Abstract
Background
Non-adherence to guideline-recommended medications is associated with an increased risk of adverse cardiovascular outcomes in patients after an acute coronary syndrome (ACS). The purpose of this systematic review was to synthesize currently available evidence on medication adherence in patients after an ACS.
Methods and Results
After a search of PubMed, PsycINFO, Health and Psychosocial Instruments, and ISI Web of Science, we included 17 studies published between January 1980 and September 2013 that measured medication adherence to guideline-recommended therapy in adults after an ACS. Adherence to 4 classes of cardiac drugs was examined at selected time points after hospital discharge. Proportion of days covered (PDC) was the most common method used to assess medication adherence. Suboptimal medication adherence was observed in all included studies, with 54% to 86% of patients having good adherence. Declines in good medication adherence with increased duration of follow-up were noted in US-based studies. Good medication adherence at 1-year was generally higher in non-US (median: 72%) than in US-based studies (median: 65%). Less than one half of included studies examined the association between possible risk factors and medication non-adherence, and there were no consistent predictors of non-adherence across all cardiac medication classes examined.
Conclusions
Post hospital discharge medication adherence to evidence-based pharmacotherapy was suboptimal among patients with an ACS. Standardized definitions and rigorous methods to longitudinally assess medication adherence and factors associated with non-adherence should be used to identify at risk patients and design interventions to enhance medication adherence and optimize patients’ long-term prognosis.
Keywords: acute coronary syndrome, medication adherence, systematic review
Introduction
Cardiovascular disease remains the leading cause of morbidity and mortality in the United States.1 The acute coronary syndromes (ACS), including unstable angina, and acute myocardial infarction (AMI) with or without ST-segment elevation, are the major forms of acute coronary heart disease (CHD) and affect approximately 1.4 million adults in the U.S. annually.2
Patients surviving an ACS are at increased risk for developing a wide range of complications, including recurrent coronary events and death, highlighting the importance of secondary prevention efforts.3 The American College of Cardiology/American Heart Association guidelines currently recommend that all patients recovering from an ACS, unless a relevant contraindication exists, be initiated on angiotensin-converting-enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARBs), beta-blockers, statins, and antiplatelet therapy for long-term treatment after an acute coronary event.4
Numerous large-scale randomized clinical trials have demonstrated the efficacy of these guideline recommended treatments in reducing the risk of recurrent ischemic events and mortality in patients after an ACS.5–8 Current evidence, however, has shown less than optimal patient adherence to physician-recommended evidence-based therapies;9 non-adherence is associated with an increased risk of cardiovascular mortality, cardiovascular hospitalizations, coronary revascularization procedures, and increased costs.10,11
Adherence is defined as the “active, voluntary, and collaborative involvement of the patient in a mutually acceptable course of behavior to produce a therapeutic result.”12A variety of methods have been used to assess adherence to medications and the reasons for poor medication adherence are often multifactorial.13 A better understanding of the barriers to more optimal adherence, and changes in adherence over time, to effective cardiac medications in patients discharged from the hospital after an ACS would help to identify patients at increased risk for poor adherence and in designing targeted intervention strategies for both patients and their health care providers. Although medication adherence is an important concern in managing patients with acute CHD on a long-term basis, medication adherence is infrequently assessed in routine clinical practice. Indeed, medication adherence has been called the “next frontier in quality improvement” in cardiovascular outcomes research.14
The purpose of this systematic review was to synthesize currently available evidence on medication adherence in patients after an ACS. Our primary objective was to examine adherence to evidence-based cardiac medications at different follow-up points in patients discharged from the hospital after an ACS. Our secondary objective was to examine factors associated with non-adherence to evidence-based medications after hospital discharge in these patients.
Methods
Search strategy
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.15 Searches to identify relevant articles were performed in PubMed, PsycINFO, Health and Psychosocial Instruments, and ISI Web of Science from January 1, 1980 to September 30, 2013. Keywords and Medical Subject Heading (MeSH) terms used in these searches included “acute coronary syndrome”, “myocardial infarction”, “heart attack”, ” unstable angina”, “STEMI”, “patient compliance”, “adherence”, “compliance”, “compliant”, “comply”, “complying”, “ complies”, “concordance”, “nonadherence”, “noncompliance”, “ noncompliant”, “noncomply”, “noncomplying”, “nonconcordance”, “medication”, “pharmacotherapy”, “therapy”, “treatment”, “drug”, “medicine”, “secondary prevention”, “beta-blocker”, “angiotensin-converting enzyme inhibitor”, “ACE inhibitor”, “angiotensin receptor blocker”, “ARB”, “statin”, “Lipid-lowering agent”, “ aspirin”, and “antiplatelet”. The bibliographies of eligible articles were searched for additional references.
Inclusion and Exclusion Criteria
Publications included in this review had to : (1) be published between January 1, 1980 and September 30, 2013; (2) have human subjects aged ≥18 years old; (3) have subjects hospitalized for an ACS; (4) have subjects prescribed at least one evidence-based medication after hospital discharge: beta-blockers, lipid-lowering agents, antiplatelet agents, and ACEIs/ARBs; (5) include a measure of medication adherence and specify its method of measurement; (6) be published in English; and (7) be published in a peer-reviewed journal.
Publications were excluded for further review if they: (1) did not specify the type of medication examined; (2) only reported inpatient or hospital discharge medication use; (3) did not have a specific follow-up time point for calculating medication adherence; (4) did not calculate medication adherence based on patients with at least one filled prescription for the drug of interest during follow-up; (5) were study summaries without original results; or (6) were review articles, opinion pieces, letters, commentaries, case reports, or case series.
Data Collection
An initial review of the titles and abstracts of all articles was performed to exclude any studies that did not meet our pre-defined inclusion criteria. Full review of all remaining studies was undertaken to determine eligibility for inclusion. One researcher (H-Y. C.) independently abstracted data from all included studies using a standardized form. Information was abstracted for study type, study country and setting, number of participants, patient’s socio-demographic characteristics (i.e., age, sex, race/ethnicity, insurance type), study condition (ACS, AMI, unstable angina), data source, drugs or therapeutic classes studied, medication adherence measure(s), reported medication adherence and cut-point used for assessing good adherence, study inclusion period, length of follow up and time point(s) of adherence assessment, and factors examined in relation to medication non-adherence.
Definition of Medication Adherence
Following the definition proposed by The International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Medication Compliance and Persistence Work Group,16 the inclusion criterion for “medication adherence” was defined as “the extent to which a patient acts in accordance with the prescribed interval and dose of a dosing regimen.”16 Studies that examined primary medication non-adherence (i.e., a patient does not fill a prescribed medication at some point during treatment) or medication persistence, defined as “the duration of time from initiation to discontinuation of therapy,”16 were not included in this review (Figure 1).
Figure 1:
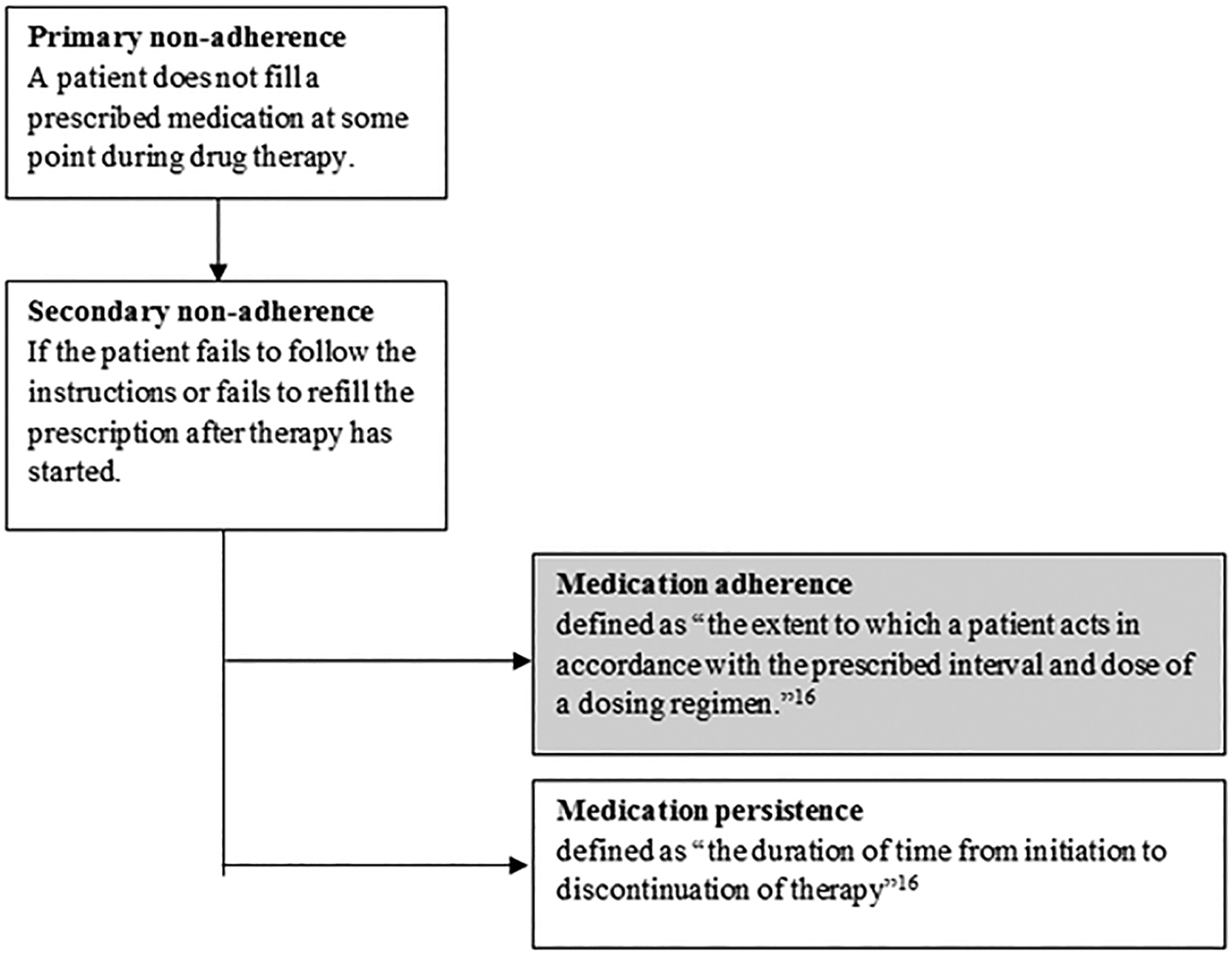
Definition of medication adherence
Measuring Good Medication Adherence
A variety of methods have been used to assess “good” medication adherence.13 Commonly used methods13 in claims-based research include the medication possession ratio (MPR; defined as “number of days of medication supplied within the refill interval/number of days in refill interval”) and the proportion of days covered (PDC; defined as “total days all drug(s) available/days in follow-up period”). Although medication adherence measures varied across the studies reviewed, they were mainly categorized at the patient level. Patients were considered as having “good” adherence to a selected medication, or class of medication, if a specified threshold (e.g., 75% or 80%) was attained. For example, a good medication adherence of 74% measured by the PDC method with an 80% cut-off means that 74% of patients achieved good medication adherence as they were covered by the prescribed medication at least 80% of days during the period of assessment.
Risk Factors
We categorized potential risk factors for medication non-adherence, following categorization by the World Health Organization,17 into 5 broad groups, including patient (e.g., demographics), socioeconomic (e.g., income), health system (e.g., reimbursement type), therapy (e.g., prior medication use, coronary procedures), and condition (e.g., comorbidity) related factors.17
Quality Assessment
The quality of each study was assessed using Downs and Black criteria,18 which evaluates study design, validity, reporting, and other study attributes in clinical trials. We modified the Downs and Black scale on the basis of prior systematic reviews19,20 to accommodate the characteristics of non-randomized observational studies. The original checklist includes 27 items with a maximum score of 32 points. Items not relevant to the objectives of this review, including criteria pertaining to randomization technique, were removed.19 In addition, we dichotomized the item assessing study statistical power into adequate or inadequate sample size. Our final modified checklist consisted of 17 items with a maximum score of 18 points awarded. For each study, a quality score was calculated by dividing the total number of points received by the total number of points for which the study was eligible to receive; this score was reported in percentages (possible range: 0 – 100%).
Results
Study Selection
A total of 1,083 articles were identified from our literature search; after 394 duplicates were removed, and 605 articles were excluded on the basis of title and abstract review, 84 articles were retrieved for more detailed assessment. Of these, 17 met our inclusion criteria (Figure 2). The most common reasons for excluding publications after full review were that they did not measure “medication adherence” as defined by ISPOR16 (n=33) or they did not provide detailed information about adherence to evidence-based cardiac medications (n=15). No additional articles were identified from the references of included articles.
Figure 2:
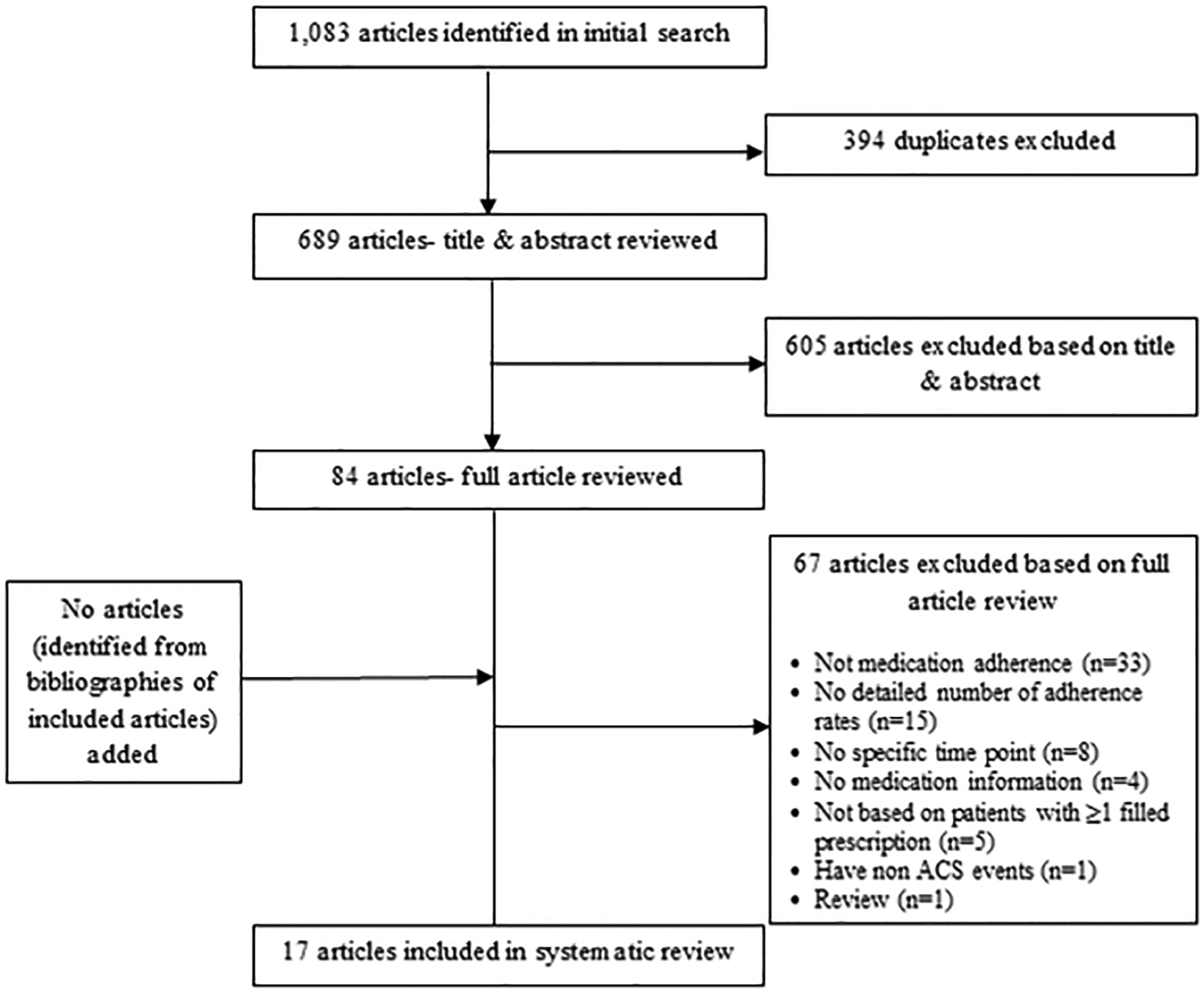
Flow chart documenting the process used to identify included articles
Study Characteristics
The characteristics of included articles are detailed in Table 1. All 17 studies included were published after 2000. The majority of included articles were retrospective cohort studies (n=14),21–25,27–29,31–34,36,37 and all but one study33 were population-based cohorts (n=13) in which patients eligible for enrollment were either residents of a particular geographic area or were from the same health care system (e.g., Medicare, Managed care organization) or insurance plan. The remaining 3 studies26,30,35 reported on samples of patients with an ACS recruited from hospital-based settings.
Table 1.
Characteristics of the 17 studies included in the systematic review of medication adherence after an acute coronary syndrome (ACS)
| Author (Year) | Type of study | Country & Setting | Sample Size | Demographics (Age/years, Sex, Race) | Health Insurance Coverage | Medication Data Source /Method | Inclusion Period | Length of Follow-up | Study Condition | Study Medication |
|---|---|---|---|---|---|---|---|---|---|---|
| Nordstrom et al. (2013)21 | Retrospective cohort | US, Population-based (covers > 20 million patients) | 1,340 | Mean age: 56.7 Men: 79.5% | Commercial or Medicare | Claims data /MPR* | Jul, 2009 – Jun, 2010 | 1 year | ACS-PCI | Prasugrel |
| Sanfelix-Gimeno et al. (2013)22 | Retrospective cohort | Spain, Population-based (Valencia community) | 7,462 | Mean age: 68.8 (Range: ≥35) Men: 70% | Universal health coverage | Claims data /PDC* | Jan, 2008 – Dec, 2008 | 9 months | ACS | ACEI/ARB, beta-blocker, statin, aspirin & clopidogrel |
| Zhang et al. (2012)23 | Retrospective cohort | US, Population-based (Medicare FFS) | 91,272 | Men: 44.7% White: 80.9% | Medicare FFS | Claims data /MPR | 2008 | Until Dec, 2009 or death | AMI | ACEI/ARB, beta-blocker, statin |
| Danchin et al. (2011)24 | Retrospective cohort | France, Population-based (covers 70% national population) | 4,939 | Mean age: 49.5 (Range <60) Men: 85% | Universal health coverage | Claims data /Prescriptions delivered | Jan – Jun 2006 | 30 months | AMI | Statin |
| Lai et al. (2011)25 | Retrospective cohort | Canada, Population-based (British Columbia) | 9,926 | Age: ≥65 Men: 59.6% | Universal health coverage | Claims data /PDC | Apr, 1994 – Jan, 2002 | 1 year | AMI | ACEI, beta-blocker, statin |
| Allen LaPointe et al. (2011)26 | Prospective cohort | US, Hospital-based (41 hospitals, CRUSADE registry) | 973 | Men: 69% | Various types | Telephone survey /Self-report | Jan, 2006 – Sep, 2007 | 3 months | NSTE ACS | ACEI/ARB, beta-blocker, lipid-lowering agent |
| Maio et al. (2011)27 | Retrospective cohort | Italy, Population-based (Regione Emilia-Romagna) | 24,367 | Age: ≥35 Men: 64% | Universal health coverage | Claims data /PDC | Jan, 2004 – Dec, 2007 | 12 months | AMI (first time) | Beta-blocker |
| Belleudi et al. (2011)28 | Retrospective cohort | Italy, Population-based (Rome) | 3,920 | Mean age: 64.6 (Range: 35–80) Men: 73.2% | Universal health coverage | Claims data /PDC | Jan, 2006 – Jun, 2007 | 1 year | AMI | ACEI/ARB, beta-blocker, statin, antiplatelet |
| Zhu et al. (2011)29 | Retrospective cohort | US, Population-based (employer-based claims database) | 10,465 | Mean age: 54.9 (Range: 18–65) Men: 77.5% | Private insurance | Claims data /MPR | Jan, 2005 – Dec, 2006 | 1 year | ACS-PCI | Clopidogrel |
| Kronish et al. (2010)30 | Prospective cohort | US, Hospital-based (3 academic hospitals in CT & NY) | 105 | Mean age: 59 (Range: 25–83) Men: 53% White 87% | N/A | MEMS* /Electronic Medication Monitor | May, 2003 – Apr, 2005 | 3 months | ACS | Aspirin |
| Tuppin et al. (2010)31 | Retrospective cohort | France, Population-based (covers 70% national population) | 11,604 | Universal health coverage | Claims data /MPR | Jan – Jun, 2006 | 30 months | AMI | ACEI/ARB, beta-blocker, statin, aspirin/clopidogrel | |
| Winkelmayer et al. (2008)32 | Retrospective cohort | US, Population-based (Medicare population, NJ & PA) | 21,484 | Mean age: 79.7 (Range: ≥65) Men: 72.6% White: 92.0% | Medicare | Claims data /PDC | 1994 – 2004 | 1 year | AMI | ACEI/ARB, beta-blocker, statin |
| Chan et al. (2008)33 | Retrospective cohort | US, Managed Care Org at Mid-Atlantic States | 387 | Mean age: 53.2 (Range: 18–64) Man: 76.2% | Private insurance | Claims data /MPR | Jan, 2002 – Dec, 2002 | 1 year | AMI | ACEI, ARB, beta-blocker, statin |
| Rasmussen et al. (2007)34 | Retrospective cohort | Canada, Population-based (Province of Ontario) | 31,455 | Age: ≥66 | Universal health coverage | Claims data /PDC | April, 1999 – May, 2003 | 1 year | AMI | Beta-blocker, statin |
| Rieckmann et al. (2006)35 | Prospective cohort (cross-lagged panel design) | US, Hospital-based (3 academic hospitals in CT & NY) | 172 | Mean age: 59.3 Men: 55.2% White: 86.6% | N/A | MEMS /Electronic Medication Monitor | May, 2003 – Apr, 2005 | 3 months | ACS | Aspirin |
| Wei et al. (2004)36 | Retrospective cohort | UK, Population-based (Tayside region, Scotland) | 865 | Mean age: 66.3 (Range: 30–93) Men: 59.4% | Universal health coverage | Claims data /PDC | Jan, 1994 – Dec, 1995 | Until Dec 1999. (Min: 4 years) | AMI (first time) | Beta-blocker |
| Simpson et al. (2003)37 | Retrospective cohort | Canada, Population-based (Province of Quebec) | 14,057 | Age: ≥65 | Universal health coverage | Claims data /PDC | Jan, 1996, – Dec, 1998 | 1 year | AMI (first time) | ACEI, beta-blocker, lipid-lowering agent, aspirin |
MPR = medication possession ratio; PDC = proportion of days covered; MEMS = medication event monitoring system
About half of the articles included were US-based studies (n=8),21,23,26,29,30,32,33,35 and the non-US studies (n=9) were from Canada25,34,37 and several European countries (i.e., UK,36 Italy,27,28 Spain,22 France24,31) with universal health coverage. Overall, 14 studies21–25,27–29,31–34,36,37 used claims-based data to estimate medication adherence. Study sample sizes ranged from 105 to 91,272 participants; 5 studies26,30,33,35,36 had <1,000 participants, 5 studies21,22,24,25,28 had 1,000 to 9,999 participants, and 7 studies23,27,29,31,32,34,37 had ≥10,000 participants. Length of patient follow-up ranged from 3 months to 5 years. Approximately three quarters of the studies included had follow-up ≥1 year (n=13);21,23–25,27–29,31–34,36,37 1 study22 had a follow-up of 9 months , and 3 studies26,30,35 had follow-up for 3 months after hospital discharge for an ACS. The time points of medication adherence reported in these investigations ranged from 3 to 30 months. All studies reported medication adherence only at one follow-up time point, with the exception of 2 studies23,27 which reported medication adherence at 2 follow-up time points (i.e., 6 months and 1 year).
Most included studies examined post-discharge medication adherence among patients hospitalized for an AMI (n=11).23–25,27,28,31–34,36,37 Ten studies21,22,24,28–30,32,33,35,36 provided data on the average age of their study sample, which ranged from 50 to 80 years, and the distribution of sex (proportion of men ranged from 45% to 85%) was reported in 14 studies.21–30,32,33,35,36 All of the non-US studies failed to report data on race/ethnicity, while 4 US studies23,30,32,35 reported that most of their participants were white. Overall, 7 studies21,24,27,29,30,35,36 examined the use of a single cardiac medication, while the other 10 studies22,23,25,26,28,31–34,37 examined adherence to multiple cardiac drugs.
Study Quality
Based on modified Downs and Black criteria, quality ratings of the 17 included studies in this review ranged from 72% to 94%, with an average score of 84%.
Medication Adherence Measures
The measures of medication adherence varied considerably across published studies. The most common methods used to calculate adherence were proportion of days covered (PDC) (n=9),22,24,25,27,28,32,34,36,37 followed by medication possession ratio (MPR) (n=5),21,23,29,31,33 electronic medication monitors (n=2),30,35 and self-reported data (n=1).26 The majority of these studies defined “good” adherence to prescribed medication using a percentage cut-off at 80% (n=13).21,24,25,27–29,30,31,32,34,35,36,37 The MPR and PDC methods were used in studies measuring adherence at time points ranging from 6 to 30 months, while self-reported measures and electronic medication monitors were used only in studies measuring adherence at 3 months after hospital discharge for an ACS (Table 2).
Table 2.
Methods for assessing medication adherence in the 17 studies included in this systematic review
| Method | Adherence cut-point | Post-discharge follow-up point | Study Reference Number |
|---|---|---|---|
| Medication Possession Ratio (MPR) | ≥75% | 6 months, 1 year | 23 |
| ≥ 80% | 1 year, 30 months | 21, 29, 31 | |
| n/a | 1 year | 33 | |
| Proportion of days covered (PDC) | ≥ 75% | 9 months | 22 |
| ≥80% | 6 months, 1 year, 30 months | 24, 25, 27, 28, 32, 34, 36, 37 | |
| Electronic Medication Monitors | ≥ 80% | 3 months | 30, 35 |
| Self-reported measure | only patients reporting that they never missed a dose were considered adherent | 3 months | 26 |
Medication Adherence
Among the 17 studies included for review, post-discharge medication adherence was calculated based on patients who survived an ACS and who had at least one filled prescription of guideline recommended medications during the period of follow-up. Overall, only 3 studies21,23,27 stated in their methods that patients with medication contraindications were excluded from their analyses. Two studies27,33 provided mean MPR or mean PDC, but did not report specific numbers of good medication adherence. Of the remaining 15 studies, the majority (n=9) examined medication adherence at a 1-year follow-up point. Within each medication class, there were at least 8 studies reporting the proportions of patients who achieved good medication adherence.
Table 3 presents the ranges of good adherence to several guideline-recommended medications from 15 studies; these findings were further subdivided and explored according to study country (US vs. non-US), follow-up time point (3, 6, 9, 12, and 30 months), and type of medication class (ACEIs/ARBs, Antiplatelet agents, Beta-blockers, and Lipid lowering agents).
Table 3.
Good adherence* to guideline recommended medications according to study setting and period of follow-up
| Time point/countries | ACEI/ARB | Antiplatelet | Beta blocker | Statin |
|---|---|---|---|---|
| 3 months† | ||||
| US | 74%26 | 76%−77%30,35 | 77%26 | 78%26 |
| Canada & European countries‡ | - | - | - | - |
| 6 months§ | ||||
| US | 68%23 | - | 70%23 | 70%23 |
| Canada & European countries‡ | - | - | - | - |
| 9 months§ | ||||
| US | - | - | - | - |
| Canada & European countries‡ | 59%22 | 75%22 | 58%22 | 68%22 |
| 1 year§ | ||||
| US | 54%−62%23,32 | 67%−69%21,29 | 64%−65%23,32 | 57%−65%23,32 |
| Canada & European countries‡ | 69%−74%25,28,37 | 71%−86%28,37 | 57%−74%25,28,34,36,37 | 66%−81%25,28,34,37 |
| 30 months§ | ||||
| US | - | - | - | - |
| Canada & European countries‡ | 77%31 | 82%31 | 68%31 | 64%−77%24,31 |
Proportion of patients who achieved “good adherence”, according to a predetermined study-specific cut-off (Table 2), to medication
All these countries have universal health coverage
Study country and duration of follow-up
Among the US studies, good medication adherence declined as the duration of follow-up to prescribed medications increased (from 3 months to 1 year), regardless of medication class. We did not observe a similar pattern among the non-US studies. However, comparing the 4 US studies21,23,29,32 to the 5 non-US studies25,28,34,36,37 at the time of the 1-year follow-up, good medication adherence was generally higher in non-US than in US-based studies.
Medication class and duration of follow-up
In comparing good medication adherence between the guideline-recommended therapies at different follow-up points, no particular medication consistently demonstrated a higher adherence during the period of follow-up at different time points. Antiplatelet agents, however, demonstrated a higher adherence than other classes of medication at 1 year (67%−69%)21,29 among the US studies, and at 9 months (75%),22 1 year (71%−86%),28,37 and 30 months (82%)31 among the non-US studies (Table 3).
Risk factors for medication non-adherence
Of the 17 studies included, only 821,22,23,25,26,29,31,34 examined possible factors associated with medication non-adherence using multivariable adjusted analyses. Among these, one study26 examined factors for non-adherence at 3 months, one study22 at 9 months, one study31 at 30 months, and 5 studies21,23,25,29,34 at the time of a 1 year follow-up visit. We focused on the latter 5 studies.21,23,25,29,34
In general, the association between various demographic and clinical factors and medication non-adherence varied between medication classes at 1 year post hospital discharge (Table 4). There were no consistent predictors of non-adherence across all cardiac medication classes examined. For example, diabetes was significantly associated with non-adherence to antiplatelet agents (OR=1.3),29 but not for beta-blockers,34 and statin therapy.34 Two studies23,25 specifically examined potential racial disparities in medication adherence and found that non-white race23 or Asian race25 was associated with an increased odds of non-adherence for ACEI/ARBs,23,25 beta-blockers,23 and statin therapy23 after adjusting for other covariates.
Table 4.
Association between examined factors and medication non-adherence at 1 year follow-up*
| Factors | ACEI/ARB | Antiplatelet | Beta-blocker | Statin |
|---|---|---|---|---|
| Patient | Young age (<55) ↑29 or NS21 | Increasing age ↑34 | Increasing age ↑34 | |
| Sex: male (NS)21 | Sex: male ↑34 | Sex: male (NS)34 | ||
| Non-white (vs. White) ↑23 | Non-white (vs. White) ↑23 | Non-white (vs. White) ↑23 | ||
| Asian (vs. Non-Asian) ↑25 | US geographic region: West ↓21 | |||
| Socioeconomic | Low income (NS)34 | Low income (NS)34 | ||
| Health System | Reimbursement type (NS)21 | |||
| Specialty of attending physician (NS)34 | Specialty of attending physician (NS)34 | |||
| Therapy | Prior use of clopidogrel ↑29 | Prior use of beta-blocker ↓34 | Prior use of statin ↓34 | |
| Prior use of beta-blockers, statins, ACE inhibitors ↓29 | ||||
| Medication received during baseline: Statin ↓21 | Concomitant statin ↓34 | Concomitant beta-blocker ↓34 | ||
| Medication received during baseline: anticoagulant ↑21 | Concomitant ACE inhibitors ↓34 | Concomitant ACE inhibitors ↓34 | ||
| Prior PCI procedure ↑21 | Post-MI revascularization ↑34 | Post-MI revascularization ↓34 | ||
| PCI without stenting during hospitalization ↑29 | ||||
| Condition | Diabetes ↑29 | Diabetes (NS)34 | Diabetes (NS)34 | |
| Atrial Fibrillation (NS)29 | Cardiac dysrhythmia ↑34 | Cardiac dysrhythmia (NS)34 | ||
| History of bleeding ↑21 | ||||
| Depression ↑21 or NS29 | Post-MI: psychiatric illnesses ↑34 | Post-MI: psychiatric illnesses ↑34 | ||
| COPD ↑29 | Post-MI: Respiratory illness ↑34 | Post-MI Respiratory illness (NS)34 | ||
| Post MI: Cancer ↑34 | Post MI: Cancer (NS)34 | |||
| Any prior hospitalization ↑29 | Increasing numbers of recurrent admissions post discharge↑34 | Increasing numbers of recurrent admissions post discharge↑34 | ||
| Renal disease (NS)29 | Acute renal failure (NS)34 | Acute renal failure (NS)34 | ||
| Chronic renal failure (NS)34 | Chronic renal failure (NS)34 | |||
| Hypertension (NS)29 | Other conditions† (NS)34 | Other conditions† (NS)34 | ||
| Charlson/Deyo Comorbidity Index (2+ vs. ≤1) (NS)21 | ||||
| UA/NSTEMI (vs. STEMI/other) (NS)29 |
↑: significantly increased; ↓ significantly decreased; NS: not significant
Other conditions include: shock, pulmonary edema, congestive heart failure, cerebrovascular disease, cancer, post-MI: ischemic heart disease, post MI: endocrine illness, post MI: apoplexia (MI=myocardial infraction)
Discussion
In this review, we found that the proportion of patients exhibiting “good” medication adherence to evidence-based pharmacotherapies ranged from 54% to 86%, depending on study and drug class, in patients after an ACS. Among the US studies with varying patient samples, good adherence to all effective cardiac medications appeared to decline as the length of follow-up after hospital discharge increased; medication adherence at 1-year follow-up was generally higher in countries where universal health coverage existed. Factors associated with non-adherence were examined in few studies, and the results varied across the medication classes assessed.
Our findings of consistent suboptimal adherence to evidence-based medications in hospital survivors of an ACS are similar to the results of a prior systematic review38 showing that approximately one third of patients with a diagnosis of CHD do not adhere to effective cardiovascular preventive treatment after a median follow-up of 24 months. This prior review, however, was restricted to studies that measured adherence by “prescription refills” only. The current review expands our understanding of long-term medication adherence in patients after an ACS by examining adherence at several specific follow-up points in studies that measured adherence using various methods. In addition, we found that in countries with universal health care, which typically provides comprehensive medication coverage and involves a low drug cost-sharing requirement, there was higher medication adherence than in the US.
Although we did not limit our search based on study design, only prospective and retrospective cohort studies met our pre-defined inclusion and exclusion criteria. During the full review process, we identified 7 studies using a randomized control trial design, but all of them were excluded based on our pre-defined exclusion criteria. While the included studies measured adherence at time points ranging from 3 to 30 months after discharge from the hospital for an ACS, most of these investigations examined medication adherence at 1-year post-hospital discharge.
In this review, the majority of the included studies used claims-based data and calculated PDC or MPR to measure medication adherence. Administrative claims databases provide a source of objective data on the occurrence of pharmacy refills of drugs; the relative efficiency of using these data for studies of adherence in large populations in a ‘real-word’ setting is highly advantageous if the data are complete. However, there are limitations to these databases, particularly the inability to determine if patients actually consumed the dispensed medication. Thus, our results based on studies using claims data may be overestimated and should be interpreted with caution. In contrast, in recent years, many pharmacy chains have introduced “$4 generic drug” programs that may have improved access to medications for low-income patients. However, without an incentive, many pharmacies do not submit claims to insurers when patients pay cash. As a result, medication adherence may be underestimated if some insured patients who have filled prescriptions with $4 medications are misclassified as nonusers of these treatments.39
In this review, we did not find a consistent factor or constellation of factors associated with non-adherence to the cardiac medications we examined. Since fewer than half of the included studies examined potential barriers to medication non-adherence, and several studies examined a limited number of possible predictors for poor adherence, our risk factor findings should be interpreted with appropriate caution. Diagnoses from claims data may not accurately reflect patients’ medical conditions, and information about patient’s socioeconomic status was limited in these databases. In addition, other important factors, such as patient’s belief in the effectiveness of medication, number of medications used, and complexity of drug regimen were not examined in the studies reviewed. Thus, studies examining medication adherence among patients discharged from the hospital for an ACS may not have been able to comprehensively evaluate important factors associated with drug non-adherence.
Clinical Implications
Despite evidence supporting the long-term effectiveness of guideline-recommended pharmacotherapy for patients discharged from the hospital after an ACS, our findings suggest that medication adherence was far from optimal, even as early as 3 months post-hospital discharge. Since the Affordable Care Act is tackling the problem of rising healthcare costs by penalizing hospitals for excess readmissions for a variety of conditions,40 including heart attack, medication management is at the core of advanced discharge planning and transitional care. Clinicians should routinely assess medication adherence in patients after an ACS during their regularly scheduled follow-up appointments and efforts directed at improving adherence should be a recognized component of patient management.41 Since current clinical practice typically initiates the first follow-up appointment in ACS patients within one or several weeks post-hospital discharge, this in-person patient/provider contact represents an important opportunity to identify medication non-adherence, and patient-specific solutions to non-adherence can be developed jointly by healthcare providers and their patients. Moreover, patients with several risk factors to non-adherence may benefit from additional support from the healthcare community (e.g., repeat telephone follow-up) in maintaining their use of evidence-based pharmacotherapy as prescribed over the full recommended duration of treatment.
Research implications
In 2008, ISPOR published their definitions for medication adherence and medication persistence.16 The definitions are geared toward future standardization in medical research to allow for more systematic comparisons across published reports. Health outcomes researchers are encouraged to adopt these working definitions which would help to facilitate health policy decisions based on consistent published evidence.
During the process of identifying literature for the current review, we found that definitions of medication adherence and medication persistence varied across published studies and were used interchangeably in some studies. We restricted our review to studies examining medication adherence as defined by ISPOR.16 Since the clinical outcomes of treatment are affected not only by how well but by how long patients take their medications, future studies reviewing the current state of medication persistence and primary non-adherence among patients after an ACS remain necessary. Since medication adherence is a complex issue, future studies, including multiple measures of primary and secondary non-adherence to prescribed treatment regimens, appear warranted to fully capture various aspects of adherence, because each of these behaviors may necessitate a different intervention. For example, as current programs of transitions of care aim to reduce 30-day hospital readmissions and mortality in patients discharged from the hospital after an ACS, it is important to monitor medication adherence during the early post-discharge period due to the high risk of recurrent cardiovascular events. However, when using claims-based data to measure medication adherence at 1-month post-hospital discharge, good medication adherence possibly will be considerably high when the prescription is for a 30-day supply. Thus, it may be more important to monitor primary adherence (i.e., patients actually fill the prescription) or use another approach to measure medication adherence (i.e., patients really take the pills as instructed) within the first month after hospital discharge.
Although a number of different methods exist for measuring medication adherence, none of these are considered to be the “gold standard”. Self-reported adherence derived from patient questionnaires and patient self-reports are simple and inexpensive. However, this method is susceptible to errors attributed to social desirability bias. Thus, a growing number of studies have used PDC or MPR methods with claims-based databases to assess medication adherence since these approaches are objective, quantifiable, and potentially generalizable for conducting population-based research, particularly in countries where universal health coverage exists. These administrative databases, however, do not capture the different reasons why certain medications were not refilled, and several likely predictors of medication adherence, including health-related behaviors, socioeconomic status, health literacy, and other barriers reflecting access to care were typically not available. Most importantly, a filled prescription does not necessarily mean that the patient took the drug at the correct frequency or in the expected manner. Future studies combining claims databases and survey questionnaires would improve our current understanding of the barriers to medication adherence.
Even though we observed a decline in medication adherence with increased duration of follow-up in the US studies, these results were primarily derived from potentially different patient samples (e.g., population-based vs. hospital-based). Also, there were a limited number of studies that examined the first few months of post-discharge adherence to effective cardiac therapies, and none presented data during the particularly high risk period for readmission within the first 30 days after hospital discharge. Longitudinal studies assessing medication adherence at serial follow-up points among ACS survivors would be valuable to understand changes in medication non-adherence during the early and subsequent high risk post-discharge periods and risk factors related to short and more extended periods of non-adherence to different treatment approaches and lifestyle interventions.
In the US, it was estimated that the avoidable cost opportunity from medication non-adherence is $105 billion annually.42 The implementation of the Affordable Care Act is gradually closing the “doughnut hole” by offering additional medication discounts for beneficiaries, which has been shown to be associated with increased adherence among patients after an AMI.43 Future research is warranted to evaluate the impact of ongoing health care reforms to better understand the association between adherence and healthcare costs and ways to enhance medication adherence.
Limitations
A number of limitations in this systematic review should be acknowledged. This review was limited to studies published in English. The extent to which our inability to review studies published in languages other than English affected our findings is unknown. Because we allowed heterogeneity of the adherence measurement methods to be included in this review, a quantitative meta-analysis was not appropriate. Our current review was limited in assessing short-term medication adherence. Furthermore, our finding that medication adherence declined as length of follow-up increased among the US-based studies should be interpreted with caution since they were based on a limited number of published studies.
Conclusions
Adherence to guideline-recommended pharmacotherapy was suboptimal in patients discharged from the hospital after an ACS. Factors associated with non-adherence were examined in a limited number of studies, and the associations varied between studies. Future studies using standardized definitions and methods are warranted to consistently measure treatment adherence and related factors to further clarity the association between potential barriers and medication non-adherence. These studies can hopefully lead to the development of innovative, patient-centered, intervention strategies which can improve the long-term medication adherence and long-term cardiovascular outcomes among patients discharged from the hospital after an ACS.
Funding Sources
No funding was secured for conducting this study.
Footnotes
Disclosures
None.
References
- 1.Mensah GA, Brown DW. An overview of cardiovascular disease burden in the United States. Health Aff. 2007; 26: 38–48. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health. What is acute coronary syndrome (ACS)? NIH MedlinePlus. 2009;4:27. Available at: http://www.nlm.nih.gov/medlineplus/magazine/issues/winter09/articles/winter09pg25-27.html Accessed Feb 19, 2014. [Google Scholar]
- 3.Rockson SG, deGoma EM, Fonarow GC. Reinforcing a continuum of care: in-hospital initiation of long-term secondary prevention following acute coronary syndromes. Cardiovasc Drugs Ther. 2007; 21:375–388. [DOI] [PubMed] [Google Scholar]
- 4.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC Jr, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation. 2004; 110: e82–e292. [PubMed] [Google Scholar]
- 5.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002; 324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox KA, Mehta SR, Peters R, Zhao F, Lakkis N, Gersh BJ, Yusuf S. Clopidogrel in unstable angina to prevent recurrent ischemic events trial. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non–ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation. 2004; 110:1202–1208. [DOI] [PubMed] [Google Scholar]
- 7.Stenestrand U, Wallentin L. Early statin treatment following acute myocardial infarction and 1-year survival. JAMA. 2001; 285:430–436. [DOI] [PubMed] [Google Scholar]
- 8.Hognestad A, Dickstein K, Myhre E, Snapinn S, Kjekshus J. Effect of combined statin and beta-blocker treatment on one-year morbidity and mortality after acute myocardial infarction associated with heart failure. Am J Cardiol. 2004; 93:603–606. [DOI] [PubMed] [Google Scholar]
- 9.Newby LK, LaPointe NMA, Chen AY, Kramer JM, Hammill BG, DeLong ER, Muhlbaier LH, Califf RM. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation. 2006; 113:203–212. [DOI] [PubMed] [Google Scholar]
- 10.Ho PM, Magid DJ, Shetterly SM, Olson KL, Maddox TM, Peterson PN, Masoudi FA, Rumsfeld JS. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008; 155:772–779. [DOI] [PubMed] [Google Scholar]
- 11.Sokol MC, Mcguigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalize statin risk and healthcare cost. Med Care. 2005; 43:521–530. [DOI] [PubMed] [Google Scholar]
- 12.Meichenbaum D, Turk DC. Facilitating Treatment Adherence: A Practitioner’s Guidebook. New York, NY: Plenum Press; 1987. [Google Scholar]
- 13.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence, its importance in cardiovascular outcomes. Circulation. 2009; 119:3028–3035. [DOI] [PubMed] [Google Scholar]
- 14.Heidenreich PA. Patient adherence: the next frontier in quality improvement. Am J Med. 2004; 117:130–132 [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009; 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: terminology and definitions. Value Health. 2008; 11:44–47. [DOI] [PubMed] [Google Scholar]
- 17.Sabaté E Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 18.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and nonrandomised studies of health care interventions. J Epidemiol Community Health. 1998; 52:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajduk AM, Kiefe CI, Person SD, Gore JG, Saczynski JS. Cognitive change in heart failure: a systematic review. Circ Cardiovasc Qual Outcomes. 2013; 6:451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pimentel CB, Lapane KL, Briesacher BA. Medicare Part D and Long-Term Care: A Systematic Review of Quantitative and Qualitative Evidence. Drugs & aging. 2013; 30:701–720. [DOI] [PubMed] [Google Scholar]
- 21.Nordstrom BL, Simeone JC, Zhao Z, Molife C, McCollam PL, Ye X, Effron MB. Adherence and persistence with prasugrel following acute coronary syndrome with percutaneous coronary intervention. Am J Cardiovasc Drugs. 2013; 13:263–271. [DOI] [PubMed] [Google Scholar]
- 22.Sanfelix-Gimeno G, Peiró S, Ferreros I, Pérez-Vicente R, Librero J, Catalá-López F, Ortiz F, Tortosa-Nácher V. Adherence to evidence-based therapies after acute coronary syndrome: a retrospective population-based cohort study linking hospital, outpatient, and pharmacy health information systems in Valencia, Spain. J Manag Care Pharm. 2013; 19:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Baik SH, Chang CC, Kaplan CM, Lave JR. Disability, race/ethnicity, and medication adherence among medicare myocardial infarction survivors. Am Heart J. 2012; 164:425–433.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danchin N, Neumann A, Tuppin P, De Peretti C, Weill A, Ricordeau P, Allemand H. Impact of free universal medical coverage on medical care and outcomes in low-income patients hospitalized for acute myocardial infarction: an analysis from the French national health insurance system. Circ Cardiovasc Qual Outcomes. 2011; 4:619–625. [DOI] [PubMed] [Google Scholar]
- 25.Lai EJ, Grubisic M, Palepu A, Quan H, King KM, Khan NA. Cardiac medication prescribing and adherence after acute myocardial infarction in Chinese and South Asian Canadian patients. BMC Cardiovasc Disord. 2011; 11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen LaPointe NM, Ou FS, Calvert SB, Melloni C, Stafford JA, Harding T, Peterson ED, Alexander KP. Association between patient beliefs and medication adherence following hospitalization for acute coronary syndrome. Am Heart J. 2011; 161:855–863 [DOI] [PubMed] [Google Scholar]
- 27.Maio V, Marino M, Robeson M, Gagne JJ. Beta-blocker initiation and adherence after hospitalization for acute myocardial infarction. Eur J Cardiovasc Prev Rehabil. 2011; 18:438–445. [DOI] [PubMed] [Google Scholar]
- 28.Belleudi V, Fusco D, Kirchmayer U, Agabiti N, Di Martino M, Narduzzi S, Davoli M, Arcà M, Perucci CA. Definition of patients treated with evidence based drugs in absence of prescribed daily doses: the example of acute myocardial infarction. Pharmacoepidemiol Drug Saf. 2011; 20:169–76. [DOI] [PubMed] [Google Scholar]
- 29.Zhu B, Zhao Z, McCollam P, Anderson J, Bae JP, Fu H, Zettler M, Lenarz L. Factors associated with clopidogrel use, adherence, and persistence in patients with acute coronary syndromes undergoing percutaneous coronary intervention. Curr Med Res Opin. 2011; 27:633–641. [DOI] [PubMed] [Google Scholar]
- 30.Kronish IM, Rieckmann N, Shimbo D, Burg M, Davidson KW. Aspirin adherence, aspirin dosage, and C-reactive protein in the first 3 months after acute coronary syndrome. Am J Cardiol. 2010; 106:1090–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuppin P, Neumann A, Danchin N, de Peretti C, Weill A, Ricordeau P, Allemand H. Evidence-based pharmacotherapy after myocardial infarction in France: adherence-associated factors and relationship with 30-month mortality and rehospitalization. Arch Cardiovasc Dis. 2010; 103:363–375. [DOI] [PubMed] [Google Scholar]
- 32.Winkelmayer WC, Levin R, Setoguchi S. Associations of kidney function with cardiovascular medication use after myocardial infarction. Clin J Am Soc Nephrol. 2008; 3:1415–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan V, Cooke CE. Pharmacotherapy after myocardial infarction: disease management versus usual care. Am J Managed Care. 2008; 14:352–358. [PubMed] [Google Scholar]
- 34.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007; 297: 177–186. [DOI] [PubMed] [Google Scholar]
- 35.Rieckmann N, Gerin W, Kronish IM, Burg MM, Chaplin WF, Kong G, Lespérance F, Davidson KW. Course of depressive symptoms and medication adherence after acute coronary syndromes: an electronic medication monitoring study. J Am Coll Cardiol. 2006; 48:2218–2222. [DOI] [PubMed] [Google Scholar]
- 36.Wei L, Flynn R, Murray GD, MacDonald TM. Use and adherence to beta-blockers for secondary prevention of myocardial infarction: who is not getting the treatment? Pharmacoepidemiol Drug Saf. 2004; 13:761–766. [DOI] [PubMed] [Google Scholar]
- 37.Simpson E, Beck C, Richard H, Eisenberg MJ, Pilote L. Drug prescriptions after acute myocardial infarction: dosage, compliance, and persistence. Am Heart J. 2003; 145:438–444. [DOI] [PubMed] [Google Scholar]
- 38.Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med. 2012; 125:882–887. [DOI] [PubMed] [Google Scholar]
- 39.Choudhry NK, Shrank WH. Four-dollar generics—increased accessibility, impaired quality assurance. N Engl J Med. 2010; 363:1885–1887. [DOI] [PubMed] [Google Scholar]
- 40.Axon RN, Williams MV. Hospital readmission as an accountability measure. JAMA. 2011; 305:504–505 [DOI] [PubMed] [Google Scholar]
- 41.Desai NR, Choudhry NK. Impediments to adherence to post myocardial infarction medications. Curr Cardiol Rep. 2013; 15:322. [DOI] [PubMed] [Google Scholar]
- 42.Avoidable Costs in U.S. Healthcare: The $200 Billion Opportunity from Using Medicines More Responsibly. Report by the IMS Institute for Healthcare Informatics. Available at: http://www.imshealth.com/deployedfiles/imshealth/Global/Content/Corporate/IMS%20Institute/RUOM-2013/IHII_Responsible_Use_Medicines_2013.pdf. Accessed February 8, 2014. [Google Scholar]
- 43.Stuart B, Davidoff A, Erten M, Gottlieb SS, Dai M, Shaffer T, Zuckerman IH, Simoni-Wastila L, Bryant-Comstock L, Shenolikar R. How Medicare Part D Benefit Phases Affect Adherence with Evidence-Based Medications Following Acute Myocardial Infarction. Health Serv Res. 2013; 48:1960–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


