Abstract
Despite the advances in the primary prevention of cervical cancer, there is an absolute increase in the incidence of cervical cancer as a result of an increase in world population. A vast majority of patients in low and low–middle income countries continue to present at a locally advanced stage, necessitating treatment with chemoradiation and brachytherapy. There is a dearth of equipment and trained professionals for the treatment of cervical cancer, especially in low and low–middle income countries. There is an urgent need to improve treatment availability and develop better treatments. Worldwide trends, however, reveal a low number of therapeutic and innovative research trials in cervical cancer. The present article elucidates the existing challenges and provides solutions to improve outcomes. The proposed strategies hinge on strengthening collaborations for global advocacy.
Keywords: Cervical cancer, global collaborations
Introduction
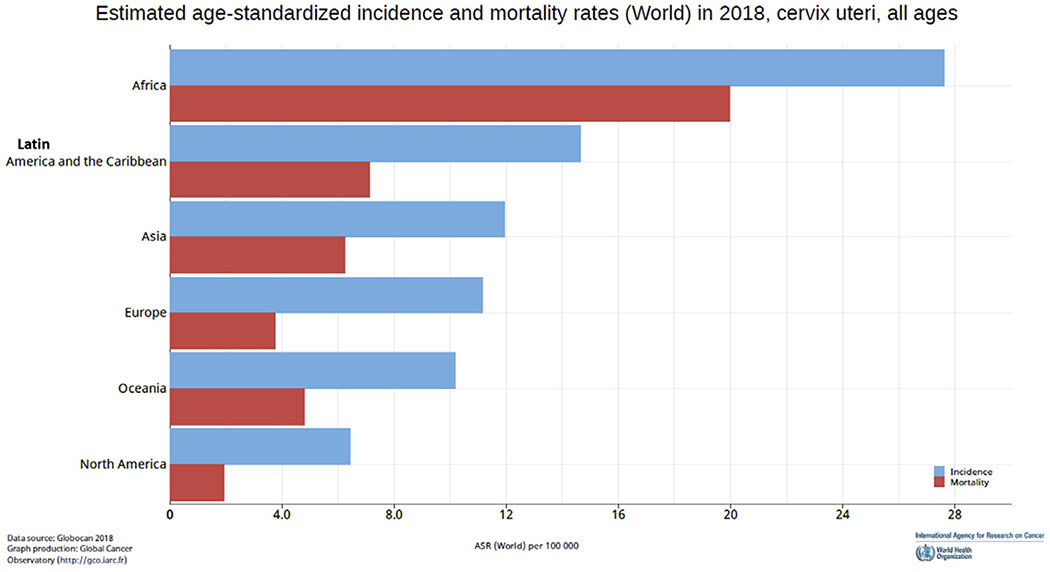
Distinct geographical disparities exist in the clinical care of women with cervical cancer, both in low–middle income countries (LMICs) and in underserved minority groups within high income countries (HICs). There is a substantial variation in the age-standardised incidence of cervical cancer (from 27.6 to 6 per 100 000) based on country-specific average per capita income and human development indices [1]. Global estimates also show the doubling of the mortality to incidence ratio if a woman is diagnosed with cervical cancer in world regions classified to have a medium to low human development index (Figure 1). These disparities may be partly related to a lack of commitment to cervical cancer as a women’s health and societal priority. Sustainable development goals (SDG 17) identified maternal mortality as one of the major indicators of women’s health [2]. This has led to a worldwide reduction in maternal mortality. Although the maternal mortality ratio has declined by 37% [3], since 2000 there has been a 17% increase in absolute mortality from cervical cancer in less than a decade (from 275 000 to 311 365 per year). As of 2018, two women die of cervical cancer each minute and the annual cervical cancer mortality exceeds maternal mortality (311 365 versus 303 000) [3,4]. These indices have led the World Health Organization (WHO) to announce the prioritisation of the elimination of cervical cancer [4]. Although Australia may be on its way to eliminate cervical cancer by 2028, it is estimated that for the rest of the world at least three to four decades will be needed before the reduction is seen if a full scale-up of prevention and vaccination is assumed [5,6]. A significant majority of patients within LMICs and underserved minorities in HICs will, therefore, continue to present with invasive locally advanced cervical cancer necessitating treatment with chemoradiation.
Fig 1.
Age-standardised incidence and mortality rates for cervical cancer in different world regions.
Global collaborations are therefore needed for effective implementation of the current standard of care for locally advanced cervical cancer. The current article discusses the existing disparities in care for cervical cancer and highlights the potential areas for East–West collaboration to improve therapeutic outcomes of locally advanced cervical cancer patients at a global level.
Access to External Radiation for Cervical Cancer
Chemoradiation and brachytherapy represent the current standard of curative care for locally advanced cervical cancer [7,8]. Low income countries (LICs) and LMICs have no or inadequate access to radiotherapy facilities. Financial investment case studies suggest that $184 million will be needed in two decades to close the demand–supply gap [9]. These estimates, however, average out radiotherapy utilisation across cancer types. Cervical cancer optimal radiotherapy utilisation is often averaged at 70%, whereas up to 85% of patients in LMICs and LICs may need radiotherapy as part of curative treatment, not only due to the advanced stage but also to compensate for a lack of surgical oncology expertise [10]. East–West collaborations are needed for country-specific or regional modelling of cost investment for radiotherapy for cervical cancer, similar to global modelling studies of cost investment into vaccination and screening [11].
A recent systematic review of the National Cancer Control Plans showed that only 58% of countries in the world have a National Cancer Control Plan and only 32–46% of LICs and LMICs mention radiotherapy as a requirement [12]. A lack of structured information to national health advisory groups may have serious implications for cost investment for the treatment of cervical cancer. The global call for the elimination of cervical cancer by the WHO Director-General [4] presents a unique opportunity to develop succinct case studies in financial investment. International and national advocacy at the radiotherapy international societies, governmental and interagency level is therefore needed and task groups for cervical cancer need to be established to undertake this initiative.
Due to the absence of radiation facilities in various world regions, the American Society of Clinical Oncology resource-stratified guidelines currently list neoadjuvant chemotherapy and surgery as therapeutic options among women who have no access to radiation facilities [13]. However, recently, a large randomised study in stage IB2–IIB patients from India failed to show the superiority of neoadjuvant chemotherapy and surgery over the standard chemoradiotherapy arm [14]. Although no survival difference was observed between the two arms, it is noteworthy that up to 40% of patients within the neoadjuvant chemotherapy and surgery arm required some form of radiation to attain equivalent overall survival. LICs that have a radiotherapy shortfall also face an acute shortage of other oncology staff to implement the resource-stratified guidelines [15]. International initiatives are therefore needed to evaluate the implementation of the proposed resource-stratified algorithms at the ground level. Furthermore, a significant proportion of advanced cancers may have minimal or no response to neoadjuvant chemotherapy. Clinical care pathways for such situations are presently undefined. Global public–private co-operation needs to be developed, akin to the Global Alliance for Vaccination and Immunization, to provide access to subsidised optimal care in nearby countries [16,17]. Regional partnerships for resource sharing and training should be developed (e.g. within the Indian subcontinent, between North and Latin America, east and west Europe) and such models should be evaluated for long-term functionality and sustainability. A recent example of these initiatives is the donation of cobalt equipment to LICs by the International Atomic Energy Agency (IAEA) through its Programme of Action for Cancer Therapy agreement with India. Tata Trusts and Radiating Hope are some examples of private philanthropy [18–21].
LMICs that have access to radiation facilities often report a shortage of adequate infrastructure to treat all patients. A national estimate for teletherapy resources for cervical cancer in India suggests that an additional 105 linear accelerators may be needed just to treat cervical cancer [22]. An international survey reports that an average of 21 fractions are routinely used for the treatment of cervical cancer [10]. Robust research trials focusing on hypofractionation (as in rectum and prostate cancer) [23–25] need to be urgently initiated to test its safety and efficacy in cervical cancer. Positive results, as for other pelvic malignancies, may substantially reduce the burden on existing facilities. In the recent past the IAEA has led resource-sparing trials for rectal cancer and established that working groups may be used to test a similar hypothesis for cervical cancer [24]. As there is a dearth of clinical infrastructure, resource-intensive procedures for external radiation (like intensity-modulated radiation therapy) should be used judiciously only if clinically indicated. International collaborative campaigns, like ‘choosing wisely’, should be used to ensure that recommended care for cervical cancer is evidence-based and cost-effective [26].
Access and Delivery of Concurrent Chemotherapy for Cervical Cancer
Strengthening the concurrent chemotherapy delivery framework within high incidence regions will be vital to improve the outcomes of cervical cancer. The use of five or more cycles of concurrent chemotherapy is associated with survival improvement within multiple clinical trials [8]. However, the implementation of five or more cycles of concurrent chemotherapy within and outside clinical trials may also be challenging [14,27–29]. An acute shortfall of adequately trained staff and a shortage of daycare beds, nursing and support staff may create ‘chemotherapy waiting lists’ [30,31]. Adequate infrastructure and health workers are also needed to manage acute complications of treatment administration. A recent study on the availability of WHO essential medicines reported frequent unavailability of cisplatin in some countries in Africa [32]. In other high incidence countries where drugs are available and subsidised for patients below the poverty line, approvals may be needed for weekly cycles [33], leading to delays in the implementation of an effective concurrent chemoradiation schedule.
Poor compliance and enhanced acute toxicity are reported in patients from a rural and underprivileged background in LMICs [34]. Audits from developing countries report 42–85% compliance to planned chemoradiation schedules, with a substantial majority of patients receiving less than five cycles of concurrent chemotherapy [29,35,36]. The coexistence of HIV infection within high incidence regions is also associated with reduced odds of receiving concurrent chemotherapy. A National Cancer Database analysis from the USA of 10 265 HIV-infected patients reported increased odds of a lack of standard treatment [37]. A recent phase II study from the AIDS malignancy consortium from Sub-Saharan Africa reported that most HIV-infected women can tolerate concurrent chemotherapy [38] and coexistence of HIV infection had no impact on 2-year survival in adequately treated patients [39]. However, other clinical audits reported significantly poor compliance and reduced overall survival, but most of them were carried out in the pre-antiretroviral therapy era or included patients not on HIV treatment [40,41].
Poor compliance with chemoradiation is also reported in underprivileged or lower social economic classes within HICs. An audit of the California Cancer Care Registry, which included 6063 patients with cervical cancer, reported only 47% adherence to guideline-based care and even within affluent societies, treatment in a low volume centre (treating <20 cases/year), low socioeconomic status, Black race and lack of insurance were independent factors that doubled the risk of mortality from cervical cancer [42]. Other studies from HICs report adverse outcomes with increasing distance from the cancer centre [43].
International global health programmes in recent years have provided much-needed collaboration to improve care for underprivileged women [44,45]. Further strengthening of the global health programme to undertake health implementation research in LMICs and underserved minorities may help to identify barriers to delivery of care while strengthening local teams. Digital mobile health approaches have been shown to improve cervical cancer screening compliance in poor rural communities with low literacy levels [46]. Evolving digital technology platforms should be evaluated to help improve treatment compliance of patients in LMICs and LICs.
Access to Brachytherapy for Cervical Cancer
Brachytherapy is an integral component of radiation treatment for cervical cancer. Unlike breast, head and neck and prostate cancer, there is no equivalent alternative for gynaecological brachytherapy and the omission of brachytherapy is associated with reduced survival [47,48]. Although the Global Task Force on Radiotherapy for Cancer Control [9] and the Global Impact of Radiation Oncology [49] initiatives focus on mapping teletherapy resources worldwide, there is a lack of initiative to map and report brachytherapy equipment for cervical cancer treatment. A recent survey by the IAEA reported a shortfall of 133 brachytherapy units within LMICs, with no brachytherapy facilities in 32/50 African facilities. Within the Commonwealth countries, a shortfall of 70 brachytherapy units is reported, of which 50 units are needed in India and Bangladesh. An investment of US$70 million for brachytherapy in Commonwealth countries could result in a saving of 315 000 lives over the next 10 years [22]. Investing in a cobalt rather than an iridium source could also reduce the long-term costs associated with high dose rate brachytherapy [50].
Recent advances in cervical brachytherapy also show improved outcomes with the integration of magnetic resonance imaging (MRI) and intracavitary and interstitial techniques [27,51]. These techniques, however, require a high investment in imaging scanners, applicators and physician, physicist and dosimetrist time. Institutions within LMICs/LICs that have access to brachytherapy infrastructure report waiting lists, with treatment times extending beyond 8 weeks. High volume centres often carry out four to eight brachytherapy procedures per day, making it challenging to carry out state of the art MRI-guided brachytherapy for all-comers [35]. Cost-effective and time-efficient alternatives to MRI brachytherapy include the use of ultrasound and computed tomography for target delineation [52–58]. The outcomes of a selected series of image-based brachytherapy that used MRI-, computed tomography- or ultrasound-based planning are encouraging (Table 1). Comparative research is needed to test the non-inferiority of computed tomography/ultrasound-based brachytherapy that may provide equivalent outcomes. High-quality studies in brachytherapy fractionation are also needed to test if abbreviated fractionation schemes provide equivalent results. The results of the IAEA randomised trial and phase I study from the Tata Memorial Centre are awaited [59,60]. Many programmes in LMICs and LICs continue to carry out X-ray-based brachytherapy or use library plans for treating repeat fractions. This may be associated with increased toxicity, as women in LMICs/LICs have a low body mass index, which can lead to a higher organ at risk dose [61–63]. Outcome studies are therefore needed for local control and toxicity. Programmes from the IAEA that help the transition from two- to three-dimensional brachytherapy within LMICs should also be considered [64].
Table 1.
Select series of image-guided brachytherapy outcomes from the Asia Pacific region
| Reference | Number | Imaging | Follow-up time | Results |
|---|---|---|---|---|
| Mahantshetty et al. [51] | 94 | MRI | 39 months | Local control rate 90% |
| Kang et al. [53] | 97 | CT | 41 months | Local control rate 97% |
| Tharavichitkul et al. [54] | 47 | CT | 26 months | Local control rate 97.9% |
| Murakami et al. [55] | 51 | CT | 39 months | Local control rate 92% |
| Narayan et al. [56] | 292 | TAUS | 4.1 years | Local control rate 87.5% |
| Tharavichitkul et al. [58] | 92 | TAUS | 41 months | Pelvic control rate 84.8% |
CT, computed tomography; MRI, magnetic resonance imaging; TAUS, transabdominal ultrasound.
Although developed countries do not have a dearth of brachytherapy equipment, a 25% reduction in the use of brachytherapy was reported from 1998 to 2009. The sharpest decline in brachytherapy in the USA corresponded to an increase in the use of external techniques and was associated with reduced survival. This may be attributed to a lack of physician incentives and increased costs. Brachytherapy accounts for 75% of the total radiotherapy treatment costs and 80% of the radiation oncologist’s time. In addition, brachytherapy results in less revenue generation and is thus potentially associated with a net loss for the institution as well as the physician [65]. Similar trends in financial reimbursements are observed in government-supported schemes within private hospitals in India, wherein the use of brachytherapy is associated with a financial loss to the provider institution [33]. Better resource- and revenue-sharing models need to be developed and tested to improve the financial sustainability of brachytherapy.
Brachytherapy requires not only a specialised infrastructure, but also qualified expertise. From 2006 to 2010, a 25% reduction in interstitial brachytherapy procedures was reported within accredited residency programmes. Residency training programmes in the USA also reported a reduction in training proficiency for brachytherapy. In a more recent survey in 2016, 40–85% of residents reported inadequate training [66,67]. Another survey from France reported that 56% of young radiation oncologists had observed a gynaecological brachytherapy procedure. However, only 12% knew how to perform the procedure [68]. A Canadian survey highlighted the need for elements of brachytherapy training to be included in the curriculum and the need to have the credentials to carry out brachytherapy [69]. In 2019, the American Board of Radiology College of Graduate Medical Education (ACGME) requirements for radiation oncology residents are being updated, with the proposal for residents to carry out at least 15 intracavitary brachytherapy procedures, of which at least five tandem-based intracavitary implants are in at least two different patients, and seven interstitial implants to complete a residency training programme. With a declining incidence of cervical cancer in HICs, coupled with less advanced stage disease, resident training may suffer due to the lack of a sufficient caseload to carry out procedures. Global education collaborations are therefore needed to preserve the skill set for brachytherapy and to develop a curriculum for brachytherapy. Collaborative brachytherapy teaching courses by various national and international professional bodies should be developed, together with dedicated fellowships to ensure the necessary skill sets for brachytherapy [70,71]. In-country training programmes or elective postings within high incidence regions may be developed to train residents in low incidence regions. Innovative training solutions, like cadaveric brachytherapy programmes, may help to close the skills gap [72].
Palliative Care in Cervical Cancer
Despite the routine use of radiation for palliation of cervical cancer, the appropriate dose schedule and fractionation is poorly defined. Various fractionation regimens, such as 20–25 Gy/five fractions, 30 Gy/10 fractions, 40 Gy/16 fractions and 10–30 Gy in 1–3 monthly fractions, have been used. However, no concrete data are available about the comparative efficacy of these regimens [73]. Trials comparing palliative fractionation schedules would help to define the optimum strategy for palliation [74]. A summary of ongoing initiatives to overcome barriers for the integration of radiotherapy into palliative care has recently been published [75]. A formal initiative is needed to appropriately define the role of palliative radiotherapy for locally advanced and metastatic cervical cancer. There are also great disparities in global morphine availability and use. The per capita consumption of morphine ranges from 0.30–0.67 mg in Africa and Asia to 24.2–55.5 mg in Europe and Latin America, reflecting disparities in access to pain control [76]. Global collaboration and advocacy will be needed to improve access to palliative care and palliative radiation for cervical cancer patients.
Collaboration in Research
Over the last two decades there has been no change in the standard of care for locally advanced cervical cancer. Although progress has been made in radiation treatment delivery, with newer techniques such as intensity-modulated radiotherapy and image-guided brachytherapy, there is a lack of level I evidence to support its routine clinical use outside clinical trials. As opposed to common cancers in HICs, such as breast cancer, with 3151 active trials currently open, cervical cancer has only 319 registered ongoing therapeutic trials across the world (Figure 2) [77–80]. Although much of the international funding is directed towards human papilloma vaccination and screening research, dedicated funding and new trials will be needed to improve therapeutics in women with cervical cancer. There are several challenges in developing research protocols in LMICs [81], however; the development of international collaborative groups can help to overcome some of these challenges. One such example is the Cervical Cancer Research Network (CCRN), a global networking effort by the Gynaecologic Cancer Intergroup. The CCRN is a multinational league of clinicians and researchers aimed at improving research and treatment in LMICs and HICs [82]. The CCRN has five active low-cost trials, some of which are aimed at improving survival and others at reducing morbidity. These alliances are complementary, as they help not only emergent nations but also HICs. International research initiatives, like the EMBRACE studies, involving more than 25 worldwide institutions, are examples of collaborative clinical trials aimed at improving the delivery of treatment for cervical cancer [27].
Fig 2.
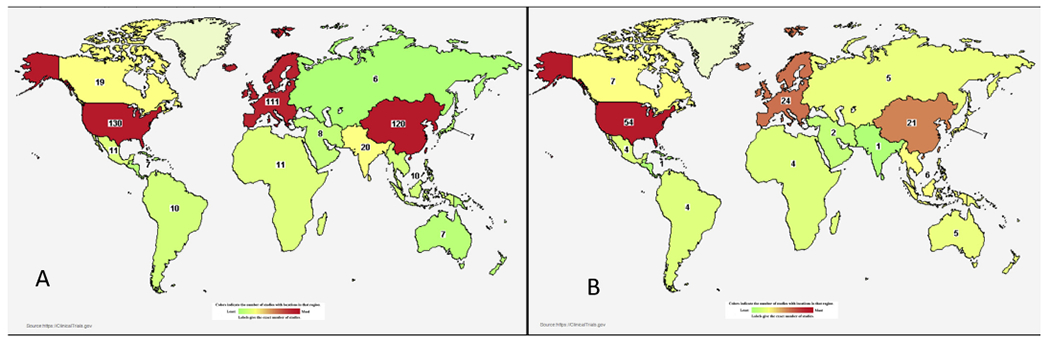
(A) Worldwide distribution of cervical cancer trials. (B) Worldwide map of industry-supported cervical cancer studies. Source: Clinicaltrials.gov (accessed 8 April 2019).
Disparities are also observed in industry funding for cervical cancer therapeutic research. Whereas common cancers in HICs have close to 60% of studies supported by the pharmaceutical industry, less than a quarter of studies in cervical cancer therapeutics have pharmaceutical funding, making new drug development particularly challenging [77–80]. Despite the availability of The Cancer Genome Atlas Report [83], there are limited studies testing molecular therapeutics. Bio RAIDS [84] and BIOEMBRACE [85] represent collaborative academic initiatives to further molecular research in cervical cancer. Although it may be ideal to test new therapeutics, including immune therapy for cervical cancer, the affordability of these therapeutics remains a practical challenge. Innovative studies [86] supported by public–private funding are presently testing repositioned drugs targeting molecular pathways to improve cervical cancer outcomes.
A summary of the aforementioned challenges and opportunities for improving access to treatment, training and research through East–West collaborations is summarised in Table 2. Moving forward, collaborations will need to involve multiple stakeholders at the national and international level. Furthermore, regional and East–West collaborations in the identified area will be necessary to promote access to treatment, training, research and optimal quality treatment for all with cervical cancer.
Table 2.
Existing challenges for the effective implementation of treatment for cervical cancer and potential solutions
| Challenges | Opportunities for collaboration | |
|---|---|---|
| Deficient external radiation resources | • No access to external radiation for treatment of locally advanced cervical cancer. • Deficient external radiation resources in reference to cervical cancer incidence. • Shortfall of radiation oncologists, medical physicists, therapists |
• Increase awareness of role of radiation therapy in the treatment of cancer at the global level. • Prepare national/regional need statements (infrastructure and staff) for common cancers, including cervical cancer, for submission to national ministry. Include source replacement and maintenance costs. • Professional radiotherapy national or regional organisations to have sessions on ‘access to cervical cancer treatment and focus on health implementation’. • Public–private co-operation or inter-government donations of equipment. • Negotiate subsidised pricing of equipment at the global level. • Potential research on resource-sparing strategies for radiation treatment. |
| Access to optimal chemotherapy | • Drug stock outs. • Shortfall of trained staff for drug administration. • Delayed approvals for making drugs available to patients under subsidy schemes. • Poor compliance to chemotherapy. • Coexistence of HIV infection. |
• Global advocacy for provision of essential medicines for cancer across countries. • Project needs for chemotherapy staff as part of resource planning for cervical cancer. • FastTrack subsidised medicines as part of a cervical cancer treatment package. • Global collaborations for staff sharing and training. • Test available digital technology to improve chemotherapy compliance and toxicity reporting in HICs and LMICs/LICs. • Collaborate to report outcomes for use and outcomes of chemotherapy within a framework of resource-stratified guidelines. • Improving patient compliance to highly active antiretroviral therapy and concurrent chemotherapy. |
| Access to brachytherapy | • Lack of adequate brachytherapy units in the LMICs. • Falling trend of brachytherapy use in developed countries. • Adequate training. • Lack of CT/MRI scanners in LMICs/LICs. |
• Develop a global taskforce for brachytherapy mapping. • Develop financial and costing models in different case scenarios, including cost of applicators, source replacements and additional staff. • Develop ‘interinstitutional brachytherapy facility sharing models’ for testing feasibility and financial sustainability. • Global curriculum in brachytherapy for gynaecological cancers, including proficiency evaluation indices. • Global ‘reverse’ fellowships for brachytherapy and training courses. • Test low-cost image-based brachytherapy (ultrasound and CT). • Advocacy to improve access to imaging scanners in LICs and LMICs. • Systematic transition from two- to three-dimensional brachytherapy. |
| Access to palliative care | • Lack of studies in optimal palliative radiation. • Access to opioids. |
• Prospective trials for testing palliative radiation regimens. • Global advocacy for improving opioid access in LMICs/LICs. |
| Barriers to research | Low number of cervical cancer studies. • Lack of collaborative groups within LMICs. • Lack of molecular driven therapeutics research. • Dearth of industry funding of cervical cancer research. |
Expand existing collaborative groups to include centres in LICs and LMICs. • Develop cervical cancer treatment and research networks within LICs and LMICs. • Global health programmes can be used for health implementation or services research. • Educate industry on global call for elimination of cervical cancer and need for philanthropic funding of research. • Develop East–West collaborations for fostering molecular research. • Drug repositioning studies. |
CT, computed tomography; HIC, high income country; LIC, low income country; LMIC, low–middle income country; MRI, magnetic resonance imaging.
Conclusion
There are a number of pressing global challenges in the delivery of optimal care for cervical cancer, including a deficit in access to external beam radiotherapy, brachytherapy, chemotherapy and palliative care. LMICs are particularly affected. There is a need for structured information for national health advisory groups to aid in rationalised cost investment planning. Global collaborations need to be established to develop the key areas for treatment, training, education and research and thereby improve therapeutic outcomes for all cervical cancer patients over the next decade.
Conflicts of interest
S. Chopra receives funding through Varian. A. Viswanathan receives funding through Springer and the NCI Task Force.
References
- [1].Globocan. Global cancer observatory 2018. Available at: http://gco.iarc.fr/today/home; 2018. Accessed 12 April 2019.
- [2].SDG 3: Good Health and Well Being. UN India. Available at: http://in.one.un.org/page/sustainable-development-goals/sdg-3-2/. Accessed 13 June 2019.
- [3].Maternal mortality. Available at: https://www.who.int/newsroom/fact-sheets/detail/maternal-mortality. Accessed 6 April 2019.
- [4].WHO. WHO Director-General calls for all countries to take action to help end the suffering caused by cervical cancer. Available at: http://www.who.int/reproductivehealth/call-to-action-elimination-cervical-cancer/en/. [Accessed 8 April 2019].
- [5].Simms KT, Steinberg J, Caruana M, Smith MA, Lew JB, Soerjomataram I, et al. Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020–99: a modelling study. Lancet Oncol 2019;20(3):394–407. [DOI] [PubMed] [Google Scholar]
- [6].Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, Bosch FX, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health 2016;4(7):e453–e463. [DOI] [PubMed] [Google Scholar]
- [7].Chopra SJ, Mathew A, Maheshwari A, Bhatla N, Singh S, Rai B, et al. National Cancer Grid of India consensus guidelines on the management of cervical cancer. J Glob Oncol 2018;4:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vale C, Tierney JF, Stewart LA, Brady M, Dinshaw K, Jakobsen A. Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration: Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol 2008;26(35):5802–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Atun R, Jaffray DA, Barton MB, Bray F, Baumann M, Vikram B, et al. Expanding global access to radiotherapy. Lancet Oncol 2015;16(10):1153–1186. [DOI] [PubMed] [Google Scholar]
- [10].Sullivan R, Alatise OI, Anderson BO, Audisio R, Autier P, Aggarwal A, et al. Global cancer surgery: delivering safe, affordable, and timely cancer surgery. Lancet Oncol 2015;16(11):1193–1224. [DOI] [PubMed] [Google Scholar]
- [11].Jit M, Brisson M, Portnoy A, Hutubessy R. Cost-effectiveness of female human papillomavirus vaccination in 179 countries: a PRIME modeling study. Lancet Glob Health 2014;2(7):e406–e414. [DOI] [PubMed] [Google Scholar]
- [12].Romero Y, Trapani D, Johnson S, Tittenbrun Z, Given L, Hohman K, et al. National cancer control plans: a global analysis. Lancet Oncol 2018;19(10):e546–e555. 10.1016/S1470-2045(18)30681-8. Epub 2018 Sep 26. [DOI] [PubMed] [Google Scholar]
- [13].Chuang LT, Temin S, Camacho R, Dueñas-Gonzalez A, Feldman S, Gultekin M, et al. Management and care of women with invasive cervical cancer: American Society of Clinical Oncology resource-stratified clinical practice guideline. J Glob Oncol 2016;2(5):311–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gupta S, Maheshwari A, Parab P, Mahantshetty U, Hawaldar R, Sastri S, et al. Neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with stage IB2, IIA, or IIB squamous cervical cancer: a randomized controlled trial. J Clin Oncol 2018;36(16):1548–1555. [DOI] [PubMed] [Google Scholar]
- [15].Yang W, Williams JH, Hogan PF, Bruinooge SS, Rodriguez GI, Kosty MP, et al. Projected supply of and demand for oncologists and radiation oncologists through 2025: an aging, better-insured population will result in shortage. J Oncol Pract 2014;10(1):39–45. [DOI] [PubMed] [Google Scholar]
- [16].Goldie SJ, O’Shea M, Campos NG, Diaz M, Sweet S, Kim SY. Health and economic outcomes of HPV 16, 18 vaccination in 72 GAVI-eligible countries. Vaccine 2008;26(32):4080–4093. [DOI] [PubMed] [Google Scholar]
- [17].Oberlin AM, Rahangdale L, Chinula L, Fuseini NM, Chibwesha CJ. Making HPV vaccination available to girls everywhere. Int J Gynecol Obstet 2018;143(3):267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Radiation Hope. https://www.radiatinghope.org/. Accessed 13 June 2019.
- [19].Cancer Care Initiative - Health. Tata trusts. Available at: https://www.tatatrusts.org/section/inside/Cancer-Care. [Accessed 8 April 2019].
- [20].Panacea: Engineering Medicine. https://www.panaceamedical.com/ssl/. Accessed 13 June 2019.
- [21].Program of Action for Cancer Therapy. https://www.iaea.org/services/key-programmes/programme-of-action-for-cancer-therapy-pact. Accessed 13 June 2019.
- [22].Minimizing Disparity in Cervical Cancer Cure Through Improved Access to Care. https://www.worldcancercongress.org/sites/congress/files/atoms/files/T3-61.pdf. Accessed 13 June 2019.
- [23].Siegel R, Burock S, Wernecke KD, Kretzschmar A, Dietel M, Loy V, et al. Preoperative short-course radiotherapy versus combined radiochemotherapy in locally advanced rectal cancer: a multi-centre prospectively randomised study of the Berlin Cancer Society. BMC Cancer 2009;9(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Resource sparing curative treatment for rectal cancer. https://clinicaltrials.gov/ct2/show/NCT01459328. Accessed 13 June 2019.
- [25].Hoffman KE, Voong KR, Levy LB, Allen PK, Choi S, Schlembach PJ, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol 2018;36(29):2943–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pramesh CS, Chaturvedi H, Reddy VA, Saikia T, Ghoshal S, Pandit M, et al. Choosing Wisely India: ten low-value or harmful practices that should be avoided in cancer care. Lancet Oncol 2019:e218–e223. 10.1016/S1470-2045(19)30092-0. [DOI] [PubMed] [Google Scholar]
- [27].Pötter R, Tanderup K, Kirisits C, de Leeuw A, Kirchheiner K, Nout R, et al. The EMBRACE II study: the outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin Transl Radiat Oncol 2018;9:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shrivastava S, Mahantshetty U, Engineer R, Chopra S, Hawaldar R, Hande V, et al. Cisplatin chemoradiotherapy vs. radiotherapy in FIGO stage IIIB squamous cell carcinoma of the uterine cervix: a randomized clinical trial. JAMA Oncol 2018;4(4):506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nandakumar A, Kishor Rath G, Chandra Kataki A, Poonamalle Bapsy P, Gupta PC, Gangadharan P, et al. Concurrent chemoradiation for cancer of the cervix: results of a multi-institutional study from the setting of a developing country (India). J Glob Oncol 2015;1(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gulia S, Sengar M, Badwe R, Gupta S. National Cancer Control Programme in India: proposal for organization of chemotherapy and systemic therapy services. J Glob Oncol 2016;3(3):271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fundytus A, Sullivan R, Vanderpuye V, Seruga B, Lopes G, Hammad N, et al.Delivery of global cancer care: an international study of medical oncology workload. J Glob Oncol 2017;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Martei YM, Chiyapo S, Grover S, Ramogola-Masire D, Dryden-Peterson S, Shulman LN, et al. Availability of WHO essential medicines for cancer treatment in Botswana. J Glob Oncol 2018;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].MPJAY. Available at: https://www.jeevandayee.gov.in/MJPJAY/FrontServlet?requestType=CommonRH&actionVal=RightFrame&page=undefined%3E%3E%3Cb%3EMJPJAY%3C/b%3E&pageName=MJPJAY&mainMenu=about&subMenu=MJPJAY. [Accessed 8 April 2019].
- [34].Harjani RR, Janaki MG, Somashekhar M, Ponni A, Alva RC, Koushik K, et al. Feasibility of concurrent chemoradiation in cervical cancer patients from rural background. Clin Ovarian Other Gynecol Cancer 2014;7(1-2):29–32. [Google Scholar]
- [35].Mittal P, Chopra S, Pant S, Mahantshetty U, Engineer R, Ghosh J, et al. Standard chemoradiation and conventional brachytherapy for locally advanced cervical cancer: is it still applicable in the era of magnetic resonance-based brachytherapy? J Glob Oncol 2018;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chopra S, Gupta M, Mathew A, Mahantshetty U, Engineer R, Lavanya G, et al. Locally advanced cervical cancer: a study of 5-year outcomes. Indian J Cancer 2018;55(1):45. [DOI] [PubMed] [Google Scholar]
- [37].Suneja G, Lin CC, Simard EP,Han X,Engels EA, Jemal A. Disparities in cancer treatment among patients infected with the human immunodeficiency virus. Cancer 2016;122(15):2399–2407. [DOI] [PubMed] [Google Scholar]
- [38].Einstein MH, Ndlovu N, Lee J, Stier EA, Kotzen J, Garg M, et al. Cisplatin and radiation therapy in HIV-positive women with locally advanced cervical cancer in sub-Saharan Africa: a phase II study of the AIDS malignancy consortium. Gynecol Oncol 2019;153(1):20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Grover S, Bvochora-Nsingo M, Yeager A, Chiyapo S, Bhatia R, MacDuffie E, et al. Impact of human immunodeficiency virus infection on survival and acute toxicities from chemoradiation therapy for cervical cancer patients in a limited-resource setting. Int J Radiat Oncol Biol Phys 2018;101(1):201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dryden-Peterson S, Bvochora-Nsingo M, Suneja G, Efstathiou JA, Grover S, Chiyapo S, et al. HIV infection and survival among women with cervical cancer. J Clin Oncol 2016;34(31):3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shrivastava SK, Engineer R, Rajadhyaksha S, Dinshaw KA. HIV infection and invasive cervical cancers, treatment with radiation therapy: toxicity and outcome. Radiother Oncol 2005;74(1):31–35. [DOI] [PubMed] [Google Scholar]
- [42].Pfaendler KS, Chang J, Ziogas A, Bristow RE, Penner KR. Disparities in adherence to National Comprehensive Cancer Network Treatment Guidelines and survival for stage IB–IIA cervical cancer in California. Obstet Gynecol 2018;131(5):899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Barrington DA, Dilley SE, Landers EE, Thomas ED, Boone JD, Straughn JM Jr, et al. Distance from a comprehensive cancer center: a proxy for poor cervical cancer outcomes? Gynecol Oncol 2016;143(3):617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].NCI Center for Global Health (CGH). National cancer institute. Available at: https://www.cancer.gov/about-nci/organization/cgh. [Accessed 8 April 2019].
- [45].Oar A, Yap ML, Rodin D, McNiven A, Papadakos J, Giuliani M. Postgraduate global health competency profile for radiation oncology. Clin Oncol 2018;30(12):810–816. [DOI] [PubMed] [Google Scholar]
- [46].Bhatt S, Isaac R, Finkel M, Evans J, Grant L, Paul B, et al. Mobile technology and cancer screening: lessons from rural India. J Glob Health 2018;8(2):020421. 10.7189/jogh.08.020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Han K, Viswanathan AN. Brachytherapy in gynecologic cancers: why is it underused? Curr Oncol Rep 2016;18(4):26. [DOI] [PubMed] [Google Scholar]
- [48].Han K, Milosevic M, Fyles A, Pintilie M, Viswanathan AN. Trends in the utilization of brachytherapy in cervical cancer in the United States. Int J Radiat Oncol Biol Phys 2013;87:111–119. [DOI] [PubMed] [Google Scholar]
- [49].Lievens Y, Gospodarowicz M, Grover S, Jaffray D, Rodin D, Torode J, et al. Global impact of radiotherapy in oncology: saving one million lives by 2035. Radiother Oncol 2017;125(2):175–177. [DOI] [PubMed] [Google Scholar]
- [50].Strohmaier S, Zwierzchowski G. Comparison of (60)Co and (192)Ir sources in HDR brachytherapy. J Contemp Brachyther 2011;3(4):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mahantshetty U, Krishnatry R, Hande V, Jamema S, Ghadi Y, Engineer R, et al. Magnetic resonance image guided adaptive brachytherapy in locally advanced cervical cancer: an experience from a tertiary cancer center in a low and middle income countries setting. Int J Radiat Oncol Biol Phys 2017;99(3):608–617. [DOI] [PubMed] [Google Scholar]
- [52].Mahantshetty U, Naga CP, Khadanga CR, Gudi S, Chopra S, Gurram L, et al. A prospective comparison of computed tomography with transrectal ultrasonography assistance and magnetic resonance imaging-based target-volume definition during image guided adaptive brachytherapy for cervical cancers. Int J Radiat Oncol Biol Phys 2018;102(5):1448–1456. [DOI] [PubMed] [Google Scholar]
- [53].Kang HC, Shin KH, Park SY, Kim JY. 3D CT-based high dose rate brachytherapy for cervical cancer: clinical impact on rectal bleeding and local control. Radiother Oncol 2010;97:507–513. [DOI] [PubMed] [Google Scholar]
- [54].Tharavichitkul E, Chakrabandhu S, Wanwilairat S, Tippanya D, Nobnop W, Pukanhaphan N, et al. Intermediate-term results of image-guided brachytherapy and high-technology external beam radiotherapy in cervical cancer: Chiang Mai University experience. Gynecol Oncol 2013;130(1):81–85. [DOI] [PubMed] [Google Scholar]
- [55].Murakami N, Kasamatsu T, Wakita A, Nakamura S, Okamoto H, Inaba K, et al. CT based three dimensional dose-volume evaluations for high-dose rate intracavitary brachytherapy for cervical cancer. BMC Cancer 2014;14(1):447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Narayan K, Van Dyk S, Bernshaw D, Khaw P, Mileshkin L, Kondalsamy-Chennakesavan S. Ultrasound guided conformal brachytherapy of cervix cancer: survival, patterns of failure, and late complications. J Gynecol Oncol 2014;25(3):206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ohno T, Noda SE, Okonogi N, Murata K, Shibuya K, Kiyohara H, et al. In-room computed tomography-based brachytherapy for uterine cervical cancer: results of a 5-year retrospective study. J Radiat Res 2016;58(4):543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tharavichitkul E, Chakrabandhu S, Klunklin P, Onchan W, Jia-Mahasap B, Wanwilairat S, et al. Intermediate-term results of trans-abdominal ultrasound (TAUS)-guided brachytherapy in cervical cancer. Gynecol Oncol 2018;148(3):468–473. [DOI] [PubMed] [Google Scholar]
- [59].Single application brachytherapy in cervical cancer. https://clinicaltrials.gov/ct2/show/NCT03110497. Accessed 13 June 2019.
- [60].CRP on Radiobiological and Clinical Studies on Viral-Induced Cancer’s Response to Radiotherapy https://clinicaltrials.gov/ct2/show/NCT00122772. Accessed 13 June 2019.
- [61].Lavigne AW, Friedman SA, Randall TC, Trimble EL, Viswanathan AN. Cervical cancer in low and middle-income countries: addressing barriers to radiotherapy delivery. Gynecol Oncol Rep 2017;22:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].NCD Risk Factor Collaboration (NCD-RISC). Trends in the adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016;387(10026):1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Boyle JM, Craciunescu O, Steffey B, Cai J, Chino J. Body mass index, dose to organs at risk during vaginal brachytherapy, and the role of three-dimensional CT-based treatment planning. Brachytherapy 2014;13(4):332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].The transition from 2D brachytherapy to 3 D high dose brachytherapy. https://www.iaea.org/publications/10705/the-transition-from-2-d-brachytherapy-to-3-d-high-dose-rate-brachytherapy. Accessed 13 June 2019.
- [65].Bauer-Nilsen K, Hill C, Trifiletti DM, Libby B, Lash DH, Lain M, et al. Evaluation of delivery costs for external beam radiation therapy and brachytherapy for locally advanced cervical cancer using time-driven activity-based costing. Int J Radiat Oncol Biol Phys 2018;100(1):88–94. [DOI] [PubMed] [Google Scholar]
- [66].Compton JJ, Gaspar LE, Shrieve DC, Wilson LD, Griem KL, Amdur RJ, et al. Resident-reported brachytherapy experience in ACGME-accredited radiation oncology training programs. Brachytherapy 2013;12(6):622–627. [DOI] [PubMed] [Google Scholar]
- [67].Marcrom SR, Kahn JM, Colbert LE, Freese CM, Doke KN, Yang JC, et al. Brachytherapy training survey of radiation oncology residents. Int J Radiat Oncol Biol Phys 2019;103(3):557–560. [DOI] [PubMed] [Google Scholar]
- [68].Fumagalli I, Faivre JC, Thureau S, Bibault JE, Diaz O, Leroy T, et al. Brachytherapy training: a survey of French radiation oncology residents. Cancer Radiother 2014;18(1):28–34. [DOI] [PubMed] [Google Scholar]
- [69].Gaudet M, Jaswal J, Keyes M. Current state of brachytherapy teaching in Canada: a national survey of radiation oncologists, residents, and fellows. Brachytherapy 2015;14(2):197–201. [DOI] [PubMed] [Google Scholar]
- [70].3rd ESTRO - AROI GYN teaching course. Available at: http://estroaroi2019.in/estro-course-overview/. Accessed 13 June 2019.
- [71].Image guided radiotherapy and chemotherapy in gynaecological cancers: Focus on MRI based adaptive brachytherapy. https://www.estro.org/Courses/IMAGE-GUIDED-RADIOTHERAPY-AND-CHEMOTHERAPY-IN-GYNA. Accessed 13 June 2019.
- [72].Alva RC, Sreenivasa KK, Gururajachar JM, Raju AP, Kumar MS, Bilimagga RS, et al. The nuances of brachytherapy taught by teachers from beyond: questionnaire-based assessment of the first cadaveric hands-on brachytherapy workshop in India. Brachytherapy 2016;15(5):593–597. [DOI] [PubMed] [Google Scholar]
- [73].van Lonkhuijzen L, Thomas G. Palliative radiotherapy for cervical carcinoma, a systematic review. Radiother Oncol 2011; 98(3):287–291. [DOI] [PubMed] [Google Scholar]
- [74].Mishra SK, Laskar S, Muckaden MA, Mohindra P, Shrivastava SK, Dinshaw KA. Monthly palliative pelvic radiotherapy in advanced carcinoma of uterine cervix. J Cancer Res Ther 2005;1(4):208. [DOI] [PubMed] [Google Scholar]
- [75].Elmore SN, Grover S, Bourque JM, Chopra S, Nyakabau AM, Ntizimira C, et al. Global palliative radiotherapy: a framework to improve access in resource-constrained settings. Ann Palliat Med 2019. Feb 23. 10.21037/apm.2019.02.02. pii: apm.2019.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Manjiani D, Paul DB, Kunnumpurath S, Kaye AD, Vadivelu N. Availability and utilization of opioids for pain management: global issues. Ochsner J 2014;14(2):208–215. [PMC free article] [PubMed] [Google Scholar]
- [77].Search of: breast cancer, NIH, U.S. Fed, industry. ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/results?cond=Breast+Cancer&term=&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&fund=0&fund=1&fund=2&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 13 June 2019.
- [78].Search of: cervix cancer, NIH, U.S. Fed, industry. ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/results?cond=Cervix+Cancer&term=&cntry=&state=&cit=&dist=&Search=Search&fund=0&fund=1&fund=2. Accessed 13 June 2019.
- [79].Search of: lung cancer, NIH, U.S. Fed, industry. ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/results?cond=lung+cancer&term=&cntry=&state&city=&dist=&Search=Search&fund=0&fund=1&fund=2. Accessed 13 June 2019.
- [80].Ramaswami R, Paulino E, Barrichello A, Nogueira-Rodrigues A, Bukowski A, St. Louis J, et al. Disparities in breast, lung, and cervical cancer trials worldwide. J Glob Oncol 2018;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Grover S, Xu M, Jhingran A, Mahantshetty U, Chuang L, Small W Jr, et al. Clinical trials in low and middle-income countries–successes and challenges. Gynecol Oncol Rep 2017;19:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Suneja G, Bacon M, Small W, Ryu SY, Kitchener HC, Gaffney DK. The cervix cancer research network: increasing access to cancer clinical trials in low- and middle-income countries. Front Oncol 2015;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].The Cancer Genome Atlas Research Network. Integrated genomic and molecular characterization of cervical cancer. Nature 2017;543(7645):378–384. 10.1038/nature21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ngo C, Samuels S, Bagrintseva K, Slocker A, Hupé P, Kenter G, et al. From prospective biobanking to precision medicine: BIO-RAIDs–an EU study protocol in cervical cancer. BMC Cancer 2015;15(1):842. 10.1186/s12885-015-1801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].BIOEMBRACE-I Protocol. https://www.embracestudy.dk/UserUpload/PublicDocuments/Docs/BIO-EMBRACE-1_Version_1.4_clean_14032018.pdf. Accessed 13 June 2019.
- [86].Radiosensitising effect of Nelfinavir in locally advanced cervical cancer. https://clinicaltrials.gov/ct2/results?cond=Nelfinavir&term=Supriya&cntry=&state=&city=&dist. Accessed 13 June 2019.