Summary
Objective
To describe patterns of benzodiazepine use as first-line treatment of status epilepticus (SE) and the association of benzodiazepine doses with response to second-line agents in patients enrolled in the Established Status Epilepticus Treatment Trial (ESETT).
Methods
Patients who failed adequate dose of benzodiazepines for the treatment of SE were enrolled in ESETT. Choice of benzodiazepine, doses given prior to administration of second-line agent, route of administration, setting, and patient weight were characterized. These were compared with guideline-recommended dosing. Logistic regression was used to determine the association of the first dose of benzodiazepine and the cumulative benzodiazepine dose with the response to second-line agent.
Results
Four-hundred-sixty patients were administered 1170 doses of benzodiazepines (669 lorazepam, 398 midazolam, 103 diazepam). Lorazepam was most frequently administered intravenously in the emergency department, midazolam intramuscularly or intravenously by the emergency medical services personnel, and diazepam rectally prior to ambulance arrival. The first dose of the first benzodiazepine (n=460) was lower than guideline recommendations in 76% of midazolam administrations and 81% lorazepam administrations. Among all administrations, > 85% of midazolam and > 76% of lorazepam were lower than recommended. Higher first or cumulative benzodiazepine doses were not associated with better outcomes or clinical seizure cessation in response to second-line medications in these benzodiazepine-refractory seizures.
Significance
Benzodiazepines as first-line treatment of SE, particularly midazolam and lorazepam, are frequently under-dosed throughout the US. This broad and generalizable cohort confirms prior single site reports that underdosing is both pervasive and difficult to remediate. ESETT ClinicalTrials.gov identifier: NCT01960075
Keywords: Benzodiazepine dose, lorazepam, midazolam, diazepam, ESETT
INTRODUCTION
The European Federation of Neurological Societies (EFNS), the Neurocritical Care Society (NCS) and the American Epilepsy Society (AES) have published evidence-based guidelines for the treatment of convulsive status epilepticus (SE).1-3 These national and international guidelines recommend the use of benzodiazepines as initial therapy and include routes of administration and doses for diazepam (DZP), midazolam (MDZ) and lorazepam (LZP) based on the patient’s weight. The guidelines emphasize the administration of benzodiazepines as a first-line treatment of SE as early as possible, as a single full dose, and by an appropriate route.1-3
The Established Status Epilepticus Treatment Trial (ESETT) was a comparative effectiveness study of fosphenytoin, levetiracetam, and valproic acid in adults and children aged two years and older with benzodiazepine-refractory SE.4,5 The primary outcome was clinical cessation of seizures with improved responsiveness at 60 minutes, without the need for additional anti-seizure medication.
Underdosing of benzodiazepines as first-line treatments have been reported in several previously published reports6-13 and pointed to an association with poor response.9,10,12,13 However, these reports typically involved fewer than three sites, small sample size, and limited characterization of use patterns. The recently completed ESETT, with its large number of sites across the US, and detailed information about first-line therapy offered the opportunity to characterize patterns of benzodiazepine use prior to enrollment in the trial. The primary objective of this report was to describe these patterns in children and adults with benzodiazepine-refractory SE. We were also able to compare these patterns with our preliminary report consisting of the first 207 ESETT patients.14 The secondary objective was to determine the association of benzodiazepine dosing with the ESETT primary outcome and clinical seizure cessation.
METHODS
Standard Protocol Approvals, Registrations, and Patient Consents
ESETT was conducted under the exception from informed-consent requirements for emergency research (FDA regulation 21 CFR 50.2412). The institutional review boards for all participating institutions approved the protocol after consultation with the local community and public disclosure.4 Patients or their legally authorized representatives were notified about enrollment in the trial by the research team as soon as possible, usually, while the patient was still in the emergency department and was asked to provide written informed consent for continued data collection through the end of the trial. ESETT was approved by institutional review boards of all participating institutions.4 ESETT is registered on ClinicalTrials.gov (identifier number NCT01960075).
Analysis of benzodiazepine dosing data
The methods used for these analyses were similar to those previously reported.14 Using pre-enrollment data from ESETT subjects, we describe benzodiazepine treatment with respect to: 1) drug choice, dose, and route of administration, 2) timing and setting in which the drugs were administered, and 3) patient weight (< or ≥ 40 kg for LZP, ≤ or > 40 kg for MDZ, and < or ≥ 66.7 kg for DZP). EFNS, NCS, and AES guidelines were used to define underdosing for our analyses.1-3 Since the AES guideline for MDZ doses in patients weighing less than 40 kgs is expressed as a fixed dose. i.e. 5 mg, that is what was used to determine adequacy of dosing instead of a mg/kg dose. The settings in which benzodiazepines were administered were categorized as: 1) Prior to emergency medical services (EMS) arrival, 2) EMS, and 3) Emergency department (ED).
Because patients could receive more than one benzodiazepine, the cumulative dose was determined using LZP equivalents to account for differences in drug potencies. For patients weighing ≥ 32 kg, 10 mg MDZ or DZP were considered equal to 4 mg LZP1-3 (Table 1). For patients weighing < 32 kg, 0.3 mg/kg of DZP IV or 0.2 mg/kg of MDZ IV or 0.3 mg/kg of MDZ IM were considered equal to 0.1 mg/kg LZP IV.1-3 The mg/kg doses were calculated by dividing the dose administered by the patient’s weight. Transmucosal benzodiazepines, e.g. rectal DZP or intranasal/buccal MDZ, given prior to EMS arrival are included in the calculation of cumulative benzodiazepine dose.
Table 1:
Guideline Recommended Doses and ESETT Protocol Eligibility Criteria (adapted from Sathe AG, Tillman H, Coles LD, et al. Underdosing of Benzodiazepines in Patients with Status Epilepticus Enrolled in Established Status Epilepticus Treatment Trial. Acad Emerg Med 2019;0(8):940–3).
| Guideline Recommended Doses per Administration* |
ESETT Eligibility Criteria for Minimally Adequate Cumulative Dose** |
|||
|---|---|---|---|---|
| Drug | Route | Dose for ≥ 32 kg Patients (mg) |
Dose for < 32 kg Patients (mg/kg) |
|
| Diazepam | IV Rectal |
0.15-0.2 mg/kg/dose, max: 10 mg/dose, may repeat dose once If IV route not available, then 0.2-0.5 mg/kg/dose, max: 20 mg/dose |
10 | 0.3 |
| Lorazepam | IV | 0.1 mg/kg/dose, max: 4 mg/dose, may repeat dose once | 4 | 0.1 |
| Midazolam | IV | IM Dosing: 10 mg for > 40 kg, 5 mg for 13-40 kg | 10 | 0.2 |
| IM | 10 | 0.3 | ||
| IN/Buccal | Dosing not specified | |||
Meierkord H et al., European Journal of Neurology, 2006; 13(5): 445-450; Brophy GM et al. ,Neurocritical Care 2012;17(1):3–23 and Glauser T et al., Epilepsy Currents, Vol. 16, No. 1 (January/February) 2016 pp. 48–61
Cut-off criteria for the transmucosal routes were the same as those for the intravenous route
The ESETT eligibility criteria required those enrolled to have been administered an adequate minimum dose of benzodiazepines without termination of SE, but there was no upper limit on the benzodiazepine dose allowed prior to enrollment. The minimum cumulative adequate dose for enrollment (4 mg LZP equivalents for those ≥ 32 kg and 0.1 mg/kg LZP equivalents for < 32 kg)14, was intended to represent the lowest doses that might be given in standard practice. The study protocol did not stipulate dose or instructions on the rate and frequency of dosing because benzodiazepine dosing occurred prior to study enrollment. ESETT sites dosed benzodiazepines as per their local standards of care and established clinical guidelines. Although benzodiazepine dosing was not dictated by the study protocol, best practices based on the guidelines were part of the study training, in part because of pre-existing published data about underdosing in clinical practice. As a component of ongoing quality improvements, we performed an analysis of pre-enrollment benzodiazepine dosing after 207 enrollments (200 unique patients).14 When this analysis indicated underdosing as compared to guidelines, the guidelines and their underlying evidence base were reemphasized, and supplemental educational tools and materials were provided to investigators to reinforce best practices at their sites.
The analyses reported here were conducted after completion of ESETT. The last patient was enrolled in December 2018 and the database was locked for analysis in April 2019. We categorized the timing of drug administration as: 1) the first administration of the first benzodiazepine and 2) all administrations of all benzodiazepines prior to enrollment including the first administration. Individual doses were compared with the guidelines to identify those doses that met recommendations. Furthermore, to account for approximation of patient weight and dose rounding at the time of administration, individual doses were also compared with 80% and 90% of the guideline-recommended dose. Data were collected from 478 enrollments in ESETT at 57 US academic and community hospitals. Among these, 16 patients (12 adults, four children) were enrolled more than once (seven in cohort 1 and nine in cohort 2), but only the data from their first enrollments were used for these analyses. Of the remaining 462 patients, the first DZP dose administered in two patients was unknown and these patients were excluded. Thus, data from 460 patients formed the basis for these analyses. Data were analyzed using R (version 3.6.1), RStudio (version 1.2.5001) and SAS (version 9.4) to compute descriptive statistics.
Time from onset of SE to first benzodiazepine dose and time from first dose to cumulative adequate dose
The difference between the adjudicated time from the onset of SE to ESETT study drug administration4 (t1) and the recorded time from the first dose of the first benzodiazepine to ESETT study drug administration (t2) was used to calculate the time, in minutes, from onset of SE to the first dose of benzodiazepine (t1-t2). In cases where t1 was adjudicated to be a range, the mean value was used. On one occasion, when the mean value of the adjudicated t1 range was lower than t2, the upper limit of the range was used. The time to cumulative adequate dose after the first dose was calculated using the recorded time for the first benzodiazepine administration and the administration to reach the cumulative adequate dose per the ESETT enrollment criteria.
Comparison of cohort 1 vs. cohort 2
Following the analysis of the initial subset of ESETT patients14, increased educational efforts to improve appropriate benzodiazepine use were implemented at investigator meetings. Dosing patterns for the first dose of the first benzodiazepine administered were compared between this first set of published data (n=200 patients: 115 adults and 85 children, cohort 1)14 versus the subsequent enrollments (n=260 patients: 121 adults and 139 children, cohort 2). We also performed time-series trend analyses.
Association of benzodiazepine doses with the response to subsequent second-line medications
The ESETT outcomes were designed to assess the safety and efficacy of second-line treatments. However, during the primary analyses, it was noted that a large number of patients received lower than recommended individual benzodiazepine doses. This provided us the opportunity to explore the association of benzodiazepine underdosing with outcomes after second-line treatment. Logistic regression modeling was used to test the association of benzodiazepine doses with ESETT primary outcome and clinical seizure cessation. The dependent variables included:
ESETT primary outcome was defined as the cessation of SE at 60 minutes after the start of the study drug infusion without additional anti-seizure medication, as determined by absence of clinically apparent seizures and improved responsiveness (1=treatment success, 0= treatment failure).
Clinical seizure-cessation at 60 minutes after the start of study drug infusion without additional use of additional antiseizure medication and with/without improved responsiveness (1= success, 0= failure)
The following benzodiazepine doses were used as primary predictors:
The first dose of the first benzodiazepine
The cumulative dose of benzodiazepines prior to enrollment
To allow adults and children to be included in the same model, both the first dose and the cumulative dose were calculated as mg/kg of LZP equivalents (continuous variable). Four logistic models were used to test the association of first dose of first benzodiazepine and cumulative dose of benzodiazepines with each of the two dependent variables of primary outcome and clinical seizure cessation. Other predictors included in all four models were age (< or ≥ 18 years), etiology (1= acute brain pathology including acute stroke/hemorrhage, central nervous system (CNS) tumor or CNS infection, 0= no acute brain pathology), and ESETT study drug (fosphenytoin, levetiracetam and valproic acid).
RESULTS
These results include 1170 benzodiazepine doses administered to 460 ESETT patients. Of these, 539 doses were administered to 224 children, while 631 doses were given to 236 adults. Although the number of benzodiazepine administrations given to patients prior to ESETT enrollment ranged from one to nine, most patients (74.2%) received 2-3 doses each. The distribution of the administrations by age group, drug, route, and setting are summarized in Table 2. Of all DZP administrations (n=103), 70.9% were given prior to EMS, of which 91.9% were by the rectal route. Of the 398 MDZ administrations, 67.7% were given by the EMS personnel mainly by IM (40.4%) or IV (35.2%) routes. In contrast 90.6% of the 669 LZP administrations were in the ED, primarily intravenously (97.2%). Twenty-five LZP administrations were via the IM route, and on four occasions LZP was given by intranasal or buccal routes.
Table 2:
Distribution of all benzodiazepine doses by route of administration, setting and age group (N=1170 in 460 patients)
| Lorazepam | Midazolam | Diazepam | Total | |||||
|---|---|---|---|---|---|---|---|---|
| N= 669 | N= 398 | N= 103 | N=1170 | |||||
| n | % | n | % | n | % | n | % | |
| Route of administration | ||||||||
| Intravenous | 638 | 95% | 159 | 40% | 21 | 20% | 818 | 70% |
| Intramuscular | 25 | 4% | 137 | 34% | 4 | 4% | 166 | 14% |
| Transmucosal* | 5 | 1% | 96 | 24% | 76 | 74% | 177 | 15% |
| Unknown | 1 | - | 6 | 2% | 2 | 2% | 9 | 1% |
| Setting | ||||||||
| Prior to EMS | 16 | 2% | 20 | 5% | 73 | 71% | 109 | 9% |
| EMS | 47 | 7% | 269 | 68% | 22 | 21% | 338 | 29% |
| ED | 606 | 91% | 109 | 27% | 8 | 8% | 728 | 62% |
| Age group | ||||||||
| Children** | 269 | 40% | 196 | 49% | 80 | 78% | 545 | 47% |
| Adult | 400 | 60% | 202 | 51% | 23 | 22% | 625 | 53% |
EMS- Emergency Medical Services; ED- Emergency Department
Transmucosal administration for diazepam was per rectum, while intranasal or buccal routes were used for lorazepam and midazolam.
The children group includes ages less than or equal to 17 years, the adult group includes those greater than 17.
The first dose of the first benzodiazepine
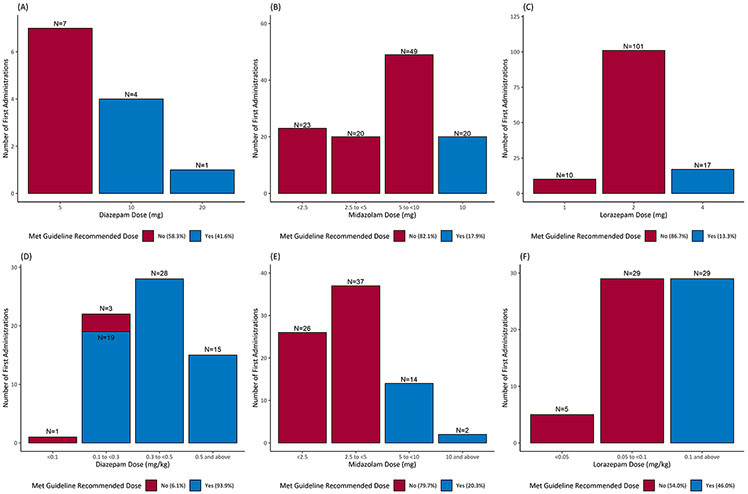
Among all first doses, 32.4% met the minimum recommendations per guidelines. Figure 1 shows the distribution of the first doses for each drug. For all patients, 85.9% of DZP, 18.9% of MDZ and 24.1% of LZP first doses met guideline recommendations. For those weighing > 40 kg, the guideline recommended MDZ dose of ≥ 10 mg was administered in 17.9% of the cases and LZP dose of ≥ 4 mg was administered in 13.3% of the cases. For patients weighing ≤ 40 kg, the recommended MDZ dose of ≥ 5 mg was used in 20.3% of the cases and LZP dose of ≥ 0.1 mg/kg was used in 46% of the cases. For DZP, patients weighing ≥ 66.7 kg received the recommended dose of ≥ 10 mg in 41.6% cases, and 93.9% of those weighing < 66.7 kg received the recommended DZP dose of ≥ 0.15 mg/kg.
Figure 1:
Distribution of first dose of the first administered benzodiazepine (DZP, MDZ or LZP) (n=460). Top panel: fixed dosing, bottom panel: weight-based dosing. A: DZP doses for those ≥ 66.7kg (IV) or ≥ 50 kg (rectal); B: MDZ doses for those > 40 kg; C: LZP doses for those ≥ 40 kg; D: DZP doses for those < 66.7 kg (IV) or < 50 kg (rectal); E: MDZ doses for those ≤ 40 kg; F: LZP doses for those < 40 kg . Categorized as met (blue) or did not meet (red) guidelines.
The first doses were also compared with 80% and 90% of the guideline-recommended doses to approximate patient weight and dose rounding. Eighty-nine percent of DZP, 23% of MDZ and 33% of LZP doses were ≥ 80% of the guideline-recommended dose; whereas, 86% percent of DZP, 19% of MDZ and 31% of LZP doses were ≥ 90% of the guideline-recommended dose.
Doses of all benzodiazepine administrations
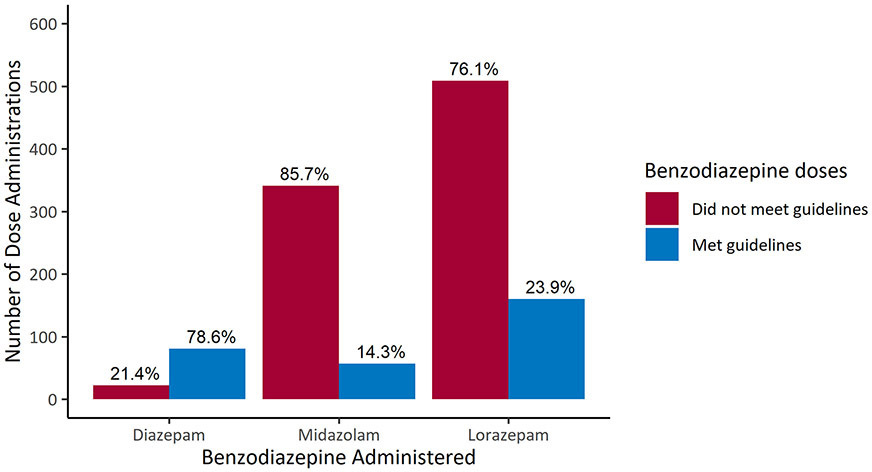
When including all benzodiazepine administrations (N=1170), 78.6% of DZP, 14.3% of MDZ and 23.9% of LZP doses met guideline recommendations (Figure 2).
Figure 2:
Administrations that met (blue) and did not meet (red) guideline recommendations among all administrations for DZP, MDZ and LZP (N=1170).
Cumulative benzodiazepine doses
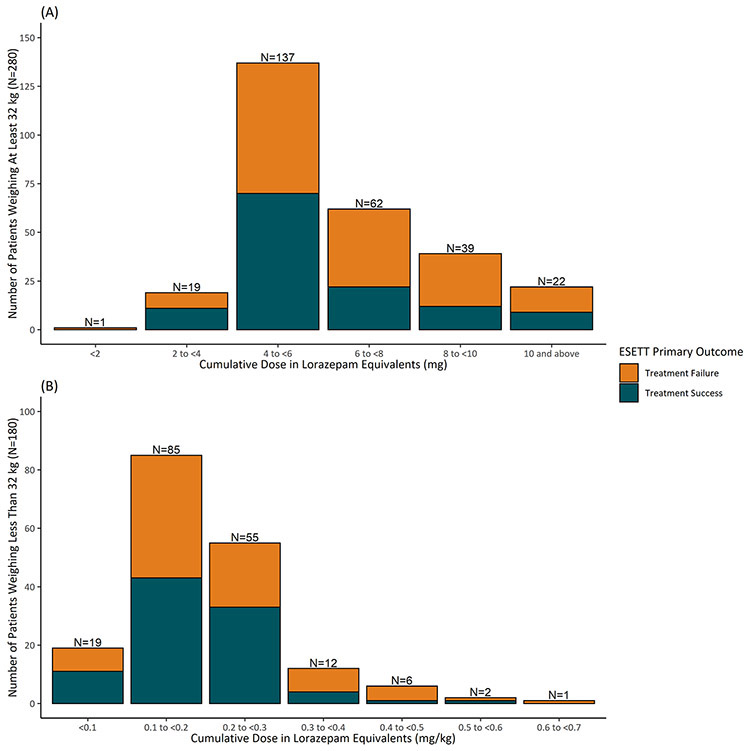
Distribution of cumulative benzodiazepines doses received prior to ESETT enrollment calculated in lorazepam equivalents shows that patients weighing ≥ 32 kg received a cumulative dose (median, interquartile range (IQR)) of 4.8 mg (4, 6.5 mg), whereas those who were < 32kg received 0.19 mg/kg (IQR: 0.14, 0.24 mg/kg) (Figure 3).
Figure 3:
Distribution of the cumulative benzodiazepine dose in lorazepam equivalents for subjects weighing ≥ 32 kg (top panel) and < 32 kg (bottom panel) as primary outcome failure (orange) or success (blue)
Time from onset of SE to the first dose of benzodiazepine
The median estimated delay from the onset of SE to the first dose of the first benzodiazepine was 27 minutes (IQR: 11, 49 minutes) (N=354).
Time to cumulative adequate benzodiazepine dose after the first dose
The median delay from the first dose of benzodiazepine to adequate cumulative dose (N=423) was six minutes (IQR: 0, 20 minutes). When analyzed by weight group, the median delay was eight minutes (IQR: 1, 21 minutes) in those weighing ≥ 32 kg (N=258), which was longer and more variable than two minutes (IQR: 0, 18 minutes) in subjects weighing < 32 kg (N=165).
Time trend of first doses
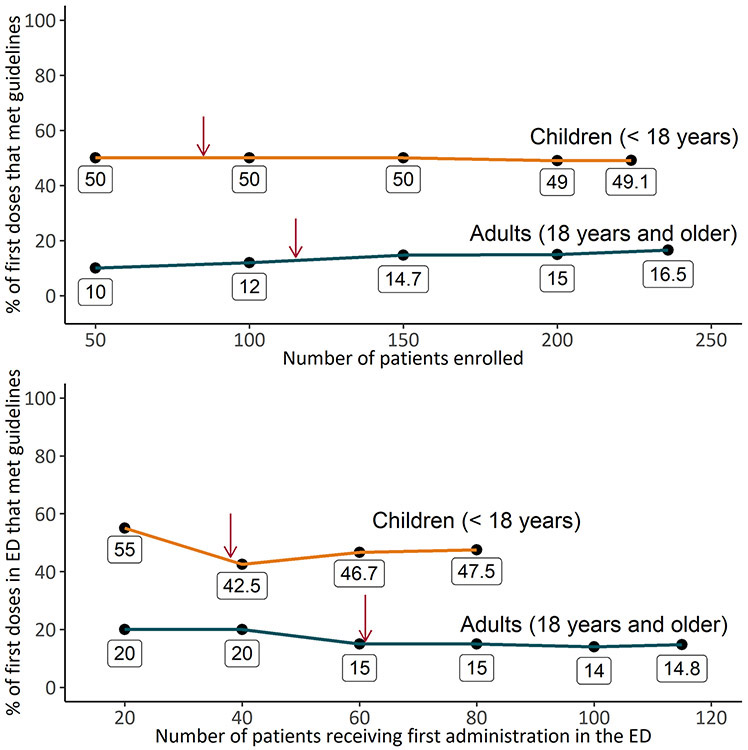
Figure 4 shows the proportion of first doses given to adults and children that met guideline recommendations during ESETT in all settings and only in the ED. In children, the proportion of first doses meeting guidelines was 110 out of 124 or 49% (95% confidence interval (CI) [43%, 56%]) in all settings and 38 out of 80 or 48% (95% CI [37%,58%]) in the ED. Similarly, for adults, the proportion of first doses meeting guidelines 39 out of 236 or 17% (95% CI [12%, 21%]) in all settings and 17 out of 115 or 15% (95% CI [8%, 21%]in the ED.
Figure 4:
Time trend of the proportion of first doses that met guidelines over the course of ESETT for children (orange) and adults (blue) for all first doses (top panel) and first doses administered in the emergency department (ED) (bottom panel). The red arrows indicate the total number of adult and children included (115 adults, 85 children) in the first cohort (top panel) and number of first doses administered in the ED (61 in adults, 38 in children) in the first cohort (bottom panel).
Comparing cohort 1 and cohort 2
Supporting information Table S1 shows the distribution of first doses of DZP, MDZ and LZP in adults and children across the two cohorts. The proportion of first doses meeting guidelines was similar between cohort 1, 56 out of 200 or 28% (95% CI [22%, 34%]), and cohort 2, 93 out of 260 or 35% (95% CI [30%, 42%]). For adults, the proportion of MDZ first doses was higher 23.6% (95% CI [12.4%, 34.9%]) in cohort 2 vs. 11.1% (95% CI [1.9%, 20.3%]) in cohort 1.
Association of benzodiazepine doses and other predictors with ESETT outcomes
Higher first or cumulative benzodiazepine doses were not associated with better outcomes or seizure cessation after subsequent second-line medications in these benzodiazepine-refractory seizures.
The first dose of the first benzodiazepine:
There was no association of a 0.1 mg/kg increase in the first dose of the first benzodiazepine with the primary outcome (adjusted odds ratio (aOR) 1.07, 95 % CI [0.63, 1.85]) or clinical seizure cessation (aOR 1.05 [0.61, 1.85]).
Cumulative benzodiazepine dose:
In this cohort of patients with benzodiazepine-refractory SE, not achieving the primary outcome of clinical seizure cessation and improved responsiveness after treatment with levetiracetam, fosphenytoin, or valproic acid was associated with higher cumulative doses of benzodiazepines given prior to the second-line drug. A 0.1 mg/kg increase in the cumulative benzodiazepine dose was associated with a decrease in the likelihood of treatment success for the primary outcome (aOR 0.71 [0.53, 0.92]). However, there was no association of cumulative benzodiazepine dose with the response to second-line antiseizure drugs as determined by clinical seizure cessation alone (aOR 0.8 [0.62, 1.04]).
Interaction of benzodiazepine dose with other predictors:
The association of age and etiology with primary outcome was significant and was unaffected by corrections for cumulative benzodiazepine dose. Age ≥ 18 years was associated with a decrease in the likelihood of treatment success (aOR 0.54 [0.33, 0.87]) after adjusting for cumulative benzodiazepine dose, etiology and ESETT study drug. Etiology of acute stroke/hemorrhage, CNS tumor or CNS infection was associated with a decrease in the likelihood of treatment success (aOR 0.42 [0.18, 0.92]) after adjusting for cumulative benzodiazepine dose, age and ESETT study drug.
DISCUSSION
Based on 460 patients ultimately enrolled in ESETT, our results show that the first dose of the first benzodiazepine as first-line treatment of SE was lower than guideline recommendations in > 80% of all MDZ cases and > 75% of all LZP cases. Reducing the guideline-recommended doses by 20% to account for the approximation of patient weight, and rounding did not meaningfully change the pattern of underdosing. Although underdosing was pervasive across the age spectrum, children received recommended doses more often than adults. The exception was DZP, mainly administered rectally to children by caregivers prior to EMS, for which most doses met guidelines. The high rates of guideline adherence for DZP may be due to the availability of a pre-packaged, easy-to-use product with preset doses based on weight and age. Similar results were obtained when all benzodiazepine doses were evaluated. When analyzing results from all 460 patients who failed first-line therapy, we observed a pattern of administering multiple, smaller than recommended benzodiazepine doses in the EMS and ED settings which confirm and extend the findings from our previous analysis of the first 200 unique enrollments14 and are consistent with other reports.6-13 With regard to route of administration and setting, MDZ was primarily administered as IM or IV by EMS personnel, while LZP was almost exclusively dosed IV in the ED. These patterns are in general agreement with published literature.2,3,15,16
Following our preliminary analyses of the first 200 patients, increased educational efforts were implemented at investigator meetings and participating clinical sites. While the proportion of MDZ first doses in adults that met guidelines showed a modest improvement, the overall trend of underdosing continued through the course of the study with only a small fraction of MDZ and LZP first doses meeting guideline recommendations.
Delay in administering appropriate therapy is thought to place patients at risk for longer seizures and poor outcomes.17-26 A multicenter, observational, prospective cohort study of 218 pediatric patients with refractory convulsive SE found that a delay of > 10 minutes between the onset of a seizure and the administration of benzodiazepine treatment was significantly associated with increased odds of death, longer duration of convulsive seizures and more frequent hypotension.19 If an initial benzodiazepine dose does not terminate a prolonged seizure, preclinical evidence suggests that higher subsequent doses may be required.27-32 This could be due to changes in benzodiazepine pharmacodynamics. Prolonged seizures result in enhanced endocytosis of synaptic GABAA receptors, thus reducing benzodiazepine potency.29-32 This internalization is associated with decreased effectiveness of DZP and, presumably, other benzodiazepines.27,28 For example, in a rat model where SE was induced using lithium chloride-pilocarpine, the DZP ED50 for terminating seizures was 10-fold higher, 40 mg/kg vs. 4.2 mg/kg, when administered after 45 minutes as compared to 10 minutes after the onset of SE.27 Furthermore, rapid receptor plasticity has been attributed to the activation of some second messengers during prolonged seizures.33 As SE continues, the activity and number of N-methyl-D-aspartate (NMDA) receptors and excitatory amino-acid synaptic concentrations increase. Hence, the administration of an adequate dose of benzodiazepines as soon as possible is recommended to terminate SE and avoid established SE.34,35 In our study, attainment of adequate cumulative benzodiazepine dose took at least 20 minutes after the first administration in approximately 25% of adults and children. Combining this time interval with the elapsed time from seizure onset to first dose (median of 27 minutes) potentially reduced the response to BZDs and highlights the importance of administering an adequate dose as soon as possible following the onset of SE.
It is not surprising, in this cohort, that responsiveness of seizures to second-line agents was not enhanced by higher doses of benzodiazepines, since the study population was, by design, those whose SE was benzodiazepine refractory. The ESETT primary outcome was a composite, which consisted of clinical seizure cessation and improved responsiveness at 60 minutes after the start of the study drug infusion. We found that even after considering the underlying brain pathology responsible for SE, higher cumulative benzodiazepine doses were significantly associated with decreased odds of primary outcome success, but not with clinical seizure cessation. Among the potential explanations for this association is that patients with more severe or persistent seizures likely required higher doses of benzodiazepines. Alternatively, the lack of association with clinical seizure cessation suggests that those with higher cumulative doses failed the primary outcome due to the lack of improved responsiveness. It is possible that many patients given higher doses of benzodiazepines did respond better to those higher doses, and thus were excluded from enrollment in ESETT and this analysis. The design of the study was to enroll participants who failed benzodiazepine therapy. Expectedly, they did not have more seizure cessation from the benzodiazepines but did have more sedation after their seizures were terminated with the second-line medication. Thus, they likely woke up slowly and did not achieve the primary outcome as often. Furthermore, there may be confounding factors that influence this finding, including the elapsed time over which the cumulative doses were administered.
There are several possible reasons for this wide-spread underdosing. Betjemann et al. found that many of the emergency medical services protocols in California did not follow evidence-based guidelines, and MDZ’s initial dose was lower than guideline-recommendations in most protocols.36 As another explanation, emergency physicians may start treating seizure emergencies with lower doses of benzodiazepines because, at the presentation in the ED, they may not have yet established whether the patient is in SE or has not yet recovered from a recent seizure. ESETT only examined benzodiazepine dosing in patients with SE who did not respond to benzodiazepines, so it was not possible to compare the dosing or outcome in patients whose seizures stopped to determine if higher benzodiazepine doses would have been helpful. Nevertheless, once the diagnosis of SE is established, randomized clinical trials make clear what dosing of benzodiazepines should be and emergency physicians should move with haste to administer adequate doses. Another factor that may be responsible for underdosing is the perceived risk of cardio-respiratory compromise associated with benzodiazepines.25,37,38 However, Alldredge et al. reported that the rate of respiratory or circulatory complications in SE patients given placebo was nearly two-fold higher (p=0.08) than those treated with benzodiazepines.15 Similarly, Guterman et al. found that higher doses of MDZ used by EMS personnel for the treatment of adult SE patients were not associated with increased need for respiratory support.13
Other deviations from best guideline-recommended practices were also observed. On 29 occasions, LZP was administered by IM, IN, or buccal routes. The pharmacokinetics of LZP following administration by these routes do not support rapid absorption and, therefore are not appropriate for SE therapy.39-42 Given the high rate of appropriate dosing of rectal DZP by caregivers, the recently approved IN DZP and IN MDZ products intended for the treatment of seizure emergencies may facilitate early administration of recommended benzodiazepine doses, particularly in adults.43
There are several limitations that affect generalizing our findings to a broader population. Our analysis is limited to SE patients who continued to have seizures despite benzodiazepine treatment. Since initial benzodiazepine underdosing is associated with treatment failure, our study population may overestimate the rate of underdosing among all patients for SE. Additionally, we were not able to precisely determine the latency between the onset of SE and initial benzodiazepine dose, a factor that affects treatment response rates. It may be that the latency for the ESETT patients was different from those who respond to initial benzodiazepine treatment. Nonetheless, benzodiazepine underdosing is particularly important in this subpopulation in whom seizures continue and may progress to refractory SE with attendant high rates of morbidity and mortality. Conversely, our analysis may underestimate the rate of underdosing because only those given an adequate cumulative benzodiazepine dose were eligible for ESETT enrollment. It is possible that eagerness to enroll subjects could bias toward lower cumulative benzodiazepine doses, but that would not be expected to impact the first dose. However, in this scenario, ESETT participating EDs would be more likely to administer larger individual doses in order to meet the minimum adequate dose sooner and should not affect EMS practice. Another minor limitation is that different routes of administration of benzodiazepines were assumed to be equivalent in the calculation of cumulative dose in LZP equivalents.
CONCLUSION
Benzodiazepines as first-line therapy for status epilepticus appear to be frequently underdosed in the EMS and ED settings regardless of drug, route of administration, or patient weight. The frequency of underdosing was far greater in adults than children. This pattern may contribute to poorer responses. This broad and generalizable cohort confirms prior single site reports that underdosing is both pervasive and difficult to remediate. Although there are published guidelines for the initial treatment of status epilepticus, additional efforts are needed to change clinical practice.
Supplementary Material
KEY POINTS.
Benzodiazepine dosing patterns as first-line treatment of status epilepticus (SE) were characterized in 478 patients enrolled in the Established Status Epilepticus Treatment Trial (ESETT)
Greater than 75% of midazolam and lorazepam first doses, administered mainly by the paramedics and in the emergency departments, respectively, were lower than guideline recommendations
Subsequent doses of midazolam and lorazepam were also lower than recommendations
It took a median of 27 minutes to receive the first benzodiazepine dose after the onset of SE and an additional six minutes to receive the cumulative adequate dose
Higher cumulative doses of benzodiazepines as first-line treatment were not associated with increased likelihood of seizure cessation after second-line therapy
ACKNOWLEDGEMENTS
Research reported in this publication was supported by National Institutes of Health, National Institutes of Neurological Disorders and Stroke under Awards U01NS088034, U01NS088023, U01NS056975, U01NS059041, and R01NS099653. (Clinical trials.gov identifier NCT01960075). We would like to acknowledge the ESETT Data and Safety Monitoring Board. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke, National Institutes of Health, or the United States Government.
Footnotes
CONFLICTS OF INTEREST
All authors were supported by the ESETT study grant from NIH/NINDS (U01NS088034). L.D. Coles reports grants from NIH/NINDS, during the conduct of the study; personal fees from Neurelis Pharmaceuticals, grants from Sollievo, outside the submitted work; H.R. Cock reports grants from NINDS, during the conduct of the study; personal fees from Sage Pharmaceuticals Ltd, Eisai Europe Ltd, UCB Pharma Ltd, UK Epilepsy Nurse Specialist Association, non-financial support from Special Products Ltd, International League Against Epilepsy, Epilepsy Certification (education) Task Force, European Academy of Neurology, personal fees from Bial and Eisai, outside the submitted work; N.B. Fountain reports grants from NINDS during the conduct of the study; grants from SK Lifesciences, Neurelis, Takeda, GW Pharma, Biogen, and UCB outside the submitted work. S. Shinnar reports grants from NINDS during the conduct of the study; personal fees from UCB Pharma, Eisai and Insys, outside the submitted work; E.S. Rosenthal reports grants from NIH/NINDS and the Department of Defense during the conduct of the study; personal fees from UCB Pharma and Ceribell, Inc., during the conduct of the study. J.C. Cloyd reports a grant from National Institute of Neurological Disorders and Stroke during the conduct of the study and personal fees from Neurelis Pharmaceuticals, grants from Sollievo, outside the submitted work; J.C. Cloyd has a patent entitled "Intranasal Drug Delivery". The remaining authors have no conflicts of interest. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Meierkord H, Boon P, Engelsen B, et al. EFNS guideline on the management of status epilepticus. Eur J Neurol. 2006; 13(5):445–50. [DOI] [PubMed] [Google Scholar]
- 2.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012; 17(1):3–23. [DOI] [PubMed] [Google Scholar]
- 3.Glauser T, Shinnar S, Gloss D, et al. Evidence-Based Guideline : Treatment of Convulsive Status Epilepticus in Children and Adults : Report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 2016; 16(1):48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapur J, Elm J, Chamberlain JM, et al. Randomized Trial of Three Anticonvulsant Medications for Status Epilepticus. N Engl J Med. 2019; 381(22):2103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamberlain JM, Kapur J, Shinnar S, et al. Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial. Lancet. 2020; :1217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langer JE, Fountain NB. A retrospective observational study of current treatment for generalized convulsive status epilepticus. Epilepsy Behav. 2014; 37(2014):95–9. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez V, Lee JW, Drislane FW, et al. Practice variability and efficacy of clonazepam, lorazepam, and midazolam in status epilepticus: A multicenter comparison. Epilepsia. 2015; 56(8):1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun J, Gau E, Revelle S, et al. Impact of non-guideline-based treatment of status epilepticus. J Neurol Sci. 2017; 382(April):126–30. [DOI] [PubMed] [Google Scholar]
- 9.Rao SK, Mahulikar A, Ibrahim M, et al. Inadequate benzodiazepine dosing may result in progression to refractory and non-convulsive status epilepticus. Epileptic Disord. 2018;20(4):265–9. [DOI] [PubMed] [Google Scholar]
- 10.Kellinghaus C, Rossetti AO, Trinka E, et al. Factors predicting cessation of status epilepticus in clinical practice: Data from a prospective observational registry (SENSE). Ann Neurol. 2019; 85(3):421–32. [DOI] [PubMed] [Google Scholar]
- 11.Trau SP, Sterrett EC, Feinstein L, et al. Institutional Pediatric Convulsive Status Epilepticus Protocol Decreases Time to First and Second Line Anti-Seizure Medication Administration. Seizure. 2020; 81(August):263–8. [DOI] [PubMed] [Google Scholar]
- 12.Vasquez A, Gaínza-Lein M, Abend NS, et al. First-line medication dosing in pediatric refractory status epilepticus. Neurology. 2020; 95(19):e2683–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guterman EL, Sanford JK, Betjemann JP, et al. Prehospital midazolam use and outcomes among patients with out-of-hospital status epilepticus. Neurology. 2020; 95(24):e3203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sathe AG, Tillman H, Coles LD, et al. Underdosing of Benzodiazepines in Patients with Status Epilepticus Enrolled in Established Status Epilepticus Treatment Trial. Acad Emerg Med. 2019; 0(8):940–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alldredge BK, Gelb AM, Isaacs SM, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001; 345(9):631–7. [DOI] [PubMed] [Google Scholar]
- 16.Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular versus Intravenous Therapy for Prehospital Status. N Engl J Med. 2012; 366(7):591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaínza-Lein M, Fernández IS, Ulate-Campos A, et al. Timing in the treatment of status epilepticus: From basics to the clinic. Seizure Eur J Epilepsy. 2018; 68:22–30. [DOI] [PubMed] [Google Scholar]
- 18.Ostendorf AP, Merison K, Wheeler TA, et al. Decreasing Seizure Treatment Time Through Quality Improvement Reduces Critical Care Utilization. Pediatr Neurol. 2018; 85:58–66. [DOI] [PubMed] [Google Scholar]
- 19.Gaínza-Lein M, Fernández IS, Jackson M, et al. Association of time to treatment with short-term outcomes for pediatric patients with refractory convulsive status epilepticus. JAMA Neurol. 2018; 75(4):410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewena S, Young S. When benzodiazepines fail: How effective is second line therapy for status epilepticus in children? Emerg Med Australas. 2006; 18(1):45–50. [DOI] [PubMed] [Google Scholar]
- 21.Betjemann JP, Lowenstein DH. Status epilepticus in adults. Lancet Neurol. 2015; 14(6):615–24. [DOI] [PubMed] [Google Scholar]
- 22.Chin RFM, Verhulst L, Neville BGR, et al. Inappropriate emergency management of status epilepticus in children contributes to need for intensive care. J Neurol Neurosurg Psychiatry. 2004; 75(11):1584–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chin RF, Neville BG, Peckham C, et al. Treatment of community-onset, childhood convulsive status epilepticus: a prospective, population-based study. Lancet Neurol. 2008; 7(8):696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madžar D, Geyer A, Knappe RU, et al. Association of seizure duration and outcome in refractory status epilepticus. J Neurol. 2016; 263(3):485–91. [DOI] [PubMed] [Google Scholar]
- 25.Uppal P, Cardamone M, Lawson JA. Outcomes of deviation from treatment guidelines in status epilepticus: A systematic review. Seizure. 2018; 58:147–53. [DOI] [PubMed] [Google Scholar]
- 26.Eriksson K, Metsäranta P, Huhtala H, et al. Treatment delay and the risk of prolonged status epilepticus. Neurology. 2005; (65):1316–8. [DOI] [PubMed] [Google Scholar]
- 27.Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci. 1997; 17(19):7532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazarati AM, Baldwin RA, Sankar R, et al. Time-dependent decrease in the effectiveness of antiepileptic drugs during the course of self-sustaining status epilepticus. Brain Res. 1998; 814(1–2):179–85. [DOI] [PubMed] [Google Scholar]
- 29.Naylor DE, Liu H, Wasterlain CG. Trafficking of GABAA receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005; 25(34):7724–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodkin HP, Joshi S, Mtchedlishvili Z, et al. Subunit-specific trafficking of GABAA receptors during status epilepticus. J Neurosci. 2008; 28(10):2527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodkin HP, Kapur J. The impact of diazepam’s discovery on the treatment and understanding of status epilepticus. Epilepsia. 2009; 50(9):2011–8. [DOI] [PubMed] [Google Scholar]
- 32.Goodkin HP, Yeh J-L, Kapur J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci. 2005; 25(23):5511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pal S, Sun D, Limbrick D, et al. Epileptogenesis induces long-term alterations in intracellular calcium release and sequestration mechanisms in the hippocampal neuronal culture model of epilepsy. Cell Calcium. 2001; 30:285–96. [DOI] [PubMed] [Google Scholar]
- 34.Wasterlain CG, Naylor DE, Liu H, et al. Trafficking of NMDA receptors during status epilepticus: Therapeutic implications. Epilepsia. 2013; 54:78–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borris DJ, Bertram EH, Kapur J. Ketamine controls prolonged status epilepticus. Epilepsy Res. 2000; 42(2–3):117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Betjemann JP, Josephson SA, Lowenstein DH, et al. Emergency Medical Services Protocols for Generalized Convulsive Status Epilepticus. JAMA. 2019; 321(12):1216–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spatola M, Alvarez V, Rossetti AO. Benzodiazepine overtreatment in status epilepticus is related to higher need of intubation and longer hospitalization. Epilepsia. 2013; 54(8):99–102. [DOI] [PubMed] [Google Scholar]
- 38.Stewart WA, Harrison R, Dooley JM. Respiratory depression in the acute management of seizures. Arch Dis Child. 2002; 87(3):225–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenblatt DJ, Divoll M, Harmatz JS, et al. Pharmacokinetic comparison of sublingual lorazepam with intravenous, intramuscular, and oral lorazepam. J Pharm Sci. 1982; 71(2):248–52. [DOI] [PubMed] [Google Scholar]
- 40.Lau SWJ, Slattery JT. Absorption of diazepam and lorazepam following intranasal administration. Int J Pharm. 1989; 54:171–4. [Google Scholar]
- 41.Anderson M, Tambe P, Sammons H, et al. Pharmacokinetics of buccal and intranasal lorazepam in healthy adult volunteers. 2012; :155–9. [DOI] [PubMed] [Google Scholar]
- 42.Wermeling DPH, Miller JL, Archer SM, et al. Bioavailability and Pharmacokinetics of Lorazepam after Intranasal , Intravenous , and Intramuscular Administration. J Clin Pharmacol. 2001; 41:1225–31. [DOI] [PubMed] [Google Scholar]
- 43.Maglalang PD, Rautiola D, Siegel RA, et al. Rescue therapies for seizure emergencies: New modes of administration. Epilepsia. 2018; 59(Suppl 2):207–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.