Abstract
Bioaerosols produced from Wastewater Treatment Plants (WWTPs) can pose health risks to plant workers and nearby inhabitants. There is a gap in air quality data for WWTPs in developing countries. The present study aimed to measure airborne bacterial and fungal concentrations in a WWTP in southwestern Iran between September 2015 and May 2016. Active sampling was conducted around operational units, and a total of 600 bacterial and fungal samples were collected. Spatial and seasonal comparisons were made. The highest average concentrations of culturable bacterial aerosol at seasonally dependent locations were, in decreasing order, 2581 ± 401 and 1952 ± 390 CFU m−3 for the selector and aeration tanks, respectively, in autumn; 1363 ± 299 CFU m−3 for the aeration tank in winter; and 1738 ± 350 CFU m−3 for the screw pump in spring. Furthermore, the predominant genera of airborne fungi isolated from the air of the WWTP in all three seasons were Cephalotrichum spp., Alternaria spp., Penicillium spp., Monilia spp., and Aspergillus spp. The results of this work emphasize the necessity of controlling WWTP workers’ exposure to bioaerosols when bacteria and fungi become aerosolized during aeration.
Keywords: Air contamination, Bioaerosols, Wastewater treatment plant, Health risk, Iran
INTRODUCTION
Vulnerable wastewater treatment plant (WWTP) workers and nearby inhabitants are exposed to bioaerosols that may contain a large variety of bacterial, viral, and fungal species (Ranalli et al., 2000; Dutkiewicz et al., 2003; Vítězová et al., 2013; Tarigan et al., 2017). Exposure to bioaerosols in WWTPs leads to occupational health risks for workers (Dutkiewicz et al., 2003; Turner et al., 2008; Uhrbrand et al., 2011). Rapid industrialization and urbanization can produce industrial and household wastewater and sludge around the world (Yassin and Almouqatea, 2010). Wastewater includes a wide variety of microorganisms, such as viruses, bacteria, fungi, protozoa, and helminthes, which arise from commercial, residential, and hospital sewage (Gerardi and Zimmerman, 2004; Oppliger et al., 2005; Fracchia et al., 2006; Filipkowska et al., 2008; Korzeniewska, 2011; Lin et al., 2016). Pathogenic microorganisms can be easily generated in aeration basins and with mechanical agitation of raw wastewater from WWTPs (Pascual et al., 2003; Sánchez-Monedero et al., 2008; Vítězová et al., 2013). Generally, many environmental factors influence the ability of microorganisms to survive in the air, the most important of which include ultraviolet radiation, microorganism species, relative humidity, and temperature (Korzeniewska et al., 2009; Korzeniewska, 2011; Goudarzi et al., 2014; Niazi et al., 2015; Goudarzi et al., 2016).
Exposure to wastewater can result in diseases such as gastrointestinal disorders, respiratory issues, skin disorders, fever, eye irritation, headaches, nausea, and fatigue (Dutkiewicz et al., 2003; Oppliger et al., 2005; Uhrbrand et al., 2011). In addition, endotoxins derived from Gram-negative bacteria cause several problems, including diarrhea, fatigue, nose irritation, respiratory symptoms, and pulmonary function decline in WWTP workers (Thorn et al., 2002; Oppliger et al., 2005; Grisoli et al., 2009; Kallawicha et al., 2015). Evidence has also shown that frequent exposure to fungal spores leads to development of hypersensitivity pneumonitis, reduction in lung function, severe asthma, organic dust toxic syndrome, airway inflammation, and respiratory disorders (Grisoli et al., 2009; Madsen et al., 2009; Rostami et al., 2009). Moreover, many studies have indicated that workers’ long-term exposure to bioaerosols could cause a wide variety of respiratory and other health disorders including sinusitis, respiratory problems, ear infections, influenza-like symptoms, and gastrointestinal ailments (Rylander, 1999; Thorn and Kerekes, 2001; Orsini et al., 2002; Smit et al., 2005; Heinonen-Tanski et al., 2009; Madsen et al., 2009; Korzeniewska, 2011; Fazlzadeh et al., 2016; Huang et al., 2017; Kallawicha et al., 2017). In general, the concentration profile of microorganisms is a useful indicator of the potential for deleterious effects due to exposure to WWTP emissions (Orsini et al., 2002; Korzeniewska, 2011).
In spite of identification of the health risks of exposure to bioaerosols, risk assessment is still difficult. Although Occupational Exposure Limits (OELs) for bioaerosols have not been determined yet (Lebrero et al., 2011; Teixeira et al., 2013), threshold concentrations have been estimated by some institutions. Such concentrations have been proposed by Swiss OELs for total cultivable bacteria (104 CFU m−3), Gram-negative bacteria (103 CFU m−3), and total fungi (103 CFU m−3) (Teixeira et al., 2013). Furthermore, concentrations of airborne bioaerosols and the degree of human exposure to bioaerosols may vary depending upon weather conditions, time of the day, season, treatment processes of wastewater and sludge, type and capacity of WWTPs, type of facilities, and performed activities (Fracchia et al., 2006; Heinonen-Tanski et al., 2009; Korzeniewska, 2011; Gotkowska-Płachta et al., 2013).
To date, few studies have focused on outdoor air quality in WWTPs in developing countries, such as Iran, and scarce information is available regarding plant workers’ risk of exposure in WWTPs. Hence, the present study aims to 1) determine the concentration of culturable bacteria and fungi aerosol in different areas of a WWTP, 2) identify the genera of fungi and percentage of Gram-positive and Gram-negative bacteria, and 3) investigate the effects of seasons and meteorology on the amount of bioaerosols.
MATERIALS AND METHODS
Study Area
This study was conducted in a WWTP located in the southwest of Iran (29°36′N, 52°32′E) (Neghab et al., 2017; Dehghani et al., 2018; Delikhoon et al., 2018). This treatment plant’s water impacts an estimated 409,000 inhabitants currently, and the coverage of inhabitants has been estimated to reach around 548,000 in future. The average inlet flow rate of this WWTP is about 930 liters per second (LPS) and it is expected to provide about 30 million cubic meters (MCM) year−1 of fresh water for irrigation. In addition, the inlet Biochemical Oxygen Demand (BOD5), Total Suspended Solids (TSS), Chemical Oxygen Demand (COD), grease and oil, and Total Phosphorus (TP) are 240 ± 4, 315 ± 6, 465 ± 9, 0.04 ± 0.01, and 3.70 ± 0.20 mg L−1, respectively. Activated sludge is the biological wastewater treatment process of this WWTP, which includes a screen bar unit, primary settling tank, selector tank, aerated tank, secondary settling tank, and chlorination unit. To date, 55 workers have been employed in this plant. A summary of the locations and sampling points in the WWTP is provided in Fig. 1 and Table 1.
Fig. 1.
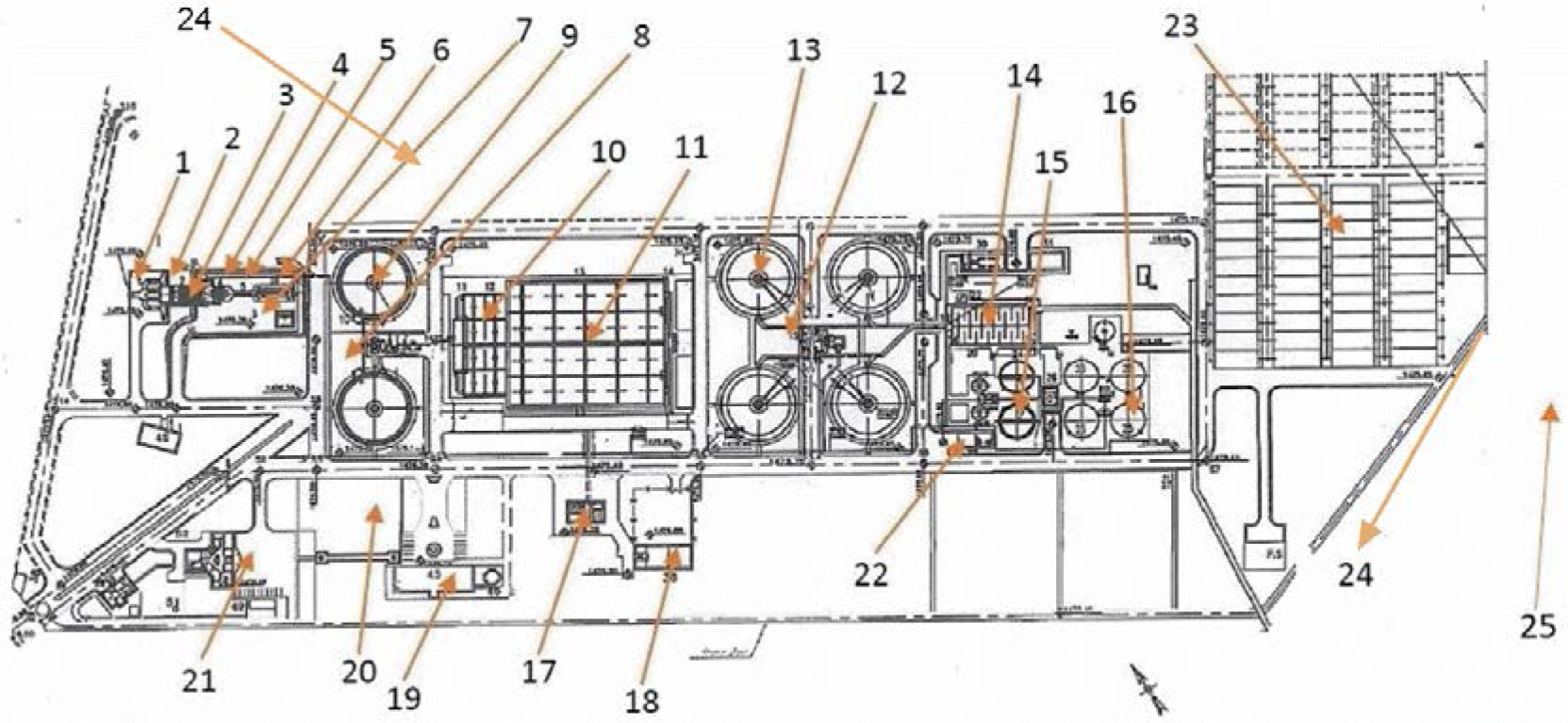
Schematic diagram of the wastewater treatment plant and sampling points (based on number: 1- coarse screen; 2- screw pump; 3- screw pump room; 4- fine screen; 5- parshall flume; 6- grit chamber; 7- blower room; 8- primary split basin; 9- primary sedimentation tank; 10- selector tank; 11- aeration tank; 12- secondary split basin; 13- secondary sedimentation tank; 14- chlorination basin; 15- sludge thickening tank; 16- anaerobic digestion tank; 17- power panel building; 18- storage building; 19- surveillance building; 20- site water supply; 21- office building; 22- chemical unit; 23- sludge drying bed; 24- WWTP wall; 25- background).
Table 1.
Description of locations and sampling points.
| Sampling point/Location | Description | |
|---|---|---|
| 1 | Coarse screen | Adjacent the screw pump, this location has two coarse screens at a 10-m distance from the screw pump |
| 2 | Screw sump | Near the screw pump room and coarse screen 12 m under the earth surface |
| 3 | Screw sump room | This location is set in front of the screw pump, this room has several windows 3-m above ground level |
| 4 | Fine screen | Adjacent the parshal flume, this location has two fine screens at a 4-m distance from the parshal flume |
| 5 | Parshall flume | Near the grit chamber, this location is used to measure water flow |
| 6 | Grit chamber | After the parshal flume, this sampling point is located at a 2-m distance from the primary split basin |
| 7 | Blower room | This location is set at the right side of the grit chamber |
| 8 | Primary split basin | In front of the grit chamber, this location divides the flow to two ways and then, the flow enters the primary sedimentation tank |
| 9 | Primary sedimentation tank | This sampling site has two circle tanks with a 40-m diameter |
| 10 | Selector tank | This location has four sections with a 12-m length and is set after the primary sedimentation tank or before the aeration tank |
| 11 | Aeration tank | Adjacent to the secondary split basin, this location has 20 surface aerations each consuming 55 kilowatts of energy with a 6-hour retention time |
| 12 | Secondary split basin | In front of the aeration tank, this location divides the flow to four ways and then, the flow enters the secondary sedimentation tank |
| 13 | Secondary sedimentation tank | This sampling site has four circle tanks with a 40-m diameter |
| 14 | Chlorination basin | Chlorination basin has 6 baffles with a 12-m length |
| 15 | Sludge thickening tank | This sampling point is set at the left side of the chlorination basin with two circle tanks with a 19-m diameter |
| 16 | Anaerobic digestion tank | It is set after the anaerobic thickening tank, this location has four tanks with a 19-m diameter |
| 17 | Power panel building | Located between the surveillance building and storage building or at a 90-m distance from the left side of the aeration tank |
| 18 | Storage building | Located after the power panel building or at a 100-m distance from the left side of the aeration tank |
| 19 | Surveillance building | Located between the power panel building and site water supply or at a 110-m distance from the left side of the selector tank |
| 20 | Site water supply | Between the office building and surveillance building, after the site water supply, at the left side of the primary sedimentation tank, or at a 90-m distance from the aeration tank |
| 21 | Office building | Near the inlet to the wastewater treatment plant |
| 22 | Chemical unit | This sampling point is set at the left side of the sludge thickening tank or near the secondary sedimentation tank |
| 23 | Sludge drying bed | Near the WWTP wall, this location is used to dewater sludge |
| 24 | WWTP wall | Wastewater treatment plant wall |
| 25 | Background | Outside the WWTP, upwind and downwind direction |
Sampling Procedures
Following EPA sampling guidelines (EPA, 2006), sampling was performed every 6 days in autumn, winter, and spring between September 2015 and May 2016 (autumn: September–November; winter: December–February; spring: March–May). In total, 25 sites were used for sampling in the WWTP (Fig. 1 and Table 1). We collected 4 bacterial samples at 4 different points at each sampling site. We collected a total of 100 samples per season owing to 4 samples at the 25 sites (25 × 4 = 100); as there were 3 seasons, we collected 300 total samples altogether. The same exact mode of operation applied to the fungi sampling, yielding 300 total fungi samples. First, the sampler was disinfected with 75% ethanol to remove any contamination. Then, a QuickTake-30 sample pump equipped with a BioStage impactor (BioStage Single-stage Impactor, SKC, Inc., USA) was used for air sampling for 2 minutes at a flow rate of 28.30 L min−1 (Li et al., 2013; Niazi et al., 2015). Calibration was performed according to BioStage Impactor Cat. Nos. 225–9611 and 225–9610. Calibration was performed for each sampling station. The sampler was located at a height of about 1.5 m above the floor (at the human breathing zone) (Breza-Boruta and Paluszak, 2007; Li et al., 2013; Niazi et al., 2015). Temperature, relative humidity, wind speed, and incidental ultraviolet radiation were also simultaneously recorded to find the relationship between bioaerosol concentration and meteorological conditions at each sampling location (Table 2) (Karra and Katsivela, 2007). Temperature (°C) and relative humidity (%) were measured using a portable instrument (Preservation Equipment Ltd, UK). Additionally, wind speed (m s−1) was recorded using a portable anemometer (Campbell Scientific, Inc., USA). Finally, ultraviolet radiation was measured with a pyranometer (Kipp and Zonen, Netherlands) and was expressed in μW cm−2. After sampling, the plates were closed and immediately transferred to the microbiology laboratory in a cold box. The fungal samples were incubated in an inverted position at 25°C for four days, while the bacterial samples were incubated at 37°C for 2 days. In addition, prepared biological indicators (stainless steel coupons containing ~106 spores of Geobacillus stearothermophilus ATCC 7953) were purchased and used for controlling the accuracy of incubator thermometer.
Table 2.
Mean, standard deviation (SD) and the comparison mean of bacteria concentration (based on CFU m−3) between different sampling locations (P-value), percentage of Gram-positive and Gram-negative bacteria, and means of temperature and relative humidity in autumn, winter, and spring.
| Sampling point | Spring | Winter | Autumn | P-value* | P-value** | ***P-value | |
|---|---|---|---|---|---|---|---|
| 1 | Coarse screen | 1324 ± 331 | 180 ± 30 | 410 ± 90 | 0.006 | 0.009 | 0.010 |
| 2 | Screw pump | 1738 ± 350 | 1129 ±200 | 233 ± 38 | 0.018 | 0.003 | 0.002 |
| 3 | Screw pump room | 1520 ± 273 | 779 ± 194 | 671 ± 134 | 0.006 | 0.002 | 0.005 |
| 4 | Fine screen | 897 ± 180 | 280 ± 40 | 449 ± 77 | 0.005 | 0.010 | 0.014 |
| 5 | Parshall flume | 1002 ± 200 | 462 ± 85 | 388 ± 65 | 0.007 | 0.006 | 0.219 |
| 6 | Grit chamber | 731 ± 118 | 133 ± 20 | 89 ± 13 | 0.002 | 0.002 | 0.013 |
| 7 | Blower room | 767 ± 145 | 122 ± 20 | 93 ± 21 | 0.003 | 0.002 | 0.093 |
| 8 | Primary split basin | 445 ± 88 | 695 ± 175 | 202 ± 39 | 0.057 | 0.007 | 0.009 |
| 9 | Primary sedimentation tank | 226 ± 38 | 98 ± 16 | 208 ± 45 | 0.003 | 0.564 | 0.012 |
| 10 | Selector tank | 423 ± 90 | 686 ± 129 | 2581 ± 401 | 0.018 | 0.001 | 0.001 |
| 11 | Aeration tank | 454 ± 83 | 1363 ± 299 | 1952 ± 390 | 0.007 | 0.004 | 0.043 |
| 12 | Secondary split basin | 76 ± 8 | 99 ± 19 | 32 ± 10 | 0.089 | 0.001 | 0.006 |
| 13 | Secondary sedimentation tank | 97 ± 18 | 517 ± 108 | 196 ± 41 | 0.004 | 0.011 | 0.006 |
| 14 | Chlorination basin | 59 ± 7 | 68 ± 8 | 45 ± 10 | 0.142 | 0.067 | 0.012 |
| 15 | Sludge thickening tank | 78 ± 8 | 197 ± 45 | 50 ± 10 | 0.012 | 0.005 | 0.006 |
| 16 | Anaerobic digestion tank | 77 ± 11 | 89 ± 13 | 34 ± 6 | 0.210 | 0.001 | 0.001 |
| 17 | Power panel building | 94 ± 12 | 165 ± 30 | 78 ± 12 | 0.012 | 0.100 | 0.006 |
| 18 | Storage building | 114 ± 17 | 172 ± 32 | 90 ± 8 | 0.027 | 0.059 | 0.012 |
| 19 | Surveillance building | 37 ± 8 | 37 ± 7 | 73 ± 15 | 0.990 | 0.010 | 0.011 |
| 20 | Site water supply | 409 ± 90 | 55 ± 9 | 58 ± 5 | 0.004 | 0.004 | 0.587 |
| 21 | Office building | 74 ± 13 | 74 ± 12 | 50 ± 11 | 0.990 | 0.031 | 0.026 |
| 22 | Chemical unit | 75 ± 14 | 299 ± 54 | 72 ± 20 | 0.003 | 0.815 | 0.002 |
| 23 | Sludge drying bed | 286 ± 60 | 101 ± 19 | 57 ± 10 | 0.006 | 0.004 | 0.011 |
| 24 | WWTP wall | 240 ± 46 | 260 ± 52 | 57 ± 7 | 0.586 | 0.004 | 0.004 |
| 25 | Background | 54 ± 11 | 30 ± 8 | 21 ± 3 | 0.014 | 0.007 | 0.106 |
| Total mean of bacteria | 473 ± 91 | 336 ± 68 | 314 ± 63 | 0.056 | 0.032 | 0.651 | |
| Total Gram-positive bacteria | 45% | 46% | 63% | ||||
| Total Gram-negative bacteria | 55% | 54% | 37% | ||||
| Total mean of humidity (%) | 41 ± 3 (9–85) | 35 ± 1(10–62) | 11 ± 1(10–28) | ||||
| Total mean of temperature (°C) | 31 ± 2(10–37) | 20 ± 1 (18–27) | 37 ± 0.80 (28–41) | ||||
The comparison mean of bacteria concentration between spring and winter.
The comparison mean of bacteria concentration between spring and autumn.
The comparison mean of bacteria concentration between winter and autumn.
Quantification and Characterization of Bioaerosols
Moreover, sabouraud dextrose agar media (Merck KGaA, Germany) containing chloramphenicol antibiotic and blood agar medium (Merck KGaA, Germany) were prepared to measure fungal and bacterial aerosols, respectively (Niazi et al., 2015). The concentration of bioaerosols (CFU m−3) was counted (with positive-hole correction) using the Biostage impactor, which has 400 holes (Macher, 1989). The slide culture method was used for examination and identification of fungal colonies. In this method, a sheet of sterile filter paper is placed in a petri dish and a sterile U-shaped glass rod is then placed on the filter paper. Then, a 5 mm square block of the medium is cut from the plate of sabouraud agar. Next, the block of agar is picked up and placed in the center of a microscope slide. Subsequently, aseptically, a sterile glass cover is put on the upper inoculated surface of the agar cube. The agar block is then incubated at room temperature for 48 hours. Finally, the slide is inspected under low power. If growth has taken place, hyphae and spores would be observed. If growth is insufficient and spores are not obvious, the fungus is allowed to grow another 24–48 hours before making the stained slides (Yoshida et al., 1989; Benson, 2007; Diba et al., 2007; Rosana et al., 2014; Cruyt et al., 2017). Furthermore, Gram staining is a common technique used to identify Gram positive and Gram negative bacteria based on their different cell wall constituents. The gram stain procedure can distinguish between Gram positive and Gram negative groups based on coloring cells red or violet (Schaad et al., 2001).
Statistical Analysis
The data were analyzed using the SPSS statistical software, version 21. At first, the Kolmogorov-Simonov test was used to assess normal distribution of quantitative variables. Quantitative variables exhibiting a normal distribution were expressed with a mean and standard deviation. Qualitative variables were also expressed using number and percentage (fungi and fungal genera, Fig. 2). Additionally, comparison of bacteria and fungi as well as Gram-positive and Gram-negative bacteria regarding normal quantitative variables was done using a Student’s t-test. The three seasons of the year were compared concerning normal qualitative variables using ANOVA test. In addition, the comparison of mean of fungi and bacteria concentration between different seasons at all sampling points was conducted using a paired t-test. Finally, Pearson correlation coefficients were used to represent the correlation between normal quantitative variables. Figures were drawn using GraphPad Prism 7.
Fig. 2.
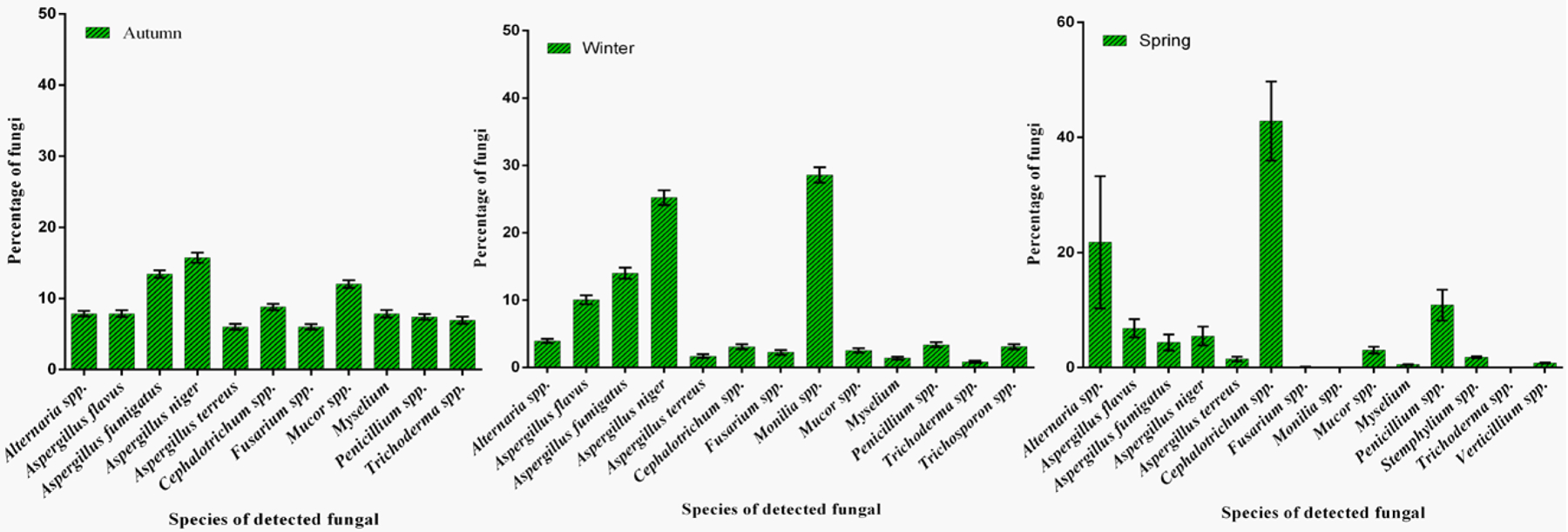
The percentages of fungi and fungal genera in autumn, winter, and spring.
RESULTS AND DISCUSSION
Meteorological Conditions
The mean ± standard deviation (SD) of temperature and relative humidity in the WWTP were, respectively, 37 ± 0.80°C and 11 ± 1% during autumn, 20 ± 1°C and 35 ± 1% during winter, and 31 ± 2°C and 41 ± 3% during spring (Table 2). Moreover, the wind speed was 1.50 ± 0.20 m s−1 in autumn, 1.60 ± 0.30 m s−1 in winter, and 2.40 ± 0.60 m s−1 in spring. Additionally, the UV index was 58.80 ± 6 μW cm−2 in autumn, 31.30 ± 3 μW cm−2 in winter, and 48.50 ± 3 μW cm−2 in spring. Pressure was also 860 ± 1.20 mb, 859 ± 1.10 mb, and 862 ± 1 mb in autumn, winter, and spring, respectively.
Concentration of Bioaerosols
The mean (± SD) concentrations of bacteria based on CFU m−3, and percentage of Gram-positive and Gram-negative bacteria in different sampling locations are summarized in Table 2. The minimum concentrations of bacteria in autumn were as follows: office building (50 ± 11 CFU m−3), secondary split basin (32 ± 10 CFU m−3), chlorination basin (45 ± 10 CFU m−3), screw pump room (38 ± 12 CFU m−3), and anaerobic digestion tank (34 ± 6 CFU m−3). Maximum concentrations in autumn were as follows: selector tank (2581 ± 401 CFU m−3), aeration tank (1952 ± 390 CFU m−3), screw pump room (671 ± 134 CFU m−3), fine screen (449 ± 77 CFU m−3), and coarse screen (410 ± 90 CFU m−3). The lowest number of bacterial aerosols in winter was observed in the surveillance building (37 ± 7 CFU m−3), site water supply (55 ± 9 CFU m−3), chlorination basin (68 ± 8 CFU m−3), and office building (74 ± 12 CFU m−3). However, the highest number of bacterial aerosols in winter was linked to the aeration tank (1363 ±299 CFU m−3) and screw pump (1129 ± 200 CFU m−3). Furthermore, the surveillance building (37 ± 8 CFU m−3) and chlorination basin (59 ± 7 CFU m−3) exhibited the lowest concentration of culturable bioaerosols in spring. In contrast, the screw pump, screw pump room, and coarse screen exhibited the highest concentration of culturable bioaerosols in this season (1738 ± 350, 1520 ± 273, and 1324 ± 331 CFU m−3, respectively). Overall, the total concentration of bacteria was higher in spring than in autumn and winter. In addition, the percentage of Gram-positive and Gram-negative bacteria were respectively 63.50% and 36.50% in autumn, 46% and 54% in winter, and 45% and 55% in spring.
Mean bacteria concentrations for different seasons at all sampling points are presented in Table 2. There was no statistically significant difference between spring and winter in sampling points such as the primary split basin (p = 0.057), secondary split basin (p = 0.089), chlorination basin (p = 0.142), anaerobic digestion tank (p = 0.210), surveillance building (p = 0.990), and office building (p = 0.990), while a statistically significant difference was observed between spring and winter at all other sampling points (p ≤ 0.001). In addition, a statistically significant difference was observed between the mean concentration of bacteria between spring and autumn (p ≤ 0.001) at all points except for the primary sedimentation tank (p = 0.564), chlorination basin (p = 0.067), power panel building (p = 0.100), storage building (p = 0.059), and chemical unit (p = 0.815). Furthermore, according to Table 2, a statistically significant difference was observed between winter and autumn (p ≤ 0.001) at all points except for the Parshall flume (p = 0.219), blower room (p = 0.093), site water supply (p = 0.587), and the background area (p = 0.106).
The mean (± SD) concentrations of fungi in different sampling locations and seasons are summarized in Table 3. Accordingly, the minimum concentration of fungi in autumn was found in the storage building, chlorination basin, secondary split basin, and sludge thickening tank, ranging from 35 ± 10 to 53 ± 19 CFU m−3. The maximum concentration of fungi in autumn was observed in the aeration tank and screw pump, ranging from 530 ± 80 to 918 ± 129 CFU m−3. In addition, the highest concentration of fungal bioaerosols in winter was related to fine screen (459 ± 60 CFU m−3), screw pump (371 ± 41 CFU m−3), and screw pump room (353 ± 42 CFU m−3). The minimum concentration of fungi in winter was found in the secondary sedimentation tank and WWTP wall, which ranged from 71 ± 18 to 88 ± 19 CFU m−3.
Table 3.
Mean, standard deviation (SD) and the comparison mean of fungi concentration (based on CFU m−3) between different sampling locations (P-value).
| Sampling point | Spring | Winter | Autumn | P-value* | P-value** | ***P-value | |
|---|---|---|---|---|---|---|---|
| 1 | Coarse screen | 707 ± 92 | 265 ± 32 | 141 ± 18 | 0.003 | 0.004 | 0.061 |
| 2 | Screw pump | 1855 ± 278 | 371 ± 41 | 918 ± 129 | 0.004 | 0.006 | 0.002 |
| 3 | Screw pump room | 2561 ± 420 | 353 ± 42 | 353 ± 41 | 0.002 | 0.001 | 0.990 |
| 4 | Fine screen | 1766 ± 355 | 459 ± 60 | 106 ± 19 | 0.005 | 0.003 | 0.006 |
| 5 | Parshall flume | 1060 ± 212 | 247 ± 27 | 159 ± 20 | 0.001 | 0.001 | 0.037 |
| 6 | Grit chamber | 1696 ± 288 | 212 ± 20 | 141 ± 19 | 0.003 | 0.003 | 0.075 |
| 7 | Blower room | 2049 ± 350 | 247 ± 32 | 141 ± 18 | 0.003 | 0.003 | 0.019 |
| 8 | Primary split basin | 1661 ± 232 | 176 ± 21 | 106 ± 20 | 0.004 | 0.004 | 0.019 |
| 9 | Primary sedimentation tank | 1837 ± 275 | 282 ± 34 | 70 ± 18 | 0.002 | 0.002 | 0.008 |
| 10 | Selector tank | 671 ± 101 | 230 ± 28 | 71 ± 19 | 0.014 | 0.006 | 0.002 |
| 11 | Aeration tank | 2101 ± 140 | 212 ± 26 | 530 ± 80 | 0.006 | 0.030 | 0.004 |
| 12 | Secondary split basin | 742 ± 126 | 212 ± 28 | 53 ± 18 | 0.011 | 0.006 | 0.002 |
| 13 | Secondary sedimentation tank | 548 ± 99 | 71 ± 18 | 124 ± 22 | 0.001 | 0.001 | 0.049 |
| 14 | Chlorination basin | 549 ± 99 | 124 ± 24 | 53 ± 19 | 0.007 | 0.004 | 0.001 |
| 15 | Sludge thickening tank | 954 ± 180 | 106 ± 18 | 35 ± 10 | 0.001 | 0.001 | 0.003 |
| 16 | Anaerobic digestion tank | 989 ± 190 | 159 ± 25 | 141 ± 22 | 0.004 | 0.004 | 0.417 |
| 17 | Power panel building | 1290 ± 222 | 124 ± 21 | 88 ± 18 | 0.005 | 0.005 | 0.141 |
| 18 | Storage building | 706 ± 139 | 123 ± 19 | 53 ± 18 | 0.001 | 0.001 | 0.087 |
| 19 | Surveillance building | 1272 ± 229 | 177 ± 27 | 260 ± 42 | 0.004 | 0.005 | 0.154 |
| 20 | Site water supply | 1166 ± 205 | 141 ± 19 | 141 ± 21 | 0.002 | 0.001 | 0.990 |
| 21 | Office building | 671 ± 114 | 159 ± 24 | 88 ± 20 | 0.004 | 0.003 | 0.019 |
| 22 | Chemical unit | 972 ± 174 | 141 ± 18 | 106 ± 19 | 0.002 | 0.002 | 0.207 |
| 23 | Sludge drying bed | 1643 ± 300 | 159 ± 22 | 159 ± 24 | 0.005 | 0.005 | 0.990 |
| 24 | WWTP wall | 954 ± 182 | 88 ± 19 | 194 ± 31 | 0.004 | 0.004 | 0.023 |
| 25 | Background | 230 ± 33 | 35 ± 11 | ± 1035 | 0.001 | 0.001 | 0.021 |
| Total mean of fungi | ± 2351307 | 212 ± 32 | 159 ± 22 | 0.004 | 0.004 | 0.154 | |
The comparison mean of fungi concentration between spring and winter.
The comparison mean of fungi concentration between spring and autumn.
The comparison mean of fungi concentration between winter and autumn.
The lowest concentration of fungal bioaerosols in spring was observed in chlorination basin, secondary sedimentation tank, and office building (549 ± 99, 548 ± 99, and 671 ± 114 CFU m−3, respectively). The highest concentration of fungal bioaerosols in spring was linked to the screw pump room, aeration tank, blower room, screw pump, and primary sedimentation tank, ranging from 2561 ± 420 to 1837 ± 275 CFU m−3. Mean fungi concentrations are compared in Table 3 for different seasons at all sampling points. A statistically significant difference was found in the mean concentration of fungal aerosol between spring and winter (p ≤ 0.001), and also between spring and autumn (p ≤ 0.001) for all sampling points. No significant difference was observed in the mean concentration of fungal aerosol between autumn and winter for most sampling points including the screw pump room (p = 0.990), anaerobic digestion tank (p = 0.321), site water supply (p = 0.990), background (p = 0.990), and sludge drying bed (p = 0.990). However, a statistically significant difference was observed in the mean concentration of fungal aerosol between autumn and winter for the other sampling points (p ≤ 0.001).
For comparison, the findings of this work revealed that fungi concentrations ranged between 35 ± 10 and 2561 ± 420 CFU m−3 and bacteria concentration ranged from 21 ± 3 to 1738 ± 350 CFU m−3 in the three seasons, which is consistent with the results of the previous studies by Kermani et al. (2016), Jahangiri et al. (2013), and Niazi et al. (2015). Furthermore, Dutkiewicz et al. (2003) reported that the concentrations of fungi and mesophilic bacteria in a municipal sewage treatment plant in eastern Poland were within ranges of 24–140 CFU m−3 and 240–7070 CFU m−3 (Dutkiewicz et al., 2003). Brandi et al. (2000) described that bioaerosol concentrations generated from a mechanical aeration system were 560 CFU m−3 for bacteria and 1100 CFU m−3 for fungi. In addition, Kermani et al. (2016) displayed that bioaerosol concentrations in the Shahrak-e-Ghods WWTP in Tehran, Iran, ranged 62–1823 CFU per plate for bacteria and 1–50 CFU per plate for fungi at a height of about 1 m above the floor. Moreover, Niazi et al. (2015) performed a study in a WWTP in Tehran and showed that the average concentrations of bacteria measured at a height of about 1.5 m in the summer and winter were 1973 and 1016 CFU m−3, respectively. Results of a study conducted by Korzeniewska et al. (2009) in a BIO-PAK WWTP at 1.3 m above the ground level showed that bioaerosol concentrations ranged between 10 and 1000 CFU m−3. All in all, though, the concentrations found in a WWTP in Iran are in the same range in comparison to other WWTPs. Additionally, Faridi et al. (2015) showed that the mean concentrations of bacteria in the ambient air of Tehran, Iran (Tohid retirement home and a school dormitory at 1.5–5 m above the ground level), in the four seasons ranged 88–336 CFU m−3 in the active method and 15–96 CFU plate−1 h−1 in the passive method, while the mean concentrations of fungi were between 53 and 265 CFU m−3 in the active method and 8 and 40 CFU plate−1 h−1 in the passive method. Furthermore, the mean concentrations of bacteria and fungi in outdoor environments in Ankara (Turkey) were 76 ± 75 and 70 ± 68 CFU m−3, respectively (Mentese et al., 2009). These similar results between our work and that of others can most likely be explained by how meteorological conditions (high relative humidity and low temperature) can protect bacteria from UV-induced inactivation. For example, in the work of Faridi et al. (2015) the concentration of bioaerosols in the morning (8:30–10:00 a.m. local time) and evening (5:30–8:30 p.m. local time) increased because of reductions in temperature, and enhancements in relative humidity. Another reason why the concentrations of bioaerosols in outdoor air were similar between this work in Shiraz and Tehran (Niazi et al., 2015) and a petrochemical WWTP in Mahshar (Iran) (Jahangiri et al., 2013) was that the duration of sampling was similar, ranging 2–10 minutes at a flow rate of 28.30 L min−1; this was in contrast to outdoor air in the works of Faridi et al. (2015), Shams-Ghahfarokhi et al. (2014), and Kermani et al. (2015), where there was more than 60 min of sampling. Another reason why the concentrations of bioaerosols in outdoor air were slightly similar to this work in Shiraz WWTP can most likely be explained by the sampling height (lower than 1.5 m for ambient air sampling and at the human breathing zone (1.5 m) for this work), meteorological conditions (high relative humidity), kinds of sampling (active and passive), duration of sampling (2–60 min and more), kinds of plastic plates (6, 9 mm diameter and so on), duration of incubation (at 25–28°C for 2 days to 3 weeks for fungi and at 35–37°C for 24–48 h for bacteria), and sampling in different seasons (Brandi et al., 2000; Jones and Harrison, 2004; Mentese et al., 2009; Darvishzadeh et al., 2013; Jahangiri et al., 2013; Shams-Ghahfarokhi et al., 2014; Basiri et al., 2015; Faridi et al., 2015; Kermani et al., 2015; Niazi et al., 2015).
The percentages of the fungi and fungal genera in WWTP air are summarized in Fig. 2. Accordingly, the predominant genera of airborne fungi identified in the air during autumn were Aspergillus niger (16%), Aspergillus fumigatus (13%), Mucor spp. (12%), and Cephalotrichum spp. (9%). In addition, the predominant genera of airborne fungi identified in the air during winter were Monilia spp. (29%), Aspergillus niger (25%), Aspergillus fumigatus (14%), and Penicillium spp. (3%). Finally, the predominant genera of airborne fungi detected in the air during spring were Cephalotrichum spp. (43%), Alternaria spp. (22%), Penicillium spp. (11%), and Aspergillus niger (5%).
The Relationship between Meteorological Conditions and Bioaerosols
The means and the standard deviation (SD) of bacterial and fungal bioaerosols based on CFU m−3 in different sampling locations in different seasons have been shown in Fig. 3. Accordingly, a significant difference was observed between different sampling locations for the concentration of bioaerosols (bacteria and fungi).
Fig. 3.
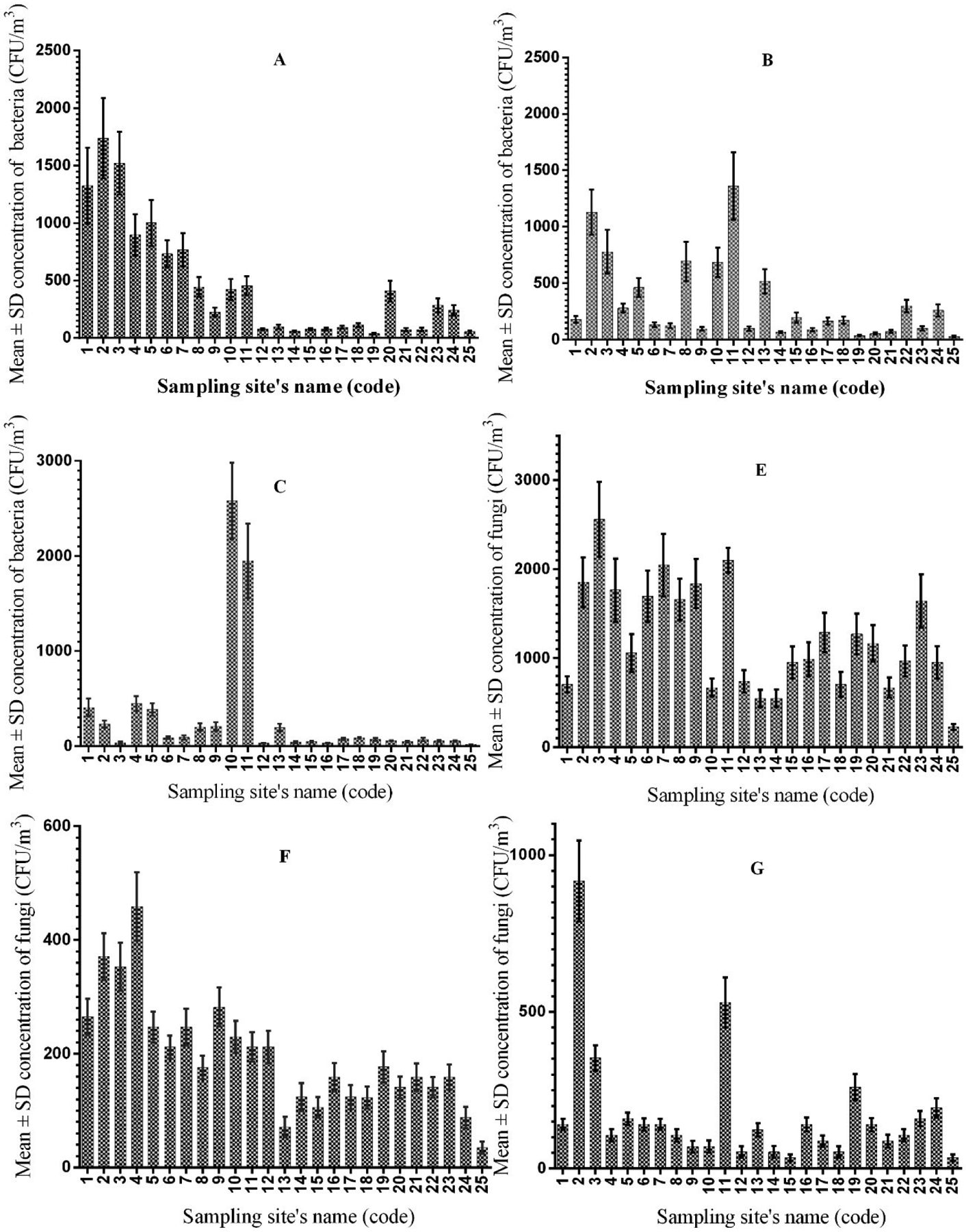
Mean and SD of bioaerosols based on CFU m−3 in different sampling locations in different seasons: (A) Concentration of bacterial aerosol in spring, (B) Concentration of bacterial aerosol in winter, (C) Concentration of bacterial aerosol in autumn, (E) Concentration of fungal aerosol in spring, (F) Concentration of fungal aerosol in winter, and (G) Concentration of fungal aerosol in autumn.
A statistically significant difference was observed between the concentration of bioaerosols in different seasons (Tables 2 and 3). Table 2 shows a statistically significant difference between the mean concentrations of bacterial aerosol in autumn and spring (p ≤ 0.001), while no significant difference was observed between autumn and winter (p = 0.651) or between winter and spring (p = 0.056). In addition, Table 3 reveals that a statistically significant difference exists between the mean concentrations of fungal aerosol when comparing winter and spring (p ≤ 0.001) and autumn and spring (p ≤ 0.001), but no significant difference was observed for the mean concentrations of bacterial aerosol between autumn and winter (p = 0.154).
The correlation between meteorological conditions and bacterial concentrations was quantified for different sampling locations in autumn, winter, and spring (Table 4). The results showed that relative humidity exhibited a significant correlation with the concentration of airborne bacteria in autumn (p < 0.05, r = 0.310) and spring (p < 0.05, r = 0.380) and no significant correlation was observed in winter (p = 0.360, r = 0.090). The results revealed a significant correlation between the concentration of bacteria and UV index in winter (p < 0.05, r = −0.270) and autumn (p < 0.05, r = −0.230) and no significant correlation was observed in spring (p > 0.05, r = 0.120). Furthermore, no significant correlation was detected between the concentration of bacteria and temperature in spring (p = 0.96, r = 0.005), winter (p = 0.080, r = 0.020) and autumn (p > 0.05, r = – 0.090). The results also revealed no significant correlation between the concentration of bacteria and wind speed and pressure in different seasons (p > 0.05). Moreover, the results showed no significant difference in the number of Gram-positive (p = 0.300) and Gram-negative bacteria (p = 0.400) in the three seasons.
Table 4.
The correlation between meteorological conditions and total concentration of bacteria and fungi in the study samples (n = 600).
| Autumn | Spring | Winter | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Te. | RH | UV | TB | TF | Te. | RH | UV | TB | TF | Te. | RH | UV | TB | TF | |||
| Te. | 1 | Te. | 1 | Te. | 1 | ||||||||||||
| RH | −0.480 | 1 | RH | −0.810 | 1 | RH | −0.710 | 1 | |||||||||
| UV | 0.580 | −0.620 | 1 | UV | 0.220 | −.050 | 1 | UV | 0.430 | −0.660 | 1 | ||||||
| TB | −0.090 | 0.310 | −0.230 | 1 | TB | 0.000 | 0.380 | 0.120 | 1 | TB | 0.020 | 0.090 | −0.270 | 1 | |||
| TF | −0.170 | −0.210 | 0.190 | 0.060 | 1 | TF | −0.080 | 0.320 | 0.100 | 0.460 | 1 | TF | −0.220 | 0.280 | −0.080 | −0.200 | 1 |
TF, total fungi; TB, total bacteria; RH, relative humidity; Te, temperature; UV, UV index
According to Table 4, a significant correlation was observed between the concentration of fungi and relative humidity in spring, autumn, and winter (p < 0.05, r = 0.320; p < 0.05, r = 0.280; and p = 0.030, r = −0.210, respectively). A significant correlation was observed between the concentration of fungi and temperature in winter (p = 0.025, r = −0.220). However, no significant correlation was found between the concentration of fungi and temperature in spring and autumn (p > 0.05, r = −0.080 and p > 0.05, r = −0.170, respectively). Moreover, the results showed no significant correlation between either wind speed, pressure or UV index with the concentration of fungi in the three seasons (p > 0.05).
WWTPs are a source of airborne bacterial contamination and constitute a source of atmospheric air pollution, which can expose workers to a biological risk (Fracchia et al., 2006; Korzeniewska et al., 2009; Korzeniewska, 2011).
Generally, many meteorological conditions have been demonstrated to influence the ability of bioaerosols to survive in the atmosphere. Among these factors, relative humidity played a key role in increasing the concentrations of airborne bacteria and fungi in the air of WWTPs (Karra and Katsivela, 2007; Korzeniewska et al., 2009; Korzeniewska, 2011).
Moreover, a previous study demonstrated that the season and weather conditions seemed to have a significant influence on the mean concentration of airborne bioaerosols in the vicinity of WWTPs (Korzeniewska et al., 2009; Niazi et al., 2015), which is in line with the findings of the present study. Our study results showed that the maximum concentration of fungi was observed in spring, since high relative humidity along with high temperature and mild wind promoted the formation of fungi. Nonetheless, Gotkowska-Płachta et al. (2013) reported higher amounts of fungi in the Ostróda WWTP (northeast of Poland) in autumn.
The findings of the current study showed no significant correlations between meteorological conditions, such as wind speed, pressure, and UV index, and the concentration of fungi. Niazi et al. (2015) also demonstrated no correlations between the concentration of fungi and wind speed, pressure, and UV index. However, our results indicated a significant correlation between the concentration of bacteria and relative humidity in autumn and spring, because high relative humidity promotes the formation of bacteria in spring (Krzysztofik, 1992).
Moreover, another study demonstrated a significant correlation between the concentration of fungi and relative humidity in winter (Niazi et al., 2015), which is in line with the findings of the present study.
The results of the present research showed that the concentration of culturable bioaerosols (bacteria and fungi) in the air depended mainly on sampling location, which is consistent with the results of the previous studies performed by Fracchia et al. (2006), Korzeniewska et al. (2009), Korzeniewska (2011), and Niazi et al. (2015).
Our study findings showed that the highest effect on the concentration of culturable bioaerosols (bacterial aerosols) was linked to the screw pump with an average of 1738 ± 350 CFU m−3 in spring (because screw pump with spiral action discharges microorganisms to the air of WWTP), the aeration tank with an average of 1363 ± 299 CFU m−3 in winter (since sewage aeration leads to discharge of larger or smaller droplets, which consist of microorganisms), and the selector tank with an average of 2581 ± 401 CFU m−3 in autumn had the highest effect on the concentration of culturable bioaerosols (bacterial aerosols).
Several reasons exist as to why the concentrations of bioaerosols (bacterial and fungi) varied in space and time. Meteorological parameters may have varied in space and time, which consequently was a limitation of our study as it is virtually impossible to hold such factors fixed in space. In addition, differences can arise due to the different types of unit processes in a WWTP and their respective variations in activity during the sampling periods. For example, mechanical mixing of wastewater by aeration, removal of suspended solids (trash) from raw wastewater by coarse screen and fine screen and pumping wastewater from 12 m under the earth surface to another unit by screw pump are very different types of processes leading to differences in measurements of bioaerosols (Table 1 and Fig. 1), which is in line with the findings of the past work (Blanchard and Syzdek, 1982; Brandi et al., 2000; Bauer et al., 2002; Pascual et al., 2003; Fernando and Fedorak, 2005; Karra and Katsivela, 2007; Heinonen-Tanski et al., 2009; Korzeniewska, 2011; Niazi et al., 2015; Cochran et al., 2016; Małecka-Adamowicz et al., 2016). Besides, seasonal factors can affect the results, and this is partly linked to the first bullet item above as meteorology can vary. For instance, enhanced rainfall intensity and humidity in spring months can lead to increased average inlet flow rate of WWTP and dry weather conditions in autumn can decrease the average inlet flow rate to the WWTP, that can influence the resulting data. Others have shown similar results (Blanchard and Syzdek, 1982; Pascual et al., 2003; Fernando and Fedorak, 2005; Karra and Katsivela, 2007; Heinonen-Tanski et al., 2009; Korzeniewska, 2011; Niazi et al., 2015).
Blanchard and Syzdek (1982) and Fernando and Fedorak (2005) described that the highest contribution to the concentration of aerosols could be ascribed to a thin surface layer of wastewater, in which inorganic and organic substances along with microorganisms were concentrated. Brandi et al. (2000) and Breza-Boruta and Paluszak (2007) reported that mechanical mixing of wastewater for aeration increased bioaerosol levels in the air. In addition, the results of the study conducted by Korzeniewska et al. (2009) in a BIO-PAK WWTP showed that bioreactor and grate chamber units were the main sources of bioaerosol emissions.
Our results indicate that equipment earlier in the process of WWTP (pretreatment and primary clarifier units and those containing moving mechanical equipment for wastewater aeration) influence pollution load and dispersion of bioaerosols more than later processes, which is consistent with past work (Blanchard and Syzdek, 1982; Pascual et al., 2003; Fernando and Fedorak, 2005; Karra and Katsivela, 2007; Heinonen-Tanski et al., 2009; Korzeniewska, 2011; Jahangiri et al., 2013; Kermani and Asl, 2015; Mirzaee et al., 2015; Niazi et al., 2015; Kermani et al., 2016). In addition, the findings show that the main parameters influencing emission of bacterial and fungi in the wastewater treatment plant in earlier processes (as compared to the later processes) were turbulence and tremor in wastewater, rainfall, relative humidity, wind speed and direction, which is consistent with past work (Pascual et al., 2003; Karra and Katsivela, 2007; Heinonen-Tanski et al., 2009; Korzeniewska, 2011; Niazi et al., 2015). Moreover, different weather conditions in Shiraz, especially in spring, with rainfall intensity with high humidity, and dry weather conditions in autumn, with low humidity, can influence dispersion of bioaerosols. Relative humidity seems to have the highest correlation with the concentration of bacteria, presumably because relative humidity in spring can protect bacteria from UV-induced inactivation as compared to autumn when there is low humidity; these findings are consistent with the results of the previous studies performed by Korzeniewska (2011) and Niazi et al. (2015).
In addition, some studies in Iran at WWTPs showed that the concentrations of fungi and bacteria in warm months (summer and spring) are 1.5–8 times more than cold months (winter) (Niazi et al., 2015; Kermani et al., 2016). Furthermore, some studies around the world reported that the concentrations of fungi and bacterial in warm months in WWTPs are more than in winter months, which is in line with previous studies (Fang et al., 2005; Oppliger et al., 2005; Breza-Boruta and Paluszak, 2007; Grisoli et al., 2009; Korzeniewska, 2011; Niazi et al., 2015; Kermani et al., 2016).
Results of the present study showed that the percentage of Gram-negative bacteria was higher compared to Gram-positive bacteria in winter since Gram-positive bacteria are more tolerant to dryness and, consequently, can survive longer in the air (Fang et al., 2005).
The Polish standard guideline for acceptable levels of number of fungi in clean air is 3000 CFU m−3 (PN-89Z-04111/03) (Michałkiewicz et al., 2011). In another guideline proposed by the Polish standard (PN-89Z-04111/02), the number of bacteria less than 1000 CFU m−3, from 1000 to 3000 CFU m−3, and more than 3000 CFU m−3 indicates no pollution, average pollution, and strong pollution, respectively (Michałkiewicz et al., 2011). Moreover, the guidelines proposed by Swiss OELs have set 1000 CFU m−3 for fungi and 10,000 CFU m−3 for total cultivable bacteria in WWTPs (Oppliger et al., 2005a). However, standard values for the concentration of bioaerosols in WWTPs have not been established in Iran and state and local agencies have no such monitoring programs yet. In our study, the concentration of bacterial aerosols in different seasons was higher compared to the recommended value by the Polish standard (1000–3000 CFU m−3) (Michałkiewicz et al., 2011) in 14% of the sampling points, while the concentration of fungi was lower than the suggested values by the Polish standard (3000 CFU m−3) (Michałkiewicz et al., 2011) and Swiss OELs (10,000 CFU m−3) (Oppliger et al., 2005) in all sampling points. In contrast, Oppliger et al. (2005) reported that more than 50% of the sewage treatment plants exceeded the recommended Swiss occupational threshold for fungi (1000 CFU m−3) in summer.
In the present research, the predominant genera of airborne fungi isolated in the air of the studied WWTP in different seasons were Cephalotrichum spp., Alternaria spp., Penicillium spp., Monilia spp., and Aspergillus spp. Similarly, Korzeniewska et al. (2009) reported that the dominant fungi types were Alternaria spp., Cladosporium spp., Penicillium spp., and Aspergillus spp. In addition, Niazi et al. (2015) and Teixeira et al. (2013) indicated that the predominant genera of airborne fungi identified in the air of WWTPs were Cladosporium spp., Penicillium spp., Aspergillus spp., and Alternaria spp. Li et al. (2011) also reported Aspergillus and Penicillium as being the most commonly identified fungi whose high concentrations in the air of WWTPs were potentially hazardous to humans. Additionally, the predominant genera of airborne fungi isolated from the background station in the three seasons were Aspergillus spp., Penicillium spp., and Myselium, while Niazi et al. (2015) reported that the predominant genera of airborne fungi identified in the background location were Cephalotrichum spp., Alternaria spp., Aspergillus spp., Penicillium spp., and Rhodotorula spp. Furthermore, the results of current study show that the genera of airborne fungi in the background location were lower than other sampling locations, which is in line with the findings of the past work (Niazi et al., 2015).
Overall, our results highlighted the necessity to control WWTP workers’ exposure to bioaerosols, especially in processing areas involving forced aeration of wastewater by mechanical agitation. The limitation of this study was that only the total concentrations of bacteria were measured. Although the concentration of airborne bacteria in the environment may serve as an indicator of microbial pollution, human pathogenic bacteria that are capable of causing illnesses even in low concentrations may still be present.
CONCLUSION
This study reports on the nature of bioaerosol at a WWTP in southwestern Iran, including detailed concentration profiles for different areas of the WWTP in different seasons. The results indicate that significant concentrations of bioaerosol (bacteria and fungi) exist in the air in the vicinity of a WWTP. In particular, the aeration tank, screw pump, and selector tank produced very high bioaerosol levels. The concentrations of bacteria and fungi were 2 and 8–10 times more abundant in spring than in winter and autumn, respectively. Moreover, increased counts of bacterial aerosols and higher species diversity of fungal bioaerosols, including Cladosporium spp., Alternaria spp., Penicillium spp., Monilia spp., and Aspergillus spp., in the air of the examined WWTP indicate that the health of workers who stay inside the plant for prolonged periods of time could be at risk. This work highlights the importance of reducing WWTP emissions of bacteria and fungi.
ACKNOWLEDGMENTS
The authors are grateful to Shiraz WWTP for providing the sampling locations. Armin Sorooshian acknowledges support from Grant 2 P42 ES04940 from the National Institute of Environmental Health Sciences (NIEHS) Superfund Research Program.
Footnotes
COMPETING INTEREST
The authors declare that they have no competing interests.
REFERENCES
- Basiri H, Godini H and Omidi-Khaniabadi Y (2015). Study of indoor and ambient air fungual bioaerosols and its relation with particulate matters in a hospital of khorramabad. yafte 17: 25–34. [Google Scholar]
- Bauer H, Fuerhacker M, Zibuschka F, Schmid H and Puxbaum H (2002). Bacteria and fungi in aerosols generated by two different types of wastewater treatment plants. Water Res. 36: 3965–3970. [DOI] [PubMed] [Google Scholar]
- Benson HJ (2007). Benson’s microbiological applications: Laboratory manual in general microbiology. McGraw-Hill Higher Education, New York. [Google Scholar]
- Blanchard DC and Syzdek LD (1982). Water-to-air transfer and enrichment of bacteria in drops from bursting bubbles. Appl. Environ. Microbiol 43: 1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandi G, Sisti M and Amagliani G (2000). Evaluation of the environmental impact of microbial aerosols generated by wastewater treatment plants utilizing different aeration systems. J. Appl. Microbiol 88: 845–852. [DOI] [PubMed] [Google Scholar]
- Breza-Boruta B and Paluszak Z (2007). Influence of water treatment plant on microbiological composition of air bioaerosol. Pol. J. Environ. Stud 16: 663–670. [Google Scholar]
- Cochran RE, Laskina O, Jayarathne T, Laskin A, Laskin J, Lin P, Sultana C, Lee C, Moore KA and Cappa CD (2016). Analysis of organic anionic surfactants in fine and coarse fractions of freshly emitted sea spray aerosol. Environ. Sci. Technol 50: 2477–2486. [DOI] [PubMed] [Google Scholar]
- Cruyt F, Sousa CA, Machado MD and Soares EV (2017). Improvement of the slide culture technique for the assessment of yeast viability. J. Inst. Brew 123: 39–44. [Google Scholar]
- Darvishzadeh N, Golbabaee F, Pourmand M, Zeini F and Rahimi Foroushani A (2013). Evaluation of bioaerosol in a hospital in Tehran. Iran. J. Health Environ 6: 23–32. [Google Scholar]
- Dehghani M, Fazlzadeh M, Sorooshian A, Tabatabaee HR, Miri M, Baghani AN, Delikhoon M, Mahvi AH and Rashidi M (2018). Characteristics and health effects of BTEX in a hot spot for urban pollution. Ecotoxicol. Environ. Saf 155: 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delikhoon M, Fazlzadeh M, Sorooshian A, Baghani AN, Golaki M, Ashournejad Q and Barkhordari A (2018). Characteristics and health effects of formaldehyde and acetaldehyde in an urban area in Iran. Environ. Pollut 242: 938–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diba K, Kordbacheh P, Mirhendi S, Rezaie S and Mahmoudi M (2007). Identification of aspergillus species using morphological characteristics. Pak. J. Med. Sci 23: 867–872. [Google Scholar]
- Dutkiewicz J, Cholewa G, Sitkowska J, Krysinska-Traczyk E, Skorska C and Prazmo Z (2003). Exposure to bioaerosols in a municipal sewage treatment plant. Ann. Agric. Environ. Med 10: 241–248. [PubMed] [Google Scholar]
- EPA (2006). Alternate 1 in 3 sampling and returns hipping schedule, http://www.epa.gov/ttn/amtic/files/ambient/pm25/2006altspec.pdf.
- Fang Z, Ouyang Z, Hu L, Wang X, Zheng H and Lin X (2005). Culturable airborne fungi in outdoor environments in Beijing, China. Sci. Total Environ 350: 47–58. [DOI] [PubMed] [Google Scholar]
- Faridi S, Hassanvand MS, Naddafi K, Yunesian M, Nabizadeh R, Sowlat MH, Kashani H, Gholampour A, Niazi S and Zare A (2015). Indoor/outdoor relationships of bioaerosol concentrations in a retirement home and a school dormitory. Environ. Sci. Pollut. Res 22: 8190–8200. [DOI] [PubMed] [Google Scholar]
- Fazlzadeh M, Sadeghi H, Bagheri P, Poureshg Y and Rostami R (2016). Microbial quality and physical-chemical characteristics of thermal springs. Environ. Geochem. Health 38: 413–422. [DOI] [PubMed] [Google Scholar]
- Fernando NL and Fedorak PM (2005). Changes at an activated sludge sewage treatment plant alter the numbers of airborne aerobic microorganisms. Water Res. 39: 4597–4608. [DOI] [PubMed] [Google Scholar]
- Filipkowska Z, Gotkowska-Plachta A, Janczukowicz W and Korzeniewska E (2008). Microbiological contamination of atmospheric air in the constructed wetland (with aerated and stabilization ponds and in its surrounding). Water Environ. Rural Areas 8: 83–98. [Google Scholar]
- Fracchia L, Pietronave S, Rinaldi M and Martinotti MG (2006). Site-related airborne biological hazard and seasonal variations in two wastewater treatment plants. Water Res. 40: 1985–1994. [DOI] [PubMed] [Google Scholar]
- Gerardi MH and Zimmerman MC (2004). Wastewater pathogens. John Wiley & Sons, New York, USA. [Google Scholar]
- Gotkowska-Płachta A, Filipkowska Z, Korzeniewska E, Janczukowicz W, Dixon B, Gołaś I and Szwalgin D (2013). Airborne microorganisms emitted from wastewater treatment plant treating domestic wastewater and meat processing industry wastes. Clean–Soil Air Water 41: 429–436. [Google Scholar]
- Goudarzi G, Shirmardi M, Khodarahmi F, Hashemi-Shahraki A, Alavi N, Ankali KA, Babaei AA, Soleimani Z and Marzouni MB (2014). Particulate matter and bacteria characteristics of the Middle East Dust (MED) storms over Ahvaz, Iran. Aerobiologia 30: 345–356. [Google Scholar]
- Goudarzi G, Soleimani Z, Sadeghinejad B, Alighardashi M, Latifi SM and Moradi M (2016). The impact of visiting hours on indoor to outdoor ratio of fungi concentration at university hospitals in Ahvaz, Iran. J. Adv. Environ. Health Res 4: 1–8. [Google Scholar]
- Grisoli P, Rodolfi M, Villani S, Grignani E, Cottica D, Berri A, Picco AM and Dacarro C (2009). Assessment of airborne microorganism contamination in an industrial area characterized by an open composting facility and a wastewater treatment plant. Environ. Res 109: 135–142. [DOI] [PubMed] [Google Scholar]
- Heinonen-Tanski H, Reponen T and Koivunen J (2009). Airborne enteric coliphages and bacteria in sewage treatment plants. Water Res. 43: 2558–2566. [DOI] [PubMed] [Google Scholar]
- Huang HL, Lee MK and Shih HW (2017). Assessment of indoor bioaerosols in public spaces by real-time measured airborne particles. Aerosol Air Qual. Res 17: 2276–2288. [Google Scholar]
- Jahangiri M, Neghab M, Kahdemain V, Rostami R, Karimi A, Aghabeigi M and Kasayee Nasab A (2013). Investigating density and type of bioaerosols in a petrochemical wastewater treatment plant: Mahshar-Iran, 2013. Iran. J. Health Environ 6: 113–122. [Google Scholar]
- Jones AM and Harrison RM (2004). The effects of meteorological factors on atmospheric bioaerosol concentrations—A review. Sci. Total Environ 326: 151–180. [DOI] [PubMed] [Google Scholar]
- Kallawicha K, Lung SCC, Chuang YC, Wu CD, Chen TH, Tsai YJ and Chao HJ (2015). Spatiotemporal distributions and land-use regression models of ambient bacteria and endotoxins in the greater Taipei area. Aerosol Air Qual. Res 15: 1448–1459. [Google Scholar]
- Kallawicha K, Chen YC, Chao HJ, Shen WC, Chen BY, Chuan YC and Guo YL (2017). Ambient fungal spore concentration in a subtropical metropolis: Temporal distributions and meteorological determinants. Aerosol Air Qual. Res 17: 2051–2063. [Google Scholar]
- Karra S and Katsivela E (2007). Microorganisms in bioaerosol emissions from wastewater treatment plants during summer at a Mediterranean site. Water Res. 41: 1355–1365. [DOI] [PubMed] [Google Scholar]
- Kermani M and Asl FB (2015). Concentration and distribution of airborne fungi in the ambient air of Milad hospital, blood transfusion organization, and shahrake ghods wastewater treatment plant in Tehran, Iran. J. Health Res. Community 1: 1–8. [Google Scholar]
- Kermani M, Dehghani A, Farzadkia M, Asl FB and Zeinalzadeh D (2016). Assessment of bioaerosol contamination in an urban wastewater treatment plant in Tehran, Iran. J. Air Pollut. Health 1: 161–170. [Google Scholar]
- Korzeniewska E, Filipkowska Z, Gotkowska-Płachta A, Janczukowicz W, Dixon B and Czułowska M (2009). Determination of emitted airborne microorganisms from a BIO-PAK wastewater treatment plant. Water Res. 43: 2841–2851. [DOI] [PubMed] [Google Scholar]
- Korzeniewska E (2011). Emission of bacteria and fungi in the air from wastewater treatment plants—A Review. Front. Biosci 3: 393–407. [DOI] [PubMed] [Google Scholar]
- Krzysztofik B (1992). Microbiology of air. Inzynieria Srodowiska (Poland). [Google Scholar]
- Lebrero R, Bouchy L, Stuetz R and Munoz R (2011). Odor assessment and management in wastewater treatment plants: A review. Crit. Rev. Env. Sci. Technol 41: 915–950. [Google Scholar]
- Li L, Gao M and Liu J (2011). Distribution characterization of microbial aerosols emitted from a wastewater treatment plant using the Orbal oxidation ditch process. Process Biochem. 46: 910–915. [Google Scholar]
- Li L, Han Y and Liu J (2013). Assessing genetic structure, diversity of bacterial aerosol from aeration system in an oxidation ditch wastewater treatment plant by culture methods and bio-molecular tools. Environ. Monit. Assess 185: 603–613. [DOI] [PubMed] [Google Scholar]
- Lin TH, Chiang CF, Lin ST and Tsai CT (2016). Effects of small-size suspended solids on the emission of Escherichia Coli from the aeration process of wastewater treatment. Aerosol Air Qual. Res 16: 2208–2215. [Google Scholar]
- Macher JM (1989). Positive-hole correction of multiple-jet impactors for collecting viable microorganisms. Am. Ind. Hyg. Assoc. J 50: 561–568. [DOI] [PubMed] [Google Scholar]
- Madsen AM, Schlünssen V, Olsen T, Sigsgaard T and Avci H (2009). Airborne fungal and bacterial components in PM1 dust from biofuel plants. Ann. Occup. Hyg 53: 749–757.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Małecka-Adamowicz M, Kubera Ł, Donderski W and Kolet K (2016). Microbial air contamination on the premises of the sewage treatment plant in Bydgoszcz (Poland) and antibiotic resistance of Staphylococcus spp. Arch. Environ. Prot 43: 58–65. [Google Scholar]
- Mentese S, Arisoy M, Rad AY and Güllü G (2009). Bacteria and fungi levels in various indoor and outdoor environments in Ankara, Turkey. Clean–Soil Air Water 37: 487–493. [Google Scholar]
- Michałkiewicz M, Pruss A, Dymaczewski Z, Jeż-Walkowiak J and Kwaśna S (2011). Microbiological air monitoring around municipal wastewater treatment plants. Pol. J. Environ. Stud 20: 1243–1250. [Google Scholar]
- Mirzaee SA, Nikaeen M, Hajizadeh Y, Nabavi BF and Hassanzadeh A (2015). Detection of Legionella spp. by a nested-PCR assay in air samples of a wastewater treatment plant and downwind distances in Isfahan. Adv. Biomed. Res 4: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neghab M, Delikhoon M, Norouzian Baghani A and Hassanzadeh J (2017). Exposure to Cooking fumes and acute reversible decrement in lung functional capacity. Int. J. Occup. Environ. Med 8: 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niazi S, Hassanvand MS, Mahvi AH, Nabizadeh R, Alimohammadi M, Nabavi S, Faridi S, Dehghani A, Hoseini M and Moradi-Joo M (2015). Assessment of bioaerosol contamination (bacteria and fungi) in the largest urban wastewater treatment plant in the Middle East. Environ. Sci. Pollut. Res 22: 16014–16021. [DOI] [PubMed] [Google Scholar]
- Oppliger A, Hilfiker S and Vu Duc T (2005). Influence of seasons and sampling strategy on assessment of bioaerosols in sewage treatment plants in Switzerland. Ann. Occup. Hyg 49: 393–400. [DOI] [PubMed] [Google Scholar]
- Orsini M, Laurenti P, Boninti F, Arzani D, Ianni A and Romano-Spica V (2002). A molecular typing approach for evaluating bioaerosol exposure in wastewater treatment plant workers. Water Res. 36: 1375–1378. [DOI] [PubMed] [Google Scholar]
- Pascual L, Pérez-Luz S, Yáñez MA, Santamaría A, Gibert K, Salgot M, Apraiz D and Catalán V (2003). Bioaerosol emission from wastewater treatment plants. Aerobiologia 19: 261–270. [Google Scholar]
- Ranalli G, Principi P and Sorlini C (2000). Bacterial aerosol emission from wastewater treatment plants: Culture methods and bio-molecular tools. Aerobiologia 16: 39–46. [Google Scholar]
- Rosana Y, Matsuzawa T, Gonoi T and Karuniawati A (2014). Modified slide culture method for faster and easier identification of dermatophytes. Microbiol. Indonesia 8: 135–139. [Google Scholar]
- Rostami R, Naddafi K, Aghamohamadi A, Saleh HN and Davil MF (2009). Survey of peanut fungal contamination and its relationship with ambient conditions in the Bazar of Zanjan. Iran. J. Environ. Health Sci. Eng 6: 295–300. [Google Scholar]
- Rylander R (1999). Health effects among workers in sewage treatment plants. Occup. Environ. Med 56: 354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Monedero M, Aguilar M, Fenoll R and Roig A (2008). Effect of the aeration system on the levels of airborne microorganisms generated at wastewater treatment plants. Water Res. 42: 3739–3744. [DOI] [PubMed] [Google Scholar]
- Schaad NW, Jones JB and Chun W (2001). Laboratory guide for identification of plant pathogenic bacteria. American Phytopathological Society (APS Press). St. Paul, MN, USA. [Google Scholar]
- Shams-Ghahfarokhi M, Aghaei-Gharehbolagh S, Aslani N and Razzaghi-Abyaneh M (2014). Investigation on distribution of airborne fungi in outdoor environment in Tehran, Iran. J. Environ. Health Sci. Eng 12: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit LA, Spaan S and Heederik D (2005). Endotoxin exposure and symptoms in wastewater treatment workers. Am. J. Ind. Med 48: 30–39. [DOI] [PubMed] [Google Scholar]
- Tarigan YG, Chen RY, Lin HC, Jung CY, Kallawicha K, Chang TP, Hung PC, Chen CY and Chao HJ (2017). Fungal bioaerosol exposure and its effects on the health of mushroom and vegetable farm workers in Taiwan. Aerosol Air Qual. Res 17: 2064–2075. [Google Scholar]
- Teixeira JV, Miranda S, Monteiro RA, Lopes FV, Madureira J, Silva GV, Pestana N, Pinto E, Vilar VJ and Boaventura RA (2013). Assessment of indoor airborne contamination in a wastewater treatment plant. Environ. Monit. Assess 185: 59–72. [DOI] [PubMed] [Google Scholar]
- Thorn J and Kerekes E (2001). Health effects among employees in sewage treatment plants: A literature survey. Am. J. Ind. Med 40: 170–179. [DOI] [PubMed] [Google Scholar]
- Thorn J, Beijer L, Jonsson T and Rylander R (2002). Measurement strategies for the determination of airborne bacterial endotoxin in sewage treatment plants. Ann. Occup. Hyg 46: 549–554. [DOI] [PubMed] [Google Scholar]
- Turner S, Hopkinson J, Oxley L, Gadd S, Healey N and Marlow P (2008). Collecting, transfer, treatment and processing household waste and recyclables. HSE Research Report RR609.
- Uhrbrand K, Schultz AC and Madsen AM (2011). Exposure to airborne noroviruses and other bioaerosol components at a wastewater treatment plant in Denmark. Food Environ. Virol 3: 130–137. [Google Scholar]
- Vítězová M, Vítěz T, Mlejnková H and Lošák T (2013). Microbial contamination of the air at the wastewater treatment plant. Acta Univ. Agric. Silvic. Mendelianae Brun 60: 233–240. [Google Scholar]
- Yassin M and Almouqatea S (2010). Assessment of airborne bacteria and fungi in an indoor and outdoor environment. Int. J. Environ. Sci. Technol 7: 535–544. [Google Scholar]
- Yoshida K, Ando M, Sakata T and Araki S (1989). Prevention of summer-type hypersensitivity pneumonitis: Effect of elimination of Trichosporon cutaneum from the patients’ homes. Arch. Environ. Health 44: 317–322. [DOI] [PubMed] [Google Scholar]


