Abstract
Objective
To determine the risk of hip fracture in persons with Alzheimer´s disease (AD) who initiated antiepileptic drugs (AEDs).
Methods
In the Medication use and AD (MEDALZ) cohort of 70,719 Finnish community dwellers with clinically verified incident AD diagnosis in 2005–2011, we identified all incident users of AEDs using national Prescription register. AEDs were classified as older (valproate, carbamazepine, clonazepam, phenytoin, levetiracetam, primidone) or newer (pregabalin, gabapentin, oxcarbazepine, lamotrigine, topiramate). We matched each user to 2 non-users. Incident hip fractures until 2015 were identified from the Care register for health care. We calculated inverse probability of treatment weighted hazard ratios (HR), with 95% confidence intervals, using Cox regression.
Results
Altogether 5522 incident users were identified and matched to 11,044 non-users (in both groups, women: 65%; median age: 81 years). Altogether 53.3% of users initiated with newer AEDs (pregabalin 79.8%, gabapentin 10.2%) while 46.7% initiated with older AEDs (valproate 67.6%, carbamazepine 13.0%). Age- and sex-adjusted IR of hip fracture per 100 person-years was 1.8 (95% CI 1.6–1.9) in non-users and 2.0 (95% CI 1.8–2.2) in users. Increased risk of hip fracture was observed in users (HR 1.17, 95% CI 1.05–1.30) compared with non-users. The risk was higher for short duration of use (<14 weeks, HR 3.64, 95% CI 2.90–4.58) than for medium duration (14 to <64 weeks, HR 1.74, 95% CI 1.48–2.05) or ≥64 weeks’ use (HR 1.23, 95% CI 1.08–1.40), compared to non-users with same follow-up time. Older AEDs had HR of 1.46 (1.03–2.08) compared with newer AEDs.
Conclusion
Our results imply that AED use is associated with an increased risk of hip fracture in people with AD. These findings prompt careful consideration before prescribing AEDs to persons with AD. Persons with AD treated with antiepileptics should be carefully monitored due to their increased risk of falling and fractures.
Keywords: antiepileptic drugs, hip fracture, Alzheimer´s disease, pharmacoepidemiology
Introduction
Antiepileptic drugs (AEDs) have been consistently associated with an increased risk of fractures, including hip fractures.1–4 In a meta-analysis of observational studies, the risk of hip fracture was almost doubled in users of AEDs compared with non-users.5 However, most studies have examined young or mid-aged adults with epilepsy, while very little evidence exists for older adults treated for indications other than epilepsy. The use of antiepileptics, especially the newer antiepileptics such as pregabalin and gabapentin, on other indications has become more common. In persons with AD, AEDs are mainly used for central neuropathic pain,6 and neuropsychiatric symptoms of cognitive disorders (eg, agitation and aggression),7 although, particularly in the case of neuropathic pain, this goes against recent recommendations due to an increased risk of falls.8 Moreover, in a recent systematic review of randomized controlled trials, valproate showed no benefit in treating dementia-related agitation and a high rate of adverse effects, including sedation was observed among valproate users.9 Still, the risk-benefit profile of most AEDs in persons with AD is still largely unclear10–13 and their adverse effects, including effects on cognition are of concern, given that cognitive status is already impaired due to AD itself.
A screening study for safety signals in a large Finnish cohort of persons with AD,14 identified an increased risk of hip fracture associated with pregabalin and valproate. In this cohort,15 use of AEDs after AD diagnosis increased from about 4% to about 8% within 5 years and the most common AEDs were new agents, such as pregabalin and gabapentin. Therefore, it is very important to confirm and further investigate the relation of AEDs with hip fracture in persons with AD, who are a well-known high-risk group for hip fracture.16 Hip fractures are indeed a major health problem in older adults,17 and strongly affect their health and well-being, substantially increasing morbidity,18 short-term mortality,19–22 and reducing autonomy and quality of life.23,24 As a result, health-care costs are also substantially increased.25–28
In this study, we compare the risk of hip fracture between AED initiators and non-initiators with AD in a nationwide Finnish cohort of persons with AD.
Methods
Study Population and Data Sources
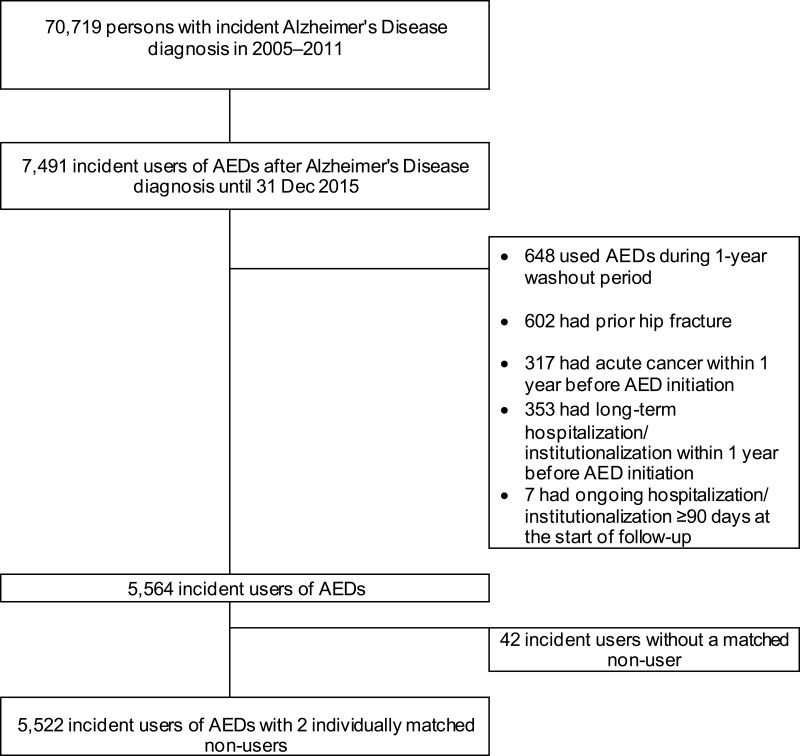
The study population was obtained from the Medication and Alzheimer’s disease (MEDALZ) cohort, which has been described in detail elsewhere.29 Briefly, the MEDALZ cohort includes community-dwelling residents of Finland who received a clinically verified diagnosis of AD from 2005 to 2011 (N=70,719) (Figure 1). Persons with diagnosis of AD were identified from the Special Reimbursement register (SRR). This register and the other sources of data are described in Table e-1. To be registered in the SRR with a diagnosis of AD, a person has to fulfil the following clinical criteria: he/she (1) had symptoms consistent with AD, (2) experienced a decrease in social capacity over a period of at least 3 months, (3) underwent a computed tomography/magnetic resonance imaging scan, (4) had possible alternative diagnoses excluded, and (5) had a diagnosis of AD made by a registered geriatrician or neurologist. The diagnosis of AD is based on the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association30 and Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria for AD.31
Figure 1.
Flow chart describing the study population.
For each member of the cohort, information on drug use, diagnoses, hospitalizations and institutionalizations were extracted from nationwide registers (Table e-1 and Figure e-1). Data on dispensed drugs were extracted from the Prescription register (years 1995–2015), on hospitalizations (including discharge diagnoses coded according to the 10th revision of the WHO International Classification of Diseases and Related Health Problems (ICD-10) from the Hospital Discharge register (1972–2015); on selected chronic diseases diagnoses from the SRR (1972–2015). Moreover, data on institutionalization (including end and start date) were obtained from the Social Insurance Institution of Finland (1972–2015), data on deaths (2005–2015) and socioeconomic status (1972–2015) from Statistics Finland.
In the MEDALZ cohort, we identified all persons who initiated AEDs after AD diagnosis (incident users) and matched each incident user to 2 non-users of AEDs.
Incident Users of AEDs
Incident users were defined as persons who had a first dispensation of an AED after AD diagnosis without having filled one within 1 year before. We restricted the study to incident users to avoid bias related to the depletion of susceptible prevalent users and under-ascertainment of earlier events.32
For incident users, the date of the first dispensation of an AED after AD diagnosis was defined as the index date. Duration of use was calculated starting from the index date until censoring (ie, until discontinuation of AED use, switch to or addition of an AED of the other group, death, start of continuous hospitalization/institutionalization lasting ≥90 days, end of the study (December 31, 2015), and is thus independent of timing of hip fracture.
AED dispensations were identified in the Prescription register through the ATC code N03A. Individual drugs were classified as older AEDs (valproate N03AG01, carbamazepine N03AF01, clonazepam N03AE01, phenytoin N03AB02, levetiracetam N03AX14, primidone N03AA03) and newer AEDs (pregabalin N03AX16, gabapentin N03AX12, oxcarbazepine N03AF02, lamotrigine N03AX09, and topiramate N03AX11).
We excluded incident users who experienced hip fracture any time between 1972 and index date, those who had an acute cancer at index date, and those who were hospitalized or institutionalized for >182 days during 1 year before or who were currently hospitalized for ≥90 days at entry date. We excluded persons with long-term hospitalization or institutionalization because drug exposure cannot be accurately measured during in-hospital stays. Definitions used for exclusion criteria are displayed in Table e-1.
Using the PREscriptions to Drug Use Periods PRE2DUP method,33 dispensation purchase data of AEDs have been transformed to drug use periods. This method constructs exposure periods and estimates the dose used during the period by considering the purchased amount in defined daily doses, recorded in the prescription register. This method models personal purchase pattern for each ATC code and considers stays in hospital and long-term care facilities (during which drug use is not recorded in the prescription register), possible stockpiling of drugs, and package information. Drug use based on PREDUP has been compared with self-reported drug use,34 and findings showed a very high agreement for central nervous system drugs.
As PREDUP modelling is based on the individual ATC code, duration of any AED use was obtained combining overlapping drug use periods of AEDs. Thus, during any AED use, a person may change AED.
Matched Non-Users
For each incident user, two non-users of AEDs were matched by sex, age (±1 year), and time since AD diagnosis (± 183 days) using incidence density sampling without replacement. For non-users, the matching date was defined as the index date. Non-users had to be alive and not hospitalized at the cohort entry date of the corresponding incident user. We applied to non-users the same exclusion criteria applied to incident users.
Follow-Up
Follow-up started on the index date (date of the first dispensation of an AED after AD diagnosis for incident users and the matched date for non-users).
In the analysis comparing incident users with non-users, each person was followed-up from index date to the date of incident hip fracture, death, start of continuous hospitalization/institutionalization lasting ≥90 days, end of the study (December 31, 2015), AED use discontinuation (for users), or AED initiation (for non-users), whichever occurred first. In the analysis comparing incident users of older and newer AEDs, the follow-up ended on the date of switch to or addition of an AED of the other group in addition to the other censoring criteria (date of incident hip fracture, death, start of continuous hospitalization/institutionalization lasting ≥90 days, or end of the study), whichever occurred first.
Outcome
Within the person-time of follow-up, we identified all persons who experienced an incident hip fracture, defined as (1) the first hospitalization with ICD-10 code for fracture of neck of femur (S72.0), pertrochanteric fracture (S72.1), or subtrochanteric fracture (S72.2), or (2) death with the same ICD-10 codes for causes.
Covariates
We ascertained at baseline characteristics that are risk factors for hip fracture and/or are associated with use of AEDs, including co-morbidities and use of drugs other than AEDs. The definitions and classifications used to measure these characteristics are described in Table e-2. Briefly, co-morbidities have been identified mainly through corresponding ICD codes in the Hospital Discharge register. Some co-morbidities were identified using additional data, for instance, dispensations of specific drugs (eg, osteoporosis was identified through hospitalization codes for osteoporosis – ICD-10 M80 and M81 – and/or dispensations of osteoporosis drugs – M05BA, M05BB, M05BC, and M05BX any time before cohort entry) or special reimbursement for chronic diseases (eg, rheumatoid arthritis was identified through hospitalization codes - ICD-10 M05, M06, M45 - and/or the special reimbursement code for this disease).
Use of drugs other than AEDs at baseline was ascertained within 12 months prior to cohort entry based on dispensations with specific ATC codes in the Prescription register.
Socioeconomic status was defined based on the highest occupational social class recorded for a person from 1972 up to 3 years prior to the AD diagnosis and it was classified into four groups by Statistics Finland. These groups corresponded to low (unemployed and students), medium (employees and lower clerical workers) and high (higher clerical workers, professionals and entrepreneurs) status. A fourth group included persons with unknown socioeconomic status or missing information.
Statistical Analysis
Age- and sex-adjusted incidence rates (IRs), with 95% confidence intervals (95% CIs), of hip fracture have been calculated using Poisson regression and expressed as number of incident cases per 100 person-years. IRs have been calculated separately in incident users and non-users as well as in incident users of older and newer AEDs.
Unadjusted and adjusted hazard ratios (HR), with 95% confidence intervals (95% CI), were estimated using Cox proportional hazard regression. The proportional hazards assumption was assessed using visual examination of hazard functions and graphic and goodness-of-fit testing using Schoenfeld residuals.
To balance the compared groups regarding baseline covariates, we applied Inverse Probability of Treatment Weighting (IPTW).35 We conducted two sensitivity analyses, one using propensity score for covariate adjustment and the other using stabilised IPTW.
We used conditional logistic regression to estimate the propensity score as the probability of receiving any AED vs none conditioned to baseline covariates. All baseline covariates (Table e-2) were included in the propensity score. IPTW was calculated in incident users as 1-propensity score and in non-users as 1/(1-propensity score).36 To quantitatively assess the degree to which IPTW weighting had removed systematic differences at baseline between incident users and non-users, we calculated unweighted and IPTW weighted standardized mean differences (SMDs) between incident users and non-users for each baseline covariate.37 New propensity scores (and, thus, IPTWs) were derived for the older vs newer AED comparison analyses based on the probability of receiving newer AED vs older AED conditional on baseline covariates.
To avoid immortal time bias in analyses assessing the risk of hip fracture per AED use duration, non-users with the same follow-up duration were used as reference category in the use/non-use analyses. In the duration-wise analyses of older vs newer AEDs, the comparisons were performed between users of older and newer AEDs with the same duration of use.
All analyses were performed using SAS statistical software, version 9.3 (SAS Institute©, Inc., Cary, NC).
Standard Protocol Approvals, Registrations, and Patient Consents
The MEDALZ study was approved by the register maintainers. According to Finnish legislation, ethics committee approval or informed consent were not required for this study because only pseudonymised register-based data were used, the study participants were not contacted and treatment was not affected by participation in the study. Data were pseudonymised before submission to the researchers.
Data Availability Statement
The data used to conduct the research are available from the corresponding author but restrictions by the register maintainers and Finnish legislation apply to the availability of these data. Therefore, the data are not publicly available without permissions of the register maintainers.
Results
A total of 5564 incident users of any AED were identified and 5522 (99.2%) of them were matched to 11,044 non-users; 42 incident users without matching non-user were excluded from further analysis (Figure 1). In both groups, about 65% were women and median age was 81 years (Table 1). At baseline, users had co-morbidities and used drugs more frequently than non-users. The largest differences were observed for opioids, benzodiazepines and related drugs, and epilepsy. These differences were balanced after IPTW, as indicated by SMD <10% between users and non-users. Distributions of stabilized IPTWs between users and non-users were comparable, with significant overlap (mean weight in users 1.00, range 0.73–7.08, mean weight in non-users 0.99, range 0.35–4.52).
Table 1.
Characteristics of Incident Users of AEDs and Matched Non-Users with AD and Standardized Mean Differences
| Users (N=5522) | Non-Users (N=11,044) | Standardized Mean Difference (%) | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | Unweighted | After IPTW | |
| Age (years)a | ||||||
| <64 | 431 | 7.8 | 1392 | 12.6 | 15.9 | 1.8 |
| 65–74 | 1360 | 24.6 | 3663 | 33.2 | ||
| 75–84 | 2909 | 52.7 | 5005 | 45.3 | ||
| 85+ | 822 | 14.9 | 984 | 8.9 | ||
| Median (25;75 percentile) | 81.1 (75.8; 85.5) | 81.0 (75.6; 85.2) | – | – | ||
| Sex | ||||||
| Men | 1941 | 35.2 | 3882 | 35.2 | 0.0 | 0.5 |
| Women | 3581 | 64.9 | 7162 | 64.9 | – | |
| Time since AD diagnosis (days)a | ||||||
| <315 days | 1373 | 24.9 | 2572 | 23.3 | 3.7 | 1.1 |
| 315 to <803 | 1382 | 25.0 | 2727 | 24.7 | 0.8 | 0.8 |
| 803 to <1465 | 1385 | 25.1 | 2759 | 25.0 | 0.2 | 0.4 |
| 1465 and above | 1382 | 25.0 | 2986 | 27.0 | 4.6 | 2.3 |
| Median (25;75 percentile) | 803 (315;1465) | 850 (344; 1533) | ||||
| Duration of follow-up (days) | ||||||
| Median (25;75 percentile) | 810.5 (293; 1504) | 973 (442; 1617) | ||||
| Health condition at baseline | ||||||
| Psychiatric and neurological | ||||||
| Epilepsy | 542 | 9.8 | 304 | 2.8 | 27.4 | 0.4 |
| Depression | 463 | 8.4 | 645 | 5.8 | 10.0 | 0.4 |
| Schizophrenia | 157 | 2.8 | 284 | 2.6 | 1.7 | 0.6 |
| Hip fracture-related | ||||||
| Vision disturbancesb | 2104 | 38.1 | 3181 | 28.8 | 19.2 | 0.3 |
| Osteoporosisc | 1036 | 18.8 | 1479 | 13.4 | 14.1 | 0.5 |
| Any fracture | 797 | 14.4 | 1241 | 11.2 | 9.2 | 0.1 |
| Head trauma | 439 | 8.0 | 687 | 6.2 | 6.0 | 1.0 |
| Rheumatoid arthritisd | 266 | 4.8 | 496 | 4.5 | 1.5 | 0.3 |
| Alcohol abuse | 186 | 3.4 | 332 | 3.0 | 1.4 | 1.1 |
| Other conditions | ||||||
| Cardiovascular diseasese | 3595 | 65.1 | 6179 | 56.0 | 18.2 | 0.2 |
| Diabetesd | 1296 | 23.5 | 2298 | 20.8 | 6.5 | 0.3 |
| Stroke | 847 | 15.3 | 1143 | 10.4 | 14.6 | 0.8 |
| Asthmad | 622 | 11.3 | 1055 | 10.0 | 5.6 | <0.1 |
| History of any cancer | 488 | 8.8 | 1110 | 10.1 | 4.1 | <0.1 |
| Chronic renal disease | 85 | 1.5 | 86 | 0.8 | 7.1 | 0.4 |
| Chronic liver disease | 27 | 0.5 | 82 | 0.7 | 2.9 | 0.9 |
| Drugs at baseline | ||||||
| CNS drugs | ||||||
| Benzodiazepines and related drugs | 2338 | 42.3 | 2859 | 25.9 | 33.1 | 0.5 |
| Antidepressants | 2307 | 41.8 | 3289 | 29.8 | 23.6 | 0.6 |
| Memantine | 2257 | 40.9 | 3970 | 36.0 | 6.2 | 0.3 |
| Antipsychotics | 1963 | 35.6 | 2698 | 24.4 | 21.0 | 1.1 |
| Opioids | 1265 | 22.9 | 1007 | 9.1 | 36.5 | 0.5 |
| Antiparkinsonians | 297 | 5.4 | 510 | 4.6 | 3.4 | 0.1 |
| Other drugs | ||||||
| Cardiovascular drugsf | 4535 | 82.1 | 8379 | 75.9 | 15.1 | 0.3 |
| PPIs | 1811 | 32.8 | 2299 | 20.8 | 26.7 | <0.1 |
| NSAIDs | 1224 | 22.2 | 1597 | 14.5 | 19.2 | 0.1 |
| Corticosteroids, systemic | 485 | 8.8 | 633 | 5.7 | 11.3 | 0.6 |
| Estrogens | 481 | 8.7 | 760 | 6.9 | 6.5 | 0.2 |
| Drugs for urinary incontinence | 313 | 5.7 | 551 | 5.0 | 3.7 | <0.1 |
| Socioeconomic status | ||||||
| Highest | 2798 | 50.7 | 4241 | 38.4 | 5.8 | 0.6 |
| Middle | 980 | 17.8 | 2187 | 19.8 | 0.2 | 0.1 |
| Lowest | 942 | 17.1 | 2606 | 23.6 | 3.0 | 0.2 |
| Unknown | 802 | 14.5 | 2010 | 18.2 | 3.8 | 1.1 |
Notes: aMatching variables. bIncludes hospital diagnosis of cataract, macular degeneration, and glaucoma. cComposite of discharge diagnosis (code M80 and M81) and dispensation data (bisphosphonates M05BA and M05BB, bone morphogenetic proteins M05BC, other drugs affecting bone structure and mineralization M05BX (eg, strontium ranelate, denosumab)). dComposite variables based on both hospitalization and special reimbursement data. eIt includes hypertension, heart failure, peripheral arterial disease, atrial fibrillation, coronary heart disease. fIt includes cardiac glycosides (C01AA), antiarrhythmics (C01B), organic nitrates (C01DA), diuretics (C03), beta blocking agents (C07), calcium channel blockers (C08), agents acting on the renin-angiotensin system (C09), lipid modifying agents (C10A, C10BA), antithrombotic agents (B01A).
Abbreviations: AD, Alzheimer’s disease; AED, antiepileptic drug; CNS, central nervous system; HF, hip fracture; IPTW, inverse probability of treatment; NSAID, nonsteroidal anti-inflammatory drugs; PPI, proton pump inhibitors.
Among incident users, 2945 (53.3%) used newer AEDs and 2577 (46.7%) used older AEDs (Table 2). The most commonly used newer AEDs were pregabalin (79.8%), gabapentin (10.2%), and oxcarbazepine (8.7%); the most commonly used older AEDs were valproic acid (67.6%), carbamazepine (13.0%), and clonazepam (10.2). Compared to users of newer AEDs, users of older AEDs were more frequently men, had shorter median time since diagnosis (656 vs 995 days), had more frequently epilepsy, and used more frequently memantine and antipsychotics (Table 2). Conversely, other co-morbidities and drugs were more common in users of newer AEDs, such as osteoporosis, vision disturbances, and opioids. The SMD exceeded 10% for 19 co-morbidities and drugs at baseline, with differences between 30% and 50% for opioids, antipsychotics, epilepsy, NSAIDs, and between 20% and 30% for PPIs, memantine, vision disturbances, and osteoporosis. These differences were balanced after IPTW, as indicated by SMDs <10% between users of newer and older AEDs for all except epilepsy (SMD 11.2%).
Table 2.
Characteristics of Incident Users of Newer and Older AEDs with AD and Mean Standardized Differences
| Usersa | Standardized Mean Difference (%) | |||||
|---|---|---|---|---|---|---|
| Newer AEDs (N=2945) | Older AEDs (N=2577) | |||||
| N | % | N | % | Unweighted | After IPTW | |
| Age (years) | ||||||
| <64 | 132 | 4.5 | 299 | 11.6 | 26.4 | 5.4 |
| 65–74 | 625 | 21.2 | 735 | 28.5 | 26.4 | 5.4 |
| 75–84 | 1678 | 57.0 | 1231 | 47.8 | 26.4 | 5.4 |
| 85+ | 510 | 17.3 | 312 | 12.1 | 26.4 | 5.4 |
| Median (25;75 percentile) | ||||||
| Sex | ||||||
| Men | 915 | 31.1 | 1026 | 39.8 | 18.4 | 2.5 |
| Women | 2030 | 68.9 | 1551 | 60.2 | ||
| Time since AD diagnosis (days) | ||||||
| <315 days | 840 | 28.5 | 533 | 20.7 | 18.3 | 0.3 |
| 315 to <803 | 828 | 28.1 | 554 | 21.5 | 15.4 | 0.2 |
| 803 to <1465 | 716 | 24.3 | 669 | 26.0 | 3.8 | 0.7 |
| 1465 and above | 561 | 19.1 | 821 | 31.9 | 29.7 | 1.2 |
| Median (25;75 percentile) | 656 (265; 1269) | 995 (402; 1697) | ||||
| Duration of follow-up (days) | ||||||
| Median (25;75 percentile) | 982.00 (437.00; 1660.00) | 578.00 (198.00; 1221.00) | ||||
| AED at start treatment (ATC) | ||||||
| Pregabalin (N03AX16) | 2349 | 79.8 | – | – | – | |
| Gabapentin (N03AX12) | 300 | 10.2 | – | – | – | |
| Oxcarbazepine (N03AF02) | 255 | 8.7 | – | – | – | |
| Lamotrigine (N03AX09) | 31 | 1.1 | – | – | – | |
| Topiramate (N03AX11) | 9 | 0.3 | – | – | – | |
| Valproic acid (N03AG01) | – | 1742 | 67.6 | – | – | |
| Carbamazepine (N03AF01) | – | 334 | 13.1 | – | – | |
| Clonazepam (N03AE01) | – | 263 | 10.2 | – | – | |
| Phenytoin (N03AB02) | – | 119 | 4.6 | – | – | |
| Levetiracetam (N03AX14) | – | 79 | 3.1 | – | – | |
| Primidone (N03AA03) | – | 4 | 0.2 | – | – | |
| Multiple AEDs | 1 | <0.1 | 36 | 1.4 | – | – |
| Health condition at baseline | ||||||
| Psychiatric and neurological | ||||||
| Depression | 265 | 9.0 | 199 | 7.7 | 4.6 | 0.6 |
| Epilepsy | 98 | 3.3 | 425 | 16.5 | 45.2 | 11.3 |
| Schizophrenia | 63 | 2.1 | 94 | 3.7 | 9.0 | 0.1 |
| Hip fracture-related | ||||||
| Vision disturbancesb | 1289 | 43.8 | 804 | 31.2 | 26.2 | 2.4 |
| Osteoporosisc | 683 | 23.2 | 346 | 13.4 | 25.5 | 3.6 |
| Any fracture | 466 | 15.8 | 326 | 12.7 | 9.1 | 0.3 |
| Head trauma | 209 | 7.1 | 223 | 8.7 | 5.8 | 2.1 |
| Rheumatoid arthritisd | 178 | 6.0 | 88 | 3.4 | 12.4 | 3.4 |
| Alcohol abuse | 78 | 2.7 | 104 | 4.0 | 7.7 | 0.7 |
| Other conditions | ||||||
| Cardiovascular diseasese | 1993 | 67.7 | 1591 | 61.7 | 12.4 | 1.1 |
| Diabetesd | 790 | 26.8 | 507 | 19.7 | 17.0 | 1.2 |
| Stroke | 411 | 14.0 | 431 | 16.7 | 7.7 | 1.1 |
| Asthmad | 393 | 13.3 | 229 | 8.9 | 14.2 | 1.3 |
| Any cancer | 271 | 9.2 | 217 | 8.4 | 2.8 | < 0.1 |
| Chronic renal disease | 52 | 1.8 | 33 | 1.3 | 4.0 | 1.8 |
| Chronic liver disease | 16 | 0.5 | 12 | 0.5 | 1.1 | 0.5 |
| Drugs at baseline | ||||||
| CNS drugs | ||||||
| Benzodiazepines and related drugs | 1265 | 43.0 | 1036 | 40.2 | 5.6 | 0.7 |
| Antidepressants | 1238 | 42.0 | 1040 | 40.4 | 3.4 | 3.2 |
| Memantine | 984 | 33.4 | 1202 | 46.6 | 27.3 | 0.8 |
| Opioids | 946 | 32.1 | 295 | 11.5 | 51.7 | 1.9 |
| Antipsychotics | 708 | 24.0 | 1197 | 46.5 | 48.3 | 3.2 |
| Antiparkinsonians | 178 | 6.0 | 118 | 4.6 | 6.5 | 1.7 |
| Other drugs | ||||||
| Cardiovascular drugsf | 2512 | 85.3 | 2019 | 78.4 | 18.1 | 2.8 |
| PPIs | 1157 | 39.3 | 644 | 25.0 | 31.0 | 3.6 |
| NSAIDs | 854 | 29.0 | 358 | 13.9 | 37.4 | 4.2 |
| Corticosteroids, systemic | 327 | 11.1 | 153 | 6.0 | 18.6 | 3. |
| Estrogens | 315 | 10.7 | 163 | 6.3 | 15.7 | 2.6 |
| Drugs for urinary frequency and incontinence | 215 | 7.3 | 104 | 4.0 | 14.2 | 3.3 |
| Socioeconomic status | ||||||
| Highest | 597 | 20.3 | 666 | 25.8 | 13.3 | 0.5 |
| Middle | 256 | 8.7 | 232 | 9.0 | 1.1 | 1.7 |
| Lowest | 1833 | 62.2 | 1481 | 57.5 | 9.7 | 0.1 |
| Unknown | 259 | 8.8 | 198 | 7.7 | 4.0 | 1.1 |
Notes: a27 persons had both a newer and an older AED and were classified as users of older AED (since they used at least one older AED). bIncludes hospital diagnosis of cataract, macular degeneration, and glaucoma. cComposite of discharge diagnosis (code M80 and M81) and dispensation data (bisphosphonates M05BA and M05BB, bone morphogenetic proteins M05BC, other drugs affecting bone structure and mineralization M05BX (eg, strontium ranelate, denosumab)). dComposite variables based on both hospitalization and special reimbursement data. eIt includes hypertension, heart failure, peripheral arterial disease, atrial fibrillation, coronary heart disease. fIt includes cardiac glycosides (C01AA), antiarrhythmics (C01B), organic nitrates (C01DA), diuretics (C03), beta blocking agents (C07), calcium channel blockers (C08), agents acting on the renin-angiotensin system (C09), lipid modifying agents (C10A, C10BA), antithrombotic agents (B01A).
Abbreviations: AD, Alzheimer’s disease; AED, antiepileptic drug; ATC, anatomical therapeutic chemical; CNS, central nervous system; HF, hip fracture; IPTW, inverse probability of treatment; NSAID, nonsteroidal anti-inflammatory drugs; PPI, proton pump inhibitors.
During follow-up, 673 (6.1%) non-users and 355 (6.4%) users experienced an incident hip fracture, with age-sex-adjusted IR (95% CI) of 1.8 (1.6–1.9) and 2.0 (1.8–2.2) per 100 person-years, respectively (Table 3). The IR was higher in users of older AEDs (2.6; 2.1–3.3) than in users of newer AEDs (1.4; 1.1–1.9).
Table 3.
Age- and Sex-Adjusted Incidence Rate (IR) and Hazard Ratio (HR), with 95% Confidence Interval (95% CI), of Hip Fracture (HF) According to Use of AED
| Incident HF (N= 1028) | Age- and Sex- Adjusted IR/100 Person-Years (95% CI) | Matched HRa (95% CI) | Propensity Score Adjusted HR (95% CI) | IPTW HRb (95% CI) | Stabilized IPTW HRc (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | ||||||||||
| Non-users | 673 | 65.5 | 1.8 (1.6; 1.9) | Reference | Reference | Reference | Reference | ||||
| Users | 355 | 34.5 | 2.0 (1.8; 2.2) | 1.24 | (1.07; 1.44) | 1.10 | (0.93; 1.29) | 1.17 | (1.05; 1.30) | 1.16 | (1.00; 1.36) |
| Duration of used, e - any AED (weeks) | |||||||||||
| Short (<14) | |||||||||||
| Non-users | 57 | 8.5 | 0.1 (0.1; 0.2) | Reference | Reference | Reference | Reference | ||||
| Users | 112 | 31.6 | 0.6 (0.5; 0.7) | 4.30 | (3.13; 5.92) | 3.98 | (2.84; 5.57) | 3.64 | (2.90; 4.58) | 3.62 | (2.65; 4.93) |
| Medium (14 to <64) | |||||||||||
| Non-users | 161 | 23.9 | 0.4 (0.3; 0.5) | Reference | Reference | Reference | Reference | ||||
| Users | 114 | 32.1 | 0.9 (0.7; 1.1) | 2.23 | (1.76; 2.84) | 1.71 | (1.32; 2.21) | 1.74 | (1.48; 2.05) | 1.74 | (1.36; 2.22) |
| Long (≥64) | |||||||||||
| Non-users | 455 | 67.6 | 1.2 (1.1; 1.4) | Reference | Reference | Reference | Reference | ||||
| Users | 129 | 36.3 | 1.7 (1.4; 2.0) | 1.47 | (1.21; 1.78) | 1.25 | (1.01; 1.54) | 1.23 | (1.08; 1.40) | 1.24 | (1.01; 1.51) |
| Type of AED | |||||||||||
| Older | 88 | 57.9 | 2.6 (2.1; 3.3) | 1.52 | (1.10; 2.10) | 1.10 | (0.77; 1.57) | 1.46 | (1.03; 2.08) | 1.37 | (1.00; 1.88) |
| Newer | 64 | 42.1 | 1.4 (1.1; 1.9) | Reference | Reference | Reference | Reference | ||||
| Duration of used, f (weeks) | |||||||||||
| Short (<14) | |||||||||||
| Older | 27 | 17.8 | 0.2 (0,4; 1.0) | 1.88 | (1.00; 3.54) | 1.51 | (0.74; 3.05) | 1.27 | (0.83; 1.95) | 1.27 | (0.69; 2.33) |
| Newer | 15 | 9.9 | 0.1 (0.1; 0.4) | Reference | Reference | Reference | Reference | ||||
| Medium (14 to <64) | |||||||||||
| Older | 31 | 20.4 | 0.9 (0.6; 1.3) | 1.60 | (0.92; 2.78) | 1.71 | (0.92; 3.18) | 2.00 | (1.33; 3.01) | 2.00 | (1.12; 3.56) |
| Newer | 21 | 13.8 | 0.4 (0.3; 0.7) | Reference | Reference | Reference | Reference | ||||
| Long (≥64) | |||||||||||
| Older | 30 | 19.7 | 1.2 (0.9; 1.8) | 1.26 | (0.75; 2.10) | 1.20 | (0.69; 2.10) | 1.06 | (0.75; 1.49) | 1.06 | (0.65; 1.72) |
| Newer | 28 | 18.4 | 0.9 (0.6; 1.3) | Reference | Reference | Reference | Reference | ||||
Notes: aAdjusted for age, sex, and time since AD diagnosis (days) by matching. bWeighted with inverse probability of treatment weights (IPTW). cWeighted with stabilized inverse probability of treatment weights (IPTW). dCut-offs based on tertile distribution of duration of AED use: 1st tertile 13.9 weeks, 2nd tertile 63.7 weeks. eReference category for each duration of any AED use group is the non-users with same follow-up duration. fReference category for each duration of older AED use group is the users of newer AEDs with same duration of use.
Incident users of AEDs had HR slightly higher than non-users (IPTW HR 1.17; 1.05–1.30) (Table 3). Among incident users, the IPTW HR was 3.64 (2.90–4.58) in those with short duration of use (<14 weeks), 1.74 (1.48–2.05) in those with medium duration (14 to <64 weeks) and 1.23 (1.08–1.40) in those who used AEDs for ≥64 weeks in comparison to non-users with same follow-up time. The results from the sensitivity analyses using stabilized weights and propensity score adjustment had larger confidence intervals but were comparable to the main analyses.
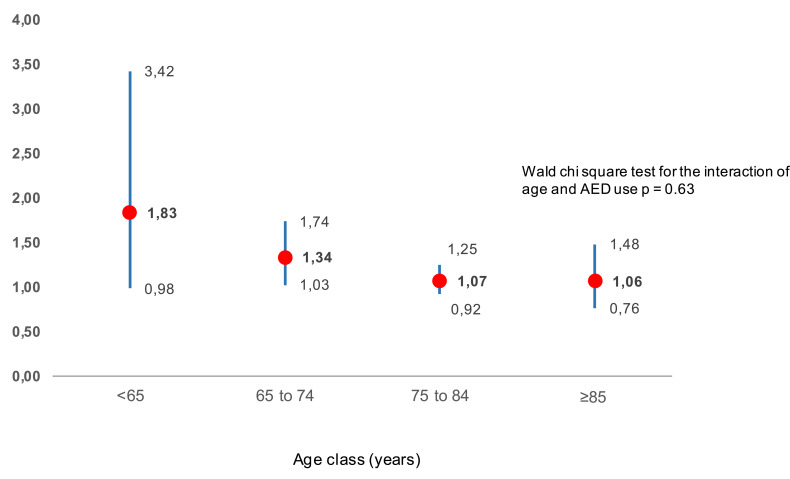
The risk was not modified by age (p for interaction between AED use and age = 0.63), but larger HR’s were observed among younger users (age <65 years) than older users (Figure 2).
Figure 2.
Hazard ratio (HR) weighted for inverse probability of AED treatment, with 95% confidence interval, of hip fracture comparing incident users and non-users by age class. The following HR point estimates are in bold: 1.83; 1.34; 1.07; 1.06.
The risk of hip fracture was higher in users of older (IPTW HR 1.46; 1.03–2.08) compared to users of newer AEDs. In users of older AEDs, the relative risk was 1.27 (0.83–1.95) in those with short duration of use, 2.00 (1.33-3-01) in those with medium and 1.06 (0.75–1.49) in those with long duration of use compared to users of new AEDs with same follow-up time. The results from the sensitivity analyses with stabilized weights or propensity score adjustment were in line with results of the main analyses, although in both sensitivity analyses the point estimates for short and medium duration were stronger, and those for old vs new comparison weaker than in the main analyses.
Discussion
In this cohort of persons with clinically confirmed AD, there was a 17% increase in the risk of hip fractures in incident users of AEDs compared with non-users. However, when duration of use was considered, higher risk increase was observed for shorter term of use (less than 14 weeks) than for longer term use. In addition, users of older AEDs had a 46% increased relative risk of hip fracture compared with users of newer AEDs.
To our knowledge, this is the first study on the risk of hip fractures associated with AEDs in persons with AD. Prior studies focused on young-adult persons with epilepsy, who are commonly treated for long periods and often in polytherapy. The increase in risk in our study has a lower magnitude than in prior studies showing a doubled risk of hip fractures associated with AEDs.5 This difference in magnitude may be explained by differences in study population regarding age and morbidity profile, as well as in indication, type of AEDs, and pattern of use. Firstly, persons with AD have a high background risk of hip fractures; therefore, the excess risk due to AEDs may be lower than in persons without cognitive disorders. Secondly, in persons with AD, AEDs are mostly used for neuropathic pain6 and to manage neuropsychiatric symptoms (eg, agitation and aggression). Consistently, in our cohort pregabalin and gabapentin were among the most commonly used AEDs, suggesting that indeed neuropathic pain and management of neuropsychiatric symptoms were the most probable indications.
The finding of a higher risk in persons with short duration of use is consistent with early adverse effects of AEDs (such as sedation, confusion, blurred vision, and ataxia) that occur mostly in the early stages of the treatment and increase the susceptibility to falls.38 Indeed, falls are the leading cause of fractures of the hip in older adults.39,40 However, the higher risk persisted also for those with medium duration of use (from 14 to <64 weeks) and longer term of use (64 weeks or longer), suggesting that early adverse events are likely not the only explanation. Indeed, sedation may also persist for longer period of time, not just in the beginning of use. Moreover, dose-related adverse effects may occur beyond early use, as when the dose is slowly and gradually increased as it should be done in vulnerable older persons to identify possible adverse effect. This is the case of hyponatremia, a risk factor for falling in elderly persons.41
This study faces methodological challenges related to the observational design and use of healthcare databases. Confounding by indication may be present in the comparison between users of AEDs and non-users, who do not have the health condition leading to pharmacotherapy, but also between newer and older AEDs. Older AEDs are mainly used to treat seizures,42 which increase the baseline risk of hip fracture. This may lead to an underestimation of the risk in users of newer AEDs if these agents are mainly used for neuropathic pain. However, newer AEDs are also used to treat neuropsychiatric symptoms (eg, hallucinations) that also increase risk of falling and fractures.
To overcome this limitation, in comparing incident users with non-users of AEDs and incident users of newer and older AEDs, we used inverse probability weighting and propensity score adjustment to balance the groups under comparison. Propensity scores were based on an extensive list of known risk factors for hip fracture, such as osteoporosis or use of psychotropic medications (eg, antidepressants, antipsychotics, benzodiazepines and related drugs), as well as of other diseases, medications and socioeconomic status to ensure that patient characteristics were captured.
Although the differences regarding these measured factors were balanced, it may be that residual confounding due to unmeasured factors persisted. Indeed, as is common in studies based on healthcare databases, direct clinical measures of certain patient characteristics, such as frailty, history of falls and degree of cognitive and functional impairment, were not available. To account for these unmeasured characteristics, proxy indicators have been accounted for. Specifically, we accounted for severity of AD by matching on time since AD diagnosis; we adjusted for prior fractures as indicators of (at least the most severe) falls and for alcohol abuse as a proxy of lifestyle habits. Alcohol abuse was defined based on related diagnoses and medications. This allowed to account alcohol abuse with impact on patient health, but likely not for less severe or more recent abuse. Moreover, we adjusted for socioeconomic status which is an indicator of both lifestyle habits and health status.
Exposure to AEDs was defined using dispensations that reflect medications redeemed at the pharmacy level, contrary to prescriptions or information extracted from medical documentation. Thus, misclassification of the exposure should be minimal if any.
We defined the outcome as hip fracture leading to hospitalization. The completeness and accuracy of registering hip fractures is generally good in data from the Finnish Health Care Register.43 Misclassification of the outcome should thus be minimal.
Conclusion
Our results imply that AED use is associated with an increased risk of hip fracture in people with AD. As the risk of hip fracture is higher in people with AD in general, prescribers need to carefully consider the risk of falling associated with antiepileptics and consider other safer options especially if prescribed to anxiety or neuropathic pain. Persons with AD treated with antiepileptics should be carefully monitored due to their increased risk of falling and fractures.
Funding Statement
AMT is funded by Academy of Finland (grants 307232 and 295334) and acknowledges strategic funding from the University of Eastern Finland. The sponsors had no role in the design, methods, data collection, analysis and preparation of paper. This study is not industry-sponsored.
Disclosure
Dr. Heidi Taipale participated in research projects funded by Janssen-Cilag and Eli Lilly with grants paid to the institution where she was employed. Dr. Jari Tiihonen has served as a consultant to Finnish Medicines Agency (Fimea), European Medicines Agency (EMA) and Janssen-Cilag, received lecture fees from Janssen-Cilag, Eli Lilly, Lundbeck, and Otsuka, is a member of advisory board in Lundbeck and has participated in research projects funded by Janssen-Cilag and Eli Lilly with grants paid to the institution where he was employed. Dr. Antti Tanskanen participated in research projects funded by Janssen-Cilag and Eli Lilly with grants paid to the institution where he was employed. Dr. Sirpa Hartikainen reports personal fees from Astellas Pharma, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Carbone LD, Johnson KC, Robbins J, et al. Antiepileptic drug use, falls, fractures, and BMD in postmenopausal women: findings from the women’s health initiative (WHI). J Bone Miner Res. 2010;25(4):873–881. doi: 10.1359/jbmr.091027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Espinosa PS, Perez DL, Abner E, Ryan M. Association of antiepileptic drugs, vitamin D, and calcium supplementation with bone fracture occurrence in epilepsy patients. Clin Neurol Neurosurg. 2011;113(7):548–551. doi: 10.1016/j.clineuro.2011.03.011 [DOI] [PubMed] [Google Scholar]
- 3.Nicholas JM, Ridsdale L, Richardson MP, Grieve AP, Gulliford MC. Fracture risk with use of liver enzyme inducing antiepileptic drugs in people with active epilepsy: cohort study using the general practice research database. Seizure. 2013;22(1):37–42. doi: 10.1016/j.seizure.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 4.Souverein PC, Webb DJ, Weil JG, Van Staa TP, Egberts AC. Use of antiepileptic drugs and risk of fractures: case-control study among patients with epilepsy. Neurology. 2006;66(9):1318–1324. doi: 10.1212/01.wnl.0000210503.89488.88 [DOI] [PubMed] [Google Scholar]
- 5.Shen C, Chen F, Zhang Y, Guo Y, Ding M. Association between use of antiepileptic drugs and fracture risk: a systematic review and meta-analysis. Bone. 2014;64:246–253. doi: 10.1016/j.bone.2014.04.018 [DOI] [PubMed] [Google Scholar]
- 6.Scherder EJ, Plooij B. Assessment and management of pain, with particular emphasis on central neuropathic pain, in moderate to severe dementia. Drugs Aging. 2012;29(9):701–706. doi: 10.1007/s40266-012-0001-8 [DOI] [PubMed] [Google Scholar]
- 7.Lanctot KL, Amatniek J, Ancoli-Israel S, et al. Neuropsychiatric signs and symptoms of Alzheimer’s disease: new treatment paradigms. Alzheimers Dement. 2017;3(3):440–449. doi: 10.1016/j.trci.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seppala LJ, Petrovic M, Ryg J, et al. STOPPFall (Screening tool of older persons prescriptions in older adults with high fall risk): a Delphi study by the EuGMS task and finish group on fall-risk-increasing drugs. Age Ageing. 2020. doi: 10.1093/ageing/afaa249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baillon SF, Narayana U, Luxenberg JS, Clifton AV. Valproate preparations for agitation in dementia. Cochrane Database Syst Rev. 2018;10:CD003945. doi: 10.1002/14651858.CD003945.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu CS, Ruthirakuhan M, Chau SA, Herrmann N, Carvalho AF, Lanctot KL. pharmacological management of agitation and aggression in Alzheimer’s disease: a review of current and novel treatments. Curr Alzheimer Res. 2016;13(10):1134–1144. doi: 10.2174/1567205013666160502122933 [DOI] [PubMed] [Google Scholar]
- 11.Gallagher D, Herrmann N. Antiepileptic drugs for the treatment of agitation and aggression in dementia: do they have a place in therapy? Drugs. 2014;74(15):1747–1755. doi: 10.1007/s40265-014-0293-6 [DOI] [PubMed] [Google Scholar]
- 12.Dolder CR, Nealy KL. The efficacy and safety of newer anticonvulsants in patients with dementia. Drugs Aging. 2012;29(8):627–637. doi: 10.1007/BF03262279 [DOI] [PubMed] [Google Scholar]
- 13.Watt JA, Goodarzi Z, Veroniki AA, et al. Safety of pharmacologic interventions for neuropsychiatric symptoms in dementia: a systematic review and network meta-analysis. BMC Geriatr. 2020;20(1):212. doi: 10.1186/s12877-020-01607-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tolppanen AM, Taipale H, Koponen M, et al. Screening approach for identifying candidate drugs and drug-drug interactions related to hip fracture risk in persons with Alzheimer disease. Pharmacoepidemiol Drug Saf. 2017;26(8):875–889. doi: 10.1002/pds.4232 [DOI] [PubMed] [Google Scholar]
- 15.Sarycheva T, Taipale H, Lavikainen P, et al. Incidence and prevalence of antiepileptic medication use in community-dwelling persons with and without Alzheimer’s disease. J Alzheimers Dis. 2018;66(1):387–395. doi: 10.3233/JAD-180594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tolppanen A-M, Lavikainen P, Soininen H, Hartikainen S. Incident hip fractures among community dwelling persons with alzheimer’s disease in a finnish nationwide register-based cohort. PLoS One. 2013;8(3):e59124. doi: 10.1371/journal.pone.0059124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanis JA, Oden A, McCloskey EV, Johansson H, Wahl DA, Cooper C. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int. 2012;23(9):2239–2256. doi: 10.1007/s00198-012-1964-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301(5):513–521. doi: 10.1001/jama.2009.50 [DOI] [PubMed] [Google Scholar]
- 19.Klop C, van Staa TP, Cooper C, Harvey NC, de Vries F. The epidemiology of mortality after fracture in England: variation by age, sex, time, geographic location, and ethnicity. Osteoporos Int. 2017;28(1):161–168. doi: 10.1007/s00198-016-3787-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsoulis M, Benetou V, Karapetyan T, et al. Excess mortality after hip fracture in elderly persons from Europe and the USA: the CHANCES project. J Intern Med. 2017;281(3):300–310. doi: 10.1111/joim.12586 [DOI] [PubMed] [Google Scholar]
- 21.Dahl C, Holvik K, Meyer HE, et al. Increased mortality in hip fracture patients living alone: a NOREPOS Study. J Bone Miner Res. 2021;36(3):480–488. doi: 10.1002/jbmr.4212 [DOI] [PubMed] [Google Scholar]
- 22.Patel R, Bhimjiyani A, Ben-Shlomo Y, Gregson CL. Social deprivation predicts adverse health outcomes after hospital admission with hip fracture in England. Osteoporos Int. 2021. doi: 10.1007/s00198-020-05768-4 [DOI] [PubMed] [Google Scholar]
- 23.Tajeu GS, Delzell E, Smith W, et al. Death, debility, and destitution following hip fracture. J Gerontol a Biol Sci Med Sci. 2014;69(3):346–353. doi: 10.1093/gerona/glt105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang VL, Sudore R, Cenzer IS, et al. Rates of recovery to pre-fracture function in older persons with hip fracture: an observational study. J Gen Intern Med. 2017;32(2):153–158. doi: 10.1007/s11606-016-3848-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bleibler F, Rapp K, Jaensch A, Becker C, Konig HH. Expected lifetime numbers and costs of fractures in postmenopausal women with and without osteoporosis in Germany: a discrete event simulation model. BMC Health Serv Res. 2014;14(1):284. doi: 10.1186/1472-6963-14-284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambrelli D, Burge R, Raluy-Callado M, Chen SY, Wu N, Schoenfeld MJ. Retrospective database study to assess the economic impact of hip fracture in the United Kingdom. J Med Econ. 2014;17(11):817–825. doi: 10.3111/13696998.2014.959588 [DOI] [PubMed] [Google Scholar]
- 27.Leal J, Gray AM, Prieto-Alhambra D, et al. Impact of hip fracture on hospital care costs: a population-based study. Osteoporos Int. 2016;27(2):549–558. doi: 10.1007/s00198-015-3277-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarride J, Adachi JD, Brown JP, Schemitsch E, Slatkovska L, Burke N. Incremental costs of fragility fractures: a population-based matched-cohort study from Ontario, Canada. Osteoporos Int. 2021. doi: 10.1007/s00198-021-05877-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tolppanen AM, Taipale H, Koponen M, et al. Cohort profile: the Finnish medication and Alzheimer’s disease (MEDALZ) study. BMJ Open. 2016;6(7):e012100. doi: 10.1136/bmjopen-2016-012100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease. Report of the NINCDS‐ADRDA Work Group* under the auspices of Department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34(7):939. doi: 10.1212/WNL.34.7.939 [DOI] [PubMed] [Google Scholar]
- 31.Bell CC. DSM-IV: diagnostic and statistical manual of mental disorders. JAMA. 1994;272(10):828–829. doi: 10.1001/jama.1994.03520100096046 [DOI] [Google Scholar]
- 32.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231 [DOI] [PubMed] [Google Scholar]
- 33.Tanskanen A, Taipale H, Koponen M, et al. From prescription drug purchases to drug use periods - a second generation method (PRE2DUP). BMC Med Inform Decis Mak. 2015;15(1):21. doi: 10.1186/s12911-015-0140-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taipale H, Tanskanen A, Koponen M, Tolppanen AM, Tiihonen J, Hartikainen S. Agreement between PRE2DUP register data modeling method and comprehensive drug use interview among older persons. Clin Epidemiol. 2016;8:363–371. doi: 10.2147/CLEP.S116160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Austin PC. Goodness-of-fit diagnostics for the propensity score model when estimating treatment effects using covariate adjustment with the propensity score. Pharmacoepidemiol Drug Saf. 2008;17(12):1202–1217. doi: 10.1002/pds.1673 [DOI] [PubMed] [Google Scholar]
- 38.Vajda FJ, Eadie MJ. The clinical pharmacology of traditional antiepileptic drugs. Epileptic Disord. 2014;16(4):395–408. doi: 10.1684/epd.2014.0704 [DOI] [PubMed] [Google Scholar]
- 39.Clement ND, Court-Brown CM. Elderly pelvic fractures: the incidence is increasing and patient demographics can be used to predict the outcome. Eur J Orthop Surg Traumatol. 2014;24(8):1431–1437. doi: 10.1007/s00590-014-1439-7 [DOI] [PubMed] [Google Scholar]
- 40.Court-Brown CM, McQueen MM. Global Forum: fractures in the Elderly. J Bone Joint Surg Am. 2016;98(9):e36. doi: 10.2106/JBJS.15.00793 [DOI] [PubMed] [Google Scholar]
- 41.Bhandari SK, Adams AL, Li BH, et al. Sub-acute more than chronic hyponatremia is associated with serious falls and hip fractures. Intern Med J. 2019. [DOI] [PubMed] [Google Scholar]
- 42.Vossel KA, Tartaglia MC, Nygaard HB, Zeman AZ, Miller BL. Epileptic activity in Alzheimer’s disease: causes and clinical relevance. Lancet Neurol. 2017;16(4):311–322. doi: 10.1016/S1474-4422(17)30044-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sund R, Nurmi-Luthje I, Luthje P, Tanninen S, Narinen A, Keskimaki I. Comparing properties of audit data and routinely collected register data in case of performance assessment of hip fracture treatment in Finland. Methods Inf Med. 2007;46(05):558–566. doi: 10.1160/ME0382 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to conduct the research are available from the corresponding author but restrictions by the register maintainers and Finnish legislation apply to the availability of these data. Therefore, the data are not publicly available without permissions of the register maintainers.