Abstract
OBJECTIVES
This study investigates the prevalence and prognostic significance of mitral regurgitation (MR) in acute decompensated heart failure (ADHF) patients.
BACKGROUND
Few studies characterize the burden of MR in heart failure.
METHODS
The ARIC (Atherosclerosis Risk In Communities) study surveilled ADHF hospitalizations for residents ≥55 years of age in 4 U.S. communities. ADHF cases were stratified by left ventricular ejection fraction (LVEF): <50% and ≥50%. Odds of moderate or severe MR in patients with varying sex and race, and odds of 1-year mortality in those with higher MR severity were estimated using multivariable logistic regression.
RESULTS
From 2005 to 2014, there were 17,931 weighted ADHF hospitalizations of which 49.2% had an LVEF <50% and 50.8% an LVEF ≥50%. Moderate or severe MR prevalence was 44.5% in those with an LVEF <50% and 27.5% in those with an LVEF ≥50%. Moderate or severe MR was more likely in females than males regardless of LVEF; LVEF <50% (odds ratio [OR]: 1.21 [95% confidence interval (CI): 1.11 to 1.33]), LVEF ≥50% (OR: 1.52 [95% CI: 1.36 to 1.69]). Among hospitalizations with an LVEF ≥50%, moderate or severe MR was less likely in blacks than whites (OR: 0.7% CI: 0.64 to 0.82]). Higher MR severity was independently associated with increased 1-year mortality in those with an LVEF <50% (OR: 1.30 [95% CI: 1.16 to 1.45]).
CONCLUSIONS
Patients with ADHF have a significant MR burden that varies with sex and race. In ADHF patients with an LVEF <50%, higher MR severity is associated with excess 1-year mortality. (J Am Coll Cardiol HF 2021;9:179–89)
Keywords: ADHF, Atherosclerosis Risk In Communities study, HF, mortality, MR
Acute decompensated heart failure (ADHF) is a leading cause of hospitalization in residents of the United States who are ≥65 years of age (1). As such, understanding the factors that contribute to poor outcomes in heart failure (HF) patients is of growing importance (2). Investigation of community-based populations has identified mitral regurgitation (MR) as the most commonly diagnosed valvular disease in the United States (3). Prior analyses have suggested that there is an association between MR and decreased survival in those with HF (4–11). In aggregate, these studies indicate that moderate or severe MR may be a predictor of mortality in both acute and chronic HF with a prevalence ranging from 17% to 53%.
MR is an emerging therapeutic target in HF with reduced left ventricular ejection fraction (LVEF) (12), necessitating better characterization of its burden in “real-world” settings. Whether there is variation in the presence of MR by sex and race is unknown, and few studies have determined the prevalence and prognostic significance of MR in HF with an LVEF ≥50% (10,11). Furthermore, the majority of available analyses investigating MR in HF primarily enrolled male participants of European ancestry and therefore have limited generalizability to a diverse U.S. population.
The ARIC (Atherosclerosis Risk In Communities) study is a biracial analysis that included investigations into ADHF admissions from 4 U.S. communities. We sought to examine ADHF hospitalizations in the ARIC study to identify sex and racial differences in the burden of MR. We also explored the association between severity of MR and 1-year mortality in ADHF hospitalizations stratified by LVEF.
METHODS
STUDY DESIGN AND POPULATION.
From 2005 through 2014, the HF Community Surveillance component of the ARIC study performed continuous, community-based surveillance of HF hospitalizations for residents ≥55 years of age in 4 geographically defined U.S. communities: Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota, suburbs; and Washington County, Maryland. Institutional review boards approved the ARIC study protocol at all participating hospitals, and no informed consent was required. Details of the HF Community Surveillance methods in the ARIC study have been previously described (13). Briefly, a stratified random sample of all eligible HF hospitalizations from 2005 to 2014 (unweighted n = 23,410) were selected based on 3 criteria: 1) International Classification of Diseases-9th Revision-Clinical Modification discharge diagnosis codes for HF or HF-related conditions (398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 415.0, 416.9, 425.4, 428.x, 518.4, 786.0x) in any position; 2) ≥55 years of age at the time of hospital discharge; and 3) home address within the boundaries of the ARIC communities. Computer algorithm, described previously (13), or 1 to 2 physicians of the ARIC Mortality and Morbidity Classification Committee then independently classified cases into 1 of 5 categories: 1) definite ADHF; 2) probable ADHF; 3) chronic stable HF; 4) HF unlikely; or 5) unclassifiable. Disagreements were adjudicated by a board-certified HF specialist serving as the chair of the Mortality and Morbidity Classification Committee. Definite and probable ADHF hospitalizations required evidence derived from signs, symptoms, imaging, treatment of an acute exacerbation, worsening or new onset of symptoms, or other decompensated circulatory state.
For the purpose of this report, definite or probable ADHF hospitalizations (unweighted n = 9,139) were eligible for inclusion (Figure 1). Of these hospitalizations, those without echocardiogram data available from their abstracted hospitalization (unweighted n = 4,702), those with any history of heart valve surgery or intervention (unweighted n = 349), those with missing LVEF data (unweighted n = 164), and those with missing MR categorization data (unweighted n = 46) were excluded in a stepwise fashion. The final sample included 3,878 (weighted n = 17,931) validated ADHF hospitalizations from 2005 to 2014.
FIGURE 1. Flow Diagram of Eligible Hospitalizations and the Final Study Sample.
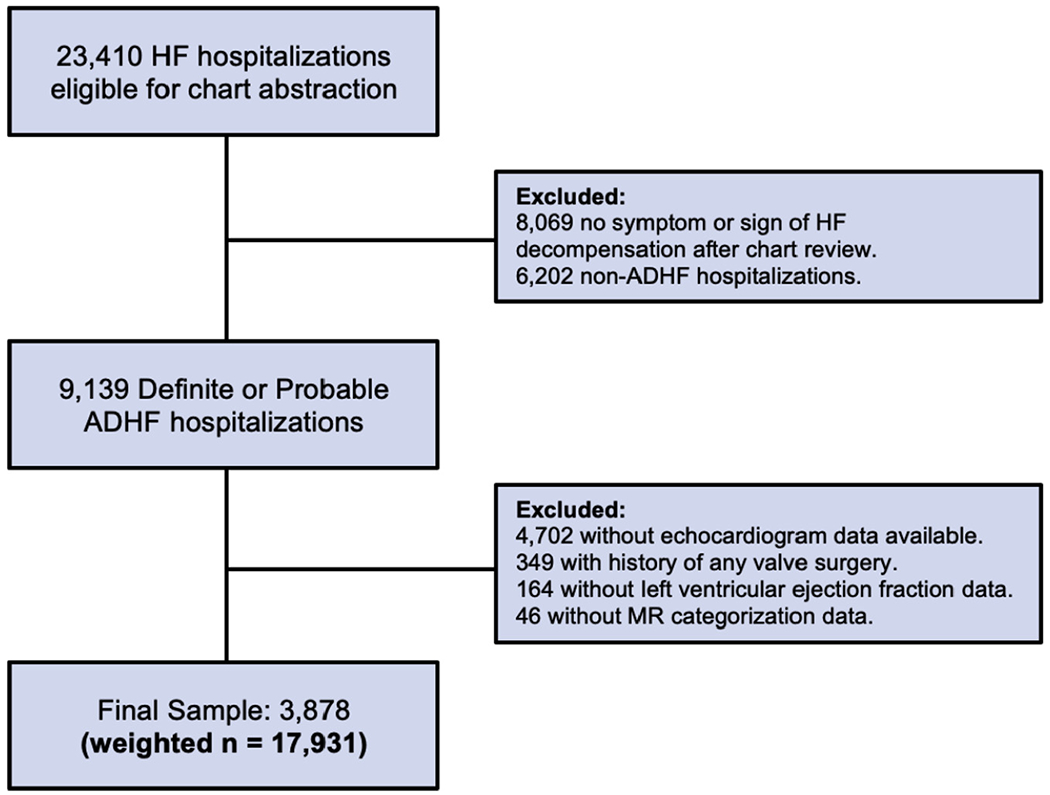
ALL values are unweighted, unless otherwise indicated. ADHF = acute decompensated heart failure; HF = heart failure; MR = mitral regurgitation.
COVARIATES, DEMOGRAPHICS, AND CLASSIFICATIONS.
Trained abstractors recorded all charted demographic and covariate data including age, sex, race, hypertension, diabetes mellitus, atrial fibrillation, myocardial infarction, coronary artery disease, tobacco smoking, hemoglobin value (g/dl), stroke, hemodialysis, chronic obstructive pulmonary disease, angiotensin-converting enzyme (ACE) inhibitor use, angiotensin receptor blocker (ARB) use, beta-blocker use, worst in-hospital blood urea nitrogen (mg/dl), and admission systolic blood pressure (mm Hg). Atrial fibrillation was defined as present based on 3 criteria: 1) past medical history; 2) electrocardiogram reading obtained during index hospitalization; or 3) International Classification of Diseases-9th Revision-Clinical Modification discharge diagnosis code 427.3. Any abstracted history of heart valve surgery or intervention was used by the present analysis for exclusion purposes. MR severity (none, mild, moderate, or severe) was abstracted from available echocardiogram reports per predefined ARIC HF Community Surveillance Study criteria (14). For the purposes of this analysis, ACE inhibitor or ARB use was combined into a single category. Anemia was defined as a hemoglobin <12 g/dl for females and <13 g/dl for males. ADHF cases were stratified into 2 groups based on LVEF recorded during their abstracted hospitalization: LVEF <50% and LVEF ≥50%. All-cause 1-year mortality outcomes after ADHF hospitalization were available through National Death Index linkage data.
STATISTICAL ANALYSIS.
The ARIC HF Community Surveillance Study implemented a stratified sampling design (13). HF hospitalizations were randomly sampled within 5 pre-specified strata: sex, race, center, discharge code group, and date of discharge. However, hospitalizations varied in their probability of being observed across these strata. Statistical weighting was necessary to account for these variations and allow the surveilled sample to estimate the intended study population. Per standard ARIC HF Community Surveillance methodology (13), hospitalizations in the present analysis were weighted by the inverse of the sampling probabilities (15). All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, North Carolina).
Demographics and characteristics of patients stratified by LVEF (LVEF <50% and LVEF ≥50%) were compared by MR severity (none or mild MR vs. moderate or severe MR). The prevalence of moderate or severe MR in 4 age categories (55 to 65, 65 to 75, 75 to 85, and ≥85 years of age) was compared by LVEF stratification (LVEF <50% and LVEF ≥50%). Categorical variables were compared using chi-square tests. Continuous variables were assessed for normality and compared using t-tests. Statistical significance was defined as p < 0.05. Sex, race, and mortality analyses were performed using multivariable logistic regression.
In sex and race analyses, higher MR severity (defined as moderate or severe MR) was modeled as the outcome variable. Sex and race were individually modeled as predictor variables to estimate each of their associations with higher MR severity in ADHF hospitalizations stratified by LVEF (LVEF <50% and LVEF ≥50%). Crude, minimally adjusted, and fully adjusted models were developed to assess these associations. Minimally adjusted models adjusted for age, sex (omitted in sex comparisons), race (omitted in race comparisons), diabetes mellitus, and hypertension. Fully adjusted models adjusted for age, sex (omitted in sex comparisons), race (omitted in race comparisons), diabetes mellitus, hypertension, tobacco smoking, atrial fibrillation, coronary artery disease, stroke, hemodialysis, anemia, and LVEF (omitted in LVEF ≥50% models). Females were compared with males in sex comparisons, and blacks with whites in race comparisons.
In mortality analyses, all-cause 1-year mortality was modeled as the outcome variable. MR severity (moderate or severe MR compared with none or mild MR) was modeled as the predictor variable to estimate the association between MR severity and all-cause 1-year mortality in ADHF hospitalizations stratified by LVEF (LVEF <50% and LVEF ≥50%). Crude, minimally adjusted, and fully adjusted models were developed to assess these associations. Minimally adjusted models adjusted for age, sex, race, diabetes mellitus, and hypertension. Fully adjusted models adjusted for age, sex, race, diabetes mellitus, hypertension, tobacco smoking, atrial fibrillation, coronary artery disease, stroke, hemodialysis, anemia, ACE inhibitor/ARB use, beta-blocker use, worst in-hospital blood urea nitrogen, admission systolic blood pressure, and LVEF (omitted in LVEF ≥50% models).
RESULTS
DEMOGRAPHICS AND CHARACTERISTICS.
Overall, 17,931 weighted ADHF hospitalizations occurred from 2005 to 2014 in those ≥55 years of age. Of all patients with ADHF, 49.2% had an LVEF <50% (44.7% female, 34.1% black) and 50.8% had an LVEF ≥50% (65.7% female, 26.8% black) (Table 1). Moderate or severe MR was present in 44.5% of those with an LVEF <50% and 27.5% of those with an LVEF ≥50% (Table 1). Among patients with an LVEF <50%, those with moderate or severe MR were older than those with none or mild MR and more likely to have a history of atrial fibrillation (Table 1). However, those with moderate or severe MR had a lower admission systolic blood pressure and were less likely to have a history of hypertension, diabetes mellitus, coronary artery disease, tobacco smoking, stroke, hemodialysis, chronic obstructive pulmonary disease, and ACE inhibitor/ARB use (Table 1). No differences in myocardial infarction, anemia, beta-blocker use, or worst in-hospital blood urea nitrogen were detected between MR comparison groups in those with an LVEF <50% (Table 1).
TABLE 1.
Demographics and Characteristics of Patients Hospitalized for ADHF, Stratified by LVEF and MR Severity
| LVEF <50% (Weighted n = 8,827) |
LVEF ≥50% (Weighted n = 9,104) |
|||||
|---|---|---|---|---|---|---|
| None or Mild MR (n = 4,901) |
Moderate or Severe MR (n = 3,926) |
p Value | None or Mild MR (n = 6,603) |
Moderate or Severe MR (n = 2,501) |
p Value | |
| Demographics | ||||||
| Age, yrs | 74.0 ± 0.3 | 76.9 ± 0.4 | <0.0001 | 75.6 ± 0.3 | 80.3 ± 0.4 | <0.0001 |
| Female | 2,068 (42.2) | 1,877 (47.8) | <0.0001 | 4,176 (63.2) | 1,807 (72.3) | <0.0001 |
| Black | 1,720 (36.4) | 1,290 (34.0) | 0.02 | 1,987 (31.3) | 453 (18.9) | <0.0001 |
| Medical history | ||||||
| Hypertension | 4,239 (86.5) | 3,259 (83.0) | <0.0001 | 5,823 (88.2) | 2,192 (87.7) | 0.49 |
| Diabetes mellitus | 2,529 (51.6) | 1,653 (42.1) | <0.0001 | 3,459 (52.4) | 970 (38.9) | <0.0001 |
| Atrial fibrillation | 1,688 (34.4) | 1,572 (40.1) | <0.0001 | 2,398 (36.3) | 1,361 (54.4) | <0.0001 |
| Myocardial infarction | 1,345 (27.5) | 1,145 (29.2) | 0.08 | 1,096 (16.6) | 486 (19.4) | 0.001 |
| Coronary artery disease | 3,262 (66.6) | 2,530 (64.4) | 0.04 | 3,319 (50.3) | 1,385 (55.4) | <0.0001 |
| Tobacco smoking | 839 (17.1) | 600 (15.3) | 0.02 | 818 (12.4) | 183 (7.3) | <0.0001 |
| Anemia | 84 (1.7) | 54 (1.4) | 0.23 | 62 (0.9) | 56 (2.3) | <0.0001 |
| Stroke | 1,017 (20.8) | 722 (18.4) | 0.005 | 1,232 (18.7) | 547 (21.9) | 0.0006 |
| Hemodialysis | 438 (9.0) | 210 (5.3) | <0.0001 | 415 (6.3) | 116 (4.6) | 0.003 |
| COPD | 1,442 (29.4) | 995 (25.3) | <0.0001 | 2,328 (35.3) | 835 (33.4) | 0.09 |
| Medications | ||||||
| ACE inhibitors/ARBs | 2,580 (56.3) | 1,961 (52.4) | 0.0003 | 2,959 (46.6) | 974 (40.4) | <0.0001 |
| Beta-blockers | 3,639 (79.3) | 2,944 (78.3) | 0.27 | 4,145 (65.2) | 1,665 (69.1) | 0.0005 |
| Labs and vitals | ||||||
| Blood urea nitrogen*, mg/dl | 43.9 ± 0.8 | 43.0 ± 0.9 | 0.45 | 40.6 ± 0.6 | 40.1 ± 1.0 | 0.67 |
| Systolic blood pressure on admission, mm Hg | 141.6 ± 1.1 | 136.9 ± 1.1 | 0.002 | 149.0 ± 0.9 | 141.4 ± 1.3 | <0.0001 |
| Mortality | ||||||
| All-cause 1-yr mortality | 1,436 (29.3) | 1,331 (33.9) | <0.0001 | 1,801 (27.3) | 752 (30.1) | 0.008 |
Values are mean ± SE or n (%). The p values in bold are significant.
Worst in-hospital blood urea nitrogen.
ACE = angiotensin-converting enzyme; ADHF = acute decompensated heart failure; ARB = angiotensin receptor blocker; COPD = chronic obstructive pulmonary disease; LVEF = left ventricular ejection fraction; MR = mitral regurgitation.
Among patients with an LVEF ≥50%, those with moderate or severe MR were older than those with none or mild MR and more likely to have a history of atrial fibrillation, myocardial infarction, coronary artery disease, anemia, stroke, and beta-blocker use (Table 1). However, those with moderate or severe MR had a lower admission systolic blood pressure and were less likely to have a history of diabetes mellitus, tobacco smoking, hemodialysis, and ACE inhibitor/ARB use (Table 1). No differences in hypertension, chronic obstructive pulmonary disease, or worst inhospital blood urea nitrogen were detected between MR comparison groups in those with an LVEF ≥50% (Table 1).
In both patients with an LVEF <50% (Figure 2A) and ≥50% (Figure 2B), the prevalence of moderate or severe MR progressively increased with age category (55 to 65, 65 to 75, 75 to 85, and ≥85 years of age). Moderate or severe MR was significantly more likely in patients with an LVEF <50% than in those with an LVEF ≥50% in all age categories (p < 0.0001 for all) (Figure 2).
Figure 2. Prevalence of Moderate or Severe MR in Patients Admitted for ADHF Stratified by Age Group.
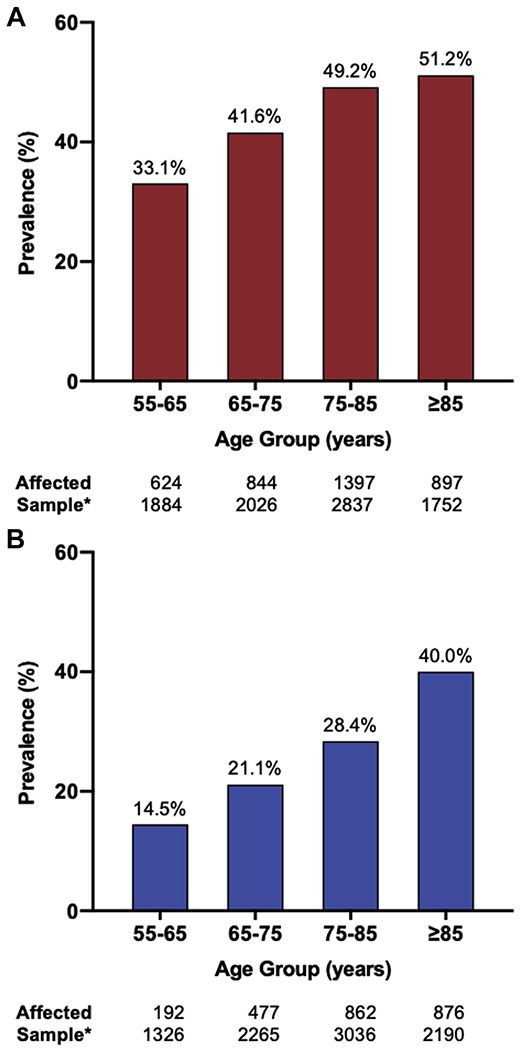
(A) ADHF patients with LVEF <50% and (B) ADHF patients with LVEF ≥50%. *Weighted ADHF hospitalizations for which age data were available. ADHF = acute decompensated heart failure; LVEF = left ventricular ejection fraction.
SEX AND RACE ANALYSES.
Table 2 summarizes the models for odds of moderate or severe MR in ADHF patients with varying sex and race, stratified by LVEF. In unadjusted analyses, moderate or severe MR was more likely in females than males in those with an LVEF <50%, and in those with an LVEF ≥50% (p < 0.0001 for both) (Tables 1 and 2). Fully adjusted models yielded similar findings with moderate or severe MR being more likely in females than males in both patients with an LVEF <50% (odds ratio [OR]: 1.21 [95% confidence interval (CI): 1.11 to 1.33]) and in those with an LVEF ≥50% (OR: 1.52 [95% CI: 1.36 to 1.69]) (Table 2, Figure 3).
TABLE 2.
Odds of Moderate or Severe MR in Patients Admitted for ADHF in Females Compared With Males and in Blacks Compared With Whites, Stratified by LVEF
| Odds of Moderate or Severe MR |
||||||
|---|---|---|---|---|---|---|
| Crude |
Minimally Adjusted* |
Fully Adjusted† |
||||
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Female‡ | ||||||
| LVEF <50% | 1.25 (1.15–1.37) | <0.0001 | 1.17 (1.07–1.27) | 0.0007 | 1.21 (1.11–1.33) | <0.0001 |
| LVEF ≥50% | 1.51 (1.37–1.67) | <0.0001 | 1.42 (1.28–1.58) | <0.0001 | 1.52 (1.36–1.69) | <0.0001 |
| Black§ | ||||||
| LVEF <50% | 0.90 (0.82–0.98) | 0.02 | 1.13 (1.02–1.24) | 0.02 | 1.08 (0.98–1.20) | 0.13 |
| LVEF ≥50% | 0.51 (0.46–0.58) | <0.0001 | 0.66 (0.58–0.74) | <0.0001 | 0.72 (0.64–0.82) | <0.0001 |
The p values in bold are significant.
Adjusted for age, sex (omitted in sex comparisons), race (omitted in race comparisons), diabetes mellitus, and hypertension.
Adjusted for age, sex (omitted in sex comparisons), race (omitted in race comparisons), diabetes mellitus, hypertension, tobacco smoking, atrial fibrillation, coronary artery disease, stroke, hemodialysis, anemia, and LVEF (omitted in LVEF ≥50% models).
Females compared with males in sex comparisons.
Blacks compared with whites in race comparisons.
CI = confidence interval; OR = odds ratio; other abbreviations as in Table 1.
Figure 3. Odds of Moderate or Severe MR in Patients With Varying Demographics, and Odds of All-Cause 1-Year Mortality in Patients With Varying MR Severity.
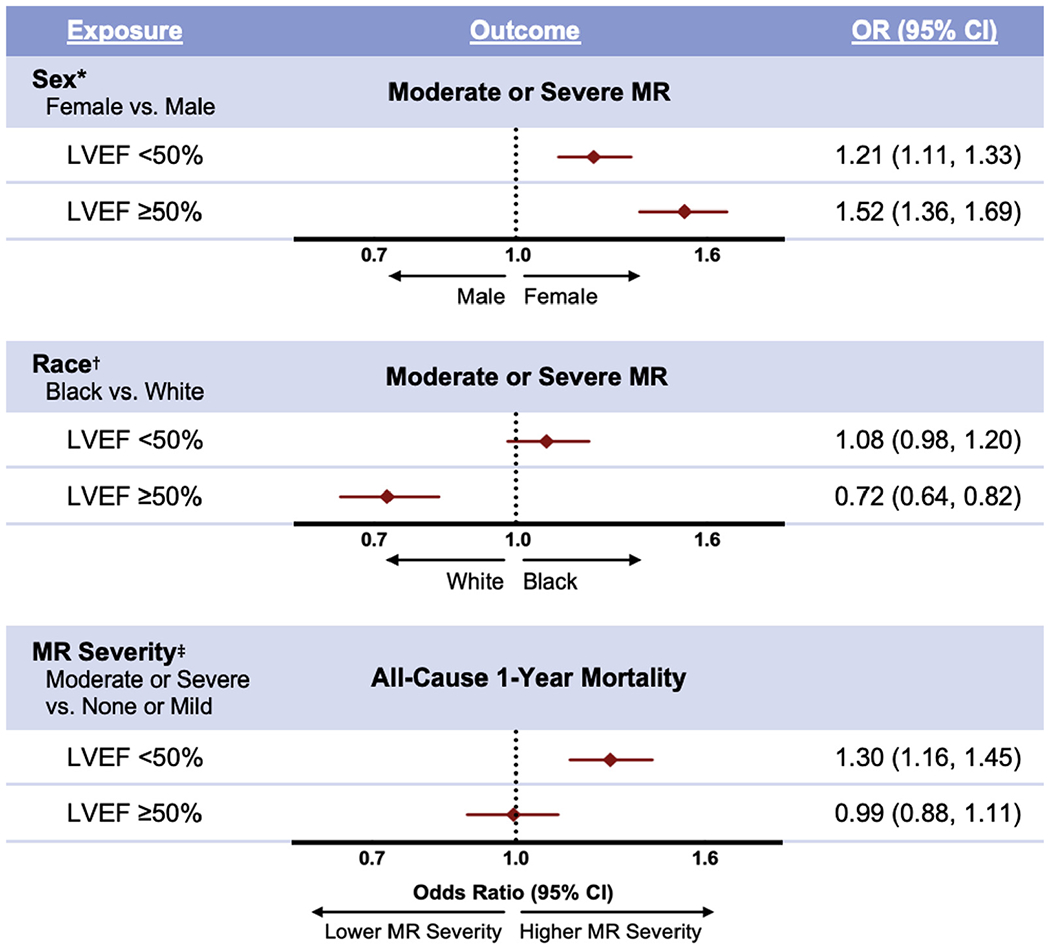
Patients were admitted for acute decompensated heart failure and were stratified by left ventricular ejection fraction. *Adjusted for age, race, diabetes mellitus, hypertension, tobacco smoking, atrial fibrillation, coronary artery disease, stroke, hemodialysis, anemia, and LVEF (omitted in LVEF ≥50% model). †Adjusted for age, sex, diabetes mellitus, hypertension, tobacco smoking, atrial fibrillation, coronary artery disease, stroke, hemodialysis, anemia, and LVEF (omitted in LVEF ≥50% model). ‡Adjusted for age, sex, race, diabetes mellitus, hypertension, tobacco smoking, atrial fibrillation, coronary artery disease, stroke, hemodialysis, anemia, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use, beta-blocker use, worst in-hospital blood urea nitrogen, admission systolic blood pressure, and LVEF (omitted in LVEF ≥50% model). CI = confidence interval; OR = odds ratio; other abbreviations as in Figures 1 and 2.
In unadjusted analyses, moderate or severe MR was more likely in whites than blacks in patients with an LVEF <50% (p = 0.02), and in those with an LVEF ≥50% (p < 0.0001) (Tables 1 and 2). In fully adjusted models, no difference in MR severity was detected between blacks and whites in patients with an LVEF <50% (OR: 1.08 [95% CI: 0.98 to 1.20]), but moderate or severe MR was more likely in whites than blacks in those with an LVEF ≥50% (OR: 0.72 [95% CI: 0.64 to 0.82]) (Table 2, Figure 3).
MORTALITY ANALYSES.
All-cause 1-year mortality after ADHF hospitalization was observed in 31.3% of patients with an LVEF <50% and 28.0% of those with an LVEF ≥50% (Table 1). Table 3 summarizes the models for odds of all-cause 1-year mortality after ADHF hospitalization in patients with higher (moderate or severe) compared with lower (none or mild) MR severity, stratified by LVEF. In unadjusted analyses, all-cause 1-year mortality was more likely in those with higher MR severity in both patients with an LVEF <50% (p < 0.0001) and those with an LVEF ≥50% (p = 0.008) (Tables 1 and 3). In fully adjusted models, all-cause 1-year mortality was more likely in patients with higher MR severity among those with an LVEF <50% (OR: 1.30 [95% CI: 1.16 to 1.45]). No difference in all-cause 1-year mortality was detected in patients with varying MR severity among those with an LVEF ≥50% (OR: 0.99 [95% CI: 0.88 to 1.11]) (Table 3, Figure 3, Central Illustration).
TABLE 3.
Odds of All-Cause 1-Year Mortality in Patients With Varying MR Severity Admitted for ADHF, Stratified by LVEF
| Odds of All-Cause 1-Year Mortality |
||||||
|---|---|---|---|---|---|---|
| Crude |
Minimally Adjusted* |
Fully Adjusted† |
||||
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| LVEF <50% | 1.24 (1.13–1.36) | <0.0001 | 1.18 (1.07–1.29) | 0.0007 | 1.30 (1.16–1.45) | <0.0001 |
| LVEF ≥50% | 1.15 (1.04–1.27) | 0.008 | 1.05 (0.94–1.17) | 0.37 | 0.99 (0.88–1.11) | 0.81 |
Patients with higher MR severity (moderate or severe MR) were compared with those with none or mild MR. The p values in bold are significant.
Adjusted for age, sex, race, diabetes mellitus, and hypertension.
Adjusted for age, sex, race, diabetes mellitus, hypertension, tobacco smoking, atrial fibrillation, coronary artery disease, stroke, hemodialysis, anemia, angiotensin-converting enzyme or angiotensin receptor blocker use, beta-blocker use, worst in-hospital blood urea nitrogen, admission systolic blood pressure, and LVEF (omitted in LVEF ≥50% models).
CENTRAL ILLUSTRATION. Prevalence, Sex and Race Differences, and Prognostic Significance of Mitral Regurgitation in Patients Admitted for Acute Decompensated Heart Failure, Stratified by Left Ventricular Ejection Fraction.
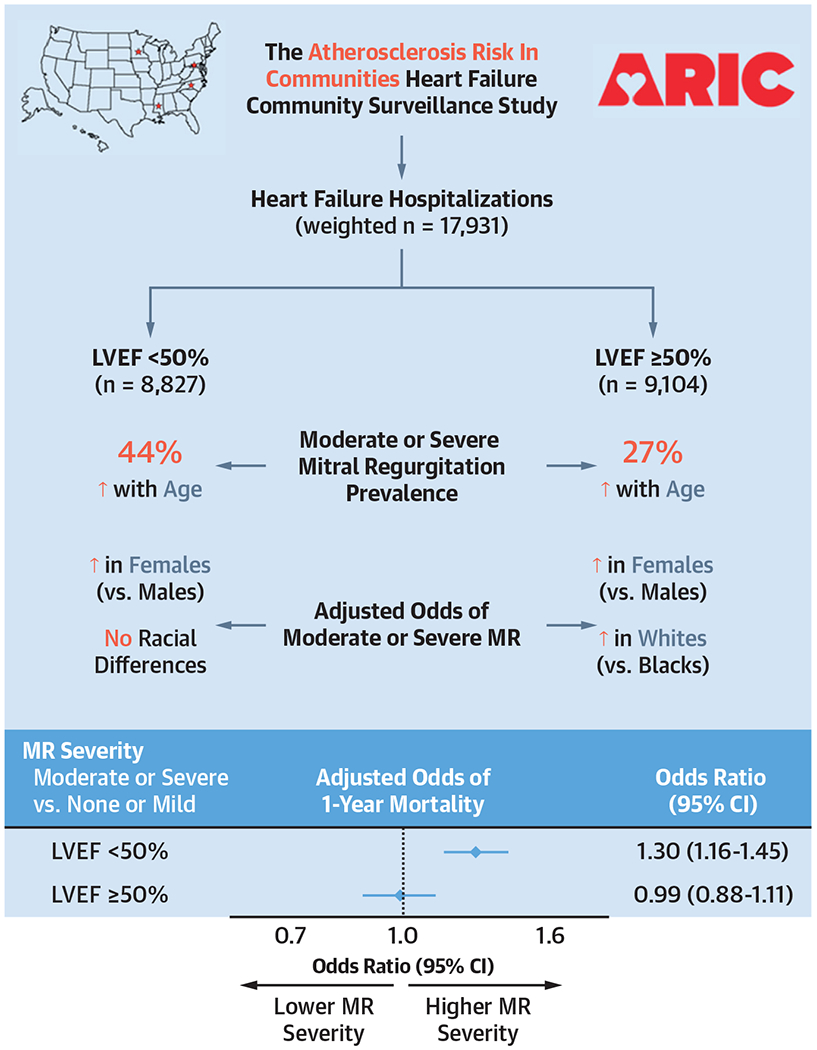
Sex analyses adjusted for age, race, diabetes mellitus, hypertension, tobacco smoking, atrial fibrillation, coronary artery disease, stroke, hemodialysis, anemia, and LVEF (omitted in LVEF ≥50% model). Race analyses adjusted for age, sex, diabetes mellitus, hypertension, tobacco smoking, atrial fibrillation, coronary artery disease, stroke, hemodialysis, anemia, and LVEF (omitted in LVEF ≥50% model). Mortality analyses adjusted for age, sex, race, diabetes mellitus, hypertension, tobacco smoking, atrial fibrillation, coronary artery disease, stroke, hemodialysis, anemia, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use, beta-blocker use, worst in-hospital blood urea nitrogen, admission systolic blood pressure, and LVEF (omitted in LVEF ≥50% model). CI = confidence interval; LVEF = left ventricular ejection fraction; MR = mitral regurgitation; OR = odds ratio.
DISCUSSION
We observed several important findings in this ARIC HF Community Surveillance Study. First, patients with ADHF had a considerable burden of moderate or severe MR, which increased with age and varied with LVEF. Second, females were more likely than males to have moderate or severe MR, regardless of LVEF. Third, among those with an LVEF ≥50%, moderate or severe MR was more likely in whites than blacks. Finally, moderate or severe MR was independently associated with mortality at 1 year after hospitalization in ADHF patients with an LVEF <50%, but not in those with an LVEF ≥50%. The findings of this study are summarized in Table 4.
Table 4.
Overview of Study Findings
| Findings in the Present Analysis of ADHF Patients |
|---|
| Moderate or severe MR prevalence among the ADHF population stratified by LVEF is: LVEF <50%: 44% LVEF ≥50%: 27% |
| Females are more likely than males to have higher MR severity, regardless of LVEF. |
| Among ADHF patients with an LVEF ≥50%, moderate or severe MR is more likely in White patients than in black patients. |
| Moderate or severe MR is independently associated with all-cause mortality at 1 yr after hospitalization in ADHF patients with an LVEF <50%, but not in those with an LVEF ≥50%. |
Abbreviations as in Table 1.
We report a prevalence of MR in patients with an LVEF <50% that falls within the range of previously reported observations in the United States and Europe (4–8,11,16). The ARIC study included hospital surveillance of ADHF admissions in a biracial population with a relatively equal representation of both sexes across all 4 U.S. field centers. Thus, our results are more reliably generalized to the U.S. population. Furthermore, the majority of prior investigations did not stratify their results by LVEF, resulting in a paucity of data on the burden of MR in HF patients with an LVEF ≥50% (10,17). We address this gap in the published reports by providing data on the prevalence of MR in a community-based U.S. population stratified by LVEF.
Known contributors to secondary MR include left ventricular remodeling due to ischemic or nonischemic cardiomyopathy, and mitral annular dilation due to atrial fibrillation (12). In the present analysis, moderate or severe MR was present to a greater degree in patients with an LVEF <50%. Additionally, myocardial infarction and coronary artery disease in those with an LVEF ≥50%, and atrial fibrillation in all patients with ADHF regardless of LVEF were more likely in those with higher MR severity. Although incapable of providing granularity to distinguish true MR etiology, these findings suggest that a substantial portion of the MR observed in this analysis may have been secondary in nature. With respect to age, older patients in our analysis had a higher moderate or severe MR burden than did their younger counterparts. Severity of MR likely worsens with increasing age and progression of HF disease course.
Few studies of patients with HF have provided evidence of sex differences in MR burden in patients stratified by LVEF. Dziadzko et al. (17) recently conducted an observational community cohort analysis of 1,294 moderate or severe MR patients in Olmsted County and found that females were more likely to have a preserved rather than a reduced LVEF. Trichon et al. (4) previously analyzed 2,057 patients with HF in the Duke Cardiovascular Databank and observed that among those with a reduced LVEF, patients with moderate or severe MR were more likely to be female. The present analysis reports that among patients with ADHF, females are more likely than males to have moderate or severe MR regardless of LVEF. Vassileva et al. (18) recently investigated >47,000 patients in the Centers for Medicare & Medicaid Services database undergoing isolated mitral valve operations and found that among patients with mitral valve pathology, females had worse outcomes than males but also presented for repair at later stages of mitral valve disease. In this setting, our finding of females being more likely than males to have moderate or severe MR in ADHF warrants further investigation to elucidate underlying mechanisms contributing to sex differences in the burden of MR.
No race differences in MR severity were detected among those with an LVEF <50%. However, Whites were more likely than Blacks to have moderate or severe MR in those with an LVEF ≥50%. Race differences in valvular pathology, specifically aortic stenosis, have become a topic of interest in the published reports. Recent analyses have evaluated cardiac and genetic risk factors that underlie the increased prevalence of aortic stenosis in White compared with black patients (19). However, no such investigations have been conducted for MR. Our study observes that among ADHF patients with an LVEF ≥50%, Whites are more likely than Blacks to have higher MR severity. Thus, we highlight the need for future studies characterizing the etiology of this race difference in MR burden.
Although a growing body of evidence suggests that MR is an independent predictor of mortality in HF patients with a reduced LVEF (12), there is limited and conflicting evidence regarding survival in HF patients with an LVEF ≥50% with higher MR severity (10,11). Our study is the largest to date in the United States that examines MR in ADHF and provides strong evidence for an independent contribution of MR to increased all-cause 1-year mortality in ADHF patients with an LVEF <50%. We also report that in ADHF patients with an LVEF ≥50%, there is no increased likelihood of all-cause 1-year mortality in those with higher as opposed to lower MR severity. Thereby, we add evidence against a significant prognostic role of MR in ADHF patients with an LVEF ≥50%.
Currently, guideline-directed medical management and cardiac resynchronization therapy hold Class I recommendations for managing MR in patients with HF (20). Surgical and transcatheter options for managing MR in patients with HF are rapidly evolving fields of investigation. The COAPT trial (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation) recently reported improved mortality in HF patients with severe secondary MR receiving transcatheter mitral valve repair as opposed to medical therapy alone (21). However, the MITRA-FR trial (Multicentre Study of Percutaneous Mitral Valve Repair MitraClip Device in Patients With Severe Secondary Mitral Regurgitation) performed a similar analysis and reported no mortality benefit for percutaneous mitral valve repair (22). The Cardiothoracic Surgical Trials Network recently conducted a trial determining that in those with moderate ischemic MR undergoing coronary artery bypass surgery, the randomized addition of surgical mitral valve repair did not improve comparative 2-year survival (23). Our study contributes an improved understanding of the prognostic implications of MR in the ADHF population. Future analyses should determine whether moderate or severe MR patients admitted for ADHF with an LVEF <50% may derive benefit from targeted MR interventions.
STUDY LIMITATIONS.
The ARIC HF Community Surveillance Study is a biracial analysis of 4 U.S. communities and does not sample other racial and ethnic demographics. Further, because 2 of the included communities, Minneapolis, Minnesota, and Washington County, Maryland, were largely made up of White patients, comparisons of Blacks and Whites in our study may be confounded by geographic location. Etiologic information to dissect causes of MR (primary, secondary, or tertiary) was not available. Left bundle branch block, a potentially meaningful covariate in an MR study, was also unavailable for analysis. Notably, MR is dynamic in that severity varies based on loading conditions. As such, MR at the onset of ADHF can improve with decongestion such that our study may overestimate MR severity (24). However, information regarding the timing of echocardiogram imaging (admission vs. discharge) was not available for analysis. Because data in the ARIC HF Community Surveillance Study were abstracted from patient records as opposed to direct measurement, there is the potential for measurement bias across ARIC study sites. Quantitative echocardiographic parameters were not available for analysis. Thus, MR severity was abstracted from echocardiogram reports as none, mild, moderate, or severe. Moderate and severe MR were subsequently analyzed as a single variable to mitigate effects stemming from potential imprecision in the distinction between these MR categories. Finally, clinically relevant data such as previous ADHF hospitalizations or readmissions were not recorded for the entire surveillance population and were therefore not analyzed.
CONCLUSIONS
Patients with ADHF in this analysis from the ARIC HF Community Surveillance Study had a significant MR burden that varied with LVEF. Moderate or severe MR was more likely in female compared with male patients. Among those with an LVEF ≥50%, moderate or severe MR was more common in Whites compared with Blacks. Notably, among ADHF patients with an LVEF <50%, moderate or severe MR was independently associated with excess 1-year mortality. Future work is needed to intensify efforts in the detection and management of MR in this high-risk population.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: Moderate or severe MR is common in patients admitted for HF exacerbations and is significantly associated with increased all-cause 1-year mortality in those with an LVEF <50%.
TRANSLATIONAL OUTLOOK: Further work is needed to better characterize the HF population at risk for MR and to intensify efforts in the detection and management of MR in this high-risk population.
ACKNOWLEDGMENTS
The authors thank the staff and participants of the ARIC study for their important contributions.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
The ARIC study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), Department of Health and Human Services, under contract nos. HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I. Dr. Qamar is supported by Daiichi Sankyo, the NIH T32 Program, and the American Heart Association Strategically Focused Research Networks in Vascular Disease grant. Dr. Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst NIH/NCATS Award UL 1TR002541. Dr. Vavalle is supported by a research grant from CSI Inc. Dr. Qamar has been an AD hoc consultant/speaker for the American College of Cardiology, Society for Cardiovascular Angiography and Interventions, Pfizer, Medscape, and the Clinical Exercise Physiology Association. Dr. Vaduganathan has served on advisory boards for Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, Cytokinetics, and Relypsa; and has participated on clinical endpoint committees for studies sponsored by Novartis and the NIH. Dr. Pandey has served as an advisory board member for Roche Diagnostics; and has also received research funding from the Texas Health Resources Clinical Scholarship, Gilead Sciences Research Scholar Program, and the National Institute of Aging GEMSSTAR Grant (1R03AG067960-01). Dr. Cavender has received non-salary research support from Amgen, AstraZeneca, Chiesi, CSL Behring, GlaxoSmithKline, and Novartis; salary research support from Novo-Nordisk; and consulting fees from Amgen, AstraZeneca, Boehringer Ingelheim, Boston Scientific, Edwards Lifesciences, and Merck. Dr. Vavalle has been a principal investigator for Abbott Medical Device Company, Boston Scientific, and Cardiac Dimensions, Inc.; has received consulting fees from Edwards Lifesciences; and has an honorarium from ZOLL Medical Corporation. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- ACE
angiotensin-converting enzyme
- ADHF
acute decompensated heart failure
- ARB
angiotensin receptor blocker
- ARIC
Atherosclerosis Risk in Communities
- CI
confidence interval
- HF
heart failure
- LVEF
left ventricular ejection fraction
- MR
mitral regurgitation
- OR
odds ratio
REFERENCES
- 1.Abdo AS. Hospital management of acute decompensated heart failure. Am J Med Sci 2017; 353:265–74. [DOI] [PubMed] [Google Scholar]
- 2.Fonarow GC, Adams KF Jr., Abraham WT, Yancy CW, Boscardin WJ, ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 2005;293:572–80. [DOI] [PubMed] [Google Scholar]
- 3.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005–11. [DOI] [PubMed] [Google Scholar]
- 4.Trichon BH, Felker GM, Shaw LK, Cabell CH, O’Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol 2003;91:538–43. [DOI] [PubMed] [Google Scholar]
- 5.Rossi A, Dini FL, Faggiano P, et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 2011;97:1675–80. [DOI] [PubMed] [Google Scholar]
- 6.Bursi F, Barbieri A, Grigioni F, et al. Prognostic implications of functional mitral regurgitation according to the severity of the underlying chronic heart failure: a long-term outcome study. Eur J Heart Fail 2010;12:382–8. [DOI] [PubMed] [Google Scholar]
- 7.Goliasch G, Bartko PE, Pavo N, et al. Refining the prognostic impact of functional mitral regurgitation in chronic heart failure. Eur Heart J 2018; 39:39–46. [DOI] [PubMed] [Google Scholar]
- 8.De la Espriella R, Santas E, Miñana G, et al. Functional mitral regurgitation predicts short-term adverse events in patients with acute heart failure and reduced left ventricular ejection fraction. Am J Cardiol 2017;120:1344–8. [DOI] [PubMed] [Google Scholar]
- 9.Wada Y, Ohara T, Funada A, et al. Prognostic impact of functional mitral regurgitation in patients admitted with acute decompensated heart failure. Circ J 2016;80:139–47. [DOI] [PubMed] [Google Scholar]
- 10.Kajimoto K, Minami Y, Otsubo S, et al. Ischemic or nonischemic functional mitral regurgitation and outcomes in patients with acute decompensated heart failure with preserved or reduced ejection fraction. Am J Cardiol 2017;120: 809–16. [DOI] [PubMed] [Google Scholar]
- 11.Pecini R, Thune JJ, Torp-Pedersen C, Hassager C, Køber L. The relationship between mitral regurgitation and ejection fraction as predictors for the prognosis of patients with heart failure. Eur J Heart Fail 2011;13:1121–5. [DOI] [PubMed] [Google Scholar]
- 12.Asgar AW, Mack MJ, Stone GW. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol 2015;65:1231–48. [DOI] [PubMed] [Google Scholar]
- 13.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail 2012;5:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atherosclerosis Risk in Communities. Surveillance Data Collection. Available at: https://sites.cscc.unc.edu/aric/surveillance-forms-data. Accessed February 1, 2020.
- 15.Mansournia MA, Altman DG. Inverse probability weighting. BMJ 2016;352:i189. [DOI] [PubMed] [Google Scholar]
- 16.Nieminen MS, Brutsaert D, Dickstein K, et al. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J 2006;27:2725–36. [DOI] [PubMed] [Google Scholar]
- 17.Dziadzko V, Clavel M-A, Dziadzko M, et al. Outcome and undertreatment of mitral regurgitation: a community cohort study. Lancet 2018;391:960–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vassileva CM, McNeely C, Mishkel G, Boley T, Markwell S, Hazelrigg S. Gender differences in long-term survival of Medicare beneficiaries undergoing mitral valve operations. Ann Thorac Surg 2013;96:1367–73. [DOI] [PubMed] [Google Scholar]
- 19.Patel DK, Green KD, Fudim M, Harrell FE, Wang TJ, Robbins MA. Racial differences in the prevalence of severe aortic stenosis. J Am Heart Assoc 2014;3:e000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70: 252–89. [DOI] [PubMed] [Google Scholar]
- 21.Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 2018;379: 2307–18. [DOI] [PubMed] [Google Scholar]
- 22.Obadia J- F, Messika-Zeitoun D, Leurent G, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018;379:2297–306. [DOI] [PubMed] [Google Scholar]
- 23.Michler RE, Smith PK, Parides MK, et al. Two-year outcomes of surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med 2016;374:1932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubo S, Kawase Y, Hata R, Maruo T, Tada T, Kadota K. Dynamic severe mitral regurgitation on hospital arrival as prognostic predictor in patients hospitalized for acute decompensated heart failure. Int J Cardiol 2018;273:177–82. [DOI] [PubMed] [Google Scholar]


