Abstract
Purpose of review
Endoscopic detection of mucosal healing has emerged as a primary therapeutic endpoint in inflammatory bowel disease. Endoscopically identified esophageal features are increasingly being utilized in diagnostic and therapeutic decisions in eosinophilic esophagitis (EoE).
Recent findings
Studies over the past 5 years have demonstrated the validity, accuracy, and clinical relevance of a systematic, endoscopic assessment of esophageal abnormalities in EoE. The initial severity of EoE endoscopic findings has important implications with regard to therapeutic options, including the need for dilations, and may be an important predictor of the effectiveness of medical therapies. Moreover, endoscopic parameters can serve as reliable therapeutic endpoints that substantiate the interpretation of currently used metrics of patient-reported symptom outcomes and eosinophil density. Finally, tools such as endosonography and functional luminal imaging probe are providing fundamental insights regarding the remodeling consequences of EoE that are the central determinants of disease complications.
Summary
Endoscopic features are having an increasing role in the diagnosis, phenotype characterization, and choice of therapies for EoE. Comprehensive assessment of therapeutics in EoE should ideally incorporate symptoms, histology, and endoscopic healing.
Keywords: endoscopy, eosinophilic esophagitis, food allergy, gastroesophageal reflux disease
INTRODUCTION
Eosinophilic esophagitis (EoE) is a chronic, immune-mediated disease characterized by esophageal symptoms associated with eosinophil-predominant inflammation localized to the esophagus in the absence of recognized causes of esophageal eosinophilia [1,2]. This definition devised by a consensus panel does not require the presence of endoscopically identified esophageal features for the diagnosis of EoE. Nevertheless, endoscopic findings are often viewed by clinicians as highly indicative of EoE, and recent data support their diagnostic utility. These findings include longitudinal furrows, white exudates (plaques), rings (trachealization), strictures, edema (mucosal pallor or decreased vascularity), narrow-caliber esophagus, and fragile or ‘crêpe-paper’ mucosa (Fig. 1) [2–5] (Table 1). Furthermore, endoscopic characteristics provide important information about the underlying phenotype of the disease and inform clinical decisions regarding the efficacy and appropriateness of therapeutic interventions. The purpose of this article is to review the available literature regarding the significance and limitations of endoscopy in the management of EoE.
FIGURE 1.
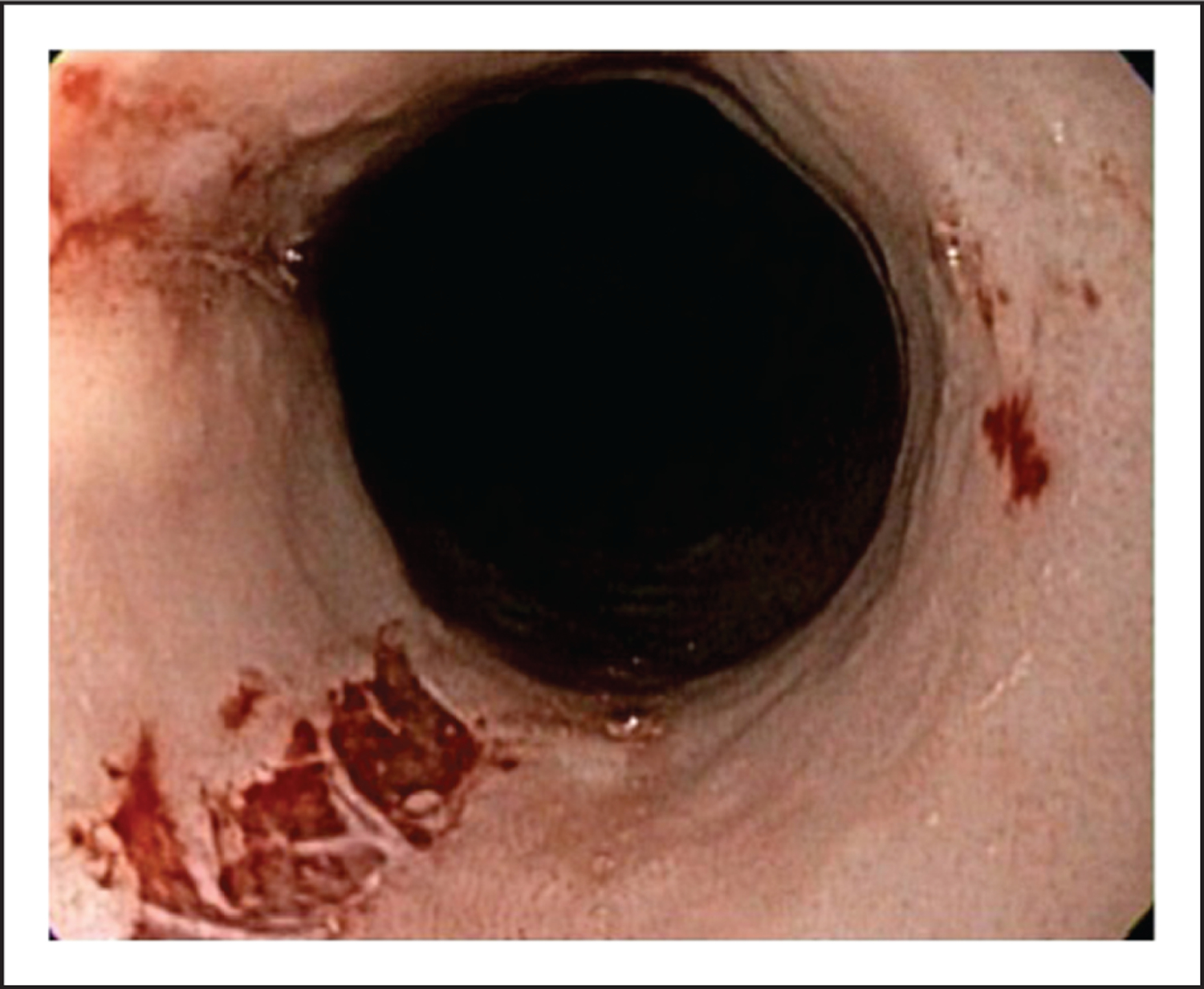
Crêpe-paper esophagus (also referred to as mucosal fragility or laceration upon passage of an endoscope). This sign has limited sensitivity in the diagnostic evaluation of eosinophilic esophagitis.
Table 1.
Endoscopic findings
| Endoscopic feature | Comment |
|---|---|
| Edema | Mucosal pallor or decreased vascularity. Best assessed either real-time or with video assessment. Vascular markings are diminished during esophageal contractions. |
| Rings | Best appreciated when esophagus is fully insufflated. May be confused with transient plications (‘feline esophagus’). |
| Exudates | Often track along furrows. May be confused with white plaques from Candida esophagitis secondary to topical steroid administration. |
| Furrows | Longitudinal lines or ‘track marks’. Blood from biopsies can fill the indentations and increase detection. |
| Strictures | Can be focal or diffuse. Precise determination of diameter is difficult. |
| Crêpe-paper esophagus | Mucosal fragility determined by sloughing or disruption resulting from passage of an endoscope. |
| Narrow-caliber esophagus | Difficult to appreciate with endoscopy. Better defined by barium esophagram or functional luminal imaging probe. |
| ‘Tug’ sign | Subjective resistance or ‘stiffness’ appreciated when taking esophageal biopsies. |
INITIAL STUDIES DESCRIBE INADEQUACIES IN THE ENDOSCOPIC ASSESSMENT OF EOSINOPHILIC ESOPHAGITIS
Early studies suggested that endoscopic parameters had limited utility in the diagnosis of EoE because of poor sensitivity and inter-observer agreement. Retrospective studies reported a normal endoscopic appearance in approximately one-third of children with EoE [2]. A 2011 study by Peery et al. [6] used self-administered, on-line assessments of endoscopic still images in patients with EoE to evaluate inter-observer agreement and found less than encouraging results: among all gastroenterologists, inter-observer agreement was fair to good for identification of rings (κ = 0.56) and furrows (κ = 0.48), but was poor for identification of exudates (κ = 0.29). Levels of agreement did not change when the analysis was stratified by practice setting or patient volume, and did not improve when narrow-band images were added to white light images. The authors concluded that, as gastroenterologists, we are not all ‘seeing the same things’ and that endoscopic findings in suspected EoE may not be reliable markers on which to base diagnostic or treatment decisions. Subsequently, a meta-analysis performed by the same group again identified considerable variability in the reported prevalence and diagnostic utility of endoscopic features of EoE [4]. The investigators reviewed a total of 80 original articles and 20 abstracts and found a pooled prevalence of 44% for rings, 21% for strictures, 9% for narrow-caliber esophagus, 48% for linear furrows, 27% for white plaques, and 41% for edema. The diagnostic sensitivity for individual features ranged from 15 to 48%, with much higher ranges for specificity (90–95%). When only prospective studies were included, one or more endoscopic abnormality typical of EoE was found in 93% of patients (versus 83% for the pooled data). This study highlighted significant variability in the reporting of endoscopic findings in EoE as well as problems created by the absence of standardization in the definitions used to identify endoscopic features.
The issue of standardization of definitions used to identify endoscopic features of EoE merits discussion (Table 1). First, variable terminology had been used in earlier reports to describe analogous findings. For example, rings have been called ‘trachealization’, ‘corrugation’, and ‘felinization’, variable terms that can create confusion and lack of consistency among endoscopists. Felinization traditionally refers to transient plications or ripples of the esophageal mucosa that occur during short duration and axial shortening events such as retching. Such plications are best appreciated in a non-distended esophagus, and they completely efface over time and with air distension (Fig. 2). In contrast, rings in EoE are fixed and best visualized during distension. Second, in the absence of standardized scoring systems, findings such as loss of vascular markings or edema are often neglected and simply not reported by endoscopists. Terminology issues affect reporting of edema because vascular markings may be reduced in the setting of mucosal inflammation or subepithelial fibrosis. Visualization of vascularity is highly dependent upon the contractile state of the esophagus, a feature that can lead to misinterpretation of static images. Third, identification of the ‘narrow-caliber esophagus’ is seriously impaired by the lack of a standardized definition. The degree of radial narrowing and axial involvement that ‘upgrades’ an esophageal stricture to a narrow-caliber esophagus is unspecified. Luminal narrowing to less than 17 mm involving more than 50% of the esophageal length have been proposed as diagnostic criteria for this entity [7]. Finally, strictures are difficult to objectively measure. Although esophageal narrowing that impairs passage of an endoscope is obvious, stenoses between 12 and 20 mm can be overlooked because of lack of focality or superimposed constriction by the upper or lower esophageal sphincter. A recent study evaluating the sensitivity and specificity of identifying narrow-caliber esophagus (defined as <21 mm in diameter) on EGD compared with detailed measurement on barium esophagram revealed poor sensitivity (14.7%) and modest specificity (79.2%) [8]. For these reasons, systematic inspection and uniform nomenclature have been suggested to optimize the diagnostic yield of endoscopy in EoE [9].
FIGURE 2.
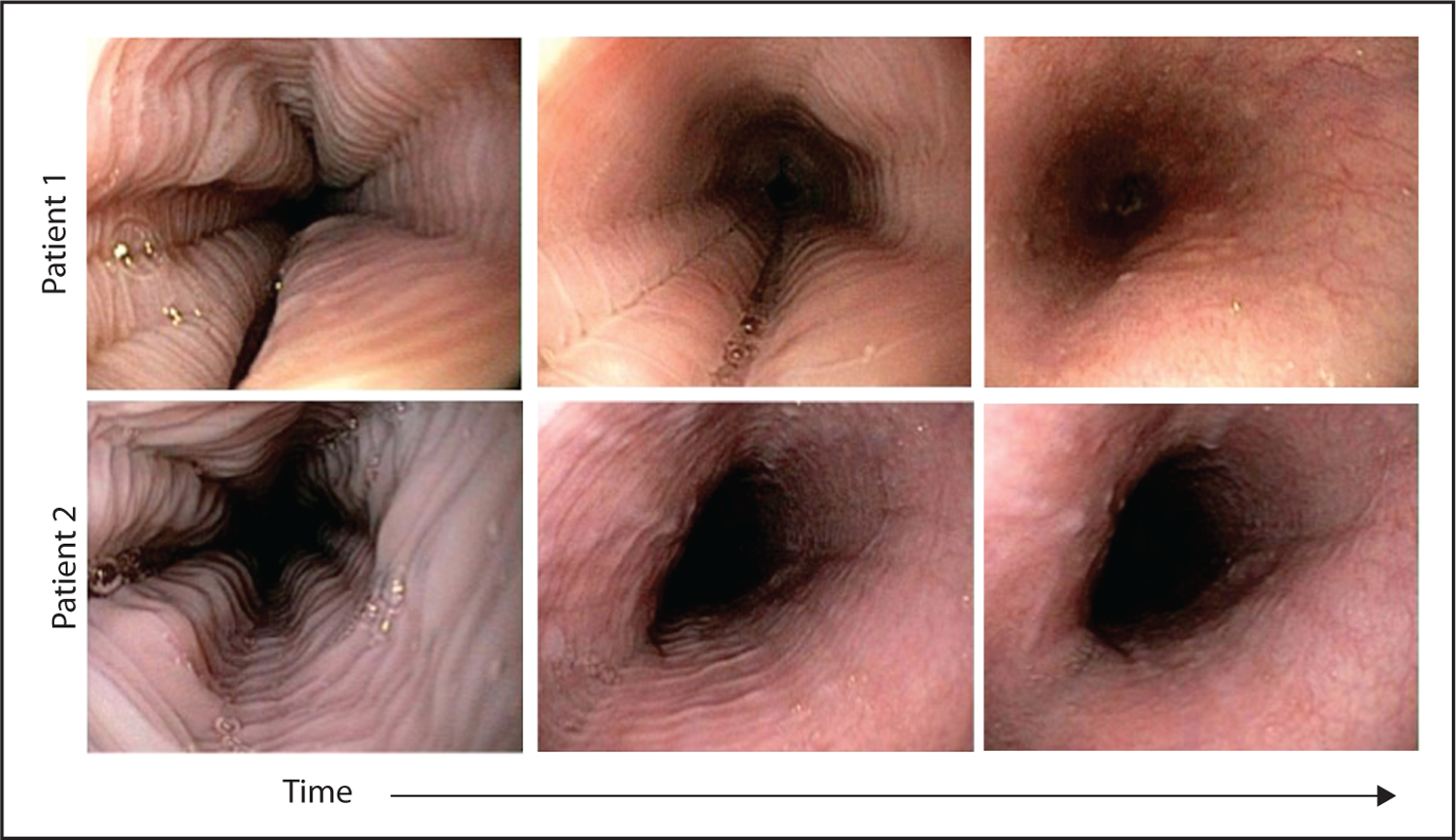
Transient esophageal rings (also known as felinization; short duration plications during belch, retching, and swallows that disappear with distension). Features can be confused with the fixed esophageal rings in eosinophilic esophagitis.
STANDARDIZATION IMPROVES THE UTILITY OF IDENTIFYING ENDOSCOPIC FEATURES IN EOSINOPHILIC ESOPHAGITIS
In light of the variability and heterogeneity of endoscopic findings in EoE noted in prior studies, a classification and grading system eventually was proposed and validated [3]. The EoE Endoscopic REFerence Scoring system, or EREFS, attempts to standardize nomenclature for the major features of EoE (edema, rings, exudates, furrows, and strictures), and also included a grading system, incorporating an assessment of severity of individual features (Fig. 3). The metric was created and validated through a process of determination of inter-observer agreement among private practice, pediatric, adult, and academic gastroenterologists. In contrast to prior studies in which still images were provided to the study participants, this study validation utilized recorded endoscopic videos. The original grading system proposed included narrow-caliber esophagus and feline esophagus as criteria, but these were removed from the modified system because agreement among endoscopists on those endoscopic features was only fair to poor. The modified grading system demonstrated good agreement for four major features of EoE (edema, rings, exudates, and furrows) (κ = 0.40– 0.54, 71–81% pairwise agreement) and the additional features of stricture and crêpe-paper esophagus (κ = 0.52 and 0.58, 79 and 92% agreement) (Fig. 3). Crêpe paper esophagus had an acceptable inter-observer agreement, but was excluded based on its very low prevalence.
FIGURE 3.
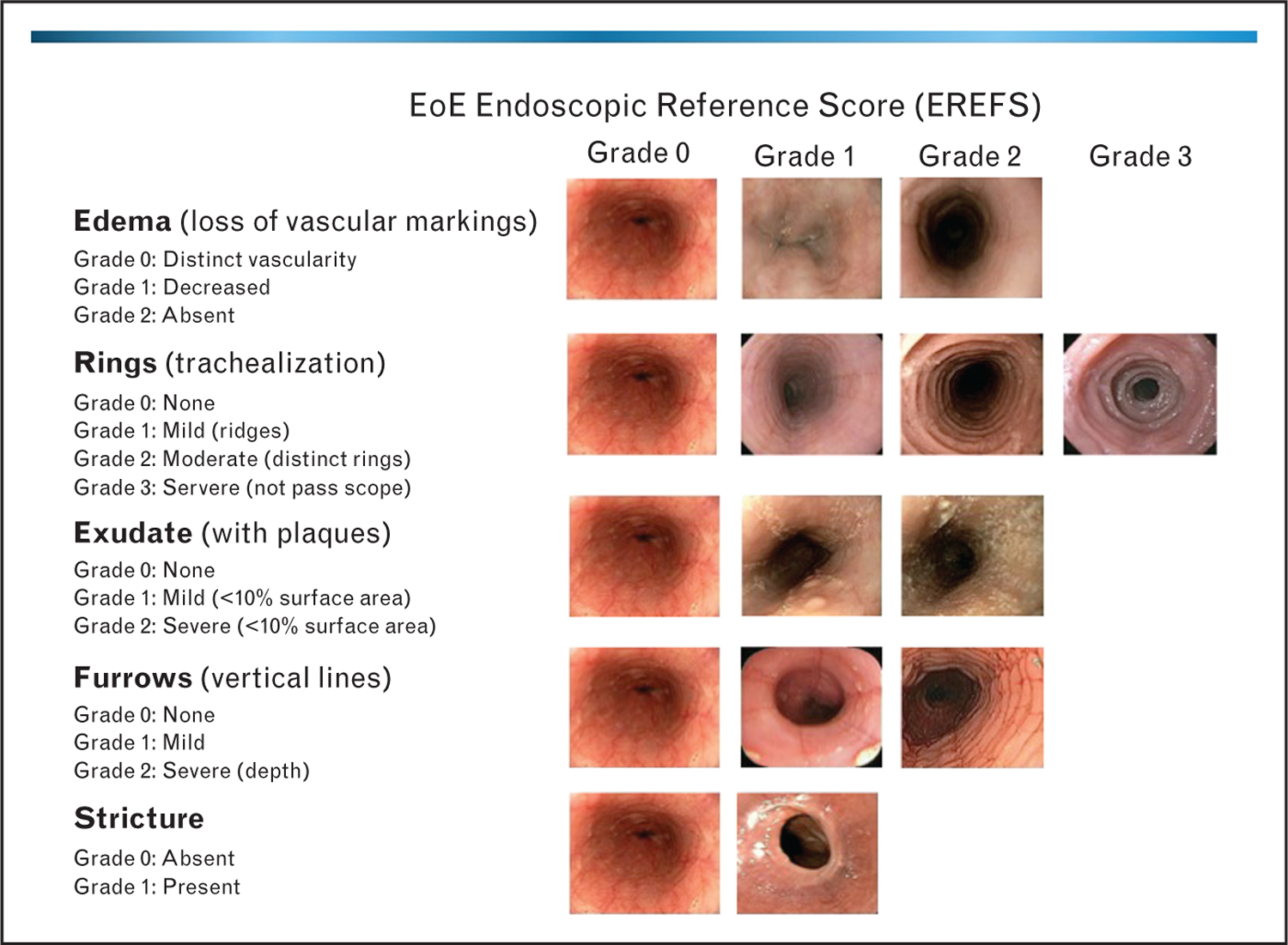
Representative endoscopic images depicting the classification and grading system for endoscopically identified esophageal features in eosinophilic esophagitis.
Since the introduction of the EREFS grading system, independent studies have further validated its performance characteristics. A European study by van Rhijn et al. [10■] assessed inter-observer agreement between four expert and four trainee endoscopists using an atlas of images from 30 different patients. This study also assessed intra-observer agreement by rescoring the images in a different order after 4 weeks. The authors found substantial inter-observer agreement for rings (κ = 0.70), exudates (κ = 0.63), and crêpe-paper esophagus (κ = 0.62), moderate for furrows (κ = 0.49) and strictures (κ = 0.54), and slight for edema (κ = 0.12). Intra-observer agreement was substantial for rings, furrows, and crêpe-paper esophagus, moderate for exudates and strictures, and less than chance for edema. No significant differences were noted between expert and trainee endoscopists. The low agreement for edema was likely affected by the use of still rather than video images.
Prospective utilization of the EREFS system has resulted in endoscopic identification of esophageal abnormalities in over 95% of EoE patients [11,12■]. This high sensitivity points to potential utility for the diagnosis. Rings and furrows are the most commonly identified abnormalities found in 70–90% of adult EoE patients. Edema is found in 60–80%, whereas exudates are present in 50–60% of adolescent and adult patients. Of note, studies have consistently demonstrated the lower detection of rings and strictures in pediatric cohorts compared with adult EoE cohorts.
Recent studies have demonstrated the clinical relevance of endoscopic severity assessment in EoE. The severity of each of the EREFS subscores was associated with patient-reported global symptom activity [13■]. Food impaction risk was also significantly associated with EREFS ring-subscore severity [14]. A recent study by Schoepfer et al. [15■] evaluated the physicians’ judgment of overall disease activity and found that gastroenterologists rate EoE activity mainly on the basis of endoscopic findings and symptoms as opposed to severity of histopathology.
‘SEEING IS BELIEVING’: EXPANDING THE SCOPE OF ENDOSCOPIC ASSESSMENT FROM DIAGNOSTIC TO THERAPEUTIC UTILITY
With recent studies supporting the accuracy of endoscopic findings in the diagnosis of EoE, it has become evident supporting their use as outcomes for the therapy of EoE. Although therapeutic trials have focused on the endpoints of symptoms and esophageal eosinophilia, experts have questioned the reliability of these important metrics as the sole determinants of successful EoE therapy [16]. Symptoms of dysphagia in EoE may improve as a result of changes in eating behaviors (e.g., avoidance of hard texture foods like meat, excessive mastication, pro-longed meal times) rather than changes in biologic activity of the disease. Using a validated patient-reported outcome instrument (eosinophilic esophagitis activity index, EEsAI), symptoms were shown to be unreliable as the primary indicator of disease activity assessed by endoscopy and pathology [17]. Dysphagia can respond dramatically to esophageal dilation without affecting the degree of esophageal eosinophilia [18]. For these reasons, histologic response has been used as an objective and reproducible measure of treatment response. However, histopathology, while central to the diagnosis of EoE, has limited correlation with patient-reported symptoms severity and physician-reported disease activity [11,17,19].
From a treatment standpoint, there is utility in grading endoscopic findings to predict response to treatment. Patients who have a predominantly ‘fibrostenotic’ pattern of injury with high-grade stenoses will benefit from esophageal dilation to alleviate dysphagia, irrespective of treatment effects on eosinophilic inflammation [18]. Trials evaluating response to corticosteroids have assessed endoscopic improvement as a secondary endpoint and have shown such improvements following treatment, particularly in the ‘inflammatory’ features of EoE including exudates and furrows [20,21]. A recent, retrospective analysis identified the presence of severe esophageal strictures that cannot be traversed with an adult endoscope as a negative predictor of histologic response to topical steroids [22]. The rate of response to steroid treatment in EoE patients with high-grade stenosis was about half that seen in EoE patients without high-grade strictures.
A recent study [12■] demonstrated that the EREFS score had a high degree of accuracy both for the diagnosis of EoE and for determining responsiveness to treatment. This study prospectively evaluated 67 patients with EoE who were treated with either topical steroids or dietary elimination, and compared their endoscopic findings to 144 control patients without EoE. The investigators found that the mean total EREFS score (range 0–9) was significantly greater in EoE patients than in control patients (3.88 versus 0.42, P > 0.001). After treatment, the score decreased significantly in EoE patients. The investigators also noted that the score correctly identified patients with EoE with a high degree of accuracy, with an area under the receiver operator characteristic curve of 0.934. Using a threshold score of 2 or greater, the EREFS has a sensitivity of 88%, specificity of 92%, positive predictive value of 84%, and negative predictive value of 94%. Another recent study was the first randomized, placebo-controlled trial of topical steroids to incorporate endoscopic outcomes determined by EREFS. In this study of oral budesonide suspension versus placebo, the EREFS scores significantly improved after treatment with budesonide but remained unchanged with placebo [11]. Each of the EREFS subscores (i.e., edema, rings, exudate, furrows, stricture) significantly improved with budesonide treatment, with the exception of stricture presence.
In summary, a growing body of literature supports the validity of systematic evaluation of EoE endoscopic features, as measured by the EREFS score, with a promising role for determining important treatment outcomes in both clinical practice and therapeutic trials. The ability of medical and diet therapies to significantly improve endoscopically visible esophageal inflammatory and structural alterations substantiates the improvements in patient-reported outcomes and histologic assessments. The emerging role of endoscopic assessment in EoE has noteworthy parallels with the movement towards endoscopic mucosal healing as a primary endpoint of therapeutics in inflammatory bowel disease.
BEYOND THE SURFACE: ENDOSCOPIC TOOLS TO EVALUATE ESOPHAGEAL REMODELING
Esophageal remodeling is a fundamental determinant of most of the major complications in EoE, including food impactions that have been associated with esophageal perforation. Thus, tools that aid in assessment of the consequences of esophageal remodeling in EoE have great potential value in disease management. The barium esophagram, as previously mentioned, can identify subtle strictures and narrow-caliber esophagus, which are features that tend to be missed on endoscopy yet have significant clinical implications. Nelson et al. found that EGD and esophagram studies had similar sensitivities for identifying remodeling consequences of EoE, but that endoscopy was superior for inflammatory mucosal features [23]. As mentioned above, Eluri et al. [22] has shown that EoE patients with severe strictures on EGD (not permitting passage of a standard endoscope) are more refractory to treatment with topical steroids, highlighting the therapeutic implication of this endoscopic finding in predicting treatment response.
The remodeling of EoE occurs beneath the squamous epithelium, and thus is generally missed in routine clinical disease activity assessment using esophageal mucosal biopsies, which often are too superficial to provide useful information on subepithelial fibrosis. Lamina propria fibrosis, while present in most patients with EoE, is not consistently assessable and seldom mentioned in routine clinical pathology reports [24]. Other modalities to assess esophageal mural remodeling consequences have been examined in EoE. In a randomized, controlled trial of nebulized budesonide, Straumann et al. [25] used endoscopic ultrasonography to measure the thickness of the esophageal wall in patients receiving treatment. When compared with controls, EoE patients had significant thickening of the esophageal submucosa and muscularis propria, with a reduction in thickness (but not normalization) after treatment. These findings confirm that the biologic effects of EoE extend well beneath the mucosa.
Another novel technique that has been used to assess esophageal mural compliance is the functional luminal imaging probe (FLIP). FLIP technology uses a multichannel electrical impedance catheter and manometric sensor surrounded by an infinitely compliant bag to measure the mechanical properties of the esophagus. An initial study using this technology showed a significant reduction in esophageal distensibility in EoE patients when compared with controls [26]. A subsequent study evaluated patients prospectively with FLIP and found that those with a history of food impactions exhibited lower esophageal distensibility than those with dysphagia alone. Decreased distensibility was also associated with increased need for dilation during a follow-up period of 4–12 months [27]. Substantiating the value of endoscopic assessment, EREFS severity was significantly correlated with distensibility parameters determined by FLIP. Specifically, higher grades of ring severity correlated stepwise with reduced distensibility metrics [14].
CONCLUSION
There have been significant advances in our understanding of the underlying mechanisms that drive the inflammatory and remodeling consequences of EoE. Over the past 5 years, numerous studies and observations have highlighted the validity, accuracy, and clinical importance of systematic endoscopic assessment of esophageal features of EoE. Initial severity of endoscopic findings has important implications with regard to therapeutic options, including the need for dilations, and may be an important predictor of the effectiveness of medical therapies. Moreover, endoscopic features can serve as reliable therapeutic endpoints, a feature of particular importance given the discord between patient-reported symptoms and histologic outcomes. Comprehensive assessment of therapeutics in EoE should ideally incorporate symptoms, histology, and endoscopic healing. Finally, tools such as endosonography and FLIP are providing important information regarding the remodeling consequences of EoE that are the central determinants of disease complications.
KEY POINTS.
Increasing numbers of studies support the validity, accuracy, and clinical relevance of systematic, endoscopic assessment of esophageal abnormalities in EoE.
Assessment of endoscopic severity is an important consideration in determining disease activity and need for dilation, and in predicting the effectiveness of medical therapies.
Endoscopic parameters can serve as important therapeutic endpoints that substantiate the interpretation of currently used metrics of patient-reported symptom outcomes and eosinophil density.
Endosonography and functional luminal imaging are providing fundamental insights regarding the remodeling consequences of EoE that are the central determinants of disease complications.
Acknowledgements
Financial support and sponsorship
I.H. has received grant support from the NIH Consortium of Eosinophilic Gastrointestinal Disease Researchers (NIH U54AI117804), which is part of the Rare Diseases Clinical Research Network, an initiative of the Office of Rare Diseases Research funded through a collaboration between NIAID, NIDDK, and NCATS, and an American Society of Gastrointestinal Endoscopy Research Award.
Footnotes
Conflicts of interest
I.H. is a consultant for Receptos, Regeneron, and Shire Pharmaceuticals. L.K. has no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011; 128:3–20. [DOI] [PubMed] [Google Scholar]
- 2.Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013; 108:679–692. [DOI] [PubMed] [Google Scholar]
- 3.Hirano I, Moy N, Heckman MG, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut 2013; 62:489–495. [DOI] [PubMed] [Google Scholar]
- 4.Kim HP, Vance RB, Shaheen NJ, Dellon ES. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastroenterol Hepatol 2012; 10:988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Straumann A, Rossi L, Simon HU, et al. Fragility of the esophageal mucosa: a pathognomonic endoscopic sign of primary eosinophilic esophagitis? Gastrointest Endosc 2003; 57:407–412. [DOI] [PubMed] [Google Scholar]
- 6.Peery AF, Cao H, Dominik R, et al. Variable reliability of endoscopic findings with white-light and narrow-band imaging for patients with suspected eosinophilic esophagitis. Clin Gastroenterol Hepatol 2011; 9:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson D, Hirano H, Carlson DA, Hirano I. Narrow-caliber esophagus of eosinophilic esophagitis: difficult to define, resistant to remedy. Gastrointest Endosc 2016. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 8.Gentile N, Katzka D, Ravi K, et al. Oesophageal narrowing is common and frequently under-appreciated at endoscopy in patients with oesophageal eosinophilia. Aliment Pharmacol Ther 2014; 40:1333–1340. [DOI] [PubMed] [Google Scholar]
- 9.Kavitt RT, Hirano H. Endoscopic assessment of eosinophilic esophagitis. Tech Gastrointest Endosc 2014; 16:20–25. [Google Scholar]
- 10.van Rhijn BD, Warners MJ, Curvers WL, et al. Evaluating the endoscopic reference score for eosinophilic esophagitis: moderate to substantial intra- and interobserver reliability. Endoscopy 2014; 46:1049–1055.■ A European study assessing intra-observer and inter-observer agreement of the EREFS system among expert and trainee endoscopists.
- 11.Hirano I, Katzka DA, Collins MH, Dellon ES. 113 randomized, double-blind, placebo controlled trial demonstrates the efficacy of oral budesonide suspension in improving endoscopically identified esophageal abnormalities in eosinophilic esophagitis. Gastroenterology 2015; 148:S-29–S-30. [Google Scholar]
- 12.Dellon ES, Cotton CC, Gebhart JH, et al. Accuracy of the eosinophilic esophagitis endoscopic reference score in diagnosis and determining response to treatment. Clin Gastroenterol Hepatol 2016; 14:31–39.■ A prospective study demonstrating that EREFS score had both a high degree of accuracy for diagnosis of EoE and significant responsiveness to treatment.
- 13.Schoepfer AM, Straumann A, Panczak R, et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology 2014; 147:1255–1266.■ An important study aimed at developing and validating a symptom-based activity index for adults with EoE. The severity of each of the EREFS subscores was associated with patient-reported global symptom activity.
- 14.Chen J, Lin Z, Pandolfino JE, et al. Su1839 endoscopically identified esophageal features of remodeling correlate with distensibility assessed by functional lumen imaging probe (FLIP) in eosinophilic esophagitis (EoE). Gastroenterology 2013; 144:S-487. [Google Scholar]
- 15.Schoepfer AM, Panczak R, Zwahlen M, et al. How do gastroenterologists assess overall activity of eosinophilic esophagitis in adult patients? Am J Gastroenterol 2015; 110:402–414.■ A recent study that evaluated physicians’ judgment of overall disease activity and found that gastroenterologists rate EoE activity mainly on the basis of endoscopic findings and symptoms as opposed to severity of histopathology.
- 16.Hirano I Therapeutic end points in eosinophilic esophagitis: is elimination of esophageal eosinophils enough? Clin Gastroenterol Hepatol 2012; 10:750–752. [DOI] [PubMed] [Google Scholar]
- 17.Safroneeva E, Straumann A, Coslovsky M, et al. Symptoms have modest accuracy in detecting endoscopic and histologic remission in adults with eosinophilic esophagitis. Gastroenterology 2016; 150:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoepfer AM, Gonsalves N, Bussmann C, et al. Esophageal dilation in eosinophilic esophagitis: effectiveness, safety, and impact on the underlying inflammation. Am J Gastroenterol 2010; 105:1062–1070. [DOI] [PubMed] [Google Scholar]
- 19.Safroneeva E, Straumann A, Coslovsky M, et al. Symptoms have modest accuracy in detecting endoscopic and histologic remission in adults with eosinophilic esophagitis. Gastroenterology 2016; 150:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dohil R, Newbury R, Fox L, et al. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology 2010; 139:418–429. [DOI] [PubMed] [Google Scholar]
- 21.Straumann A, Conus S, Degen L, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology 2010; 139:1526–1537. [DOI] [PubMed] [Google Scholar]
- 22.Eluri S, Runge TM, Cotton CC, et al. The extremely narrow-caliber esophagus is a treatment-resistant subphenotype of eosinophilic esophagitis. Gastrointest Endosc 2015. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 23.Moy N, Miller F, Hirano I. Limited accuracy of UGI series in the diagnosis of eosinophilic esophagitis in adults. Gastroenterology 2010; 138:S-178. [Google Scholar]
- 24.Hirano I, Aceves SS. Clinical implications and pathogenesis of esophageal remodeling in eosinophilic esophagitis. Gastroenterol Clin North Am 2014; 43:297–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straumann A, Conus S, Degen L, et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2011; 9:400–490. [DOI] [PubMed] [Google Scholar]
- 26.Kwiatek MA, Hirano I, Kahrilas PJ, et al. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology 2011; 140:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicodeme F, Hirano I, Chen J, et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2013; 11:1101–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]


