Abstract
Background:
Growing evidence suggests that there may be a sex-specific biological risk for Alzheimer’s disease (AD). Individuals with autosomal dominant AD due to a mutation (E280A) in Presenilin-1 (PSEN1) are genetically determined to develop early-onset dementia and thus, have few age-related risk factors for AD that are known to vary by sex (i.e., cardiovascular disease, menopause, life expectancy).
Objective:
Investigate sex differences in markers of cognition and neurodegeneration in autosomal dominant AD.
Methods:
We conducted a retrospective study in 19 cognitively-unimpaired PSEN1 mutation carriers (age range 20–44; 11 females), 11 symptomatic carriers (age range 42–56; 8 females), and 23 matched non-carriers family members (age range 20–50; 13 females). We examined hippocampal volume ratio, CERAD Total Score and CERAD Word List (i.e., Learning, Delayed Recall, and Recognition). Mann-Whitney U tests, Spearman correlations and regression models were conducted.
Results:
There were no differential associations between age, CERAD Total Score, CERAD Word List – Learning, Delayed Recall, Recognition, and hippocampal volume ratio in male and female carriers and non-carriers. Cognitively-unimpaired female carriers showed better CERAD Total scores and CERAD Word List – Learning than cognitively-unimpaired male carriers, despite having similar hippocampal volume ratios. The interaction of sex and hippocampal volume ratio did not predict cognitive performance across groups.
Conclusions:
Our preliminary findings suggest that cognitively-unimpaired female carriers showed a verbal memory reserve, while, as disease progresses, female carriers did not exhibit a cognitive susceptibility to AD-related neurodegeneration. Future studies with larger samples of autosomal dominant AD are warranted to further understand sex differences in AD-related clinical and pathological markers.
Keywords: sex, Alzheimer’s disease, Familial Alzheimer Disease (FAD), cognition, memory, atrophy
INTRODUCTION
Epidemiological studies suggest that females are disproportionately affected by Alzheimer’s disease (AD) [1]. Although AD incidence increases with age [2] and more females survive to late-life than males [3], there may also be a sex-specific biological risk for AD. Growing evidence suggests that females have greater AD pathology burden, as evidenced by postmortem studies [4, 5], in vivo cerebrospinal fluid (CSF) [6] and neuroimaging studies [7, 8], and that they are more susceptible to AD-related pathology. As levels of AD-related pathology increase, females show greater tau accumulation [7], faster hippocampal volume loss [8], and faster cognitive decline [9] and progression [5] than males. Possible mechanisms underlying sex differences in biological risk have been suggested, including endocrinological [10], cardiovascular [1, 11], or heightened inflammatory responses [12].
However, previous studies investigating sex differences in AD-related pathology and cognitive performance have yielded mixed findings. For instance, Caldwell and colleagues [13] did not find sex differences in hippocampal volume among cognitively normal older adults, while other studies showed that cognitively normal females had greater hippocampal volumes than their male counterparts [14, 15]. Notably, Sundermann and colleagues [15, 16] found that among individuals with mild cognitive impairment, females had better verbal memory than men despite similar levels of medial and inferior temporal glucose metabolism, and hippocampal volume, suggesting that females may have a sex-specific form of cognitive reserve. Thus, sex differences in clinical and pathological manifestations of AD are not well understood.
To our knowledge, no study to date has investigated sex differences in individuals with autosomal dominant AD. Our group follows individuals from the world’s largest autosomal dominant AD kindred due to a single mutation (E280A) in the Presenilin1 gene (PSEN1). Importantly, this mutation is fully penetrant and not sex-linked, meaning that males and females are equally affected. PSEN1 mutation carriers are genetically determined to develop early-onset dementia, and although the pathogenesis of autosomal dominant AD is different from late-onset AD, these conditions have similar clinical, cognitive, and physiological profiles [17]. PSEN1 mutation carriers have a well-characterized clinical profile, with mild cognitive impairment symptoms emerging at a median age of 44 years old (95% C.I.= 43, 45 years) and dementia at 49 years old (95% C.I.= 49, 50 years) [18, 19]. AD-pathological changes have also been characterized in PSEN1 mutation carriers, with elevated cortical amyloid accumulation 15 years before clinical symptom onset (i.e., mild cognitive impairment) and elevated levels of tau in medial temporal lobe regions (i.e., entorhinal cortex and inferior temporal lobe) 6 years before clinical symptom onset [17, 20, 21].
Thus, this extraordinary cohort allows us to investigate sex differences along the AD trajectory from preclinical to clinical stages. Moreover, PSEN1 carriers are virtually destined to develop early-onset dementia and thus, have few age-related factors that confer risk for AD and are known to vary by sex such as cardiovascular disease [1, 11, 22], or hormonal changes [10, 23]. Lastly, examining sex differences in autosomal dominant AD limits common methodological challenges that may confound research into sex differences in sporadic AD, such as survival bias due to differences in mortality among males and females [24].
For the purpose of this study, we leveraged data from the Alzheimer’s Prevention Initiative Biomarker study [20] to retrospectively examine sex differences in global cognition, verbal memory (i.e., learning, delayed recall and recognition in a list-learning task), and hippocampal volume ratio in PSEN1 mutation carriers. We hypothesized that cognitively intact female and male mutation carriers would not differ in hippocampal volume or global cognition, while cognitively intact female mutation carriers would exhibit better verbal memory. When examining sex differences across disease stages by also including symptomatic carriers, we further hypothesized that female mutation carriers would exhibit worse global cognition, verbal memory delayed recall, and lower hippocampal volume than male mutation carriers.
METHODS
Participants
Thirty PSEN1 mutation carriers (age range: 20–56, mean age: 37.8 +/− 10.6 years; 63% females) and twenty-three matched non-carrier family members (age range: 20–50, mean age: 32.9 +/− 8.8 years; 61% females) from the Alzheimer’s Prevention Initiative Biomarker study [20] were retrospectively analyzed. Individuals were recruited from the Alzheimer’s Prevention Initiative registry, an effort to locate, enroll, genotype, and perform medical and cognitive evaluations of PSEN1 E280A family members living in the region of Antioquia, Colombia [25].
Inclusion criteria included an age range of 18 to 60 years. To ensure a broad age distribution among cognitively unimpaired mutation carriers and non-carriers, individuals were enrolled into 18–34 years and 35–60 years age groups. Mutation carriers and non-carriers were matched for sex, age, and educational level within the two age groups [17, 20]. Cognitively unimpaired participants (n=19 carriers) had to show no cognitive impairment on a standard cognitive battery as defined by cutoff scores on the Spanish version of the Consortium to Establish a Registry for AD battery (CERAD) [26], a Clinical Dementia Rating (CDR) global score of zero and a Mini-Mental State Examination (MMSE) score of at least 28. Symptomatic mutation carriers were defined as having a CDR of ≥ 0.5 and MMSE score between 27 and 18, indicating a clinical diagnosis of mild cognitive impairment (7 carriers, 5 females; mean age: 46 +/− 4.8 years) or mild dementia (4 carriers, 3 females, mean age: 50.5 +/− 1.9 years) according to the National Institute on Aging-Alzheimer’s Association diagnostic criteria terminology [27, 28]. Demographic and clinical characteristics of the sample are described in Table 1.
Table 1.
Demographic, cognitive and clinical characteristics of sample.
| Non-carriers | Cognitively Unimpaired Carriers | Symptomatic Carriers | All Carriers | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M(SD) | pa | M(SD) | pb | M(SD) | M(SD) | pc | |||||
| Male n = 9 | Female n = 13 | Male n = 8 | Female n = 11 | Male n = 3 | Female n = 8 | Male n = 11 | Female n = 19 | ||||
| Age (years) | 30.89 (8.81) | 34.21 (8.88) | .449 | 31.13 (9.75) | 32.82 (8.33) | .545 | 47.00 (3.00) | 47.87 (5.08) | 35.45 (11.10) | 39.16 (10.34) | .838 |
| Education (years) | 12.00 (3.16) | 10.83 (3.41) | .498 | 12.13 (3.23) | 12.36 (2.06) | .075 | 6.00 (2.65) | 9.75 (3.58) | 10.45 (4.11) | 11.26 (3.02) | .121 |
| MMSE | 29.67 (.50) | 29.77 (.44) | .459 | 29.88 (.35) | 29.91 (.30) | .904 | 25.00 (1.00) | 23.88 (4.05) | 28.55 (2.34) | 27.37 (3.97) | 1.00 |
| CERAD Total | 79.44 (6.08) | 80.79 (7.14) | .430 | 79.75 (6.34) | 88.00 (9.18) | .047* | 45.67 (5.77) | 39.12 (11.19) | 70.45 (16.98) | 67.42 (26.65) | .303 |
| CERAD Word List – Learning | 18.56 (2.51) | 19.79 (3.21) | .280 | 19 (2.51) | 21.64 (2.91) | .030* | 7.67 (2.52) | 5.88 (3.27) | 15.91 (5.80) | 15 (8.53) | .469 |
| CERAD Word List – Delayed Recall | 7.00 (1.32) | 7.43 (1.50) | .496 | 7.38 (.52) | 8.00 (1.61) | .230 | 2.00 (1.00) | .88 (1.13) | 5.91 (2.59) | 5.00 (3.87) | .138 |
| CERAD Word List – Recognition | 9.78 (.67) | 9.86 (.53) | .747 | 9.88 (.35) | 9.91 (.30) | .816 | 6.00 (1.00) | 4.25 (3.37) | 8.82 (1.89) | 7.53 (3.56) | .301 |
| Hippocampal Volume × 103 | 5.52 (.19) | 5.68 (.48) | .378 | 5.46 (.58) | 5.66 (.31) | .56 | 3.72 (.45) | 4.22 (1.11) | 4.98 (.96) | 5.05 (1.03) | .540 |
Note: M = Mean; SD = Standard Deviation; MMSE = Mini Mental State Exam; CERAD = Consortium to Establish a Registry for Alzheimer’s Disease.
p-value calculated for Mann-Whitney test for males versus females in matched non-carriers.
p-value calculated for Mann-Whitney test for males versus females in cognitively intact PSEN1 mutation carriers.
p-value calculated for Mann-Whitney test for males versus females across all PSEN1 mutation carriers (including cognitively unimpaired and symptomatic carriers).
p<0.05.*. Group differences significant at the 0.05 level (2-tailed).
Procedure
All participants or their legal representatives provided informed consent before enrollment into the registry. Ethics approval was obtained from the University of Antioquia Ethics Committee Review Board. Participants and investigators acquiring data were blind to genetic status.
Clinical and Neuropsychological Test
Clinical and neuropsychological evaluations were performed in Spanish at the Universidad de Antioquia (Colombia), which included the MMSE [29], CDR [30], and the Spanish version of the CERAD battery [26] that was previously adapted for this Colombian population. The CERAD Word List task is divided into learning, delayed recall, and recognition. First, participants are shown 10 cards with a word. CERAD Word List – Learning is calculated as the sum of words correctly recalled over three trials (30 points possible for total score). After a 7-minute delay, participants are asked to recall the 10 words shown (CERAD Word List – Delayed Recall; 10 points possible for total score). Finally, participants are shown a list of 20 words (10 target words and 10 distractors). CERAD Word List – Recognition is calculated as the sum of correctly identified targets and distractors minus 10. We also calculated a CERAD Total score, as previously reported [31], by aggregating six CERAD subtests including semantic fluency (Animals), Boston Naming Test (15 items), Word List- Learning, World List - Delayed Recall, Word List - Recognition, and Constructional Praxis Copy.
Brain Imaging
Structural magnetic resonance imaging was performed at Hospital Pablo Tobon Uribe in Medellín, Colombia. As previously described [17], volumetric magnetic resonance imaging data were acquired on a 1.5-T imaging system (Avanto; Siemens) with a T1-weighted, magnetization-prepared, rapid-acquisition, gradient-echo pulse sequence (echo time, minimum full; flip angle, 8°; number of excitations, 1; field of view, 22 cm; imaging matrix, 192 × 192 pixels; and section thickness, 1.2 mm). All images were reviewed for quality and compliance in accord with the Alzheimer’s Disease Neuroimaging Initiative recommendations [32]. All images were visually inspected to verify ROI characterization. Hippocampal volume to total intracranial volume ratios were characterized from bilateral hippocampus volumes in each participant’s T1-weighted magnetic resonance image using a software package (FreeSurfer 5.1; http://surfer.nmr.mgh.harvard.edu) [33, 34].
Statistical Analyses
Analyses were carried out using SPSS Version 24. Nonparametric Mann-Whitney U test and Spearman correlations were conducted, as data were not normally distributed. Fisher’s Z-test was used to compare correlation coefficients. We used regression analyses to characterize associations between sex, hippocampal volume, and the interaction term of sex by hippocampal volume as the predictor variables and cognitive performance as the dependent variable in cognitively unimpaired PSEN1 mutation carriers. We then conducted the same regression analyses in all PSEN1 mutation carriers (i.e., cognitively unimpaired and symptomatic carriers) to characterize these associations along the disease trajectory. All analyses were also conducted in non-carriers.
RESULTS
Markers of cognition and neurodegeneration in male and female carriers
Table 1 shows demographic, cognitive and clinical characteristics. Among cognitively unimpaired carriers, males and females did not differ on age, years of education, MMSE scores or hippocampal volume ratio. Cognitively unimpaired females showed better CERAD Total scores and CERAD Word List – Learning than cognitively unimpaired males, while there were no other differences in cognitive measures. There were no sex differences in demographics, cognitive measures or hippocampal volume ratio among symptomatic male and female carriers and male and female non-carriers.
Association of age with markers of cognition and neurodegeneration in male and female carriers
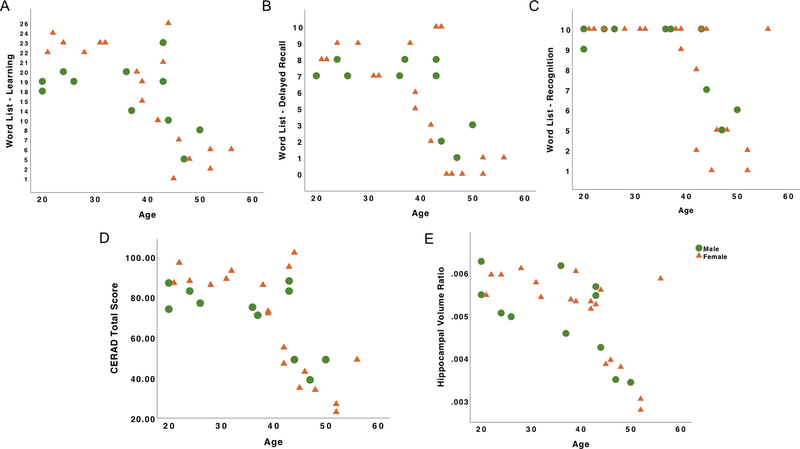
In this population, age is commonly used as a proxy to measure disease progression because carriers get closer to the estimated age of symptom onset as they age [18, 19]. We found that, among cognitively unimpaired male and female carriers, age was not associated with hippocampal volume ratio, CERAD Word List- Learning, Delayed Recall, Recognition, or CERAD Total scores (Table 2).
Table 2.
Correlation of age, cognitive measures and neurodegeneration in cognitively unimpaired PSEN1 mutation carriers, across all PSEN1 mutation carriers and non-carriers.
| Cognitively Unimpaired Carriers | ||||||
|---|---|---|---|---|---|---|
| Male n = 8 | Female n = 11 | |||||
| rs | pa | rs | pb | Z | pc | |
| CERAD Total | .15 | .720 | .05 | .894 | .18 | .859 |
| CERAD Word List – Learning | .27 | .512 | −.27 | .427 | .97 | .331 |
| CERAD Word List – Delayed Recall | .34 | .407 | .16 | .644 | .34 | .735 |
| CERAD Word List – Recognition | .50 | .206 | .16 | .644 | .68 | .496 |
| Hippocampal Volume | −.23 | .586 | −.43 | .189 | .40 | .692 |
| All Carriers | ||||||
| Male n = 11 | Female n = 19 | |||||
| rs | pa | rs | pb | Z | pc | |
| CERAD Total | −.55 | .081 | −.68 | .001* | .49 | .626 |
| CERAD Word List – Learning | −.52 | .103 | −.76 | .000* | .97 | .332 |
| CERAD Word List – Delayed Recall | −.51 | .107 | −.66 | .002* | .53 | .595 |
| CERAD Word List – Recognition | −.58 | .060 | −.62 | .005* | .14 | .885 |
| Hippocampal Volume | −.71 | .015* | −.66 | .002* | −.22 | .827 |
| Non-Carriers | ||||||
| Male n = 9 | Female n = 14 | |||||
| rs | pa | rs | pb | Z | pc | |
| CERAD Total | −.01 | .983 | −.02 | .931 | .02 | .984 |
| CERAD Word List – Learning | .23 | .558 | .23 | .427 | .00 | 1.00 |
| CERAD Word List – Delayed Recall | −.23 | .553 | .06 | .825 | −.58 | .562 |
| CERAD Word List – Recognition | .00 | 1.00 | −.38 | .181 | .78 | .430 |
| Hippocampal Volume | −.13 | .732 | −.19 | .507 | .12 | .903 |
Note: CERAD = Consortium to Establish a Registry for Alzheimer’s Disease; Hippocampal Volume = Hippocampal Volume Ratio; rs = Spearman’s rho coefficient; Z = Z statistics.
p-value calculated for Spearman correlation in males.
p-value calculated for Spearman correlation in females.
p-value calculated for Fisher exact test comparing Spearman’s rho in males and females.
p<0.05.*. Group differences significant at the 0.05 level (2-tailed).
Across all carriers, greater age in both male and females was associated with lower hippocampal volume (Males: rs = −.71, p = .015; Females: rs = −.66, p = .002). Older age was significantly associated with worse CERAD Word List – Learning (rs = −.76, p = .000), Delayed Recall (rs = −.66, p = .002), Recognition (rs = −.62, p = .005), and CERAD Total score (rs = −.68, p = .001) in female carriers. In contrast, while greater age was also associated with worse CERAD Word List – Learning, Delayed Recall, Recognition, or CERAD Total score in male carriers, these associations did not reach significance. The difference between the magnitudes of these associations was not statistically significant.
Among male and female non-carriers, age was not correlated with hippocampal volume ratio, CERAD Word List – Learning, Delayed Recall, Recognition, and CERAD Total score.
Sex, and markers of cognition and neurodegeneration
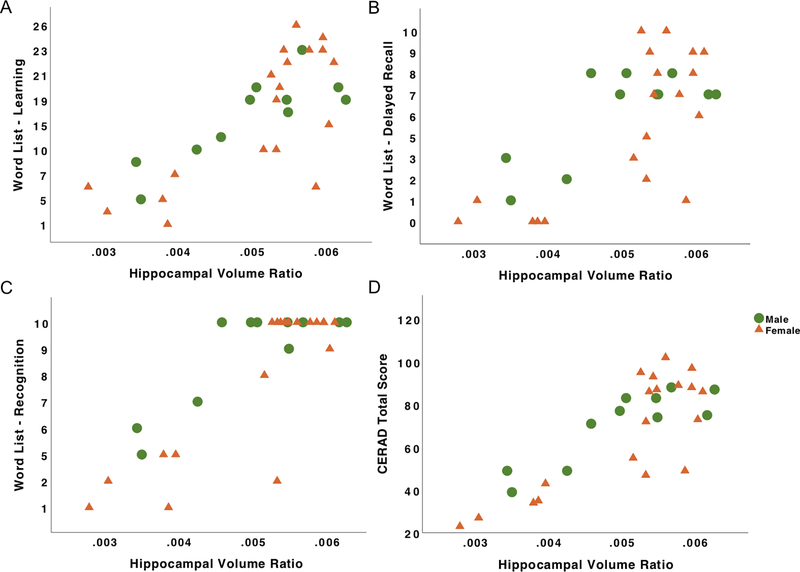
Among cognitively unimpaired carriers, hippocampal volume ratio, sex and the interaction term of sex and hippocampal volume ratio did not predict performance on the CERAD Word List- Learning, Delayed Recall, Recognition, or CERAD Total score (Table 3). Sex showed a trend towards significance in predicting CERAD Total scores (β = .58, p = .072), wherein cognitively unimpaired female carriers showed higher scores than cognitively unimpaired male carriers.
Table 3.
Regression estimates of the effect of sex and neurodegeneration on cognition in PSEN1 mutation carriers
| Cognitively Unimpaired Carriers n = 19 | All Carriers n = 30 | ||||||
|---|---|---|---|---|---|---|---|
| Outcome Variable | Predictors | β | pa | Effect Size (Cohen’s f) | β | pb | Effect Size (Cohen’s f) |
| CERAD Word List - Learning | Hippocampal Volume | .35 | .215 | .27 | 3.33 | .003* | 1.27 |
| Sex | .54 | .093 | .53 | −.58 | .568 | .10 | |
| Sex × Hippocampal Volume | −.26 | .468 | .19 | .44 | .661 | .09 | |
| CERAD Word List- Delayed Recall | Hippocampal Volume | −.15 | .617 | .12 | 2.60 | .015* | 1.09 |
| Sex | .23 | .510 | .26 | −.97 | .341 | .19 | |
| Sex × Hippocampal Volume | .08 | .845 | .05 | .17 | .476 | .14 | |
| CERAD Word List- Recognition | Hippocampal Volume | −.02 | .958 | .17 | .53 | .007* | 1.52 |
| Sex | .31 | .382 | .06 | −.18 | .096 | .39 | |
| Sex × Hippocampal Volume | −.37 | .362 | .24 | .33 | .078 | .36 | |
| CERAD Total Score | Hippocampal Volume | .25 | .372 | .16 | .65 | .002* | 1.45 |
| Sex | .59 | .072 | .55 | −.06 | .586 | .12 | |
| Sex × Hippocampal Volume | −.27 | .460 | .20 | .20 | .315 | .20 | |
Note. CERAD= Consortium to Establish a Registry for Alzheimer’s Disease; Hippocampal Volume = Hippocampal Volume Ratio.
p-value calculated for regression model in cognitively unimpaired PSEN1 mutation carriers.
p-value calculated for regression model across all PSEN1 mutation carriers (including cognitively unimpaired and symptomatic carriers).
p<0.05.*. Group differences significant at the 0.05 level (2-tailed).
Across all carriers, lower hippocampal volume ratio predicted worse performance on the CERAD Word List- Learning, Delayed Recall, Recognition, and CERAD Total scores (Table 3). Sex did not predict performance on the CERAD Word List- Learning, Delayed Recall, Recognition or CERAD Total scores across all carriers. The interaction term of sex and hippocampal volume ratio did not predict performance on the CERAD Word List- Learning, Delayed Recall, or CERAD Total scores, while it showed a trend towards significance in predicting CERAD Word List- Recognition scores (β = .33, p = .078).
Across non-carriers (Table 4), hippocampal volume ratio, sex, and the interaction term of sex and hippocampal volume ratio did not predict CERAD Word List- Learning, Delayed Recall, Recognition, or CERAD Total scores.
Table 4.
Regression estimates of the effect of sex and neurodegeneration on cognition in non-carriers.
| Outcome Variable | Predictors | β | pa | Effect Size (Cohen’s f) |
|---|---|---|---|---|
| CERAD Word List - Learning | Hippocampal Volume | .23 | .772 | .03 |
| Sex | .28 | .465 | .21 | |
| Sex × Hippocampal Volume | −.24 | .793 | .06 | |
| CERAD Word List- Delayed Recall | Hippocampal Volume | −.31 | .698 | .04 |
| Sex | .00 | .994 | .15 | |
| Sex × Hippocampal Volume | .42 | .648 | .11 | |
| CERAD Word List- Recognition | Hippocampal Volume | −.83 | .306 | .04 |
| Sex | −.23 | .541 | .07 | |
| Sex × Hippocampal Volume | .93 | .310 | .24 | |
| CERAD Total Score | Hippocampal Volume | −.03 | .968 | .14 |
| Sex | .00 | .991 | .10 | |
| Sex × Hippocampal Volume | .20 | .824 | .05 | |
Note. CERAD= Consortium to Establish a Registry for Alzheimer’s Disease; Hippocampal Volume = Hippocampal Volume Ratio.
p-value calculated for regression model in non-carriers.
p<0.05.*. Group differences significant at the 0.05 level (2-tailed).
DISCUSSION
Growing evidence suggests that females may be more susceptible to AD-related pathology [5, 7–9, 35]. However, sex differences in clinical and pathological manifestations of AD remain poorly understood. To our knowledge, this is the first study to investigate sex differences in markers of cognition and neurodegeneration in carriers of an autosomal dominant Alzheimer’s disease mutation who are destined to develop early-onset AD by midlife [18, 19]. We leveraged data from the world’s largest kindred with a single mutation (E280A) in the Presenilin1 gene (PSEN1). Importantly, PSEN1 carriers develop mild cognitive impairment at a median age of 44 years and dementia at a median age of 49 years [18], and thus have few age-related risk factors for AD at symptom onset that are known to vary by sex (i.e., life expectancy, cardiovascular disease, hormonal changes).
In autosomal dominant AD, age is commonly used as a proxy of disease progression, because as carriers age, they get closer to their age of estimated symptom onset. Therefore, we examined the relationship between age and markers of cognition and neurodegeneration. We found that among cognitively unimpaired male and female carriers, age was not associated with markers of cognition or neurodegeneration, perhaps due to limited age range in this group. When examining this relationship across all carriers, older age was associated with lower hippocampal volume in both male and female carriers, while older age was associated with worse global cognition and verbal memory in female carriers only. Of note older age was also associated with worse cognition and verbal memory in male carriers but these associations did not reach significance. Nonetheless, the magnitude of these associations was not statistically different among male and female carriers. This finding may be driven by the larger number of symptomatic female carriers that result in a greater range of cognitive performance.
We examined sex differences in markers of cognition and preliminary findings show that cognitively unimpaired female carriers showed better global cognition and verbal memory learning than cognitively unimpaired male carriers. This finding is consistent with previous extensive literature showing a verbal memory advantage for females [36]. However, we did not find a difference in word list delayed recall or recognition variables, perhaps due to restricted range in performance. With regard to the sex difference on global cognition, it is important to note that global cognition was measured using the CERAD total score, an aggregate of six CERAD subtests that also includes word list learning, thus likely contributing to the effect found. Non-carrier females also showed slightly better cognitive performance than non-carrier males, although differences were not statistically significant. Again, restricted range of performance in the CERAD may have limited our ability to detect differences, particularly among cognitively unimpaired individuals. In contrast, when examining all carriers by also including symptomatic carriers, cognitive performances did not differ among males and females. This may suggest that this verbal memory advantage may only be present in earlier stages of the disease, as previously shown [37], although further examination in samples with larger number of symptomatic carriers is required.
We also examined sex differences in markers of neurodegeneration and found that males and females had similar levels of hippocampal volume ratio among cognitively unimpaired and symptomatic carriers. This finding does not support our hypothesis, as we posited that as disease progresses and pathology accumulates, female carriers would exhibit worse levels of neurodegeneration. However, it is consistent with previous reports that found no sex-specific differences in hippocampal volume preservation among cognitively normal individuals [13]. Nonetheless, it is important to highlight that previous studies show great variability in findings wherein some studies found that females showed greater brain resilience to tau deposition [38], while others found that females showed lower brain resilience [35]. Further research is needed to clarify whether females show greater resilience or susceptibility to AD-related pathology than males, and whether this effect varies across disease stages.
With regard to the effect of sex moderating the relationship between neurodegeneration and cognitive performance, we found that there was no interaction between sex and hippocampal volume ratio in predicting cognitive performance among cognitively unimpaired carriers. Similarly, across all carriers, sex by hippocampal volume interaction did not predict verbal memory learning, delayed recall, or global cognition. However, there was a trend towards significance, wherein female carriers with lower hippocampal volume ratio had worse verbal memory recognition than male carriers. Overall, contrary to our hypotheses, our findings show no evidence that females with this autosomal dominant AD mutation have greater cognitive susceptibility to AD-related neurodegeneration compared to male carriers. Our ability to detect such an effect may have been limited by the relatively low number of symptomatic carriers, particularly with mild dementia. Alternatively, the sex differences previously found in sporadic AD may be linked to factors that are not relevant in this unique cohort of autosomal dominant AD, such as older age [2, 19], cardiovascular disease [1, 11, 22], menopause [10, 23], or survival bias (i.e., mortality differences in males and females) [24].
This study has limitations and thus, findings should be interpreted with caution. First, this is a retrospective, cross-sectional study with a small sample size and relatively few symptomatic carriers, which may have resulted in limited power to detect sex differences, particularly in later stages of the disease. Additionally, there were a larger number of females in the sample and a greater number of symptomatic female carriers. The generalizability of these findings to others at risk for autosomal dominant AD or late-onset sporadic AD requires further investigation. However, despite the small sample size/power limitations, which are generally inherent in autosomal dominant AD research, this cohort offers a unique opportunity to examine sex differences using neuroimaging methods with fewer age- and sex-related confounds than in research with sporadic AD (i.e., mortality/life expectancy, cardiovascular disease, or menopause).
Summary
In conclusion, this is the first study to investigate sex differences in autosomal dominant AD due to a single mutation (E280A) in the PSEN1 gene. We examined the effect of sex on markers of cognition and neurodegeneration. Our findings suggest that cognitively unimpaired female carriers had better global cognition and verbal memory learning than males, despite having similar hippocampal volume ratios. Moreover, there was no evidence that, as disease progresses, female carriers had a cognitive susceptibility to AD-related neurodegeneration. Notably, these findings are preliminary and interpretation warrants caution. Future studies with larger samples of autosomal dominant AD and longitudinal time points are warranted to further understand sex differences in AD-related clinical and pathological markers to inform detection, prevention, and development of AD treatments.
Figure 1. Relations among age, cognition and neurodegeneration in PSEN1 male and female carriers.
Note. A, Verbal memory learning as a function of age. B, Verbal memory delayed recall as a function of age. C, Verbal memory recognition as a function of age. D, CERAD Total score as a function of age. E, Hippocampal volume ratio as a function of age. Green dots represent male PSEN1 mutation carriers and orange triangles represent female PSEN1 mutation carriers.
Figure 2. Relations among neurodegeneration and cognition in PSEN1 male and female carriers.
Note. A, Verbal memory learning as a function of hippocampal volume ratio. B, Verbal memory delayed recall as a function of hippocampal volume ratio. C, Verbal memory recognition as a function of hippocampal volume ratio. D, CERAD Total scores as a function of hippocampal volume ratio. Green dots represent male PSEN1 mutation carriers and orange triangles represent female PSEN1 mutation carriers.
Acknowledgements
This work was supported by Banner Alzheimer’s Foundation, Anonymous Foundation, Colciencias (Grant #111554531651) and the State of Arizona. Dr. Vila-Castelar received funding from the Alzheimer’s Association (2019-AARF-644631). Mr. Fox-Fuller reports support from the NIH National Institute on Aging National Research Service Awards (1F31AG062158-01A1). Dr. Quiroz received funding from NIH National Institute of Aging (DP5OD019833).
Footnotes
Conflict of Interest/Disclosure Statement
The authors have no conflict of interest to report.
REFERENCES
- [1].Chêne G, Beiser A, Au R, Preis SR, Wolf PA, Dufouil C, Seshadri S (2015) Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimer’s & Dementia 11, 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hebert LE, Weuve J, Scherr PA, Evans DA (2013) Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80, 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Arias E, Heron M, Xu J (2017) United States life tables, 2013. National Vital Statistics Reports 66, 1–64. [PubMed] [Google Scholar]
- [4].Oveisgharan S, Arvanitakis Z, Yu L, Farfel J, Schneider JA, Bennett DA (2018) Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathologica 136, 887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA (2005) Sex differences in the clinical manifestations of Alzheimer disease pathology. Archives of General Psychiatry 62, 685–691. [DOI] [PubMed] [Google Scholar]
- [6].Hohman T, Dumitrescu L, Barnes L, Thambisetty M, Beecham G, Kunkle B, Gifford K, Bush W, Chibnik L, Mukherjee S (2018) Alzheimer’s Disease Genetics Consortium and the Alzheimer’s Disease Neuroimaging Initiative Sex-Specific Association of Apolipoprotein E with Cerebrospinal Fluid Levels of Tau. JAMA Neurology 75, 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Buckley RF, Mormino EC, Rabin JS, Hohman TJ, Landau S, Hanseeuw BJ, Jacobs HIL, Papp KV, Amariglio RE, Properzi MJ, Schultz AP, Kirn D, Scott MR, Hedden T, Farrell M, Price J, Chhatwal J, Rentz DM, Villemagne VL, Johnson KA, Sperling RA (2019) Sex Differences in the Association of Global Amyloid and Regional Tau Deposition Measured By Positron Emission Tomography in Clinically Normal Older Adults. JAMA Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Koran MEI, Wagener M, Hohman TJ (2017) Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging and Behavior 11, 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Buckley RF, Mormino EC, Amariglio RE, Properzi MJ, Rabin JS, Lim YY, Papp KV, Jacobs HIL, Burnham S, Hanseeuw BJ, Dore V, Dobson A, Masters CL, Waller M, Rowe CC, Maruff P, Donohue MC, Rentz DM, Kirn D, Hedden T, Chhatwal J, Schultz AP, Johnson KA, Villemagne VL, Sperling RA (2018) Sex, amyloid, and APOE epsilon4 and risk of cognitive decline in preclinical Alzheimer’s disease: Findings from three well-characterized cohorts. Alzheimer’s & Dementia 14, 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pike CJ, Carroll JC, Rosario ER, Barron AM (2009) Protective actions of sex steroid hormones in Alzheimer’s disease. Frontiers in Neuroendocrinology 30, 239–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pankratz VS, Roberts RO, Mielke MM, Knopman DS, Jack CR, Geda YE, Rocca WA, Petersen RC (2015) Predicting the risk of mild cognitive impairment in the Mayo Clinic Study of Aging. Neurology 84, 1433–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fisher DW, Bennett DA, Dong H (2018) Sexual dimorphism in predisposition to Alzheimer’s disease. Neurobiology of Aging 70, 308–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Caldwell JZ, Cummings JL, Banks SJ, Palmqvist S, Hansson O (2019) Cognitively normal women with Alzheimer’s disease proteinopathy show relative preservation of memory but not of hippocampal volume. Alzheimer’s Research & Therapy 11, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jack CR, Wiste HJ, Weigand SD, Knopman DS, Vemuri P, Mielke MM, Lowe V, Senjem ML, Gunter JL, Machulda MM (2015) Age, sex, and APOE ε4 effects on memory, brain structure, and β-amyloid across the adult life span. JAMA Neurology 72, 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sundermann EE, Biegon A, Rubin LH, Lipton RB, Mowrey W, Landau S, Maki PM, Initiative AsDN (2016) Better verbal memory in women than men in MCI despite similar levels of hippocampal atrophy. Neurology 86, 1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sundermann EE, Maki PM, Rubin LH, Lipton RB, Landau S, Biegon A, Initiative AsDN (2016) Female advantage in verbal memory: Evidence of sex-specific cognitive reserve. Neurology 87, 1916–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fleisher AS, Chen K, Quiroz YT, Jakimovich LJ, Gomez MG, Langois CM, Langbaum JB, Roontiva A, Thiyyagura P, Lee W (2015) Associations between biomarkers and age in the presenilin 1 E280A autosomal dominant Alzheimer disease kindred: a cross-sectional study. JAMA neurology 72, 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Acosta-Baena N, Sepulveda-Falla D, Lopera-Gómez CM, Jaramillo-Elorza MC, Moreno S, Aguirre-Acevedo DC, Saldarriaga A, Lopera F (2011) Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer’s disease: a retrospective cohort study. The Lancet Neurology 10, 213–220. [DOI] [PubMed] [Google Scholar]
- [19].Fuller JT, Cronin-Golomb A, Gatchel JR, Norton DJ, Guzmán-Vélez E, Jacobs HI, Hanseeuw B, Pardilla-Delgado E, Artola A, Baena A (2019) Biological and Cognitive Markers of Presenilin1 E280A Autosomal Dominant Alzheimer’s Disease: A Comprehensive Review of the Colombian Kindred. The Journal of Prevention of Alzheimer’s Disease 6, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fleisher AS, Chen K, Quiroz YT, Jakimovich LJ, Gomez MG, Langois CM, Langbaum JB, Ayutyanont N, Roontiva A, Thiyyagura P (2012) Florbetapir PET analysis of amyloid-β deposition in the presenilin 1 E280A autosomal dominant Alzheimer’s disease kindred: a cross-sectional study. The Lancet Neurology 11, 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Quiroz YT, Sperling RA, Norton DJ, Baena A, Arboleda-Velasquez JF, Cosio D, Schultz A, Lapoint M, Guzman-Velez E, Miller JB (2018) Association between amyloid and tau accumulation in young adults with autosomal dominant Alzheimer disease. JAMA neurology 75, 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lau ES, Paniagua SM, Guseh JS, Bhambhani V, Zanni MV, Courchesne P, Lyass A, Larson MG, Levy D, Ho JE (2019) Sex differences in circulating biomarkers of cardiovascular disease. Journal of the American College of Cardiology 74, 1543–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rocca WA, Bower JH, Maraganore D, Ahlskog J, Grossardt B, De Andrade M, Melton Lr (2007) Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 69, 1074–1083. [DOI] [PubMed] [Google Scholar]
- [24].Mayeda ER (2019) Invited commentary: examining sex/gender differences in risk of Alzheimer disease and related dementias—challenges and future directions. American Journal of Epidemiology 188, 1224–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rios-Romenets S, Lopez H, Lopez L, Hincapie L, Saldarriaga A, Madrigal L, Piedrahita F, Navarro A, Acosta-Uribe J, Ramirez L (2017) The Colombian Alzheimer’s Prevention Initiative (API) Registry. Alzheimer’s & Dementia 13, 602–605. [Google Scholar]
- [26].Aguirre-Acevedo D, Gomez R, Moreno S, Henao-Arboleda E, Motta M, Muñoz C, Arana A, Pineda D, Lopera F (2007) Validity and reliability of the CERAD-Col neuropsychological battery. Revista de Neurologia 45, 655–660. [PubMed] [Google Scholar]
- [27].Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McKhann G, Knopman D, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [30].Morris JC (1993) The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43, 2412–2414. [DOI] [PubMed] [Google Scholar]
- [31].Aguirre-Acevedo DC, Lopera F, Henao E, Tirado V, Muñoz C, Giraldo M, Bangdiwala SI, Reiman EM, Tariot PN, Langbaum JB (2016) Cognitive decline in a Colombian kindred with autosomal dominant Alzheimer disease: A retrospective cohort study. JAMA Neurology 73, 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack C, Jagust W, Trojanowski JQ, Toga AW, Beckett L (2005) The Alzheimer’s disease neuroimaging initiative. Neuroimaging Clinics 15, 869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jack CR Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJ, L. Whitwell J, Ward C (2008) The Alzheimer’s disease neuroimaging initiative (ADNI): MRI methods. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine 27, 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fischl B, Sereno MI, Dale AM (1999) Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. Neuroimage 9, 195–207. [DOI] [PubMed] [Google Scholar]
- [35].Ramanan VK, Castillo AM, Knopman DS, Graff-Radford J, Lowe VJ, Petersen RC, Jack CR, Mielke MM, Vemuri P (2019) Association of Apolipoprotein E ɛ4, Educational Level, and Sex With Tau Deposition and Tau-Mediated Metabolic Dysfunction in Older Adults. JAMA Network Open 2, e1913909–e1913909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Siedlecki KL, Falzarano F, Salthouse TA (2019) Examining Gender Differences in Neurocognitive Functioning Across Adulthood. Journal of the International Neuropsychological Society 25, 1051–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Digma LA, Madsen JR, Rissman RA, Jacobs DM, Brewer JB, Banks SJ (2020) Women can bear a bigger burden: Ante-and post-mortem evidence for reserve in the face of tau. Brain Communications 2, fcaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ossenkoppele R, Lyoo CH, Jester-Broms J, Sudre CH, Cho H, Ryu YH, Choi JY, Smith R, Strandberg O, Palmqvist S (2020) Assessment of Demographic, Genetic, and Imaging Variables Associated With Brain Resilience and Cognitive Resilience to Pathological Tau in Patients With Alzheimer Disease. JAMA Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]