Abstract
Purpose
Inferior alveolar nerve (IAN) injury is one of the most serious complications after extraction of impacted lower third molars. Photobiomodulation (PBM) therapy has been noted to reduce pain and inflammation while promoting tissue healing. This study examined the efficacy of PBM therapy tested in a case series of patients with postoperative IAN injury.
Material and methods
20 patients with post-extraction IAN injury were involved in this study and divided into two groups. In the study group, PBM therapy (808-nm laser, 16 mW, 3 J/cm2) was used every other day for 2 weeks solely on post-extraction sockets in 10 patients diagnosed with IAN injury. In the control group, mecobalamine was prescribed to 10 patients with IAN injury. Objective and subjective recovery of IAN paresthesia was evaluated using clinical neurosensory testing and visual analog score.
Results
All patients showed improvement in both objective and subjective examination. Notably, the visual analog score was significantly improved after PBM treatment compared to the mecobalamine treatment (p < 0.05).
Conclusion
PBM therapy with 808-nm laser appears to be an effective approach to manage paresthesia post-IAN injury following impacted third molar surgery. Given the limited sample size in this study, large-scale, placebo-controlled, multi-center randomized controlled trials are needed for further validation of this innovative treatment.
Keywords: Inferior alveolar nerve injury, LM3 extraction, Photobiomodulation, Extraction socket irradiation, Paresthesia
Introduction
Inferior alveolar nerve (IAN) injury is one of the most serious complications during extraction of impacted lower third molars [1, 2]. The incidence can be more than 10% in high-risk individuals [3, 4]. Although most paresthesia is temporary, the changes in sensation in the oral-facial region may interfere with speaking, chewing, and social interactions. The period needed for rehabilitation can range from several months to 2 years, and persistent paresthesia for longer than 6 months is considered to be unacceptably high morbidity. Therefore, early intervention is essential for better quality of life. The major therapeutic strategy for IAN injury is conservative treatment, such as neurotrophic supplements and physical therapy [5]. Disappointingly, the recovery period is still very lengthy [6]. Patients suffer from a drawn-out treatment schedule and also from psychological stress.
Photobiomodulation (PBM) is a non-invasive, safe and effective, and non-pharmaceutical modality for the treatment of many injuries and conditions. It has demonstrated positive effects on the repair process for neuromuscular and peripheral nerve injuries, using red and/or near-infrared (NIR) light [7–10]. A large number of reports have shown a positive outcome for PBM in diseases and injuries related to the nervous system (both central and peripheral) [7–11]. Three discrete mechanisms of PBM have been described. Among them, the most commonly accepted hypothesis is that cytochrome c oxidase (CCO; unit IV in the mitochondrial respiratory chain) can absorb light in the red and the NIR spectral regions. It is proposed that photon absorption can dissociate inhibitory nitric oxide from the CCO enzyme, leading to an increase in electron transport, a rise in mitochondrial membrane potential (MMP), and more ATP production [12–14]. The other two mechanisms described involve photosensitive membrane channels and an extracellular latent growth factor complex, TGF-β1 [15]. It has been proved that the PBM could promote axonal growth and nerve regeneration in spinal cord and peripheral nerve injuries in vivo [16, 17]. PBM has also been proposed as a useful adjunctive treatment modality for IAN paresthesia, especially for the patients after dental alveolar surgery [7–10]. However, the clinical therapeutic effects of PBM appear to depend on the precise device treatment parameters (wavelength, power density, energy density, and time) that still remain unclear. The site of IAN injury after third molar surgery is located deep, posterior part of the mandible, where the cortical bone may be too thick for the light to penetrate easily. Therefore, the present study tested a new approach by delivering the light solely through the socket after third molar extraction in a case series of 10 patients diagnosed with IAN paresthesia and compared this group with a control group of 10 patients receiving standard treatment (oral mecobalamine).
Materials and methods
Clinical study
This study included 20 consecutive patients who had suffered a postoperative IAN injury after undergoing lower third molar surgery in Peking University School and Hospital of Stomatology by the same surgeon. The study was approved by the IRB (PKUSSIRB-201728065), all procedures were in accordance with the Declaration of Helsinki, and all subjects provided informed consent prior to inclusion in the study.
Grouping and treatment
Subjects were assigned into two groups based on a random number table with the first being the study group (10 cases, treated with PBM) and the other the control group (10 patients, treated with oral mecobalamine (Eisai China Inc. Shanghai, China), 0.5 mg, three times per day).
Assessment of IAN paresthesia
IAN paresthesia was evaluated by both objective (clinical neurosensory test; CNT) and subjective (visual analog scale; VAS) test. The CNT and VAS were completed just before surgery, during each of the PBM treatment sessions and again on days 14, 20, and 30. The results of the CNT and VAS during each period tested were averaged together for all patients and plotted in a linear fashion for the entire testing period.
CNT test
All patients underwent a complete preoperative CNT including two-point discrimination test (TPD) and light touch (LT) with Semmes-Weinstein monofilaments (Von Frey filaments) (Fig. 1). The CNT was performed by a single examiner who was not involved with the surgical procedures. The CNT was performed bilaterally over a 1-cm area on the labiomental fold. Following explanation of the neurosensory tests to the subjects, CNT was performed on the contralateral side to confirm that the patient understood the test.
Fig. 1.
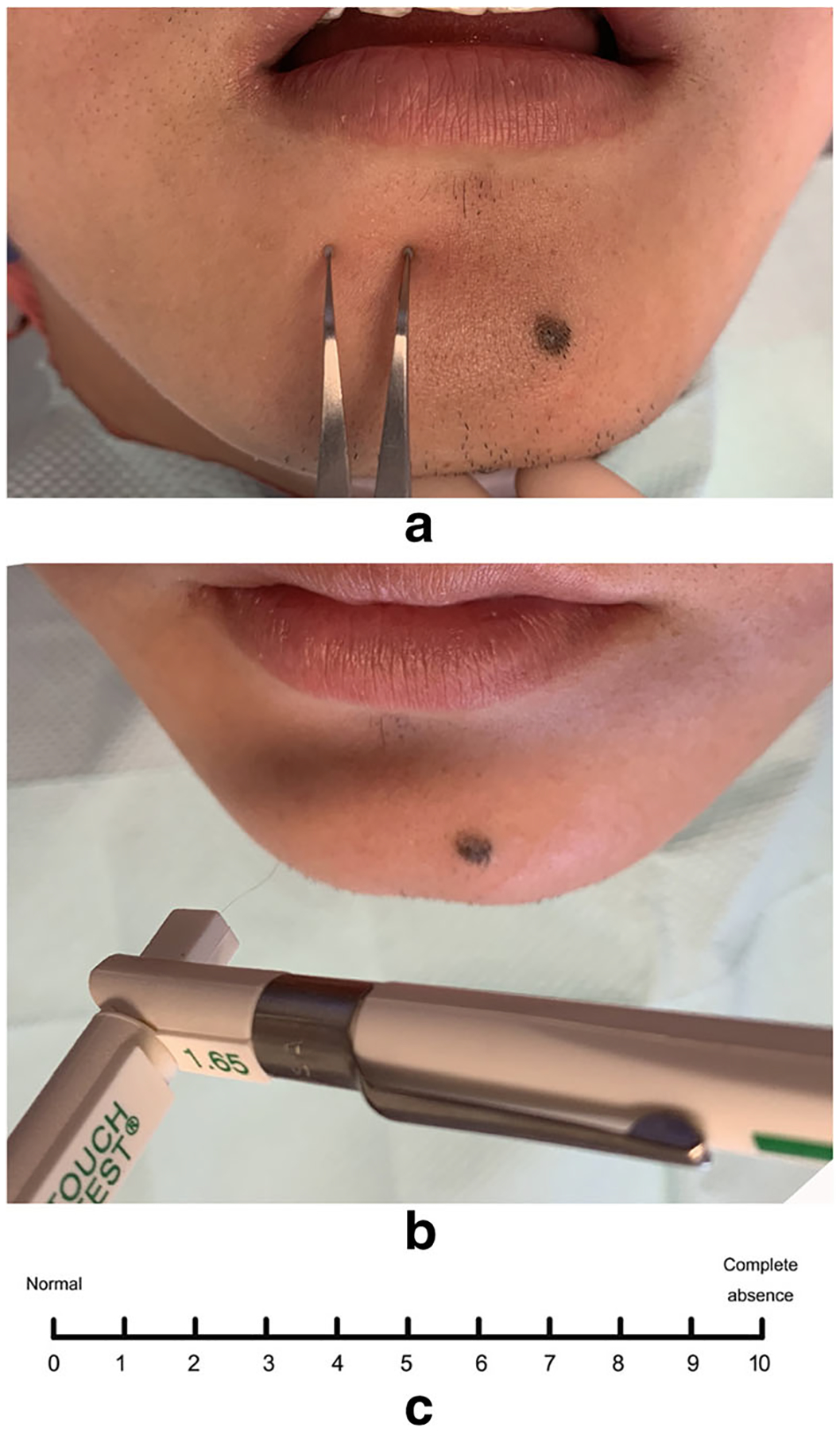
Objective and subjective evaluation for IAN injury. a Two-points discrimination test with a Boley gauge with blunt points; b light touch test with Semmes-Weinstein monofilaments; c table for VAS test
TPD test
TPD was performed using a Boley gauge with blunt points, intended to elicit a non-painful response. The smallest number of millimeters of separation that could be discerned consistently was used as the discrimination value for this test, and the preoperative value was regarded as the baseline. Nerve injury could be established when the distance during following up was 2 mm greater than the preoperative value [16, 17].
LT test
LT was performed with the Semmes-Weinstein monofilaments, and each monofilament corresponded to a specific amount of pressure (0.008–1 g). The monofilaments were placed perpendicular to the skin and pressed until the filament began to deform, after that the threshold was recorded. Preoperative value of the Semmes-Weinstein monofilaments was set as the baseline, and any increased amount of pressure after extraction was considered abnormal.
VAS
The subjective assessment of neurosensory deficit was determined using VAS. All the patients complained of paresthesia in the lower lip and the teeth on the involved side, and the VAS score before treatment was greater than 5. The subjective neurosensory assessment was performed using a 10-cm, 10° VAS with divisions at 1-cm intervals (Fig. 1c). The number 10 meant complete absence of sensation, and 0 meant fully normal sensation. Patients were asked to make an “x” on the line at each testing session. An improvement in the VAS score greater than 3 between the baseline value and the value 30 days after treatment was regarded as a clinically relevant improvement, and the improvement rate was used for statistical analysis.
Diagnosis of the paresthesia
Patients with either objective or subjective abnormality were diagnosed as IAN paresthesia. All the paresthesia was diagnosed on the second postoperative day.
PBM treatments
The protocol for PBM treatment was as follows. A near-infrared continuous wave laser (808 nm, Laserwave, China) was used in this study with the outlined parameters (Table 1). The unit consisted of a control console and a handheld laser probe connected to the console by a cable. The diameter of the probe at the point of laser delivery was 2 mm. The unit delivers 50-mW total power output at 808 nm with a spot size of approximately 3.14 cm2 and incident power density at 16 mW/cm2. The device was set to deliver 3 J/cm2 per treatment site by treating for 188 s. The treatments were performed at a single point through the extraction socket. Before PBM, patients were asked to rinse their mouth using 0.2% chlorhexidine for sterilization. The sockets were also rinsed to remove any food debris. As the clot in the socket contracted 24 h after extraction, it was easy to insert the laser probe into the socket. Initially, the socket was anaesthetized with 4% articaine so that the probe could be inserted into the bottom of the socket without any discomfort to the patient, after which light was delivered within the socket. During the PBM treatments, the operator attempted to treat the IAN injury site located at the center of the laser treatment spot. Each irradiation lasted for about 3 min (188 s) and a total of seven treatment sessions were performed (once every two days) between postoperative days 2 to 14 (Fig. 2).
Table 1.
PBM parameters
| Manufacturer | Laser wave, China |
|---|---|
| Model identifier | 808 nm Model 1 |
| Year produced | 2016 |
| Number of emitters | 1 |
| Wavelength [nm] | 808 |
| Pulse mode [CW or Hz] | CW |
| Beam spot size at target [cm2] | 3.14 |
| Irradiance at target [mW/cm2] | 16 |
| Exposure duration [s] | 188 |
| Radiant exposure [J/cm2] | 3 |
| Radiant energy [J] | 9.42 |
| Number of points irradiated | 1 |
| Area irradiated [cm2] | 3.14 |
| Application technique | In contact with extraction socket |
| Number of treatment sessions | 7 |
| Frequency of treatment sessions | Once every 2 days |
| Total radiant energy [J] | 65.94 |
Fig. 2.
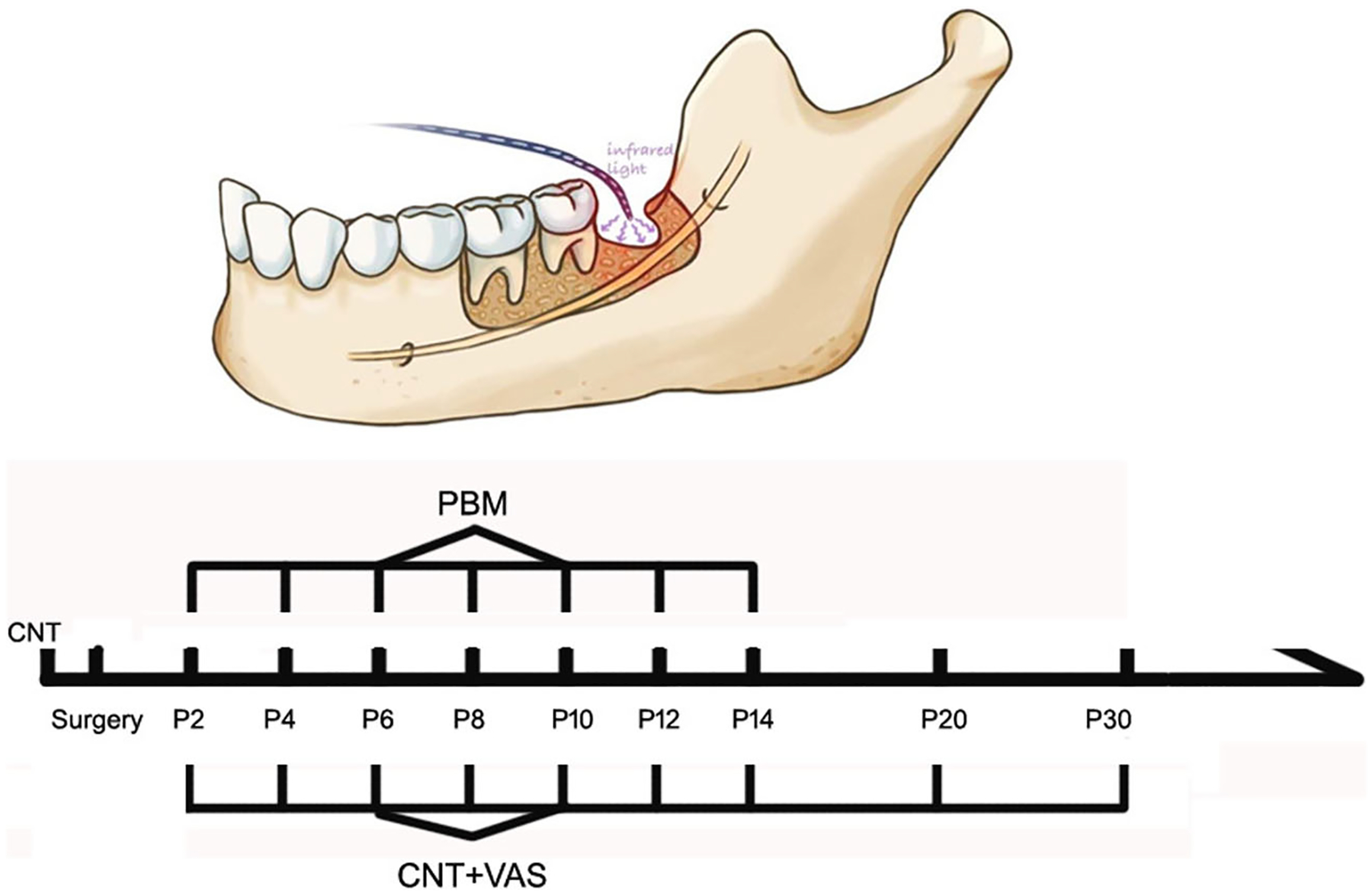
Protocol for PBM and timescale for treatment and follow-up. NIR laser (808 nm, 3 J/cm2) was delivered from postoperative days 2 to 14 with a total of 7 sessions. CNT and VAS scores were evaluated at every follow-up visit. Besides, CNT value before surgery was regarded as the baseline
Statistical analysis
SPSS19.0 was used to perform t test to evaluate the statistical significance of VAS results (p < 0.05). The Fisher’s exact test was used to evaluate the difference in therapeutic effects on IAN injury between the two groups.
Results
Subject demographics and baseline assessments
A total of 20 patients were divided into the experimental group (10) and the control group (10) according to the treatment applied. The presence of the injury was detected on the second postoperative day. All the surgical procedures were carried out by the same surgeon. No significant difference was detected in subjects’ age and the extent of the IAN injury (VAS on postoperative second day and CNT) between the two groups at baseline (Table 2).
Table 2.
Patient age and VAS score in each group
| Age | VAS score1 | Rate2 | ||
|---|---|---|---|---|
| P2 | P30 | |||
| Control | 33.3 ± 9 | 6.8 ± 1.3 | 5.1 ± 0.88 | 40% |
| Mecobalamine group | ||||
| Treatment | 34.1 ± 6.8 | 6.6 ± 1.3 | 1.1 ± 1.14 | 90% |
| PBM group | ||||
| p value | 0.82 | 0.73 | <0.001 | 0.057 |
Avariation of VAS greater than 3 was regarded as a significant improvement by subjective evaluation. The preoperative VAS was 0 in all subjects of the two groups
The rate = patients with significant improvement in a group/number of patients in this group. The rate in this table was calculated 30 days after extraction
Subjective IAN paresthesia assessments
The peak value of VAS was detected on the postoperative second day in both groups, and no significant difference was detected between groups (Table 2). The VAS values in the PBM treatment group decreased steadily with clinically significant improvement (a decrease greater than 3) evident after six to seven sessions of treatment (Fig. 3 and Table 2). On the contrary, the VAS score decreased more slowly in the control group (oral mecobalamine) compared to the experimental PBM group (p < 0.05).
Fig. 3.
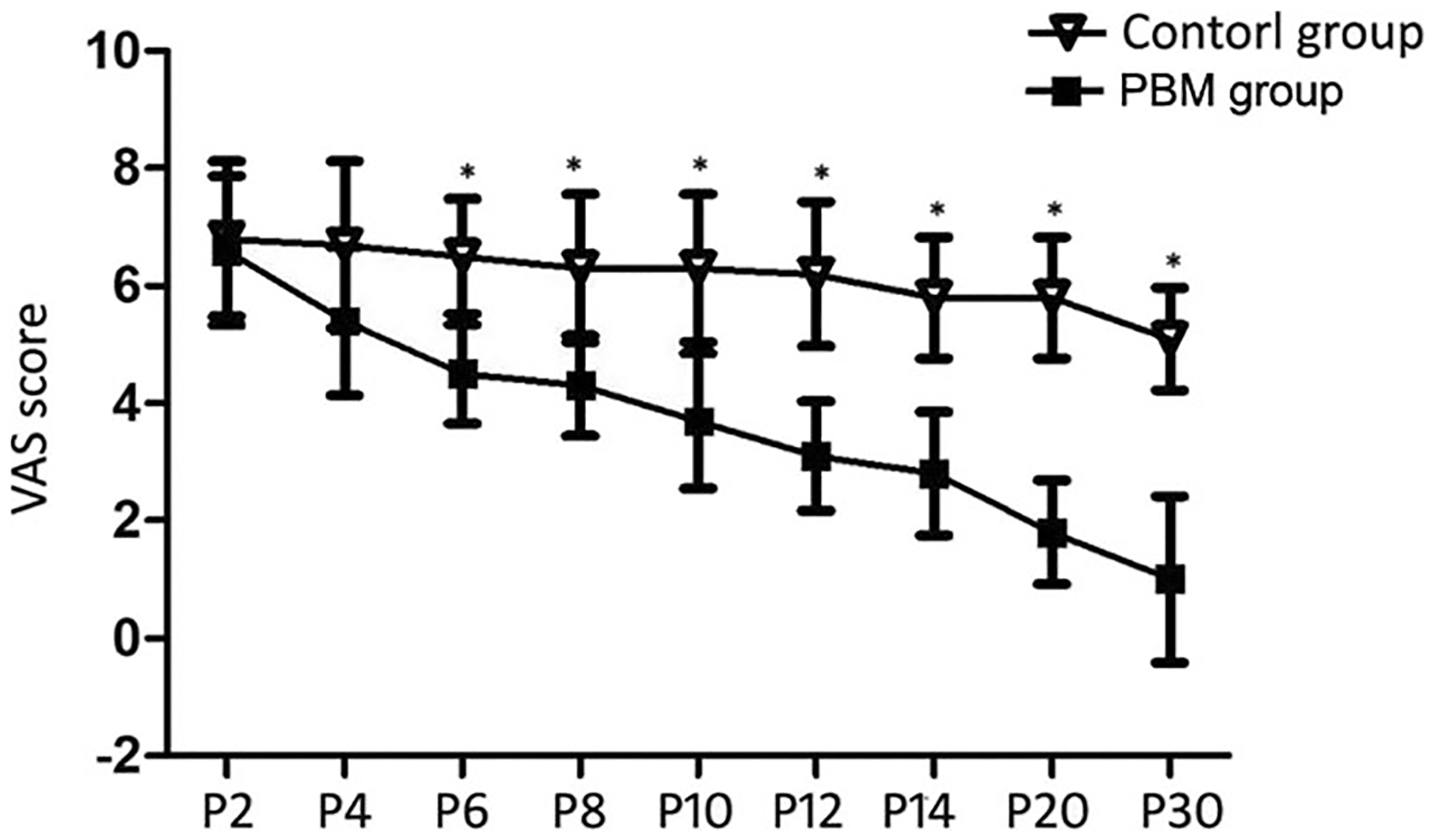
The time course of VAS score for 30 days after treatment in both groups. *Significant difference was detected between the two groups, p < 0.05
Objective IAN paresthesia assessments
An objective abnormality as reported by the CNT test was only detected in a proportion of the patients in both groups (Table 3). Specifically, the peak value of TPD in both groups was detected on postoperative second day (11.3 mm in control group and 11.2 mm in PBM group respectively, p = 0.87). Besides, the initial value of LT before extraction in these case series was 0.008 g, and the peak value was detected on the postoperative second day with a value of 0.02 g in both groups. Most of the objective deficits in the experimental group resolved completely by 30 days post-extraction compared with the control group where at least half the patients had deficits remaining.
Table 3.
Results of TPD and LT before and after PBM treatment in each group
| IAN injury1 | ||||
|---|---|---|---|---|
| TPD2 | LTΔ3 | |||
| P2 | P30 | P2 | P30 | |
| Control | 7/10 | 6/10 | 5/10 | 5/10 |
| Mecobalamine group | ||||
| Treatment | 7/10 | 3/10 | 4/10 | 1/10 |
| PBM group | ||||
| p value | 1 | 0.37 | 1 | 0.14 |
Objective IAN injury was detected in part of the patients in two groups
TPD distances on follow-up day of 2 mm or greater than preoperative values were considered abnormal. Data showed the patients with abnormality in each group
LTΔ values on the follow-up day greater than the preoperative value were considered abnormal. Data shows the fraction of patients with abnormality in each group. The baseline LT of these patients was 0.008 g, and the average value after injury in each group was 0.02 g
Discussion
IAN injury is one of the most serious complications occurring after the third molar extractions, and the recovery period usually takes several months especially in patients over 30 years of age [1–4, 18, 19]. The nerve injury is commonly attributed to acute compression of adjacent teeth, and a routinely used drug, mecobalamin, has been recommended to improve neurological function after peripheral nerve damage, especially crush injuries [19, 20]. The dosage of mecobalamin for clinical effectiveness is 0.5–6 mg/day, and no significant therapeutic advantage has been observed beyond this range [21]. Generally, the most commonly used dose was 0.5–1.5 mg/day administered orally.
The present study was undertaken to evaluate the therapeutic effect of PBM therapy as compared to the current standard of care, mecobalamin. The data in this study noted that the therapeutic effect of PBM delivering through the socket was more effective than a routine dose of mecobalamin. A major highlight of our approach is the use of the laser fiber directly inserted into the extraction tooth socket to ensure uniform and consistent treatment of the nerve damage. Hence, the putative nerve injured part is directly exposed to PBM treatments through the extraction socket; we assumed the socket would not only reduce energy dissipation, but also focus on the target more precisely. Further, postoperative healing of the socket should also be taken into consideration for intraoral PBM treatments. As the organization of the clot within the socket by deposition of granulation tissue occurs approximately 24 h after tooth extraction. Therefore, IAN irradiation though the extraction socket as early as 48 h after extraction could improve the efficiency and precision of the treatment without interfering with the crucial healing process within the socket.
Moreover, the swelling, pain around the IAN tissue was more severe on postoperative days 1 to 3 and PBM has been reported to be efficient in reducing postoperative swelling and pain [22, 23]. Eslamian et al. [25] reported that 810 nm PBM could significantly relieve the pain caused by orthodontic elastomeric separators 6 h to 3 days after teeth seperation. Eshghpour et al. [27] compared the effect of 660 nm and 810 nm PBM in management of the post-extraction complications in a randomized, double-blinded, split-mouth study. The result showed that both wavelengths were effective in reducing postoperative pain and swelling. These results may explain the tendency toward relatively rapid recovery after the first two sessions of irradiation. In accordance with previous research, our results suggest that the therapeutic efficiency and time for subjective rehabilitation could be enhanced significantly with early PBM treatments post-surgery.
Although the consensus on the time of PBM treatment on IAN injury is still unclear, early intervention has been supported by other studies [7, 25–27]. Miloro et al. treated the IAN injury after SSO with PBM taking place 6 h after SSO, and significant recovery was observed as early as 14 days after irradiation [7]. Mohajerani et al. also applied the PBM one day after SSO and dramatic improvement in both subjective and objective examination was observed after 2 years follow-up [31].
Phototherapy involves the application of PBM to sites of injury to stimulate cellular processes, mitochondrial metabolism, and notably to speed up wound healing and reduce inflammation, swelling, and pain. Many studies have demonstrated the beneficial effects of PBM with NIR wavelength on bactericidal effects, tissue regeneration, functional recovery, and improved healing, as well as the modulation of inflammatory cytokines and growth factors [10, 21, 23, 29–34]. Fekrazad et al. [36] reported that 810-nm PBM could stimulate the proliferation and differentiation of bone marrow mesenchymal stem cells in vitro. Yucesoy et al. [37] established a mental nerve injury model by partly suturing the nerve in rat and improved healing with markedly larger number of Schwann cells after 808-nm PBM treatment was observed compared to the control group.
Photon energy must be absorbed by photoacceptors inside the cell, which results in a photochemical effect. In 2008, Karu et al. [13] demonstrated that the redox state of Cox was influenced by red and NIR light. It was also postulated by Karu [12] that irradiation intensified the transfer of electrons in Cox by making more electrons available, resulting in accelerated oxidative phosphorylation. A number of in vitro studies on cells of different origin using wavelengths ranging from 632 to 980 nm have suggested that PBM can modulate cellular processes such as ATP production, cyclic AMP, and MMP. It is a non-invasive, nonthermal treatment and has the ability to modulate a wide variety of biological processes. Some researchers believe that the thermal effect of low-energy lasers is beneficial to tissue regeneration [35–37]. Lingamaneni et al. [38] found that surface epithelialization was much better when 810-nm PBM was applied in patients with inflammatory type gingival enlargement after gingivectomy or gingivoplasty. In our study, we used a 808-nm diode laser, which produces relatively little heat because we used only a lower output power of 50 mW/cm2, so we believe that PBM will activate mitochondrial function and promote local pain reduction and tissue regeneration.
In addition to the biological absorption characteristics of the NIR, tissue penetration is also crucial for the clinical scenario in this study [38]. As shown this study, we chose to use 808-nm PBM with a power of 50 mW/cm2 and total energy of 21 J/cm2 over seven sessions. Overall, both the total energy and the number of treatment sessions were less than those reported in other studies. However, we did not observe any reduced effectiveness and we attribute this to the direct, intraoral treatments through the socket that was likely most efficient for PBM dose delivery.
PBM treatment with red or NIR wavelengths has been proposed as a useful adjunctive treatment modality for trigeminal nerve paresthesia. Much research interest has focused on the treatment of IAN injury and subsequent paresthesia of the lower lip and chin after sagittal split osteotomy (SSO) and other dental alveolar surgeries [6–10]. PBM treatments are performed intraorally and/or extraorally onto the mental foramen and along the pathway of the IAN passing along the jaw. It has been reported that PBM was effective for the treatment of acute IAN injury producing resolution within 6 months while, in some cases, it was noted to be effective in IAN whose duration was longer than 6 months [10]. However, unlike the mental nerve injury, IAN injury post-third molar extracts are located deep at the posterior aspect of the mandible surrounded by thick cortical bone. Hence, we chose to treat with a NIR laser at 808 nm to enable optimal light penetration. Führer-Valdivia et al. demonstrated the therapeutic effects of 810-nm PBM on IAN injury caused after SSO [31]. Moreover, Khullar et al. reported that the 820-nm wavelength could promote nerve injury recovery in patients whose injury had lasted longer than 6 months [10].
The results in this study demonstrated both subjective and objective improvements in IAN paresthesia that improved steadily after PBM therapy. Although the incidence of IAN injury is relatively common during maxillofacial surgery, there is no accepted standard protocol for evaluating and grading the injury. The use of a light touch (LT) with Semmes-Weinstein monofilaments (also known as on Frey filament test) and the Two-Points Discrimination (TBD) test are frequently used in studies. The subjective questionnaire in the form of a VAS score is also an effective method for standardizing or quantifying symptoms and complaints of the patients [39, 40]. It is prudent to point out that the extent of injury is also directly correlated with the skill of the surgeon performing the procedures. To enable appropriate comparison in this study, all extractions were performed by a skilled surgeon with over 10 years of experience. Consistent with this, no significant differences were noted between both objective and subjective CNTs between the two groups on the postoperative second day.
In the present study, 4% articaine was used for blocking anesthesia during teeth extraction. IAN injury was diagnosed on the second postoperative day in the present study. It has been reported that the half-lives of elimination (t½α and t½β) of articaine are 0.6 and 2.5 h [41]. Therefore, evaluating the IAN injury 48 h after surgery could not only detected the injury, but also help to the early treatment.
Conclusions
In conclusion, our study has introduced a novel approach to deliver light to treat IAN injury after LM3 extraction. As the light was directly delivered through the socket, some draw-backs of PBM such as poor penetration through bony tissues could be avoided. Besides, the treatment efficacy and accuracy could be improved significantly because the irradiation site coincides with the injury site. Due to the limited sample size in our study, further large-scale randomized controlled clinical trials are needed for verification.
Funding information
Michael R Hamblin was funded by US NIH Grants R01AI050875 and R21AI121700. Yuguang Wang is funded by the following funds: The Clinical Characteristics Application of Research Projects in Capital (Z181100001718186), Smart Medical Discipline Construction Project in Peking University Health Science Center (BMU2018ZHYL013) and New Technology and New Therapy Project in Peking University School of Stomatology (PKUSSNCT-16A06).
Footnotes
Ethical approval The study was approved by the IRB of School and Hospital of Stomatology, Peking University, Beijing, China (PKUSSIRB-201728065), and all procedures were in accordance with the Declaration of Helsinki,
Informed consent Informed consent was obtained from all individual participants included in the study.
Conflict of interest Michael R Hamblin declares the following potential conflicts of interest: Scientific Advisory Boards: Transdermal Cap Inc, Cleveland, OH, USA; BeWell Global Inc, Wan Chai, Hong Kong; Hologenix Inc. Santa Monica, CA, USA; LumiThera Inc, Poulsbo, WA, USA; Vielight, Toronto, Canada; Bright Photomedicine, Sao Paulo, Brazil; Quantum Dynamics LLC, Cambridge, MA, USA; Global Photon Inc, Bee Cave, TX, USA; Medical Coherence, Boston MA, USA; NeuroThera, Newark DE, USA; JOOVV Inc, Minneapolis-St. Paul MN, USA; AIRx Medical, Pleasanton CA, USA; FIR Industries, Inc. Ramsey, NJ, USA; UVLRx Therapeutics, Oldsmar, FL, USA; Ultralux UV Inc, Lansing MI, USA; Illumiheal & Petthera, Shoreline, WA, USA; MB Lasertherapy, Houston, TX, USA; ARRC LED, San Clemente, CA, USA; Varuna Biomedical Corp. Incline Village, NV, USA; Niraxx Light Therapeutics, Inc, Boston, MA, USA. Consulting; Lexington Int, Boca Raton, FL, USA; USHIO Corp, Japan; Merck KGaA, Darmstadt, Germany; Philips Electronics Nederland B.V. Eindhoven, Netherlands; Johnson & Johnson Inc, Philadelphia, PA, USA; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany. Stockholdings: Global Photon Inc, Bee Cave, TX, USA; Mitonix, Newark, DE, USA. The other authors declare that they have no competing interest.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lopes V, Mumenya R, Feinmann C, Harris M (1995) Third molar surgery: an audit of the indications for surgery, post-operative complaints and patient satisfaction. Br J Oral Maxillofac Surg 33:33–35 [DOI] [PubMed] [Google Scholar]
- 2.Nguyen E, Grubor D, Chandu A (2014) Risk factors for permanent injury of inferior alveolar and lingual nerves during third molar surgery. J Oral Maxillofac Surg 72:2394–2401 [DOI] [PubMed] [Google Scholar]
- 3.Ueda M, Nakamori K, Shiratori K, Igarashi T, Sasaki T, Anbo N, Kaneko T, Suzuki N, Dehari H, Sonoda T, Hiratsuka H (2012) Clinical significance of computed tomographic assessment and anatomic features of the inferior alveolar canal as risk factors for injury of the inferior alveolar nerve at third molar surgery. J Oral Maxillofac Surg 70:514–520 [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa T, Ri S, Shigeta T, Akashi M, Imai Y, Kakei Y, Shibuya Y, Komori T (2013) Risk factors associated with inferior alveolar nerve injury after extraction of the mandibular third molar–a comparative study of preoperative images by panoramic radiography and computed tomography. Int J Oral Maxillofac Surg 42:843–851 [DOI] [PubMed] [Google Scholar]
- 5.Nogami S, Yamauchi K, Shiiba S, Kataoka Y, Hirayama B, Takahashi T (2015) Evaluation of the treatment modalities for neurosensory disturbances of the inferior alveolar nerve following retromolar bone harvesting for bone augmentation. Pain Med 16: 501–512 [DOI] [PubMed] [Google Scholar]
- 6.Kushnerev E, Yates JM (2015) Evidence-based outcomes following inferior alveolar and lingual nerve injury and repair: a systematic review. J Oral Rehabil 42:786–802 [DOI] [PubMed] [Google Scholar]
- 7.Miloro M, Repasky M (2000) Low-level laser effect on neurosensory recovery after sagittal ramus osteotomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 89:12–18 [DOI] [PubMed] [Google Scholar]
- 8.Ozen T, Orhan K, Gorur I, Ozturk A (2006) Efficacy of low level laser therapy on neurosensory recovery after injury to the inferior alveolar nerve. Head Face Med 2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colella G, Cannavale R, Vicidomini A, Lanza A (2007) Neurosensory disturbance of the inferior alveolar nerve after bilateral sagittal split osteotomy: a systematic review. J Oral Maxillofac Surg 65:1707–1715 [DOI] [PubMed] [Google Scholar]
- 10.Khullar SM, Emami B, Westermark A, Haanaes HR (1996) Effect of low-level laser treatment on neurosensory deficits subsequent to sagittal split ramus osteotomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 82:132–138 [DOI] [PubMed] [Google Scholar]
- 11.Andreo L, Soldera CB, Ribeiro BG, de Matos PRV, Bussadori SK, Fernandes KPS, Mesquita-Ferrari RA (2017) Effects of photobiomodulation on experimental models of peripheral nerve injury. Lasers Med Sci 32:2155–2165 [DOI] [PubMed] [Google Scholar]
- 12.Karu TI (2010) Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life 62: 607–610 [DOI] [PubMed] [Google Scholar]
- 13.Karu TI (2008) Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem Photobiol 84:1091–1099 [DOI] [PubMed] [Google Scholar]
- 14.Wu S, Zhou F, Wei Y, Chen WR, Chen Q, Xing D (2014) Cancer phototherapy via selective photoinactivation of respiratory chain oxidase to trigger a fatal superoxide anion burst. Antioxid Redox Signal 20:733–74615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arany PR (2016) Craniofacial wound healing with photobiomodulation therapy: new insights and current challenges. J Dent Res 95:977–984 [DOI] [PubMed] [Google Scholar]
- 16.Veronez S, Assis L, Del Campo P, de Oliveira F, de Castro G, Renno AC, Medalha CC (2017) Effects of different fluences of low-level laser therapy in an experimental model of spinal cord injury in rats. Lasers Med Sci 32:343–349 [DOI] [PubMed] [Google Scholar]
- 17.Anders JJ, Moges H, Wu X, Erbele ID, Alberico SL, Saidu EK, Smith JT, Pryor BA (2014) In vitro and in vivo optimization of infrared laser treatment for injured peripheral nerves. Lasers Surg Med 46:34–4518. [DOI] [PubMed] [Google Scholar]
- 18.Kjolle GK, Bjornland T (2013) Low risk of neurosensory dysfunction after mandibular third molar surgery in patients less than 30 years of age. A prospective study following removal of 1220 mandibular third molars. Oral Surg Oral Med Oral Pathol Oral Radiol 116:411–417 [DOI] [PubMed] [Google Scholar]
- 19.Kim JW, Cha IH, Kim SJ, Kim MR (2012) Which risk factors are associated with neurosensory deficits of inferior alveolar nerve after mandibular third molar extraction? J Oral Maxillofac Surg 70: 2508–2514 [DOI] [PubMed] [Google Scholar]
- 20.Pol R, Gallesio G, Riso M, Ruggiero T, Scarano A, Mortellaro C, Mozzati M (2016) Effects of superpulsed, low-level laser therapy on neurosensory recovery of the inferior alveolar nerve. J Craniofac Surg 27:1215–1219 [DOI] [PubMed] [Google Scholar]
- 21.Kazancioglu HO, Ezirganli S, Demirtas N (2014) Comparison of the influence of ozone and laser therapies on pain, swelling, and trismus following impacted third-molar surgery. Lasers Med Sci 29: 1313–1319 [DOI] [PubMed] [Google Scholar]
- 22.Ferrante M, Petrini M, Trentini P, Perfetti G, Spoto G (2013) Effect of low-level laser therapy after extraction of impacted lower third molars. Lasers Med Sci 28:845–849 [DOI] [PubMed] [Google Scholar]
- 23.Eslamian L, Borzabadi-Farahani A, Hassanzadeh-Azhiri A, Badiee MR, Fekrazad R (2014) The effect of 810-nm low-level laser therapy on pain caused by orthodontic elastomeric separators. Lasers Med Sci 29:559–564 [DOI] [PubMed] [Google Scholar]
- 24.Eshghpour M, Ahrari F, Takallu M (2016) Is low-level laser therapy effective in the management of pain and swelling after mandibular third molar surgery? J Oral Maxillofac Surg 74:1322.e1–8 [DOI] [PubMed] [Google Scholar]
- 25.Xuan W, Vatansever F, Huang L, Wu Q, Xuan Y, Dai T, Ando T, Xu T, Huang YY, Hamblin MR (2013) Transcranial low-level laser therapy improves neurological performance in traumatic brain injury in mice: effect of treatment repetition regimen. PLoS ONE 8: e53454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang YY, Hamblin MR (2013) Transcranial low-level light therapy produces neuroprotection, neurogenesis and BDNF after TBI in mice. Conference on Mechanisms for Low-light Therapy VIII. [Google Scholar]
- 27.Naeser MA, Zafonte R, Krengel MH, Martin PI, Frazier J, Hamblin MR, Knight JA, Meehan WP 3rd, Baker EH (2014) Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. Journal of Neurotrauma 31:1008–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohajerani SH, Tabeie F, Bemanali M, Tabrizi R (2017) Effect of low-level laser and light-emitting diode on inferior alveolar nerve recovery after sagittal split osteotomy of the mandible: a randomized clinical trial study. J Craniofac Surg 28:e408–e411 [DOI] [PubMed] [Google Scholar]
- 29.Keshri GK, Gupta A, Yadav A, Sharma SK, Singh SB (2016) Photobiomodulation with pulsed and continuous wave near-infrared laser (810 nm, Al-Ga-As) augments dermal wound healing in immunosuppressed rats. PLoS One 11:e0166705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.das Neves MF, Dos Reis MC, de Andrade EA, Lima FP, Nicolau RA, Arisawa EÂ, Andrade AO, Lima MO (2016) Effects of low-level laser therapy (LLLT 808 nm) on lower limb spastic muscle activity in chronic stroke patients. Lasers Med Sci 31:1293–1300 [DOI] [PubMed] [Google Scholar]
- 31.Fuhrer-Valdivia A, Noguera-Pantoja A (2014) Ramirez-Lobos V. & Sole-Ventura P. Low-level laser effect in patients with neurosensory impairment of mandibular nerve after sagittal split ramus osteotomy. Randomized clinical trial, controlled by placebo. Med Oral Patol Oral Cir Bucal 19:e327–e334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai S, Xiao G, Dong N, Liu F, He S, Guo Q (2018) Bactericidal effect of a diode laser on Enterococcus faecalis in human primary teeth-an in vitro study. BMC Oral Health 18:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fekrazad R, Asefi S, Baghaban Eslaminejad M, Taghiyar L, Bordbar S, Hamblin MR (2019) Photobiomodulation with single and combination laser wavelengths on bone marrow mesenchymal stem cells: proliferation and differentiation to bone or cartilage. Lasers Med Sci 34:115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yucesoy T, Kutuk N, Canpolat DG, Alkan A (2017) Comparison of ozone and photo-biomodulation therapies on mental nerve injury in rats. J Oral Maxillofac Surg 75:2323–2332 [DOI] [PubMed] [Google Scholar]
- 35.El Nawam H, El Backly R, Zaky A, Abdallah A (2019) Low-level laser therapy affects dentinogenesis and angiogenesis of in vitro 3D cultures of dentin-pulp complex. Lasers Med Sci 27 [DOI] [PubMed] [Google Scholar]
- 36.Borzabadi-Farahani A (2017) The adjunctive soft-tissue diode laser in orthodontics. Compend Contin Educ Dent 38(eBook 5):e18–e31 [PubMed] [Google Scholar]
- 37.Lingamaneni S, Mandadi LR, Pathakota KR (2019) Assessment of he aling following low-level laser irradiation after gingivectomy operations using a novel soft tissue healing index: a randomized, double-blind, split-mouth clinical pilot study. J Indian Soc Periodontol 23:53–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hudson DE, Hudson DO, Wininger JM, Richardson BD (2013) Penetration of laser light at 808 and 980 nm in bovine tissue samples. Photomedicine & Laser Surgery 31:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poort LJ, van Neck JW, van der Wal KG (2009) Sensory testing of inferior alveolar nerve injuries: a review of methods used in prospective studies. J Oral Maxillofac Surg 67:292–300 [DOI] [PubMed] [Google Scholar]
- 40.Jääskeläinen SK, Teerijoki-Oksa T, Virtanen A, Tenovuo O, Forssell H (2004) Sensory regeneration following intraoperatively verified trigeminal nerve injury. Neurology 62:1951–1957 [DOI] [PubMed] [Google Scholar]
- 41.Vree TB, Gielen MJ (2005) Clinical pharmacology and the use of articaine for local and regional anaesthesia. Best Pract Res Clin Anaesthesiol 19:293–308 [DOI] [PubMed] [Google Scholar]


