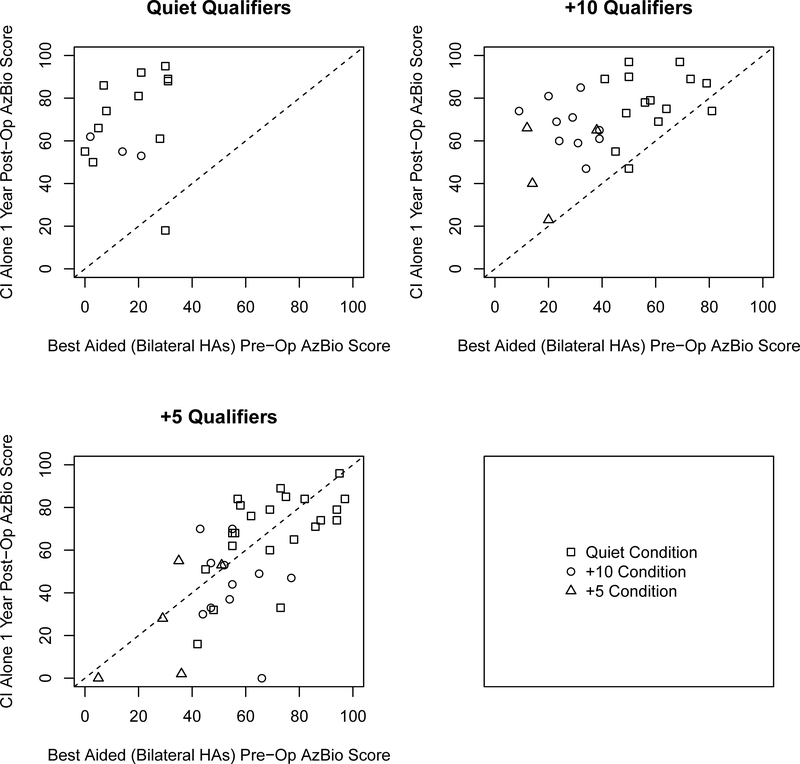
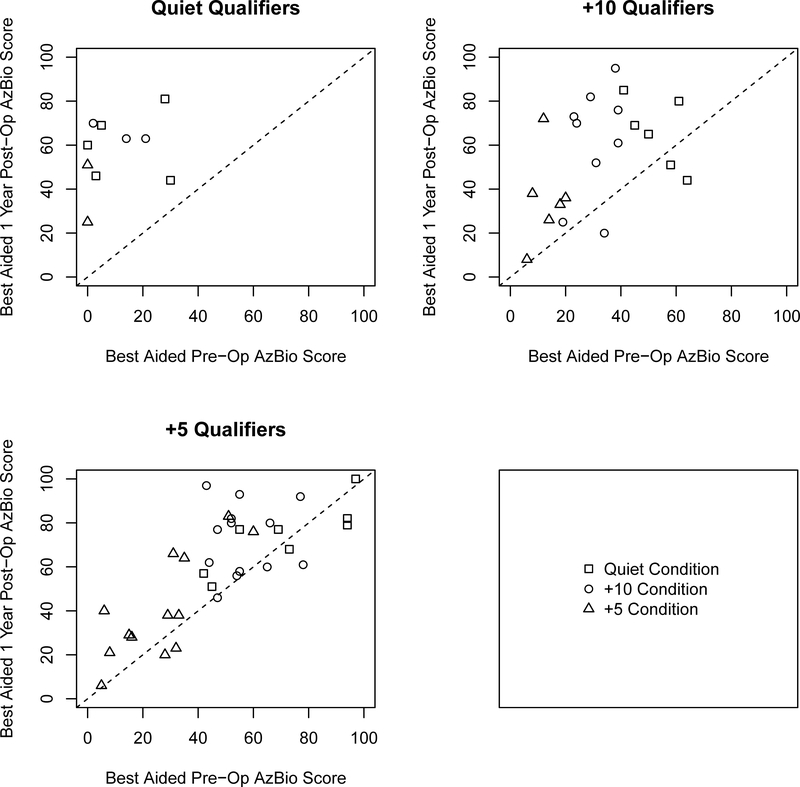
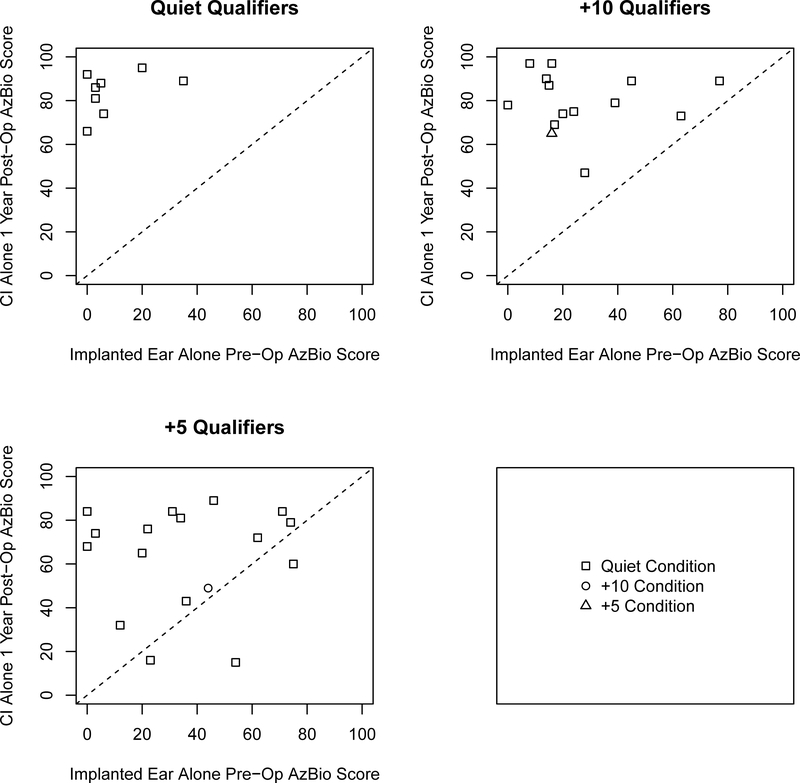
To examine individual variability amongst the three groups we plotted the CI alone post-operative CNC scores as a function of the performance of the aided CNC scores of the ear to be implanted for all three groups (Figure 1) and plotted the changes in AzBio sentence recognition for CI alone and best-aided postoperative scores as a function of best-aided pre-operative scores (shown in Figures 2 and 3 respectively) as well as the CI alone as a function of the implant-ear alone (Figure 4). Overall, the figures indicate that individuals qualifying in quiet or at +10 dB SNR (Groups 1 and 2) improve from pre-operative to postoperative testing, however the amount of improvement is dependent upon test condition, pre-operative performance, and contribution of the non-implanted ear. These factors are particularly important to consider for individuals qualifying at +5 dB SNR (Group 3) as improvement from pre- to postoperative testing was both dependent upon test condition and the consideration of the non-implanted ear (i.e., use of a contralateral hearing aid).
Figure 1.
Scatter plot comparing CI alone postoperative CNC scores as a function of the aided operative ear CNC scores for all three groups. Shapes represent candidacy group. Data falling below the dotted diagonal line indicate decrement in score one year postoperative. Data falling above the dotted diagonal line indicate improvement in score since implantation.
Figure 2.
Scatter plots comparing one-year CI alone postoperative AzBio sentence scores as a function of pre-operative AzBio sentence scores in the best aided condition. Panels represent candidacy group and shape represents the testing condition evaluated. Data falling below the dotted diagonal line indicate decrement in score one year postoperative. Data falling above the dotted diagonal line indicate improvement in score since implantation.
Figure 3.
Scatter plots comparing one-year best-aided postoperative AzBio sentence scores as a function of pre-operative AzBio sentence scores in the best aided condition. Panels represent candidacy group and shape represents the testing condition evaluated.
Figure 4.
Scatter plots comparing one-year CI alone postoperative AzBio sentence scores as a function of pre-operative AzBio sentence scores with the implanted ear alone. Panels represent candidacy group and shape represents the testing condition evaluated.
On linear regression analysis adjusting for age (one-way ANOVA) comparing postoperative performance in quiet across groups we found no difference on CNC testing [0.10 (−0.22, 0.42); p=0.547], CI alone AzBio testing [−0.25 (−0.68, 0.18); p=0.247] or best aided AzBio testing [−0.20 (−0.71, 0.31); p=0.435]. On linear regression analysis adjusting for the effect of EAS usage (Fisher’s exact test) comparing postoperative performance in quiet across groups we found no difference on CNC testing [2.12 (−12.78, 17.02); p=0.780], CI alone AzBio testing [EAS: −7.61 (−28.49, 13.26); p=0.475] or best aided AzBio testing [EAS: −0.50 (−20.90, 19.90); p= 0.962].
Discussion
On some level any individual, including those with normal hearing, if testing with a low enough SNR could be deemed a CI candidate. Ultimately it is the comparison of an individual’s preoperative best aided performance with their postoperative best-aided (CI with or without contralateral hearing aid) using identical testing conditions and materials that will provide the most meaningful audiological assessment of whether a CI improved speech recognition. With more studies advocating CI candidacy criteria expansion (2, 8, 15–17) as well as clinics moving towards candidacy determination based on +5 dB speech-in-noise testing (11) there is an increasing need to evaluate patient outcomes for individuals who only qualify for a CI in noise, particularly at +5 dB SNR. To date, few studies have compared outcomes of individuals who have qualified with speech material in quiet versus those who qualified with speech materials presented in noise. The current findings expand on those of Mudery et al. (2016) who examined the postoperative outcomes for older adults who met CI candidacy criteria in noise (AzBio presented at +10 dB SNR or +5 dB SNR), but not in quiet. The authors found that postoperative AzBio scores for the implanted ear improved, on average for both testing in quiet and in noise (11). However, 2 of the 15 patients displayed some degree of decrement in performance, warranting further investigation of expected outcomes for individuals qualifying for a CI in noise. Importantly this study did not evaluate those individuals qualifying in +10 versus +5 dB SNR as individual groups. In our study we found that in the best aided condition there was one patient in Group 3 who had a decrement in performance exceeding the AzBio critical difference when tested in quiet (greater than 23.3% when calculated using any starting level with one list as specified by Schafer et al., 2012). In the best-aided condition no patients from any group exhibited a decrement exceeding the critical difference when tested in +10 dB or +5 dB SNR. These findings suggest that, though uncommon on the whole, a decrement in performance postoperatively can occur in some patients.
The current study examined similar questions, seeking to specifically compare individuals qualifying in the +5 dB SNR condition. We found that on average, CNC scores improved at the one-year appointment for all qualifying groups. However, when examining improvements in AzBio sentence scores, we saw more variability in improvement across qualifying groups. In particular we observed insignificant improvement in the best-aided condition for noise qualifiers (Group 2 and Group 3) when tested postoperatively in quiet, likely due to the higher pre-operative quiet scores in these individuals. When examining postoperative outcomes with the CI alone compared to best-aided pre-operative results, we saw insignificant change for noise qualifiers (Groups 2 and 3) in the +5 dB SNR testing condition as well as the quiet and +10 dB SNR testing conditions for the +5 dB SNR qualifiers (Group 3). However, comparisons of postoperative CI alone outcomes compared to pre-operative results of the implant-ear alone showed significant improvements in all testing conditions for all three qualifying groups.
Although an average improvement in CNC scores when comparing preoperative testing of the aided operative ear to CI alone postop was observed across qualifying groups, 6 of the 58 patients exhibited some degree of decrement in CNC scores (Figure 1). Importantly, five of these six individuals who had decreased performance had qualified in the +5 dB SNR condition only (Group 3). For Group 3 (+5 dB qualifiers), although evaluations of AzBio sentence recognition in the best-aided condition (Table 2 and Figure 3) showed a significant improvement in the +10 and +5 dB SNR testing conditions (likely due to aidable residual hearing in the contralateral ear for this group), evaluations of AzBio sentence recognition in the CI alone condition showed no significant change and a non-significant trend suggesting an average decrease in all conditions (Table 2 and Figure 2), likely due to their higher pre-operative scores, particularly scores in quiet. However, comparing postoperative CI alone outcomes to pre-operative results of the implant-ear alone for Group 3 showed significant improvements in the quiet testing condition (Table 2 and Figure 4, the +10 and +5 testing conditions are not listed as a reflection of the available data).
The question of whether individuals who qualified in noise (Groups 2 and 3), and more specifically the +5 dB qualifying group (Group 3) improved post-implantation depends on the consideration of the contralateral ear. Individuals in Group 3 showed significant asymmetry in CNC word recognition between ears [mean asymmetry was 22.6 percentage points, 95% CI: (15.0, 30.2)] compared to CNC word recognition asymmetry of the quiet qualifiers (Group 1) [mean asymmetry was 8.4 percentage points, 95% CI: (4.1, 12.7), p<0.05]. This finding in combination with the significant improvement in AzBio scores when comparing best-aided postoperative to best-aided pre-operative results (Figure 2) along with CI alone to pre-operative implanted ear alone results (Figure 4) suggests the importance of considering the status of the non-implanted ear and plan of treatment for that ear in candidacy decisions.
When assessing individuals both for CI candidacy and tracking performance at follow-up appointments, it is important to assess patients with materials that are ecologically valid. This includes decisions both regarding words versus sentences, and also the level of noise that is presented. Studies classifying real-world SNRs based on recordings of everyday listening situations of hearing aid users with an average PTA between 25 and 60 dB HL have found that for speech in babble noise, the median SNR was slightly below 5 dB (9, 10). This information would suggest that testing and qualifying CI users with material presented at 5 dB SNR is a valid assessment. However, if CI recipients are avoiding environments with low SNRs or are not practicing listening in noise, this may be an unrealistic test condition for this population. Specific listening environments and their SNRs are also important considerations for individuals qualifying only at +5 dB SNR. If an individual only qualifies for a CI in noise, but does not find themself in noisy environments in their everyday life, a CI may not be a good solution given the risk of speech recognition in quiet declining postoperatively.
In both the current study and within the literature, speech recognition outcomes for CI recipients vary (2, 6, 19). In the past, duration of hearing loss and pre-operative sentence recognition have been established as significant predictors of postoperative word recognition (6, 16, 17). While other findings have observed attention to spectral structure, phonemic sensitivity, and the combination of both bottom-up and top-down processes to be important predictors of speech recognition (21, 23–24). This wide range of factors and variability in outcomes emphasizes both the need for utilizing a uniform, comprehensive test battery (2) as well as the need for counseling patients, particularly those qualifying in more aggressive SNRs such as +5 dB, regarding realistic expectations for outcomes and the risk of potential decrement in speech recognition in quiet, as well as the need to match listening needs with the qualifying candidacy condition (3, 8, 20).
One component of CI outcomes that was not addressed in the present study and is not included in the MSTB (7) is subjective measures of perceived benefit. Previous studies have found disagreement between objective measures of postoperative speech perception and subjective measures of perceived performance (24, 25), emphasizing the importance of tracking this element of CI benefit. Tracking outcomes in perception of benefit, quality of life changes, listening effort, and/or communication partner perception of benefit may offer a more comprehensive picture of progress in CI patients, which will become increasingly important as CI candidacy criteria expands and more individuals with higher pre-operative scores qualify for a CI.
The current design was a retrospective study, which by nature exhibits limitations not found in prospective studies. These constraints include incomplete data sets, due to the progressive nature of the AzBio testing (follow-up testing in quiet was tested first and noise conditions were only tested if a score of 75% or greater was achieved). This progressive nature of testing also introduced a sampling bias as +10 dB SNR testing only included individuals who scored 75% or greater in quiet and +5 dB SNR testing only included individuals who scored 75% or greater in +10 dB SNR testing. The relatively low sample size, particularly for Groups 1 and 2 should also be taken into account when interpreting results. This study also evaluated patient performance outcomes at one time point, discounting results of individuals who may have plateaued in performance later than one year, or individuals who did not perform well during this particular appointment.
One important consideration in interpreting these data lies in how performance may change over time. Specifically our patients who qualified for a CI in only the +5 dB SNR condition likely will experience progression in the loss of their acoustic hearing over time had they not undergone implantation. Their pre-operative testing is therefore likely capturing their best performance which is ostensibly only going to worsen over time. As such comparing post-CI performance, whether one year later (as in this study) or henceforth, to their pre-operative, optimal performance data introduces some bias as to how well they would currently be performing with hearing aids had they not elected to undergo implantation. This bias cannot be easily controlled for however should be kept in mind when scrutinizing whether CI in patients with more residual hearing preop (such as our Group 3) is indeed in the patients’ best interest, which ultimately is the primary focus of this study.
Conclusion
The evidence of the current study suggests that for individuals with higher pre-operative scores (e.g., individuals qualifying at +5 dB SNR but not at +10 dB SNR), postoperative objective outcomes can be variable. While our findings indicate that some individuals qualifying at +5 dB SNR experienced a decrement in their postoperative speech recognition with the CI only, many individual’s speech recognition improved and improvement in all test conditions was observed when considering postoperative testing results in the best-aided condition. While individuals qualifying for a CI only in the +5 dB SNR condition may derive significant benefit from implantation, speech understanding outcomes can be more variable for this group. As such, the status of the contralateral ear as well as daily listening needs should be considered in candidacy and additional pre-operative counseling and caution should be strongly emphasized in this population prior to implantation.
Contributor Information
Emily M.H. Lundberg, Department of Speech-Language-Hearing Sciences, University of Colorado at Boulder, Boulder, Colorado
Darcy Strong, UCHealth, Hearing and Balance Clinic, University of Colorado Hospital.
Melinda Anderson, UCHealth, Hearing and Balance Clinic, University of Colorado Hospital; Department of Otolaryngology, University of Colorado School of Medicine.
Alexander M. Kaizer, Department of Biostatistics and Informatics, Colorado School of Public Health, University of Colorado – Anschutz Medical Campus, Aurora, Colorado
Samuel Gubbels, UCHealth, Hearing and Balance Clinic, University of Colorado Hospital; Department of Otolaryngology, University of Colorado School of Medicine.
References
- 1.Roche JP, Hansen MR. On the horizon: Cochlear implant technology. Otolaryngol Clin North Am 2015;48:1097–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlson ML, Sladen DP, Gurgel RK, et al. Survey of the American Neurotology Society on cochlear implantation: Part 1, candidacy assessment and expanding indications. Otol Neurotol 2018;39:e12–e19. [DOI] [PubMed] [Google Scholar]
- 3.Sorkin DL. Cochlear implantation in the world’s largest medical device market: Utilization and awareness of cochlear implants in the United States. Cochlear Implants International 2013;14(1):S4–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorkin DL, Buchman CA. Cochlear implant access in six developed countries. Otol Neurotol 2016;37(2):e161–e164. [DOI] [PubMed] [Google Scholar]
- 5.Blamey P, Artieres F, Baskent D, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: An update with 2251 patients. Audiol Neurotol 2013;18:36–47. [DOI] [PubMed] [Google Scholar]
- 6.Holden LK, Finley CC, Firszt JB, et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear 2013;34:342–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minimum Speech Test Battery (MSTB) for Adult Cochlear Implant Users; 2011. Available at: http://www.auditorypotential.com/MSTBfiles/MSTBManual2011-06-20%20.pdf. Accessed June 25, 2019.
- 8.Holder JT, Reynolds SM, Suderhaus LW, et al. Current profile of adults presenting for preoperative cochlear implant evaluation. Trends in Hearing 2018;22:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smeds K, Wolters F, Rung M. Estimation of signal-to-noise ratios in realistic sound scenarios. J Am Acad Audiol 2015;26(2):183–196. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Stangl E, Chipara O, et al. Characteristics of real-world signal to noise ratios and speech listening situations of older adults with mild to moderate hearing loss. Ear Hear 2017;39(2):293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mudery JA, Francis R, McCrary H, et al. Older individuals meeting Medicare cochlear implant candidacy criteria in noise but not in quiet: Are these patients improved by surgery? Otol Neurotol 2016;38:187–191. [DOI] [PubMed] [Google Scholar]
- 12.Byrne D, Dillon H, Ching T, et al. NAL-NL1 procedure for fitting nonlinear hearing aids: characteristics and comparisons with other procedures. J Am Acad Audiol 2001;12:37–51. [PubMed] [Google Scholar]
- 13.Centers for Medicare and Medicaid Services (CMS). National coverage determination (NCD) for cochlear implantation (50.3). Publication No. 100–3. 2005. Available at https://www.cms.gov/Medicare/Coverage/Coverage-with-Evidence-Development/Cochlear-Implantation-.html [PubMed]
- 14.Gifford R Cochlear implant patient assessment: Evaluation of candidacy, performance, and outcomes. 2013. Plural Publishing: San Diego, California. [Google Scholar]
- 15.Gifford RH, Dorman MF, Shallop JK, et al. Evidence for the expansion of adult cochlear implant candidacy. Ear Hear 2010;31:186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin FR, Chien WW, Li L, et al. Cochlear implantation in older adults. Medicine 2012;91:229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubinstein JT, Parkinson WS, Tyler RS, et al. Residual speech recognition and cochlear implant performance: Effects of implantation criteria. Am J Otol 1999;20:445–452. [PubMed] [Google Scholar]
- 18.Sladen DP, Gifford RH, Haynes D, et al. Evaluation of a revised indication for determining adult cochlear implant candidacy. Laryngoscope 2017;127:2368–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang E, Coelho DH. Beyond sentence recognition in quiet for older adults: Implications for cochlear implant candidacy. Otol Neurotol 2018;39:979–986. [DOI] [PubMed] [Google Scholar]
- 20.Brant JA, Eliades SJ, Kaufman H, et al. AzBio speech understanding performance in quiet and noise in high performing cochlear implant users. Otol Neurotol 2018;39:571–575. [DOI] [PubMed] [Google Scholar]
- 21.Moberly AC, Lowenstein JH, Nittrouer S. Word recognition variability with cochlear implants: The degradation of phonemic sensitivity. Otol Neurotol 2016; 37:470–477. [DOI] [PubMed] [Google Scholar]
- 22.Gifford RH, Shallop JK, Peterson AM. Speech recognition materials and ceiling effects: Considerations for cochlear implant programs. Audiol Neurotol 2008;13:193–205. [DOI] [PubMed] [Google Scholar]
- 23.Moberly AC, Lowenstein JH, Nittrouer S. Word recognition variability with cochlear implants: “Perceptual attention” versus “auditory sensitivity”. Ear Hear 2016;37:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofkens-Van den Brandt A, Martens G, Gilles A, et al. Auditory performances in older and younger adult cochlear implant recipients: Use of the HEARRING registry. Otol Neurotol 2019;40(8):e787–e795. [DOI] [PubMed] [Google Scholar]
- 25.Wackym PA, Runge-Samuelson CL, Firszt JB, et al. More challenging speech-perception tasks demonstrate binaural benefit in bilateral cochlear implant users. Ear Hear 2007;28(2):80S–85S. [DOI] [PubMed] [Google Scholar]
- 26.Schafer EC, Pogue J, Milrany T. List equivalency of the AzBio sentence test in noise for listeners with normal-hearing sensitivity or cochlear implants. J Am Acad Audiol 2012;23(7):501–9. [DOI] [PubMed] [Google Scholar]