Abstract
Objective:
Cortical spreading depression (SD) is an intense depolarization underlying migraine aura. Despite the weight of evidence linking SD to the pain phase of migraine, controversy remains over a causal role of SD in cephalgia because of the invasive nature of previous SD induction methods. To overcome this problem, we employed a novel minimally invasive optogenetic SD induction method and examined the effect SD on behavior.
Methods:
Optogenetic SD was induced as a single event or repeatedly every other day for 2 weeks. Endpoints including periorbital and hindpaw mechanical allodynia, mouse grimace, anxiety, and working memory were examined in male and female mice.
Results:
A single SD produced bilateral periorbital mechanical allodynia that developed within 1 hour and resolved within 2 days. Sumatriptan prevented periorbital allodynia when administered immediately after SD. Repeated SDs also produced bilateral periorbital allodynia that lasted 4 days and resolved within 2 weeks after the last SD. In contrast, the hindpaw withdrawal thresholds did not change after repeated SDs suggesting that SD-induced allodynia was limited to the trigeminal region. Moreover, repeated SDs increased mouse grimace scores 2 days after the last SD, while single SD did not. Repeated SD also increased thigmotaxis scores as a measure of anxiety. In contrast, neither single nor repeated SDs affected visuospatial working memory. We did not detect sexual dimorphism in any endpoint.
Interpretation:
Altogether, these data show a clinically congruent causal relationship between SD, trigeminal pain and anxiety behavior possibly reflecting SD modulation of hypothalamic, thalamic and limbic mechanisms.
INTRODUCTION
Cortical spreading depression (SD), a wave of depolarization propagating at a rate of 2–5 mm/min, is the most likely culprit responsible for migraine aura.1 Despite evidence suggesting that SD is linked to the pain phase of migraine, controversy remains over a causal role of SD in cephalgia.2, 3 Arguing for a role, the vast majority (90%) of migraine aura attacks are accompanied by headache, and aura onset tends to occur before (78–93%) headache onset.4, 5 Furthermore, migraine aura is more frequently associated with severe cutaneous allodynia as compared to migraine without aura.6–10 Conversely, the most common form of migraine occurs without a perceived aura and in a small percentage of patients, aura can occur after headache onset or without headache altogether, suggesting that SD may not be the trigger for cephalgia.
Several animal studies suggest SD activates trigeminal nociceptive pathways. SD increases c-fos expression in the trigeminal nucleus caudalis (TNC),11 increases firing rates of TNC neurons with meningeal receptive fields2, 12 and single unit activity of meningeal afferents.13 SD activation of TNC neurons is sensitive to meningeal nerve transection11 and anti-migraine medications.11, 12 Moreover, several studies reported SD induced trigeminal pain behaviour. For example, SDs elicited by application of 3M KCl onto the dura produced periorbital allodynia in male Wistar rats.14 Similar reductions in periorbital mechanical thresholds were observed in female Sprague Dawley rats following cortical injection of 1M KCl.15 However, activation of trigeminal nociceptors and allodynia observed in these studies may have been confounded by invasive cranial surgery, exposure to hyperosmolar KCl or direct stimulation of dural or calvarial nociceptors.3, 16 For example, in one study, the number of KCl injections, but not of actual SDs, was associated with c-fos expression in TNC.17 Others also observed that SD was not necessary for cutaneous allodynia to develop after KCl application.18 Therefore, the highly invasive nature of SD induction methods precluded conclusive demonstration of a causal link between SD and trigeminal nociceptor activation.19, 20
To overcome this barrier, we employed optogenetics to induce SDs in a minimally invasive fashion without damaging the skull or underlying dura to test whether single or recurrent SD events trigger trigeminal pain behaviour in mice.21–23 Given the high prevalence of anxiety and cognitive dysfunction in migraineurs, we further examined anxiety and working memory.
METHODS
Animals:
Experiments were approved by the MGH Institutional Animal Care and Use Committee and performed in accordance with the US Public Health Service’s Policy on Humane Care and Use of Laboratory Animals. Male and female transgenic mice (4–12 months, 23–35 g) expressing channelrhodopsin-2-YFP fusion protein under the thymus cell antigen-1 promoter (B6.Cg-Tg(Thy1-COP4/EYFP)9Gfng/J; Jackson Laboratories, Bar Harbor, ME, USA) were housed with 12hr/12hr light-dark cycle and given food and water ad libitum. Animals were randomly divided into sham and SD groups.
Optogenetic SD induction:
Mice were anesthetized with isoflurane (5% induction, 1–2% maintenance in 70% N2O, 30% O2) and placed in a stereotactic frame. A single midline incision of the skin was made to expose the skull which was otherwise left intact. A glass coverslip was affixed to the skull in all mice using cyanoacrylate and C&B metabond (Parkell Inc., Edgewood, NY, USA). SD induction started 2 days later as described previously.23 Under brief isoflurane anesthesia (1–2%) and light head immobilization in a stereotactic frame, motor cortex was stimulated with 470nM light for 10sec at 1mW increments (max 10mW) every 2min using a 400mm optical fiber-LED light source (Thorlabs, Newton, NJ, USA; Figure 1A) over the right hemisphere until a single SD was detected. This protocol does not cause light or heat injury even after repeated exposures.22 A webcam (OT-HD, Opti-TekScope, Chandler, AZ, USA) interfaced with MATLAB was used to create intrinsic optical signal difference images every 2 seconds. SDs were identified as a characteristic change in reflectance propagating across the cortex (Figure 1A). The sensitivity and specificity of this method to reliably detect SDs has been validated.23 Animals in the single SD group underwent a single SD event (n=32; Figure 1B). Animals in the repeated SD group received a single SD induced every other day for 2 weeks (n=32; Figure 1C). Sham animals for the single (n=31) and repeated (n=28) SD groups were handled in a similar fashion as SD animals, they underwent glass coverslip placement and had similar exposures to isoflurane anesthesia and handling. In the repeated SD group, we induced SD every other day to model a high attack frequency based on the previously reported average of 12 aura attacks per month (range 2–60) in patients experiencing > 2 attacks per month.24
Figure 1. Optogenetic SD and behavioral testing.
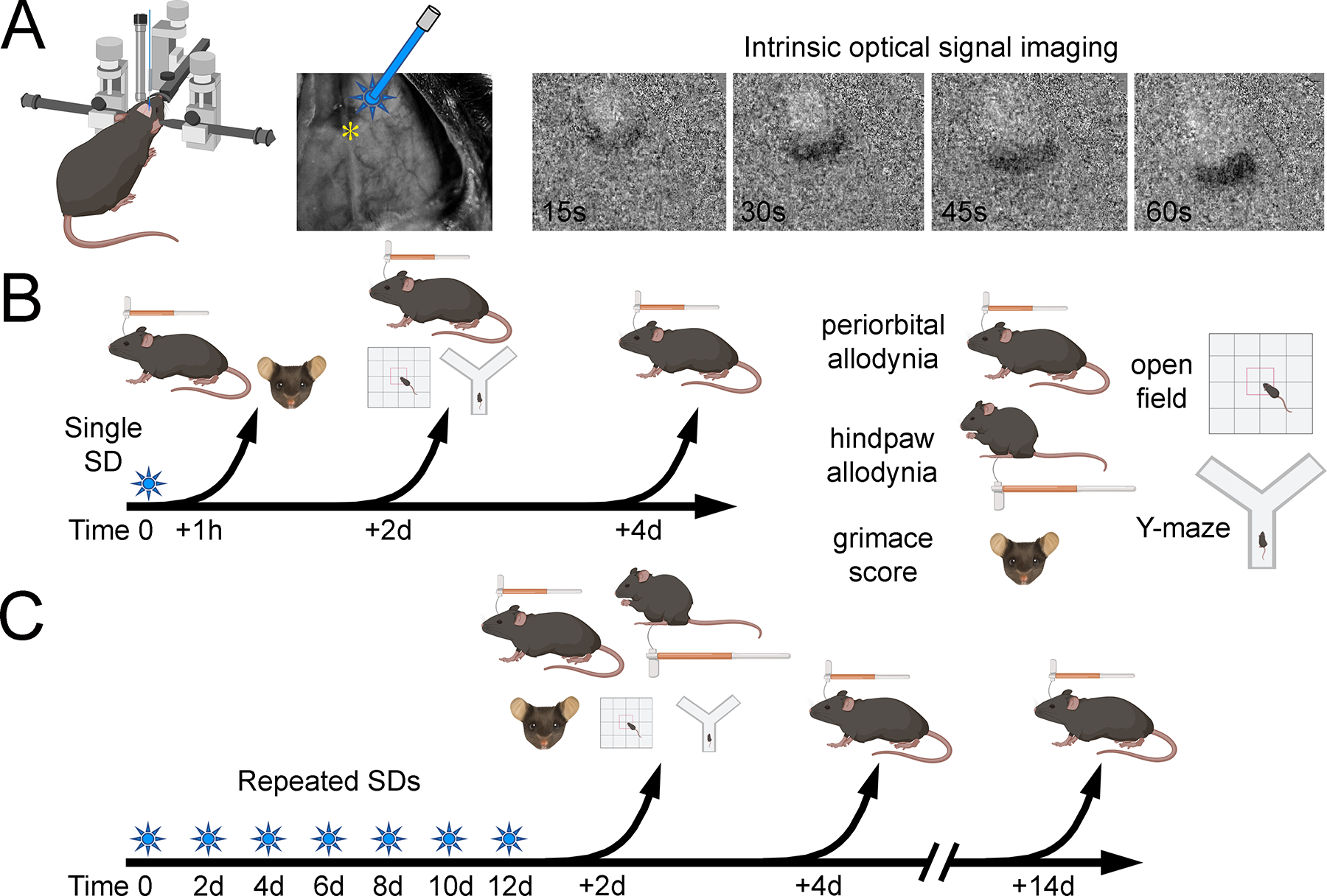
(A) Motor cortex was stimulated with 470 nM wavelength light using an optical fiber for 10 seconds at 1mW power increments every 2 minutes until an SD occurred (max 10mW). SD was visualized through a glass coverslip using intrinsic optical signal imaging acquired at 0.5 Hz (*, bregma). Each image was subtracted from the baseline image to demonstrate SD propagation over the dorsal cortex. (B) In the single SD paradigm, animals were assessed 1 hour, 2 days or 4 days after SD. Individual readouts are shown on the right, including periorbital and hindpaw mechanical allodynia using von Frey monofilaments, and anxiety-like behavior and working memory using open field and Y maze, respectively. (C) In the repeated SD paradigm, SDs were induced once every other day for 14 days and animals were assessed 2 days, 4 days or 14 days after the last SD.
Allodynia testing:
Tests were carried out during daylight and after acclimation to the behavioral room and apparatuses. Mechanical thresholds were tested by an evaluator blinded to group allocation with sequential ascending calibrated von Frey monofilaments using a method previously published.25, 26 Periorbital allodynia was defined as a reduction in periorbital mechanical threshold. Mice were gently placed in a restrainer device large enough to allow for movement of the forepaw and hindpaw with an opening that allowed access to the periorbital region. Calibrated von Frey monofilaments (Stoelting Co, Wood Dale, IL, USA) between 0.008 and 0.4 grams were applied perpendicular to the surface just superior-medial to the right and left eye with a force that allowed the monofilament to bend. The force producing head withdrawal, vigorous turn, or scratching in more than 50% of 5 trials was considered the threshold. Periorbital thresholds were determined 1 hour, 2 and 4 days after SD in the single SD group and 2, 4 and 14 days after the last SD in the repeated SD group (Figure 1B, C). To prevent habituation upon repeated testing, separate groups were studied for each time point. Hindpaw allodynia was tested 2 days after the last SD in a clear chamber with a grid floor. Filaments with bending force between 0.4 and 6 grams were applied to the central-ventral hindpaw. The force producing brisk withdrawal or shaking in more than 50% of 5 trials was considered the threshold. Ipsilateral and contralateral thresholds did not differ and were averaged for all analyses.
In a separate group of mice, we tested the efficacy of sumatriptan on periorbital allodynia. Sumatriptan succinate (Sigma Aldrich, St Louis, MO) was dissolved in 0.9% saline to make a 1mg/mL stock solution. Working solutions of 0.1mg/mL were prepared just prior to experimental testing. Animals received intraperitoneal injection of either 600 ug/kg sumatriptan (n=8) or an equivalent volume of 0.9% saline vehicle (n=7) immediately following a single SD. Periorbital allodynia was tested 1 hour after the SD. Sumatriptan dose was chosen based on efficacy in reversing pain behavior in prior animal models of migraine.27
Mouse grimace score:
To assess signs of spontaneous pain, four to six clear random front facing frames were taken from videos of animals prior to periorbital testing 1 hour after the last SD in the single SD group and 2 days after the last SD in the repeated SD group. Facial grimace was scored by two investigators blinded to allocation with high interrater reliability (kappa statistic = 0.876, 95%CI 0.71–1.0). Mouse grimace was scored as previously described.28, 29 Action units of orbital and whisker tightening, cheek and nose bulge were given a score of 0 for not present, 1 for moderate, and 2 for severe for each screenshot. The scores were averaged across action units for each frame. The score for all frames were then averaged to calculate the grimace score per animal. In the sham group for repeated SD comparison, 4 animals had side facing videos and front facing images were not available for grimace score quantification.
Open field test:
All mice were studied for anxiety behavior prior to allodynia testing. To measure SD induced anxiety behavior, mice were placed in an arena (12×14 inches) 2 days following the last SD and allowed to explore for 5 minutes in dim light (164 lux) with a lightmeter used to standardize lighting conditions. An open source MATLAB automated toolbox tracked mouse movement.30 Heat maps, path tracings, total distance traveled, and thigmotaxis score (time spent in the outer perimeter/total time) were compared between groups. Thigmotaxis scores approaching 1 indicate higher levels of anxiety.
Y-maze test:
A subset of mice were studied for working memory prior to allodynia testing on the same day. To assess working memory, mice were placed for 5 minutes in a Y-maze with three arms separated by 120 degrees and distinct visual cues. Correct alternation rate was defined as the number of ABC or CBA sequence arm entries divided by total number of arms entered minus two and expressed as percentage. Data from one animal was excluded due to persistent jumping out of the Y-maze apparatus.
Statistical analyses:
In the absence of prior experience in our lab, the initial sample size was estimated empirically from published data to achieve a power of 0.8 and alpha of 0.05. The sample size was calculated based on the ability to detect an effect size of 50% assuming a standard deviation of 0.03g in periorbital mechanical threshold (minimum estimated sample size = 7 mice per group)25, assuming a standard deviation of 40% for thigmotaxis score (minimum estimated sample size = 12 mice per group)31, 32, and a standard deviation of 15% for correct percent alternation in Y maze (minimum estimated sample size = 6 mice per group)33. For grimace behavior, prior studies indicate adequate power (0.800) to detect a mean difference score of 0.5 with a standard deviation of 0.28 using a minimum estimated sample size = 7 mice per group.29 An interim analysis was performed to revise the sample sizes needed based on the preliminary standard deviation. If more than 20 animals per group were needed to detect a difference (i.e. futility), that particular test was dropped from the protocol, as was the case for the Y maze. Data were analyzed using GraphPad Prism v8 (La Jolla, CA). Data in text are expressed as median with 25th, 75th percentile or mean +/− SEM. For allodynia, the impact of single SD or repeated SD and time on mechanical threshold was tested using a two-way ANOVA on rank transformed data followed by Bonferonni post-hoc analysis. Data were rank transformed based on the skewed distribution on visual inspection of box plots, which resulted in a better fit of the QQ plot of predicted and actual residuals.
Regression analysis on rank transformed data was used to evaluate the relationship between independent variables sex, SD versus sham group, time, and single versus repeated SD, on periorbital allodynia. For two group comparisons, Mann Whitney U nonparametric analysis was used for data that were not normally distributed and unpaired t tests for parametric data. Two-tailed p<0.05 was considered statistically significant.
RESULTS
Single SD triggers periorbital allodynia
Periorbital mechanical thresholds were measured bilaterally in male and female mice 1 hour, 2 days or 4 days after a single SD or sham procedure (Figure 1B). Despite unilateral SD induction, ipsilateral and contralateral mechanical thresholds did not differ; therefore, right and left mechanical thresholds were averaged in each animal for all analyses. A single SD triggered periorbital allodynia [F=3.587, p=0.0652 for SD; F=1.388, p=0.2607 for time; F=4.381, p=0.0187 for interaction; two-way ANOVA; Figure 2]. Post-hoc comparisons showed a significant reduction in periorbital mechanical threshold at 1 hour (p=0.0035) which normalized within 2 days (p>0.9999 at 2 and 4 days). In a separate group of mice, the migraine-abortive 5HT1B/1D receptor agonist sumatriptan (600 μg/kg i.p. immediately after SD) restored periorbital mechanical thresholds compared with vehicle when tested 1 hour after a single SD (Mann-Whitney U=6.500, p=0.0104; Figure 2, right panel). To control for potential direct effects of optogenetic light exposure, we also exposed wild type mice (i.e. not expressing Chr2) to 7mW optogenetic illumination and did not observe any SD or an effect on periorbital mechanical thresholds compared with non-stimulated wild type mice (n=4/group, Mann-Whitney U=6.00, p=0.7714, Figure 2)
Figure 2. Single SD produces acute periorbital mechanical allodynia.
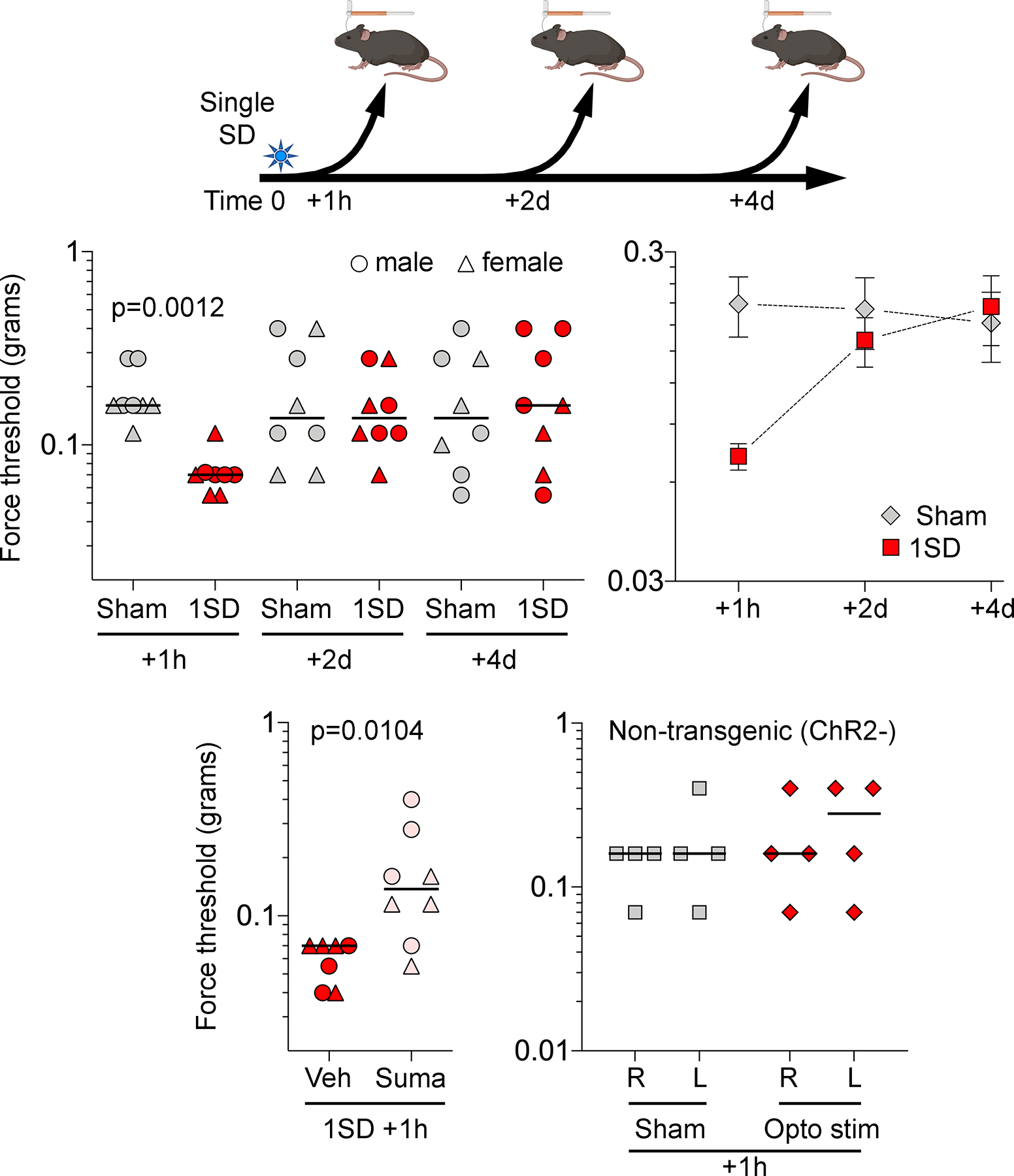
Experimental timeline is shown on top. Left top panel shows individual log force data (grams) from females (triangle) and males (circles) along with the median. A single SD (1SD) reduced periorbital mechanical thresholds 1 hour after SD as compared with sham controls. This effect was lost 2 and 4 days after SD (two-way ANOVA on ranks). Right top panel shows group averages of the same dataset to illustrate the time course of periorbital allodynia (mean ± SEM). Left bottom panel shows normalization of the reduced mechanical thresholds 1 hour after a single SD by sumatriptan (600 μg/kg, intraperitoneal immediately after SD) compared with vehicle (Mann-Whitney U test). Right bottom panel shows single 10 sec, 7mW 470nM optogenetic light stimulation in wild type mice did not produce periorbital mechanical allodynia (Mann-Whitney U test p=0.7714).
Repeated SD and evoked mechanical nociceptive behavior
Cutaneous allodynia is associated with migraine aura and can occur not only during but also in between attacks.9, 34, 35 To explore whether repeated attacks of SD increased the duration of cutaneous allodynia, we examined the effect of repeated SD on periorbital mechanical thresholds compared to sham animals starting at 2 days when the effect of a single SD has already resolved (Figure 1C). Repeated SD produced a longer lasting periorbital allodynia as compared to single event SD [F=24.02, p<0.0001 for SD; F=4.038, p=0.0232 for time; F=5.490, p=0.0068 for interaction; two-way ANOVA; Figure 3]. Post-hoc analysis showed a significant SD-induced reduction in periorbital mechanical threshold at 2 days (p<0.001) and 4 days (p=0.0242). This effect recovered by 14 days (p>0.9999). In contrast, repeated SDs did not change hindpaw mechanical thresholds when tested 2 days after the last SD (Mann-Whitney U=71, p=0.2478; Figure 3) suggesting that SD-induced allodynia was trigeminal-specific.
Figure 3. Repeated SDs induce lasting periorbital mechanical allodynia.
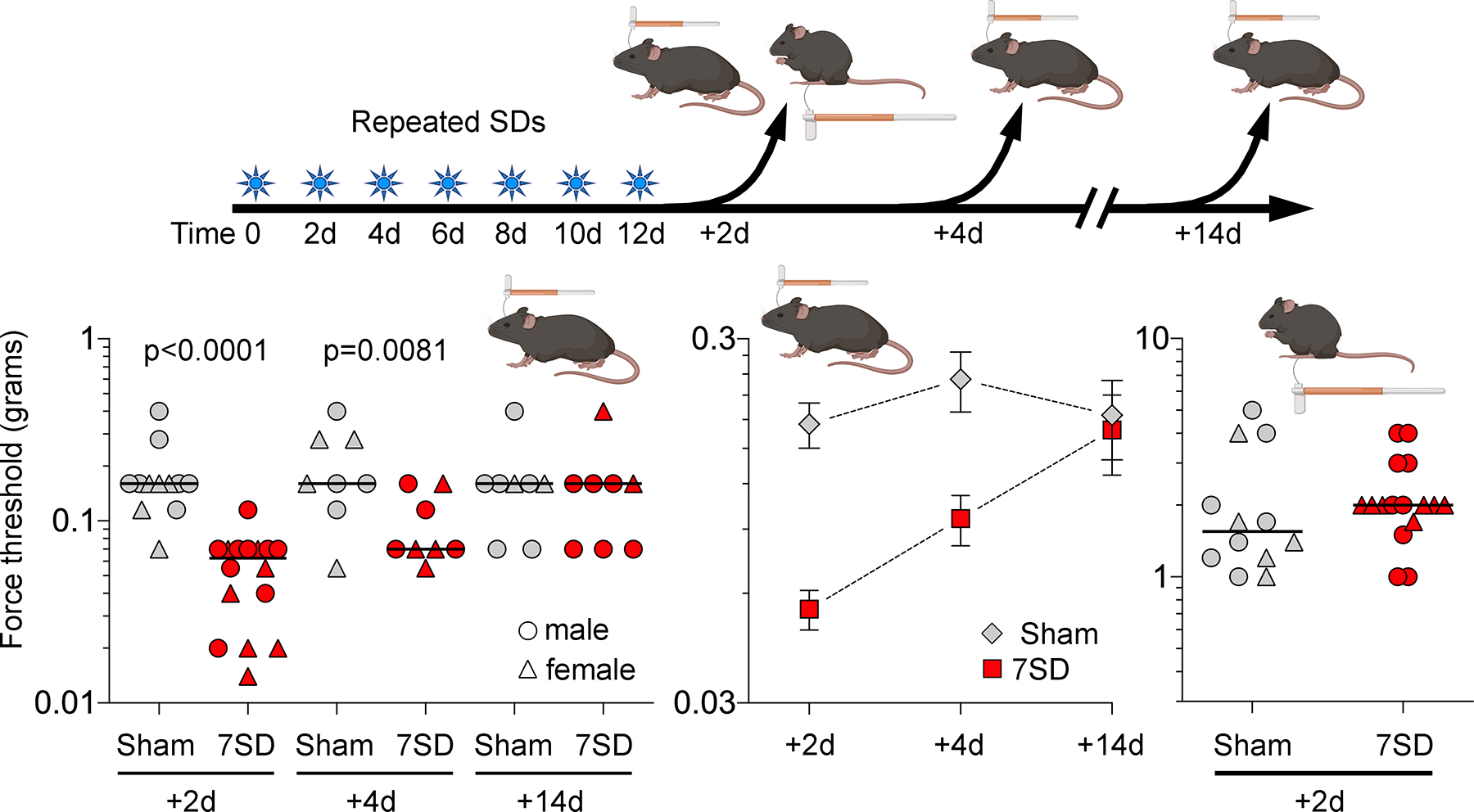
Left panel shows individual log force data (grams) from females (triangle) and males (circles) along with the median. Seven SDs once every other day (7SD) reduced periorbital mechanical thresholds 2 and 4 but not 14 days after the last SD compared with sham controls (two-way ANOVA on ranks). Middle panel shows group averages of the same dataset to better illustrate the time course of periorbital allodynia (mean ± SEM). Right panel shows unchanged mechanical hindpaw thresholds in repeated SD and sham groups tested 2 days following the last SD (Mann-Whitney U test).
SD and spontaneous mouse grimace behavior
In addition to evoked pain thresholds, we also used grimace score of changes in facial expression as a validated measure of spontaneous pain29 (Figure 4). We found no change in grimace following a single SD (t=0.4588, p=0.6534, df=14). In contrast, recurrent SDs significantly increased the grimace score when measured 2 days after the last SD (t=2.343, p=0.0286, df=22). We excluded 4 animals in the repeated sham group because a clear front-facing headshot was not available from the video. The median periorbital mechanical thresholds in these animals did not differ from the 8 repeated sham animals included in the grimace score analysis (0.16 [25th-75th percentile: 0.13–0.16] and 0.16 [25th-75th percentile: 0.13–0.25], respectively).
Figure 4. SD induced facial grimace.
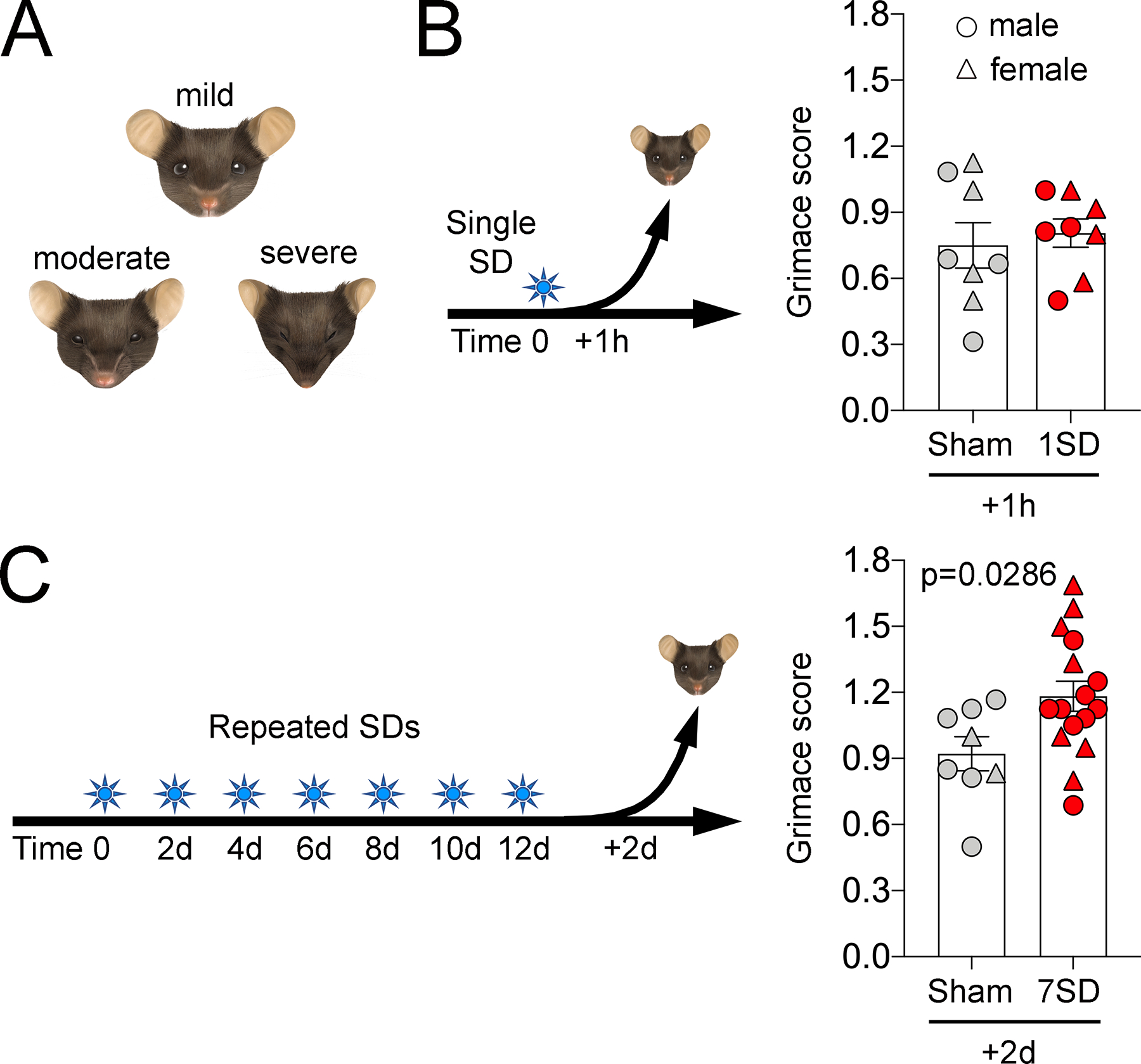
(A) Facial features of animals with mild, moderate and severe discomfort are illustrated. (B) Single SD (1SD) produced no change in mean mouse grimace score as compared to sham animals when assessed 1 hour later. (C) Repeated SDs (7SD) increased mean mouse grimace score. Individual data points from females (triangle) and males (circles) as well as mean ± SEM are shown (t-test).
Sex influences on SD evoked and spontaneous nociceptive behavior
To test sexual dimorphism in mechanical periorbital thresholds we pooled the data across all groups in exploratory analyses. We found no difference in sham groups suggesting that thresholds did not differ between males and females in the absence of SD (Mann-Whitney U=279.5, p=0.3315; Figure 5, left panel). In SD-induced allodynic groups, however, we found a strong trend towards lower thresholds in females (F=3.703, p=0.0653 for sex; two-way ANOVA; Figure 5, right panel). Given this trend, we performed a multivariable regression on periorbital thresholds controlling for sex, sham versus SD, single versus repeated SD, and time between SD and allodynia testing (Table 1). Both SD exposure and recurrent SD were significantly associated with periorbital allodynia, whereas the time between SD and allodynia testing was associated with recovery from periorbital allodynia. After accounting for these relationships, we did not find an association between sex and periorbital mechanical thresholds (Table 1). We also did not find sexual dimorphism on grimace scores (F=0.7060 p=0.4070; two-way ANOVA).
Figure 5. Sexual dimorphism in periorbital mechanical allodynia in sham and SD groups.
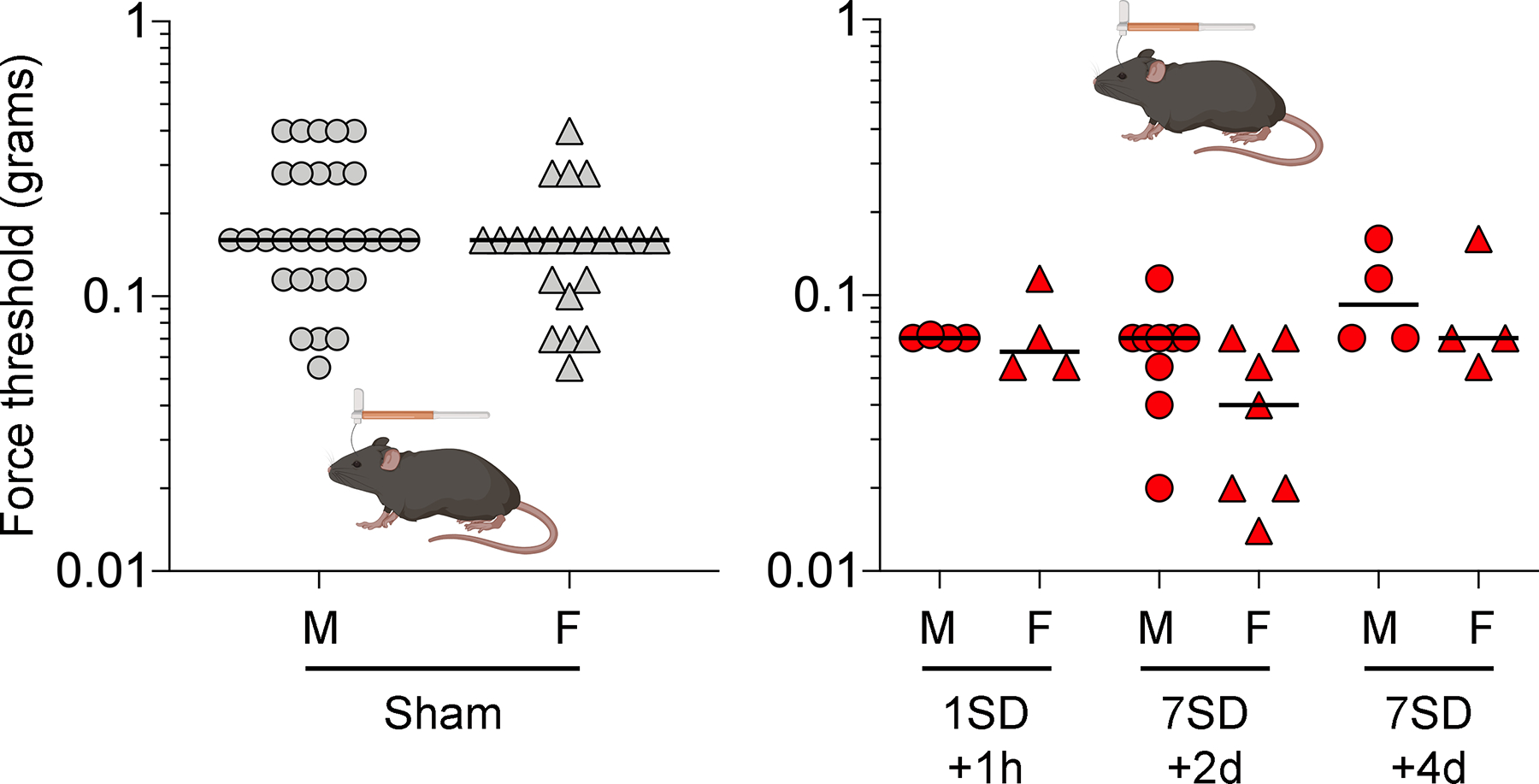
Individual log force data (grams) in previous figures are reorganized to display males (circles) and females (triangle) separately in sham and single (1SD) or repeated SD (7SD) arms. There were no significant differences in pooled periorbital mechanical thresholds between males and females in pooled sham (left panel, Mann-Whitney U test) or SD (right panel, two-way ANOVA on ranks) groups.
Table 1. Regression analysis for sexual dimorphism.
A multivariable regression analysis was performed on periorbital thresholds controlling for sex, sham versus SD, single versus repeated SD, and time between SD and allodynia testing. The overall model was significant (F 9.244, p<0.001). Any SD exposure and recurrent SDs were significantly associated with lower periorbital mechanical thresholds. Time between the last SD and allodynia testing was associated with recovery from periorbital allodynia. After accounting for these relationships, there was no association between sex and periorbital mechanical thresholds.
| 95% CI | ||||||
|---|---|---|---|---|---|---|
| Beta | STE | t | Significance | Lower | Upper | |
| Constant | 72.42 | 5.59 | 12.96 | <0.0001 | 61.34 | 83.51 |
| Sex | −6.56 | 5.27 | 1.24 | 0.2159 | −17.02 | 3.89 |
| Sham vs. SD | −25.61 | 5.16 | 4.96 | <0.0001 | −35.85 | −15.37 |
| Time from last SD | 1.52 | 0.68 | 2.25 | 0.0264 | 0.18 | 2.86 |
| Single vs. repeated SD | −14.88 | 5.67 | 2.63 | 0.010 | −26.12 | 3.64 |
SD induced changes in anxiety and working memory
In the open field test to examine anxiety-like behavior after a single SD, neither the total distance traveled nor the thigmotaxis score differed between SD and sham groups (t=0.1418, p=0.8893, df=14 for total distance traveled; t=0.7725, p=0.4527, df=14 for thigmotaxis scores; Figure 6A, B). In contrast, repeated SDs significantly increased thigmotaxis scores compared with the sham group (t=2.597, p=0.0153, df=26) suggesting increased anxiety-like behavior (Figure 6B). Neither single nor repeated SDs affected the spatial working memory tested using a Y-maze 2 days after the last SD (t=0.3692, p=0.7175, df=14 for single SD and t=1.242, p=0.2312, df=18 for repeated SD in total arm entries; t=0.1894, p=0.8525, df=14 for single SD and t=1.206, p=0.2444, df=18 for repeated SD in percent correct alternation; Figure 6C). Male and female mice did not differ in thigmotaxis scores (F=2.527, p=0.1206, two way-ANOVA) or percent alternations (F=1.713, p=0.2017, two way-ANOVA).
Figure 6. Repeated SDs induce anxiety-like behavior but do not impact working memory.
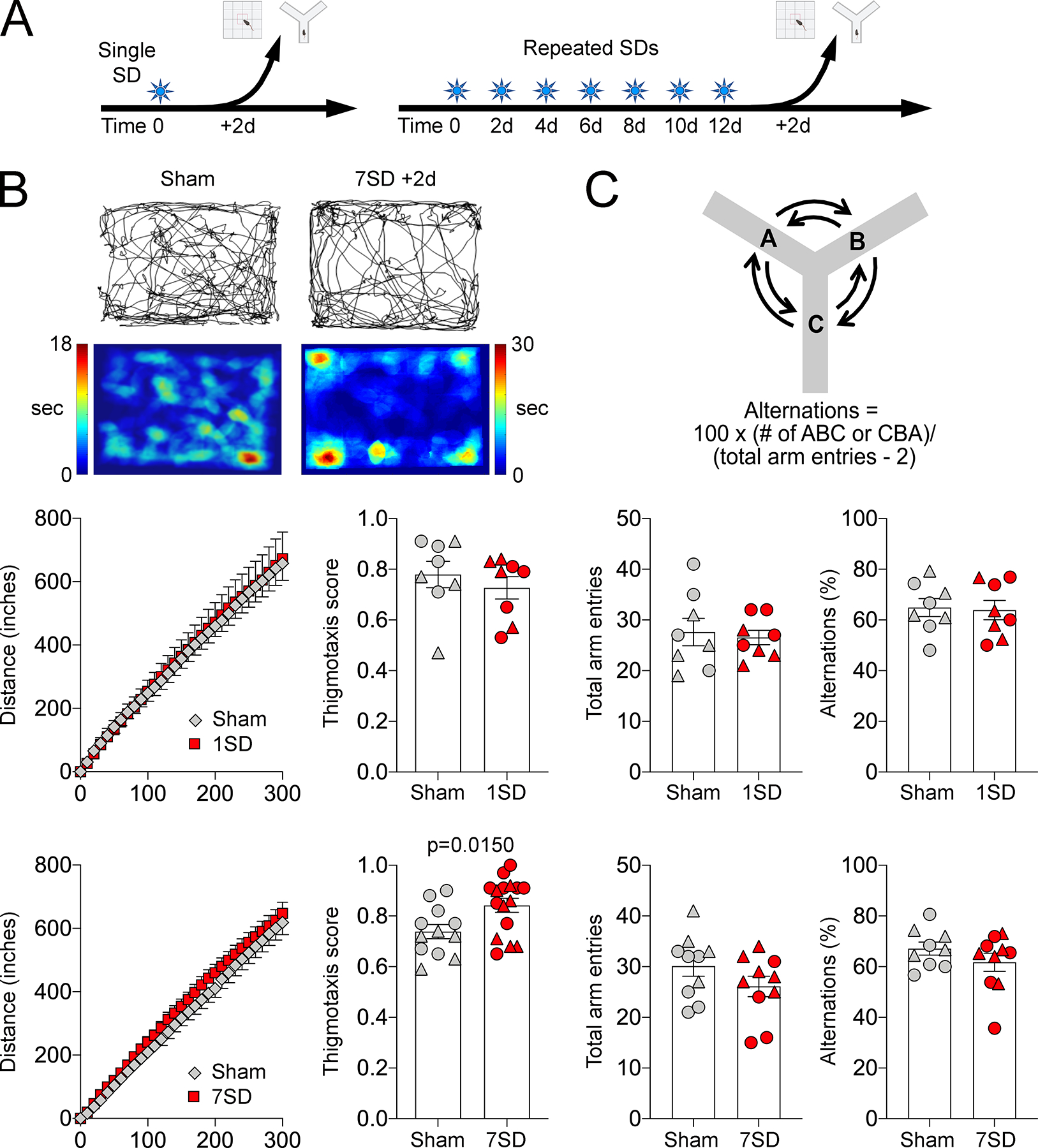
(A) Anxiety-like behavior and working memory were tested using open field thigmotaxis scores and Y-maze alternations, respectively, 2 days after a single (1SD) or repeated SDs (7SD). (B) Upper panel shows representative exploratory paths and heatmaps from a sham and a repeated SD animal. Individual data for total distance traveled and thigmotaxis scores from females (triangle) and males (circles) are shown along with mean ± SEM after a single (middle panel) or repeated SDs (lower panel). Total distance traveled was not affected by SD. Repeated, but not a single, SD increased thigmotaxis scores. (C) Upper panel shows the calculation of percent correct alternations. Individual data for total arm entries and correct alternations from females (triangle) and males (circles) are shown along with mean ± SEM after a single (middle panel) or repeated SDs (lower panel). Sham and SD groups were compared using t-tests.
Discussion
Here, we show for the first time using a minimally-invasive optogenetic approach a causal link between SD and a robust trigeminal pain behavior. Cephalgic allodynia occurs as part of the migraine pain experience in 70–80% of those with migraine.8, 36 While it is not possible to directly measure a subjective symptom such as migraine headache in mice, cutaneous allodynia of the periorbital region is well accepted as a sensory discriminative test used to assay nociceptive pathways that are relevant for migraine pain.37 We show that a single SD can trigger the pain phenotype within an hour, which resolves within 2 days. We also show that repeated SDs produce a more robust pain phenotype lasting at least several days, accompanied by facial grimace as a sign of spontaneous discomfort. Perhaps more importantly, we show that SD-induced trigeminal pain behavior is aborted by a triptan, underscoring the clinical congruence of this model. Inhibition of acute pain behavior by sumatriptan, which activates 5HT1D receptors on central nerve terminals of meningeal nociceptors38, 39, suggests that optogenetic SD-induced trigeminal pain is mediated by activation of meningeal nociceptors40, 41, consistent with prior electrophysiological studies of SD-induced increases in single unit activity of meningeal afferents.42 Altogether, these data establish single or repeated optogenetic SDs as a new model to examine trigeminal pain pathways with relevance to migraine pain states, and as a screening tool for abortive or preventive migraine therapeutics.
Previous studies on SD-induced pain behavior used more invasive and potentially injurious induction methods casting doubt on whether the observed pain behavior was the result of SD or experimental injury.14, 15, 18, 43 For example, some studies applied high concentration KCl (3M) directly on the meninges after a craniotomy to induce multiple SDs14,38 and in one study allodynia was studied only 30 minutes later.43 Such invasive approaches can easily confound any impact of SD on pain behavior, especially when examined acutely after the surgical procedure. Our optogenetic approach does not require a craniotomy, cortical injury or application of hyperosmolar solutions on the meninges or cortex. The method completely obviates the need to breech the skull, permits a recovery period to avoid the confounding effects of minor surgery, and allows testing for weeks and possibly months.
Mechanical allodynia is a consequence of trigeminovascular neuronal sensitization in the brainstem trigeminal nucleus.44 Interestingly, we found bilateral periorbital allodynia despite unilateral SD stimulation. Unilateral SD can induce bilateral activation of brain regions relevant for pain. For example, unilateral SD increased c-fos expression bilaterally in periaqueductal gray45 and paraventricular hypothalamus.46 Indeed, migraine-associated cutaneous allodynia develops in a stepwise fashion with progressive sensitization of higher-order (e.g. bilateral) neuronal populations.47 Patients often describe an initial allodynia ipsilateral to cephalgia that later spreads to also involve the contralateral side.8 In one cohort, almost 80% of episodic migraine patients experienced cutaneous allodynia during attacks, and 85% of those reported allodynia in at least one other area outside of the ipsilateral head (33% mechanical allodynia and 55% cold and heat allodynia of the contralateral head).8 Similarly in a different study of 6 allodynic migraine patients, 4 reported sensitivity to cotton swab application to the contralateral face.48 Moreover, a majority (79%) of allodynic migraine patients had aura8 suggesting an association between aura and allodynia.
Repeated SDs also produced signs of anxiety as an affective response to pain where animals avoided an open field (i.e. thigmotaxis scores). These findings are congruent with the clinical observation that migraine is associated with psychiatric disturbances including depression and anxiety disorders.49 Indeed, phobic and panic symptoms and anxiety sensitivity index scores were most strongly associated with migraine aura.50 Anxiety may become manifest as a result of repeated migraine attacks, and thus is a chronic rather than episodic manifestation. Our data support this possibility for anxiety-like behavior. Importantly, the uniform illumination of the open field under dim light (164 lux) makes thigmotaxis upon repeated SDs unlikely to be caused by photophobia. Mechanistically, repeated SDs may modulate cortico-limbic circuits involved in anxiety and fear, such as the anterior cingulate cortex, amygdala, or periaqueductal gray.51–53 It is important to note that SD rarely propagates into subcortical tissues in wild type mice under isoflurane anesthesia;54 therefore, SD-induced anxiety behavior in this paradigm is unlikely to be a direct effect on subcortical centers. Moreover, the very short wavelength of the optogenetic light stimulus (470 nm) precludes deep penetration into the brain tissue for direct activation of subcortical centers.55
Facial expression of discomfort is likely a phylogenetically conserved behavior observed across mammalian species that reflects the negative affective component of pain. This affective pain response likely has specific neuroanatomical substrates, such as limbic and subcortical structures including the mediodorsal thalamus, hypothalamus, anterior cingulate cortex, amygdala and insular cortex.56–58 Specifically, mouse grimace may involve activation of the insular cortex. In one study, ablation of anterior cingulate cortex or amygdala had no effect on visceral pain related grimace while ablation of the rostral anterior insula reduced mouse grimace.29 Therefore, grimace scores may be directly modulated by SDs propagating into the insular cortex as well.
We did not observe overt sexual dimorphism in this model of SD-induced cephalgia, although there was a trend for more severe allodynia in females in pooled analysis. Although females may have higher SD susceptibility compared with males,59–61 we used identical SD burden in both sexes as the denominator. These data suggest that sexual dimorphism in migraine may reflect upstream differences in cortical excitability rather than downstream nociception. Of course, estrus cycle stage may still influence SD-induced allodynia, which we did not examine in this study.
Our study has limitations. First, SD in rodents may produce a more severe pain phenotype because in rodents SD almost always propagates throughout the entire ipsilateral cortex. In humans, SD limited to a small region of the brain may trigger mild headache or none at all. Second, pain thresholds and processing in congenic strains may differ from outbred strains that are more representative of human genetic variability.62 Third, although minimally invasive, the model does require a skin incision a couple days prior. A review of literature suggests that studies with a scalp incision, such as the one used to implant the coverslip a few days prior to testing in our study, generally yield lower thresholds than those without an incision.25, 63–68 In addition, lower absolute thresholds in our study may also be related to the ascending rather than up-down method of allodynia assessment that we used to reduce testing times and avoid sensitization.26, 37, 69 Regardless of the baseline thresholds, however, SD potently induced periorbital allodynia. The model also involves multiple brief exposures to gas anesthesia in the repeated SD cohort. The control (i.e. non-SD) thresholds in the single anesthesia cohort (Figure 2) did not differ from the repeated anesthesia cohort (Figure 3), suggesting that repeated anesthesia does not affect the thresholds. Moreover, sham (i.e. non-SD) groups are exposed to the same anesthesia protocol. Lastly, in the chronic model we repeated SD induction every other day for 2 weeks, to mirror the frequency of attacks in patients experiencing more than 2 attacks per month.24 The impact of less frequent but protracted SD exposure on pain behavior remains to be tested.
Taken together, our data support optogenetic SD-induced allodynia and grimace score as a novel model of aura-related evoked and spontaneous affective trigeminal pain behavior. Modulation of pain as well as anxiety behavior with SD burden underscores its utility to study migraine severity as well. We believe the model is robust and malleable for the exploration of mechanisms of migraine in relation to SD and can be adopted to test abortive and prophylactic drugs, neuromodulation and the impact of other comorbid factors like sleep deprivation, stress, hormones and circadian periodicity on migraine-relevant biology. Future studies may also examine other aspects of migraine pain related behavior including photophobia, phonophobia and other complex social and aversive behaviors.
Acknowledgements:
We thank Isra Tamim for the illustration showing mouse grimace scores. Support is provided to A.H. by the Phyllis and Jerome Lyle Rappaport Foundation, Building Interdisciplinary Careers in Women’s Health Award - (NIH, 5K12HD051959), and the Training in Research for Academic Neurologists to Sustain Careers and Enhance the Numbers of Diverse Scholars (TRANSCENDS) - NINDS/NIH Award through the American Academy of Neurology (R25 NS098999-02); to D.Y.C by the NIH (R25NS065743, , KL2TR002542, and K08NS112601)), American Heart Association (18POST34030369), Brain Aneurysm Foundation, AVM Foundation and Heitman Foundation; to T.T. by Keio University Global Research Institute (KGRI) Pre-Start-up Grant; and to C.A. by the NINDS/NIH (R01NS102969), Foundation Leducq, Heitman Foundation, and the Ellison Foundation Awards.
Footnotes
Potential Conflicts of interest: There are no conflicts of interest to disclose.
References
- 1.Ayata C Cortical spreading depression triggers migraine attack: pro. Headache. 2010. April;50(4):725–30. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, Burstein R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol. 2011. May;69(5):855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goadsby PJ. Migraine, aura, and cortical spreading depression: why are we still talking about it? Ann Neurol. 2001. January;49(1):4–6. [DOI] [PubMed] [Google Scholar]
- 4.Viana M, Sances G, Linde M, et al. Clinical features of migraine aura: Results from a prospective diary-aided study. Cephalalgia. 2017. September;37(10):979–89. [DOI] [PubMed] [Google Scholar]
- 5.Russell MB, Olesen J. A nosographic analysis of the migraine aura in a general population. Brain. 1996. April;119 ( Pt 2):355–61. [DOI] [PubMed] [Google Scholar]
- 6.Lipton RB, Bigal ME, Ashina S, et al. Cutaneous allodynia in the migraine population. Ann Neurol. 2008. February;63(2):148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovati C, D’Amico D, Rosa S, et al. Allodynia in different forms of migraine. Neurol Sci. 2007. May;28 Suppl 2:S220–1. [DOI] [PubMed] [Google Scholar]
- 8.Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. 2000. May;47(5):614–24. [PubMed] [Google Scholar]
- 9.LoPinto C, Young WB, Ashkenazi A. Comparison of dynamic (brush) and static (pressure) mechanical allodynia in migraine. Cephalalgia. 2006. July;26(7):852–6. [DOI] [PubMed] [Google Scholar]
- 10.Lipton RB, Fanning KM, Buse DC, et al. Identifying Natural Subgroups of Migraine Based on Comorbidity and Concomitant Condition Profiles: Results of the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache. 2018. July;58(7):933–47. [DOI] [PubMed] [Google Scholar]
- 11.Moskowitz MA, Nozaki K, Kraig RP. Neocortical spreading depression provokes the expression of c-fos protein-like immunoreactivity within trigeminal nucleus caudalis via trigeminovascular mechanisms. J Neurosci. 1993. March;13(3):1167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melo-Carrillo A, Noseda R, Nir RR, et al. Selective Inhibition of Trigeminovascular Neurons by Fremanezumab: A Humanized Monoclonal Anti-CGRP Antibody. J Neurosci. 2017. July 26;37(30):7149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci. 2010. June 30;30(26):8807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filiz A, Tepe N, Eftekhari S, et al. CGRP receptor antagonist MK-8825 attenuates cortical spreading depression induced pain behavior. Cephalalgia. 2019. March;39(3):354–65. [DOI] [PubMed] [Google Scholar]
- 15.Cottier KE, Galloway EA, Calabrese EC, et al. Loss of Blood-Brain Barrier Integrity in a KCl-Induced Model of Episodic Headache Enhances CNS Drug Delivery. eNeuro. 2018. Jul-Aug;5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosaras B, Jakubowski M, Kainz V, Burstein R. Sensory innervation of the calvarial bones of the mouse. J Comp Neurol. 2009. July 20;515(3):331–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingvardsen BK, Laursen H, Olsen UB, Hansen AJ. Possible mechanism of c-fos expression in trigeminal nucleus caudalis following cortical spreading depression. Pain. 1997. September;72(3):407–15. [DOI] [PubMed] [Google Scholar]
- 18.Fioravanti B, Kasasbeh A, Edelmayer R, et al. Evaluation of cutaneous allodynia following induction of cortical spreading depression in freely moving rats. Cephalalgia. 2011. July;31(10):1090–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sukhotinsky I, Dilekoz E, Wang Y, et al. Chronic daily cortical spreading depressions suppress spreading depression susceptibility. Cephalalgia. 2011. December;31(16):1601–8. [DOI] [PubMed] [Google Scholar]
- 20.Schneider AM, Behar M. A Chronic Preparation for Spreading Cortical Depression. J Exp Anal Behav. 1964. September;7:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houben T, Loonen IC, Baca SM, et al. Optogenetic induction of cortical spreading depression in anesthetized and freely behaving mice. J Cereb Blood Flow Metab. 2017. May;37(5):1641–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung DY, Sadeghian H, Qin T, et al. Determinants of Optogenetic Cortical Spreading Depolarizations. Cereb Cortex. 2018. February 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung DY, Sugimoto K, Fischer P, et al. Real-time non-invasive in vivo visible light detection of cortical spreading depolarizations in mice. J Neurosci Methods. 2018. September 5;309:143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turk WE, Uiterwijk A, Pasmans R, Meys V, Ayata C, Koehler PJ. Aspirin Prophylaxis for Migraine with Aura: An Observational Case Series. Eur Neurol. 2017;78(5–6):287–9. [DOI] [PubMed] [Google Scholar]
- 25.Elliott MB, Oshinsky ML, Amenta PS, Awe OO, Jallo JI. Nociceptive neuropeptide increases and periorbital allodynia in a model of traumatic brain injury. Headache. 2012. June;52(6):966–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deuis JR, Dvorakova LS, Vetter I. Methods Used to Evaluate Pain Behaviors in Rodents. Front Mol Neurosci. 2017;10:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A. Characterization of a novel model of chronic migraine. Pain. 2014. February;155(2):269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akintola T, Raver C, Studlack P, Uddin O, Masri R, Keller A. The grimace scale reliably assesses chronic pain in a rodent model of trigeminal neuropathic pain. Neurobiol Pain. 2017. August;2:13–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langford DJ, Bailey AL, Chanda ML, et al. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010. June;7(6):447–9. [DOI] [PubMed] [Google Scholar]
- 30.Patel TP, Gullotti DM, Hernandez P, et al. An open-source toolbox for automated phenotyping of mice in behavioral tasks. Front Behav Neurosci. 2014;8:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seibenhener ML, Wooten MC. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp. 2015. February 6(96):e52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailey KR, Crawley JN. Anxiety-Related Behaviors in Mice. In: nd, Buccafusco JJ, editors. Methods of Behavior Analysis in Neuroscience. Boca Raton (FL)2009. [Google Scholar]
- 33.King DL, Arendash GW. Behavioral characterization of the Tg2576 transgenic model of Alzheimer’s disease through 19 months. Physiol Behav. 2002. April 15;75(5):627–42. [DOI] [PubMed] [Google Scholar]
- 34.Tietjen GE, Brandes JL, Peterlin BL, et al. Allodynia in migraine: association with comorbid pain conditions. Headache. 2009. October;49(9):1333–44. [DOI] [PubMed] [Google Scholar]
- 35.Lovati C, D’Amico D, Bertora P, et al. Acute and interictal allodynia in patients with different headache forms: an Italian pilot study. Headache. 2008. February;48(2):272–7. [DOI] [PubMed] [Google Scholar]
- 36.Oshinsky ML. Insights from experimental studies into allodynia and its treatment. Curr Pain Headache Rep. 2006. June;10(3):225–30. [DOI] [PubMed] [Google Scholar]
- 37.Harriott AM, Strother LC, Vila-Pueyo M, Holland PR. Animal models of migraine and experimental techniques used to examine trigeminal sensory processing. J Headache Pain. 2019. August 29;20(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harriott AM, Gold MS. Serotonin type 1D receptors (5HTR) are differentially distributed in nerve fibres innervating craniofacial tissues. Cephalalgia. 2008. September;28(9):933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harriott AM, Scheff NN, Gold MS. The complex actions of sumatriptan on rat dural afferents. Cephalalgia. 2012. July;32(10):738–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levy D, Jakubowski M, Burstein R. Disruption of communication between peripheral and central trigeminovascular neurons mediates the antimigraine action of 5HT 1B/1D receptor agonists. Proc Natl Acad Sci U S A. 2004. March 23;101(12):4274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Storer RJ, Goadsby PJ. Microiontophoretic application of serotonin (5HT)1B/1D agonists inhibits trigeminal cell firing in the cat. Brain. 1997. December;120 ( Pt 12):2171–7. [DOI] [PubMed] [Google Scholar]
- 42.Melo-Carrillo A, Strassman AM, Nir RR, et al. Fremanezumab-A Humanized Monoclonal Anti-CGRP Antibody-Inhibits Thinly Myelinated (Adelta) But Not Unmyelinated (C) Meningeal Nociceptors. J Neurosci. 2017. November 1;37(44):10587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karatas H, Erdener SE, Gursoy-Ozdemir Y, et al. Spreading depression triggers headache by activating neuronal Panx1 channels. Science. 2013. March 1;339(6123):1092–5. [DOI] [PubMed] [Google Scholar]
- 44.Oshinsky ML. Sensitization and ongoing activation in the trigeminal nucleus caudalis. Pain. 2014. July;155(7):1181–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borysovych Bogdanov V, Bogdanova OV, Lombard A, et al. Cortical spreading depression decreases Fos expression in rat periaqueductal gray matter. Neurosci Lett. 2015. January 12;585:138–43. [DOI] [PubMed] [Google Scholar]
- 46.Iqbal Chowdhury GM, Liu Y, Tanaka M, Fujioka T, Ishikawa A, Nakamura S. Cortical spreading depression affects Fos expression in the hypothalamic paraventricular nucleus and the cerebral cortex of both hemispheres. Neurosci Res. 2003. February;45(2):149–55. [DOI] [PubMed] [Google Scholar]
- 47.Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000. August;123 ( Pt 8):1703–9. [DOI] [PubMed] [Google Scholar]
- 48.Cooke L, Eliasziw M, Becker WJ. Cutaneous allodynia in transformed migraine patients. Headache. 2007. April;47(4):531–9. [DOI] [PubMed] [Google Scholar]
- 49.Peres MFP, Mercante JPP, Tobo PR, Kamei H, Bigal ME. Anxiety and depression symptoms and migraine: a symptom-based approach research. J Headache Pain. 2017. December;18(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smitherman TA, Davis RE, Walters AB, Young J, Houle TT. Anxiety sensitivity and headache: diagnostic differences, impact, and relations with perceived headache triggers. Cephalalgia. 2015. July;35(8):710–21. [DOI] [PubMed] [Google Scholar]
- 51.Sellmeijer J, Mathis V, Hugel S, et al. Hyperactivity of Anterior Cingulate Cortex Areas 24a/24b Drives Chronic Pain-Induced Anxiodepressive-like Consequences. J Neurosci. 2018. March 21;38(12):3102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis M The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–75. [DOI] [PubMed] [Google Scholar]
- 53.Akcali D, Sayin A, Sara Y, Bolay H. Does single cortical spreading depression elicit pain behaviour in freely moving rats? Cephalalgia. 2010. October;30(10):1195–206. [DOI] [PubMed] [Google Scholar]
- 54.Eikermann-Haerter K, Yuzawa I, Qin T, et al. Enhanced subcortical spreading depression in familial hemiplegic migraine type 1 mutant mice. J Neurosci. 2011. April 13;31(15):5755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aravanis AM, Wang LP, Zhang F, et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. Journal of neural engineering. 2007. September;4(3):S143–56. [DOI] [PubMed] [Google Scholar]
- 56.Borsook D, Veggeberg R, Erpelding N, et al. The Insula: A “Hub of Activity” in Migraine. Neuroscientist. 2016. December;22(6):632–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao YJ, Ren WH, Zhang YQ, Zhao ZQ. Contributions of the anterior cingulate cortex and amygdala to pain- and fear-conditioned place avoidance in rats. Pain. 2004. July;110(1–2):343–53. [DOI] [PubMed] [Google Scholar]
- 58.Maizels M, Aurora S, Heinricher M. Beyond neurovascular: migraine as a dysfunctional neurolimbic pain network. Headache. 2012. Nov-Dec;52(10):1553–65. [DOI] [PubMed] [Google Scholar]
- 59.Brennan KC, Romero Reyes M, Lopez Valdes HE, Arnold AP, Charles AC. Reduced threshold for cortical spreading depression in female mice. Ann Neurol. 2007. June;61(6):603–6. [DOI] [PubMed] [Google Scholar]
- 60.Eikermann-Haerter K, Dilekoz E, Kudo C, et al. Genetic and hormonal factors modulate spreading depression and transient hemiparesis in mouse models of familial hemiplegic migraine type 1. J Clin Invest. 2009. January;119(1):99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eikermann-Haerter K, Baum MJ, Ferrari MD, van den Maagdenberg AM, Moskowitz MA, Ayata C. Androgenic suppression of spreading depression in familial hemiplegic migraine type 1 mutant mice. Ann Neurol. 2009. October;66(4):564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mogil JS. The genetic mediation of individual differences in sensitivity to pain and its inhibition. Proc Natl Acad Sci U S A. 1999. July 6;96(14):7744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Macolino CM, Daiutolo BV, Albertson BK, Elliott MB. Mechanical allodynia induced by traumatic brain injury is independent of restraint stress. J Neurosci Methods. 2014. April 15;226:139–46. [DOI] [PubMed] [Google Scholar]
- 64.Daiutolo BV, Tyburski A, Clark SW, Elliott MB. Trigeminal Pain Molecules, Allodynia, and Photosensitivity Are Pharmacologically and Genetically Modulated in a Model of Traumatic Brain Injury. J Neurotrauma. 2016. April 15;33(8):748–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Z, Wang Q, Wang C, Xu X, Yu H. Tetramethylpyrazine attenuates periorbital allodynia and neuroinflammation in a model of traumatic brain injury. J Inflamm (Lond). 2017;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ben Aissa M, Tipton AF, Bertels Z, et al. Soluble guanylyl cyclase is a critical regulator of migraine-associated pain. Cephalalgia. 2018. July;38(8):1471–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moye LS, Novack ML, Tipton AF, Krishnan H, Pandey SC, Pradhan AA. The development of a mouse model of mTBI-induced post-traumatic migraine, and identification of the delta opioid receptor as a novel therapeutic target. Cephalalgia. 2019. January;39(1):77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Logu F, Landini L, Janal MN, et al. Migraine-provoking substances evoke periorbital allodynia in mice. J Headache Pain. 2019. February 14;20(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vuralli D, Wattiez AS, Russo AF, Bolay H. Behavioral and cognitive animal models in headache research. J Headache Pain. 2019. January 31;20(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]


